Published online Jul 26, 2023. doi: 10.4252/wjsc.v15.i7.751
Peer-review started: April 8, 2023
First decision: April 25, 2023
Revised: May 15, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 26, 2023
Processing time: 107 Days and 21.8 Hours
Zinc (Zn) is the second most abundant trace element after Fe, present in the human body. It is frequently reported in association with cell growth and proliferation, and its deficiency is considered to be a major disease contributing factor.
To determine the effect of Zn on in vitro growth and proliferation of human umbilical cord (hUC)-derived mesenchymal stem cells (MSCs).
hUC-MSCs were isolated from human umbilical cord tissue and characterized based on immunocytochemistry, immunophenotyping, and tri-lineage differentiation. The impact of Zn on cytotoxicity and proliferation was determined by MTT and Alamar blue assay. To determine the effect of Zn on population doubling time (PDT), hUC-MSCs were cultured in media with and without Zn for several passages. An in vitro scratch assay was performed to analyze the effect of Zn on the wound healing and migration capability of hUC-MSCs. A cell adhesion assay was used to test the surface adhesiveness of hUC-MSCs. Transcriptional analysis of genes involved in the cell cycle, proliferation, migration, and self-renewal of hUC-MSCs was performed by quantitative real-time polymerase chain reaction. The protein expression of Lin28, a pluripotency marker, was analyzed by immunocytochemistry.
Zn at lower concentrations enhanced the rate of proliferation but at higher concentrations (> 100 µM), showed concentration dependent cytotoxicity in hUC-MSCs. hUC-MSCs treated with Zn exhibited a significantly greater healing and migration rate compared to untreated cells. Zn also increased the cell adhesion rate, and colony forming efficiency (CFE). In addition, Zn upregulated the expression of genes involved in the cell cycle (CDC20, CDK1, CCNA2, CDCA2), proliferation (transforming growth factor β1, GDF5, hypoxia-inducible factor 1α), migration (CXCR4, VCAM1, VEGF-A), and self-renewal (OCT4, SOX2, NANOG) of hUC-MSCs. Expression of Lin28 protein was significantly increased in cells treated with Zn.
Our findings suggest that zinc enhances the proliferation rate of hUC-MSCs decreasing the PDT, and maintaining the CFE. Zn also enhances the cell adhesion, migration, and self-renewal of hUC-MSCs. These results highlight the essential role of Zn in cell growth and development.
Core Tip: Zinc (Zn) efficiently increased the proliferation of mesenchymal stem cells (MSCs) with a significant increase in the rate of population doublings, and at higher concentrations, it was cytotoxic. MSCs of the test group maintained a higher frequency of mesenchymal progenitors. Upregulation of genes involved in cell cycle, proliferation, pluripotency, and migration, was also observed in test groups. Zn increased the migration capability of MSCs and cell adhesion ability of MSCs. Overall, the results showed that Zn at lower concentrations supported cell growth, division, and proliferation of MSCs while maintaining the MSC-specific characteristics, including pluripotency, migration, and cell adhesion.
- Citation: Sahibdad I, Khalid S, Chaudhry GR, Salim A, Begum S, Khan I. Zinc enhances the cell adhesion, migration, and self-renewal potential of human umbilical cord derived mesenchymal stem cells. World J Stem Cells 2023; 15(7): 751-767
- URL: https://www.wjgnet.com/1948-0210/full/v15/i7/751.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i7.751
Preclinical and clinical research supports regenerative medicine as a promising therapeutic approach to address degenerative disorders, including cardiovascular diseases, dermal wounds, rheumatic diseases, traumas, immune system diseases, neuronal diseases, and cancers[1,2]. Cell-based therapy approach in regenerative medicine utilizes undifferentiated stem cells and possesses the potential to proliferate clonally and differentiate into other types of cells[3-5]. Among these stem cells, mesenchymal stem cells (MSCs) are the multipotent stem cells that act as immune modulators, lack HLA-DR, and down-regulate the antigen-presenting and co-stimulatory molecules[6]. These immune privilege properties make MSCs a potential candidate in regenerative medicine[7-9]. Currently, more than 1000 registered clinical studies involve MSCs for the regeneration of tissues and organs[10].
MSCs from perinatal sources, such as umbilical cord tissue, umbilical cord blood, and amniotic fluid, are highly proliferative with a sustained tendency of differentiation[11-13]. Though MSCs can be isolated from various body tissues, their low number has created a need for in vitro expansion. Clinically, 20 to 100 × 106 MSCs are required for a single therapeutic dose, which minimally takes 4 to 8 wk for in vitro expansion[13]. The expansion period and the quality of MSCs depend on the isolation and cell culture protocols. In addition, the biological properties of MSCs are influenced by the patient’s clinical history, including age and genetic makeup[14]. Non-physiological conditions, including cell density, oxygen level, passage number, culture medium ingredients, and senescence are also being reported to alter biological characteristics of MSC like stemness, morphological features, migration, and proliferative potential[13].
Zinc (Zn) is a trace element that is widely reported for its role in growth and proliferation of cells, namely osteoblasts (MC3T3-E1 cells)[15], endothelial cells[16], smooth muscle cells[17], CD8+T cells[18,19]. Its deficiency is directly proportional to stem cell proliferation[20]. Plasma contains more than 1% of the total Zn[21]. Biologically, Zn is involved in cell catalytic, structural, and regulatory functions[22]. It acts as a cofactor of more than 300 enzymes[23].
Based on the role of Zn in cell growth and proliferation, we hypothesized that the addition of Zn in the growth medium would significantly increase the growth and proliferation rate of MSCs, which will be helpful in their in vitro expansion for therapeutic purposes. To the best of our knowledge, no study has addressed the effects of Zn on human umbilical cord (hUC)-MSCs. Thus, for the first time, we anticipate the potential role of Zn in the proliferation, migration, morphology, doubling time, and gene expression profile of hUC-MSCs.
Human umbilical cord tissue samples were collected during elective C-sections of healthy donors under formal consent from Zainab Panjwani Memorial Hospital, Karachi. The independent ethical committee of PCMD, ICCBS, University of Karachi, for human subjects has approved the protocol (IEC-009-UCB-2015). The samples were collected in phosphate buffer saline (PBS) containing 0.5% EDTA as an anticoagulant, and processed as reported previously[24,25]. The cord tissue was washed with PBS to remove blood clots and debris. After washing, the tissue was chopped into 3 mm3 pieces or explants and transferred into T75 culture flask containing 10-12 mL Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1 mmol/L sodium pyruvate. The flask was incubated at 37 °C in a humidified incubator set at 5% CO2. The culture medium was changed at the interval of three days. After 10-15 days, an outgrowth of cells was noted from the explants, that was allowed to grow and proliferate. hUC-MSCs up to passage 5 were used in the experiments.
MSCs were characterized based on tri-lineage differentiation, immunophenotyping, and immunocytochemistry.
Tri-lineage differentiation: For differentiation towards adipogenic, chondrogenic, and osteogenic lineages, the cells at passage P3 were cultured in a 6-well plate and grown in DMEM until they achieved 60%-70% confluency. Subsequently, DMEM was replaced with an adipogenic induction medium (200 µM indomethacin, 1 µM dexamethasone, and 10 µM insulin), osteogenic induction medium (10 µM beta glycerophosphate, 50 µM ascorbic phosphate, and 0.1 µM dexa
Immunophenotyping: Immunophenotyping was performed for cell surface markers CD73, Vimentin, CD105, and CD45 (Cat No. 562245, BD Bioscience). MSCs were incubated in blocking solution with primary antibodies at recommended dilutions for 2 h at 37 °C, and finally with secondary antibody Alexa Flour 546 of 1:200 dilution for 1 h. Cells were analyzed on a flow cytometer (BD FACS Celesta, Becton Dickson, United States).
Immunocytochemistry: MSCs were cultured in a chamber slide with a density of 5000 cells per well. Cells were fixed with 4% PFA for 10 min at room temperature (RT) and then permeabilized with 0.1% Triton X-100. Blocking was performed with 2% bovine serum albumin for 20 min at RT. Primary antibodies for MSC antigens CD73 (550256, BD Pharmingen, United States), CD117 (32-9000, Zymed Laboratories, Inc., United States), CD29 (MAB-1981, Chemicon International, United States), CD105 (560839, BD Pharmingen, United States), Stro1 (14-6688-82, Molecular Probes, Invitrogen, United States), Vimentin (V6389, Sigma-Aldrich, Inc., United States), Lin28 (PA1-096, Molecular Probes, Invitrogen, United States), and CD45 (CBL415, BD Pharmingen, United States) were added at recommended dilutions and kept at 4 °C overnight. Following PBS washing, cells were incubated with Alexa fluor-546 secondary antibody having 1:200 dilutions for 1 h at 37 °C. Nuclei were stained with DAPI for 15 min. Finally, cells were mounted and visualized under a fluorescent microscope (NiE, Nikon, Japan).
MTT assay was performed, taking different concentrations of ZnCl2 in complete DMEM to determine minimal toxic concentration. MSCs (2000 cells) per well were seeded in a 96-well plate with 200 µL complete DMEM. The medium was aspired, and cells were incubated with 5 µM, 10 µM, 20 µM, 30 µM, 50 µM, 100 µM, 250 µM, and 500 µM concentrations of ZnCl2 for overnight. The next day, the medium was removed, and cells were incubated with 200 µL of MTT dye (0.5 gm/mL stock) for 4 h and incubated at 37 °C with 5% CO2. Then, the MTT solution was discarded, and 200 µL of DMSO was added to dissolve formazan crystals formed by metabolically active cells. The plate was kept on an orbital shaker for 5 min at RT, and absorbance was measured at 570 nm by spectrophotometer (SpectraMax, United States).
To analyze MSC proliferation, 2000 cells per well were seeded in a 96-well plate and incubated with different concentrations of ZnCl2 for 24 h, 48 h, and 72 h. After each time point, the medium was removed, and 0.02% Alamar blue solution was added to each well. Fluorescence intensity was measured using a fluorescence spectrophotometer with excitation at 560nm and emission at 600nm (Varioskan LUX, Thermo Scientific). The relative fluorescence unit (RFU) was calculated by the given formula given below:
Difference between treated and untreated (%) = (RFU of treated groups/RFU of untreated group) × 100
To analyze the proliferation rate of hUC-MSCs, 10000 cells per well were seeded in a 24-well plate and incubated with 20 µM ZnCl2. When cells attained 70%-80% confluency, they were detached and counted by hemocytometer for the number of population doubling (NPD), and population doubling time (PDT) by the formulae given below:
NPD = 3.33 × log (Nt/Ni)
PDT = t × log (2)/[log (Nt) – log (Ni)]
Where, Ni = no. of cells plated; Nt = no. of cells harvested; ti = initial day (0 d) and t (harvesting day).
MSCs were seeded at 500 cells per cm2 in a T25 culture flask. After two weeks of incubation, cells were fixed with 4% PFA, followed by incubation with 0.5% crystal violet prepared in 10% methanol. Cells were washed with distilled water, and colonies were visualized at 40 × magnification under bright field microscope. A cluster of 100 cells was considered a colony. Results were analyzed by SPSS software.
Cells were harvested for RNA isolation, 1 mL Trizol reagent (15596026, Invitrogen) was added, followed by the addition of 500 µL chloroform for phase separation and centrifugation at 12000 rpm. The aqueous layer was collected and incubated with 1 mL absolute ethanol for 2 h at -80 °C. The suspension was centrifuged, and the pellet was rinsed with 70% ethanol. Finally, the pellet was reconstituted in 15 µL nuclease-free water, quantified by nanodrop spectrophotometer, and stored at -20 °C. cDNA was synthesized using 1 μg RNA by RevertAid first-strand cDNA synthesis kit (K1622, Thermo Scientific, United States). All the primers were designed by http://www.ncbi.nlm.nih.gov/tool/primerblast and synthesized commercially. quantitative real-time polymerase chain reaction (qPCR) amplification was performed in a triplicate manner using qPCR master mix (A600A, Promega, Madison, WI, United States) for the genes mentioned in Table 1.
| S. No | Genes | Primer sequence (5’-3’) | Annealing temperature (ºC) |
| 1 | GAPDH | (F) CCAGAACATCATCCCTGCCT | 58 |
| (R) CCTGCTTCACCACCTTCTTG | |||
| 2 | CDC20 | (F) TCCTGAGTCTGACCATGAGC | 58 |
| (R) CTGAGGTGATGGGTTGGTCT | |||
| 3 | CDK1 | (F) GGGGTCAGCTCGTTACTCAA | 58 |
| (R) CACTTCTGGCCACACTTCAT | |||
| 4 | CCNA2 | (F) CCAGGAGAATATCAACCCGGA | 58 |
| (R) GGAACGGTGACATGCTCATC | |||
| 5 | CDCA2 | (F) TCTGGGAGTAAGCCTGTCTG | 58 |
| (R) AGGAGGTAATTCGGCATGCT | |||
| 6 | TGFβ1 | (F) CAAGGCACAGGGGACCAG | 58 |
| (R) CAGGTTCCTGGTGGGCAG | |||
| 7 | GDF5 | (F) CACATCCCAAGAGCCCCTTC | 58 |
| (R) GCCCAGGTGAGGAGAAATGG | |||
| 8 | HIF1α | (F) CTCGAGATGCAGCCAGATCT | 58 |
| (R) CCAGAAGTTTCCTCACACGC | |||
| 9 | 45203 | (F) GATGTCAGGGCTCTTTGTCCA | 54 |
| (R) TACTCTCCCCAGCTTGCTTTG | |||
| 10 | SOX-2 | (F) CCCTGCAGTACAACTCCATGAC | 54 |
| (R) GGTAGTGCTGGGACATGTGAAG | |||
| 11 | NANOG | (F) AAGGCAAACAACCCACTTCTG | 54 |
| (R) CCAGTTGTTTTTCTGCCACCT | |||
| 12 | CXCR 4 | (F) ACTGAGAAGCATGACGGACA | 54 |
| (R) TCATCTGCCTCACTGACGTT | |||
| 13 | VCAM1 | (F) CCTGGGAAGATGGTCGTGAT | 54 |
| (R) AGATGTGGTCCCCTCATTCG | |||
| 14 | VEGF-A | (F) AGCGCAAGAAATCCCGGT | 54 |
| (R) CCCTCCGGACCCAAAGTG | |||
| 15 | MMP2 | (F) TTGACGGTAAGGACGGACTC | 54 |
| (R) AGCGGAATGGAAACTTGCAG |
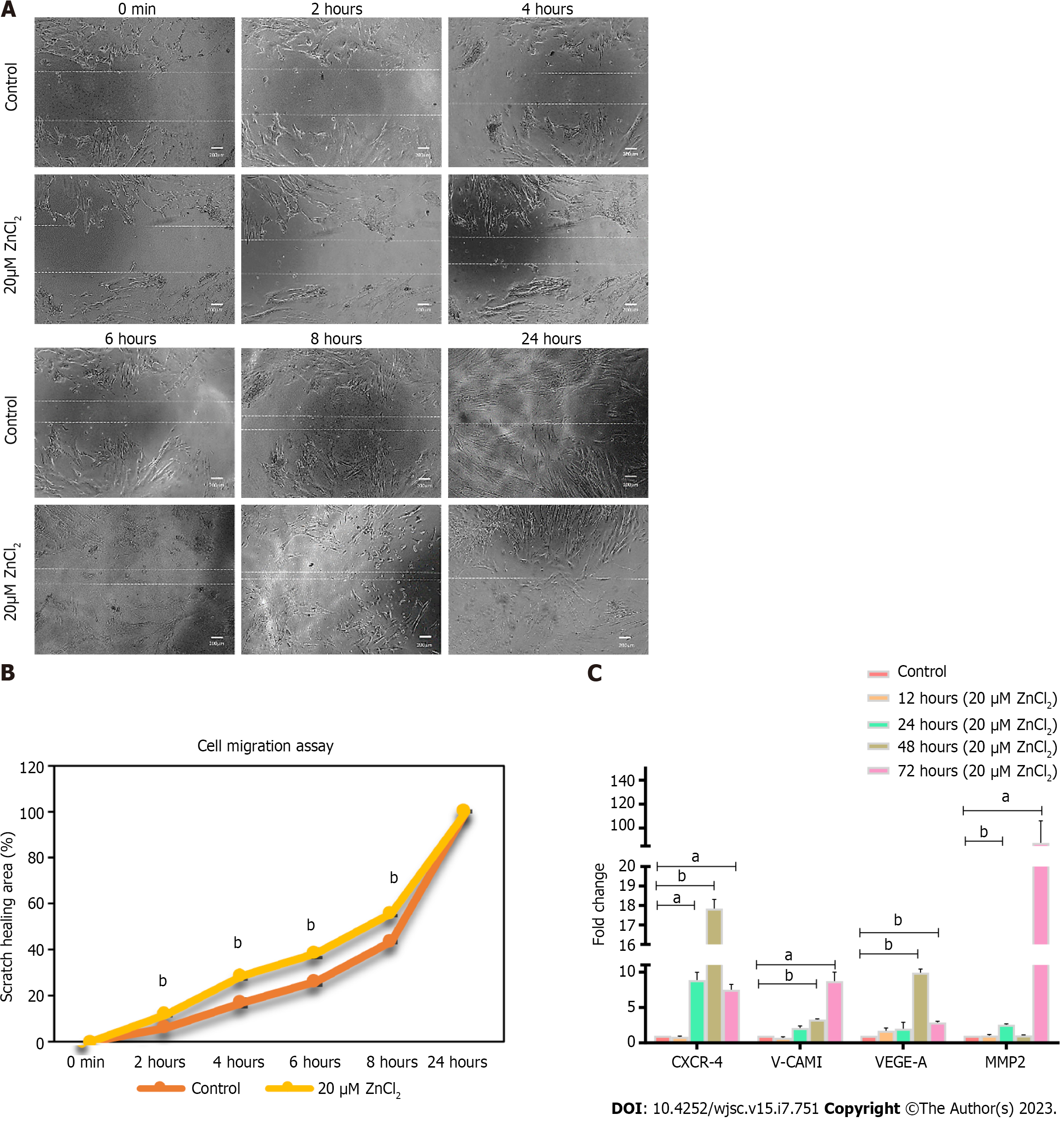
For cell migration, 4000 cells per well were seeded in a 24-well plate containing DMEM and incubated for 24 h. After monolayer formation, the scratch was developed using a 100 µL pipette tip, and cells were incubated with 20 µM ZnCl2. Migration of cells was observed at 2 h, 4 h, 6 h, 8 h, and 24 h of scratch development. Images were captured at different time points under a phase-contrast microscope (Eclipse Ts2, Nikon, Japan) and migration was measured in percentage with the help of the following formula:
% of MSCs migration = [ (hi − Δh) ÷ hi] × 100%
Where, hi = the area of scratch measured immediately after scratching, and Δh = is the area of scratch measured at 2, 4, 6, 8, and 24 h.
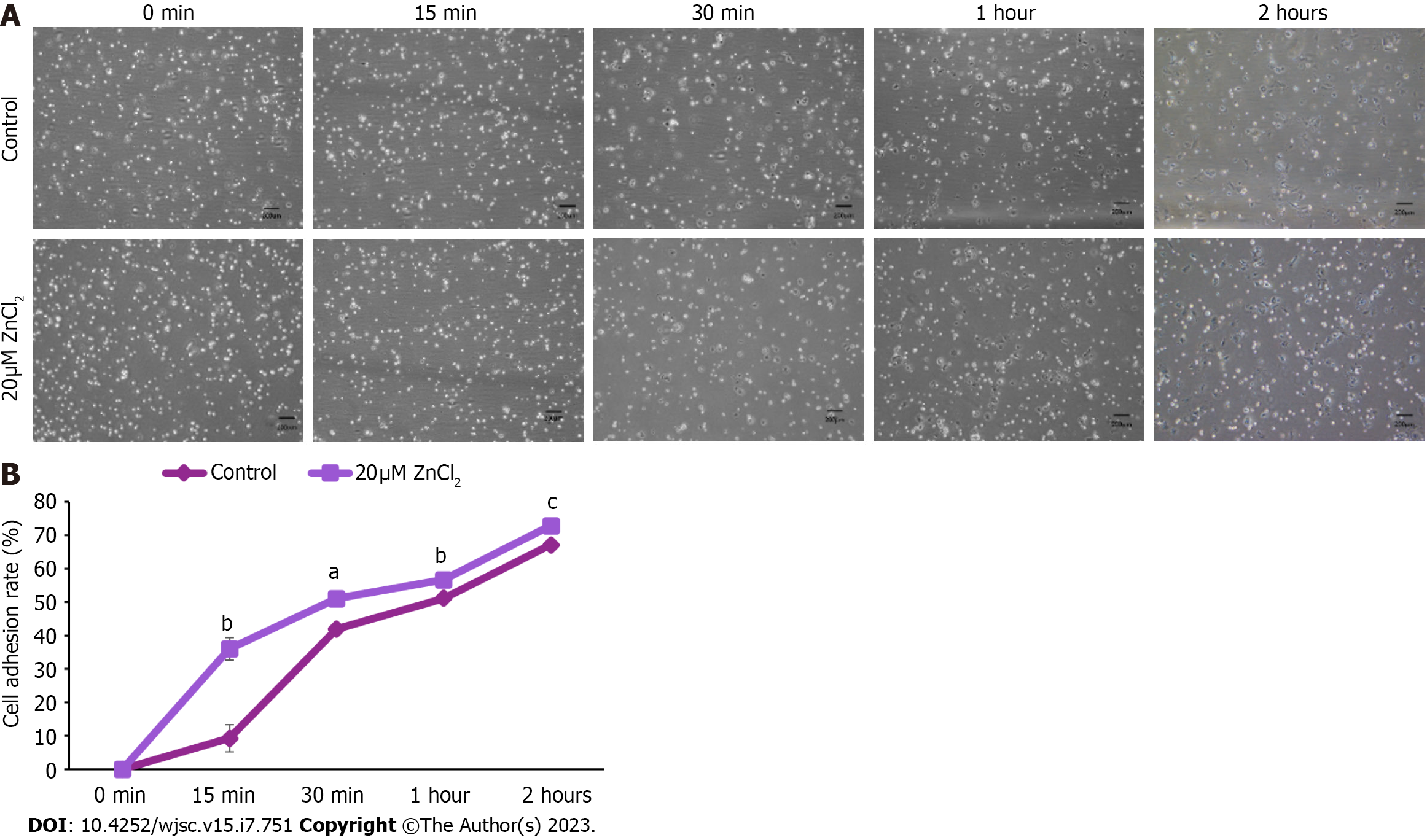
To analyze cell adherence in control and test groups, MSCs were harvested, seeded in a culture flask, and cell adhesion was determined by observing MSCs at different time points, i.e., at 0 min (immediately after seeding), after 15 min, 30 min, 1 h, and 2 h. Cell adhesion was evaluated by the given formula:
%Percentage = no. of cells adhered on the flask surface/no. of floating cells in media.
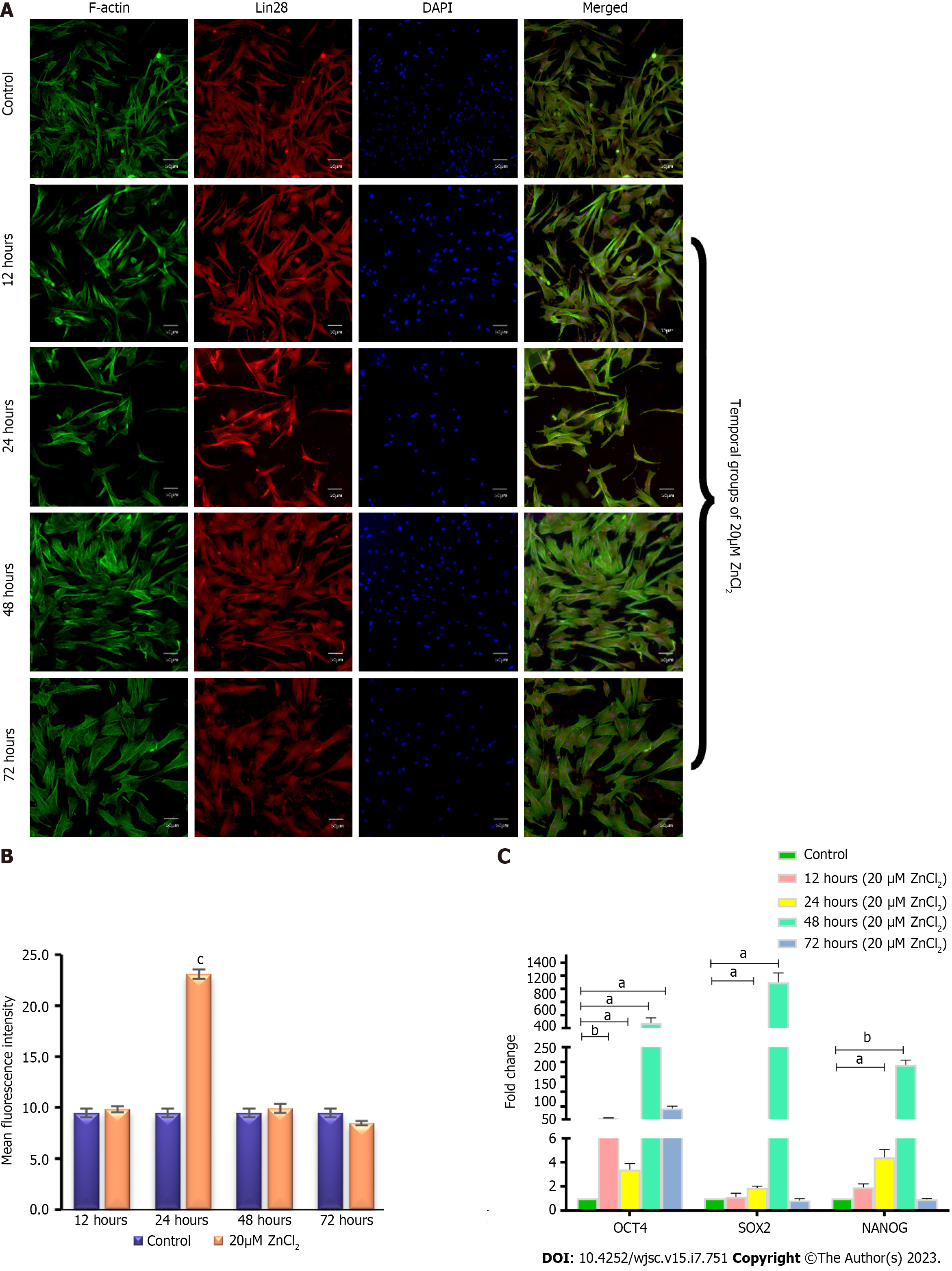
To analyze the pluripotency marker after 20 µM ZnCl2 treatment, MSCs were incubated with the primary antibody of Lin28 at 1:100 dilution and kept at 4 °C overnight. After PBS washing, cells were incubated with Alexa fluor-546 secondary antibody of 1:200 dilutions for 1 h at 37 °C. Nuclei were stained with DAPI for 15 min and then visualized under a fluorescent microscope (NiE, Nikon, Japan).
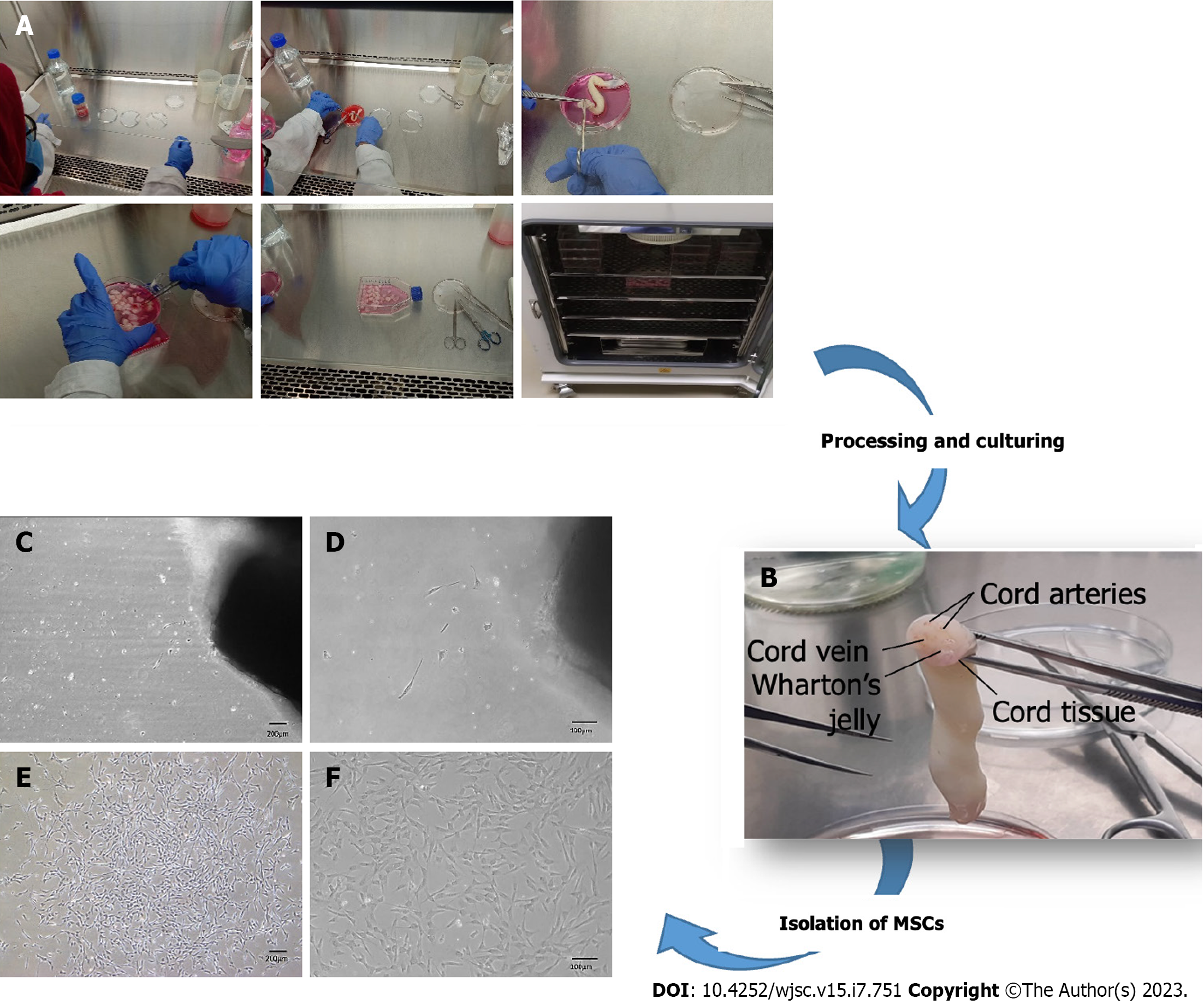
MSCs were isolated from human umbilical cord tissue by the explant method (Figure 1A). Human umbilical cord tissue comprises one vein, two arteries, and Wharton's jelly (Figure 1B). MSCs started to outgrow the cord tissue after 10-15 d of cord processing. At this stage, cells adhered to the flask surface and appeared as trigonal shape (Figure 1C and D). After approximately 25 d, cells were observed to be interconnected with each other by their extensions, formed colonies, and exhibited elongated fibroblast-like morphology, which was termed P0 (passage zero) MSCs (Figure 1E and F).
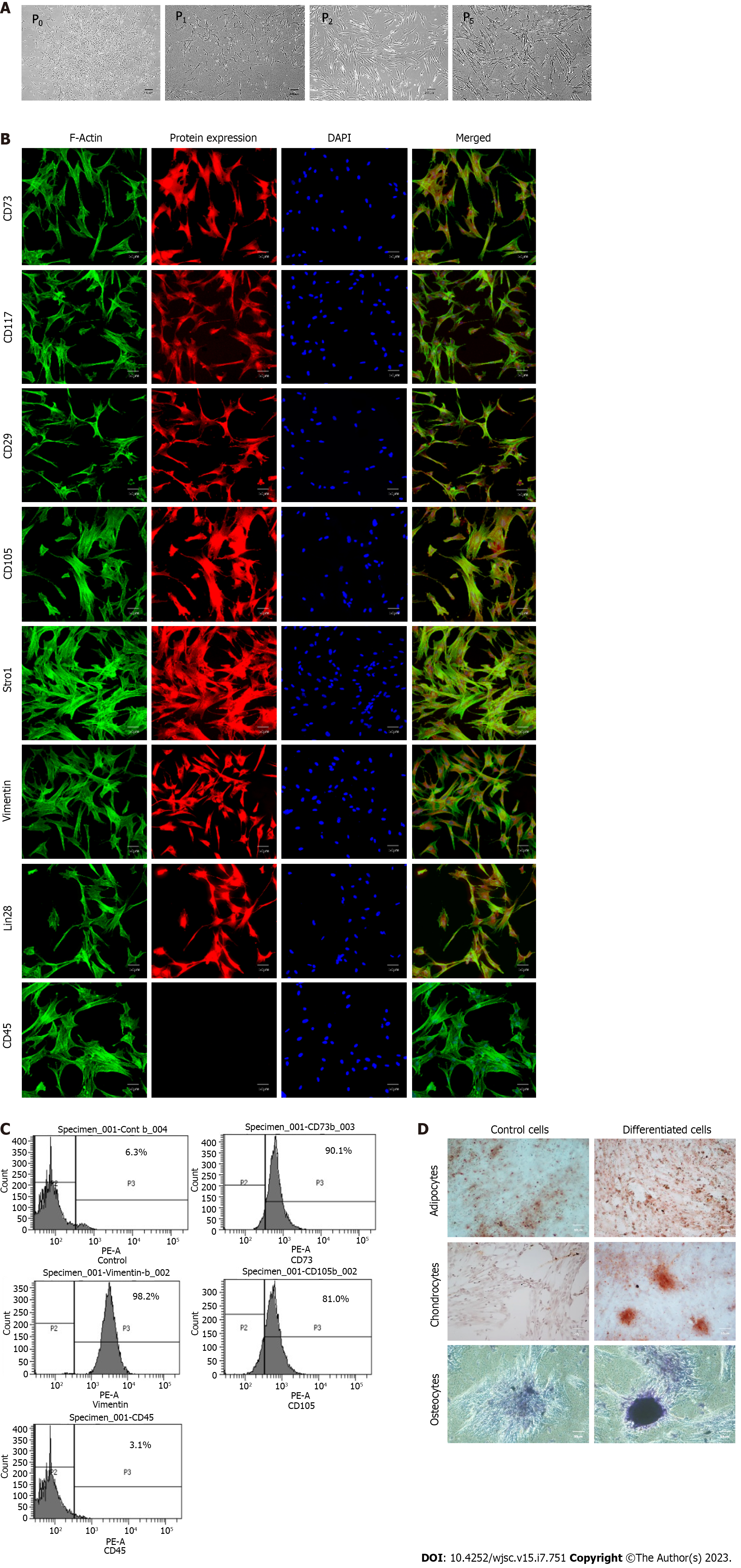
Cells isolated from the umbilical cord possessed adhesive properties and exhibited fibroblast-like morphology (Figure 2A). The isolated population of cells showed positive expression for MSCs specific markers, including CD73, CD117, CD29, CD105, Stro1, Vimentin, and Lin28, while negative for CD45, which is a hematopoietic marker (Figure 2B). Immunophenotypic analysis showed positive expression for CD73 (90%), vimentin (98%), and CD105 (80%), and less than 4% population was positive for CD45 (Figure 2C). The isolated cells successfully differentiated into adipogenic, osteogenic, and chondrogenic lineages as confirmed by Oil red O, Alizarine red S, and Alcine blue staining (Figure 2D).
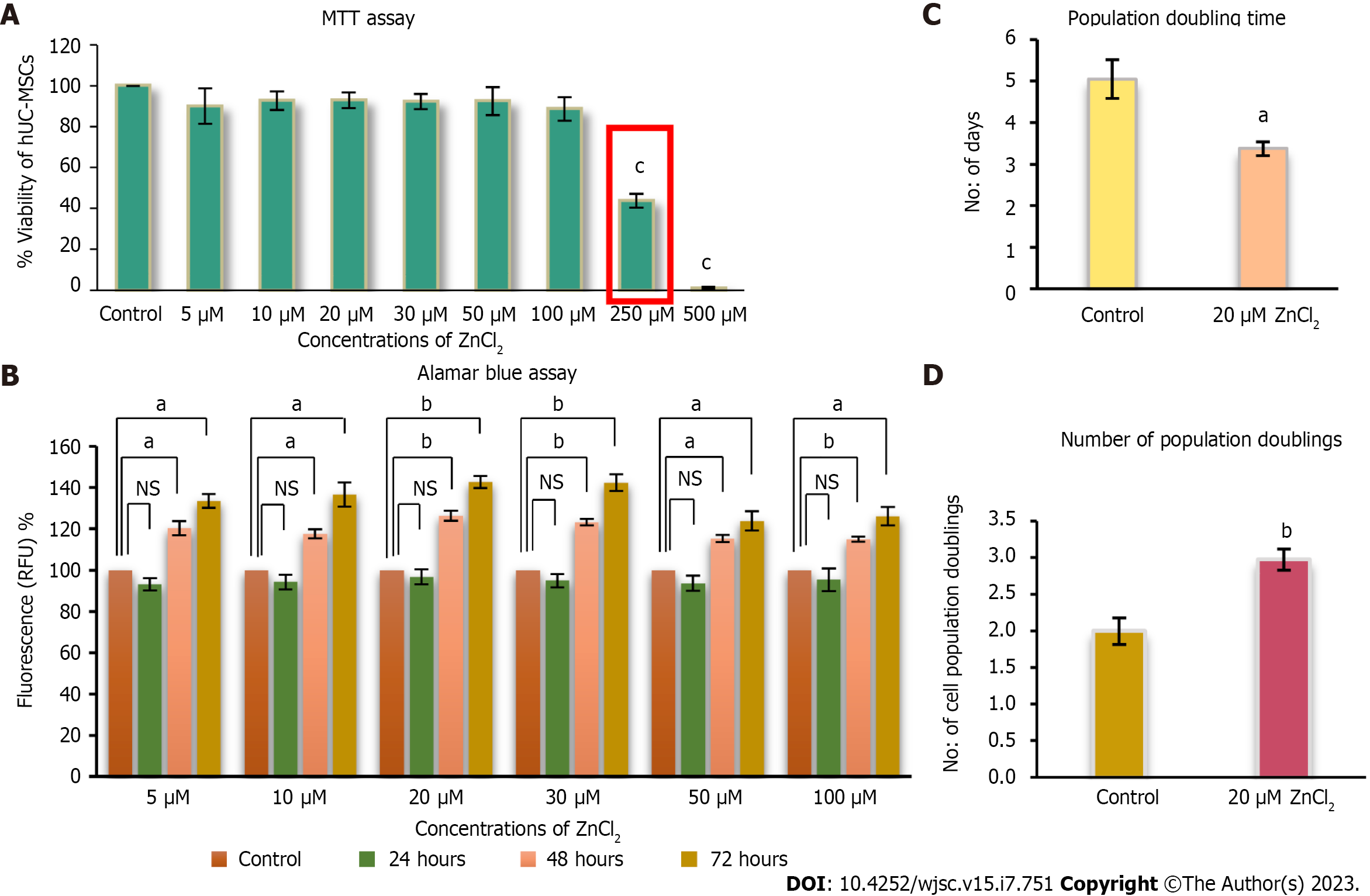
ZnCl2 concentrations ranging from 5 µM-100 µM did not show any cytotoxic effect on cells (Figure 3A and B). ZnCl2 was found to be significantly cytotoxic above 100 µM concentration, and its cytotoxicity was found to be higher in a concentration-dependent manner. The IC50 of ZnCl2 was found at 250 µM concentration (Figure 3A). For proliferation analysis, MSCs were treated with 5-50 µM ZnCl2 for 24, 48, and 72 h. Fluorescence intensity was measured for each group at the respective time after incubating with Alamar blue for 4 h. Results of each temporal group were analyzed by one-way ANOVA between control and test groups. All test groups observed significantly increased proliferation after 48 and 72 h (Figure 4A and B). MSCs in passage 5 were analyzed for NPD and PDT. After 10 d, MSCs of the control and treated group (20 µM ZnCl2), were counted, and significantly higher NPD was observed in 20 µM ZnCl2 treated group (Figure 3C), while PDT reduced significantly in the treated group in contrast to the control group (Figure 3D).
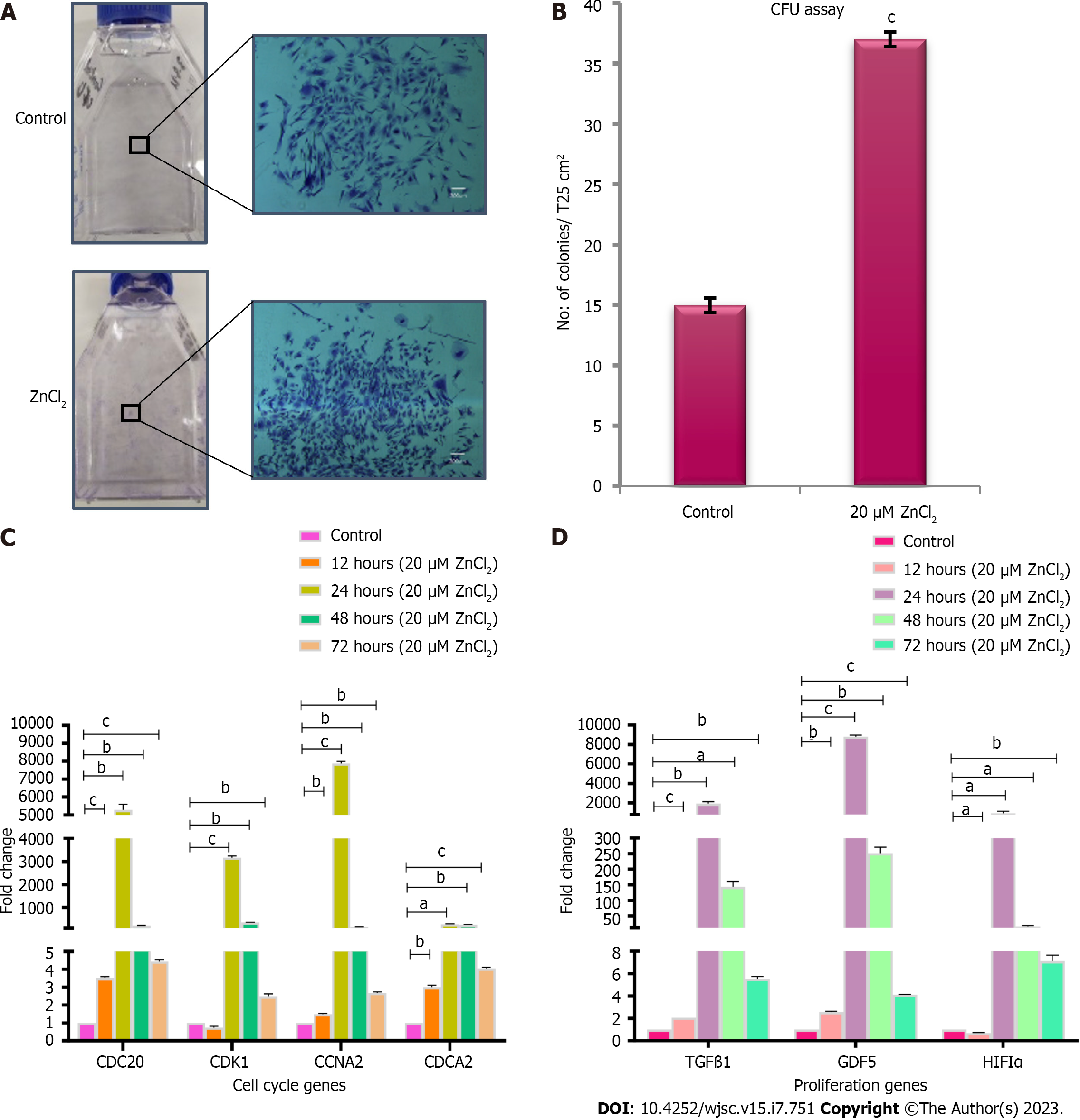
To analyze the presence of mesenchymal progenitors, colony forming unit (CFU) assay was performed. Colonies of hUC-MSCs were observed after 2 wk of cell seeding. The test group supplemented with 20 µM ZnCl2, exhibited a significantly higher number of colonies than the control group (Figure 4A and B). To analyze the transcriptional changes of 20 µM ZnCl2 treated MSCs to the untreated MSCs, fold change regulation (2-ΔΔCt) of cell cycle and proliferation genes was analyzed at 12, 24, 48, and 72 h. Expression of cell cycle genes, i.e., CDC20, CCNA2, and CDCA2, was comparatively increased in all temporal groups of 20µM ZnCl2, while CDK1 was upregulated after 24, 48, and 72 h (Figure 4C). Among proliferation genes, expression of TGFβ1 and GDF5 was significantly increased in all temporal groups of 20 µM ZnCl2, whereas hypoxia-inducible factor 1α (HIF-1α) exhibited a significant upregulation after 24, 48, and 72 h (Figure 4D).
ZnCl2 was also found to facilitate the migration of hUC-MSCs (Figure 5A) and significantly increased (bP ≤ 0.01) migration was observed in 20 µM ZnCl2 treated groups for 2-8 h, while scratch was completely healed after 24 h in both groups, i.e., control and ZnCl2 treated groups. The percentage of area healed is plotted against time (Figure 5B). Gene expression analysis of migration factors showed upregulation of CXCR-4 at all the time points, i.e., 24, 48, and 72 h, while V-CAM1, VEGF-A was significantly upregulated after 48 and 72 h. MMP-2 was found to be upregulated after 24 and 72 h (Figure 5C).
To analyze the effect of ZnCl2 on the adhesiveness of hUC-MSCs, in vitro cell adhesion assay, was performed. A significant increase in the cell adhesion rate of hUC-MSCs in the 20 µM ZnCl2 treated group was noted at 15 min, 30 min,1 h, and 2 h, compared to the control group (Figure 6A). Cell adhesion rate (%) is plotted against time (Figure 6B).
Immunostaining: Immunostaining of 20 µM ZnCl2 treated MSCs showed positive expression of pluripotency marker Lin28 at all the time points (Figure 7A). The quantified mean fluorescence intensity showed a significant increase in expression at 24 h (Figure 7B).
Gene expression analysis: Genes expression analysis showed that OCT4 was significantly upregulated in all temporal groups (12, 24, 48, and 72 h), of 20 µM ZnCl2. In contrast, the expression of SOX2 and NANOG was significantly higher in temporal groups of 24 and 48 h and then fell to the level of control in 72 h (Figure 7C).
Zn is among the essential trace elements that is ubiquitously found in the human body. According to prior investigations, it is one of the trace elements that is majorly involved in cell growth and proliferation[16,17,26]. It stimulates cell division and proliferation by regulating enzymes such as deoxythymidine kinase and/or by regulating hormones including insulin-like growth factor-I (IGF-I) and nerve growth factors[23,27]. Zn acts as a signal molecule for both extracellular and intracellular pathways. It upregulates PI3 kinase pathway which activates AKT in fibroblast cells and transactivates EGF receptor by Src pathway in epithelial cells of the airway. It also regulates the ERK1/2 pathway with the induction of p21 (CiP/WAF1), and cyclin D1 in colonocytes[21]. Studies reported the role of Zn on the proliferation of adipose derived MSCs[23,26]. A recent study reported the effect of Zn on human umbilical cord derived MSCs and showed its importance in MSC survival and differentiation properties. The study suggested that Zn could enhance antioxidative properties of MSCs by regulating Nrf2/Sirt3 signaling pathway[28]. In the present study, we analyzed the effect of ZnCl2 on hUC-MSCs in the context of its proliferation and growth, pluripotency, migration, and cell adhesion properties.
ZnCl2 is reported to have a biphasic effect on the viability and proliferation of the cells and exhibited dose-dependent response, which varies for different cell lines[29]. Most of the studies found ZnCl2 cytotoxic above 100 µM in different dividing cells, including coronary artery endothelial cells, vascular smooth muscle cells, and even MSCs[16,17,23]. Our findings showed cytotoxicity of ZnCl2 above 100 µM, which is in line with the previous study[23]. The proliferation profile of MSCs reported by previous studies gave a bell shape biphasic curve whose peaks vary for ZnCl2 concentrations for different cells. We also obtained a bell shape biphasic curve representing an increase in MSC proliferation with increase of ZnCl2 concentration up to 30 µM, and then gradually decreasing upon further increase. However, up to 100 µM concentration of ZnCl2 gave significantly increased proliferation of MSCs in test groups as compared to control. Increased population doublings (NPD) and reduced PDT were observed in the treatment group in contrast to control MSCs.
MSCs are the progenitor cells that proliferate rapidly[30,31]. MSCs retain their multipotent ability, but the expanded cultured population of MSCs shows heterogeneous morphology of cells with variations in the frequency of early progenitor cells[30]. It is reported that human umbilical cord-derived MSCs exhibit the highest percentage of colony-forming units in contrast to the MSCs derived from bone marrow, adipose tissue, and dental pulp[32]. By CFU assay, we analyzed the impact of Zn on the colony-forming potential of MSCs derived from the human umbilical cord. Our results revealed that ZnCl2 maintains a high pool of early progenitor cells in the MSC population, as many colonies were found in the treated group. ZnCl2 treated group also exhibited denser colonies with spindle-shaped morphology which represented the rapidly self-replicating cells[30]. MSCs of the control group showed broad morphology with less dense colonies, which reflected slow self-replicating cells[30].
Gene expression analysis of treated MSCs exhibited a significant increase in cell cycle genes, including CDK1, CDC20, CCNA2, and CACD2. The level of CDK1 remains constant in entire cell division, but its activity depends on cyclins that are synthesized at different cell cycle stages[33]. CCNA2 or cyclin A2 is important for G1/S and G2/M transition, thus responsible for cell cycle progression[34]. CDK1 and CDC20 are the key mitotic regulators[35,36]. CDC20 is a cofactor of CPC/C (anaphase-promoting complex/Cyclosome), by activating CPC/C, CDC20 regulates the activity of CDK1 and promotes chromosome segregation by degrading securin, thus responsible for anaphase transition[36]. Whereas CDCA2 is essential for retaining chromatin structure in post-mitotic nuclei and regulates DNA damage responses for mitotic to interphase transition[37]. Upregulation of these genes required at different stages of the cell cycle showed progression of the cell cycle in the test group.
Few growth factors are pleiotropic and are found to be involved in multiple biological functions. For instance, TGFβ is one of them, and previous studies have reported the role of TGFβ in MSCs proliferation[38,39], and differentiation towards chondrogenic lineage; however, it inhibits terminal differentiation[39]. TGFβ1 is found to promote hUC-MSCs proliferation without affecting the phenotype of MSCs[38]. Hence, our results are in the context of these findings as the ZnCl2 treated group showed a significant increase in TGFβ1 expression and stemness markers like OCT4, SOX2, and NANOG.
Few studies have reported the effect of Zinc on the expression of HIF1-α, but there is inconsistency, as some studies on cancer cells proved down-regulation of HIF1-α caused by Zinc[40,41], while in keratinocytes and prostate cells, Zinc is found to stabilize HIF1-α expression[41,42]. Our findings showed enhanced expression of HIF1-α in ZnCl2 treatment group which is in agreement with later studies[41,42]. Overall, the significantly increased expression of TGFβ1, GDF5, and HIF1-α suggested the increased proliferation and multipotency of MSCs in the test group.
Migration is an important factor that should be considered for better therapeutic effects of MSCs in regeneration and cell therapy. Studies reported that Zn enhances the migratory capability of cells[16,17,23,43]. For example, endothelial cells and smooth muscle exhibited enhanced migration and cell adhesion when treated with ZnCl2. These cells showed upregulation of focal adhesion molecules, including integrin, vinculin, actin, ICAM1, and VEGFA[16,17]. We analyzed the effect of Zn on the migration ability of MSCs by in vitro scratch assay, which showed a significant increase in MSC migration after ZnCl2 treatment. These results were further confirmed by gene expression analysis of migration factors, including CXCR-4, V-CAM1, VEGF-A, and MMP2. These are involved in chemotaxis, cell rolling, and cell invasion and play their role in different phases of cell migration[44]. CXCR-4 and VEGF-A are the early migration markers, where CXCR-4 is a chemokine receptor, and VEGF is a growth factor, and both are involved in chemotaxis and trafficking of cells. V-CAM1 is a cell adhesion molecule, whereas MMP2 is required for cell invasion[44]. Here we analyzed these genes to estimate the migration ability of MSCs after ZnCl2 treatment, and we found that after 12 h, only VEGF-A showed high expression as compared to control, and after 24 h, all four migration markers exhibited significantly increased expression. After 48 h, CXCR-4, V-CAM1, and VEGF-A showed significantly increased expression, and after 72 h all of these genes showed significantly high expression, whereas MMP2 showed the highest expression. Overall, up-regulation of these genes suggested that Zn also enhanced the expression of migration markers and promoted MSC migration, where fluctuations in their regulation may relate to their role at different stages of cell migration[44]. Studies reported that Zn promotes cell adhesion at lower concentrations in different cell types. Endothelial cells of coronary artery and smooth muscle cells exhibited increased cell adhesion ability when cultured in a ZnCl2 supplemented medium[16,17]. We also found a considerably enhanced cell adhesion rate of MSCs when treated with 20 µM ZnCl2.
Lin28, a pluripotency marker, was analyzed for its protein expression. It is an RNA binding protein that was first characterized in C.elegans as a heterochronic gene and was found to control developmental timing. In mammals, two homologs, including Lin28a and Lin28b are present in the cytoplasm as well as in the nucleus[45]. Structurally, Lin28 comprises the N-terminal cold shock domain and two zinc-binding motifs (CCHCx2) located at C-terminal. Lin28 is highly expressed in ESCs, while upon differentiation, its expression is suppressed by miRNAs, namely Let-7 and Lin-4[45]. It is involved in post-translational modifications of specific subsets of mRNAs. In skeletal muscles, it binds to IGF-2 mRNA[46], while in mouse ESCs it is reported to bind with mRNAs of cell cycle-specific genes[47,48]. OCT4 mRNA is also reported as one of the targets of Lin28 for post-translational modifications[49]. Our results showed positive expression of Lin28 in all temporal groups, however, its expression exhibited fluctuations, i.e., after 24 h of treatment, it was significantly enhanced, and then at later time points, the expression decreased gradually and became approximately similar to control group. It might be because of its post-translational role. That is why it showed higher expression before, i.e., after 24 h than other pluripotency genes that exhibited their higher expression mainly after 48 and 72 h. Altogether, these factors make regulatory circuitry which is regulated through feed-forward loop and autoregulatory mechanism[49], and the fluctuations in their expression at time points may reflect this regulatory circuitry.
OCT4, SOX2, and NANOG, are the transcription factors that are conserved across mammalian species[50]. All three are highly expressed in ESCs and are responsible for the pluripotency and self-renewal of ESCs[51]. These transcription factors regulate gene expression in a highly cooperative fashion[52]. OCT4 and SOX2 form heterodimers and bind to specific promoters on DNA[53]. NANOG is a homeobox family gene that also forms heterodimer and regulates other pluripotency genes in feedback mechanism[53]. These are also found to have similar role in adult stem cells, including MSCs[11,50,54]. A study carried out by Hu et al[55], 2016, saw a significant increase in the expression of OCT4, SOX2, and NANOG in mouse ESCs when treated with ZnCl2 for 48 h. The study further reported that as ESCs in humans and mice share the same key regulators, i.e., OCT4, SOX2, NANOG[55], Zn might exert similar effects regarding the acquaintance and maintenance of the pluripotency in human stem cells. Mnatsakanyan et al[56], 2019 also showed significantly increased expression of SOX2 in mouse ESC after long-term treatment, i.e., 30 days with ZnCl2[56]. In our study, gene expression analysis of all three genes OCT4, SOX2, NANOG showed a significant increase in the test group after 24 and 48 h. After 12 and 72 h of treatment, SOX2 and NANOG did not exhibit any significant difference compared to the control, while OCT4 represented a significant increase in all temporal groups.
Limitations of this study is that growth kinetics is analyzed by treating cells up to three days with zinc. Further temporal study is required to evaluate the long term effects of zinc. Also the signaling pathways that zinc utilizes to produce its effects are needed to be explored via protein quantification. An in vivo experimental evaluation is also needed to further support the in vitro findings.
At lower concentrations, Zn efficiently increased the proliferation of MSCs with a significant increase in the rate of population doublings, and at higher concentrations, it was cytotoxic. MSCs of the test group maintained a higher frequency of mesenchymal progenitors. Upregulation of genes involved in cell cycle, proliferation, pluripotency, and migration, was also observed in test groups. Zn increased the migration capability of MSCs and cell adhesion ability of MSCs. Overall, the results showed that Zn at lower concentrations supported cell growth, division, and proliferation of MSCs while maintaining the MSC-specific characteristics, including pluripotency, migration, and cell adhesion. Hence, Zinc could be a suitable constituent for cell culture medium that would help to enhance the quantity, quality, and rate of in vitro proliferation of MSCs for clinical and industrial-scale production. Though fewer studies are reported on Zinc effects on MSCs, further investigations are required to understand the mechanism that Zn utilizes to induce its effects.
Zinc (Zn) is the second most abundant trace element after Fe, present in the human body. It is frequently reported in association with cell growth and proliferation, and its deficiency is considered to be a major disease contributing factor.
Trace elements are required to be supplemented in culturing media that will help to promote proliferation, cell division, and cellular growth, which will result in in vitro expansion of mesenchymal stem cells (MSCs) to achieve a desired number of cells for therapeutic dose.
In the present study, we aimed to determine the effect of Zn on in vitro growth and proliferation of human umbilical cord (hUC)-derived MSCs.
hUC-MSCs were isolated from human umbilical cord tissue and characterized based on immunocytochemistry, immunophenotyping, and tri-lineage differentiation. The impact of Zn on cytotoxicity and proliferation was determined by MTT and Alamar blue assay. To determine the effect of Zn on population doubling time (PDT), hUC-MSCs were cultured in media with and without Zn for several passages. An in vitro scratch assay was performed to analyze the effect of Zn on the wound healing and migration capability of hUC-MSCs. A cell adhesion assay was used to test the surface adhesiveness of hUC-MSCs. Transcriptional analysis of genes involved in the cell cycle, proliferation, migration, and self-renewal of hUC-MSCs was performed by quantitative real-time polymerase chain reaction. The protein expression of Lin28, a pluripotency marker, was analyzed by immunocytochemistry.
Zn at lower concentrations enhanced the rate of proliferation but at higher concentrations (> 100 µM), showed concentration dependent cytotoxicity in hUC-MSCs. hUC-MSCs treated with Zn exhibited a significantly greater healing and migration rate compared to untreated cells. Zn also increased the cell adhesion rate, and colony forming efficiency (CFE). In addition, Zn upregulated the expression of genes involved in the cell cycle (CDC20, CDK1, CCNA2, CDCA2), proliferation (TGFβ1, GDF5, hypoxia-inducible factor 1α), migration (CXCR4, VCAM1, VEGF-A), and self-renewal (OCT4, SOX2, NANOG) of hUC-MSCs. Expression of Lin28 protein was significantly increased in cells treated with Zn.
Our findings suggest that zinc enhances the proliferation rate of hUC-MSCs decreasing the PDT, and maintaining the CFE. Zn also enhances the cell adhesion, migration, and self-renewal of hUC-MSCs. These results highlight the essential role of Zn in cell growth and development.
Zinc at lower concentrations supported cell growth, division, and proliferation of MSCs while maintaining the MSC-specific characteristics, including pluripotency, migration, and cell adhesion. This will lead to produce large number of MSCs required for in vivo implanation in shorter period of time.
| 1. | Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci USA. 2015;112:14452-14459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 557] [Article Influence: 50.6] [Reference Citation Analysis (2)] |
| 2. | Trohatou O, Roubelakis MG. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram. 2017;19:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv Pharm Bull. 2015;5:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Fathi E, Farahzadi R. Isolation, Culturing, Characterization and Aging of Adipose Tissue-Derived Mesenchymal Stem Cells: A Brief Overview. Braz Arch Biol Technol. 59. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019;6:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 7. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13033] [Article Influence: 685.9] [Reference Citation Analysis (12)] |
| 8. | Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H, Minayi N, Movassagh Pour A. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013;3:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 9. | Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 10. | Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Kang CM, Kim H, Song JS, Choi BJ, Kim SO, Jung HS, Moon SJ, Choi HJ. Genetic Comparison of Stemness of Human Umbilical Cord and Dental Pulp. Stem Cells Int. 2016;2016:3453890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Donders R, Bogie JFJ, Ravanidis S, Gervois P, Vanheusden M, Marée R, Schrynemackers M, Smeets HJM, Pinxteren J, Gijbels K, Walbers S, Mays RW, Deans R, Van Den Bosch L, Stinissen P, Lambrichts I, Gyselaers W, Hellings N. Human Wharton's Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev. 2018;27:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Drela K, Stanaszek L, Nowakowski A, Kuczynska Z, Lukomska B. Experimental Strategies of Mesenchymal Stem Cell Propagation: Adverse Events and Potential Risk of Functional Changes. Stem Cells Int. 2019;2019:7012692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Estrada JC, Torres Y, Benguría A, Dopazo A, Roche E, Carrera-Quintanar L, Pérez RA, Enríquez JA, Torres R, Ramírez JC, Samper E, Bernad A. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4:e691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Seo HJ, Cho YE, Kim T, Shin HI, Kwun IS. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2010;4:356-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 16. | Ma J, Zhao N, Zhu D. Endothelial Cellular Responses to Biodegradable Metal Zinc. ACS Biomater Sci Eng. 2015;1:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Ma J, Zhao N, Zhu D. Bioabsorbable zinc ion induced biphasic cellular responses in vascular smooth muscle cells. Sci Rep. 2016;6:26661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133:1452S-1456S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, Meydani SN. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016;103:942-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Levenson CW, Morris D. Zinc and neurogenesis: making new neurons from development to adulthood. Adv Nutr. 2011;2:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Hara T, Takeda TA, Takagishi T, Fukue K, Kambe T, Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017;67:283-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 22. | Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013;18:144-157. [PubMed] |
| 23. | Moon MY, Kim HJ, Choi BY, Sohn M, Chung TN, Suh SW. Zinc Promotes Adipose-Derived Mesenchymal Stem Cell Proliferation and Differentiation towards a Neuronal Fate. Stem Cells Int. 2018;2018:5736535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Aslam S, Khan I, Jameel F, Zaidi MB, Salim A. Umbilical cord-derived mesenchymal stem cells preconditioned with isorhamnetin: potential therapy for burn wounds. World J Stem Cells. 2020;12:1652-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (2)] |
| 25. | Khalid S, Ekram S, Ramzan F, Salim A, Khan I. Co-regulation of Sox9 and TGFβ1 transcription factors in mesenchymal stem cells regenerated the intervertebral disc degeneration. Front Med (Lausanne). 2023;10:1127303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Fathi E, Farahzadi R. Zinc Sulphate Mediates the Stimulation of Cell Proliferation of Rat Adipose Tissue-Derived Mesenchymal Stem Cells Under High Intensity of EMF Exposure. Biol Trace Elem Res. 2018;184:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130:1500S-1508S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 557] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 28. |
Lu XD, Lin YF, Lin XY, Zhang Q, Wang ZH, Mi XG, Wang RB, Zhang XF, Luan X, Liu Y, Li B, Tan Y, Fang YQ. Anti-Oxidative Effect of Zinc in Human Umbilical Cord Mesenchymal Stem Cells.
Biophys. Reports. 2 142-151 2021, |
| 29. | Salesa B, Sabater I Serra R, Serrano-Aroca Á. Zinc Chloride: Time-Dependent Cytotoxicity, Proliferation and Promotion of Glycoprotein Synthesis and Antioxidant Gene Expression in Human Keratinocytes. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Pochampally R. Colony forming unit assays for MSCs. Methods Mol Biol. 2008;449:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Bertolo A, Capossela S, Fränkl G, Baur M, Pötzel T, Stoyanov J. Oxidative status predicts quality in human mesenchymal stem cells. Stem Cell Res Ther. 2017;8:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Fabre H, Ducret M, Degoul O, Rodriguez J, Perrier-Groult E, Aubert-Foucher E, Pasdeloup M, Auxenfans C, McGuckin C, Forraz N, Mallein-Gerin F. Characterization of Different Sources of Human MSCs Expanded in Serum-Free Conditions with Quantification of Chondrogenic Induction in 3D. Stem Cells Int. 2019;2019:2186728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Mendenhall MD, Hodge AE. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1191-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Müller GA, Engeland K. The central role of CDE/CHR promoter elements in the regulation of cell cycle-dependent gene transcription. FEBS J. 2010;277:877-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Hein JB, Nilsson J. Interphase APC/C-Cdc20 inhibition by cyclin A2-Cdk2 ensures efficient mitotic entry. Nat Commun. 2016;7:10975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Qiao R, Weissmann F, Yamaguchi M, Brown NG, VanderLinden R, Imre R, Jarvis MA, Brunner MR, Davidson IF, Litos G, Haselbach D, Mechtler K, Stark H, Schulman BA, Peters JM. Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc Natl Acad Sci USA. 2016;113:E2570-E2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 37. | Feng Y, Qian W, Zhang Y, Peng W, Li J, Gu Q, Ji D, Zhang Z, Wang Q, Zhang D, Sun Y. CDCA2 promotes the proliferation of colorectal cancer cells by activating the AKT/CCND1 pathway in vitro and in vivo. BMC Cancer. 2019;19:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14:1681-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 40. | Nardinocchi L, Pantisano V, Puca R, Porru M, Aiello A, Grasselli A, Leonetti C, Safran M, Rechavi G, Givol D, Farsetti A, D'Orazi G. Zinc downregulates HIF-1α and inhibits its activity in tumor cells in vitro and in vivo. PLoS One. 2010;5:e15048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Pan R, Chen C, Liu WL, Liu KJ. Zinc promotes the death of hypoxic astrocytes by upregulating hypoxia-induced hypoxia-inducible factor-1alpha expression via poly(ADP-ribose) polymerase-1. CNS Neurosci Ther. 2013;19:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Yun YJ, Li SH, Cho YS, Park JW, Chun YS. Survivin mediates prostate cell protection by HIF-1alpha against zinc toxicity. Prostate. 2010;70:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Ninsontia C, Phiboonchaiyanan PP, Chanvorachote P. Zinc induces epithelial to mesenchymal transition in human lung cancer H460 cells via superoxide anion-dependent mechanism. Cancer Cell Int. 2016;16:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Shojaeian A, Mehri-Ghahfarrokhi A, Banitalebi-Dehkordi M. Migration Gene Expression of Human Umbilical Cord Mesenchymal Stem Cells: A Comparison between Monophosphoryl Lipid A and Supernatant of Lactobacillus acidophilus. Int J Mol Cell Med. 2019;8:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 45. | Ma F, Zhou Z, Li N, Zheng L, Wu C, Niu B, Tang F, He X, Li G, Hua J. Lin28a promotes self-renewal and proliferation of dairy goat spermatogonial stem cells (SSCs) through regulation of mTOR and PI3K/AKT. Sci Rep. 2016;6:38805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Polesskaya A, Cuvellier S, Naguibneva I, Duquet A, Moss EG, Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21:1125-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Xu B, Huang Y. Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element. Nucleic Acids Res. 2009;37:4256-4263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 49. | Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38:1240-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 50. | Carlin R, Davis D, Weiss M, Schultz B, Troyer D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Pierantozzi E, Gava B, Manini I, Roviello F, Marotta G, Chiavarelli M, Sorrentino V. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3597] [Cited by in RCA: 3416] [Article Influence: 162.7] [Reference Citation Analysis (15)] |
| 53. | Shariati F, Favaedi R, Ramazanali F, Ghoraeian P, Afsharian P, Aflatoonian B, Aflatoonian R, Shahhoseini M. Increased expression of stemness genes REX-1, OCT-4, NANOG, and SOX-2 in women with ovarian endometriosis vs normal endometrium: A case-control study. Int J Reprod Biomed. 2018;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Ruiz G, Valencia-González HA, Pérez-Montiel D, Muñoz F, Ocadiz-Delgado R, Fernández-Retana J, Pérez-Plasencia C, Reséndis-Antonio O, Gariglio P, García-Carrancá A. Genes Involved in the Transcriptional Regulation of Pluripotency Are Expressed in Malignant Tumors of the Uterine Cervix and Can Induce Tumorigenic Capacity in a Nontumorigenic Cell Line. Stem Cells Int. 2019;2019:7683817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Hu J, Yang Z, Wang J, Yu J, Guo J, Liu S, Qian C, Song L, Wu Y, Cheng J. Zinc Chloride Transiently Maintains Mouse Embryonic Stem Cell Pluripotency by Activating Stat3 Signaling. PLoS One. 2016;11:e0148994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Mnatsakanyan H, Sabater I Serra R, Salmeron-Sanchez M, Rico P. Zinc Maintains Embryonic Stem Cell Pluripotency and Multilineage Differentiation Potential via AKT Activation. Front Cell Dev Biol. 2019;7:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmadabad HN, Iran; Shen Y, China S-Editor: Li L L-Editor: A P-Editor: Zhang XD