Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.617
- This article has been corrected.
- See: World J Stem Cells. Feb 26, 2026; 18(2): 116824
Peer-review started: March 29, 2023
First decision: April 25, 2023
Revised: April 28, 2023
Accepted: May 25, 2023
Article in press: May 25, 2023
Published online: June 26, 2023
Processing time: 89 Days and 10.3 Hours
Bone marrow-derived mesenchymal stem cells (MSCs) show podocyte-protective effects in chronic kidney disease. Calycosin (CA), a phytoestrogen, is isolated from Astragalus membranaceus with a kidney-tonifying effect. CA preconditioning enhances the protective effect of MSCs against renal fibrosis in mice with unilateral ureteral occlusion. However, the protective effect and underlying mechanism of CA-pretreated MSCs (MSCsCA) on podocytes in adriamycin (ADR)-induced focal segmental glomerulosclerosis (FSGS) mice remain unclear.
To investigate whether CA enhances the role of MSCs in protecting against podocyte injury induced by ADR and the possible mechanism involved.
ADR was used to induce FSGS in mice, and MSCs, CA, or MSCsCA were administered to mice. Their protective effect and possible mechanism of action on podocytes were observed by Western blot, immunohistochemistry, immunofluorescence, and real-time polymerase chain reaction. In vitro, ADR was used to stimulate mouse podocytes (MPC5) to induce injury, and the supernatants from MSC-, CA-, or MSCsCA-treated cells were collected to observe their protective effects on podocytes. Subsequently, the apoptosis of podocytes was detected in vivo and in vitro by Western blot, TUNEL assay, and immunofluorescence. Overexpression of Smad3, which is involved in apoptosis, was then induced to evaluate whether the MSCsCA-mediated podocyte protective effect is associated with Smad3 inhibition in MPC5 cells.
CA-pretreated MSCs enhanced the protective effect of MSCs against podocyte injury and the ability to inhibit podocyte apoptosis in ADR-induced FSGS mice and MPC5 cells. Expression of p-Smad3 was upregulated in mice with ADR-induced FSGS and MPC5 cells, which was reversed by MSCCA treatment more significantly than by MSCs or CA alone. When Smad3 was overexpressed in MPC5 cells, MSCsCA could not fulfill their potential to inhibit podocyte apoptosis.
MSCsCA enhance the protection of MSCs against ADR-induced podocyte apoptosis. The underlying mechanism may be related to MSCsCA-targeted inhibition of p-Smad3 in podocytes.
Core Tip: Calycosin (CA)-pretreated mesenchymal stem cells (MSCsCA) enhanced the protective effect of MSCs against adriamycin (ADR)-induced podocyte injury in vitro and in vivo by inhibiting apoptosis, accompanied by more reversal of the upregulated expression of p-Smad3 after ADR induction. Smad3 overexpression eliminated the inhibitory effect of MSCsCA on podocyte apoptosis, suggesting that MSCsCA inhibit podocyte apoptosis by targeting p-Smad3. These results broaden our understanding of the potential of MSCs pretreated with herbal extract and provide new theories for possible therapeutic mechanisms for ADR-induced focal segmental glomerulosclerosis.
- Citation: Hu QD, Tan RZ, Zou YX, Li JC, Fan JM, Kantawong F, Wang L. Synergism of calycosin and bone marrow-derived mesenchymal stem cells to combat podocyte apoptosis to alleviate adriamycin-induced focal segmental glomerulosclerosis. World J Stem Cells 2023; 15(6): 617-631
- URL: https://www.wjgnet.com/1948-0210/full/v15/i6/617.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i6.617
Focal segmental glomerulosclerosis (FSGS) is the most common primary glomerulopathy and the dominant pathological type of chronic kidney disease (CKD)[1,2], associated with high albuminuria and end-stage renal disease (ESRD) with a poor prognosis[3,4]. FSGS is linked with injury or even depletion of podocytes, manifested by the gradual disappearance of podocyte-specific markers such as podocin[5,6]. As podocyte injury plays a critical role in FSGS progression, protecting podocytes is promising to prevent ESRD in patients with FSGS[7].
Apoptosis of podocytes has been widely studied in previous studies[8-10], and inhibition of podocyte apoptosis has been reported to delay FSGS progression[11]. Podocyte apoptosis is characterized by the loss of Bcl-2 protein and the increase of Bax protein[12,13]. Recently, Smad3-related pathways have been reported to be involved in podocyte apoptosis[14]. However, the underlying mechanism remains unclear, and no specific effective treatment can prevent podocyte apoptosis.
Mesenchymal stem cells (MSCs) are multipotent stem cells that exhibit varying potential for multilineage cell differentiation as well as the capacity for self-renewal[15]. Therefore, using MSCs to treat various diseases is worth exploring[16-18]. MSCs treat diabetic nephropathy by protecting podocytes[19-21], and bone marrow-derived MSC (BMSC) transplantation can attenuate FSGS progression in a rat model of FSGS[22,23]. In addition, the protective effects of MSC derivatives or exosomes on podocytes have also been reported[24,25]. However, the application of MSCs is also limited. For instance, MSCs may be losing their biological function after being isolated and cultured for a long time. After infusion, MSCs must face harsh environments with various stressors such as inflammation, hypoxia, high acidity, or reduced energy reserve. On this account, preconditioning, genetic modification, and delivering MSCs with biomaterials have been developed[26]. Thus, it is important to explore how MSCs can overcome adverse microenvironments to enhance their therapeutic benefits.
Calycosin (CA), a phytoestrogen with a kidney-tonifying effect, is isolated from Astragalus membranaceus. It has been reported that CA is the top component of potentially active compounds for the treatment of nephrotic syndrome[27]. Moreover, CA has also been found to be an active ingredient in the treatment of adriamycin (ADR) nephropathy using network pharmacology combined with transcriptomics[28]. Our research group used Ca-pretreated MSCs to treat mice with unilateral ureteral occlusion (UUO) and found that they improved renal fibrosis and inhibited necrosis of renal tubular epithelial cells more than normal MSCs did[29]. However, the protective effect on podocytes and their mechanism of action remain unknown.
In rodents, ADR can induce rapid podocyte injury characterized by massive foot process effacement and glomerulosclerosis, which serves as a model of FSGS[30,31]. In the present study, we compared the antiapoptotic efficacy of CA-pretreated MSCs (MSCsCA) to that of MSCs or CA in a mouse model of FSGS induced with ADR and in vitro, as well as the possible mechanisms of action involved.
The C57BL/6 mice utilized in this investigation were bought from Chengdu Dashuo Biotechnology Co., LTD. in China. They were male, 8 wk old, and weighed 22-25 g. All the mice were kept in a specific disease-free space with 12 h of light and dark cycles and had free access to water and food. The mice were randomly divided into the following six groups: Normal control group; ADR injection group; ADR with Dulbecco’s modified Eagle’s medium (DMEM; 200 μL) injection (ADR + DMEM); ADR with 200 μL MSCs (106 cells/mL) (ADR + MSCs); ADR with 200 μg/mL CA (106 cells/mL) (ADR + CA); and ADR with MSCs preconditioned with 200 μg/mL CA (106 cells/mL) (ADR + MSCsCA). For ADR-induced FSGS, the mice were injected with 10 mg/kg ADR (Shenzhen Main Luck Pharmaceuticals Inc.) via the tail vein. The normal control mice were injected with vehicle (saline). MSCs, CA dissolved in DMEM, and MSCsCA were injected via the tail vein 4 wk after ADR injection once weekly. Since both MSCs and CA are soluble in DMEM, mice in the ADR + DMEM group were given an equal volume of normal DMEM as the solvent control. All mice were killed at 8 wk after ADR injection. All animal experiments were carried out in accordance with the recommendations of the Institute of Nutrition and Health’s Animal Care and Utilization Committee, and were approved by the Southwest Medical University’s Animal Ethics Committee (No. 20210223-024).
As previously described, MSCs were isolated from the leg bone marrow of male C57BL/6 mice aged 6-8 wk[32]. Briefly, cells were grown at 37 °C and 5% CO2 in DMEM Petri plates with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, United States), 1 g/L glucose, and 1% penicillin-streptomycin (Beyotime, Shanghai, China). At 24 h, the medium was changed to remove the non-adherent cells. The MSCs were passed once 90% confluence was reached. As described previously[26], anti-CD29 (102205; Biolegend), anti-CD90 (ab24904; Abcam, Cambridge, MA, United States), and anti-CD11b (101205; Biolegend, San Diego, CA, United States) antibodies were used to label MSCs, and the purity of the MSCs was analyzed using a BD FACSVerse (Becton, Dickinson and Company, Franklin Lakes, NJ, United States).
CA (≥ 94% purity) was purchased from Cayman Chemical Company (Ann Arbor, MI, United States). The stoste used for MSC pretreatment included full medium and CA (200 g/mL) dissolved in DMEM as previously described[29]. After incubation for 72 h, the MSCs and MSCsCA were injected into mice, and the supernatants were used to treat mouse podocytes (MPC5) for 48 h.
Random urine samples were collected, followed by determining the albumin concentration with a mouse albumin ELISA kit (Sangon Biotech, China) and creatinine with a creatinine assay kit (Nanjing Jiancheng, Jiangsu Province, China). The urine albumin-creatinine ratio was calculated by dividing the urine albumin concentration by the creatinine concentration.
Mouse kidneys were fixed in 4% neutral formaldehyde followed by paraffin embedding. The paraffin sections were rehydrated in a graded ethanol series and subjected to hematoxylin-eosin (HE) staining (Beyotime, Shanghai, China) as previously described[33].
The sections underwent antigen retrieval in 0.01 M citric acid solution (pH 6.0) in a microwave oven for 10 min after deparaffinization and rehydration. To inhibit endogenous peroxidase, the slices were incubated with 5% H2O2 for 15 min. The sections were then further blocked for 30 min at room temperature with 5% bovine serum albumin (BSA), and then incubated overnight at 4 °C with anti-p-Smad3 antibody (C25A9; Cell Signaling Technology, Danvers, MA, United States). The slices were treated with secondary antibodies for 1 h at room temperature following PBS washing. Images were recorded with a light microscope (Eclipse 80i; Nikon, Japan).
TUNEL assay was used to evaluate podocyte apoptosis in the kidneys after ADR induction, as previously described[34]. After the mouse kidneys were fixed in 4% neutral formaldehyde followed by paraffin embedding, the paraffin sections were used for staining. Podocyte apoptosis was measured through the utilization of a One-step TUNEL In Situ Apoptosis Assay Kit (AF488; Green) (E-CK-A321; Elabscience, China). The images were captured with an orthotopic fluorescence microscope (DM4B; Leica, Germany).
Prof. San-Tao Ou (Department of Nephrology, Southwest Medical University) kindly donated the conditionally immortalized MPC5 cell line. The cells were grown at 33 °C in RPMI-1640 medium supplemented with 10 IU/mL recombinant interferon and 10% FBS. After the MPC5 cells were cultured at 37 °C for 14 d to induce differentiation, the differentiated cells were treated with different concentrations of ADR for 24 h. A Smad3 overexpressing MPC5 cell line was established with the Smad3 overexpression plasmid pcDNA3.1-Smad3 which was described previously[35].
After treatment, the MPC5 cells or frozen sections were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.25% Triton X-100 (in PBS), and blocked with 5% BSA for immunofluorescence. After that, the frozen sections or MPC5 cells were incubated with anti-podocin (BA0290; Boster, Wuhan, China), anti-Bax (AF0120; Affinity, United States), and anti-Bcl-2 (AF6139; Affinity) antibodies at 4 °C overnight. After washing with PBS, the frozen sections or MPC5 cells were incubated with Alexa Fluor 594 Donkey anti-mouse/rabbit secondary antibodies (Thermo Fisher Scientific, Waltham, MA, United States) for 1 h at room temperature. The nuclei were stained with 4¢,6-diamidino-2-phenylindole (Sangon Biotech). Images were captured with a fluorescence microscope (EVOS FL Auto, Thermo Fisher Scientific, United States).
TRIzol reagent (Invitrogen, Carlsbad, CA, United States) was used to separate total RNA from cells or kidneys, and a Reverse Transcription Kit (Promega, Madison, WI, United States) was used to obtain cDNA. Using Master Mixture (TaKaRa, Dalian, China) and LightCycler 480 equipment (Roche, Germany), the podocin mRNA expression levels were assessed. The internal control used was GAPDH. Using 2-ΔΔCt analysis, the relative expression of the target gene was standardized to GAPDH expression. The primer sequences used are shown in Supplementary Table 1.
Using RIPA lysis buffer (Beyotime), total proteins were extracted from kidneys or cells. The protein concentrations were determined with a BCA protein assay kit (Beyotime). Proteins were transferred onto polyvinylidene difluoride membranes after being separated by 12% SDS-PAGE. Then the membranes were incubated with anti-podocin (BA0290; Boster), anti-p-Smad3 (C25A9; Cell Signaling Technology), anti-Smad3 (C67H9; Cell Signaling Technology), anti-Bax (AF0120; Affinity), anti-Bcl-2 (AF6139; Affinity), and anti-GAPDH (AB0037; Abways, China) antibodies at 4°C overnight. The membranes were treated with the relevant secondary antibody at room temperature for 1 h after being rinsed with Tris-buffered saline with Tween (TBST). The protein bands were depicted with an enhanced ECL kit (Boster) and a chemiluminescence imaging system (ChemiScope 6200; Clinx, China). ImageJ software (NIH, Bethesda, MD, United States) was used to calculate the band gray intensity.
The cells were digested with trypsin-EDTA solution (C0201; Beyotime), collected in a centrifuge tube, and centrifuged for 5 min at 1800 rpm, and the supernatant was discarded. The cells were resuspended with 1 mL precooled PBS. According to the Annexin V-FITC/PI Apoptosis Detection kit’s instructions (Vazyme, Nanjing, China), the prepared propyl iodide staining solution was added to the cells and incubated at 37 °C for 10 min without light. Red fluorescence was detected at an excitation wavelength of 488 mm and light scattering was detected with a BD FACSVerse (Becton, Dickinson).
The mean and standard deviation of the data are displayed. Using SPSS 21.0 software (IBM Corp., Chicago, IL, United States), one-way analysis of variance was used to compare the data. P < 0.05 was considered statistically significant.
To investigate whether CA pretreatment enhances the protective effect of MSCs on podocyte injury in ADR-induced FSGS mice, we treated mice with MSCs, CA, or MSCsCA. Due to MSCs, CA, and MSCsCA being dissolved in DMEM, a DMEM group was separately designed as the solvent control group to exclude the protective effect of DMEM-containing nutrients on podocytes (Figure 1A). The identification of MSCs, the chemical formula of CA, and its appropriate concentration can be found in our previous research[26]. Eight weeks after ADR injection, increased urinary albumin excretion was detected in ADR-treated mice, and MSCsCA reversed this more significantly than MSCs or CA alone. However, there was no difference between the DMEM group and the model group (Figure 1B). HE staining showed that glomerular atrophy and FSGS were prominent in the ADR and DMEM groups, but MSCsCA treatment reversed this change and was superior to MSCs and CA treatment (Figure 1C). Immunostaining, real-time quantitative polymerase chain reaction (RT-PCR), and Western blot analysis showed that the expression of podocin, a podocyte-specific marker, was significantly reduced in the ADR and DMEM groups; however, MSCsCA treatment best restored its expression (Figure 1D-G). The above evidence indicated that MSCsCA treatment better protected podocytes from ADR injury in FSGS mice.
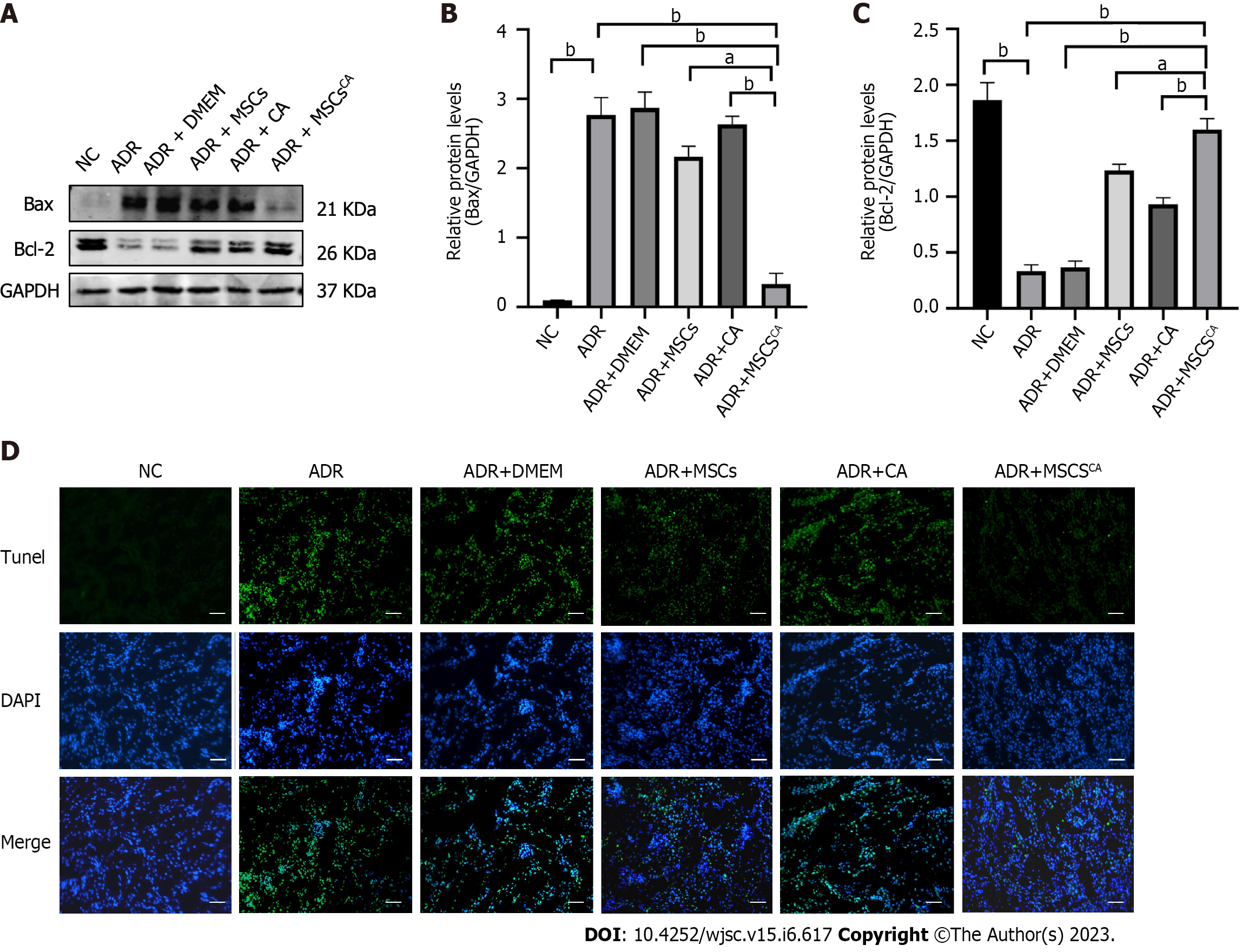
To determine the effect of MSCsCA on renal cell apoptosis, Western blot and TUNEL assay were performed. Expression of Bax protein as an apoptosis marker was significantly increased in the ADR and DMEM groups compared with the normal group, and their levels were reduced after MSC or CA treatment (Figure 2A and B). MSCsCA reduced ADR-induced Bax protein expression more significantly than MSCs or CA. The changing trend in Bcl-2 protein expression was opposite to that of Bax in each group (Figure 2A and C). TUNEL assay showed an obvious increase in the brightness and range of green fluorescence in the ADR group, which was weakened by MSCsCA treatment (Figure 2D). The above data indicated that MSCsCA enhanced the antiapoptotic effect of MSCs on kidney cells of ADR-induced FSGS mice.
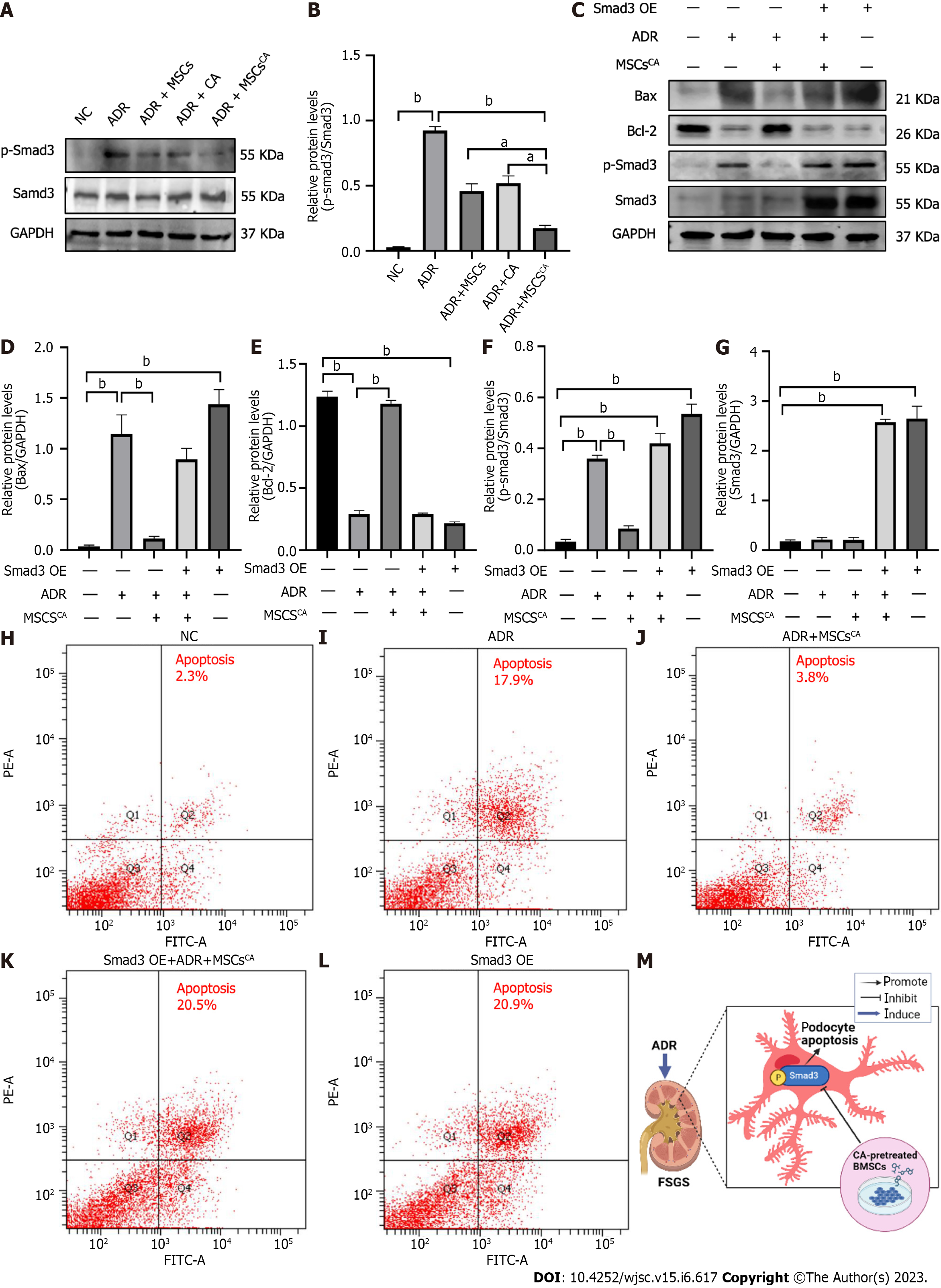
It has been reported that the Smad3 protein is involved in podocyte apoptosis[13], so we examined the effect of MSCsCA on the expression of Smad3 and p-Smad3 proteins. As expected, we found by Western blot and immunohistochemistry that MSCsCA treatment significantly reversed the upregulation of p-Smad3 in ADR-treated mouse renal podocytes, and the effect was superior to that of MSC and CA treatment (Figure 3). The above evidence suggested that p-Smad3 was involved in the ADR-induced injury of podocytes and the recovery after MSCsCA treatment.
To further demonstrate the enhanced potential of MSCsCA to protect podocytes from ADR injury, we cultured and treated MPC5 cells. Immunofluorescence staining, RT-PCR, and Western blot showed that 1.2 μM/mL ADR decreased the expression of podocin mRNA and protein, while the expression was significantly promoted by treatment with conditioned medium from MSCs, or CA. Importantly, conditioned medium from MSCsCA further elevated the expression of podocin mRNA and protein compared with the ADR group (Figure 4). Therefore, MSCsCA protected podocytes from ADR injury better than MSCs or CA alone.
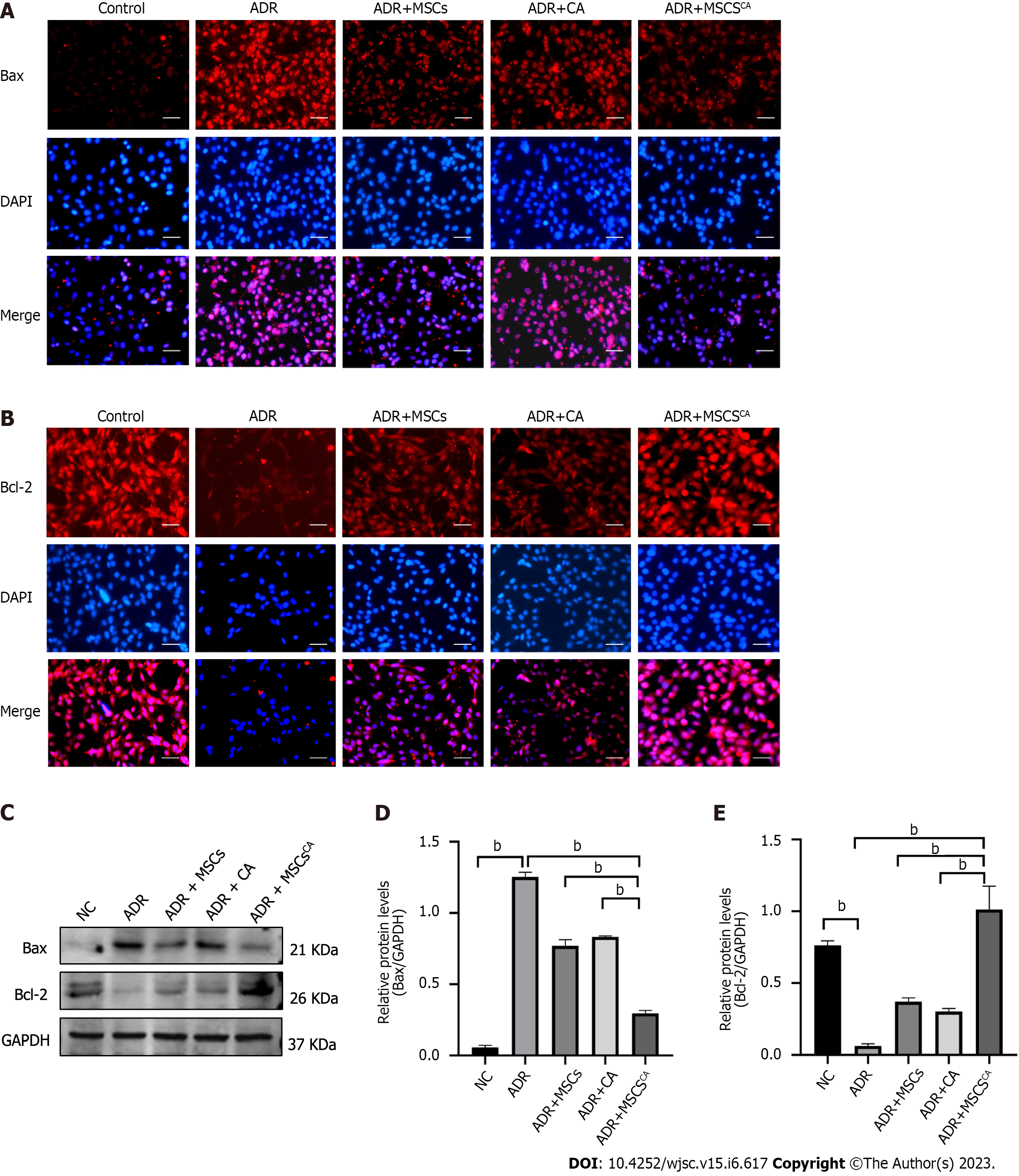
Immunofluorescence staining and Western blot demonstrated that the protein level of Bax was upregulated in MPC5 cells treated with ADR, but its expression was significantly inhibited by treatment with a conditioned medium from MSCs, or CA alone (Figure 5). The effects of the conditioned medium from MSCsCA were more pronounced. The trend for Bcl-2 protein expression was the opposite. These findings revealed that Ca-pretreated MSCs enhanced the inhibitory effect of MSCs on podocyte apoptosis.
As described previously, p-Smad3 is involved in ADR-induced FSGS mice. Further experiments were conducted to explore whether MSCsCA inhibit podocyte apoptosis by targeting p-Smad3. Expression of p-Smad3 in MPC5 cells was markedly elevated by ADR stimulation and subsequently significantly downregulated by MSCsCA treatment. The upregulated expression of p-Smad3 was also reversed by MSCs or CA, but to a lesser extent (Figure 6A and B). When Smad3 accompanied by p-Smad3 in MPC5 cells was overexpressed, Bax protein expression was upregulated but Bcl-2 protein expression was downregulated. Meanwhile, MSCsCA treatment no longer showed a protective effect against ADR-induced podocyte apoptosis compared with the group without Smad3 overexpression (Figure 6C-G). Flow cytometry was used to detect apoptosis, and it was found that the apoptosis rate of MPC5 cells was significantly increased after ADR induction compared with the normal group, and MSCsCA reversed this increase. However, after overexpression of Smad3, the apoptosis rate was increased compared with the normal group and the model group regardless of whether MSCsCA were administered. This means that treatment with MSCsCA did not improve the apoptosis of podocytes with Smad3 overexpression (Figure 6H-L). The graphical abstract (created in BioRender.com) is shown in Figure 6M. The above evidence suggested that MSCsCA improved podocyte apoptosis through targeted inhibition of p-Smad3.
Increasing evidence has shown that MSCs and derived extracellular vesicles can ameliorate renal deterioration in CKD[36,37]. However, because of a hostile environment with several stresses such as inflammation, high acidity, hypoxia, and depleted energy reserve, few MSCs survive in vivo after intravenous or direct local injection[38-40]. The question of whether preconditioning BMSCs can shield them from the damaging environment at the injury site and enhance their functionality has drawn more attention in research. These pretreatments involve the application of supportive materials, cytokines, and natural or synthetic chemicals[41-44]. Researchers have been investigating the preconditioning of MSCs using Chinese herbal medicine or its primary monomer components. There is evidence that resveratrol-pretreated adipose-derived stem cells show increased regenerative capacity in a rat model of diabetes-induced cardiomyopathy[45]. Further research has shown that preconditioning MSCs obtained from umbilical cords with the active ingredient of a Chinese herb, triptolide, primed MSCs to be activated and inhibited the immune response before being delivered[46]. Previous results from our group have also shown that CA-pretreated BMSCs show enhanced antifibrotic activity in UUO mice and inhibit tubular epithelial cell necrosis[29]. Therefore, we investigated whether MSCsCA enhance podocyte protection. Similar to previous studies, MSCsCA protected podocytes from ADR-induced apoptosis, both in vivo and in vitro, which means that they may be a potential therapy for FSGS.
CA is the top ingredient in Astragalus, which is one of the most widely used herbs in Chinese medicine to treat kidney disease[47-49]. The effectiveness of CA in CKD has been confirmed in recent years[50-52]. However, whether its combination with MSCs can enhance their efficacy in treating CKD remains to be seen. It has been shown that human MSCs are stimulated to enhance osteogenesis and mineralization by CA-7-O-glucoside obtained from Astragalus membranaceus[53]. This result gave us confidence and we also identified the advantages and potential of MSCsCA in the treatment of FSGS, which extends the application of CA and MSCs in FSGS.
The main pathological manifestations of FSGS are podocyte injury and the therapeutic options for FSGS are limited, requiring further research and exploration. Therefore, we explored the mechanism of podocyte injury. Podocyte apoptosis is the main type of podocyte injury, which includes podocyte dedifferentiation, autophagy, and epithelial-mesenchymal transformation[54]. Podocyte apoptosis is caused by many factors, including drugs, infection, and immune disorders[55-57]. ADR is one of the drugs that causes podocyte apoptosis due to its pharmacological action and distribution[58]. However, how to protect podocytes from ADR needs further research to find more effective targeted drugs.
Smad3 is involved in apoptosis, and podocytes are no exception[59,60]. Activation of Smad3 and its related pathway proteins induces podocyte apoptosis[14,61]. The canonical Smad pathway is a crucial regulatory route in the etiology of renal inflammation and fibrosis, according to earlier research. Major receptor-associated Smads include Smad2 and Smad3. Mad-homology 2 domain is located at the C-terminus of Smad3, which has unique phosphorylation sites and sequences triggered by transforming growth factor (TGF)-β1. The binding of phosphorylated Smad3 to TGF-β1 signaling receptors promotes fibrosis[62]. Our study showed that MSCsCA significantly downregulated the expression of p-Smad3 in the kidneys of ADR-induced FSGS mice and ADR-induced MPC5 cells. Subsequently, we overexpressed Smad3 in MPC5 cells and confirmed that MSCsCA targeted inhibition of p-Smad3 to improve podocyte apoptosis using rescue experiments. This provides a new possible mechanism and target for preventing podocyte apoptosis by MSCsCA.
There were some limitations to this study. Although we have revealed that MSCsCA improve podocytes apoptosis by inhibiting Smad3 signaling, this study still has certain limitations and the underlying mechanism deserves further exploration. First, how does MSCsCA intervene in the Smad3 signal, directly or indirectly? We speculate that CA may activate the anti-apoptotic activity of MSCs or affect the differentiation, mobilization, and homing of BMSCs as well as the abundance of beneficial exosomes, but the main mechanism and responsible factors are still unknown. Second, it is still unclear which molecules in podocytes respond to the activity of MSCs and what are their potential relationship with Smad3. Understanding these mechanisms is conducive in expanding the application of MSCsCA, and we will answer each question one by one in future research.
This study showed that MSCsCA improve ADR-induced podocyte apoptosis by targeting Smad3 inhibition, and are superior to MSCs or CA. Thus, our study provides a new perspective on the synergistic application of MSCs and a new theory for the mechanism of improvement of podocyte apoptosis.
Focal segmental glomerulosclerosis (FSGS) has become a global public health problem due to its high incidence and lack of treatment. Prevention of podocyte apoptosis is essential in the treatment of FSGS. Bone marrow-derived mesenchymal stem cells (BMSCs) have been found to protect podocytes, but have some limitations, such as low survival rate in vivo and poor homing function. In our previous study, calycosin (CA)-pretreated BMSCs enhanced the antifibrotic activity in kidneys compared with BMSCs. Therefore, CA-pretreated MSCs are expected to be a new method to protect podocytes in the treatment of FSGS.
Although MSCs have been confirmed to improve podocyte apoptosis in mice, their availability and effectiveness in vivo are limited. Currently, there is still a lack of effective therapeutic methods for FSGS, and their mechanism of action is not clear.
To evaluate the therapeutic effect of CA-pretreated BMSCs in a mouse model of adriamycin (ADR)-induced FSGS in vivo and MPC5 cells in vivo.
MSCsCA were compared with MSCs or CA to observe their inhibitory effects on podocyte apoptosis in mice with ADR-induced FSGS in vivo and ADR-treated MPC5 cells in vitro, to explore the possible mechanism by which MSCsCA improves podocyte apoptosis.
In vivo results showed that MSCsCA reduced podocyte apoptosis, improved podocyte injury and depletion, alleviated glomerulosclerosis and albuminuria, and downregulated p-Smad3 expression in ADR-induced FSGS mice, which were superior to MSCs and CA. Similar to in vivo studies, MSCsCA alleviated ADR-induced apoptosis of MPC5 cells more significantly than MSCs and CA. Through rescue experiments, we found that the potential of MSCsCA to protect podocytes may be realized through targeted inhibition of p-Smad3 expression.
MSCsCA improve ADR-induced podocyte apoptosis by targeting Smad3 inhibition, which are superior to MSCs or CA.
Our findings provide a new potential strategy for the treatment of FSGS.
| 1. | Wheeler DC, Jongs N, Stefansson BV, Chertow GM, Greene T, Hou FF, Langkilde AM, McMurray JJV, Rossing P, Nowicki M, Wittmann I, Correa-Rotter R, Sjöström CD, Toto RD, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2022;37:1647-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Rajasekeran H, Reich HN, Hladunewich MA, Cattran D, Lovshin JA, Lytvyn Y, Bjornstad P, Lai V, Tse J, Cham L, Majumder S, Bowskill BB, Kabir MG, Advani SL, Gibson IW, Sood MM, Advani A, Cherney DZI. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Renal Physiol. 2018;314:F412-F422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Peev V, Hahm E, Reiser J. Unwinding focal segmental glomerulosclerosis. F1000Res. 2017;6:466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Shabaka A, Tato Ribera A, Fernández-Juárez G. Focal Segmental Glomerulosclerosis: State-of-the-Art and Clinical Perspective. Nephron. 2020;144:413-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Margos G, Fingerle V, Oskam C, Stevenson B, Gofton A. Comment on: Gupta, 2019, distinction between Borrelia and Borreliella is more robustly supported by molecular and phenotypic characteristics than all other neighbouring prokaryotic genera: Response to Margos' et al. "The genus Borrelia reloaded" (PLoS One 13(12): e0208432). PLoS One 14(8):e0221397. Ticks Tick Borne Dis. 2020;11:101320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Agrawal V, Prasad N, Jain M, Pandey R. Reduced podocin expression in minimal change disease and focal segmental glomerulosclerosis is related to the level of proteinuria. Clin Exp Nephrol. 2013;17:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Campbell KN, Tumlin JA. Protecting Podocytes: A Key Target for Therapy of Focal Segmental Glomerulosclerosis. Am J Nephrol. 2018;47 Suppl 1:14-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Liu XQ, Jiang L, Li YY, Huang YB, Hu XR, Zhu W, Wang X, Wu YG, Meng XM, Qi XM. Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol Sin. 2022;43:96-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, Liang W, Ding G. Sirt6 Suppresses High Glucose-Induced Mitochondrial Dysfunction and Apoptosis in Podocytes through AMPK Activation. Int J Biol Sci. 2019;15:701-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 10. | Xu J, Deng Y, Wang Y, Sun X, Chen S, Fu G. SPAG5-AS1 inhibited autophagy and aggravated apoptosis of podocytes via SPAG5/AKT/mTOR pathway. Cell Prolif. 2020;53:e12738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Chen A, Feng Y, Lai H, Ju W, Li Z, Li Y, Wang A, Hong Q, Zhong F, Wei C, Fu J, Guan T, Liu B, Kretzler M, Lee K, He JC. Soluble RARRES1 induces podocyte apoptosis to promote glomerular disease progression. J Clin Invest. 2020;130:5523-5535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Li Y, Xu L, Shi J, Yu X, Wang X, Li X, Jiang H, Yang T, Yin X, Du L, Lu Q. Quercetin Attenuates Podocyte Apoptosis of Diabetic Nephropathy Through Targeting EGFR Signaling. Front Pharmacol. 2021;12:792777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Zhang L, Liu S, Yao B, Zhang H, Liang S, Ma J, Liang X, Shi W. Sam68 mediates high glucose‑induced podocyte apoptosis through modulation of Bax/Bcl‑2. Mol Med Rep. 2019;20:3728-3734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Guo W, Gao H, Pan W, Yu P, Che G. High glucose induces Nox4 expression and podocyte apoptosis through the Smad3/ezrin/PKA pathway. Biol Open. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Thanaskody K, Jusop AS, Tye GJ, Wan Kamarul Zaman WS, Dass SA, Nordin F. MSCs vs. iPSCs: Potential in therapeutic applications. Front Cell Dev Biol. 2022;10:1005926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Xiang J, Zhang F, Liu L, Hu C. MSCs can be a double-edged sword in tumorigenesis. Front Oncol. 2022;12:1047907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 18. | Kwon DG, Kim MK, Jeon YS, Nam YC, Park JS, Ryu DJ. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Khalilpourfarshbafi M, Hajiaghaalipour F, Selvarajan KK, Adam A. Mesenchymal Stem Cell-Based Therapies against Podocyte Damage in Diabetic Nephropathy. Tissue Eng Regen Med. 2017;14:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Liu J, Zhang Q, Wang W, Liu Q, Liu S, Song Y, Wang X, Zhang Y, Li S, Yang X, Lv S, Liu G. Human umbilical cord mesenchymal stem cells attenuate podocyte injury under high glucose via TLR2 and TLR4 signaling. Diabetes Res Clin Pract. 2021;173:108702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Sun J, Zhao F, Zhang W, Lv J, Yin A. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway. J Cell Mol Med. 2018;22:4840-4855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Yang RC, Zhu XL, Wang J, Wan F, Zhang HQ, Lin Y, Tang XL, Zhu B. Bone marrow mesenchymal stem cells attenuate the progression of focal segmental glomerulosclerosis in rat models. BMC Nephrol. 2018;19:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Li Y, Liu Q, Ou ST, Wu WH, Gan LW. Research on mechanism of MAPK signal pathway induced by BMSCs for the proteinuria of rat's kidney, glomerulosclerosis and activity of RAS. Eur Rev Med Pharmacol Sci. 2021;25:795-803. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Zhao T, Jin Q, Kong L, Zhang D, Teng Y, Lin L, Yao X, Jin Y, Li M. microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis. J Bioenerg Biomembr. 2022;54:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Duan Y, Luo Q, Wang Y, Ma Y, Chen F, Zhu X, Shi J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J Biol Chem. 2020;295:12868-12884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 27. | Feng D, Li XR, Wang ZY, Gu NN, Zhang SX, Li CF, Chen Y, Ma ZQ, Lin RC, Zhang HG, Zhao C. Integrated UPLC-MS and Network Pharmacology Approach to Explore the Active Components and the Potential Mechanism of Yiqi Huoxue Decoction for Treating Nephrotic Syndrome. Front Pharmacol. 2021;12:775745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Li AP, He SS, Zhang WN, Zhang LC, Liu YT, Li K, Qin XM. Exploration the active compounds of Astragali Radix in treatment of adriamycin nephropathy by network pharmacology combined with transcriptomic approach. J Ethnopharmacol. 2020;258:112537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Hu Q, Zhu B, Yang G, Jia J, Wang H, Tan R, Zhang Q, Wang L, Kantawong F. Calycosin pretreatment enhanced the therapeutic efficacy of mesenchymal stem cells to alleviate unilateral ureteral obstruction-induced renal fibrosis by inhibiting necroptosis. J Pharmacol Sci. 2023;151:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Dou Y, Shang Y, Shen Y, Qu J, Liu C, Cao J. Baicalin alleviates adriamycin-induced focal segmental glomerulosclerosis and proteinuria by inhibiting the Notch1-Snail axis mediated podocyte EMT. Life Sci. 2020;257:118010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Liu G, He L, Yang X, Tang L, Shi W, She J, Wei J. MicroRNA-155-5p Aggravates Adriamycin-Induced Focal Segmental Glomerulosclerosis through Targeting Nrf2. Nephron. 2023;147:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 786] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 33. | Dong L, Li JC, Hu ZJ, Huang XR, Wang L, Wang HL, Ma RCW, Lan HY, Yang SJ. Deletion of Smad3 protects against diabetic myocardiopathy in db/db mice. J Cell Mol Med. 2021;25:4860-4869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Xing L, Fang J, Zhu B, Wang L, Chen J, Wang Y, Huang J, Wang H, Yao X. Astragaloside IV protects against podocyte apoptosis by inhibiting oxidative stress via activating PPARγ-Klotho-FoxO1 axis in diabetic nephropathy. Life Sci. 2021;269:119068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Peng Z, Guo HY, Li YQ, Li JC, Yang XH, Liu J, Hu QD, Wang HL, Wang L. The Smad3-dependent microRNA let-7i-5p promoted renal fibrosis in mice with unilateral ureteral obstruction. Front Physiol. 2022;13:937878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Grange C, Skovronova R, Marabese F, Bussolati B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 37. | Yun CW, Lee SH. Potential and Therapeutic Efficacy of Cell-based Therapy Using Mesenchymal Stem Cells for Acute/chronic Kidney Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Chen Y, Shen H, Ding Y, Yu Y, Shao L, Shen Z. The application of umbilical cord-derived MSCs in cardiovascular diseases. J Cell Mol Med. 2021;25:8103-8114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Wang C, Tian C, Cai D, Jiang H, Zhang W, Liu S, Peng L, Hu X. BDNF-overexpressing MSCs delivered by hydrogel in acute ischemic stroke treatment. Ann Transl Med. 2022;10:1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 40. | Nie WB, Zhang D, Wang LS. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des Devel Ther. 2020;14:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 42. | Li C, Lv H, Du Y, Zhu W, Yang W, Wang X, Wang J, Chen W. Corrigendum to "Biologically modified implantation as therapeutic bioabsorbable materials for bone defect repair" [Regen Ther 19 (2022) 9-23]. Regen Ther. 2023;22:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 43. | Fatima A, Malick TS, Khan I, Ishaque A, Salim A. Effect of glycyrrhizic acid and 18β-glycyrrhetinic acid on the differentiation of human umbilical cord-mesenchymal stem cells into hepatocytes. World J Stem Cells. 2021;13:1580-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 44. | Ulpiano C, da Silva CL, Monteiro GA. Mesenchymal Stromal Cells (MSCs): A Promising Tool for Cell-Based Angiogenic Therapy. Curr Gene Ther. 2021;21:382-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Chen TS, Chuang SY, Shen CY, Ho TJ, Chang RL, Yeh YL, Kuo CH, Mahalakshmi B, Kuo WW, Huang CY. Antioxidant Sirt1/Akt axis expression in resveratrol pretreated adipose-derived stem cells increases regenerative capability in a rat model with cardiomyopathy induced by diabetes mellitus. J Cell Physiol. 2021;236:4290-4302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | He H, Takahashi A, Mukai T, Hori A, Narita M, Tojo A, Yang T, Nagamura-Inoue T. The Immunomodulatory Effect of Triptolide on Mesenchymal Stromal Cells. Front Immunol. 2021;12:686356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Zhang HW, Lin ZX, Xu C, Leung C, Chan LS. Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014;CD008369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Han C, Jiang YH, Li W, Liu Y. Astragalus membranaceus and Salvia miltiorrhiza ameliorates cyclosporin A-induced chronic nephrotoxicity through the "gut-kidney axis". J Ethnopharmacol. 2021;269:113768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Yoshino T, Horiba Y, Mimura M, Watanabe K. Oral Astragalus Root Supplementation for Mild to Moderate Chronic Kidney Disease: A Self-Controlled Case-Series. Front Pharmacol. 2022;13:775798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Huang D, Shen P, Wang C, Gao J, Ye C, Wu F. Calycosin plays a protective role in diabetic kidney disease through the regulation of ferroptosis. Pharm Biol. 2022;60:990-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 51. | Yosri H, El-Kashef DH, El-Sherbiny M, Said E, Salem HA. Calycosin modulates NLRP3 and TXNIP-mediated pyroptotic signaling and attenuates diabetic nephropathy progression in diabetic rats; An insight. Biomed Pharmacother. 2022;155:113758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 52. | Zhang N, Guan C, Liu Z, Li C, Yang C, Xu L, Niu M, Zhao L, Zhou B, Che L, Wang Y, Xu Y. Calycosin attenuates renal ischemia/reperfusion injury by suppressing NF-κB mediated inflammation via PPARγ/EGR1 pathway. Front Pharmacol. 2022;13:970616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Park KR, Park JE, Kim B, Kwon IK, Hong JT, Yun HM. Calycosin-7-O-β-Glucoside Isolated from Astragalus membranaceus Promotes Osteogenesis and Mineralization in Human Mesenchymal Stem Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Dai H, Liu Q, Liu B. Research Progress on Mechanism of Podocyte Depletion in Diabetic Nephropathy. J Diabetes Res. 2017;2017:2615286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 55. | Yu S, Ren Q, Yu L, Tan J, Xia ZK. Role of autophagy in Puromycin Aminonucleoside-induced podocyte apoptosis. J Recept Signal Transduct Res. 2020;40:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Hall G, Wyatt CM. Mechanisms of Proteinuria in HIV. Front Med (Lausanne). 2021;8:749061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 57. | Bao W, Xia H, Liang Y, Ye Y, Lu Y, Xu X, Duan A, He J, Chen Z, Wu Y, Wang X, Zheng C, Liu Z, Shi S. Toll-like Receptor 9 Can be Activated by Endogenous Mitochondrial DNA to Induce Podocyte Apoptosis. Sci Rep. 2016;6:22579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Qu G, He T, Dai A, Zhao Y, Guan D, Li S, Shi H, Gan W, Zhang A. miR-199b-5p mediates adriamycin-induced podocyte apoptosis by inhibiting the expression of RGS10. Exp Ther Med. 2021;22:1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 59. | Lee JH, Mellado-Gil JM, Bahn YJ, Pathy SM, Zhang YE, Rane SG. Protection from β-cell apoptosis by inhibition of TGF-β/Smad3 signaling. Cell Death Dis. 2020;11:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 60. | Qiu M, Li T, Wang B, Gong H, Huang T. miR-146a-5p Regulated Cell Proliferation and Apoptosis by Targeting SMAD3 and SMAD4. Protein Pept Lett. 2020;27:411-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Song S, Shi C, Bian Y, Yang Z, Mu L, Wu H, Duan H, Shi Y. Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-β1/Smad3 axis in diabetic kidney disease. Cell Death Dis. 2022;13:663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 62. | Wu W, Wang X, Yu X, Lan HY. Smad3 Signatures in Renal Inflammation and Fibrosis. Int J Biol Sci. 2022;18:2795-2806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jena MK, India; Sheykhhasan M, Iran S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhang XD