Published online Apr 26, 2024. doi: 10.4252/wjsc.v16.i4.334
Peer-review started: December 4, 2023
First decision: January 30, 2024
Revised: February 20, 2024
Accepted: March 12, 2024
Article in press: March 12, 2024
Published online: April 26, 2024
Processing time: 143 Days and 1.3 Hours
Wound repair is a complex challenge for both clinical practitioners and resear
Core Tip: In this work, we provided a comprehensive review of the current development and application of biological scaffolds for stem cells in wound healing, emphasizes the scaffolds’ capacity to facilitate stem cell adhesion, proliferation, differentiation, and paracrine function, identifies the pivotal characteristics of scaffolds that contribute to enhanced cellular activities.
- Citation: Xiang JY, Kang L, Li ZM, Tseng SL, Wang LQ, Li TH, Li ZJ, Huang JZ, Yu NZ, Long X. Biological scaffold as potential platforms for stem cells: Current development and applications in wound healing. World J Stem Cells 2024; 16(4): 334-352
- URL: https://www.wjgnet.com/1948-0210/full/v16/i4/334.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i4.334
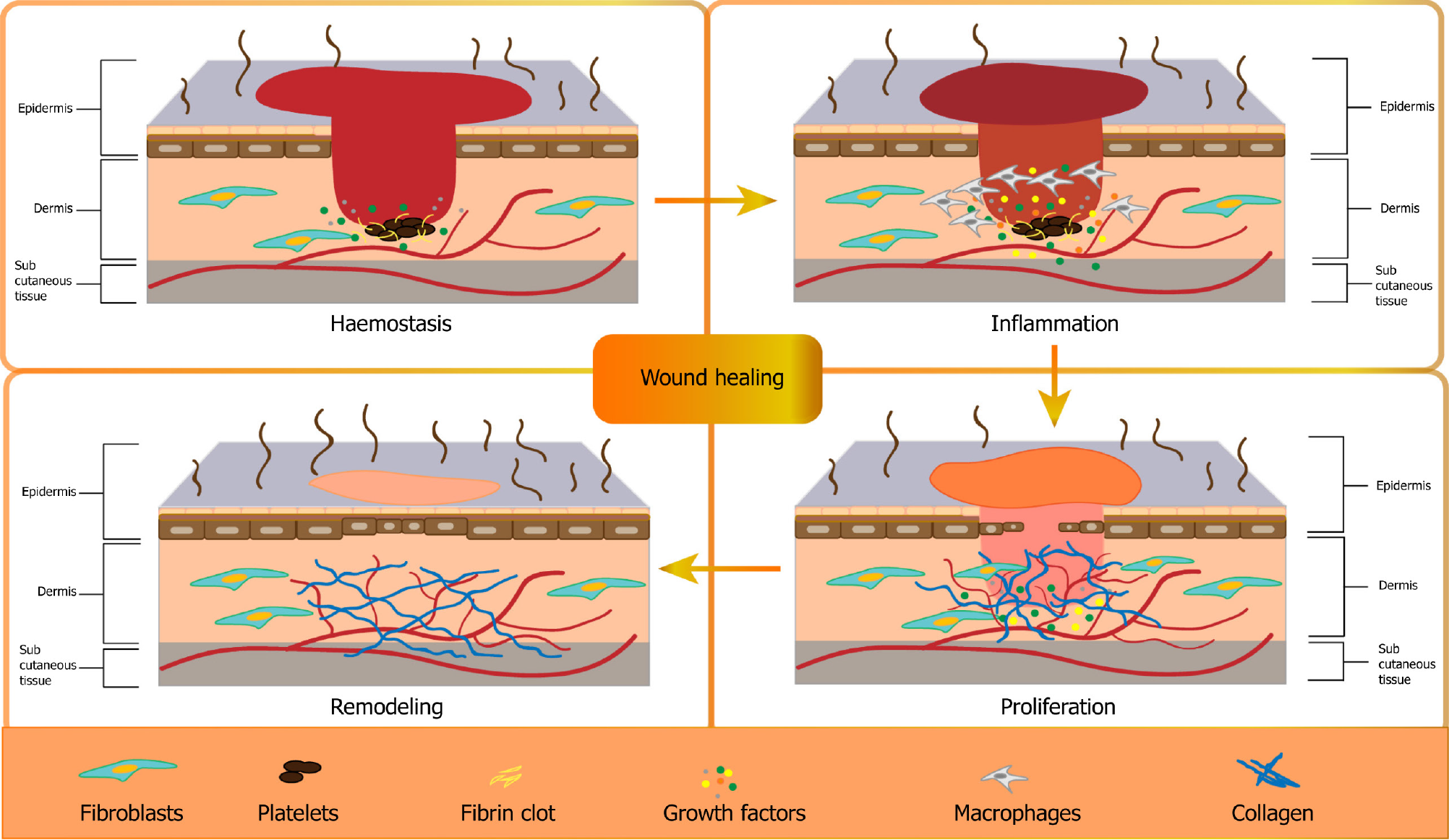
As the body’s largest organ, the skin is critically important for safeguarding the body, and any disruption in its integrity can result in acute or chronic wounds[1]. The intricate process of wound healing involves various stages and necessitates proper coordination[2,3]. Wound healing is a complex biological process involving four main phases: Hemostasis, inflammation, proliferation, and remodeling[2] (Figure 1). Hemostasis is initiated by platelet activation and the release of growth factors such as platelet-derived growth factor (PDGF), transforming growth factors (TGFs), fibroblast growth factors (FGFs), and vascular endothelial growth factor (VEGF). Inflammation is characterized by fibrin clot formation and the release of chemotactic factors that attract leukocytes, particularly neutrophils, to the wound site[2,4]. Monocytes differentiate into macrophages (Mø) and secrete cytokines that are essential for the proliferative healing phase. Lymphocytes also contribute to this process by producing interleukin-2, which recruits fibroblasts[5]. Epithelialization involves the migration and proliferation of keratinocytes, aided by EGF, keratinocyte growth factors, and TGF-α[6]. Angiogenesis, triggered by endothelial cell migration and tube formation, is supported by angiogenic factors such as VEGF and PDGF. Finally, in the remodeling phase, inflammatory cells depart, and fibroblasts continue to synthesize collagen[7,8].
Numerous conventional and regenerative studies have been dedicated to achieving effective wound therapies, aiming to reduce health costs and ensure successful scar healing and long-term relief[9,10]. In recent years, regenerative medicine and tissue engineering therapy combined with stem cells and biomaterial scaffolds have gained popularity[11-13]. The challenges of wound healing in the field of medicine have prompted the development of new approaches that combine the use of stem cells and biomaterials[9,14,15]. Although stem-cell-based therapy has emerged as a novel strategy, showing significant potential in promoting skin regeneration, it also faces challenges such as poor survival and differentiation of the transplanted cells, which tissue engineering seeks to address[9,16]. Biomaterials, which are known for their ability to control stem cell functions and enhance their regenerative potential, have garnered attention. Biological scaffolds play a crucial role in facilitating the adhesion of stem cells by providing surfaces for cell attachment, proliferation, differentiation, and paracrine functions[17,18]. The combination of stem cells with specifically designed novel biomaterials results in different effects on the engineered skin after wounding. This review provides a comprehensive overview of the current developments and applications of biological scaffolds for stem cells and their derivatives in wound healing. It highlights the effect of scaffolds on stem cell behavior and identifies the key characteristics of scaffolds responsible for improved cell activity when used in combination with biomaterials. In addition, future research directions in skin tissue engineering focusing on further clinical applications and customized designs are discussed.
Cellulose: Cellulose, a pivotal biopolymer, is an essential component of plant cell walls, fungi, and algae and is widely utilized in industries such as paper, tissue engineering, biosensors, agriculture, and water purification owing to its structural benefits[19-21]. Unlike plant cellulose, which contains impurities such as lignin and hemicellulose, bacterial cellulose (BC) produced by strains such as Acetobacter and Sarcina ventriculi stands out in the biomedical field because of its purity, crystallinity, and superior physical properties[22-27]. The unique nanofibril network of BC ensures high porosity and surface area, promoting exceptional liquid absorption and retention. Its biocompatibility, transparency, hydrophilicity, and non-toxicity make it ideal for medical applications, especially in tissue engineering[26,28,29].
Cellulose-based scaffolds maintain an optimally moist environment, effectively absorb exudates, and facilitate the removal of debris, including necrotic tissue and fibrinous coatings, which are crucial aspects of wound care[28]. Furthermore, BC-based dressings act as a barrier, effectively preventing infections by blocking the infiltration of bacteria into the wound site, thus minimizing the risk of infection[30,31]. One study by Soliman et al[32] assessed the impact of a GO-cellulose nanocomposite on skin wound healing. The GO-cellulose-treated groups displayed thick granulation tissue and intense collagen deposition. In another study, Keskin et al[33] created a novel natural nanocomposite for skin tissue engineering by incorporating keratin into the BC produced by Acetobacter xylinum. Furthermore, cellulose materials can be functionalized or combined with other substances to enhance their properties, support soft-tissue regeneration, and reduce the risk of infection. Mao et al[34] developed innovative nanocomposite hydrogels based on BC, gelatin, and selenium nanoparticles for wound-healing applications. The hydrogel exhibited superior antioxidant and anti-inflammatory capabilities and outstanding antibacterial activity. In the field of hard tissue engineering, Cao et al[35] crafted a nanocomposite scaffold by reinforcing BC with chitosan (CS) and nano-hydroxyapatite (nHA), enhancing its mechanical strength, degradation rate, and water retention compared to a CS/nHA-only scaffold. An in-vivo experiment on rat skull defects demonstrated the ability of the scaffold to stimulate bone formation. Gu et al[36] developed BC/gelatin methacryloyl (GelMA) composite hydrogels characterized by high porosity and an interconnected structure, making them ideal for cartilage tissue engineering. Furthermore, chondrocytes encapsulated in GelMA/BC hydrogels not only flourished but also retained their natural phenotype, highlighting the potential of the composite for cartilage regeneration applications.
Alginates: Alginates are naturally occurring polysaccharides derived from brown marine algae and bacteria and are widely used as wound dressings[37]. When an alginate-containing dressing comes into contact with the wound exudate, it initiates an ion exchange process, leading to an interaction between calcium ions within the alginate and sodium ions present in the blood or exudate. As a result, the alginate fibers undergo swelling, partial dissolution, and gel formation when a sufficient number of calcium ions are replaced by sodium ions. Their application in wound healing leverages the natural biopolymer properties of alginates to fulfill various functions in wound care[38]. However, alginates exhibit a notable solubility in water, which may affect their stability in specific applications. Furthermore, alginates tend to swell when exposed to water, potentially constraining their practicality in certain cases[39-41]. In a recent study, Zhang et al[42] developed sodium alginate-CS oligosaccharide-zinc oxide, a novel composite hydrogel, through a spontaneous schiff base reaction. It offers a moist and antibacterial environment and displays wound healing effects. Similarly, in another study by Mndlovu et al[43], a three-step approach involving partial crosslinking, freeze-drying, and pulverization was employed to fabricate a particulate, partially crosslinked, and transferulic acid-loaded CS-alginate interpolymer complex with enhanced wound-healing capabilities. This bioplatform can be used as a bioactive delivery system. When applied to a wound in the form of sprinkles, it undergoes in-situ hydrogel formation upon exposure to fluids, ultimately enhancing the wound healing process. Moreover, alginate-based hydrogel carriers, ranging from injectable to bioprinting materials, can encapsulate bioactive cells and molecules. This technology can be directly applied to damaged tissues and supports tissue repair, both in vivo and in vitro[44].
Hyaluronic acid: Hyaluronic acid (HA), a naturally occurring glycosaminoglycan found in the skin, plays a pivotal role in wound healing. HA-based hydrogels have been extensively researched for application in wound dressing because of their inherent properties, such as biocompatibility, ability to promote fibroblast proliferation for the formation of connective tissue, and capacity to facilitate keratinocyte migration, aiding in re-epithelialization[45]. However, the mechanical strength of HA is relatively low, which renders it unsuitable for certain applications that require high strength. Moreover, its solubility in specific solvents is limited and may be subject to restrictions based on specific conditions[46,47]. Orellana et al[48] utilized a freeze-drying technique to create sponges comprising CS/alginate with the addition of HA, resulting in a microporous structure conducive to cell adhesion and proliferation. Bai et al[49] engineered a hydrogel by combining natural macromolecules, gallic acid-grafted quaternized CS, and oxidized HA. The hydrogel displayed remarkable antioxidant properties and beneficial effects on cell migration in vitro. Additionally, the HA in the hydrogel exhibits pro-angiogenic effects, supporting the development of new blood vessels that are crucial for tissue repair and regeneration.
CS: CS, a cationic polysaccharide derived from chitin, is widely used owing to its remarkable therapeutic attributes, rendering it an ideal choice for wound dressings. Its advantages include antibacterial properties, hemostatic effects, analgesic attributes, biodegradability, and compatibility with blood[50]. However, CS exhibits relatively low strength and toughness, which may render it unsuitable for applications requiring excellent mechanical properties. Additionally, its solubility in aqueous solutions is limited and is subject to specific conditions[51,52]. Carboxymethyl-CS (CMC), a water-soluble and biocompatible CS derivative, fosters cell interactions, leading to successful cell growth, tissue regeneration, and wound healing[53].
CS and its derivatives have garnered substantial attention owing to their roles in promoting wound healing and fostering a healing-conducive environment. Recently, Xu et al[54] reported a multifunctional cryogel composed of CS, silver, and tannic acid (CS/Ag/TA). The prepared CS/Ag/TA cryogel not only exhibited consistent stability and compressibility but also demonstrated significant antibacterial and hemostatic capabilities, achieving hemostasis in less than 20 s. Additionally, Li et al[55] introduced in situ injectable, self-healing, antibacterial, hemostatic, and biocompatible hydrogels derived from a CMC hybrid. This hydrogel showcases outstanding antibacterial properties that were primarily attributed to the inherent antibacterial characteristics of CMC. The hydrogel adhered tightly to biological tissues and exhibited excellent in vivo hemostatic performance. In the field of bone-tissue repair, Saravanan et al[56] developed a thermosensitive CS hydrogel with graphene oxide that demonstrated biocompatibility and metabolic activity in mesenchymal stem cells (MSCs). It enhanced osteogenic differentiation, and is a promising platform for bone regeneration. Additionally, CS serves as an effective adhesive agent for enhancing the clinical outcomes of dental restorations. Diolosà et al[57] demonstrated that modified CS, when incorporated into the “etch-and-rinse” adhesive system, significantly boosts the longevity of dental restorations.
Collagen: Collagen, a crucial protein in the extracellular matrix (ECM) of connective tissues, plays a central role in wound healing. When applied to wounds, collagen dressings foster a moist environment that actively supports various aspects of the healing process[58]. However, collagen possesses relatively modest mechanical properties, rendering it unsuitable for certain high-strength applications. It may also cause allergies. In addition, collagen exhibits limited solubility in water and requires specific solvent conditions[59-62]. Jiang et al[63] developed a collagen fibril hydrogel that significantly promoted wound healing and showed remarkable potential for aiding skin tissue regeneration. Another study by Chen et al[64] compared the efficacy of a pullulan-collagen dressing with that of two commercially available wound dressings. This dressing was found to accelerate wound closure and promote tissue healing, characterized by less dense, more randomly aligned, and shorter collagen fibers. Ramanathan et al[65] fabricated a highly interconnected porous dressing material incorporating a novel collagen variant (COL-SPG) for efficient wound healing. They created a three-dimensional (3D) sponge scaffold matrix that mimicked the function of the ECM and featured a highly interconnected structure with excellent biocompatibility, thus facilitating cell adhesion and attachment.
Gelatin: Gelatin, a derivative of collagen, exhibits substantial promise in wound healing owing to its ability to promote tissue regeneration and closure. In the field of tissue engineering, gelatin hydrogels and scaffolds function as templates for cell adhesion, growth, and differentiation and can serve as carriers for growth factors, drugs, or cells[66]. Gelatin exhibits low solubility in cold water and requires specific temperature and pH conditions for dissolution. Moreover, gelatin is a potential risk factor for allergic reactions and is susceptible to loss of structural stability at elevated temperatures, thus limiting its application in certain scenarios[67-70]. In one study by Shan et al[71], electrospun nanofibers comprising a combination of gelatin and silk fibroin were loaded with astragaloside IV. This dressing effectively increased the number of microvessels and regulated collagen deposition by controlling the drug release rate. In vivo experiments revealed that the dressing accelerated wound healing and inhibited scar formation by stimulating wound closure, enhancing angiogenesis, regulating newly formed collagen types, and improving collagen organization. In another study, Li et al[72] employed poly (L-lactic-co-caprolactone)/gelatin/EGCG core-shell nanofiber membranes with sustained drug release capabilities using coaxial electrospinning technology. Experiments confirmed that this core-shell-structured dressing displayed excellent biocompatibility, antibacterial properties, and antioxidant abilities.
Silk fibroin: Silk fibroin, a natural protein sourced from silkworm cocoons, has immense potential for wound healing. Its unique attributes make it a promising biomaterial for tissue regeneration and wound closure. However, the extraction and processing of silk fibroin are relatively complex and require high production standards. The current high production cost of silk fibroin restricts its large-scale application[73,74]. Previous studies have demonstrated its role in enhancing fibroblast proliferation and migration, collagen secretion, and expediting wound contraction[75,76]. In the field of tissue engineering, silk fibroin is used to construct 3D scaffolds that mimic the natural ECM, facilitating cellular attachment and growth. Certain investigators have explored the impact of varying concentrations of silk fibroin protein on the microstructure of 3D scaffolds. Utilizing the freeze-drying technique, Zhang et al[77] generated silk fibroin scaffolds with varying pore sizes, ranging from 50 to 300 micrometers. An optimized pore architecture within silk fibroin scaffolds was found to modulate the bioactivity of bone marrow-derived MSCs (BMSCs) expressing bone morphogenetic protein 7, leading to enhanced cell proliferation and ECM production. When loaded with growth factors or bioactive molecules, these silk fibroin materials increase cellular activity and facilitate tissue repair. In a separate study, Qu et al[78] fabricated porous silk fibroin microspheres loaded with bFGF and featuring interior-out channels using high-voltage electrostatic differentiation, followed by lyophilization. The encapsulated bFGF exhibited a sustained release pattern over 13 d, avoiding abrupt release. L929 cells demonstrated robust adhesion and generated a collagen-like fibrous matrix on the surface of SF microspheres.
Fibrous protein: Fibrin, a fibrous protein, plays a crucial role in the coagulation and tissue repair processes. The primary function of fibrin is the formation of blood clots. When a wound occurs, the body’s clotting cascade results in the conversion of fibrinogen to fibrin, creating a mesh-like structure that entraps blood cells and platelets. This clot staunches bleeding and initiates the wound healing process[79]. Fibrin is an attractive delivery vector owing to its structural and mechanical properties, as well as its inherent biological features. It offers several advantages: It can be injected and polymerized in situ and can be easily functionalized by incorporating therapeutic molecules into the fibrinogen or thrombin component in the fibrin formulation[80]. However, the extraction and processing of fibroin involve intricate procedures that require meticulous control, imposing restrictions on its widespread application[81,82]. Losi et al[83] designed a fibrin-based scaffold incorporating poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with VEGF and bFGF. These scaffolds, enriched with growth factors, spurred complete re-epithelialization, advanced granulation tissue maturation, and augmented collagen deposition. Alphonsa et al[84] successfully integrated antimicrobial drugs (ci
| Biopolymer | Sources | Function and characteristics in wound healing | Merits | Demerits | Ref. |
| Cellulose | Specific bacterial strains | Keep a moist environment. Absorb the exudates. Blocking bacterial infiltration | High purity. Good mechanical properties. Porous structure. High hydrophilic properties | No antimicrobial. Slow biodegradability | [26-31] |
| Plants (main sources: Cotton and woods) | Support tissue regeneration. Enhances cell attachment and proliferation | Good mechanical properties. Porous structure | Impurities available. Low solubility. No antimicrobial. Slow biodegradability | [22-26] | |
| Alginates | Brown marine algae and bacteria | Keep a moist environment. Absorb the exudates. Converted into a gel to cover the wound surface | Haemostatic. Form a hydrogel network on the surface of the wound | Poor mechanical properties. Tendency to swell. Not suitable for dry wounds | [37-42] |
| Hyaluronic acid | Animal origin | Good biocompatibility. Promote keratinocyte migration. Promote fibroblasts proliferation. Promote angiogenesis | Flexible. Highly biocompatible | Low mechanical strength. Low solubility | [45-48] |
| Chitosan | Exoskeleton of crabs, insects, fungal cell wall | Antibacterial properties. Hemostasis effect | Antimicrobial. Haemostatic | Low strength and toughness. Low solubility in aqueous solutions | [49-55] |
| Collagen | Bovine, porcine etc. | Keep a moist environment. Promote cell migration, proliferation, differentiation and angiogenesis. Regulate growth factors and cytokines | Cost effective. High water holding capacity | Has a risk of allergies. Exhibits restricted solubility in water | [58-65] |
| Gelatin | Extracted from the collagen | Keep a moist environment. Promote cell adhesion | Cost effective. High water holding capacity | Has a risk of allergies. Exhibits low solubility in cold water. Susceptible to losing structural stability at elevated temperatures | [66-72] |
| Silk fibroin | Silkworm cocoons | Excellent mechanical strength and flexibility. Enhances cell adhesion, migration, and proliferation | Good mechanical strength and flexibility. Good biocompatibility | Complex extraction and processing | [73-78] |
| Fibrous protein | Plasma | Keep a moist environment. Promotes cell growth and adhesion | Can be injected and polymerizes in situ. Can be functionalized into the fibrinogen or thrombin component in the fibrin formulation | Complex extraction and processing | [79-85] |
Synthetic scaffold: Synthetic polymers play a pivotal role in wound repair and tissue regeneration by providing a wide spectrum of functionalities with precisely defined and customizable chemical and mechanical properties.
Poly(vinyl alcohol) (PVA) is a biodegradable, semicrystalline synthetic polymer extensively utilized in drug delivery and tissue engineering applications, notably for its mechanical attributes. PVA hydrogels maintain a moist wound environment, making them valuable for wound dressings and controlled drug-release systems[86]. In a particular study, El-Attar et al[87] employed repetitive freeze-thawing to blend silk sericin with PVA. This hydrogel exhibited excellent hydrophilicity and swelling behavior owing to the effective blending of PVA with sericin, forming a porous structure.
Poly L-lactic acid (PLLA) and PLGA are biodegradable synthetic polymers commonly used to deliver cells to wounds[88,89]. In a study, Kim et al[88] developed an RGD-g-PLLA biosynthetic scaffold, showcasing its potential as an in vivo targeted delivery vehicle for endothelial progenitor cells that promotes vascular regeneration in murine dermal wound models. Another study by Chen et al[90] involved the fabrication of PLGA scaffolds seeded with extracorporeal shockwave-treated BMSCs, demonstrating the ability of the scaffolds to support BMSC attachment, proliferation, and differentiation and their significant potential in tissue regeneration.
Other biodegradable synthetic polymers, such as polycaprolactone (PCL) and polyethylene glycol (PEG), have also found applications in regenerative therapies. PCL is a biodegradable scaffold material used in tissue engineering for wound healing and bone regeneration. In a related study by Unalan et al[91], PCL electrospun fiber mats loaded with peppermint essential oil were created to confer antibacterial properties to the fibers for wound-healing applications. Moreover, PEG is a versatile polymer utilized in wound dressings. Kwak et al[92] designed PEG hydrogels to encapsulate M2-exos to maximize their therapeutic effects in cutaneous wound healing. These hydrogels offer adjustable degradation times by controlling crosslinking density, thus presenting a potential tool for regulating the local polarization state of Mø, which is a critical factor in tissue homeostasis and wound repair.
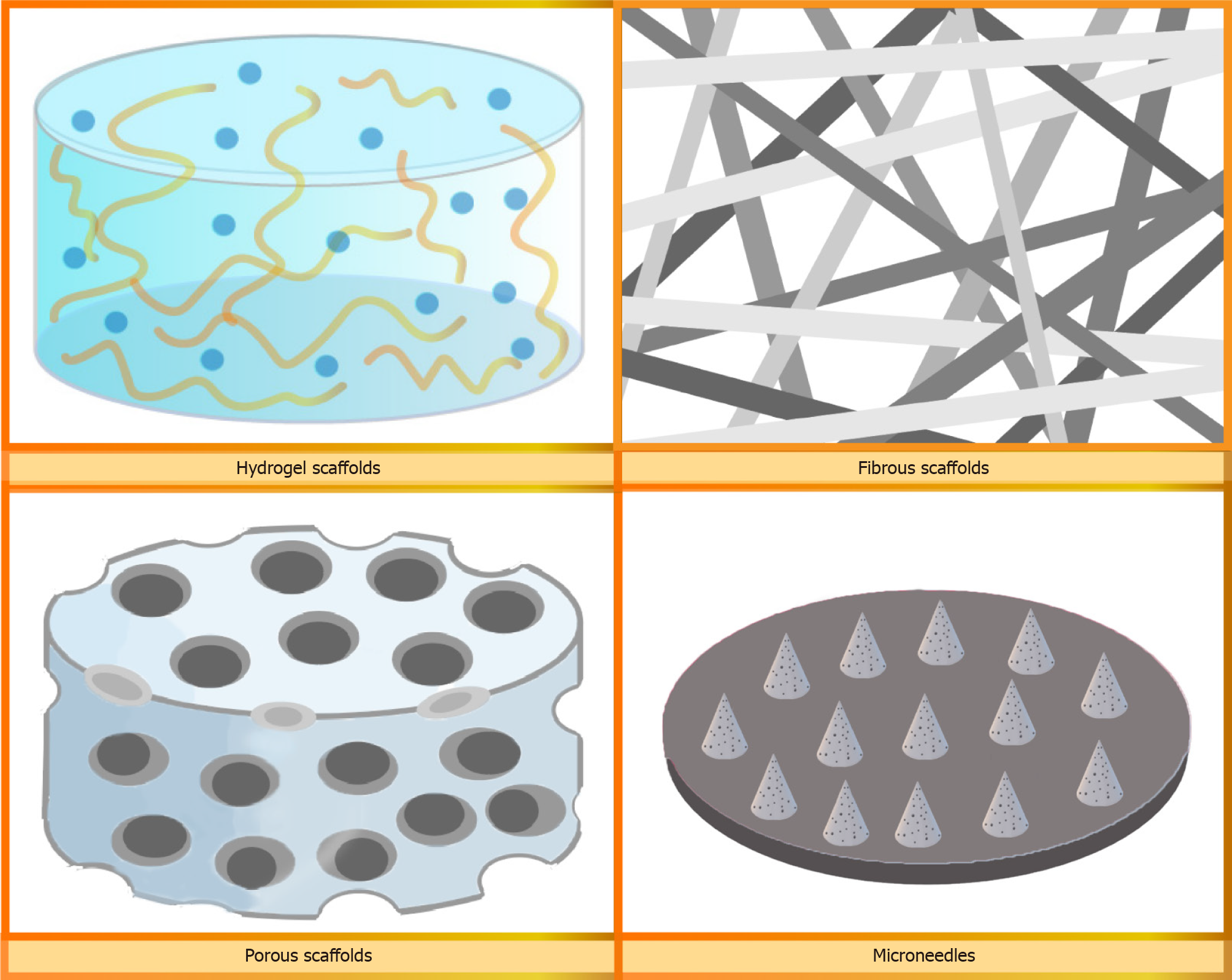
Hydrogel: Hydrogel scaffolds have attracted significant attention because of their tremendous potential as biomaterials for tissue engineering applications (Figure 2). These hydrogels are composed of structural supports made from bioactive compounds and exhibit a remarkable ability to regulate molecular-level biofunctions and provide adequate mechanical strength. Moreover, they create an ECM-like environment that enhances cellular activity[93]. Hydrogels can be synthesized using both natural and synthetic polymers. Naturally derived hydrogels offer several advantages over synthetic polymer scaffolds in terms of their biocompatibility, cellular interactions, and degradation. However, their limited mechanical strength restricts their range of applications. This drawback can be overcome in synthetic polymer-based hydrogels by controlling the structure or functional groups of the polymer chains[94]. Biocompatible hydrogels are extensively used in tissue engineering applications such as wound healing, bone regeneration, drug delivery, and cell delivery[95]. Liu et al[96] developed a hemostatic and anti-inflammatory hydrogel using ultraviolet light on a dual-cross-linked methacryloyl-substituted Bletilla striata polysaccharide and gelatin. This hydrogel was shown to regulate the M1/M2 phenotype of Mø, enhance the proliferation and migration of fibroblasts in vitro, and expedite angiogenesis. In another study conducted by Zhou et al[97], an injectable, biocompatible, and thermosensitive hydrogel called Pluronic F-127 hydrogel was utilized to encapsulate ADSC-derived exosomes (ADSC-exos) and was applied to a full-thickness cutaneous wound in mice. Their findings revealed that administering ADSC-exos to PF-127 improved the effectiveness of exosome delivery, preserved the bioactivity of ADSC-exos, and optimized their performance.
Fibrous scaffolds: Fibrous scaffolds are primarily fabricated using electrospinning to produce 3D constructs consisting of fibers at the microscale or nanoscale to closely mimic the architecture of natural human tissues[98] (Figure 2). Electrospinning is a technique that utilizes electrostatic forces to generate continuous fibers from biocompatible materials[99]. The orientation of fibrous scaffolds is determined based on specific application requirements, as it can influence cell adhesion, proliferation, differentiation, and paracrine functions[100]. Fibrous scaffolds have been used in diverse areas of tissue engineering, including skin, bone, cartilage, and neural tissue engineering[98]. Hsieh et al[101] incorporated HA into electrospun PLGA/gelatin fibrous membrane scaffolds to create an environment for 3D culture and delivery of ADSCs. The resulting scaffold exhibited favorable cytocompatibility, and the ADSCs cultured on the scaffold demonstrated increased expression of genes associated with angiogenesis. In a recent study, Zhao et al[102] produced 3D photocrosslinkable gelatin-based fibrous scaffolds with adjustable physical and biological properties. These fibrous scaffolds not only supported cell viability and adhesion but also facilitated cell migration and proliferation, thereby expediting the regeneration and formation of cutaneous tissues.
Porous scaffolds: Sponge scaffolds with high porosity and a uniformly interconnected pore network are created using natural or synthetic polymers through a range of techniques, such as porogen leaching, gas foaming, and freeze-drying[103]. Furthermore, the porous structure of sponge scaffolds closely resembles the composition of the ECM (Figure 2), and their ability to absorb and retain water allows for the absorption of exudate from the wound bed. This creates an advantageous environment that promotes cell migration and proliferation[104]. In a recent study by Li et al[105], a fish collagen-based sponge was explored as a substitute for the dura mater after surgical resection in rabbits. A rabbit dural defect model demonstrated that the scaffolds prevented the adhesion of brain tissue, thereby reducing the risk of inflammation, promoting fibroblast growth, and enhancing tissue regeneration and healing. In another investigation by Lian et al[106], a sponge-like scaffold with hierarchical and interconnected pores was fabricated using low-temperature deposition modeling printing. These sponges improved the adhesion, retention, survival, and ingrowth of MSCs and facilitated cell-material interactions. Furthermore, the paracrine functions of the cultured MSCs on the sponges were enhanced, leading to a significant secretion of upregulated immunomodulatory, angiogenic, and osteogenic factors.
Microneedle: Microneedles, a drug delivery system under development consisting of an array of tiny needles (Figure 2), have attracted considerable attention because of their minimally invasive nature, user-friendly operation, capability to deliver drugs in a targeted manner, and capacity to carry diverse payloads[107,108]. The unique structure of micro
ADSCs exhibit the ability to self-renew and differentiate into various cell types, including bone, cartilage, and fat cells[112]. Capitalizing on their potential holds great promise for expediting the wound healing process[113]. Additionally, cell-free derivatives of ADSCs, such as conditioned media, exosomes, and other bioactive components, have emerged as viable alternatives to conventional ADSC-based therapies[114]. Zhou et al[115] isolated exosomes from human ADSCs and explored the combined application of ADSCs and ADSC-exo to enhance the healing of cutaneous wounds. Their findings demonstrated that the systemic administration of ADSCs and ADSC-exo effectively stimulated cell proliferation, suppressed apoptosis and inflammation, and improved skin elasticity and barrier integrity. Furthermore, Zheng et al[116] revealed the protective effects of ADSC-exos against cellular oxidative damage. These exosomes enhanced cell proliferation and migration while reducing apoptosis. Subsequent investigations revealed significant enrichment of miR-378 in these exosomes. Significantly, experiments confirmed that the introduction of an miR-378 mimic could replicate the protective effects observed with ADSC-exos, whereas the application of miR-378 inhibitors to ADSCs nullified these protective properties.
BMSCs, sourced from bone marrow, represent the most prevalent stem cell type in the realms of cell therapy and tissue engineering[117]. When harnessed for wound healing applications, BMSCs release growth factors and cytokines, orchestrating fundamental processes such as angiogenesis, inflammation mitigation, and collagen synthesis, which are all pivotal for effective tissue restoration[118]. Ding et al[119] preconditioned BMSC-derived exosomes with deferoxamine (DFO-exosomes). Their study revealed that DFO-exosomes employed in cell-free therapies exhibited heightened pro-angiogenic potential by triggering the PI3K/AKT signaling pathway through miR-126-mediated PTEN downregulation, thus stimulating in vitro angiogenesis. Gondaliya et al[120] explored the combination of an miR-155 inhibitor and exosomes derived from BMSCs in a diabetic wound model. Their findings showed the synergistic effects of miR-155-inhibitor-loaded MSC-derived exosomes, including augmented keratinocyte migration, restoration of FGF-7 levels, and decreased inflammation. Furthermore, treatment with these exosomes ameliorated collagen deposition, angiogenesis, and re-epithelialization in diabetic wounds.
Derived from umbilical cord tissue, human umbilical cord-derived MSCs (hUC-MSCs) possess remarkable differentiation potential and can give rise to fibroblasts, endothelial cells, and immunomodulatory cells. hUC-MSCs are highly sought after because of their advantageous attributes, including low immunogenicity, immunomodulatory properties, non-invasive procurement, straightforward in vitro expansion, and ethical compliance[121]. Zhang et al[122] developed a PLGA-based scaffold to generate tissue sheets using hUC-MSCs. In vitro immunostaining revealed the formation of robust hUC-MSC sheets with a rich ECM. In a diabetic mouse model, transplantation of hUC-MSC tissue sheets significantly expedited wound healing compared with hUC-MSC injection or PLGA fibers alone. These results indicate that culturing hUC-MSCs on PLGA scaffolds is a promising approach for diabetic wound treatment. Xue et al[123] engineered spherical hUC-MSCs, which were subsequently integrated into self-assembled hydrogels. This strategy significantly accelerated wound healing, resulting in a shorter healing duration.
In addition to ADSCs, BMSCs, and hUC-MSCs, various other types of MSCs, such as epidermal stem cells (EPSCs), fetal dermal MSCs (FD-MSCs), and human amniotic fluid-derived stem cells, also play a significant role in wound healing and regeneration.
EPSCs, located in the basal layer of the epidermis and hair follicle bulges, are multipotent cells with diverse functions for maintaining epidermal integrity. These functions include epidermal formation, differentiation, maintenance of homeostasis, re-epithelialization, and the promotion of epidermal regeneration[124]. Pan et al[125] devised an electrospun micro/nanofiber scaffold using PCL and cellulose acetate (CA) to culture and transplant EPSCs to preserve their stemness and prevent differentiation. The results revealed that the PCL + CA micro/nanofiber scaffold effectively inhibited EPSC differentiation by activating YAP, which in turn suppressed the Notch signaling pathway[126].
In the field of regenerative medicine, FD-MSCs represent a novel and multipotent subset of MSCs derived from the fetal skin. FD-MSCs can counteract d-galactose-induced senescence in adult dermal fibroblasts (ADFs), primarily through their paracrine effects[125]. Costa et al[127] examined the therapeutic potential of exosomes released by FD-MSCs in the context of standard wound healing and explored their impact on ADFs. Their findings showed that FD-MSC exosomes effectively stimulated the proliferation, migration, and secretion of ADFs, accompanied by activation of the Notch signaling pathway following exosome treatment[128].
Human amniotic fluid-derived stem cells (hAFSCs), obtained from human amniotic fluid, play a crucial role in the wound healing process. They exhibit remarkable self-renewal capabilities and are amenable to large-scale expansion[127]. Zhang et al[129] investigated the therapeutic potential of hAFSC-derived exosomes (hAFSC-exos) in full-thickness skin wounds. The results showed that hAFSC-exos expedited wound healing and promoted hair follicle, nerve, and vessel regeneration. Furthermore, the hAFSC-exos mitigated excessive myofibroblast aggregation and ECM production. Table 2 summarizes the MSCs introduced above and their application in wound healing.
| MSCs | Sources | Application in wound healing |
| ADSCs | Adipose | Zhou et al[115] demonstrated the systemic administration of ADSCs and ADSC-exosomes effectively stimulated cell proliferation, suppressed cell apoptosis and inflammation, and improved skin elasticity and barrier integrity. Zheng et al[116] unveiled the protective effects of ADSC-exosomes on cells against oxidative damage. These exosomes enhanced cell proliferation and migration while reducing apoptosis |
| BMSCs | Bone marrow | Ding et al[119] preconditioned BMSC-exosomes with deferoxamine exhibited heightened proangiogenic. Gondaliya et al[120] explored the combination of a miR-155 inhibitor with BMSC-exosomes, showcased augmented keratinocyte migration, restoration of FGF-7 levels, and decreased inflammation |
| hUC-MSCs | Umbilical cord tissue | Zhang et al[122] devised a scaffold to generate tissue sheets using hUC-MSCs which significantly expedited wound healing. Xue et al[123] engineered spherical hUC-MSCs, which were subsequently integrated into self-assembling hydrogels |
| EPSCs | Epidermis | Pan et al[125] devised an electrospun micro/nanofiber scaffold to culture and transplant EPSCs with the aim of preserving their stemness and preventing differentiation |
| FD-MSCs | Fetal dermal | Costa et al[127] found out the exosomes from FD-MSCs effectively stimulated adult dermal fibroblasts’ proliferation, migration and secretion |
| hAFSCs | Human amniotic fluid | Zhang et al[129] showed that hAFSC-exosomes expedited wound healing, promoting hair follicle, nerve, and vessel regeneration |
Adhesion and proliferation: Stem cell therapy, when combined with various biomaterials, is a promising avenue for enhancing wound healing outcomes. This approach leverages the unique regenerative properties of stem cells as well as the structural and functional support offered by biomaterials[130].
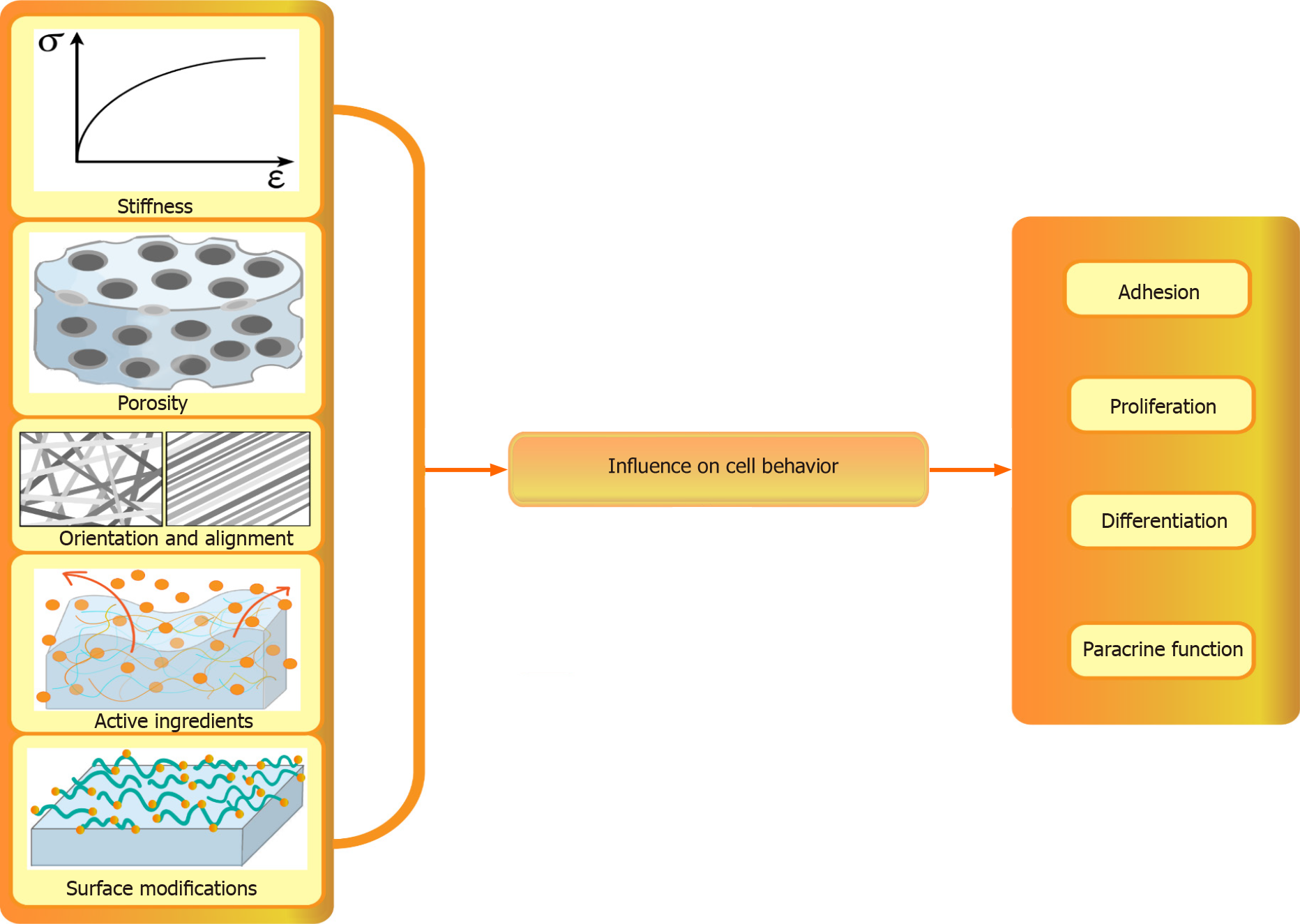
Biological scaffolds play a crucial role in facilitating stem cell adhesion by providing surfaces for cell attachment and spread (Figure 3). In a recent study, Martins et al[131] characterized pectin/CS (PT/CS) membranes and discovered that their surface wettability and swelling properties significantly promoted stem cell attachment. These findings demonstrate the potential of these novel PT/CS membranes to enhance anchorage and adhesion and support the growth of human stem cells, which makes them promising materials for tissue engineering applications. In another study, GelMA and dialdehyde-functionalized polyurethane were combined to create a double-crosslinking system, which was then loaded onto human MSCs (hMSCs). Within three days, cells within the 3D-printed hydrogel exhibited noticeable proliferation, resulting in an increased cell count. Moreover, the scaffold was nontoxic and provided a favorable environment for immediate cell growth. These results highlight the potential of 3D-printed materials as scaffolds for cartilage tissue engineering[132].
Differentiation: Moreover, biological scaffolds can influence stem cell differentiation (Figure 3). Cantu et al[133] conducted a study to examine the immunophenotype of Mø and the differentiation of MSCs in relation to established and experimental collagen-based biomaterials used for MSC entrapment. The results showed that MSCs cocultured with Møs exhibited reduced chondrocyte differentiation but enhanced osteoblast differentiation. Additionally, MSC differentiation into adipocytes was significantly enhanced when entrapped within a gelatin/PEG-based matrix[133]. In another study by Ferlin et al[134], scaffolds with two distinct and well-controlled pore geometries were fabricated to investigate their effects on MSC enrichment and differentiation. Their findings revealed that scaffolds with an ordered cubic pore geometry were conducive to both MSC enrichment from unprocessed bone marrow and MSC differentiation, which resulted in increased gene expression during adipogenesis and chondrogenesis processes.
Paracrine function: Furthermore, biological scaffolds have a significant effect on the paracrine functions of stem cells (Figure 3). They regulate the release of growth factors and cytokines, which play crucial roles in tissue repair and regeneration. Qazi et al[135] investigated whether the 3D culture in different microenvironments affected the secretion pattern of MSCs. The results showed that MSCs seeded on scaffolds exhibited an enhanced secretion profile compared with MSCs encapsulated in hydrogels. These scaffold-seeded MSCs exerted beneficial paracrine effects on various myoblast functions, including migration and proliferation. The increased paracrine effects of scaffold-seeded cells were partly attributed to N-cadherin-mediated cell-cell interactions during culture. Su et al[136] fabricated electrospun scaffolds composed of random, aligned, and mesh-like fibers. The paracrine behavior of ADSCs on these scaffolds was investigated and compared to that of conventional cell cultures on microplates. Their findings revealed that ADSCs cultured on electrospun fibers produced significantly higher levels of anti-inflammatory and pro-angiogenic cytokines. This suggests that the scaffold architecture can influence the paracrine behavior of ADSCs, potentially enhancing their therapeutic effects.
Effect of characteristics of scaffolds over cell behavior: Biological materials play critical roles in the regulation of various aspects of stem cell behavior, including growth, adhesion, proliferation, and paracrine activity. The careful selection of suitable biomaterials has a significant impact on these cellular processes in stem cell applications. Furthermore, biological materials also have the ability to influence stem cell differentiation and the secretion of signaling molecules, thereby affecting their therapeutic potential in tissue regeneration and regenerative medicine[137]. Factors such as stiffness, porosity, material alignment, and surface chemistry have been explored to gain a better understanding of the interactions between stem cells and biomaterials.
Stiffness: The mechanical properties of biomaterials, such as rigidity or stiffness, play crucial roles in regulating the interactions between stem cells and their microenvironments (Figure 3). Stiffness can be changed by varying the amount, concentration, percentage, and ratio[138]. The stiffness of the HA-tyramine system, which is covalently enzymatically cross-linked via an oxidative coupling reaction catalyzed by horseradish peroxidase and hydrogen peroxide (H2O2), can be adjusted by varying the amount or concentration of H2O2[139]. Several matrices become stiffer with increasing crosslinking. Sun et al[140] developed 3D gelatin scaffolds with distinct stiffnesses using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)-mediated crosslinking. The mechanical strength of the scaffolds was significantly increased by the EDC treatment. Moreover, the photopolymerization parameters also affect the stiffness of the materials. Allen et al[141] examined the impact of light intensity and exposure time on scaffold properties, such as shear modulus and micropore structure, and found that light intensity and polymerization time directly impact the scaffold stiffness and micropore structure, which in turn influence the cell loading capacity of the scaffold.
Stem cells can sense and respond to the stiffness of the substrate on which they are cultured, which influences their adhesion, spreading, and differentiation. Nam et al[142] examined the specific effects of scaffold stiffness on the behavior of embryonic mesenchymal progenitor cells. Scaffolds with identical microstructures and surface chemistries but different mechanical properties were used. The modulus of the core-shell fibers (30.6 MPa) was more than four times that of pure PCL (7.1 MPa). The results showed that the lower-modulus PCL fibers provided a more suitable microenvironment for chondrogenesis, whereas the stiffer core-shell PES-PCL fibers supported enhanced osteogenesis[142]. Ye et al[143] used PEG hydrogels as the matrix to prevent nonspecific protein adsorption. They also allowed cell-adhesive arginine (glycine) peptides to covalently bind onto hydrogel surfaces in the form of well-defined nanoarrays to control specific cell adhesion. This approach allowed separation of the effects of matrix stiffness and surface chemistry, clearly demonstrating that matrix stiffness is a potent regulator of stem cell differentiation. Similarly, Chu et al[144] fabricated four types of scaffolds with controlled stiffness and fiber size to investigate their potential to induce the differentiation of AFSCs. This study revealed that physical cues such as mechanical properties, topographical features, and geometrical factors have a profound impact on the growth and differentiation of cultured stem cells and that scaffold stiffness regulates AFSC differentiation.
Porosity: The topographical features of biomaterial surfaces play crucial roles in governing the interactions between stem cells and their microenvironments. Stem cells are highly sensitive to surface morphology, and cues from the substrate, such as biomaterial porosity and the arrangement or alignment of biomaterials, can influence the adhesion, spreading, and activation of intracellular signaling pathways. Ultimately, these factors impact cell growth and differentiation[145,146].
Pore sizes in biomaterials can be categorized into nanosize (nano-roughness, < 100 nm), micropore size (micro-roughness, 100 nm-100 μm), and macropore size (macro-roughness, 100 μm-millimeters)[147]. Pore size affects various cellular processes (Figure 3). Nanopore-sized membranes have been shown to act as an interim synthetic ECM with which cells interact before forming new tissues[148], while macropores play a crucial role in cell proliferation, differentiation, migration, and angiogenesis[145].
Zhang et al[149] developed a 3D fibrous collagen scaffold for potential pulp regeneration. The influence of the various pore sizes of these scaffolds on the proliferation and odontoblastic differentiation of human dental pulp cells (hDPCs) was investigated. Scaffolds with larger mean pore sizes of 65 and 145 μm improved hDPC ingrowth and proliferation, with the 65-μm scaffold group showing the highest level of odontogenic gene expression. Furthermore, Wang et al[150] explored the optimal pore structure and pore size of a Ti6Al4V porous scaffold for bone defect repair and the promotion of vascularization. The results demonstrated that cell proliferation ability and viability increased gradually as the pore size of the scaffold increased. Additionally, cells exhibited a stronger ability to vascularize on scaffolds with irregular pore sizes than on those with regular pore sizes. Tytgat et al[151] found that the spatial distribution of MSCs is dependent on the scaffold pore size, with larger pores leading to a more uniform spatial distribution of adipogenically differentiated cells. Wang et al[152] prepared a biomimetic fibroblast-loaded artificial dermis composed of three-layer scaffolds with different pore sizes. The outer layers of the scaffolds had larger pores than the middle layers. These scaffolds significantly promoted wound healing by facilitating granulation tissue formation and wound re-epithelialization.
Orientation and alignment: Besides the pore sizes of the biomaterials, stem cells are also influenced by the spatial orientation and alignment of these biomaterials (Figure 3). These factors play a significant role in determining cell adhesion, spreading, and activation of intracellular signaling pathways, ultimately affecting cell growth and differentiation.
The topographic orientation of biomaterials closely resembles the structure of the ECM and can generally be categorized as random, aligned, or featuring a specific pattern arrangement[153-156]. A recent study by Xu et al[157] showed that the topographic cues of materials can directly influence cellular orientation, potentially laying the groundwork for further differentiation. Moreover, aligned scaffolds have been found to expedite bone and fiber regeneration. Similarly, Rinoldi et al[158] designed a 3D system comprising stem cells and highly aligned hydrogel yarns. The aligned orientation of the fibers, combined with mechanical stimulation, led to a significantly preferred longitudinal cell orientation and enhanced the expression of collagen types I and III. Furthermore, the combination of biochemical and mechanical stimulations promotes the expression of specific tenogenic markers, indicative of efficient cell differentiation towards the tendons. In another study, electrospun scaffolds consisting of fibers arranged in random, aligned, and mesh-like patterns were fabricated, and the paracrine behavior of ADSCs on these scaffolds was compared with that of conventional microplate cell cultures. The findings revealed that ADSCs cultured on electrospun fibers produced significantly higher levels of anti-inflammatory and pro-angiogenic cytokines than those cultured on microplates. This demonstrates that the fibrous topography of scaffolds is a critical material property that modulates the paracrine function of cells[136]. In addition, Chen et al[159] pioneered the development of 3D nanofiber scaffolds with radial and vertical alignments for the transplantation of BMSCs. BMSC-laden 3D scaffolds have demonstrated the ability to stimulate granulation tissue formation, angiogenesis, and collagen deposition while also steering immune responses in a pro-regenerative direction. Andalib et al[160] fabricated both uniaxially aligned and randomly distributed nanofibers from PLLA and assessed the behavior of MSCs on these nanofibers. Their findings shed light on the role of focal adhesion kinase, a key molecular sensor in the focal adhesion complex, in governing the shape of MSC on nanofibers.
Surface modifications: To enhance biocompatibility, cell adhesion, and cell-biomaterial interactions, the surface chemistry of biomaterials must be appropriately modified[161,162] (Figure 3). This can be achieved through surface functionalization or modification. Surface functionalization involves altering the atoms or molecules on a biomaterial surface using physical or chemical methods. Alternatively, bioactive molecules can be coated on the surfaces[163]. Physical modification primarily involves the application of a biomimetic material or functional group to the surface of a biomaterial without altering its chemical characteristics. Common approaches for modifying biomaterials include coating them with collagen, integrin, fibronectin, and molecules resembling the ECM, such as CS or gelatin[164-166]. In a recent study by Murschel et al[167], a system was developed to enhance the physical adsorption of VEGF onto poly(allylamine)-functionalized polystyrene. They found that VEGF, when tagged with amphiphilic peptides, exhibited specific adsorption through coiled-coil interactions. This method effectively preserves the bioactivity of VEGF and allows its controlled release over several days. These results highlight the improved adsorption achieved by utilizing the electrostatic and hydrophobic forces on organic substrates. In a separate investigation conducted by Sadeghi et al[166], a non-woven fibrous substrate made of PLGA was subjected to hydrolysis using different concentrations of NaOH. This process aimed to enhance the presence of carboxyl and hydroxyl groups on the fiber surface. To further enhance the bioactivity, the activated substrates were coated with a collagen solution. Subsequent analyses revealed a significant increase in the proliferation of HaCaT cells after the collagen coating. This suggests that proper chemical bonding and crosslinking of collagen on the substrate surface result in sufficient stability and greatly improved bioactivity.
Polydopamine (PDA), a natural bioadhesive polymer, can regulate cell behavior and influence focal adhesion behavior at the biomaterial interface[168], as highlighted in a recent study by Wang et al[169]. This study demonstrated that PDA coating significantly enhanced the roughness, hydrophilicity, and protein absorption capacity of the film, which promoted the adhesion and migration of MSCs onto biomaterial surfaces. In another investigation conducted by Wan et al[170], a PDA-mediated surface modification of decellularized intestinal scaffolds was combined with ADSC to promote intestinal wound healing. The PDA-coated scaffold effectively supported the growth and proliferation of ADSCs and enhanced their secretory activity for paracrine effects.
In the field of biomaterial modification, layer-by-layer (LBL) deposition is a prominent fabrication technique. In this technique, alternating layers with opposite charges self-assemble because of electrostatic interactions between them[171]. da Câmara et al[172] utilized the LBL deposition approach to assemble tanfloc (TN), a cationic tannin-derivative polymer, with heparin and chondroitin sulfate. The assembly of 11 layers was found to show the highest rate of cell proliferation. Furthermore, when TN was used as the terminal layer of the scaffold, the spread of ADSCs was promoted. This suggests that the surface charge and terminal layer play crucial roles in creating the microenvironmental niche that influences cellular responses.
Active ingredients: Biomaterials can replicate the ECM structure of the ECM, thereby inducing cell adhesion, proliferation, and angiogenesis, which contribute to wound healing. Furthermore, biomaterials can carry active ingredients such as drugs, peptides, and growth factors. These components can influence cell growth, migration, and secretion, thereby further enhancing their role in promoting wound healing[173,174] (Figure 3).
Chen et al[175] designed and analyzed a unique active drug delivery vector that effectively addressed the challenges of high-efficiency loading and the simultaneous and controlled release of Mg2+ and curcumin. Their findings highlighted that Mg2+ released from the hydrogels bolstered the proliferation of BMSCs, whereas curcumin mitigated the damage to BMSCs from H2O2 exposure and reduced apoptotic cells and nuclear condensation. Elango et al[176] created a PVA retinol hydrogel and discovered that the cell proliferation effect of the PVA hydrogel was significantly enhanced by incorporating 0.1 to 0.5 wt% of retinol. They also found that retinol promotes the differentiation of MSCs into neural cells. Further research by Fadera et al[177] led to the development of injectable hydrogel systems loaded with stromal cell-derived factor-1α, which recruited ASCs and boosted ASC migration. Tao et al[178] utilized hepatocyte growth factor- and 5α-DHT-gelatin microspheres for treating human ADSCs and explored the reconstruction of sebaceous glands. They found that these gelatin microspheres stimulated ADSC migration and tube formation. In addition to drugs and cellular factors, bioactive peptides can significantly enhance stem cell adhesion, proliferation, and targeted differentiation. These peptides can be immobilized on culture plates or attached to scaffolds, such as hydrogels or synthetic matrices[174]. Jose et al[179] investigated the ability of glycine-histidine-lysine (GHK) to boost the secretion of pro-angiogenic factors from human MSC in culture and when covalently linked to alginate hydrogels. They observed a dose-dependent increase in the VEGF concentration in the media conditioned with GHK-treated MSC, which amplified endothelial cell proliferation, migration, and tubule formation. These findings indicate that the pro-angiogenic potential of MSC can be significantly augmented by presenting GHK with a biodegradable carrier. Gallagher et al[180] conducted a systematic review of the impact of the arginine-glycine-aspartate (RGD) peptide on hMSCs in a HA hydrogel under standard and ischemic culture conditions. They found that under standard culture conditions, RGD notably increased the spread of hMSC and the release of VEGF and monocyte chemoattractant factor-1.
To tackle the complex challenges of wound repair, stem cell-based therapy has emerged as a promising strategy aimed at enhancing wound healing and promoting skin regeneration. However, the use of stem cells in this context presents several challenges. Recently, the critical role of combining stem cells and biomaterials in the wound healing process has been recognized. Both natural and synthetic biomaterials can be deliberately crafted for the purpose of treating wounds, considering their physical and chemical properties. The structural properties of a well-designed biomaterial or scaffold can create a protective and sometimes stimulatory environment for stem cells, imitating the natural ECM and controlling different aspects of stem cell behavior such as adhesion, proliferation, differentiation, and paracrine activities.
Incorporating stem cells into organized biomaterials improves their ability to repair impaired skin tissue and enhance crucial wound-healing factors, such as epithelialization, granulation tissue formation, vascularization, and angiogenesis. The use of biomaterials can help overcome the barriers associated with stem cell-based therapies. This comprehensive review examines the development and application of biological scaffolds for stem cells and their derivatives, emphasizing their roles in supporting stem cell adhesion, proliferation, differentiation, and paracrine functions. It also highlights the effect of scaffolds on stem cell behavior and identifies the key characteristics of scaffolds responsible for improved cell activity when combined with biomaterials.
Despite yielding promising outcomes in non-clinical studies, the availability of biomaterials tailored for stem cell-based treatments in clinical settings is limited. Consequently, additional clinical trials employing biomaterials are required to clarify the influence of biomaterial characteristics on the healing, restoration, and regeneration of tissues in human subjects. Moreover, there is a growing need for customized biomaterial designs to meet various patient requirements, which entails adjusting the physical, chemical, and structural properties of materials to align with specific medical needs. This personalized approach ensures that the biomaterial optimally interacts with the patient’s distinctive physiological environment, thereby fostering efficient tissue repair and regeneration. By considering factors such as biocompatibility, degradation rates, mechanical strength, and the capacity to support cell growth and differentiation, tailored biomaterials can effectively meet a wide range of patient needs, including wound healing, tissue regeneration, and organ repair.
| 1. | Kolimi P, Narala S, Nyavanandi D, Youssef AAA, Dudhipala N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 258] [Reference Citation Analysis (0)] |
| 2. | Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 1003] [Article Influence: 167.2] [Reference Citation Analysis (0)] |
| 3. | Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 561] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 4. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3887] [Cited by in RCA: 3404] [Article Influence: 212.8] [Reference Citation Analysis (0)] |
| 5. | Lee YS, Wysocki A, Warburton D, Tuan TL. Wound healing in development. Birth Defects Res C Embryo Today. 2012;96:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 618] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 7. | Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015;5:a023267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 458] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 8. | Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1868] [Article Influence: 266.9] [Reference Citation Analysis (0)] |
| 9. | Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 338] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 10. | Bai Q, Han K, Dong K, Zheng C, Zhang Y, Long Q, Lu T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int J Nanomedicine. 2020;15:9717-9743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Nii T, Katayama Y. Biomaterial-Assisted Regenerative Medicine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Legrand JMD, Martino MM. Growth Factor and Cytokine Delivery Systems for Wound Healing. Cold Spring Harb Perspect Biol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma. 2018;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Monika P, Chandraprabha MN, Rangarajan A, Waiker PV, Chidambara Murthy KN. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front Nutr. 2021;8:791899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 15. | Farahani M, Shafiee A. Wound Healing: From Passive to Smart Dressings. Adv Healthc Mater. 2021;10:e2100477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 415] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 16. | Sorice S, Rustad KC, Li AY, Gurtner GC. The Role of Stem Cell Therapeutics in Wound Healing: Current Understanding and Future Directions. Plast Reconstr Surg. 2016;138:31S-41S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Sung TC, Wang T, Liu Q, Ling QD, Subbiah SK, Renuka RR, Hsu ST, Umezawa A, Higuchi A. Cell-binding peptides on the material surface guide stem cell fate of adhesion, proliferation and differentiation. J Mater Chem B. 2023;11:1389-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 18. | Yu D, Wang J, Qian KJ, Yu J, Zhu HY. Effects of nanofibers on mesenchymal stem cells: environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J Zhejiang Univ Sci B. 2020;21:871-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Aoudi B, Boluk Y, Gamal El-Din M. Recent advances and future perspective on nanocellulose-based materials in diverse water treatment applications. Sci Total Environ. 2022;843:156903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Ullah MW, Ul-Islam M, Khan S, Kim Y, Park JK. Structural and physico-mechanical characterization of bio-cellulose produced by a cell-free system. Carbohydr Polym. 2016;136:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Ali F, Khan SB, Kamal T, Alamry KA, Asiri AM, Sobahi TRA. Chitosan coated cotton cloth supported zero-valent nanoparticles: Simple but economically viable, efficient and easily retrievable catalysts. Sci Rep. 2017;7:16957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Bae SO, Shoda M. Production of bacterial cellulose by Acetobacter xylinum BPR2001 using molasses medium in a jar fermentor. Appl Microbiol Biotechnol. 2005;67:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Avcioglu NH. Bacterial cellulose: recent progress in production and industrial applications. World J Microbiol Biotechnol. 2022;38:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Rajwade JM, Paknikar KM, Kumbhar JV. Applications of bacterial cellulose and its composites in biomedicine. Appl Microbiol Biotechnol. 2015;99:2491-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (2)] |
| 25. | Naomi R, Bt Hj Idrus R, Fauzi MB. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Ul-Islam M, Khan S, Ullah MW, Park JK. Comparative study of plant and bacterial cellulose pellicles regenerated from dissolved states. Int J Biol Macromol. 2019;137:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Moniri M, Boroumand Moghaddam A, Azizi S, Abdul Rahim R, Bin Ariff A, Zuhainis Saad W, Navaderi M, Mohamad R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials (Basel). 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Ahmed J, Gultekinoglu M, Edirisinghe M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol Adv. 2020;41:107549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Khalid A, Khan R, Ul-Islam M, Khan T, Wahid F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr Polym. 2017;164:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 30. | Kaewnopparat S, Sansernluk K, Faroongsarng D. Behavior of freezable bound water in the bacterial cellulose produced by Acetobacter xylinum: an approach using thermoporosimetry. AAPS PharmSciTech. 2008;9:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Paleczny J, Brożyna M, Junka A, Bartoszewicz M, Dudek-Wicher R. Modifications of bacterial cellulose in wound care. Polim Med. 2021;51:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Soliman M, Sadek AA, Abdelhamid HN, Hussein K. Graphene oxide-cellulose nanocomposite accelerates skin wound healing. Res Vet Sci. 2021;137:262-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Keskin Z, Sendemir Urkmez A, Hames EE. Novel keratin modified bacterial cellulose nanocomposite production and characterization for skin tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;75:1144-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Mao L, Wang L, Zhang M, Ullah MW, Liu L, Zhao W, Li Y, Ahmed AAQ, Cheng H, Shi Z, Yang G. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv Healthc Mater. 2021;10:e2100402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 35. | Cao S, Li Q, Zhang S, Liu K, Yang Y, Chen J. Oxidized bacterial cellulose reinforced nanocomposite scaffolds for bone repair. Colloids Surf B Biointerfaces. 2022;211:112316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Gu L, Li T, Song X, Yang X, Li S, Chen L, Liu P, Gong X, Chen C, Sun L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen Biomater. 2020;7:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Zhang H, Cheng J, Ao Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar Drugs. 2021;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 38. | Zhang M, Zhao X. Alginate hydrogel dressings for advanced wound management. Int J Biol Macromol. 2020;162:1414-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 39. | Abka-Khajouei R, Tounsi L, Shahabi N, Patel AK, Abdelkafi S, Michaud P. Structures, Properties and Applications of Alginates. Mar Drugs. 2022;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 40. | Rosiak P, Latanska I, Paul P, Sujka W, Kolesinska B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Aderibigbe BA, Buyana B. Alginate in Wound Dressings. Pharmaceutics. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 467] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 42. | Zhang M, Qiao X, Han W, Jiang T, Liu F, Zhao X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr Polym. 2021;266:118100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 43. | Mndlovu H, Kumar P, du Toit LC, Choonara YE. In Situ Forming Chitosan-Alginate Interpolymer Complex Bioplatform for Wound Healing and Regeneration. AAPS PharmSciTech. 2022;23:247. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Hernández-González AC, Téllez-Jurado L, Rodríguez-Lorenzo LM. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr Polym. 2020;229:115514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 333] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 45. | Lam J, Truong NF, Segura T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014;10:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 46. | Graça MFP, Miguel SP, Cabral CSD, Correia IJ. Hyaluronic acid-Based wound dressings: A review. Carbohydr Polym. 2020;241:116364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 542] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 47. | Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol. 2016;86:917-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 48. | Orellana SL, Giacaman A, Pavicic F, Vidal A, Moreno-Villoslada I, Concha M. Relevance of charge balance and hyaluronic acid on alginate-chitosan sponge microstructure and its influence on fibroblast growth. J Biomed Mater Res A. 2016;104:2537-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Bai Q, Gao Q, Hu F, Zheng C, Chen W, Sun N, Liu J, Zhang Y, Wu X, Lu T. Chitosan and hyaluronic-based hydrogels could promote the infected wound healing. Int J Biol Macromol. 2023;232:123271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 50. | Wang CH, Cherng JH, Liu CC, Fang TJ, Hong ZJ, Chang SJ, Fan GY, Hsu SD. Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 51. | Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 486] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 52. | Abd El-Hack ME, El-Saadony MT, Shafi ME, Zabermawi NM, Arif M, Batiha GE, Khafaga AF, Abd El-Hakim YM, Al-Sagheer AA. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int J Biol Macromol. 2020;164:2726-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 469] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 53. | Shariatinia Z. Carboxymethyl chitosan: Properties and biomedical applications. Int J Biol Macromol. 2018;120:1406-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 460] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 54. | Xu G, Xu N, Ren T, Chen C, Li J, Ding L, Chen Y, Chen G, Li Z, Yu Y. Multifunctional chitosan/silver/tannic acid cryogels for hemostasis and wound healing. Int J Biol Macromol. 2022;208:760-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | Li H, Cheng F, Wei X, Yi X, Tang S, Wang Z, Zhang YS, He J, Huang Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater Sci Eng C Mater Biol Appl. 2021;118:111324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 56. | Saravanan S, Vimalraj S, Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed Pharmacother. 2018;107:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Diolosà M, Donati I, Turco G, Cadenaro M, Di Lenarda R, Breschi L, Paoletti S. Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules. 2014;15:4606-4613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Sharma S, Rai VK, Narang RK, Markandeywar TS. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022;290:120096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 59. | Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online. 2019;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 60. | Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2946] [Cited by in RCA: 2461] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 61. | Lucey P, Goldberg DJ. Complications of collagen fillers. Facial Plast Surg. 2014;30:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Peng YY, Glattauer V, Ramshaw JA, Werkmeister JA. Evaluation of the immunogenicity and cell compatibility of avian collagen for biomedical applications. J Biomed Mater Res A. 2010;93:1235-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Jiang H, Zheng M, Liu X, Zhang S, Wang X, Chen Y, Hou M, Zhu J. Feasibility Study of Tissue Transglutaminase for Self-Catalytic Cross-Linking of Self-Assembled Collagen Fibril Hydrogel and Its Promising Application in Wound Healing Promotion. ACS Omega. 2019;4:12606-12615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 64. | Chen K, Sivaraj D, Davitt MF, Leeolou MC, Henn D, Steele SR, Huskins SL, Trotsyuk AA, Kussie HC, Greco AH, Padmanabhan J, Perrault DP, Zamaleeva AI, Longaker MT, Gurtner GC. Pullulan-Collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Repair Regen. 2022;30:397-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Ramanathan G, Singaravelu S, Muthukumar T, Thyagarajan S, Perumal PT, Sivagnanam UT. Design and characterization of 3D hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;72:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Echave MC, Saenz del Burgo L, Pedraz JL, Orive G. Gelatin as Biomaterial for Tissue Engineering. Curr Pharm Des. 2017;23:3567-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 67. | Salahuddin B, Wang S, Sangian D, Aziz S, Gu Q. Hybrid Gelatin Hydrogels in Nanomedicine Applications. ACS Appl Bio Mater. 2021;4:2886-2906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 68. | Afewerki S, Sheikhi A, Kannan S, Ahadian S, Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng Transl Med. 2019;4:96-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 69. | Bogdanovic J, Halsey NA, Wood RA, Hamilton RG. Bovine and porcine gelatin sensitivity in children sensitized to milk and meat. J Allergy Clin Immunol. 2009;124:1108-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Lied GA, Lund KB, Storaas T. Intraoperative anaphylaxis to gelatin-based hemostatic agents: a case report. J Asthma Allergy. 2019;12:163-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Shan YH, Peng LH, Liu X, Chen X, Xiong J, Gao JQ. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int J Pharm. 2015;479:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 72. | Li A, Li L, Zhao B, Li X, Liang W, Lang M, Cheng B, Li J. Antibacterial, antioxidant and anti-inflammatory PLCL/gelatin nanofiber membranes to promote wound healing. Int J Biol Macromol. 2022;194:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Zheng H, Zuo B. Functional silk fibroin hydrogels: preparation, properties and applications. J Mater Chem B. 2021;9:1238-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 74. | Liu Q, Liu H, Fan Y. Preparation of silk fibroin carriers for controlled release. Microsc Res Tech. 2017;80:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Aramwit P, Kanokpanont S, Nakpheng T, Srichana T. The effect of sericin from various extraction methods on cell viability and collagen production. Int J Mol Sci. 2010;11:2200-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 76. | Aramwit P, Palapinyo S, Srichana T, Chottanapund S, Muangman P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch Dermatol Res. 2013;305:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 77. | Zhang Y, Fan W, Ma Z, Wu C, Fang W, Liu G, Xiao Y. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010;6:3021-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 78. | Qu J, Wang L, Niu L, Lin J, Huang Q, Jiang X, Li M. Porous Silk Fibroin Microspheres Sustainably Releasing Bioactive Basic Fibroblast Growth Factor. Materials (Basel). 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Kearney KJ, Ariëns RAS, Macrae FL. The Role of Fibrin(ogen) in Wound Healing and Infection Control. Semin Thromb Hemost. 2022;48:174-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 80. | Rajangam T, An SS. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int J Nanomedicine. 2013;8:3641-3662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Brown AC, Barker TH. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014;10:1502-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 82. | Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 641] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 83. | Losi P, Briganti E, Errico C, Lisella A, Sanguinetti E, Chiellini F, Soldani G. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9:7814-7821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 84. | Alphonsa BM, Sudheesh Kumar PT, Praveen G, Biswas R, Chennazhi KP, Jayakumar R. Antimicrobial drugs encapsulated in fibrin nanoparticles for treating microbial infested wounds. Pharm Res. 2014;31:1338-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Mendez JJ, Ghaedi M, Sivarapatna A, Dimitrievska S, Shao Z, Osuji CO, Steinbacher DM, Leffell DJ, Niklason LE. Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials. 2015;40:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Jin SG. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem Asian J. 2022;17:e202200595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 87. | El-Attar AA, El-Wakil HB, Hassanin AH, Bakr BA, Almutairi TM, Hagar M, Elwakil BH, Olama ZA. Silver/Snail Mucous PVA Nanofibers: Electrospun Synthesis and Antibacterial and Wound Healing Activities. Membranes (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Kim KL, Han DK, Park K, Song SH, Kim JY, Kim JM, Ki HY, Yie SW, Roh CR, Jeon ES, Kim DK, Suh W. Enhanced dermal wound neovascularization by targeted delivery of endothelial progenitor cells using an RGD-g-PLLA scaffold. Biomaterials. 2009;30:3742-3748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2117] [Cited by in RCA: 2529] [Article Influence: 180.6] [Reference Citation Analysis (0)] |
| 90. | Chen Y, Xu J, Huang Z, Yu M, Zhang Y, Chen H, Ma Z, Liao H, Hu J. An Innovative Approach for Enhancing Bone Defect Healing Using PLGA Scaffolds Seeded with Extracorporeal-shock-wave-treated Bone Marrow Mesenchymal Stem Cells (BMSCs). Sci Rep. 2017;7:44130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Unalan I, Slavik B, Buettner A, Goldmann WH, Frank G, Boccaccini AR. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front Bioeng Biotechnol. 2019;7:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 92. | Kwak G, Cheng J, Kim H, Song S, Lee SJ, Yang Y, Jeong JH, Lee JE, Messersmith PB, Kim SH. Sustained Exosome-Guided Macrophage Polarization Using Hydrolytically Degradable PEG Hydrogels for Cutaneous Wound Healing: Identification of Key Proteins and MiRNAs, and Sustained Release Formulation. Small. 2022;18:e2200060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 93. | Ho TC, Chang CC, Chan HP, Chung TW, Shu CW, Chuang KP, Duh TH, Yang MH, Tyan YC. Hydrogels: Properties and Applications in Biomedicine. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 452] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 94. | Patel DK, Jung E, Priya S, Won SY, Han SS. Recent advances in biopolymer-based hydrogels and their potential biomedical applications. Carbohydr Polym. 2024;323:121408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 95. | Mahmood A, Patel D, Hickson B, DesRochers J, Hu X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 96. | Liu J, Qu M, Wang C, Xue Y, Huang H, Chen Q, Sun W, Zhou X, Xu G, Jiang X. A Dual-Cross-Linked Hydrogel Patch for Promoting Diabetic Wound Healing. Small. 2022;18:e2106172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 97. | Zhou Y, Zhang XL, Lu ST, Zhang NY, Zhang HJ, Zhang J. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13:407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 98. | Stocco TD, Bassous NJ, Zhao S, Granato AEC, Webster TJ, Lobo AO. Nanofibrous scaffolds for biomedical applications. Nanoscale. 2018;10:12228-12255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Xue J, Wu T, Dai Y, Xia Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem Rev. 2019;119:5298-5415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3036] [Cited by in RCA: 1994] [Article Influence: 284.9] [Reference Citation Analysis (7)] |
| 100. | Krishna L, Dhamodaran K, Jayadev C, Chatterjee K, Shetty R, Khora SS, Das D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res Ther. 2016;7:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 101. | Hsieh CF, Chen CH, Kao HH, Govindaraju DT, Dash BS, Chen JP. PLGA/Gelatin/Hyaluronic Acid Fibrous Membrane Scaffold for Therapeutic Delivery of Adipose-Derived Stem Cells to Promote Wound Healing. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 102. | Zhao X, Sun X, Yildirimer L, Lang Q, Lin ZYW, Zheng R, Zhang Y, Cui W, Annabi N, Khademhosseini A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017;49:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 103. | Chatzimitakos TG, Stalikas CD. Sponges and Sponge-Like Materials in Sample Preparation: A Journey from Past to Present and into the Future. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Doillon CJ. Porous collagen sponge wound dressings: in vivo and in vitro studies. J Biomater Appl. 1988;2:562-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Li Q, Mu L, Zhang F, Sun Y, Chen Q, Xie C, Wang H. A novel fish collagen scaffold as dural substitute. Mater Sci Eng C Mater Biol Appl. 2017;80:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Lian M, Sun B, Han Y, Yu B, Xin W, Xu R, Ni B, Jiang W, Hao Y, Zhang X, Shen Y, Qiao Z, Dai K. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration. Biomaterials. 2021;274:120841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 107. | Lyu S, Dong Z, Xu X, Bei HP, Yuen HY, James Cheung CW, Wong MS, He Y, Zhao X. Going below and beyond the surface: Microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration. Bioact Mater. 2023;27:303-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 108. | Zhang X, Wang Y, Chi J, Zhao Y. Smart Microneedles for Therapy and Diagnosis. Research (Wash D C). 2020;2020:7462915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 109. | Xu J, Xu D, Xuan X, He H. Advances of Microneedles in Biomedical Applications. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 110. | Ma W, Zhang X, Liu Y, Fan L, Gan J, Liu W, Zhao Y, Sun L. Polydopamine Decorated Microneedles with Fe-MSC-Derived Nanovesicles Encapsulation for Wound Healing. Adv Sci (Weinh). 2022;9:e2103317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 111. | Wu X, Huang D, Xu Y, Chen G, Zhao Y. Microfluidic Templated Stem Cell Spheroid Microneedles for Diabetic Wound Treatment. Adv Mater. 2023;35:e2301064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 112. | Calabrese EJ. Hormesis and adult adipose-derived stem cells. Pharmacol Res. 2021;172:105803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 113. | Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 114. | Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 115. | Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST, Cheng J, Fu Y, Lin L, Zhang NY, Li PX, Zhang J. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 116. | Zheng T, Shao W, Tian J. Exosomes derived from ADSCs containing miR-378 promotes wound healing by targeting caspase-3. J Biochem Mol Toxicol. 2021;35:e22881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 117. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 727] [Article Influence: 103.9] [Reference Citation Analysis (36)] |
| 118. | Dama G, Du J, Zhu X, Liu Y, Lin J. Bone marrow-derived mesenchymal stem cells: A promising therapeutic option for the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2023;195:110201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 119. | Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed Res Int. 2019;2019:9742765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 120. | Gondaliya P, Sayyed AA, Bhat P, Mali M, Arya N, Khairnar A, Kalia K. Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol Pharm. 2022;19:1294-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 121. | Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 122. | Zhang J, Qu X, Li J, Harada A, Hua Y, Yoshida N, Ishida M, Sawa Y, Liu L, Miyagawa S. Tissue Sheet Engineered Using Human Umbilical Cord-Derived Mesenchymal Stem Cells Improves Diabetic Wound Healing. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 123. | Xue J, Sun N, Liu Y. Self-Assembled Nano-Peptide Hydrogels with Human Umbilical Cord Mesenchymal Stem Cell Spheroids Accelerate Diabetic Skin Wound Healing by Inhibiting Inflammation and Promoting Angiogenesis. Int J Nanomedicine. 2022;17:2459-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 124. | Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K. Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther. 2019;10:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 125. | Pan S, Gong S, Zhang J, Jia S, Wang M, Pan Y, Wang X, Jiang D. Anti-aging effects of fetal dermal mesenchymal stem cells in a D-galactose-induced aging model of adult dermal fibroblasts. In Vitro Cell Dev Biol Anim. 2021;57:795-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 126. | Lin Z, Zhao C, Lei Z, Zhang Y, Huang R, Lin B, Dong Y, Zhang H, Li J, Li X. Epidermal stem cells maintain stemness via a biomimetic micro/nanofiber scaffold that promotes wound healing by activating the Notch signaling pathway. Stem Cell Res Ther. 2021;12:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 127. | Costa A, Quarto R, Bollini S. Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 128. | Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, Wang M, Shao M, Wang X, Jiang D. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019;2019:2402916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 129. | Zhang Y, Yan J, Liu Y, Chen Z, Li X, Tang L, Li J, Duan M, Zhang G. Human Amniotic Fluid Stem Cell-Derived Exosomes as a Novel Cell-Free Therapy for Cutaneous Regeneration. Front Cell Dev Biol. 2021;9:685873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 130. | Riha SM, Maarof M, Fauzi MB. Synergistic Effect of Biomaterial and Stem Cell for Skin Tissue Engineering in Cutaneous Wound Healing: A Concise Review. Polymers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 131. | Martins JG, Camargo SEA, Bishop TT, Popat KC, Kipper MJ, Martins AF. Pectin-chitosan membrane scaffold imparts controlled stem cell adhesion and proliferation. Carbohydr Polym. 2018;197:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 132. | Cheng QP, Hsu SH. A self-healing hydrogel and injectable cryogel of gelatin methacryloyl-polyurethane double network for 3D printing. Acta Biomater. 2023;164:124-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 133. | Cantu DA, Hematti P, Kao WJ. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cells Transl Med. 2012;1:740-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Ferlin KM, Prendergast ME, Miller ML, Kaplan DS, Fisher JP. Influence of 3D printed porous architecture on mesenchymal stem cell enrichment and differentiation. Acta Biomater. 2016;32:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 135. | Qazi TH, Mooney DJ, Duda GN, Geissler S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials. 2017;140:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 136. | Su N, Gao PL, Wang K, Wang JY, Zhong Y, Luo Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 137. | Zhao C, Tan A, Pastorin G, Ho HK. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv. 2013;31:654-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 138. | Lv H, Wang H, Zhang Z, Yang W, Liu W, Li Y, Li L. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 139. | Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33:3835-3845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 140. | Sun H, Zhu F, Hu Q, Krebsbach PH. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials. 2014;35:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 141. | Allen BN, Wendland RJ, Thompson JD, Tucker BA, Worthington KS. Photopolymerization Parameters Influence Mechanical, Microstructural, and Cell Loading Properties of Rapidly Fabricated Cell Scaffolds. ACS Biomater Sci Eng. 2023;9:2663-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 142. | Nam J, Johnson J, Lannutti JJ, Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 2011;7:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 143. | Ye K, Wang X, Cao L, Li S, Li Z, Yu L, Ding J. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015;15:4720-4729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 144. | Chu G, Yuan Z, Zhu C, Zhou P, Wang H, Zhang W, Cai Y, Zhu X, Yang H, Li B. Substrate stiffness- and topography-dependent differentiation of annulus fibrosus-derived stem cells is regulated by Yes-associated protein. Acta Biomater. 2019;92:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 145. | Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1649] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 146. | Salmasi S, Kalaskar DM, Yoon WW, Blunn GW, Seifalian AM. Role of nanotopography in the development of tissue engineered 3D organs and tissues using mesenchymal stem cells. World J Stem Cells. 2015;7:266-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 147. | Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013-4024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 148. | Smith IO, Liu XH, Smith LA, Ma PX. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 149. | Zhang Q, Yuan C, Liu L, Wen S, Wang X. Effect of 3-dimensional Collagen Fibrous Scaffolds with Different Pore Sizes on Pulp Regeneration. J Endod. 2022;48:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 150. | Wang C, Xu D, Lin L, Li S, Hou W, He Y, Sheng L, Yi C, Zhang X, Li H, Li Y, Zhao W, Yu D. Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration. Mater Sci Eng C Mater Biol Appl. 2021;131:112499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 151. | Tytgat L, Kollert MR, Van Damme L, Thienpont H, Ottevaere H, Duda GN, Geissler S, Dubruel P, Van Vlierberghe S, Qazi TH. Evaluation of 3D Printed Gelatin-Based Scaffolds with Varying Pore Size for MSC-Based Adipose Tissue Engineering. Macromol Biosci. 2020;20:e1900364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 152. | Wang Y, Xu R, Luo G, Lei Q, Shu Q, Yao Z, Li H, Zhou J, Tan J, Yang S, Zhan R, He W, Wu J. Biomimetic fibroblast-loaded artificial dermis with "sandwich" structure and designed gradient pore sizes promotes wound healing by favoring granulation tissue formation and wound re-epithelialization. Acta Biomater. 2016;30:246-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 153. | Kim J, Bae WG, Kim YJ, Seonwoo H, Choung HW, Jang KJ, Park S, Kim BH, Kim HN, Choi KS, Kim MS, Choung PH, Choung YH, Chung JH. Directional Matrix Nanotopography with Varied Sizes for Engineering Wound Healing. Adv Healthc Mater. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 154. | Yang CY, Huang WY, Chen LH, Liang NW, Wang HC, Lu J, Wang X, Wang TW. Neural tissue engineering: the influence of scaffold surface topography and extracellular matrix microenvironment. J Mater Chem B. 2021;9:567-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 155. | Gaharwar AK, Nikkhah M, Sant S, Khademhosseini A. Anisotropic poly (glycerol sebacate)-poly (ϵ-caprolactone) electrospun fibers promote endothelial cell guidance. Biofabrication. 2014;7:015001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 156. | Gerberich BG, Bhatia SK. Tissue scaffold surface patterning for clinical applications. Biotechnol J. 2013;8:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Xu X, Zhou Y, Zheng K, Li X, Li L, Xu Y. 3D Polycaprolactone/Gelatin-Oriented Electrospun Scaffolds Promote Periodontal Regeneration. ACS Appl Mater Interfaces. 2022;14:46145-46160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 158. | Rinoldi C, Costantini M, Kijeńska-Gawrońska E, Testa S, Fornetti E, Heljak M, Ćwiklińska M, Buda R, Baldi J, Cannata S, Guzowski J, Gargioli C, Khademhosseini A, Swieszkowski W. Tendon Tissue Engineering: Effects of Mechanical and Biochemical Stimulation on Stem Cell Alignment on Cell-Laden Hydrogel Yarns. Adv Healthc Mater. 2019;8:e1801218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 159. | Chen S, Wang H, Su Y, John JV, McCarthy A, Wong SL, Xie J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020;108:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 160. | Andalib MN, Lee JS, Ha L, Dzenis Y, Lim JY. Focal adhesion kinase regulation in stem cell alignment and spreading on nanofibers. Biochem Biophys Res Commun. 2016;473:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 161. | Jiao YP, Cui FZ. Surface modification of polyester biomaterials for tissue engineering. Biomed Mater. 2007;2:R24-R37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 162. | Richbourg NR, Peppas NA, Sikavitsas VI. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J Tissue Eng Regen Med. 2019;13:1275-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 163. | Rana D, Ramasamy K, Leena M, Jiménez C, Campos J, Ibarra P, Haidar ZS, Ramalingam M. Surface functionalization of nanobiomaterials for application in stem cell culture, tissue engineering, and regenerative medicine. Biotechnol Prog. 2016;32:554-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 164. | Yang L, Yaseen M, Zhao X, Coffey P, Pan F, Wang Y, Xu H, Webster J, Lu JR. Gelatin modified ultrathin silk fibroin films for enhanced proliferation of cells. Biomed Mater. 2015;10:025003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Bierbaum S, Hintze V, Scharnweber D. Functionalization of biomaterial surfaces using artificial extracellular matrices. Biomatter. 2012;2:132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 166. | Sadeghi AR, Nokhasteh S, Molavi AM, Khorsand-Ghayeni M, Naderi-Meshkin H, Mahdizadeh A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater Sci Eng C Mater Biol Appl. 2016;66:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 167. | Murschel F, Zaimi A, Noel S, Jolicoeur M, De Crescenzo G. Specific Adsorption via Peptide Tags: Oriented Grafting and Release of Growth Factors for Tissue Engineering. Biomacromolecules. 2015;16:3445-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 168. | Yazdi MK, Zare M, Khodadadi A, Seidi F, Sajadi SM, Zarrintaj P, Arefi A, Saeb MR, Mozafari M. Polydopamine Biomaterials for Skin Regeneration. ACS Biomater Sci Eng. 2022;8:2196-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 169. | Wang J, Chen Y, Zhou G, Mao C, Yang M. Polydopamine-Coated Antheraea pernyi (A. pernyi) Silk Fibroin Films Promote Cell Adhesion and Wound Healing in Skin Tissue Repair. ACS Appl Mater Interfaces. 2019;11:34736-34743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 170. | Wan J, Wu T, Wang K, Xia K, Yin L, Chen C. Polydopamine-modified decellularized intestinal scaffolds loaded with adipose-derived stem cells promote intestinal regeneration. J Mater Chem B. 2022;11:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 171. | Richardson JJ, Björnmalm M, Caruso F. Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science. 2015;348:aaa2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 867] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 172. | da Câmara PCF, Balaban RC, Hedayati M, Popat KC, Martins AF, Kipper MJ. Novel cationic tannin/glycosaminoglycan-based polyelectrolyte multilayers promote stem cells adhesion and proliferation. RSC Adv. 2019;9:25836-25846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 173. | Alven S, Aderibigbe BA. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 174. | Abdal Dayem A, Lee SB, Lim KM, Kim A, Shin HJ, Vellingiri B, Kim YB, Cho SG. Bioactive peptides for boosting stem cell culture platform: Methods and applications. Biomed Pharmacother. 2023;160:114376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 175. | Chen B, Liang Y, Zhang J, Bai L, Xu M, Han Q, Han X, Xiu J, Li M, Zhou X, Guo B, Yin Z. Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg(2+)/curcumin from injectable self-healing hydrogels. Theranostics. 2021;11:5911-5925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 176. | Elango J, Zamora-Ledezma C, Negrete-Bolagay D, Aza PN, Gómez-López VM, López-González I, Belén Hernández A, De Val JEMS, Wu W. Retinol-Loaded Poly(vinyl alcohol)-Based Hydrogels as Suitable Biomaterials with Antimicrobial Properties for the Proliferation of Mesenchymal Stem Cells. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 177. | Fadera S, Cheng NC, Young TH, Lee IC. In vitro study of SDF-1α-loaded injectable and thermally responsive hydrogels for adipose stem cell therapy by SDF-1/CXCR4 axis. J Mater Chem B. 2020;8:10360-10372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 178. | Tao K, Bai X, Ji P, Zhang Y, Cao T, Han F, Zhang Z, Guan H, Hu D. A Composite of Hepatocyte Growth Factor- and 5α-Dihydrotestosterone-Gelatin Microspheres with Adipose-Derived Stem Cells Enhances Wound Healing. Skin Pharmacol Physiol. 2022;35:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 179. | Jose S, Hughbanks ML, Binder BY, Ingavle GC, Leach JK. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014;10:1955-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 180. | Gallagher LB, Dolan EB, O'Sullivan J, Levey R, Cavanagh BL, Kovarova L, Pravda M, Velebny V, Farrell T, O'Brien FJ, Duffy GP. Pre-culture of mesenchymal stem cells within RGD-modified hyaluronic acid hydrogel improves their resilience to ischaemic conditions. Acta Biomater. 2020;107:78-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagdalian AA, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL