Published online Jan 26, 2024. doi: 10.4252/wjsc.v16.i1.7
Peer-review started: November 15, 2023
First decision: December 17, 2023
Revised: December 18, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 26, 2024
Processing time: 67 Days and 19.8 Hours
Mesenchymal stromal/stem cells (MSCs) have garnered significant attention in the field of regenerative medicine due to their remarkable therapeutic potential. MSCs play a pivotal role in maintaining tissue homeostasis and possess diverse functions in tissue repair and recovery in various organs. These cells are characterized by easy accessibility, few ethical concerns, and adaptability to in vitro cultures, making them a valuable resource for cell therapy in several clinical conditions. Over the years, it has been shown that the true therapeutic power of MSCs lies not in cell engraftment and replacement but in their ability to produce critical paracrine factors, including cytokines, growth factors, and exosomes (EXOs), which modulate the tissue microenvironment and facilitate repair and regeneration processes. Consequently, MSC-derived products, such as condi
Core Tip: Mesenchymal stromal/stem cells (MSCs) offer important therapeutic effects in the field of regenerative medicine. Their key role lies in the production of paracrine factors that modulate tissue environments and allow their repair following insults. Recently, MSC-derived products such as exosomes and conditioned media are replacing whole MSCs in clinical applications. In this regard, to optimize the results of MSC-based treatment, researchers have explored priming strategies in order to enhance MSC properties. Realizing the full potential of MSC therapy depends on identifying the right tissue source and developing priming strategies specific to the disease being treated.
- Citation: Miceli V. Use of priming strategies to advance the clinical application of mesenchymal stromal/stem cell-based therapy. World J Stem Cells 2024; 16(1): 7-18
- URL: https://www.wjgnet.com/1948-0210/full/v16/i1/7.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i1.7
Over the years, mesenchymal stromal/stem cells (MSCs) have emerged as an important therapeutic tool in the field of regenerative medicine[1-4]. These versatile multipotent adult stromal/stem cells play a crucial role in maintaining tissue homeostasis under both physiological and pathological conditions. In fact, MSCs possess the remarkable ability to influence their surroundings by differentiating, attracting supporting cells, and orchestrating central processes for tissue regeneration[5,6]. Together, the multifaceted potential of MSCs shed light on their role as key regulatory elements in the complex mechanisms governing tissue repair/recovery in several tissues, including the intestine[7], skin[8], and skeletal muscle[6], where MSCs exhibit diverse functions, either supporting high cellular turnover or facilitating regeneration following injury.
These discoveries offer a strong motivation for investigating the potential of MSCs as a cellular therapeutic product to enhance tissue injury responses in various diseases[9-14]. MSCs show high accessibility, minimal ethics-related concerns, and great adaptability to in vitro cultures for expansion[15]. Moreover, these cells possess immune privilege attributed to their low expression of CD40, CD80, CD86, and major histocompatibility complex I (MHC I), along with the absence of MHC II expression[16,17]. These attributes make these cells a highly valuable resource for developing new cell therapies in the field of regenerative medicine.
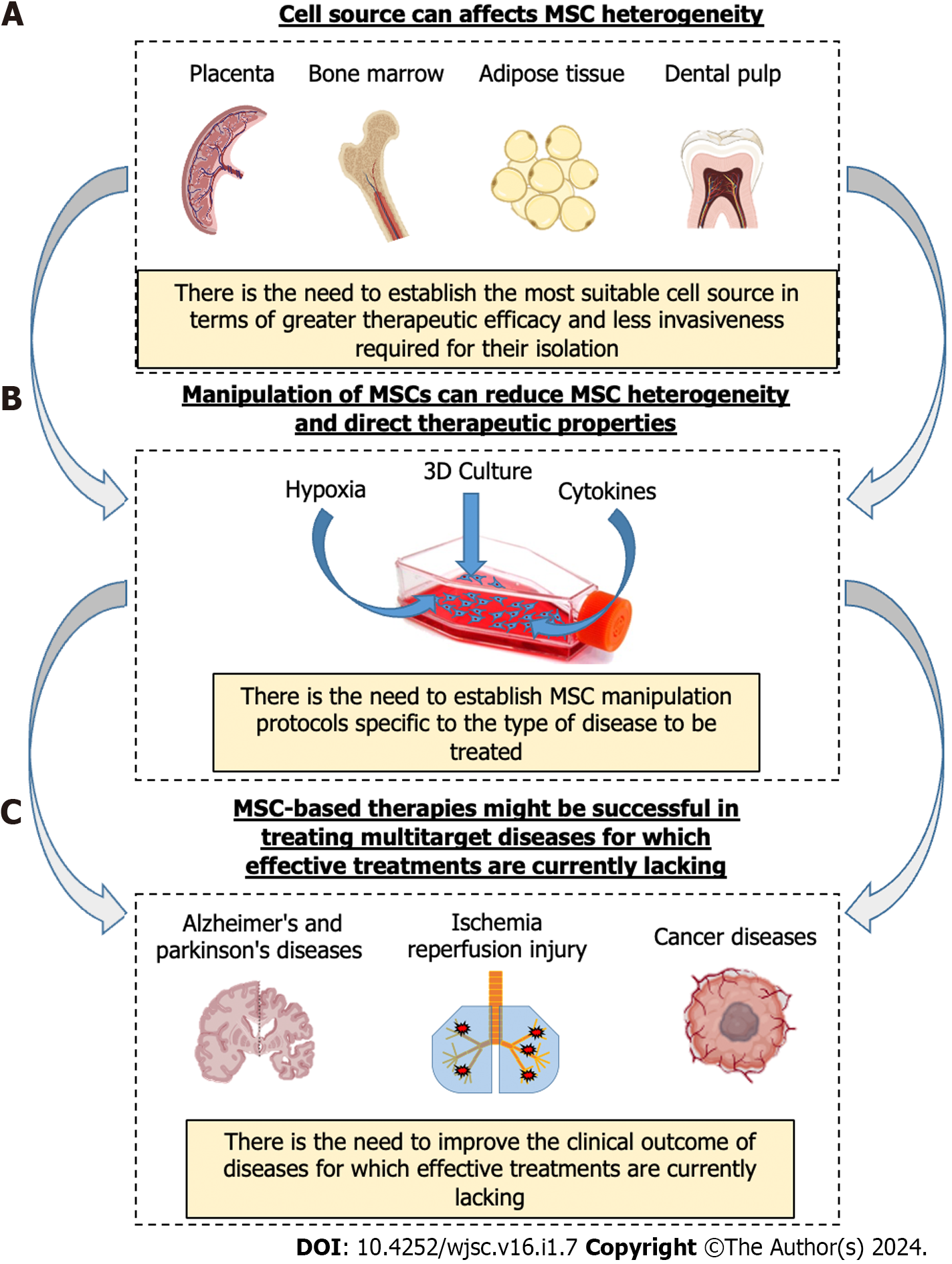
MSCs are present in various tissues, including bone marrow[18], adipose tissue[19], umbilical cord[14], dental pulp[20], and placenta[21]. In these diverse tissue environments, MSCs interact with different cell types, such as epithelial cells, endothelial cells, immune cells, and stromal cells, showing immunomodulatory, angiogenic, pro-trophic, and anti-oxidative properties[22-25]. Their adaptability and therapeutic potential make them promising candidates for addressing a wide range of clinical disorders, including cardiovascular, neurodegenerative, immune, lung, liver, kidney, and orthopedic diseases. Notably, it has become increasingly evident that the true therapeutic power of MSC therapies lies not in engraftment and cell replacement but rather in their ability to produce critical paracrine factors that modulate the tissue microenvironment and facilitate repair and regeneration processes. Indeed, these cells are able to produce crucial functional factors, such as cytokines, growth factors, and exosomes (EXOs), which can mediate their therapeutic effects[26-28]. Hence, given the regenerative potential and trophic properties inherent in certain MSC-derived products, such as the conditioned medium and/or EXOs, these products have arisen as potential therapeutic tools with a wide range of applications. Consequently, they are undergoing extensive evaluation for potential medical use[9,12,29-32]. The clinical utilization of MSC-derived products must be considered for their advantages, particularly in contrast to concerns related to the prolonged use of MSCs and the associated risks of infectious disease transmission, such as viruses present in transplanted allogeneic cells[33].
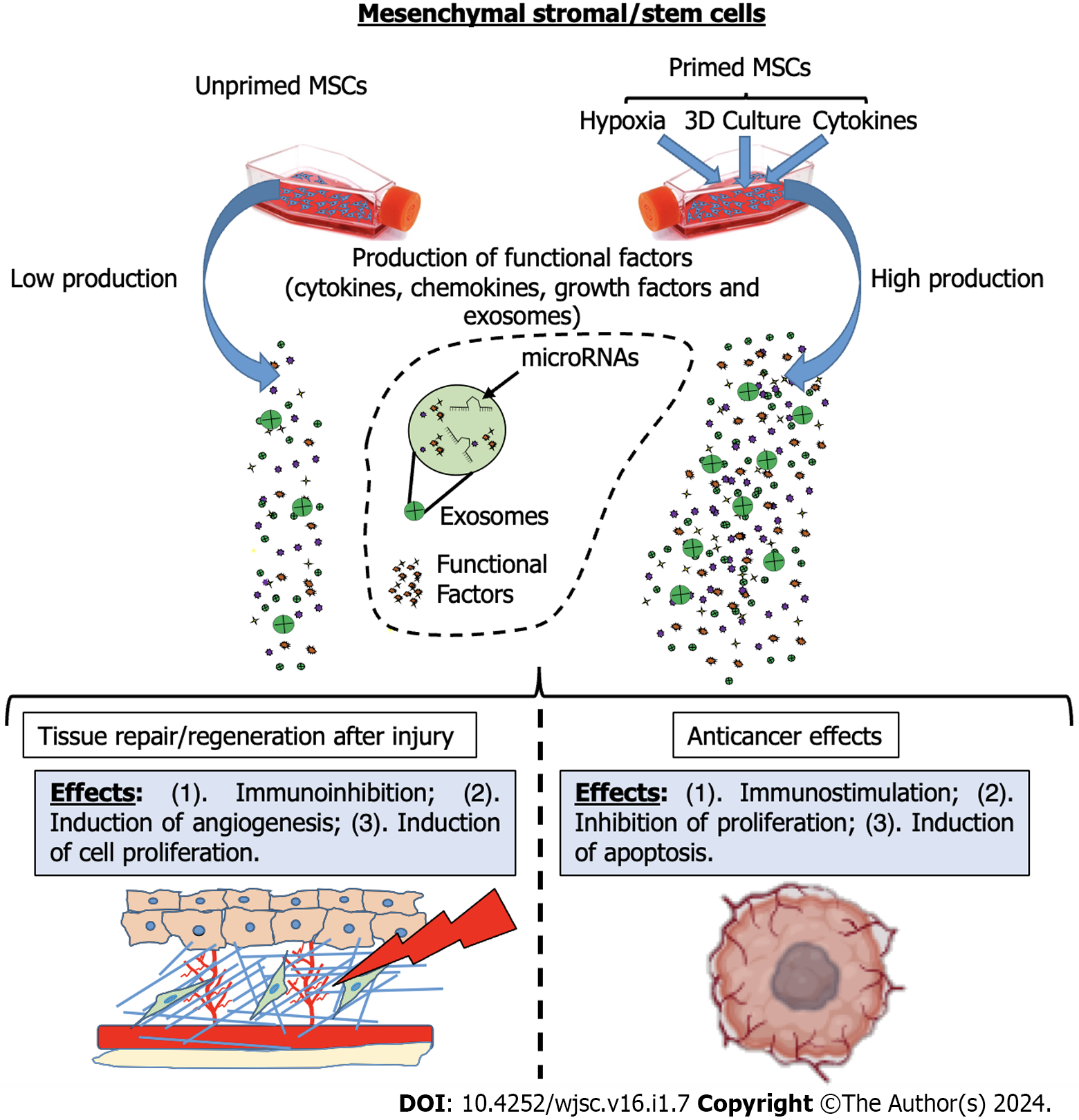
However, the therapeutic landscape of MSCs is not without its challenges and controversies. The efficacy of MSC-based treatments has yielded variable results in clinical trials, reflecting the complexity of intrinsic differences between cell-based products and a lack of standardized methods for MSC production that affects their potency[34-39]. The effects of MSCs vary based on the tissue source and the methods employed in their production and administration[35,40,41]. Several studies have demonstrated that the composition of the MSC secretome can be modulated through the preconditioning of MSCs with cytokine treatments and hypoxia. Additionally, cultivating MSCs under specific culture systems, such as three-dimensional (3D) conditions, also influences their secretome. In response to MSC “priming”, the production of factors is switched towards a greater functional phenotype that results in an increase in MSC therapeutic effects[3,27,42].
The field of research on MSCs is still very complex and is constantly evolving, emphasizing that the road to consolidating the use of MSCs as an effective cell therapy for various pathologies is still quite long. In this regard, promising approaches are being studied, among which MSC priming certainly represents one of the most hopeful strategies.
In the last decade, the concept of priming or preconditioning MSCs has gained credibility as a means to enhance MSC therapeutic potential by modulating the secretion of paracrine factors and tailoring their actions to specific medical conditions[3,27]. Similar to immune cells[43], MSCs have been shown to memorize a stimulus after transitioning to a new environment[44]. In this regard, MSCs can be primed to generate a short-term-memory effect and, mimicking microenvironmental stimuli, this strategy may be used in vitro to avoid the need for in vivo activation of the MSCs when aiming towards specific therapeutic activities. This approach has been widely explored in the context of immunomodulation[45,46], tissue regeneration[47,48], and even cancer interactions[49], with each priming strategy offering a unique set of advantages and applications.
One of the principal priming strategies involves exposing MSCs to inflammatory molecules. Numerous studies reveal that the immunosuppressive properties of MSCs are not intrinsic but require priming by inflammatory factors. In fact, depending on the specific inflammatory conditions, the MSC phenotype can be polarized into MSC type 1, characterized by pro-inflammatory properties, or MSC type 2, with immunosuppressive capabilities[50]. Various strategies have been implemented to modulate and enhance the secretion of immunomodulatory molecules in MSCs. The treatment of MSCs with inflammatory cytokines, including interferon-γ, interleukin (IL)-1α/β, IL-6, tumor necrosis factor (TNF)-α, and IL-17, is shown to significantly enhance their immunomodulatory properties. This priming approach increases the production and secretion of key functional factors such as hepatocyte growth factor (HGF), transforming growth factor (TGF)-β, IL-6, prostaglandin E2 (PGE2), leukemia inhibitory factor (LIF), granulocyte colony-stimulating factor, IL-10, macrophage inflammatory protein (MIP)-1α, indoleamine 2,3-dioxygenase (IDO), intercellular adhesion molecule, programmed death ligand (PDL)1-2, monocyte chemoattractant protein (MCP)-1, monokine induced by interferon-gamma, interferon-gamma-inducible protein 10, and MIP-1β. These factors, in turn, empower MSCs with enhanced paracrine immunomodulatory properties, making them potent inhibitors of T cell proliferation and activators of anti-inflammatory M2 macrophage polarization[27]. Moreover, treatment with inflammatory cytokines is shown to improve the immunomodulatory capabilities of extracellular vesicles (EVs) derived from MSCs, further highlighting the versatility of this priming strategy in the context of immunoregulation[45,51].
Priming with hypoxia represents another pivotal approach to enhancing MSC functionality. Hypoxic preconditioning of MSCs is shown to stimulate the secretion of essential growth factors, such as vascular endothelial growth factor (VEGF) and HGF, which are crucial for angiogenesis and tissue regeneration[52]. Under hypoxic conditions, MSCs activate signaling pathways, including the HIF-1α-GRP78-Akt axis, leading to the overproduction of pro-angiogenic factors[53]. This approach yields significant benefits in various acute injuries, including ischemia-reperfusion injury (IRI), renal injury, and myocardial infarction[3]. Moreover, hypoxic preconditioning is effective in promoting hepatic tissue regeneration, with increased expression of factors such as HGF and VEGF[48,54]. This is particularly advantageous in cases of liver injury and fibrosis. Hypoxic MSCs also exhibit the ability to secrete functional EVs capable of stimulating tissue remodeling, contributing to tissue repair in cerebral tissue[55]. In addition, hypoxic MSC-derived EVs show enhanced activity both in vitro and in vivo, especially in promoting angiogenesis on human brain microvascular endothelial cells. Interestingly, this effect appears to be mediated by microRNA (miRNA)-612[56]. Therefore, several functional factors produced by hypoxia-primed MSCs are found to play a crucial role in stimulating angiogenic and regenerative activities, making this priming strategy a valuable tool to enhance MSC therapeutic effects for tissue recovery after acute injury.
Priming through 3D culture techniques offers an alternative approach to enhancing MSC therapeutic properties. This strategy involves the generation of MSC spheroids, which closely mimic the in vivo MSC niche and boost the functional phenotypic profile of MSCs. These spheroids exhibit superior trophic and immunomodulatory functionalities, driven by the paracrine secretion of functional factors with anti-inflammatory, angiogenic, anti-fibrotic, anti-apoptotic, and mitogenic properties[30,51,57-59]. Comparative studies show that 3D culture of MSCs can modify their transcriptome profile, leading to the overexpression of genes that regulate proliferation, differentiation, immunomodulation, and angiogenic processes[60]. These spheroids are found to secrete a plethora of regenerative and immunomodulatory factors, including stromal cell-derived factor-1α, growth-regulated oncogene α, MCP-1/3, IL-4, IL-10, EGF, LIF, placental growth factor-1, VEGF-A/D, HGF, insulin-like growth factor 1, TNFAIP6, stanniocalcin 1, PDGFB, TGF-β, PGE2, and IDO. Such factors are involved in promoting tissue repair and regeneration, making 3D-cultured MSCs valuable for various applications in regenerative medicine[27].
The application of these priming strategies is not limited to basic research. They have found practical utility in the treatment of various clinical conditions (Table 1). For instance, in the context of chronic immune-related disorders, MSCs primed with pro-inflammatory cytokines demonstrate enhanced immunomodulatory properties, making them more effective in diseases such as colitis, autoimmune encephalomyelitis, and graft-versus-host disease (GVHD)[61,66,102]. Notably, the priming of MSCs with IL-1β shows promise in alleviating the side effects of sepsis, primarily by inducing macrophage polarization toward an anti-inflammatory M2 phenotype[103]. Similarly, the use of TNF-α-primed MSCs attenuates symptoms of GVHD and peritonitis, with a demonstrated reduction in pro-inflammatory cytokines and an increase in anti-inflammatory factors[67]. Moreover, the efficacy of MSCs primed with 3D culture conditions is evident in the treatment of diseases characterized by unresolved inflammation, as these spheroids overexpress TSG-6 and exhibit a more significant impact in reducing inflammation[92].
| MSCs | Priming treatments | Model/disease | Therapeutic effects | Ref. |
| Priming with inflammatory molecules | ||||
| BM-MSCs | IFN-γ | In vivo model of chronic colitis | Attenuation of inflammation | [61] |
| UC-MSCs | TNF-α | In vivo model of intrauterine adhesion | Reduction of inflammation and endometrium fibrosis | [62] |
| BM-MSCs | IFN-γ | In vivo models of acute radiation syndrome | Protection from radiation-induced lethality | [63] |
| UC-MSCs | IL-1β | In vivo model of chronic colitis | Attenuation of inflammation | [64] |
| BM-MSCs | IL-25 | In vivo model of chronic colitis | Attenuation of inflammation | [65] |
| BM-MSCs and CB-MSCs | IFN-γ | In vivo model of GVHD | Reduction of the symptoms of GVHD | [66] |
| UC-MSCs | IFN-γ; TNF-α | In vivo model of GVHD | Reduction of the clinical symptoms | [67] |
| BM-MSCs | IL-6 | In vivo model of liver fibrosis | Reduction of liver injury | [68] |
| UC-MSCs | IL-1β | In vivo model of sepsis | Increase in survival rate | [69] |
| CB-MSCs | IFN-γ | In vivo model of acute kidney injury | Reduction of kidney injury | [70] |
| AdMSCs | TNF-α | In vivo model of wound healing | Acceleration of wound closure and angiogenesis | [71] |
| Priming with hypoxia | ||||
| BM-MSCs | Hypoxia | In vivo model of traumatic brain injury | Improved neurogenesis and cognitive function | [47] |
| AdMSCs | Hypoxia | In vivo model of hepatectomy | Enhanced liver regeneration | [48] |
| UC-MSCs | Hypoxia | In vivo model of spinal cord injury | Improved axonal preservation | [52] |
| AdMSCs | Hypoxia | In vivo model of hindlimb ischemia | Improvement of angiogenesis | [53] |
| BM-MSCs | Hypoxia | In vivo model of hepatectomy | Enhanced liver regeneration | [54] |
| BM-MSCs | Hypoxia | In vivo model of pulmonary fibrosis | Increased survival rate | [72] |
| BM-MSCs | Hypoxia | In vivo model of hindlimb ischemia | Improvement of angiogenesis | [73] |
| AdMSCs | Hypoxia | In vivo model of hindlimb ischemia | Improvement of functional recovery | [74] |
| BM-MSCs | Hypoxia | In vivo model of radiation-induced lung injury | Improvement of antioxidant ability | [75] |
| BM-MSCs | Hypoxia | In vivo model of lung IRI | Attenuation of lung injury | [76] |
| AdMSCs | Hypoxia | In vivo model of acute kidney injury | Improvement of renal function | [77] |
| AdMSCs | Hypoxia | In vivo model of acute kidney injury | Attenuation of kidney injury | [78] |
| PMSCs | Hypoxia | In vivo model of scar formation | Reduction of scar formation | [79] |
| AF-MSCs | Hypoxia | In vivo model of wound healing | Acceleration of wound healing | [80] |
| BM-MSCs | Hypoxia | In vivo model of wound healing | Acceleration of wound healing | [81] |
| BM-MSCs | Hypoxia | In vivo model of hindlimb ischemia | Improvement of muscle fiber regeneration | [82] |
| DP-MSCs | Hypoxia | In vivo model of dental pulp injury | Regeneration of dental pulp | [83] |
| BM-MSCs | Hypoxia | In vivo model of cerebral ischemia | Enhanced angiogenesis and neurogenesis | [84] |
| BM-MSCs | Hypoxia | In vivo model of ischemic cortex | Reduction of infarct volume | [85] |
| BM-MSCs | Hypoxia | In vivo model of myocardial infarction | Reduction of cardiac fibrosis | [86] |
| BM-MSCs | Hypoxia | In vivo model of myocardial infarction | Improvement cardiac functions | [87] |
| BM-MSCs | Hypoxia | In vivo model of myocardial infarction | Prevention of apoptosis in cardiomyocytes | [88] |
| BM-MSCs | Hypoxia | In vivo model of myocardial infarction | Increased cardiomyocyte proliferation and function | [89] |
| BM-MSCs | Hypoxia | In vivo model of myocardial infarction | Improved cardiac repair | [90] |
| BM-MSCs | Hypoxia | In vivo IRI model of myocardium | Reduction of IRI | [91] |
| Priming with 3D culture | ||||
| BM-MSCs | 3D culture | In vivo model of peritonitis | Attenuation of inflammation | [92] |
| UC-MSCs | 3D culture | In vivo model of arthritis | Attenuation of systemic arthritic manifestations | [93] |
| CB-MSCs | 3D culture | In vivo model of hindlimb ischemia | Improvement of cell survival and angiogenesis | [94] |
| AdMSCs | 3D culture | In vivo model of hindlimb ischemia | Improvement of angiogenesis | [95] |
| AdMSCs | 3D culture | In vivo model of acute kidney injury | Amelioration of renal function | [96] |
| AdMSCs | 3D culture | In vivo model of disc degeneration | Induction of disc repair | [97] |
| BM-MSCs | 3D culture | In vivo model of bilateral calvarial defects | Induction of bone regeneration | [98] |
| SMSCs | 3D cultures | In vivo model of osteochondral defects | Induction of cartilage regeneration | [99] |
| BM-MSCs | 3D culture | In vivo model of myocardial infarction | Promotion of cardiac repair | [100] |
| BM-MSCs | 3D cultures | In vivo model of myocardial infarction | Improvement of cardiac function | [101] |
The therapeutic potential of MSCs also extends to the treatment of acute injuries, where priming strategies can play a crucial role in boosting their regenerative capabilities. For instance, in cases of acute myocardial injury, hypoxic preconditioning significantly improves blood flow recovery, influences heart remodeling, and enhances the regeneration of ischemic tissues[87,88]. These effects are attributed to the increased production of pro-survival and pro-angiogenic factors by hypoxia-primed MSCs, including HIF-1α, ANGPT1, VEGF, Flk-1, Bcl-2, and Bcl-xL[87]. Hypoxic MSCs demonstrate enhanced integration into damaged tissues, with improved survival, proliferation, and regenerative effects[74]. In parallel, 3D-cultured MSCs show potential in both bone and cartilage repair, highlighting their capacity to stimulate tissue regeneration across various contexts[98,99].
In recent years, the interaction between MSCs and cancer has also garnered considerable attention. Indeed, MSCs represent a crucial actor in the tumor microenvironment due to their ability to modulate the function/survival of both immune cells and tumor cells, with the final effects of promoting or inhibiting cancer[104]. Numerous studies have investigated the molecular mechanisms involved in the MSC-based modulation of tumor immunity, revealing that MSCs might either support or suppress tumor progression since many MSC factors can be produced differently in the tumor microenvironment[104-106]. For instance, the cross-talk between MSCs and M1/M2 macrophages plays a pivotal role in regulating tumor progression[107]. MSCs are shown to promote the shift from anti-tumorigenic M1 macrophages to pro-tumorigenic M2 macrophages, contributing to immune evasion and tumor growth[108]. Moreover, the capacity of MSCs to express immune checkpoint molecules, including PDL1, further intensifies their role in immunosuppression, facilitating the evasion of host immune responses by cancer cells[109]. On the other hand, various studies indicate that utilizing MSC-derived EVs housing anti-tumorigenic miRNAs might offer a novel therapeutic opportunity for MSC-based tumor therapy[110].
In summary, priming strategies represent a versatile approach to managing the therapeutic potential of MSCs, tailoring their secreted factors and interactions to diverse clinical conditions. These strategies show great promise in regenerative medicine, immune-related disorders, and the complex interplay between MSCs and cancer (Figure 1). Through exposure to inflammatory molecules, hypoxic environments, 3D culture conditions, or other new priming strategies, MSCs can be transformed into highly specialized therapeutic tools, extending the possibilities for their application in various clinical settings and expanding our understanding of the dynamic role of MSCs in health and disease. The ongoing research in this field promises further advancements in the optimization of MSC-based therapies, offering new hope for patients suffering from a wide range of pathologies.
While research on MSCs is booming, as are their clinical applications, it is becoming increasingly important to understand the multiple properties of MSCs and how these can be optimally modulated to achieve the desired therapeutic effects. The use of MSC therapy, unfortunately, suffers from intrinsic biological variability, both due to the source and inter-subject variability. On the other hand, these therapies might prove to be decisive in the treatment of certain so-called multifactorial pathologies where multiple molecular targets are involved, as in the case of inflammatory-related diseases[111], including Alzheimer’s and Parkinson’s diseases[112,113], cancer[114], IRI[13,115], and others. Due to the ability of MSCs to produce multiple functional factors capable of acting simultaneously on multiple targets, cell therapies based on the use of MSCs might be successful in the treatment of some such acute and chronic diseases for which effective treatments are currently lacking (Figure 2).
However, to achieve this goal, it will be necessary to understand how to modulate MSCs according to the specific dysfunction to be treated. In fact, while MSC immune inhibitory and pro-angiogenic effects may be suitable for various diseases in the field of regenerative medicine, the same properties might be disadvantageous in the treatment of some tumors. In the case of immune-mediated diseases such as GVHD or liver cirrhosis, MSCs with pronounced immunomodulatory capabilities might show enhanced therapeutic efficacy. Also, in the context of wound healing, MSCs displaying a well-balanced array of therapeutic attributes, encompassing immunomodulation, trophic stimulation, and angiogenic promotion, may be more efficacious.
It is true that MSCs from various sources possess unique therapeutic properties, but it is unthinkable that they can be extracted from any tissue and used as they are for various types of diseases. The only way to build an effective cell therapy based on MSCs is to first establish the most suitable source in terms of therapeutic efficacy with the least invasive strategy required for their isolation. Subsequently, appropriate in vitro manipulation strategies should be studied to promote their expansion and trigger specific therapeutic functions in order to establish MSC manipulation protocols specific to the type of disease to be treated. Our future goal should be to unlock the full potential of MSCs, fostering a deeper appreciation of their remarkable therapeutic capabilities and actively contributing to the ongoing progress of regenerative medicine.
| 1. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 782] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 2. | Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Miceli V, Zito G, Bulati M, Gallo A, Busà R, Iannolo G, Conaldi PG. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J Stem Cells. 2023;15:400-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Szydlak R. Mesenchymal stem cells in ischemic tissue regeneration. World J Stem Cells. 2023;15:16-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1852] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 6. | Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019;27:2029-2035.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 7. | Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 1000] [Article Influence: 76.9] [Reference Citation Analysis (2)] |
| 8. | Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 9. | Chinnici CM, Russelli G, Bulati M, Miceli V, Gallo A, Busà R, Tinnirello R, Conaldi PG, Iannolo G. Mesenchymal stromal cell secretome in liver failure: Perspectives on COVID-19 infection treatment. World J Gastroenterol. 2021;27:1905-1919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Cittadini E, Brucculeri AM, Quartararo F, Vaglica R, Miceli V, Conaldi PG. Stem cell therapy in the treatment of organic and dysfunctional endometrial pathology. Minerva Obstet Gynecol. 2022;74:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Gao G, Fan C, Li W, Liang R, Wei C, Chen X, Yang Y, Zhong Y, Shao Y, Kong Y, Li Z, Zhu X. Mesenchymal stem cells: ideal seeds for treating diseases. Hum Cell. 2021;34:1585-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Miceli V, Bertani A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Miceli V, Bulati M, Gallo A, Iannolo G, Busà R, Conaldi PG, Zito G. Role of Mesenchymal Stem/Stromal Cells in Modulating Ischemia/Reperfusion Injury: Current State of the Art and Future Perspectives. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Russo E, Corrao S, Di Gaudio F, Alberti G, Caprnda M, Kubatka P, Kruzliak P, Miceli V, Conaldi PG, Borlongan CV, La Rocca G. Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Ferrin I, Beloqui I, Zabaleta L, Salcedo JM, Trigueros C, Martin AG. Isolation, Culture, and Expansion of Mesenchymal Stem Cells. Methods Mol Biol. 2017;1590:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. 2013;91:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1234] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 18. | Walter SG, Randau TM, Hilgers C, Haddouti EM, Masson W, Gravius S, Burger C, Wirtz DC, Schildberg FA. Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Rathinasabapathy A, Bruce E, Espejo A, Horowitz A, Sudhan DR, Nair A, Guzzo D, Francis J, Raizada MK, Shenoy V, Katovich MJ. Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis. Br J Pharmacol. 2016;173:2859-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3448] [Article Influence: 132.6] [Reference Citation Analysis (1)] |
| 21. | Papait A, Vertua E, Magatti M, Ceccariglia S, De Munari S, Silini AR, Sheleg M, Ofir R, Parolini O. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 23. | Poggi A, Zocchi MR. Immunomodulatory Properties of Mesenchymal Stromal Cells: Still Unresolved "Yin and Yang". Curr Stem Cell Res Ther. 2019;14:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 2020;9:985-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 25. | Tao H, Han Z, Han ZC, Li Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016;2016:1314709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 26. | Chang C, Yan J, Yao Z, Zhang C, Li X, Mao HQ. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv Healthc Mater. 2021;10:e2001689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 27. | Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 28. | Russo E, Alberti G, Corrao S, Borlongan CV, Miceli V, Conaldi PG, Di Gaudio F, La Rocca G. The Truth Is Out There: Biological Features and Clinical Indications of Extracellular Vesicles from Human Perinatal Stem Cells. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Alberti G, Russo E, Corrao S, Anzalone R, Kruzliak P, Miceli V, Conaldi PG, Di Gaudio F, La Rocca G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Miceli V, Bertani A, Chinnici CM, Bulati M, Pampalone M, Amico G, Carcione C, Schmelzer E, Gerlach JC, Conaldi PG. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Miceli V, Chinnici CM, Bulati M, Pampalone M, Amico G, Schmelzer E, Gerlach JC, Conaldi PG. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. Biochem Biophys Res Commun. 2020;522:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Schmelzer E, Miceli V, Chinnici CM, Bertani A, Gerlach JC. Effects of Mesenchymal Stem Cell Coculture on Human Lung Small Airway Epithelial Cells. Biomed Res Int. 2020;2020:9847579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal Stromal Cells and Viral Infection. Stem Cells Int. 2015;2015:860950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Fričová D, Korchak JA, Zubair AC. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regen Med. 2020;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 497] [Article Influence: 82.8] [Reference Citation Analysis (1)] |
| 36. | Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019;2019:9628536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 385] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 37. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1068] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 38. | Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 40. | Cai S, Fan C, Xie L, Zhong H, Li A, Lv S, Liao M, Yang X, Su X, Wang Y, Wang H, Wang M, Huang P, Liu Y, Wang T, Zhong Y, Ma L. Single-cell RNA sequencing reveals the potential mechanism of heterogeneity of immunomodulatory properties of foreskin and umbilical cord mesenchymal stromal cells. Cell Biosci. 2022;12:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 41. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1281] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 42. | Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Correction to: Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. Trained immunity - basic concepts and contributions to immunopathology. Nat Rev Nephrol. 2023;19:23-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 264] [Reference Citation Analysis (0)] |
| 44. | Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol. 2014;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 45. | Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, Conaldi PG. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-γ Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front Immunol. 2020;11:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 46. | Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 680] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 47. | Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond). 2013;124:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 48. | Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl Med. 2016;5:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Xuan X, Tian C, Zhao M, Sun Y, Huang C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021;21:595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 50. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 975] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 51. | Bulati M, Gallo A, Zito G, Busà R, Iannolo G, Cuscino N, Castelbuono S, Carcione C, Centi C, Martucci G, Bertani A, Baiamonte MP, Chinnici CM, Conaldi PG, Miceli V. 3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Zhilai Z, Biling M, Sujun Q, Chao D, Benchao S, Shuai H, Shun Y, Hui Z. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Lee JH, Yoon YM, Lee SH. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 54. | Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, Zhang M, He J, Zheng S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013;4:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Gregorius J, Wang C, Stambouli O, Hussner T, Qi Y, Tertel T, Börger V, Mohamud Yusuf A, Hagemann N, Yin D, Dittrich R, Mouloud Y, Mairinger FD, Magraoui FE, Popa-Wagner A, Kleinschnitz C, Doeppner TR, Gunzer M, Meyer HE, Giebel B, Hermann DM. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res Cardiol. 2021;116:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 56. | Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y, Duan D, Hu Z, Chen P, Lu M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 2021;19:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 57. | Lo Nigro A, Gallo A, Bulati M, Vitale G, Paini DS, Pampalone M, Galvagno D, Conaldi PG, Miceli V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front Med (Lausanne). 2021;8:746298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Miceli V, Pampalone M, Vella S, Carreca AP, Amico G, Conaldi PG. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019;2019:7486279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 59. | Zito G, Miceli V, Carcione C, Busà R, Bulati M, Gallo A, Iannolo G, Pagano D, Conaldi PG. Human Amnion-Derived Mesenchymal Stromal/Stem Cells Pre-Conditioning Inhibits Inflammation and Apoptosis of Immune and Parenchymal Cells in an In Vitro Model of Liver Ischemia/Reperfusion. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Gallo A, Cuscino N, Contino F, Bulati M, Pampalone M, Amico G, Zito G, Carcione C, Centi C, Bertani A, Conaldi PG, Miceli V. Changes in the Transcriptome Profiles of Human Amnion-Derived Mesenchymal Stromal/Stem Cells Induced by Three-Dimensional Culture: A Potential Priming Strategy to Improve Their Properties. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 62. | Li J, Pan Y, Yang J, Wang J, Jiang Q, Dou H, Hou Y. Tumor necrosis factor-α-primed mesenchymal stem cell-derived exosomes promote M2 macrophage polarization via Galectin-1 and modify intrauterine adhesion on a novel murine model. Front Immunol. 2022;13:945234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 63. | Chinnadurai R, Bates PD, Kunugi KA, Nickel KP, DeWerd LA, Capitini CM, Galipeau J, Kimple RJ. Dichotomic Potency of IFNγ Licensed Allogeneic Mesenchymal Stromal Cells in Animal Models of Acute Radiation Syndrome and Graft Versus Host Disease. Front Immunol. 2021;12:708950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 65. | Cheng W, Su J, Hu Y, Huang Q, Shi H, Wang L, Ren J. Interleukin-25 primed mesenchymal stem cells achieve better therapeutic effects on dextran sulfate sodium-induced colitis via inhibiting Th17 immune response and inducing T regulatory cell phenotype. Am J Transl Res. 2017;9:4149-4160. [PubMed] |
| 66. | Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, Sung KW, Koo HH, Yoo KH. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine. 2018;28:261-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 67. | Liu W, Yuan F, Bai H, Liu Y, Li X, Wang Y, Zhang Y. hUC-MSCs Attenuate Acute Graft-Versus-Host Disease through Chi3l1 Repression of Th17 Differentiation. Stem Cells Int. 2022;2022:1052166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 68. | Nasir GA, Mohsin S, Khan M, Shams S, Ali G, Khan SN, Riazuddin S. Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med. 2013;11:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 69. | Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017;35:1208-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 389] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 70. | Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, Kankuri E, Mervaala E, Laitinen S. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 72. | Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, Kuo HP, Chong KY. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2015;6:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 73. | Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 74. | Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2016;7:e2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 75. | Li B, Li C, Zhu M, Zhang Y, Du J, Xu Y, Liu B, Gao F, Liu H, Cai J, Yang Y. Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability. Cell Physiol Biochem. 2017;44:1295-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Liu YY, Chiang CH, Hung SC, Chian CF, Tsai CL, Chen WC, Zhang H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS One. 2017;12:e0187637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Overath JM, Gauer S, Obermüller N, Schubert R, Schäfer R, Geiger H, Baer PC. Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp Cell Res. 2016;342:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Zhang W, Liu L, Huo Y, Yang Y, Wang Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. Biomed Res Int. 2014;2014:462472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Du L, Lv R, Yang X, Cheng S, Ma T, Xu J. Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation. Life Sci. 2016;149:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Jun EK, Zhang Q, Yoon BS, Moon JH, Lee G, Park G, Kang PJ, Lee JH, Kim A, You S. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci. 2014;15:605-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, Liu X, Qi S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9:e96161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 82. | Leroux L, Descamps B, Tojais NF, Séguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamazière JM, Dufourcq P, Couffinhal T, Duplàa C. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 83. | Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, Ma PX. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016;33:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 85. | Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF, Wei L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22:977-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 86. | Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 87. | Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 478] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 88. | Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 491] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 89. | Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y, Cheng H, Kong M, Zhang F, Chen Q, Sun J, Li Q, Jin J, Chen L, Wang C, Zhan H, Fan Y, Yang Q, Yu L, Wu R, Liang J, Zhu J, Jin Y, Lin Y, Yang F, Jia L, Zhu W, Chen J, Yu H, Zhang J, Wang J. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circ Res. 2016;118:970-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 90. | Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, Shen H, Zhang GG, Bai YP. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163-6177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 91. | Park H, Park H, Mun D, Kang J, Kim H, Kim M, Cui S, Lee SH, Joung B. Extracellular Vesicles Derived from Hypoxic Human Mesenchymal Stem Cells Attenuate GSK3β Expression via miRNA-26a in an Ischemia-Reperfusion Injury Model. Yonsei Med J. 2018;59:736-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724-13729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 780] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 93. | Miranda JP, Camões SP, Gaspar MM, Rodrigues JS, Carvalheiro M, Bárcia RN, Cruz P, Cruz H, Simões S, Santos JM. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front Immunol. 2019;10:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 94. | Bhang SH, Lee S, Shin JY, Lee TJ, Kim BS. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18:2138-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 95. | Lee JH, Han YS, Lee SH. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells. Biomol Ther (Seoul). 2016;24:260-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Xu Y, Shi T, Xu A, Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med. 2016;20:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 97. | Muttigi MS, Kim BJ, Kumar H, Park S, Choi UY, Han I, Park H, Lee SH. Efficacy of matrilin-3-primed adipose-derived mesenchymal stem cell spheroids in a rabbit model of disc degeneration. Stem Cell Res Ther. 2020;11:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Suenaga H, Furukawa KS, Suzuki Y, Takato T, Ushida T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J Mater Sci Mater Med. 2015;26:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Suzuki S, Muneta T, Tsuji K, Ichinose S, Makino H, Umezawa A, Sekiya I. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res Ther. 2012;14:R136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | You Y, Kobayashi K, Colak B, Luo P, Cozens E, Fields L, Suzuki K, Gautrot J. Engineered cell-degradable poly(2-alkyl-2-oxazoline) hydrogel for epicardial placement of mesenchymal stem cells for myocardial repair. Biomaterials. 2021;269:120356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 101. | Wang CC, Chen CH, Hwang SM, Lin WW, Huang CH, Lee WY, Chang Y, Sung HW. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 102. | Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 103. | Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264:118658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 104. | Yuan J, Wei Z, Xu X, Ocansey DKW, Cai X, Mao F. The Effects of Mesenchymal Stem Cell on Colorectal Cancer. Stem Cells Int. 2021;2021:9136583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Sun Z, Zhang J, Li J, Li M, Ge J, Wu P, You B, Qian H. Roles of Mesenchymal Stem Cell-Derived Exosomes in Cancer Development and Targeted Therapy. Stem Cells Int. 2021;2021:9962194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, Li L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 107. | Harrell CR, Volarevic A, Djonov VG, Jovicic N, Volarevic V. Mesenchymal Stem Cell: A Friend or Foe in Anti-Tumor Immunity. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 108. | Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, Vinta A, Buckanovich RJ, di Magliano MP. Mesenchymal Stem Cells Promote Pancreatic Tumor Growth by Inducing Alternative Polarization of Macrophages. Neoplasia. 2016;18:142-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 109. | Liu Z, Mi F, Han M, Tian M, Deng L, Meng N, Luo J, Fu R. Bone marrow-derived mesenchymal stem cells inhibit CD8(+) T cell immune responses via PD-1/PD-L1 pathway in multiple myeloma. Clin Exp Immunol. 2021;205:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 110. | Harrell CR, Jovicic N, Djonov V, Volarevic V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 111. | Hwang SH, Wecksler AT, Wagner K, Hammock BD. Rationally designed multitarget agents against inflammation and pain. Curr Med Chem. 2013;20:1783-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 112. | Bajda M, Guzior N, Ignasik M, Malawska B. Multi-target-directed ligands in Alzheimer's disease treatment. Curr Med Chem. 2011;18:4949-4975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 113. | Youdim MB, Kupershmidt L, Amit T, Weinreb O. Promises of novel multi-target neuroprotective and neurorestorative drugs for Parkinson's disease. Parkinsonism Relat Disord. 2014;20 Suppl 1:S132-S136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 114. | Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr Med Chem. 2008;15:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 363] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 115. | Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D; CARDIOPROTECTION COST Action (CA16225). Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 558] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoyagi K, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD