INTRODUCTION
As a chronic liver disease, metabolic dysfunction-associated fatty liver disease (MASLD) affects approximately one-third of the global population[1]. The rising prevalence of MASLD is associated with an array of comorbidities, including type 2 diabetes mellitus, cardiovascular disease, obesity, and insulin resistance. The precise pathogenesis of this disease remains unclear. Previous studies have indicated that the two-hit theory may provide an explanation for the pathogenesis of this disease[2]. Firstly, the accumulation of lipids and the development of insulin resistance result in a reduction in the degradation of fatty acids, which in turn leads to the formation of hepatic steatosis. Subsequently, the liver becomes susceptible to inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress. Based on these factors, reactive oxygen species is triggered to increase, inducing fatty hepatitis, fibrosis, and cirrhosis.
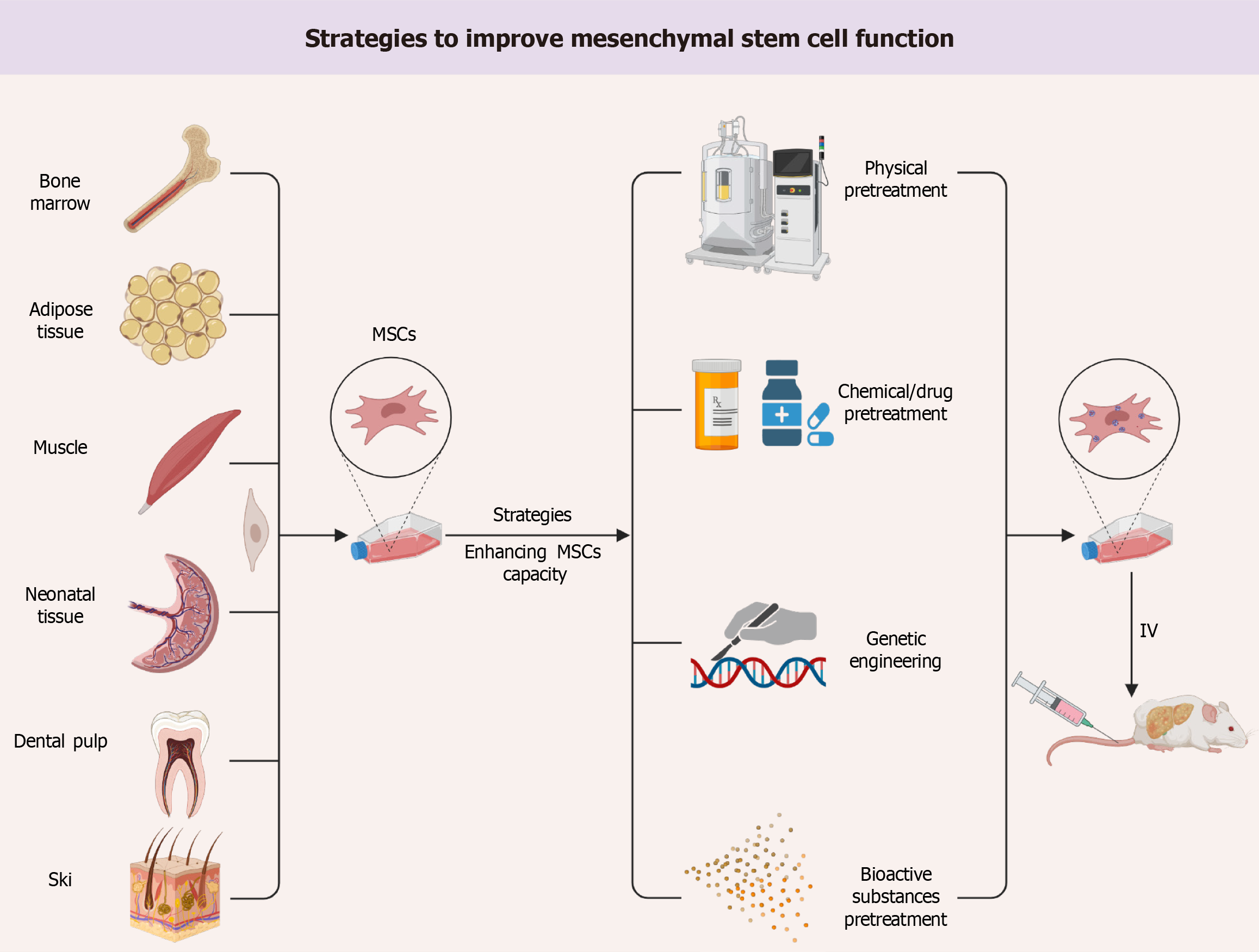
However, as research progresses, it becomes evident that this theory is unable to fully summarize the pathogenesis of the disease. Despite the preclinical studies of this disease involving multiple targets, few drugs have been applied to the clinic due to the complexity of its pathogenesis. Mesenchymal stem cells (MSCs) are adult pluripotent cells with high self-renewal, multidirectional differentiation, good biocompatibility, low immunogenicity, and paracrine function. The pleiotropic effects of MSCs are potentially more advantageous relative to drug therapy and have received much attention in the study of innovative therapeutic approaches for liver disease. Recently, Jiang et al[3] presented the same study in the World Stem Cell Journal. We agree with the authors’ findings that MSCs and paracrine secretions exhibited regulation of glycolipid metabolism, anti-inflammatory, anti-apoptotic, amelioration of oxidative stress, anti-fibrotic, and stimulation of angiogenesis and tissue regeneration in damaged liver in different experimental models of liver diseases. However, a key issue in the clinical application of MSCs is how to maintain their stability and efficacy after systemic administration. Current stem cell preconditioning protocols allow to improve their therapeutic efficacy to overcome the limitations of MSCs, i.e., migration rate, survival, implantation and paracrine activity. Therefore, we discuss strategies to enhance the effectiveness and stability of MSCs in the treatment of liver disease, including physical pretreatment, drug or chemical pretreatment, pretreatment with bioactive substances, and genetic engineering (Figure 1).
Figure 1 Strategies to improve mesenchymal stem cell function.
MSC: Mesenchymal stem cell; IV: Intravenous injection.
STRATEGIES TO ENHANCE THE THERAPEUTIC POTENTIAL OF MSC
Physical pretreatment
Physical preconditioning is the most prevalent method of manipulation of MSCs to counteract the harsh microenvironment (improve survival and function after transplantation). Among them, hypoxia initiation is the more studied treatment operation. Compared to normoxic cultured MSCs, oxygen solubility in vivo and oxygen content in liver tissue and circulatory system were extremely low. MSCs amplification is difficult to adapt to drastic oxygen pressure changes. Short-term hypoxic exposure upregulates Sug1 and downregulates HSP90α levels, resulting in increased immunogenicity of MSCs[4,5]. Hypoxia or hypoxia and reoxygenation have been shown to acclimatize MSCs to changes in oxygen in defined microenvironments in vitro while promoting their pluripotency and enhancing their cellular activity and therapeutic efficacy[6]. Hypoxia initiation increased the expression of mediators of anti-inflammatory and regenerative responses in MSCs [including interleukin (IL)-6, tumor necrosis factor-alpha, hepatocyte growth factor, vascular endothelial growth factor, Jagged 1 and prostaglandin E synthase], promoting liver regeneration and inhibiting hepatocyte death[7-9]. Hypoxic priming also prevents MSCs senescence by upregulating the polypyrimidine tract-binding protein 1/phosphoinositide 3-kinase-mediated autophagy and downregulating p16INK4A, p53, and p21[10,11].
Other physical methods used for triggering include three-dimensional culture, electrical stimulation, serum deprivation preconditioning, and more. Compared to the traditional two-dimensional culture of bone marrow MSC, the three-dimensional porous scaffold structure increases the surface area for cells to interact with the extracellular matrix. In addition, the three-dimensional culture more closely resembles the conditions of the natural extracellular matrix, which provides a better environment for cell attachment and growth, resulting in a substantial increase in MSCs yield[12]. Electrical stimulation of MSC enhanced their proliferation, migration, differentiation and scaffold adhesion[13]. In addition, it has been found that simultaneous pretreatment of MSCs with oxidative and serum deprivation stress greatly improves their survival under adverse conditions and increases the stability of the graft[14]. In conclusion, most studies have demonstrated that physical pretreatment improves the outcome of MSCs therapy for liver disease. It has the potential to be safely to translated into the clinic.
Drug or chemical pretreatment
In vitro, pretreatment of MSCs with drugs or chemicals enhances the therapeutic efficacy of MSCs in liver diseases by repairing the lost functions through modulation of various pathways. Among them, oxidative stress and inflammation are important factors affecting the survival of transplanted MSCs[15]. MSCs pretreated with curcumin significantly downregulated the ASK-JNK-BAX gene expression involved in mitochondrial stress and apoptosis[16]. And, curcumin pretreated MSCs improved liver fibrosis and prevented MASLD recurrence compared to MSCs[16]. Similarly, baicalin-pretreated MSCs ameliorated liver injury by activating p62/Kelch-like ECH-associated protein 1/nuclear factor-erythroid 2-related factor 2 signaling and inhibiting oxidative burst, inflammation, and lipid peroxidation-induced iron death[17]. Eugenol pretreated MSCs inhibited hepatic fibrosis and promoted hepatocyte regeneration and survival by inhibiting the inducible nitric oxide synthase pathway and modulating the transforming growth factor (TGF)-β/small mother against decapentaplegic pathway[18-20]. In addition, several antioxidant drugs and anti-inflammatory agents have been used to overcome oxidative stress and inflammation thereby improving MSCs survival in vitro. For example, low-dose antioxidant (reduced glutathione and melatonin) treatments were able to inhibit reactive oxygen species generation thereby maintaining stemness and multidirectional differentiation potential during long-term in vitro passaging[21]. One study using NIR-II fluorescent nanoparticles to track MSCs viability also confirmed that glutathione (antioxidant) and dexamethasone (anti-inflammatory) improved stem cell implantation efficiency and enhanced MSCs for liver fibrosis[22]. Another tracking experiment showed that more vitamin E-pretreated MSCs were transplanted into rat livers[22]. Vitamin E pretreated MSCs reduced the expression of oxidative stress-related genes (Cyp2e1, Hif1-alpha, and Il-1beta) as well as liver fibrosis-related gene markers (Tgf-beta1, alpha-Sma, and Col1alpha1), which prevented Carbon tetrachloride 4-induced oxidative stress, and improve their tolerance to unfavorable ecological niches in fibrotic livers[23]. Considering that some antioxidant and anti-inflammatory agents (e.g., vitamin E and reduced glutathione) have been used clinically in patients with MASLD, drug or chemical pretreatment is a promising clinical strategy.
Bioactive substance pretreatment
Initiating MSCs with bioactive substances is also one of the pretreatment strategies to improve their function involving growth factors, cytokines, trophic factors, hormones, vitamins, and others[15]. Compared to unmodified MSCs, TGF-β1 pretreated MSCs showed maximal inhibition of TGFβ-small mother against decapentaplegic 2/3 signaling and expression of fibrosis markers (E-cadherin, α-smooth muscle actin, type I collagen-alpha 1) in activated hepatic stellate cells[24]. Zhang et al[25] found that using interferon-γ for priming MSCs promoted indoleamine 2,3-dioxygenase (IDO) secretion which leads to better therapeutic effects in the liver injury model. On the one hand, IDO enhanced the AMP-activated protein kinase-mechanistic target of rapamycin autophagy axis and induced protective autophagy in hepatocytes; on the other hand, IDO increased Treg counts and boosted MSCs' ability to induce immune tolerance[16]. In addition, tumor necrosis factor α[26,27], interleukin(IL)-1[27,28], vitamin E[22], melatonin[21], and oxytocin hormones[29] can enhance cell proliferation, survival, migration, and homing of MSCs by inducing signaling pathways such as mitogen-activated protein kinases, mechanistic target of rapamycin, protein kinase B/extracellular signal-regulated kinase 1/2, nuclear factor-kappaB and IL-1R1/myeloid differentiation primary response 88.
Genetic engineering
The homing of MSCs is primarily the result of interactions between ligands and receptors. Correcting or modifying the gene expression of receptors/ligands on MSCs is a potential way to increase homing rates within target tissues. MSCs were modified by overexpression of migration-related genes [CC chemokine receptor 2, chemokine receptor 4, chemokine (C-X-C motif) ligand 9 and mesenchymal-epithelial transition factor] to enhance their homing ability. Overexpression migration related genes [CC chemokine receptor 2, chemokine receptor 4, chemokine (C-X-C motif) ligand 9 as well as mesenchymal-epithelial transition factor] have been used to enhance MSCs mobilization, homing and engraftment[30]. MSCs genetically modified with protein kinase B 1 have a survival advantage and stronger immunomodulatory effect both in vitro and in vivo, thus suggesting the therapeutic potential for amelioration of liver injuries[31]. Another editing method is by promoting exosome overexpression in MSCs thereby enhancing their reproduction and homing and treatment. For example, overexpression of miR-27b can inhibit directional migration of primary cultured chemokine receptor 4-positive murine MSCs by down-regulating stromal cell-derived factor-1α levels[32]. In conclusion, these genetic engineering strategies hold great promise for enhancing MSCs homing, survival, and therapeutic capabilities.
CONCLUSION
Currently, stem cell-based therapies are showing promise in both preclinical and clinical settings. However, the field is still evolving and further research will be required in the future before it can be used as a conventional treatment modality for MASLD. The first challenges addressed are MSC transplantation use and long-term therapeutic outcomes. Pretreatment of MSCs by physical pretreatment, drugs and chemicals, bioactive substances, and gene modification prior to administration improves the in vitro and in vivo function of MSCs, thereby highly enhancing the repairing efficacy for liver disease models. These modified MSCs will be more promising than cells from naive MSCs.
ACKNOWLEDGEMENTS
The authors would like to thank all members of the Tianjin Institution of Acupuncture and Moxibustion who provided us with critical comments and assistance.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited Manuscript; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade C, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade C
P-Reviewer: Deng Y; Mubarak M S-Editor: Wang JJ L-Editor: A P-Editor: Zhang L