Published online Mar 26, 2024. doi: 10.4252/wjsc.v16.i3.267
Peer-review started: October 26, 2023
First decision: December 17, 2023
Revised: December 30, 2023
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: March 26, 2024
Processing time: 151 Days and 0.6 Hours
The bone remodeling during orthodontic treatment for malocclusion often requires a long duration of around two to three years, which also may lead to some complications such as alveolar bone resorption or tooth root resorption. Low-intensity pulsed ultrasound (LIPUS), a noninvasive physical therapy, has been shown to promote bone fracture healing. It is also reported that LIPUS could reduce the duration of orthodontic treatment; however, how LIPUS regulates the bone metabolism during the orthodontic treatment process is still unclear.
To investigate the effects of LIPUS on bone remodeling in an orthodontic tooth movement (OTM) model and explore the underlying mechanisms.
A rat model of OTM was established, and alveolar bone remodeling and tooth movement rate were evaluated via micro-computed tomography and staining of tissue sections. In vitro, human bone marrow mesenchymal stem cells (hBMSCs) were isolated to detect their osteogenic differentiation potential under compre
The force treatment inhibited the osteogenic differentiation potential of hBMSCs; moreover, the expression of osteogenesis markers, such as type 1 collagen (COL1), runt-related transcription factor 2, ALP, and osteocalcin (OCN), decreased. LIPUS could rescue the osteogenic differentiation of hBMSCs with increased expression of osteogenic marker inhibited by force. Mechanically, the expression of LaminA/C, F-actin, and YAP1 was downregulated after force treatment, which could be rescued by LIPUS. Moreover, the osteogenic differentiation of hBMSCs increased by LIPUS could be attenuated by YAP siRNA treatment. Consistently, LIPUS increased alveolar bone density and decreased vertical bone absorption in vivo. The decreased expression of COL1, OCN, and YAP1 on the compression side of the alveolar bone was partially rescued by LIPUS.
LIPUS can accelerate tooth movement and reduce alveolar bone resorption by modulating the cytoskeleton-Lamin A/C-YAP axis, which may be a promising strategy to reduce the orthodontic treatment process.
Core Tip: Low-intensity pulsed ultrasound can promote local alveolar bone remodeling and reduce vertical alveolar bone resorption and consequent gingival recession by regulating the osteogenic ability of bone marrow mesenchymal stem cells by upregulating the expression and nuclear translocation of Yes-associated protein decreased by mechanical stress via affecting the cytoskeleton and nuclear skeleton.
- Citation: Wu T, Zheng F, Tang HY, Li HZ, Cui XY, Ding S, Liu D, Li CY, Jiang JH, Yang RL. Low-intensity pulsed ultrasound reduces alveolar bone resorption during orthodontic treatment via Lamin A/C-Yes-associated protein axis in stem cells. World J Stem Cells 2024; 16(3): 267-286
- URL: https://www.wjgnet.com/1948-0210/full/v16/i3/267.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i3.267
Orthodontic treatment for malocclusion usually lasts 2-3 years, which brings great challenges to patient compliance and increases the risk of many complications, such as alveolar bone resorption, gingivitis, and other destructive diseases of the periodontal supporting tissue[1]. The alveolar bone loss caused by long-term orthodontic treatment may be due to imbalanced osteogenic and osteoclast activity.
Low-intensity pulsed ultrasound (LIPUS) usually refers to a pulse-emitted ultrasonic wave with an intensity between 30 and 100 mW/cm2, which is a noninvasive physical mechanical energy source[2]. It is delivered in the form of an acoustic wave and applied to tissue and cells to regulate biochemical functions[3]. As revealed by several clinical trials and animal experiments in vivo, LIPUS can reduce the fracture healing time[4] and effectively treat delayed fracture union[5] and bone nonunion[6], and is safer and noninvasive than other treatments.
LIPUS has also gained attention in the field of orthodontics. In vivo, LIPUS can increase the distance of the teeth movement[7]; a retrospective clinical study also showed that LIPUS reduced the duration of invisible treatment by an average of 49%[8]. In a clinical study, buccal alveolar bone thickness and height did not respond to LIPUS during maxillary arch expansion[9]. In a tooth movement model in rats, LIPUS application increased the number of osteoclasts on the compression side[10]. The increased osteoclasts may lead to alveolar bone resorption and periodontal supporting tissue destruction. It is unclear whether LIPUS regulates alveolar bone remodeling.
Bone marrow mesenchymal stem cells (BMSCs), as bone marrow-derived stem cells, exhibit self-renewal capacity and multiple differentiation potential and can differentiate into multiple types of cells, tissues, and organs under proper conditions[11]. LIPUS could regulate MSC growth[12] and promote chondrogenesis of MSCs seeded on three-dimensionally (3D) printed scaffolds[13]. Besides, MSCs encapsulated in hydrogels of certain stiffness show enhanced osteogenesis ability under LIPUS[14]. Few studies have reported the effect of LIPUS on cells loaded in compression. Whether LIPUS could regulate the property of MSCs under compression force to control the alveolar bone remodeling during orthodontic treatment and the underlying mechanism need to be investigated.
Here, we show that LIPUS could accelerate the orthodontic tooth movement (OTM) and increase alveolar bone density and decreased vertical bone absorption via the Lamin A/C-Yes-associated protein (YAP) axis, suggesting that LIPUS is a promising strategy to accelerate the orthodontic treatment with little side effects.
The human jawbone tissue sampling protocol gained approval from the Ethical Guidelines of Hospital of Stomatology, Peking University (No. PKUSSIRB-202385020) and was carried out after informed consent was obtained. BMSCs were isolated and cultured following the previously reported protocol[15]. Briefly, we obtained a small piece of cortical bone located above the tooth crown, which required removal during the extraction of the donor’s impacted third molars. The bone was carefully sectioned using a scalpel and subsequently digested using collagenase type I (2 mg/mL; Worthington Biochem, Lakewood, NJ, United States) and dispase II (4 mg/mL; Roche Diagnostic, Indianapolis, IN, United States) for an hour at 37 °C. Single-cell suspensions from the jaw bone were subsequently acquired using 70-μm cell strainers (BD Bioscience, United States). Then, single cells were seeded onto 100-mm dishes at 1 to 1.5 × 106 cells/mL. BMSCs were isolated and cultured following the previously reported protocol[15]. BMSCs were cultivated in Minimum Essential Medium α (VivaCell, Shanghai, China) supplemented with 15% fetal bovine serum (Gibco, Grand Island, NY, United States) and 1% penicillin-streptomycin solution (Cytiva, Shanghai, China) at 37 °C with 5% CO2, and the medium was changed at 3-d intervals. The expression of stem cell surface markers in BMSCs was characterized by flow cytometry according to the manufacturer’s protocol (BD Bioscience).
Before transfection, the cell culture medium was changed to standard conditions without penicillin-streptomycin solution. The cells were then transfected with control small interfering RNA (siRNA) or YAP1 siRNA (Ribobio, Guangzhou, China) via a riboFECT™ CP Transfection Kit (Ribobio) in accordance with the manufacturer’s instructions. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR), Western blotting, and immunofluorescence staining were subsequently performed to measure the knockdown rate.
BMSCs (1 × 105/mL) were seeded onto 6-well plates and cultured until the cell confluence reached 70%-80% before the medium was changed to osteogenic differentiation medium supplemented with L-ascorbic acid (50 μg/mL; Sigma, Missouri, United States), β-glycerophosphate (10 mmol/L; Sigma), and dexamethasone (0.1 μM; Sigma). During osteogenic induction of stem cells, the induction differentiation medium was changed every 3 d.
After induction for 14 d, alkaline phosphatase (ALP) staining (Beyotime, Shanghai, China) were conducted in accordance with the protocol, and so did alizarin red staining (Sigma) after induction for 21 d. For the quantification of mineralization, we dissolved red matrix sediment in 10% cetyl-pyridinium chloride (Macklin, Shanghai, China), and the absorbance of the solution was measured to determine the degree of mineralization quantitatively[16].
Compression force was applied to the BMSCs to mimic stress during orthodontic movement[17]. Briefly, a rounded glass sheet (30 mm in diameter) was placed over cell layers close to confluence in a 6-well plate. Stainless steel beads were placed above the glass sheet to adjust the static pressure to 1 g/cm2. The cells were under static compression for 24 h.
In this study, the LIPUS device was obtained from the Institute of Acoustics (Chinese Academy of Sciences, Beijing, China). The device has circular transducers with an area of 9.07 cm2 to match the area of a well in a 6-well plate. The cells were stimulated with LIPUS following the following specifications: 1.5 MHz frequency, 0.2 pulse duration ratio, 30 mW/cm2 incident intensity, and 1.0 kHz repetition rate. Stimulation was applied for 20 min every day in vivo and in vitro until the rats and cells were harvested, and the control group and force group were treated by pseudo-LIPUS. In vivo, the rats under anesthesia were placed at a constant location, after which the transducer was pressed against the side of the cheek closest to the maxillary first molar. In vitro, we attached the transducer to the bottom of the plate corresponding to the well[18].
After 7 d of osteogenic induction, we utilized TRIzol reagent (Invitrogen, California, United States) to extract total cellular RNA following the previous protocol[19]. Table 1 displays all the primers used. cDNA synthesis kits (Takara Bio, Tokyo, Japan) were used to prepare first-strand cDNA from RNA through reverse transcription in accordance with the protocol. A ViiaTM 7 Real-time PCR System (Applied Biosystems, Washington, Rhode Island, United States) was used for qRT-PCR, which was carried out in triplicate.
| Gene | Primer sequence | |
| GAPDH | Forward | TCATTGACCTCAACTACATG |
| Reverse | TCGCTCCTGGAAGATGGTGAT | |
| RUNX2 | Forward | TGGTTACTGTCATGGCGGGTA |
| Reverse | TCTCAGATCGTTGAACCTTGCTA | |
| ALP | Forward | ACTGGTACTCAGACAACGAGAT |
| Reverse | ACGTCAATGTCCCTGATGTTATG | |
| COL1a1 | Forward | GTGCGATGACGTGATCTGTGA |
| Reverse | CGGTGGTTTCTTGGTCGGT | |
| OCN | Forward | CACTCCTCGCCCTATTGGC |
| Reverse | CAGCAGAGCGACACCCTAGAC | |
| YAP1 | Forward | AGAATCAGTCAGAGTGCTCCAGTG |
| Reverse | CGCAGCCTCTCCTTCTCCATC | |
After 7 d of osteogenic induction, RIPA buffer containing 1 mM phenylmethanesulfonyl fluoride (Solarbio, Beijing, China) was added for cell lysis, followed by centrifugation at 12000 × g for 20 min at 4 °C. Thereafter, the supernatant was quantified with a BCA assay (Beyotime). Total protein was added to loading buffer, and the mixture was subsequently boiled for 10 min at 100 °C. Thereafter, the samples were stored at -20 °C. Equal amounts of total protein (30 μg) were loaded on sodium dodecyl sulfate-polyacrylamide gels and separated prior to electroblotting onto polyvinylidene difluoride membranes (Millipore, Billerica, Massachusetts, United States). After blocking with 5% bovine serum albumin, the membranes were incubated overnight on a shaker at 4 °C. The primary antibodies used were rabbit anti-type 1 collagen (COL1) and anti-osteocalcin (OCN) (1:500; Abcam, Cambridge, Massachusetts, United States), rabbit anti-ALP (1:500; Invitrogen), and mouse anti-GAPDH (1:1000; ProteinTech, Cook, Illinois, United States). Finally, HRP-labeled secondary antibodies (Zsgb-Bio, Beijing, China) were added for another 1-h incubation, followed by visualization via enhanced chemiluminescence (NCM Biotech, Suzhou, China). ImageJ software was used for band intensity analysis.
After 7 d of osteogenic induction, 4% paraformaldehyde (PFA) was added for 15 min to fix the cells, after which the cells were rinsed with phosphate buffered saline (PBS) three times prior to 10 min of permeabilization with 0.5% Triton X-100 and washing with PBS. Thereafter, 5% BSA was added to the block cells for an hour before they were incubated with primary antibodies (1:200; Abcam) against YAP1, Lamin A/C, and F-actin at 4 °C overnight. The cells were washed with PBS before further incubation with Alexa Fluor 488- and Alexa Fluor 594-labeled antibodies (1:200; Invitrogen) for one hour. Finally, medium containing DAPI was added to the mount cells after washing. Images were captured with an inverted confocal microscope (Olympus, Tokyo, Japan).
Male SD rats (6 wk old) weighing 200 ± 20 g were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed under laboratory conditions (12 h light/dark cycle, 21 ± 2 °C, 50% humidity, ad libitum access to food and water). All animal protocols utilized in the present work were approved by the Ethics Committee for Animal Experiments at Peking University Health Science Center (No. LA2022288) and were designed to minimize animal pain or discomfort.
In total, 27 male rats were randomized to control (n = 3), force (day 7 and day 14) (n = 6 per group), or force + LIPUS group (day 7 and day 14) (n = 6 per group). Before establishing the experimental OTM model, each rat was administered pentobarbital sodium (40 mg/kg of body weight) for anesthesia. In those latter two groups, the OTM model was established as described in our previous study[20]. A stainless steel ligation wire (0.025 mm in diameter; Tomy International, Inc., Tokyo, Japan) was used to ligate a closed-coil spring (Tomy International, Inc., Tokyo, Japan) to the maxillary first molar and incisor neck. The spring offers 50 g of force to move the maxillary first molar mesially. To enhance the retaining force and prevent the device from falling off, a 0.5 mm deep groove was made by a slow speed mill near the gingival margin of the maxillary incisor to accommodate the ligature wire, which was subsequently filled with flowing resin (3 M, Minnesota, United States). All animals were sacrificed with an overdose of pentobarbital sodium (150 mg/kg) for tissue collection.
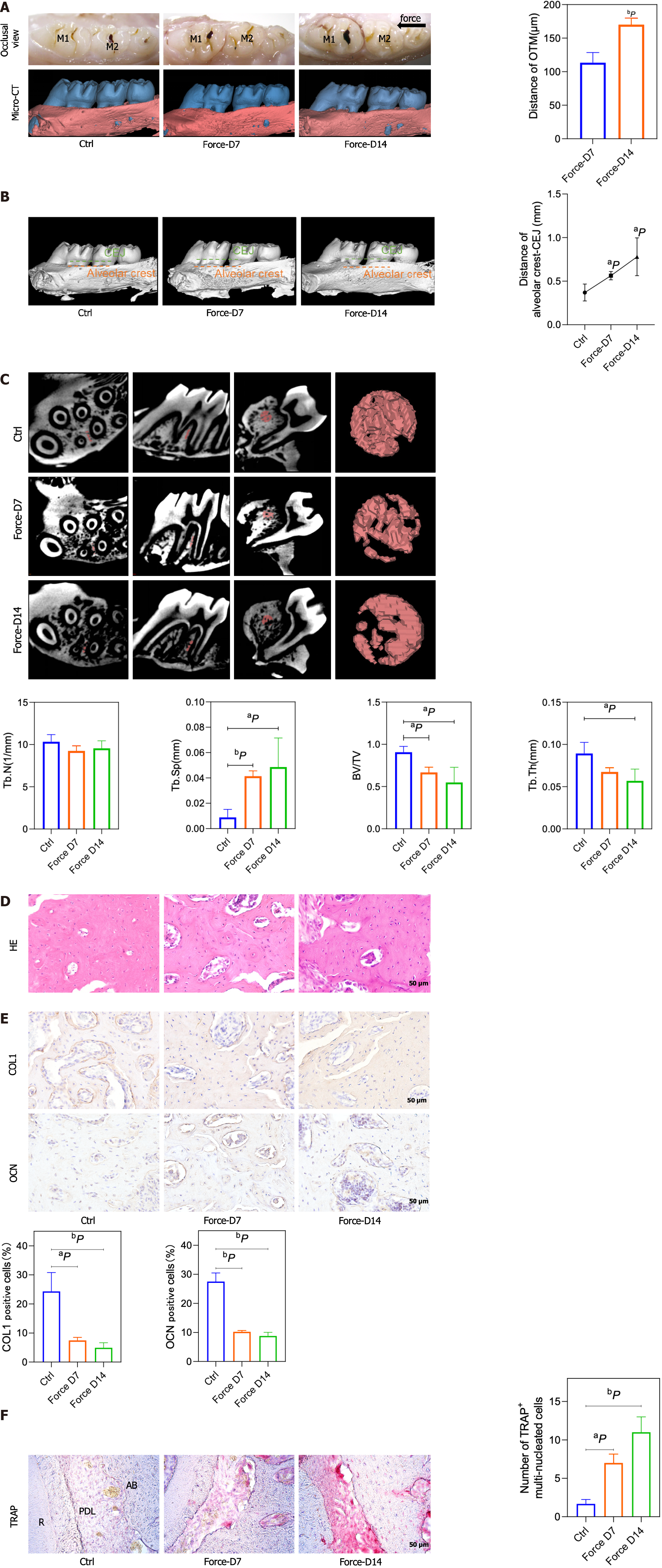
A microcomputed tomography scanner was used to scan maxillary samples at 8.82 μm resolution, 500 μA tube current, 60 kV tube voltage, and 1500 ms exposure time. Inveon Research Workplace software (Siemens, Munich, Germany) together with Mimics Research 21.0 software (Materialise, Leuven, Belgium) was used for raw data reconstruction. With respect to the reconstructed 3D model, the shortest distance from the first to second molar crown was considered the tooth movement distance (Figure 1B). A straight plane was made at the cementum-enamel boundary (CEJ) on the first molar’s distal buccal side, and the farthest distance between it and the parallel line tangent to alveolar crest resorption was measured to assess the reduction in alveolar bone height. In addition, Inveon Research Workplace software was used to evaluate the BV/TV, Tb.Th, Tb.N, Tb.Sp, and BS/BV in the chosen regions of interest (ROIs) (Figure 1C) by the reviewer, who was blinded to the groupings. All the samples were analyzed thrice to obtain the means.
We set the experimental period at 7 and 14 d following the establishment of the tooth movement device. The rats were sacrificed with an overdose of pentobarbital sodium, after which the maxilla was dissected and immersed in 4% paraformaladehyde. For hematoxylin and eosin (HE), tartrate-resistant acid phosphatase (TRAP), and immunohistochemical staining, the trimmed tissues were decalcified with 5% ethylenediaminetetraacetic acid disodium for 15 d, followed by ethanol dehydration and paraffin embedding. Thereafter, the samples were cut into 5-μm vertical serial sections with a rotary microtome (RM2125RT, Leica, Heidelberger, Germany).
Sections were stained with an HE staining kit (Sigma, Missouri, United States) or a TRAP staining kit (Solarbio, Beijing, China) following the protocols. Immunohistochemical staining was performed as follows. After xylene deparaffinization and ethanol rehydration, 0.125% trypsin and 20 μg/mL proteinase K solution were added to the sections, which were incubated at 37 °C for a 30-min period. Endogenous peroxidase activity was blocked with 3% H2O2 for a 30-min period at room temperature. The sections were subsequently washed with PBS, blocked with 5% BSA for an hour, and incubated with polyclonal anti-rabbit COL1 and OCN antibodies (1:200; Abcam) overnight at 4 °C, followed by another 1-h incubation with HRP-labeled secondary goat anti-rabbit IgG (Zsgb-Bio, Beijing, China) at room temperature. Diaminobenzidine (Zsgb-Bio) was used for visualization in accordance with the protocol. After hematoxylin counterstaining, gradient ethanol, and xylene dehydration, the sections were mounted using neutral resins.
The data are presented as the mean ± SD and were analyzed with GraphPad Prism 7 software (GraphPad, Inc., La Jolla, CA, United States). Every assay was carried out thrice. Student’s t test was used to evaluate intergroup differences, while one-way analysis of variance (ANOVA) with multiple comparisons was used to evaluate differences among multiple groups. P < 0.05 indicated statistical significance.
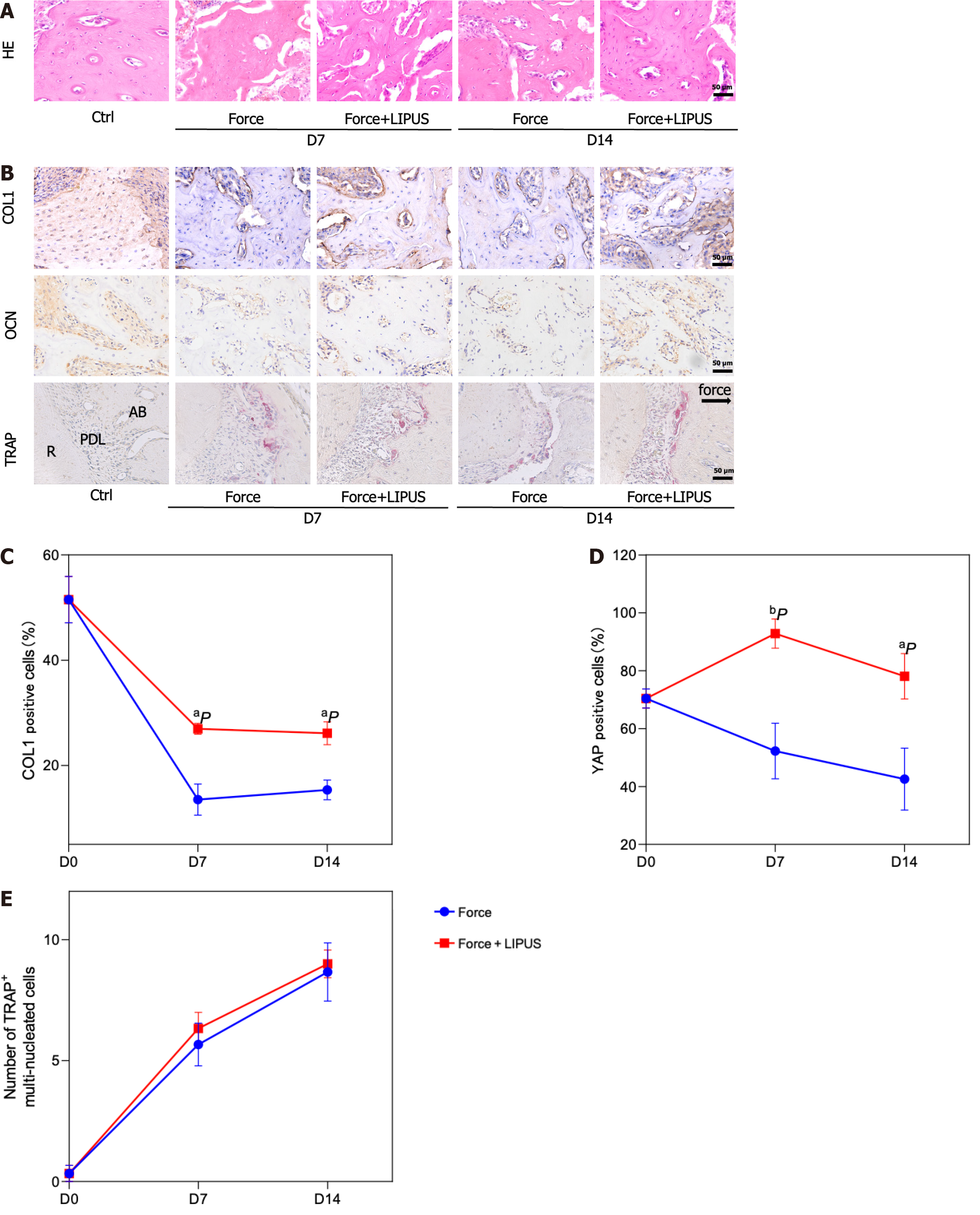
We detected the movement distance of the maxillary first molars on the 7th d and 14th d after force application and found that the movement distance increased with time under the effect of 50 g of force (P < 0.01, n = 3) (Figure 1A), suggesting that the rat OTM model was successfully constructed. Moreover, the distance between the alveolar crest and CEJ significantly increased on day 7 and day 14, showing that orthodontic force led to vertical resorption of alveolar bone beginning on day 7 (Figure 1B). We selected the alveolar bone mesial to the medial 1/3 of the first molar’s distal buccal root with a certain thickness as the ROI (Figure 1C). Micro-computed tomography results showed that at 7 and 14 d of tooth movement, the alveolar bone BV/TV and Tb.Th decreased after force application, while the BS/BV and Tb.Sp increased after force application (Figure 1C). This result showed that the bone density decreased on the compression side after orthodontic force treatment. HE staining and immunohistochemical staining revealed a decreased number of cells positive for the osteogenic markers COL1 and OCN in alveolar bone on the pressure side (Figure 1D and E), with a greater number of TRAP-positive cells on the pressure side near the alveolar bone edge (Figure 1F).
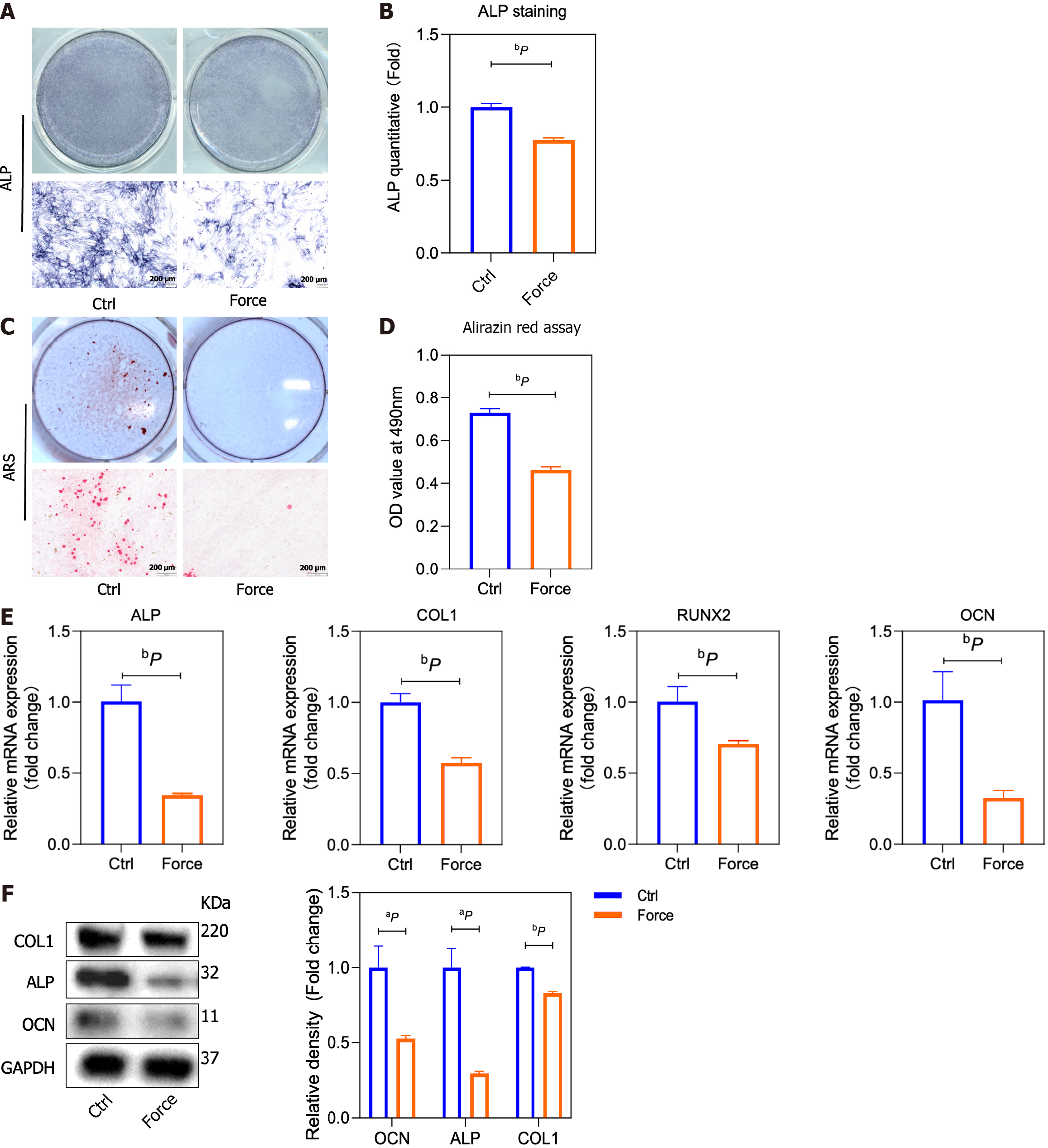
Static pressure was used in vitro to simulate alveolar bone on the pressurized side. Compared to those in the control group, the osteogenic differentiation of the BMSCs in the force group was decreased, as evidenced by the ALP and alizarin red staining results (Supplementary Figure 1 and Figure 2A-D). Correspondingly, the expression levels of the osteogenesis-related markers ALP, COL1, runt-related transcription factor 2 (RUNX2), and OCN in the force group significantly decreased (Figure 2E and F) compared to the control ones, as detected by qRT-PCR and Western blot.
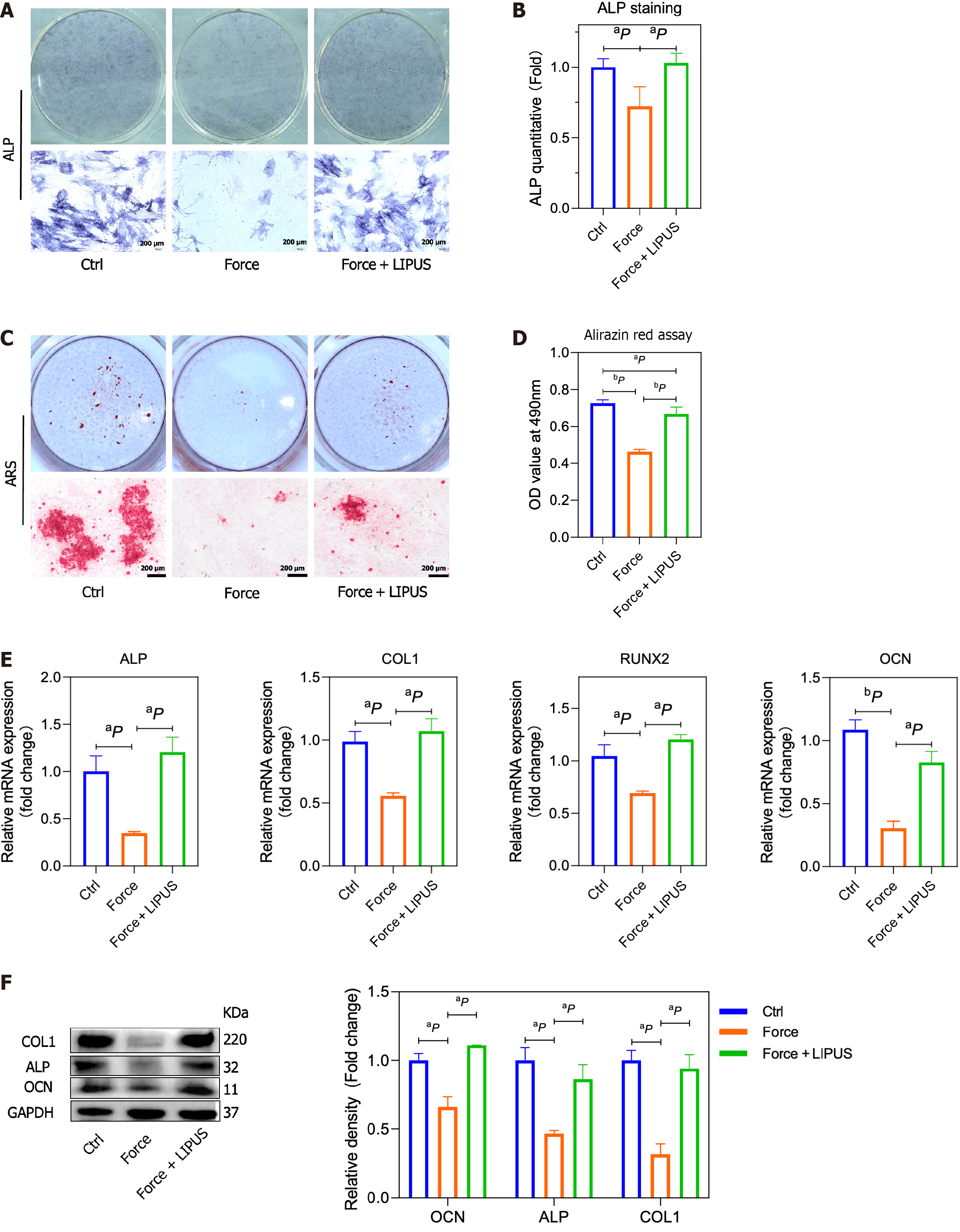
The force treatment decreased the osteogenic differentiation of BMSCs, while LIPUS could rescue the impaired osteogenic differentiation of BMSCs caused by force treatment (Figure 3A-D), as shown by ALP and alizarin red staining. Moreover, the expression of ALP, COL1, RUNX2, and OCN was accordingly increased after LIPUS treatment compared with the force group, as assessed by qRT-PCR (Figure 3E) and Western blot (Figure 3F).
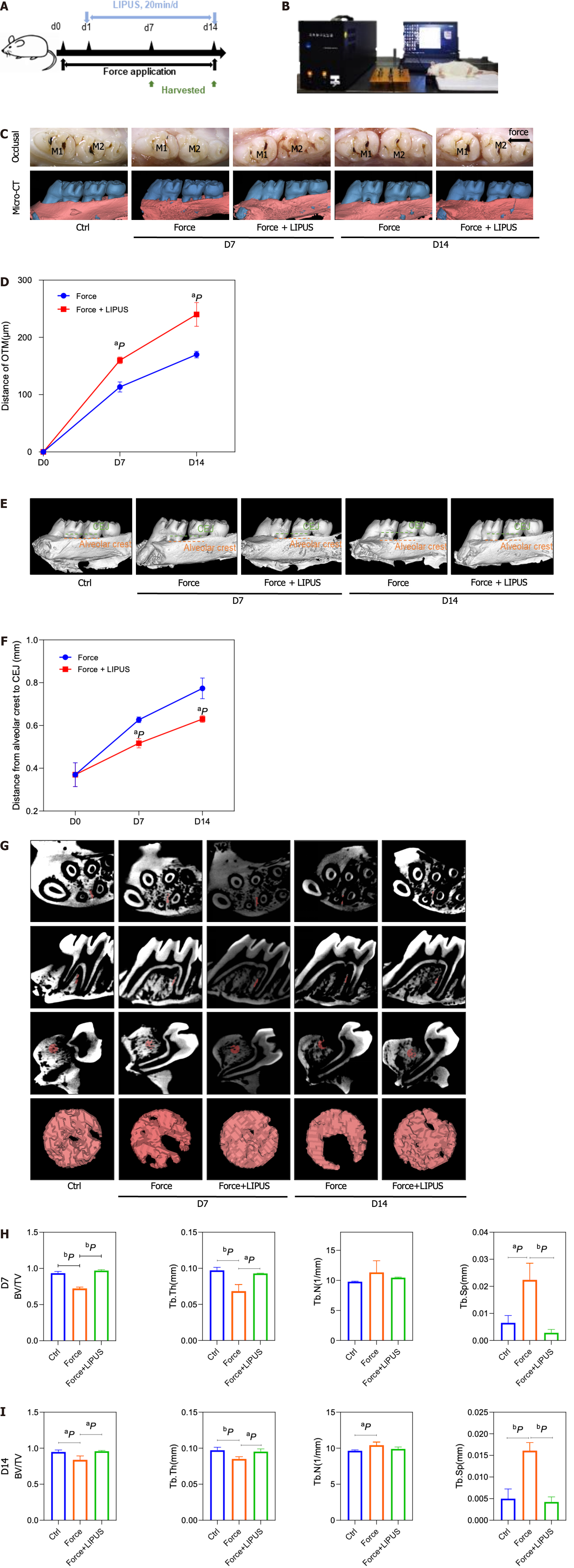
The movement distance of the first molar in the force + LIPUS group was greater than that of the force group on the 7th and 14th d after force application (P < 0.05) (Figure 4A-D). Moreover, the height of alveolar bone resorption was also reduced in the LIPUS treatment group (P < 0.05) (Figure 4E and F). ROI measurements of the corresponding sites indicated that the BV/TV and Tb.Th of the force + LIPUS group were higher than those of the force group, while the BS/BV and Tb.Sp were decreased (P < 0.05, n = 3) (Figure 4G-I), indicating that LIPUS promoted alveolar bone formation. Furthermore, the expression of COL1 and OCN was increased on the compressed side in the LIPUS treatment group compared with the force group (Figure 5A-D). However, no significant differences in TRAP-positive cell numbers were observed between the force group and the force + LIPUS group (Figure 5B and E).
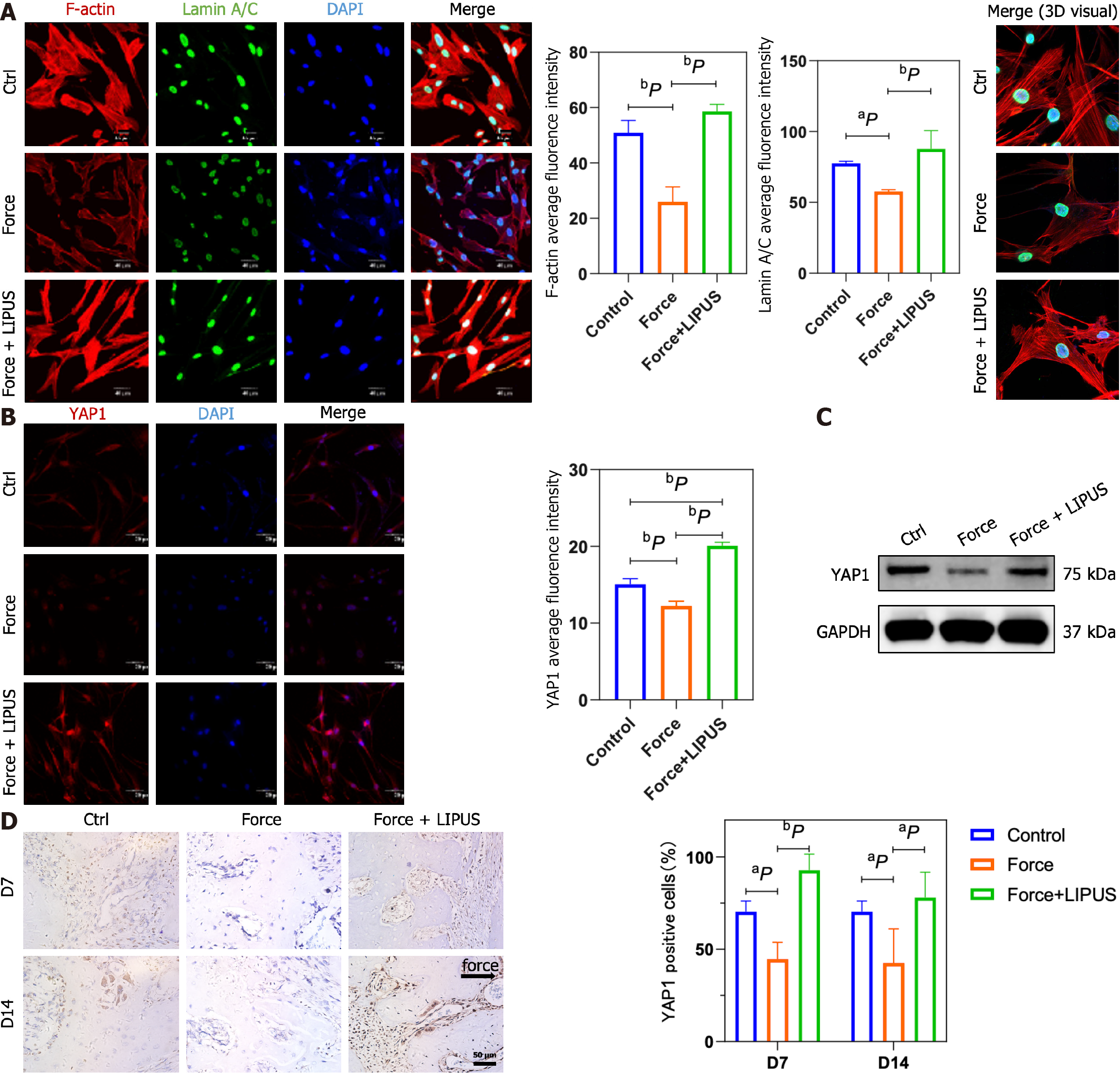
To explore the role of LIPUS in regulating the cytoskeleton in BMSCs, we performed F-actin and Lamin A/C immunofluorescence co-staining in vitro. The results showed that the compressive force treatment downregulated the expression of F-actin, disrupted the cytoskeleton, and inhibited the Lamin A/C ratio in BMSCs, while LIPUS effectively reversed these changes (Figure 6A). Lamin A/C was reported to mediate the YAP1 nuclear localization by regulating nuclear stiffness[21,22]. Next, we analyzed the expression of YAP1. The results showed that the expression of YAP1 was decreased by force treatment, whereas LIPUS restored the expression of YAP1 and increased the nuclear localization of YAP1, as shown by immunofluorescence staining and Western blot (Figure 6B and C). Consistently, the expression of YAP1 was decreased in the force group, while its expression was increased in the LIPUS treatment group in vivo, as analyzed by immunochemical staining (Figure 6D).
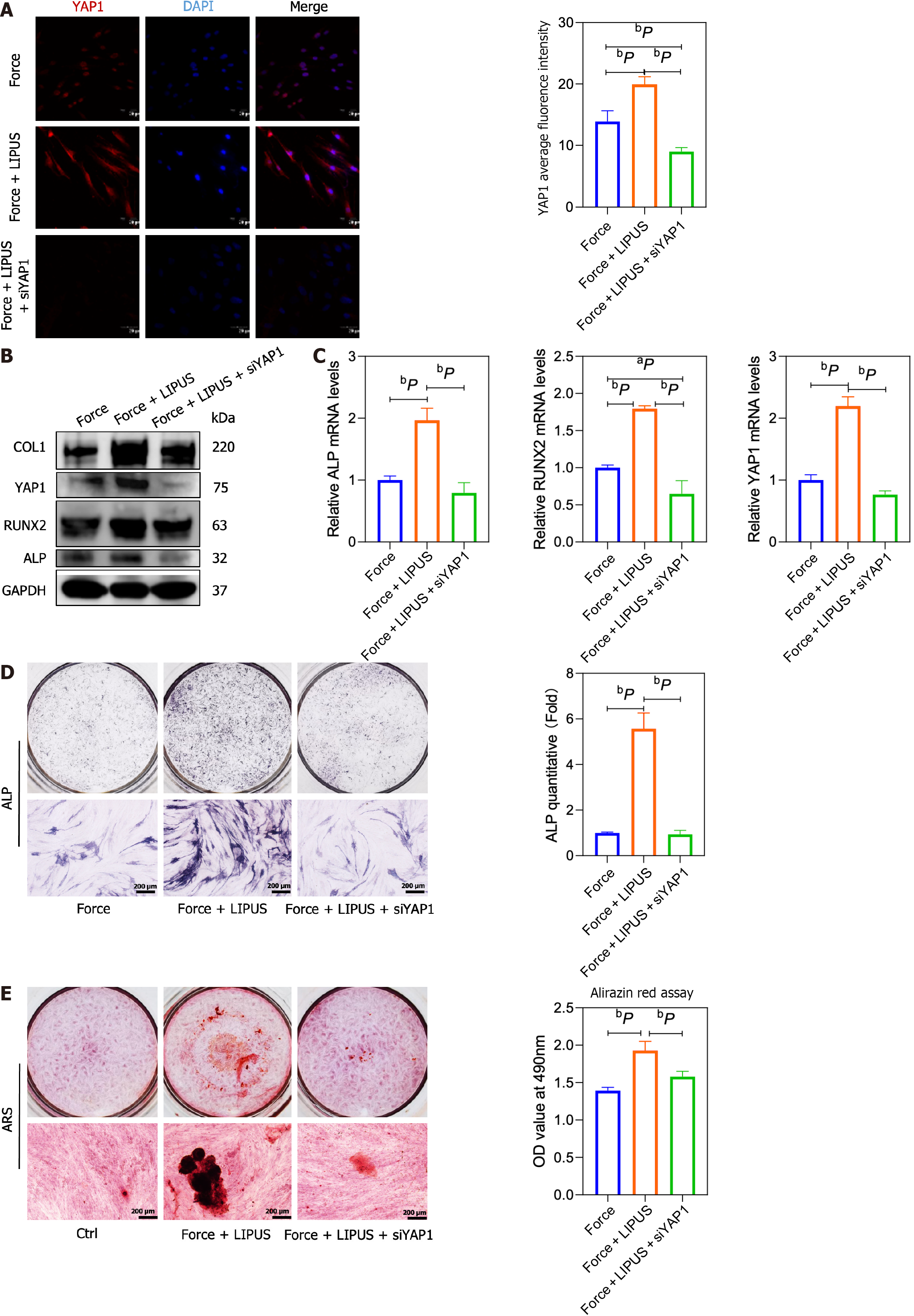
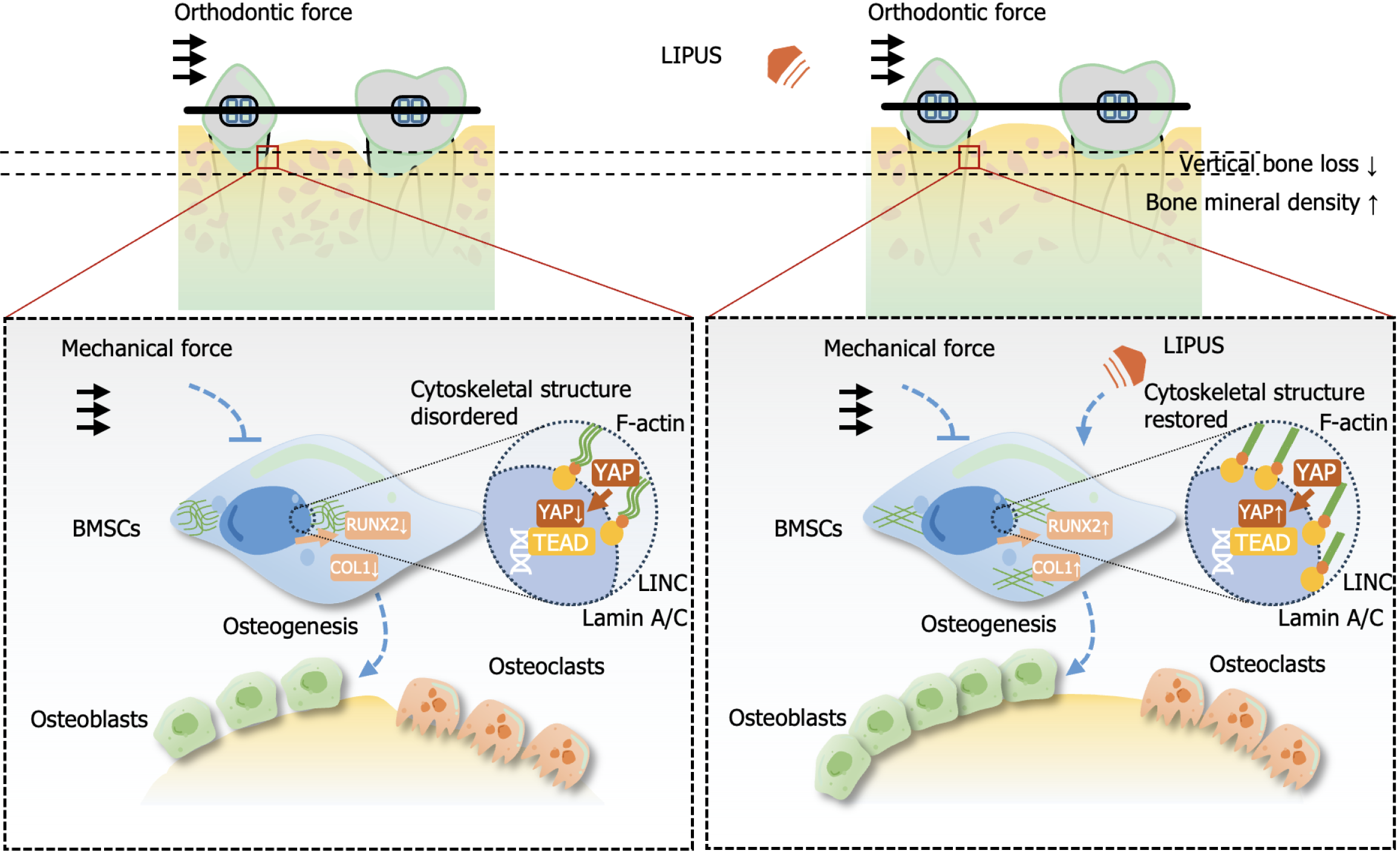
To verify the role of YAP1 on the property of BMSCs, we used YAP1 siRNA to treat BMSCs (Figure 7A), and the results showed that the expression of osteogenic differentiation-related markers ALP, COL1, RUNX2 and OCN increased by LIPUS treatment could be blocked by YAP1 siRNA treatment (Figure 7B and C). The osteogenic differentiation of BMSCs increased by LIPUS treatment could also be inhibited by YAP1 siRNA treatment, as assessed by ALP and alizarin red staining (Figure 7D and E). Our results demonstrated that LIPUS can accelerate tooth movement and reduce alveolar bone resorption by modulating the cytoskeleton-Lamin A/C-YAP axis (Figure 8).
The process of orthodontic treatment is a bone remodeling process. The force applied to drive tooth movement in some extent led to some adverse reactions, such as root resorption and bone mineral density decline. LIPUS has been reported to alleviate chondrocyte damage in temporomandibular disorders[23], reduce root resorption[24], and enhance bone remodeling[10]. However, there is a lack of research on the effect of LIPUS on the aesthetic problem of gingival recession due to the loss of alveolar bone height during orthodontic treatment. Changes in alveolar bone morphology affect the aesthetic effect, safety, and stability[25] of orthodontic treatment. Clinical studies have shown that alveolar bone height loss often leads to insufficient periodontal supporting tissue, decreased dental stability, and aesthetic problems in the anterior teeth[26]. Our experiments showed that LIPUS could effectively ameliorate the aesthetic and health problems caused by alveolar bone height loss by reducing vertical alveolar bone resorption and improving the morphology of alveolar bone remodeling, and filled the research gap in the relevant field.
Clinical studies have used cone beam computed tomography to assess changes in periodontal bone mineral density during orthodontic treatment. On average, the alveolar bone mineral density decreases by 24% after 7 mo of orthodontic treatment, while the region with the largest reduction in bone mineral density seems to be related to the direction of tooth movement[27,28]. Consistently, in the present study, we found that bone mineral density decreased at 7 and 14 d after stress. Mechanical stress can modulate osteoclasts and osteoblasts, thereby promoting bone reconstruction[29,30]. Excessive compressive force can cause stem cell apoptosis[31], aggravate mitochondrial dysfunction, ATP consumption, and oxidative stress in stem cells[32], and inhibit the proliferation, colony formation, and migration of stem cells[33]. Bone mineral density is positively correlated with OCN protein level[34]. In our study, static pressure on BMSCs inhibited the mRNA and protein expression of osteogenic differentiation markers such as COL1 and OCN, which partly explained the reduction in alveolar bone mineral density around moving teeth under orthodontic force.
Previous studies have shown that LIPUS plays a role in bone metabolism and remodeling by regulating osteogenic and osteoclast activity[35-37]. In our study, on day 7 and day 14, the number of osteoclasts in the force group was not significantly different from that in the force + LIPUS group, indicating that LIPUS may promote bone remodeling by promoting osteoclast activity but not by increasing the number of osteoclasts. As shown in numerous clinical trials and animal studies, LIPUS promotes osteogenesis, resulting in improved and quicker fracture healing[38,39]. It can also stimulate condylar growth and increase mandibular ramus height in rabbits[40,41]. LIPUS is effective in various cell processes, such as growth, differentiation[42,43], extracellular matrix formation, and mineralization of osteoblasts[44], and involves multiple signaling pathways, such as the hedgehog and TRPM7 pathways[45,46]. In addition to osteoblasts, LIPUS can promote the differentiation of adipose-derived stromal cells[47], mesenchymal stem cells[48], periodontal ligament stem cells[49], and rat osteosarcoma cell lines[50] and increase the expression of RUNX2 and COL1. LIPUS stimulation of BMSCs can enhance cell activity, promote osteogenic differentiation, and increase COL1 and OCN gene expression[51]. To date, few reports have focused on the role of LIPUS in cellular functions under loading conditions. In our study, LIPUS increased COL1, RUNX2, and OCN expression at the mRNA and protein levels, and rescued the inhibitory effect of compression force on the osteoblastic differentiation of BMSCs, demonstrating a positive effect on alveolar bone remodeling during orthodontic processing at the cellular level.
The way that mechanical signals can be transmitted from the plasma membrane to the nucleus directly via the cytoskeleton is considered to be an important mechanical signal transduction pathway[52]. The nucleus is considered a key mechanoreceptor that can directly influence chromatin organization, epigenetic modifications, and gene expression[53]. Lamin A/C, as LMNA-encoded Lamin proteins, participate in nuclear mechanics[54] and the transduction of mechanical signals[55,56], thereby regulating the fate of stem cells. The lincRNAs (linkers of cytoskeleton and nucleoskeleton) complex links Lamin A/C to the cytoskeleton, thereby mediating the transmission of mechanical signals from the cytoskeleton to the nucleus[57]. The Lamin A level is known to increase during osteogenic differentiation of MSCs but decrease during adipogenic differentiation[58,59]. In our study, Lamin A/C and F-actin were consistently downregulated after compression force application, and the cytoskeleton was more disordered under compression. To our knowledge, our study is the first to show that LIPUS increases Lamin A/C and F-actin expression and reorders the cytoskeleton under compression, thus reversing the decrease in osteogenesis of BMSCs induced by static force.
As a transcriptional coactivator protein, YAP1 is closely associated with changes in the mechanical state of cellular microenvironments[60]. YAP1 can relocate to the nucleus from the cytoplasm, interact with the TEA domain[61], and promote transcription. In mechanical transduction, it is also a downstream signal for the assembly and contraction of actin filaments[62]. Pressure can promote F-actin depolymerization and lead to cytoplasmic translocation of YAP1[63]. YAP1 was found to participate in multiple cellular activities. For example, YAP1 was reported to be activated during inflammation in endothelial cells induced by lipopolysaccharide[64] and was essential for epithelial cell proliferation[65]. In the inflammatory microenvironment in periodontitis, YAP1 expression and nuclear translocation are decreased[66]. In the force group, the YAP1 level decreased, while in the force + LIPUS group, the YAP1 level increased both in vitro and in vivo, suggesting that YAP1 exerts a crucial effect on the regulation of BMSC osteogenesis by mechanical force and LIPUS and might be a downstream effector of the cytoskeleton and nuclear skeleton.
This study has some limitations. Osteoblasts and osteoclasts jointly participate in the process of bone metabolism. This study mainly focused on the effect of LIPUS on the osteogenic function of stem cells, and whether LIPUS could regulate the crosstalk of osteoclasts and osteoblasts is unclear, which needs further investigation. In addition, the underlying mechanism of how LIPUS controls cytoskeleton changes remains unclear.
In summary, LIPUS can promote local alveolar bone remodeling, increase bone mineral density, and reduce vertical alveolar bone resorption and consequent gingival recession by regulating the osteogenic ability of BMSCs. In terms of mechanism, LIPUS upregulates the expression and nuclear translocation of YAP, which is decreased by mechanical stress through effects on the cytoskeleton and nuclear skeleton, thereby affecting the osteogenic differentiation of BMSCs.
The bone remodeling during orthodontic treatment for malocclusion often requires a long duration, which also may lead to some complications such as alveolar bone resorption. Low-intensity pulsed ultrasound (LIPUS), a noninvasive physical therapy, has been shown to promote bone fracture healing and reduce the duration of orthodontic treatment; however, how LIPUS regulates the bone metabolism during the orthodontic treatment process is still unclear.
How to shorten the orthodontic treatment duration and reduce the side effects caused by orthodontic treatment such as alveolar bone resorption has become a very important clinical problem. LIPUS, as a non-invasive physical therapy, has been reported to promote the fracture healing process, and may also play a good role in orthodontic treatment.
This study was to investigate the effects of LIPUS on bone remodeling in an orthodontic tooth movement (OTM) model and explore the underlying mechanisms.
We established a rat model of OTM, and alveolar bone remodeling and tooth movement rate were evaluated via micro-computed tomography and staining of tissue sections. In vitro, human bone marrow mesenchymal stem cells (hBMSCs) were isolated to detect their osteogenic differentiation potential under compression and LIPUS stimulation and to investigate the underlying mechanisms.
The force treatment inhibited the expression of osteogenesis markers and osteogenic differentiation potential of hBMSCs, which could be rescued by LIPUS treatment. Mechanically, the expression of LaminA/C, F-actin, and Yes-associated protein (YAP1) was downregulated after force application, which could be rescued by LIPUS treatment. Moreover, the osteogenic differentiation of MSCs increased by LIPUS treatment could be attenuated by YAP small interfering RNA treatment. Consistently, LIPUS increased alveolar bone density and decreased vertical bone absorption in vivo. The decreased expression of type 1 collagen, osteocalcin, and YAP1 on the compression side of the alveolar bone was partially rescued by the LIPUS treatment.
By regulating the cytoskeleton-Lamin A/C-YAP axis, LIPUS can effectively accelerate tooth movement and reduce bone resorption. Therefore, LIPUS can be used as an effective auxiliary method for orthodontic treatment.
These results may provide an adjunctive treatment strategy for orthodontic treatment and enrich the theoretical basis for LIPUS application.
| 1. | Kirschneck C, Fanghänel J, Wahlmann U, Wolf M, Roldán JC, Proff P. Interactive effects of periodontitis and orthodontic tooth movement on dental root resorption, tooth movement velocity and alveolar bone loss in a rat model. Ann Anat. 2017;210:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Hu B, Zhang Y, Zhou J, Li J, Deng F, Wang Z, Song J. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS One. 2014;9:e95168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Liu Z, Xu J, E L, Wang D. Ultrasound enhances the healing of orthodontically induced root resorption in rats. Angle Orthod. 2012;82:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Chung SL, Pounder NM, de Ana FJ, Qin L, Sui Leung K, Cheung WH. Fracture healing enhancement with low intensity pulsed ultrasound at a critical application angle. Ultrasound Med Biol. 2011;37:1120-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Naruse K, Uchino M, Hirakawa N, Toyama M, Miyajima G, Mukai M, Urabe K, Uchida K, Itoman M. 4. The Low-Intensity Pulsed Ultrasound (LIPUS) Mechanism and the Effect of Teriparatide on Fracture Healing. J Orthop Trauma. 2016;30:S3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Zura R, Della Rocca GJ, Mehta S, Harrison A, Brodie C, Jones J, Steen RG. Treatment of chronic (>1 year) fracture nonunion: heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS). Injury. 2015;46:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Alazzawi MMJ, Husein A, Alam MK, Hassan R, Shaari R, Azlina A, Salzihan MS. Effect of low level laser and low intensity pulsed ultrasound therapy on bone remodeling during orthodontic tooth movement in rats. Prog Orthod. 2018;19:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Kaur H, El-Bialy T. Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS). J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Bahammam M, El-Bialy T. Effect of Low-Intensity Pulsed Ultrasound (LIPUS) on Alveolar Bone during Maxillary Expansion Using Clear Aligners. Biomed Res Int. 2022;2022:4505063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Arai C, Kawai N, Nomura Y, Tsuge A, Nakamura Y, Tanaka E. Low-intensity pulsed ultrasound enhances the rate of lateral tooth movement and compensatory bone formation in rats. Am J Orthod Dentofacial Orthop. 2020;157:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 12. | Xie S, Jiang X, Wang R, Xie S, Hua Y, Zhou S, Yang Y, Zhang J. Low-intensity pulsed ultrasound promotes the proliferation of human bone mesenchymal stem cells by activating PI3K/AKt signaling pathways. J Cell Biochem. 2019;120:15823-15833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Aliabouzar M, Lee SJ, Zhou X, Zhang GL, Sarkar K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol Bioeng. 2018;115:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Assanah F, Grassie K, Anderson H, Xin X, Rowe D, Khan Y. Ultrasound-derived mechanical stimulation of cell-laden collagen hydrogels for bone repair. J Biomed Mater Res A. 2023;111:1200-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Pettersson LF, Kingham PJ, Wiberg M, Kelk P. In Vitro Osteogenic Differentiation of Human Mesenchymal Stem Cells from Jawbone Compared with Dental Tissue. Tissue Eng Regen Med. 2017;14:763-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Zhan X, Li S, Cui Y, Tao A, Wang C, Li H, Zhang L, Yu H, Jiang J, Li C. Comparison of the osteoblastic activity of low elastic modulus Ti-24Nb-4Zr-8Sn alloy and pure titanium modified by physical and chemical methods. Mater Sci Eng C Mater Biol Appl. 2020;113:111018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Wang J, Jiao D, Huang X, Bai Y. Osteoclastic effects of mBMMSCs under compressive pressure during orthodontic tooth movement. Stem Cell Res Ther. 2021;12:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Zhu Y, Ai D, Zhou M, Li H, Fu Y, Song J. Low-intensity pulsed ultrasound regulates osteoblast-osteoclast crosstalk via EphrinB2/EphB4 signaling for orthodontic alveolar bone remodeling. Front Bioeng Biotechnol. 2023;11:1192720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Wang H, Li Y, Liu S, Chen J, Ying H. miR-24 and miR-122 Negatively Regulate the Transforming Growth Factor-β/Smad Signaling Pathway in Skeletal Muscle Fibrosis. Mol Ther Nucleic Acids. 2018;11:528-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Xin J, Zhan X, Zheng F, Li H, Wang Y, Li C, Jiang J. The effect of low-frequency high-intensity ultrasound combined with aspirin on tooth movement in rats. BMC Oral Health. 2023;23:642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Koushki N, Ghagre A, Srivastava LK, Molter C, Ehrlicher AJ. Nuclear compression regulates YAP spatiotemporal fluctuations in living cells. Proc Natl Acad Sci U S A. 2023;120(28) e2301285120. [PMID:37399392 DOI: 10.1073/pnas.2301285120]. |
| 22. | Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171:1397-1410.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 1035] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 23. | Yang T, Liang C, Chen L, Li J, Geng W. Low-Intensity Pulsed Ultrasound Alleviates Hypoxia-Induced Chondrocyte Damage in Temporomandibular Disorders by Modulating the Hypoxia-Inducible Factor Pathway. Front Pharmacol. 2020;11:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | El-Bialy T, Farouk K, Carlyle TD, Wiltshire W, Drummond R, Dumore T, Knowlton K, Tompson B. Effect of Low Intensity Pulsed Ultrasound (LIPUS) on Tooth Movement and Root Resorption: A Prospective Multi-Center Randomized Controlled Trial. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Ahn HW, Moon SC, Baek SH. Morphometric evaluation of changes in the alveolar bone and roots of the maxillary anterior teeth before and after en masse retraction using cone-beam computed tomography. Angle Orthod. 2013;83:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Zheng Y, Zhu C, Zhu M, Lei L. Difference in the alveolar bone remodeling between the adolescents and adults during upper incisor retraction: a retrospective study. Sci Rep. 2022;12:9161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Chang HW, Huang HL, Yu JH, Hsu JT, Li YF, Wu YF. Effects of orthodontic tooth movement on alveolar bone density. Clin Oral Investig. 2012;16:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Hsu JT, Chang HW, Huang HL, Yu JH, Li YF, Tu MG. Bone density changes around teeth during orthodontic treatment. Clin Oral Investig. 2011;15:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Carter DR. Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int. 1984;36 Suppl 1:S19-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 282] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol. 2000;19:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 169] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Hu Y, Huang L, Shen M, Liu Y, Liu G, Wu Y, Ding F, Ma K, Wang W, Zhang Y, Shao Z, Cai X, Xiong L. Pioglitazone Protects Compression-Mediated Apoptosis in Nucleus Pulposus Mesenchymal Stem Cells by Suppressing Oxidative Stress. Oxid Med Cell Longev. 2019;2019:4764071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Li Z, Chen S, Ma K, Lv X, Lin H, Hu B, He R, Shao Z. CsA attenuates compression-induced nucleus pulposus mesenchymal stem cells apoptosis via alleviating mitochondrial dysfunction and oxidative stress. Life Sci. 2018;205:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Liang H, Chen S, Huang D, Deng X, Ma K, Shao Z. Effect of Compression Loading on Human Nucleus Pulposus-Derived Mesenchymal Stem Cells. Stem Cells Int. 2018;2018:1481243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Roomi AB, Mahdi Salih AH, Noori SD, Nori W, Tariq S. Evaluation of Bone Mineral Density, Serum Osteocalcin, and Osteopontin Levels in Postmenopausal Women with Type 2 Diabetes Mellitus, with/without Osteoporosis. J Osteoporos. 2022;2022:1437061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Sasaki K, Takeshita N, Fukunaga T, Seiryu M, Sakamoto M, Oyanagi T, Maeda T, Takano-Yamamoto T. Vibration accelerates orthodontic tooth movement by inducing osteoclastogenesis via transforming growth factor-β signalling in osteocytes. Eur J Orthod. 2022;44:698-704. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Feres MFN, Kucharski C, Diar-Bakirly S, El-Bialy T. Effect of low-intensity pulsed ultrasound on the activity of osteoclasts: An in vitro study. Arch Oral Biol. 2016;70:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Berber R, Aziz S, Simkins J, Lin SS, Mangwani J. Low Intensity Pulsed Ultrasound Therapy (LIPUS): A review of evidence and potential applications in diabetics. J Clin Orthop Trauma. 2020;11:S500-S505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | He R, Zhou W, Zhang Y, Hu S, Yu H, Luo Y, Liu B, Ran J, Wu J, Wang Y, Chen W. Combination of low-intensity pulsed ultrasound and C3H10T1/2 cells promotes bone-defect healing. Int Orthop. 2015;39:2181-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | El-Bialy T, El-Shamy I, Graber TM. Growth modification of the rabbit mandible using therapeutic ultrasound: is it possible to enhance functional appliance results? Angle Orthod. 2003;73:631-639. [PubMed] [DOI] [Full Text] |
| 41. | Erdogan O, Esen E, Ustün Y, Kürkçü M, Akova T, Gönlüşen G, Uysal H, Cevlik F. Effects of low-intensity pulsed ultrasound on healing of mandibular fractures: an experimental study in rabbits. J Oral Maxillofac Surg. 2006;64:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Li L, Yang Z, Zhang H, Chen W, Chen M, Zhu Z. Low-intensity pulsed ultrasound regulates proliferation and differentiation of osteoblasts through osteocytes. Biochem Biophys Res Commun. 2012;418:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Man J, Shelton RM, Cooper PR, Landini G, Scheven BA. Low intensity ultrasound stimulates osteoblast migration at different frequencies. J Bone Miner Metab. 2012;30:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Tassinary JAF, Lunardelli A, Basso BS, Dias HB, Catarina AV, Stülp S, Haute GV, Martha BA, Melo DADS, Nunes FB, Donadio MVF, Oliveira JR. Low-intensity pulsed ultrasound (LIPUS) stimulates mineralization of MC3T3-E1 cells through calcium and phosphate uptake. Ultrasonics. 2018;84:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Matsumoto K, Shimo T, Kurio N, Okui T, Ibaragi S, Kunisada Y, Obata K, Masui M, Pai P, Horikiri Y, Yamanaka N, Takigawa M, Sasaki A. Low-intensity pulsed ultrasound stimulation promotes osteoblast differentiation through hedgehog signaling. J Cell Biochem. 2018;119:4352-4360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Yao H, Zhang L, Yan S, He Y, Zhu H, Li Y, Wang D, Yang K. Low-intensity pulsed ultrasound/nanomechanical force generators enhance osteogenesis of BMSCs through microfilaments and TRPM7. J Nanobiotechnology. 2022;20:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 47. | Chen C, Zhang T, Liu F, Qu J, Chen Y, Fan S, Chen H, Sun L, Zhao C, Hu J, Lu H. Effect of Low-Intensity Pulsed Ultrasound After Autologous Adipose-Derived Stromal Cell Transplantation for Bone-Tendon Healing in a Rabbit Model. Am J Sports Med. 2019;47:942-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Costa V, Carina V, Fontana S, De Luca A, Monteleone F, Pagani S, Sartori M, Setti S, Faldini C, Alessandro R, Fini M, Giavaresi G. Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation. J Cell Physiol. 2018;233:1558-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Li H, Deng Y, Tan M, Feng G, Kuang Y, Li J, Song J. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res Ther. 2020;11:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Takayama T, Suzuki N, Ikeda K, Shimada T, Suzuki A, Maeno M, Otsuka K, Ito K. Low-intensity pulsed ultrasound stimulates osteogenic differentiation in ROS 17/2.8 cells. Life Sci. 2007;80:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Lim K, Kim J, Seonwoo H, Park SH, Choung PH, Chung JH. In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering. Biomed Res Int. 2013;2013:269724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Zhang B, Yang Y, Keyimu R, Hao J, Zhao Z, Ye R. The role of lamin A/C in mesenchymal stem cell differentiation. J Physiol Biochem. 2019;75:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Killaars AR, Walker CJ, Anseth KS. Nuclear mechanosensing controls MSC osteogenic potential through HDAC epigenetic remodeling. Proc Natl Acad Sci U S A. 2020;117:21258-21266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Dahl KN, Kalinowski A. Nucleoskeleton mechanics at a glance. J Cell Sci. 2011;124:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Philip JT, Dahl KN. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J Biomech. 2008;41:3164-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G. Cellular Mechanotransduction: From Tension to Function. Front Physiol. 2018;9:824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 679] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 57. | Stroud MJ. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiomyopathy. Biophys Rev. 2018;10:1033-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1466] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 59. | Bermeo S, Vidal C, Zhou H, Duque G. Lamin A/C Acts as an Essential Factor in Mesenchymal Stem Cell Differentiation Through the Regulation of the Dynamics of the Wnt/β-Catenin Pathway. J Cell Biochem. 2015;116:2344-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | La Verde G, Artiola V, Pugliese M, La Commara M, Arrichiello C, Muto P, Netti PA, Fusco S, Panzetta V. Radiation therapy affects YAP expression and intracellular localization by modulating lamin A/C levels in breast cancer. Front Bioeng Biotechnol. 2022;10:969004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 61. | Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1392] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 62. | Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4707] [Cited by in RCA: 4423] [Article Influence: 294.9] [Reference Citation Analysis (5)] |
| 63. | Gao J, He L, Zhou L, Jing Y, Wang F, Shi Y, Cai M, Sun J, Xu H, Jiang J, Zhang L, Wang H. Mechanical force regulation of YAP by F-actin and GPCR revealed by super-resolution imaging. Nanoscale. 2020;12:2703-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Pavel M, Renna M, Park SJ, Menzies FM, Ricketts T, Füllgrabe J, Ashkenazi A, Frake RA, Lombarte AC, Bento CF, Franze K, Rubinsztein DC. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat Commun. 2018;9:2961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 65. | Lin C, Yao E, Zhang K, Jiang X, Croll S, Thompson-Peer K, Chuang PT. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (13)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Roomi AB, Iraq S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang XD