Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.385
Peer-review started: January 12, 2023
First decision: January 31, 2023
Revised: February 17, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: May 26, 2023
Processing time: 134 Days and 5.3 Hours
Spinal cord injury (SCI) is a devastating condition with complex pathological mechanisms that lead to sensory, motor, and autonomic dysfunction below the site of injury. To date, no effective therapy is available for the treatment of SCI. Recently, bone marrow-derived mesenchymal stem cells (BMMSCs) have been considered to be the most promising source for cellular therapies following SCI. The objective of the present review is to summarize the most recent insights into the cellular and molecular mechanism using BMMSC therapy to treat SCI. In this work, we review the specific mechanism of BMMSCs in SCI repair mainly from the following aspects: Neuroprotection, axon sprouting and/or regeneration, myelin regeneration, inhibitory microenvironments, glial scar formation, immunomodulation, and angiogenesis. Additionally, we summarize the latest evidence on the application of BMMSCs in clinical trials and further discuss the challenges and future directions for stem cell therapy in SCI models.
Core Tip: In this work, we review the specific mechanism of bone marrow-derived mesenchymal stem cell (BMMSC) in spinal cord injury (SCI) repair mainly from the following aspects: Neuroprotection, neuronal circuit, axon sprouting and or regeneration, myelin regeneration, inhibitory microenvironment, glial scar formation, immunomodulation, and angiogenesis. Additionally, we also summarize the latest evidence on application of BMMSC in clinical trials and further discuss the challenges and future directions for stem cell therapy in SCI models.
- Citation: Huang LY, Sun X, Pan HX, Wang L, He CQ, Wei Q. Cell transplantation therapies for spinal cord injury focusing on bone marrow mesenchymal stem cells: Advances and challenges. World J Stem Cells 2023; 15(5): 385-399
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/385.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.385
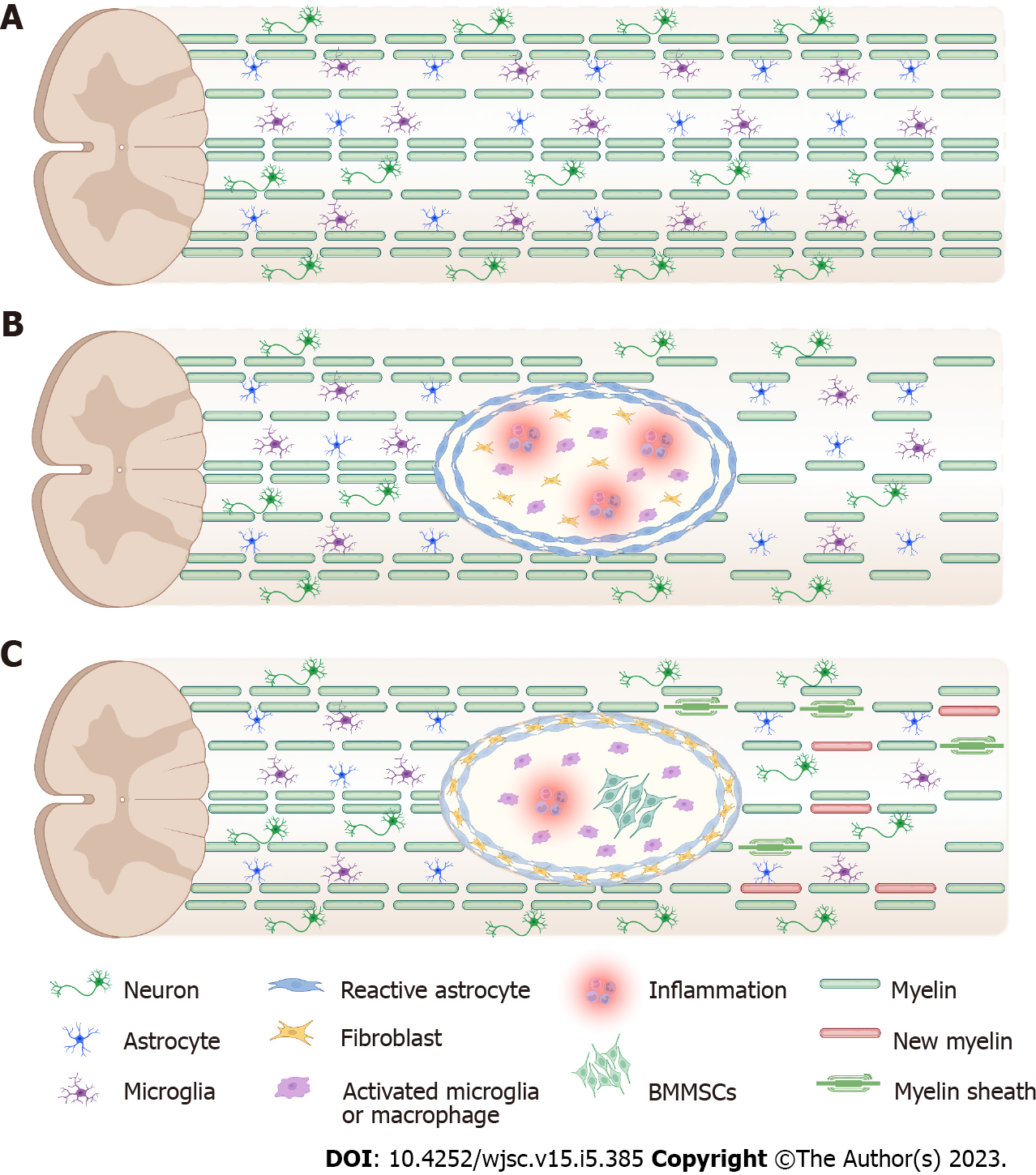
Spinal cord injury (SCI) is a serious neurological disorder that often results in paralysis during the reproductive years, causing temporary or permanent changes in normal motor, sensory, and autonomic functions, with significant impacts on individuals, families, and socioeconomic systems[1]. It has been reported that more than 27 million patients worldwide experience long-term disability due to SCI[2], with 541 cases per 100000 people[3]. Complex pathophysiology and time sensitivity in particular limit the therapeutic effects of SCI[4]. In incomplete SCI, there is less hemorrhage in the gray matter and no change in the white matter 3 h after injury; 6-10 h after injury, the hemorrhagic foci gradually expand, and the neurological tissue becomes edematous, which gradually subsides after 24-48 h. As the degree of incomplete SCI differs between mild and severe injuries, milder injuries only have small foci of necrosis in the center, and most of the nerve fibers are preserved. Severe injuries may have foci of necrosis and softening in the center of the spinal cord and are replaced by gliosis or scarring, and only a small portion of the nerve fibers are preserved. Most posttraumatic tissue degeneration is caused by multiple secondary injuries, including blood-spinal cord barrier (BSCB) disruption, free radical formation, ion imbalance, apoptosis, demyelination, and inflammatory response (Figure 1). Spontaneous recovery occurs within a limited time window because the subacute phase of SCI is thought to be detrimental to axonal regeneration and functional recovery[5]. Currently, clinical treatment includes surgical decompression, stabilization of the spinal cord, relief of spasticity and rehabilitation care, which consists primarily of supportive care and injury management. Frustratingly, the effectiveness of these treatments is limited because they are not effective in stimulating repair of the injured spinal cord.
The spinal cord consists of the gray and white matter, which contains nerve cell bodies and ascending and descending fibers. Thus, the different locations and levels of SCI can cause various degrees of disability, from partial loss of motor or sensory function to complete paralysis below the injured location. The resulting lifelong devastating deficits associated with impaired mobility (weakness or paralysis), sensation and autonomic dysfunction, and disabled neurological conditions lead to permanent and severe impacts on patients’ daily lives and their caregivers.
Pathophysiology following SCI comprises interrelated multicellular, multimolecular interactions and multiphasic events[6]. Classically, the pathophysiology of SCI is divided into two phases: Primary injury and secondary injury. SCI commonly occurs after sudden trauma because of direct and immediate mechanical insult to the spinal cord from vertebral fractures and dislocation with features of bone fragments and spinal ligament tearing. This was accompanied by extensive bleeding with further compression and interruption of the spinal cord blood supply. Thus, the primary injury mainly includes compression, contusion, shear, laceration, and acute stretching. This is followed by disruption of the neural parenchyma, shearing of the axonal network, destruction of the glial membrane, and vascular disruption[7,8]. Secondary injuries consist of ischemia, spinal cord ischemia-reperfusion injury, vascular dysfunction, edema, excitotoxicity, formation of free radicals, glial and neuronal apoptosis, and the inflammatory response[7]. This secondary damage is divided into three phases: The acute phase, which is accompanied by vascular and cell membrane damage and the secretion of different proinflammatory factors, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), with microglial activation. The subacute phase is accompanied by edema and vascular damage, inflammatory cytokine and glutamate secretion, astrogliosis, and demyelination for a few days. The chronic phase has symptoms such as the formation of a cavity in the spinal cord[8]. Based on further study of the spinal cord microenvironment, pathophysiological changes are divided into tissue, cellular and molecular levels. The tissue level involves hemorrhage and ischemia, glial scar formation, demyelination and remyelination[9]. The cellular level involves the activation of astrocytes, the differentiation of endogenous neural stem cells, oligodendrocyte progenitors and microglia, and the infiltration of macrophages. The molecular level involves the expression of neurotrophic factors and their pro-peptides, cytokines, chemokines, and ion imbalance. There is an imbalance between promoting and inhibiting growth molecules in the microenvironment of SCI, where growth inhibitors occupy the dominant position.
Cell transplantation has come to the forefront in SCI regenerative strategies due to its potential neuroprotective effects. Many cell types have been widely investigated in SCI treatment, including oligodendrocyte precursor cells, Schwann cells, olfactory ensheathing cells, neural stem cells, and mesenchymal stem cells (MSCs)[10,11]. MSCs have the capacity for self-renewal and multilineage differentiation potential and can differentiate into osteoblasts, adipocytes, and chondrocytes[12]. Moreover, MSCs express surface markers (CD105, CD73 and CD90) and do not show expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, or human leukocyte antigen type DR surface molecules[13]. MSCs mainly exist in bone marrow and can also be isolated from other tissues and organs, such as umbilical cord, adipose tissue and muscle. Among these, bone marrow-derived MSCs (BMMSCs) are the most widely studied cell type in SCI application due to their low immunogenicity, easy isolation, few ethical concerns and reduced tumorigenesis risks[14]. According to the current research progress at home and abroad, BMMSC-based treatment has extraordinary prospects in the field of SCI.
In this review, we will summarize the applications of BMMSCs in SCI based on the most frequently proposed mechanisms: Neuroprotection, neuronal circuit, axon sprouting and/or regeneration, myelin regeneration, inhibitory microenvironments, glial scar formation, immunomodulation, and angiogenesis. A better understanding of these mechanisms could allow the identification of more targeted therapies.
Neurons are postmitotic, without the ability to proliferate, and strategies developed to promote neuronal protection and regeneration have long-term benefits. Neuroprotective measures are crucial not only for the preservation of further injury for optimal neuron survivability but also for the restoration of injured nerve cells during pathological progression. BMMSCs reestablish the injured spinal cord via neuroprotection, neural regeneration, and remyelination in SCI[15]. MSCs can release growth and neurotrophic factors, including brain-derived neurotrophic factor (BDNF)[16], vascular endothelial growth factor (VEGF)[17], glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), fibroblast growth factor (FGF), neurotrophin-3, and epidermal growth factor, which can enhance regeneration and repair in damaged tissues[18,19]. miR-22, regulated by Gasdermin D mRNA, plays a role in inhibiting the occurrence of pyroptosis[20]. miR-22-modified BMMSCs suppress pyroptosis-mediated inflammation and neuronal injury during SCI[20]. Moreover, MSC-derived exosomes enhance the survival of neurons and repair of nerve fibers by inhibiting Nod1 inflammasome activation, suppressing pyroptosis in pericytes, preserving the integrity of the BSCB[21], inhibiting neuronal apoptosis through the Wnt/beta-catenin signaling pathway[22] and eventually improving functional recovery. The overexpression of miR-338-5p in exosomes was shown to profoundly increase the expression levels of neurofilament 200 and growth-associated protein-43 and decrease those of myelin-associated glycoprotein (MAG) and glial fibrillary acidic protein (GFAP), which provided neuroprotective effects through the cannabinoid receptor 1/Rap1/Akt pathway after acute SCI[23]. cAMP-mediated Rap1 activation plays an important role in apoptosis reduction and neuronal survival induced by the PI3K/Akt pathway[23].
Despite the inhibitory environment in the adult mammalian central nervous system, neuronal-intrinsic mechanisms are sufficient to support significantly extensive axonal growth and synapse formation after SCI, resulting in the formation of new neuronal circuits that restore electrophysiological activity and behavior[24]. When stem cells are cotransplanted with a supportive fibronectin matrix containing growth factors, axons form connections with host axons over significantly long distances. Even when crossing the inhibitory white matter, they elongate rapidly at a rate of 1-2 mm per day[24]. In addition, axons from the host spine regenerate into neural stem cell grafts, and this bidirectional growth contributes to the recovery of hindlimb mobility[24]. BMMSCs were initially thought to be similar to neural stem cells in their ability to multidirectionally differentiate into neurons and glial cells, but this theory was gradually discarded. In fact, the mechanism of action of BMMSCs may be cell fusion or trans-differentiation rather than cell differentiation[25]. In summary, transplanted BMMSCs after SCI exhibit significant autocrine and paracrine activities, which in turn stimulate the proliferation and differentiation of other cells and themselves, promoting tissue repair and functional recovery[26].
The axon is a unique cellular structure that maintains communication between neurons[27]. An important cause of persistent dysfunction after SCI is axonal disruption, and therefore promoting axonal regeneration and plasticity is very promising. However, previous studies have shown that the percentage of injected MSCs aggregating in the CNS is 0.75%-18.5%[28] and 6.7%[29], and thus it is conceivable that only a fraction of the cells reach the site of SCI. The Nakano et al[30] found that bone marrow stromal cell transplantation via cerebrospinal fluid was effective in acute, subacute and chronic spinal cord injuries in rats, and although the transplanted cells did not survive more than 7 d, a large number of axons crossed longitudinally through the astrocyte-deficient connective tissue. Transplantation via the CSF is a more clinically preferred modality because it does not cause secondary injury to preserved spinal cord tissue. From another perspective, Okuda et al[31] demonstrated that transplantation of BMMSC sheets after SCI prompted Tuj-1-positive axons to span a specially designed cell sheet without requiring a scaffold, offering a permissive microenvironment for damaged axons[31].
BMMSC therapy has been shown to play a positive role in rodent models of SCI[32], but evidence of its long-term therapeutic efficacy and effectiveness in human clinical trials is limited. Gene modification of MSCs, for example, by overexpression of neurotrophic or growth factors, is one of the ways to enhance their well-known beneficial effects. Overexpression of IL-13, an inducer of M2 microglia/macrophages, in BMMSCs significantly ameliorates axonal retraction caused by axonal-attacking macrophages[33]. In addition, the combination of neurotrophic factors and physical stimuli is often used to enhance the effects of BMMSCs. Cografting stromal-derived factor-1-overexpressing BMMSCs with neural stem cells (NSCs) at day 9 after SCI resulted in better axonal density enhancement than BMMSCs alone[34]. Furthermore, physical factor therapy has also shown a synergistic effect, as low-intensity pulsed ultrasound-optimized BMMSCs transplanted one week after SCI showed a good promotion effect on axonal regeneration[5].
Although the transplanted cells can reach the site of injury, they cannot survive long enough to integrate with the host spinal cord tissue, thus showing that these cells do not act as scaffolds for tissue repair. The use of biomaterials can provide transplanted cells with an environment closer to their physiological state, maintaining and regulating stem cell properties and protecting them from the harsh local microenvironment. Permissive bridging material allows nerve fibers with regenerative growth potential or collateral sprouting to pass through nonpermissive physical spaces[35]; therefore, tissue-engineered grafts loaded with cells and growth factors have become popular as bridging therapy for SCI. After spinal cord injuries in rats and dogs, NT-3/fibroin-coated gelatin sponge scaffolds were shown to continuously release NT-3 for up to 28 d, maintain the cell activity of MSCs, promote axonal regeneration, and attenuate the inflammatory response[36]. The multichannel poly lactic-co-glycolic acid scaffolds implanted with Schwann cells and BMMSCs were shown to effectively connect the injury gap of rats with complete SCI, and the cell combination strategy promoted the survival and neuron-like characteristics of BMMSCs and finally facilitated axonal regeneration and functional recovery[37]. The short half-life and rapid clearance challenges posed by the innate immune system have hampered the popularity of extracellular vesicle therapy[38]. Wang et al[39] synthesized an F127-polycitrate-polyethyleneimine hydrogel (FE) with sustainable and long-term extracellular vesicle release (FE@EVs), and its in situ administration after SCI inhibited the inflammatory response and promoted myelination and axonal regeneration. There is no shortage of biomaterials in clinical trials for cancer, but no clinical trials on biomaterials for SCI repair have been registered on the ClinicalTrials.gov website. Possible reasons for this include inconsistency between injury models from preclinical studies and actual injury models in the clinic (thoracic SCI models are often used in preclinical studies, but cervical SCI is more common in humans[40]), unpredictable residual degradation products in the body, and potentially low payloads[41]. Caution is needed in drawing conclusions about axonal regeneration because the current consensus is that it refers to regrowth of axons after transection[42], whereas there is a significant degree of axonal preservation in incomplete spinal cord injuries, and the best model for exploring axonal regeneration is complete SCI.
Demyelination in traumatic SCI can lead to loss of function, and poor myelination of preserved nerve fibers may lead to permanent functional impairment. Myelin loss is accompanied by oligodendrocyte apoptosis, and replacement of lost oligodendrocytes and myelin improves conductivity and protects axons from degeneration[43]. The process of transplanted cells producing myelin around axons that have lost myelin sheaths is a mechanism that enhances recovery after SCI[43]. Conditioned medium from MSCs not only improves the survival of oligodendrocytes in vitro in culture but also increases levels of Olig2, a transcription factor that plays a key role in the differentiation of oligodendrocytes, in SCI[44]. Intravenous infusion of MSCs during the chronic phase in a severely injured SCI model promotes remyelination of axons[45]. BMMSCs can act as catalysts for the differentiation of NSCs into oligodendrocytes by regulating Id2 and Olig1/2[46]. However, the genetically engineered cells showed a more satisfactory therapeutic effect than the original cells. BMMSCs secrete various trophic factors, including VEGF, insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), basic FGF, GDNF[47,48], and certain extracellular matrix molecules, such as laminin and type IV collagen[49]. Given the positive effects of IGF-1 on oligodendrocyte differentiation and survival during normal development[50], IGF-1-overexpressing BMMSCs better protect the integrity of myelin sheaths[51]. Similarly, GDNF is a potential target for axon enhancement and is directly involved in axonogenesis and dopaminergic neuronal differentiation via the Ras/MARK pathway and P13K signaling pathway[52].
In addition to the abovementioned cytotrophic factors, myelination-related graft factors can also be used as targets for genetic engineering. Silencing Nogo-66 receptor expression was shown to promote neurite outgrowth after BMMSC differentiation and increase myelinated nerve fibers after SCI[53]. There are benefits from the effects of neuroprotective drugs themselves, which have antioxidant effects, inhibit intracellular calcium overload, regulate γ-aminobutyric acid receptors and inhibit apoptosis, and combining these drugs can also improve the therapeutic effect of BMMSCs. Currently, the combination of BMMSCs and propofol, a neuroprotective anesthetic, has dramatically increased the number of myelinated and nonmyelinated fibers after SCI[54]. Transplanted cells with the ability to myelinate do not always promote functional recovery, and sufficient myelination needs to occur to achieve significant results[55].
The inflammatory response is a critical component in the secondary injury cascade, which can persist for weeks to months after SCI, during which microglia/macrophages and leukocytes are recruited to the injury site. A certain inflammatory response in the injured spinal cord is required for clearing neurotoxic cellular debris and limiting tissue damage. However, macrophage clearance can promote regeneration after SCI. In addition, overactivation of the inflammatory response can aggravate tissue damage[56]. In the acute phase of SCI, these cells produce proinflammatory cytokines such as IL-6, IL-β, and TNF-α. Reactive oxygen species (ROS), matrix-metalloproteinase (MMP), and inducible nitrous oxide synthase are released by neutrophils, macrophages and microglia, which can exacerbate the inflammatory response[57]. Microglia/macrophages have been regarded as an important cell type in the innate and adaptive immune responses after SCI[58]. Homeostatic macrophages are the main phenotype in the normal spinal cord, but resting macrophages are activated into different phenotypes. Some macrophages produce proinflammatory cytokines that aggravate inflammation and inhibit axon regeneration, while other macrophages produce anti-inflammatory cytokines that promote functional recovery, such as IL-10 and transforming growth factor-β (TGF-β)[59]. Therefore, immunomodulatory therapeutic approaches are mainly focused on inducing macrophage polarization from the proinflammatory phenotype to the anti-inflammatory phenotype following SCI.
Many studies have shown that BMMSC transplantation after SCI can exert therapeutic effects by attenuating detrimental inflammation or enhancing beneficial inflammation. Yagura et al[60] found that human BMMSC implantation significantly increased CCL5 expression and enhanced macrophage polarization. Similarly, another study analyzed the tissue expression levels of IL-1β, TNF-α, and Toll-like receptor 4 (TLR4) in a rat SCI model after intravenous BMMSC injection[61]. This study demonstrated that BMMSCs could attenuate spinal cord inflammation by inhibiting the TLR4-mediated signaling pathway and decreasing the expression levels of IL-1β and TNF-α. Similar situations were observed in another study that investigated the efficacy of grafted BMMSCs after SCI[62]. Interestingly, most recent studies have focused on the anti-inflammatory roles of exosomes derived from BMMSCs in SCI treatment[20,63,64]. For example, Sheng et al[63] confirmed that the application of BMMSC-derived exosomes promoted the phagocytosis ability of macrophages by upregulating the expression of MARCO, an important phagocytic receptor. In addition to modulating the phagocytic capacity of macrophages, BMMSC-derived exosomes could also affect the balance of macrophage polarization through the nuclear factor-kappaB pathway[65].
BMMSC transplantation exerts immunoregulatory effects following SCI mainly by inducing the formation of anti-inflammatory immune cells, modulating the expression levels of TLRs, and inhibiting the inflammatory response in the injured spinal cord, thereby promoting functional recovery[61]. Immediately after SCI, the organism enters an immunosuppressive state due to shock and stress. The first cells to mobilize at the injured site are the myeloid cells of the innate immune system, such as neutrophils and macrophages, which phagocytose debris. Then, adaptive immune cells such as B- and T-lymphocytes are recruited to the injured spinal cord[66,67]. As previously described, microglia, which participate in the clearance of apoptotic and myelin debris, are resident immune cells[68]. In the acute stage after SCI, microglia extend their processes toward the injured site and are biased toward the activated subtype, which leads to a further loss of neurons and promotes scar formation[69]. During this period, although microglia have beneficial effects on tissue recovery by clearing cellular and myelin debris, they have also been reported to aggravate secondary tissue damage and axonal retraction[67,70]. Homeostatic microglia in the injured spinal cord may play a protective role in tissue repair. Therefore, therapeutic strategies for targeting immunomodulation should be directed at modulating cytokine levels and other factors in the microenvironment and balancing activated/homeostatic microglia levels.
In mammals, damaged axons in the spinal cord are unable to regenerate at the lesion site and to reestablish synaptic connections with their destination due to “natural barriers” and diminished intrinsic regenerative capacity[71,72]. This barrier consists of a lumen and a nonpermissive environment. Nogo-A, MAG and oligodendrocyte myelin glycoprotein (OMgp) are well-defined myelin breakdown products[73], but knockdown of the MAG and OMgp genes did not lead to neuronal growth after SCI, suggesting that Nogo-A plays a dominant role in inhibiting axonal regeneration, while MAG and OMgp play secondary roles[74]. In addition, several types of cells, including astrocytes, glial cells, and microglia/macrophages, migrate to the center of the injury, leading to the formation of glial scars and impeding the progression of axonal growth cones[75]. Glial scarring does not always play a negative role; on the one hand, it initiates the injury repair process, limits lesion expansion and inhibits the spread of the inflammatory response, and on the other hand, it acts as a physical barrier to nerve regeneration[76].
Few oligodendrocyte markers were found to be expressed in SCI centers transplanted with BMMSCs, while the number of axons was significantly increased, indicating that the transplanted cells provided a suitable environment for the regeneration and neural differentiation of endogenous neural stem cells[77]. BDNF in overexpressed BMMSCs further promotes axonal remyelination by affecting oligodendrocytes[78]. Zhao et al[79] found that the association of BDNF-overexpressing BMMSCs with platelet-rich plasma promoted more axonal remyelination and oligodendrocytes, probably due to astrocyte migration to the lesion region and increased graft BDNF-BMSCs, which could provide a favorable substrate for stabilizing regenerating axons in an inhibitory environment. However, damaging properties of the microenvironment (such as increased ROS) result in extensive stem cell death and dysfunction, severely impairing stem cell therapy for SCI.
The spinal injury scar is generally classified into two components: The lesion core and the lesion border[80]. The lesion core is primarily composed of stromal-derived fibroblasts and inflammatory immune cells and is commonly regarded as the fibrotic scar. The lesion border is formed by microglia and reactive astrocytes, which surround the core and are generally considered the glial scar. The glial scar (mainly astrocytic) strongly upregulates the expression of intermediate filament proteins such as vimentin, GFAP, and chondroitin sulfate proteoglycans (CSPGs)[9]. At the acute stage after SCI, the glial scar separates healthy tissue from inflammatory cells and limits the spread of inflammation. However, the glial scar creates a long-lasting physical and molecular barrier that hinders axon regeneration and outgrowth during the chronic period[81]. Over the past decade, accumulating evidence has attributed the failure of axon regeneration to diminished intrinsic neuronal plasticity and the presence of glial scars and myelin-associated growth inhibitors.
Numerous experimental studies in SCI animal models have shown that BMMSC transplantation can suppress glial scar formation. Okuda et al[31] explored whether BMMSC sheets are permissive for injured nerve fiber elongation and the extension of astrocyte processes. They found that GFAP-positive astrocyte processes penetrated into the BMMSC-transplanted site, which is an indicator of a permissive environment for axon outgrowth[31]. Another study showed that transplantation of human BMMSCs in an SCI rat model largely reduced the inflammatory reaction and the expression of collagen type IV, one of the markers of fibrotic scars[82]. The application of biomaterials to improve the survival rate and efficacy of implanted cells has attracted attention in recent years. Some biomaterials are primarily used as scaffolds to support the growth of BMMSCs[83,84], while a few biomaterials are capable of modulating the harsh microenvironment following SCI[85,86]. For instance, Li et al[84] investigated the efficacy of a ROS-responsive hydrogel cotransplanted with BMMSCs in a rat transection SCI model. They performed double immunofluorescence staining to identify the formation of two distinct scars: The glial scar was labeled by GFAP, and the fibrotic scar was labeled by platelet-derived growth factor receptor-β. These results revealed that this BMMSC-encapsulated hydrogel could significantly alleviate the formation of both glial and fibrotic scars at the injury site[84]. In addition, CSPGs, which are enriched in glial scars and secreted by reactive astrocytes, are potent inhibitors of axonal outgrowth. Takeuchi et al[87] found that SCI mice treated with chondroitinase ABC, a kind of CSPG-digesting enzyme, can promote axon regeneration and improve functional outcome. Indeed, another study explored the efficacy of chondroitinase ABC plus BMMSCs in the repair of SCI. Notably, the application of chondroitinase ABC combined with BMMSCs significantly reduced GFAP expression at the injury site, and the scar tissue area was much smaller than that in the model group[88].
To date, the specific molecular mechanisms of scar formation have been widely studied. TGF-β initiates signaling after binding to transmembrane type I and type II receptors, and then the type I receptor leads to the recruitment and phosphorylation of Smad2 and Smad3 proteins[89]. After the formation of a heteroprotein complex together with the co-Smad protein SMAD4, this complex can translocate into the nucleus, where it acts as a transcription factor to regulate target gene expression[90]. There is a growing body of evidence to suggest that the TGF-β/Smad signaling pathway plays an important role in collagen deposition[91]. Studies have shown that administration of BMMSCs after SCI can inhibit scar formation by downregulating TGF-β and collagen expression[92,93]. Furthermore, studies have shown that the activation and proliferation of astrocytes can be suppressed after inhibiting the JAK/STAT3 or JNK/c-Jun pathway, thus reducing scar formation and promoting functional recovery after SCI[94]. Kim et al[95] suggested that acute transplantation of BMMSCs can modulate astrogliosis through the MMP2/STAT3 pathway.
In summary, BMMSC transplantation can suppress glial scar formation and provide a favorable environment for axon regeneration after SCI. However, there has been some debate in the field on the role of scar formation in recovery following SCI[96,97]. Although the glial scar has an important protective role in separating healthy tissue from pathology after injury, it has been acknowledged that scar formation has an inhibitory role, as it is associated with failed axon regrowth. Recent evidence suggests that the phenotypes of reactive glial cells are considered the key regulators of the dual nature of the spinal injury scar[98]. In addition, the opposing roles of the scar matrix cannot be ignored, which contains beneficial molecules that are required for the formation of scar borders and inhibitory molecules such as CSPGs, tenascin, ephin B2, and slit proteins[99]. Therefore, therapeutic strategies for targeting the spinal injury scar should be directed at reducing scar formation or blocking inhibitory molecules associated with the scar.
After SCI, immediate loss of spinal vascular support occurs, which induces local hypoxia around the injury epicenter, followed by a series of molecular cascades that lead to increased microvascular permeability and BSCB disruption. Angiogenesis, which is the process of forming new vasculature, plays an important role in the proliferation phase of wound healing[100,101]. Therefore, stabilizing the provisional vessels and forming a permanent vascular network are necessary for tissue repair following SCI. Angiogenesis is a multistep process that requires endothelial proliferation and differentiation, crosstalk among endothelial cells and extracellular matrix components, and the interplay of multiple proangiogenic and antiangiogenic factors[102]. VEGF, which is an important proangiogenic factor, is upregulated after hypoxia stimulus and induces blood vessel morphogenesis by binding to the VEGF receptor. Other critical angiogenesis-related proteins include FGF, TGF-β, angiopoietin-1, and angiopoietin-2[103].
Accumulating evidence has documented that BMMSC engraftment can promote angiogenesis and vascular stability in the treatment of different diseases, especially ischemic diseases, such as myocardial infarction and cerebral infarction[104-109]. Similarly, enhancement of angiogenesis by implanted BMMSCs has been demonstrated after SCI in animals[108]. In addition, in vitro studies also confirmed that BMMSC transplantation promotes vascular formation and vasoprotection[110,111]. In general, there is a close relationship between angiogenesis and enhanced functional recovery following SCI. Emerging evidence has shown that improved angiogenesis and BSCB integrity can promote motor function recovery[112,113]. A recent study found strong correlations between the level of angiogenesis and the number of surviving BMMSCs at the injury site. This study demonstrated that the expression of occludin and ZO-1 was significantly upregulated, which indicates the maturation and sealing of newly formed vasculature[114]. BMMSCs can produce FGF and VEGF-A, which can enhance the proliferation, migration, and vascular tube formation of microvascular endothelial cells[115]. BMMSCs also secrete specific factors, including IGF-1, HGF, VEGF, NGF, and TGF-β1, which can provide a favorable environment for angiogenesis after SCI[116]. For instance, Cantinieaux et al[117] investigated the efficacy of conditioned medium from BMMSCs in SCI treatment in a rat model and found that blood vessels displayed larger diameters in the conditioned medium-treated group, indicating enhanced regional blood perfusion at the lesion epicenter.
BMMSC engraftment can promote revascularization, enhance blood supply and increase BSCB integrity, which will attenuate secondary injury and promote axon growth, thereby improving functional recovery following SCI. Improved functional outcome after SCI is closely related to successful revascularization. First, a well-vascularized injury site can provide a regeneration-permissive microenvironment for the transplanted cells to survive. Additionally, blood vessels may act as a scaffold to guide transplanted cell migration and axon sprouting after injury. Emerging evidence has demonstrated a significant interaction between vascular regrowth and nerve repair. For example, some neurotrophins, such as NGF and NT-3, can control the sympathetic innervation of blood vessels, and VEGF-A, secreted by neurons and glial cells, can enhance vascular regrowth. To date, treatments based on revascularization for SCI include gene modulation, proangiogenic factor administration, cell therapy and biomaterial application. However, many aspects of the process of blood vessel formation remain unclear, and the therapeutic effect is limited. Therapeutic strategies for targeting angiogenesis after injury should be focused on the identification of combined strategies.
During past decades, cell transplantation has been regarded as a promising therapy after SCI. There have been not only many animal and preclinical studies but also a considerable number of clinical studies, and several systematic reviews/meta-analyses have proven the effects of cell transplantation in patients with SCI[118-122]. Among them, MSC transplantation is the most widely used and promising therapeutic approach for treating SCI. MSCs are mainly derived from adipose tissue and bone marrow sources and are accordingly divided into adipose tissue-derived MSCs and BMMSCs. The main stem cell type used in clinical trials to treat SCI is the BMMSC, which has lower immunogenicity and a widely available source and has been proven to be overall safe, well tolerated and valid in SCI patients, with a particular effectiveness in chronic and complete injuries.
After assessing the relevant literature, we found 38 clinical studies containing 1090 participants that provided overall evidence of the safety and efficacy of BMMSC cell transplantation for SCI patients, which was mainly represented by ASIA score improvement in at least one segment, and both sensory and motor improvements were observed according to different previous studies. Some studies have shown that up to 70% of patients with complete cervical SCI and 33% of patients with thoracic SCI could recover at least one spinal cord level within 1 year after injury by spontaneous recovery[123,124]. Chhabra et al[125] indicated that most of the spontaneous neurological recovery in AISA subjects was likely to occur within the zone of partial preservation, which was less likely related to cell therapy. Due to the complicated process of neuroregeneration, minor therapeutic effects at the anatomical/histological level were difficult to detect in clinical trials, which might be ignored. Some novel assessments may provide further insights into the recovery of neuroregeneration after SCI in future clinical research. Furthermore, an increasing number of studies have tended to certify the same efficacy on bladder and gastrointestinal functions by slightly improved maximum capacity and decreased bladder pressure and residual urine volume, which are still unsatisfactory.
Considering the various therapy effects, there were related issues: Ambiguities in the selection of patients, timing of intervention, injection doses and routes of stem cell transplantation in different clinical trials. The optimal dose of cell transplantation has not yet been determined, and cell numbers between 106-108 seemed to be more beneficial[126-128]. Transplant routes included intrathecal, scaffold-loaded, intralesional, venous, arterial, and subdural administration, with intrathecal injection as the most widely used.
However, there were still some potential adverse events (AEs) observed, such as neuropathic pain, muscle spasm, and fever. Some of these AEs were slight and without further injury, while other more potentially serious AEs required a longer follow-up visit. Most of the current studies have a small number of samples, are low quality, lack control groups, and represent single-arm, early-stage clinical trials with the main purpose of evaluating the safety of stem cells. Nonetheless, prospective, well-designed randomized trials in larger cohorts with extensive follow-up are still awaited to confirm and update the findings.
Based on the results of previous studies, the effects that can be achieved with a single BMMSC treatment are limited, and combination therapy is an important future development direction. Combination therapy using various molecules or factors (including gene modulation, etc.) can enhance the effect of cellular therapy and achieve multi-effective and superimposed effects. In addition, most preclinical studies are currently designed with observation periods of 4 and 8 wk, and longer observation periods are important for the clinical translation of stem cell therapy to explore and address certain adverse effects when possible. Finally, given the current encouraging preclinical trial results, some treatments have been translated into clinical practice. BMMSC transplantation has been shown to be safe in SCI patients, and partial efficacy has been seen in some cases, but most clinical studies are still in phases I and II, and the results of phase III trials have extraordinary implications for the clinical translation of stem cell therapy for SCI. In conclusion, although many problems and challenges remain, researchers have been working to optimize preclinical studies and actively translate them to the clinic, and these efforts will pave the way for the field of SCI.
| 1. | Liau LL, Looi QH, Chia WC, Subramaniam T, Ng MH, Law JX. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1347] [Article Influence: 192.4] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, Alahdab F, Amit AML, Bärnighausen TW, Beghi E, Beheshti M, Chavan PP, Criqui MH, Desai R, Dhamminda Dharmaratne S, Dorsey ER, Wilder Eagan A, Elgendy IY, Filip I, Giampaoli S, Giussani G, Hafezi-Nejad N, Hole MK, Ikeda T, Owens Johnson C, Kalani R, Khatab K, Khubchandani J, Kim D, Koroshetz WJ, Krishnamoorthy V, Krishnamurthi RV, Liu X, Lo WD, Logroscino G, Mensah GA, Miller TR, Mohammed S, Mokdad AH, Moradi-Lakeh M, Morrison SD, Shivamurthy VKN, Naghavi M, Nichols E, Norrving B, Odell CM, Pupillo E, Radfar A, Roth GA, Shafieesabet A, Sheikh A, Sheikhbahaei S, Shin JI, Singh JA, Steiner TJ, Stovner LJ, Wallin MT, Weiss J, Wu C, Zunt JR, Adelson JD, Murray CJL. Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study. JAMA Neurol. 2021;78:165-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 420] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 4. | Huang L, Fu C, Xiong F, He C, Wei Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021;30:963689721989266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Ning GZ, Song WY, Xu H, Zhu RS, Wu QL, Wu Y, Zhu SB, Li JQ, Wang M, Qu ZG, Feng SQ. Bone marrow mesenchymal stem cells stimulated with low-intensity pulsed ultrasound: Better choice of transplantation treatment for spinal cord injury: Treatment for SCI by LIPUS-BMSCs transplantation. CNS Neurosci Ther. 2019;25:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 873] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 7. | Guest J, Datta N, Jimsheleishvili G, Gater DR Jr. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Shende P, Subedi M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed Pharmacother. 2017;91:693-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, Zhou H, Ning G, Kong X, Feng S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27:853-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 398] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 10. | Mukhamedshina Y, Shulman I, Ogurcov S, Kostennikov A, Zakirova E, Akhmetzyanova E, Rogozhin A, Masgutova G, James V, Masgutov R, Lavrov I, Rizvanov A. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Kostennikov A, Kabdesh I, Sabirov D, Timofeeva A, Rogozhin A, Shulman I, Rizvanov A, Mukhamedshina Y. A Comparative Study of Mesenchymal Stem Cell-Derived Extracellular Vesicles' Local and Systemic Dose-Dependent Administration in Rat Spinal Cord Injury. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Gugjoo MB, Amarpal, Fazili MR, Shah RA, Sharma GT. Mesenchymal stem cell: Basic research and potential applications in cattle and buffalo. J Cell Physiol. 2019;234:8618-8635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Hu L, Yin C, Zhao F, Ali A, Ma J, Qian A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 317] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 14. | Assunção Silva RC, Pinto L, Salgado AJ. Cell transplantation and secretome based approaches in spinal cord injury regenerative medicine. Med Res Rev. 2022;42:850-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 430] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 16. | Gao X, Han Z, Huang C, Lei H, Li G, Chen L, Feng D, Zhou Z, Shi Q, Cheng L, Zhou X. An anti-inflammatory and neuroprotective biomimetic nanoplatform for repairing spinal cord injury. Bioact Mater. 2022;18:569-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Liu X, Xu W, Zhang Z, Liu H, Lv L, Han D, Liu L, Yao A, Xu T. Vascular Endothelial Growth Factor-Transfected Bone Marrow Mesenchymal Stem Cells Improve the Recovery of Motor and Sensory Functions of Rats With Spinal Cord Injury. Spine (Phila Pa 1976). 2020;45:E364-E372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Menezes K, Nascimento MA, Gonçalves JP, Cruz AS, Lopes DV, Curzio B, Bonamino M, de Menezes JR, Borojevic R, Rossi MI, Coelho-Sampaio T. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS One. 2014;9:e96020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, Rizvanov AA. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14:227-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Sheng Y, Zhou X, Wang J, Shen H, Wu S, Guo W, Yang Y. MSC derived EV loaded with miRNA-22 inhibits the inflammatory response and nerve function recovery after spinal cord injury in rats. J Cell Mol Med. 2021;25:10268-10278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Zhou Y, Wen LL, Li YF, Wu KM, Duan RR, Yao YB, Jing LJ, Gong Z, Teng JF, Jia YJ. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022;17:194-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 22. | Li C, Jiao G, Wu W, Wang H, Ren S, Zhang L, Zhou H, Liu H, Chen Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-catenin Signaling Pathway. Cell Transplant. 2019;28:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Zhang A, Bai Z, Yi W, Hu Z, Hao J. Overexpression of miR-338-5p in exosomes derived from mesenchymal stromal cells provides neuroprotective effects by the Cnr1/Rap1/Akt pathway after spinal cord injury in rats. Neurosci Lett. 2021;761:136124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 704] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 25. | Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol Med. 2017;23:831-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Lv B, Zhang X, Yuan J, Chen Y, Ding H, Cao X, Huang A. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Winter CC, He Z, Jacobi A. Axon Regeneration: A Subcellular Extension in Multiple Dimensions. Cold Spring Harb Perspect Biol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Nakano N, Nakai Y, Seo TB, Homma T, Yamada Y, Ohta M, Suzuki Y, Nakatani T, Fukushima M, Hayashibe M, Ide C. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS One. 2013;8:e73494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Okuda A, Horii-Hayashi N, Sasagawa T, Shimizu T, Shigematsu H, Iwata E, Morimoto Y, Masuda K, Koizumi M, Akahane M, Nishi M, Tanaka Y. Bone marrow stromal cell sheets may promote axonal regeneration and functional recovery with suppression of glial scar formation after spinal cord transection injury in rats. J Neurosurg Spine. 2017;26:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Song SJ, Ziegler R, Arsenault L, Fried LE, Hacker K. Asian student depression in American high schools: differences in risk factors. J Sch Nurs. 2011;27:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Dooley D, Lemmens E, Vangansewinkel T, Le Blon D, Hoornaert C, Ponsaerts P, Hendrix S. Cell-Based Delivery of Interleukin-13 Directs Alternative Activation of Macrophages Resulting in Improved Functional Outcome after Spinal Cord Injury. Stem Cell Reports. 2016;7:1099-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Stewart AN, Kendziorski G, Deak ZM, Brown DJ, Fini MN, Copely KL, Rossignol J, Dunbar GL. Co-transplantation of mesenchymal and neural stem cells and overexpressing stromal-derived factor-1 for treating spinal cord injury. Brain Res. 2017;1672:91-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Chhabra HS, Sarda K. Clinical translation of stem cell based interventions for spinal cord injury - Are we there yet? Adv Drug Deliv Rev. 2017;120:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Li G, Che MT, Zhang K, Qin LN, Zhang YT, Chen RQ, Rong LM, Liu S, Ding Y, Shen HY, Long SM, Wu JL, Ling EA, Zeng YS. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Yang EZ, Zhang GW, Xu JG, Chen S, Wang H, Cao LL, Liang B, Lian XF. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol Sin. 2017;38:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 462] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 39. | Wang C, Wang M, Xia K, Wang J, Cheng F, Shi K, Ying L, Yu C, Xu H, Xiao S, Liang C, Li F, Lei B, Chen Q. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact Mater. 2021;6:2523-2534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 40. | Dvorak MF, Noonan VK, Fallah N, Fisher CG, Rivers CS, Ahn H, Tsai EC, Linassi AG, Christie SD, Attabib N, Hurlbert RJ, Fourney DR, Johnson MG, Fehlings MG, Drew B, Bailey CS, Paquet J, Parent S, Townson A, Ho C, Craven BC, Gagnon D, Tsui D, Fox R, Mac-Thiong JM, Kwon BK. Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J Neurotrauma. 2014;31:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Song YH, Agrawal NK, Griffin JM, Schmidt CE. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev. 2019;148:38-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 43. | Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1031] [Cited by in RCA: 1294] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 44. | Tsai MJ, Liou DY, Lin YR, Weng CF, Huang MC, Huang WC, Tseng FW, Cheng H. Attenuating Spinal Cord Injury by Conditioned Medium from Bone Marrow Mesenchymal Stem Cells. J Clin Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Morita T, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Oka S, Oshigiri T, Takebayashi T, Yamashita T, Kocsis JD, Honmou O. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Song P, Xia X, Han T, Fang H, Wang Y, Dong F, Zhang R, Ge P, Shen C. BMSCs promote the differentiation of NSCs into oligodendrocytes via mediating Id2 and Olig expression through BMP/Smad signaling pathway. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Hill RL, Zhang YP, Burke DA, Devries WH, Zhang Y, Magnuson DS, Whittemore SR, Shields CB. Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J Neurotrauma. 2009;26:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, Aronowski J, Savitz SI. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88:2869-2876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Gu Y, Wang J, Ding F, Hu N, Wang Y, Gu X. Neurotrophic actions of bone marrow stromal cells on primary culture of dorsal root ganglion tissues and neurons. J Mol Neurosci. 2010;40:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Dyer AH, Vahdatpour C, Sanfeliu A, Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 51. | Allahdadi KJ, de Santana TA, Santos GC, Azevedo CM, Mota RA, Nonaka CK, Silva DN, Valim CXR, Figueira CP, Dos Santos WLC, do Espirito Santo RF, Evangelista AF, Villarreal CF, Dos Santos RR, de Souza BSF, Soares MBP. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res Ther. 2019;10:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Lu Y, Gao H, Zhang M, Chen B, Yang H. Glial Cell Line-Derived Neurotrophic Factor-Transfected Placenta-Derived Versus Bone Marrow-Derived Mesenchymal Cells for Treating Spinal Cord Injury. Med Sci Monit. 2017;23:1800-1811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Li Z, Zhang Z, Zhao L, Li H, Wang S, Shen Y. Bone marrow mesenchymal stem cells with Nogo-66 receptor gene silencing for repair of spinal cord injury. Neural Regen Res. 2014;9:806-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Zhou YJ, Liu JM, Wei SM, Zhang YH, Qu ZH, Chen SB. Propofol promotes spinal cord injury repair by bone marrow mesenchymal stem cell transplantation. Neural Regen Res. 2015;10:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Plemel JR, Chojnacki A, Sparling JS, Liu J, Plunet W, Duncan GJ, Park SE, Weiss S, Tetzlaff W. Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia. 2011;59:1891-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Fehlings MG, Nguyen DH. Immunoglobulin G: a potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol. 2010;30 Suppl 1:S109-S112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330-9341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 58. | Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 590] [Article Influence: 53.6] [Reference Citation Analysis (7)] |
| 59. | Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435-13444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 1734] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 60. | Yagura K, Ohtaki H, Tsumuraya T, Sato A, Miyamoto K, Kawada N, Suzuki K, Nakamura M, Kanzaki K, Dohi K, Izumizaki M, Hiraizumi Y, Honda K. The enhancement of CCL2 and CCL5 by human bone marrow-derived mesenchymal stem/stromal cells might contribute to inflammatory suppression and axonal extension after spinal cord injury. PLoS One. 2020;15:e0230080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Han D, Wu C, Xiong Q, Zhou L, Tian Y. Anti-inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biochem Biophys. 2015;71:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 318] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 63. | Sheng X, Zhao J, Li M, Xu Y, Zhou Y, Xu J, He R, Lu H, Wu T, Duan C, Cao Y, Hu J. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Accelerate Functional Recovery After Spinal Cord Injury by Promoting the Phagocytosis of Macrophages to Clean Myelin Debris. Front Cell Dev Biol. 2021;9:772205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Nakazaki M, Morita T, Lankford KL, Askenase PW, Kocsis JD. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10:e12137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 65. | Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H, Guan P, Lu F, Luo Y, Tan G, Yu P, Chen D, Deng C, Sun Y, Zhou L, Ning C. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci (Weinh). 2022;9:e2105586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 66. | Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron. 2015;87:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 67. | Salvador AFM, Kipnis J. Immune response after central nervous system injury. Semin Immunol. 2022;59:101629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 68. | Chio JCT, Xu KJ, Popovich P, David S, Fehlings MG. Neuroimmunological therapies for treating spinal cord injury: Evidence and future perspectives. Exp Neurol. 2021;341:113704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 69. | Yong HYF, Rawji KS, Ghorbani S, Xue M, Yong VW. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol. 2019;16:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 70. | Tran AP, Warren PM, Silver J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol Rev. 2018;98:881-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 636] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 71. | Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 72. | Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci U S A. 2011;108:E99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 73. | Moreno-Flores MT, Avila J. The quest to repair the damaged spinal cord. Recent Pat CNS Drug Discov. 2006;1:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 75. | Wilems TS, Sakiyama-Elbert SE. Sustained dual drug delivery of anti-inhibitory molecules for treatment of spinal cord injury. J Control Release. 2015;213:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1219] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 77. | Gu W, Zhang F, Xue Q, Ma Z, Lu P, Yu B. Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology. 2010;30:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31:14182-14190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 79. | Zhao T, Yan W, Xu K, Qi Y, Dai X, Shi Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy. 2013;15:792-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 80. | Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 527] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 81. | Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res. 2015;218:15-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 82. | Kim M, Kim KH, Song SU, Yi TG, Yoon SH, Park SR, Choi BH. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J Tissue Eng Regen Med. 2018;12:e1034-e1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Raynald, Shu B, Liu XB, Zhou JF, Huang H, Wang JY, Sun XD, Qin C, An YH. Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats. CNS Neurosci Ther. 2019;25:951-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Li Z, Zhao T, Ding J, Gu H, Wang Q, Wang Y, Zhang D, Gao C. A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact Mater. 2023;19:550-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 85. | García E, Rodríguez-Barrera R, Buzoianu-Anguiano V, Flores-Romero A, Malagón-Axotla E, Guerrero-Godinez M, De la Cruz-Castillo E, Castillo-Carvajal L, Rivas-Gonzalez M, Santiago-Tovar P, Morales I, Borlongan C, Ibarra A. Use of a combination strategy to improve neuroprotection and neuroregeneration in a rat model of acute spinal cord injury. Neural Regen Res. 2019;14:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Ogle ME, Doron G, Levy MJ, Temenoff JS. Hydrogel Culture Surface Stiffness Modulates Mesenchymal Stromal Cell Secretome and Alters Senescence. Tissue Eng Part A. 2020;26:1259-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 87. | Takeuchi K, Yoshioka N, Higa Onaga S, Watanabe Y, Miyata S, Wada Y, Kudo C, Okada M, Ohko K, Oda K, Sato T, Yokoyama M, Matsushita N, Nakamura M, Okano H, Sakimura K, Kawano H, Kitagawa H, Igarashi M. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun. 2013;4:2740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 88. | Zhang C, He X, Li H, Wang G. Chondroitinase ABC plus bone marrow mesenchymal stem cells for repair of spinal cord injury. Neural Regen Res. 2013;8:965-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 89. | Seoane J, Gomis RR. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb Perspect Biol. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 90. | Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2613] [Cited by in RCA: 2593] [Article Influence: 185.2] [Reference Citation Analysis (2)] |
| 91. | Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-β-mediated induction of chondroitin sulfate proteoglycans. J Neurochem. 2011;119:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Lv C, Zhang T, Li K, Gao K. Bone marrow mesenchymal stem cells improve spinal function of spinal cord injury in rats via TGF-β/Smads signaling pathway. Exp Ther Med. 2020;19:3657-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Xu JH, Xu SQ, Ding SL, Yang H, Huang X, Shi HF. Bone marrow mesenchymal stem cells alleviate the formation of pathological scars in rats. Regen Ther. 2022;20:86-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 94. | Shen D, Wang X, Gu X. Scar-modulating treatments for central nervous system injury. Neurosci Bull. 2014;30:967-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Kim C, Kim HJ, Lee H, Lee SJ, Lee ST, Yang SR, Chung CK. Mesenchymal Stem Cell Transplantation Promotes Functional Recovery through MMP2/STAT3 Related Astrogliosis after Spinal Cord Injury. Int J Stem Cells. 2019;12:331-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1402] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 97. | Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 522] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 98. | Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3268] [Cited by in RCA: 5829] [Article Influence: 647.7] [Reference Citation Analysis (0)] |
| 99. | Kabdesh IM, Mukhamedshina YO, Arkhipova SS, Sabirov DK, Kuznecov MS, Vyshtakalyuk AB, Rizvanov AA, James V, Chelyshev YA. Cellular and Molecular Gradients in the Ventral Horns With Increasing Distance From the Injury Site After Spinal Cord Contusion. Front Cell Neurosci. 2022;16:817752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Okon EB, Streijger F, Lee JH, Anderson LM, Russell AK, Kwon BK. Intraparenchymal microdialysis after acute spinal cord injury reveals differential metabolic responses to contusive versus compressive mechanisms of injury. J Neurotrauma. 2013;30:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1106] [Cited by in RCA: 1110] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 102. | Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 599] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 103. | Kundi S, Bicknell R, Ahmed Z. The role of angiogenic and wound-healing factors after spinal cord injury in mammals. Neurosci Res. 2013;76:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Xiao Y, Zhang Y, Li Y, Peng N, Liu Q, Qiu D, Cho J, Borlongan CV, Yu G. Exosomes Derived From Mesenchymal Stem Cells Pretreated With Ischemic Rat Heart Extracts Promote Angiogenesis via the Delivery of DMBT1. Cell Transplant. 2022;31:9636897221102898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Shen H, Gu X, Wei ZZ, Wu A, Liu X, Wei L. Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp Neurol. 2021;337:113542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 106. | Yao Z, Liu H, Yang M, Bai Y, Zhang B, Wang C, Yan Z, Niu G, Zou Y, Li Y. Bone marrow mesenchymal stem cell-derived endothelial cells increase capillary density and accelerate angiogenesis in mouse hindlimb ischemia model. Stem Cell Res Ther. 2020;11:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, Sun L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 108. | Zeng X, Zeng YS, Ma YH, Lu LY, Du BL, Zhang W, Li Y, Chan WY. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant. 2011;20:1881-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 109. | Fan L, Zhang C, Yu Z, Shi Z, Dang X, Wang K. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and osteogenesis in rabbit femoral head osteonecrosis. Bone. 2015;81:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 110. | Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551-4558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 418] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 111. | Sorrell JM, Baber MA, Caplan AI. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tissue Eng Part A. 2009;15:1751-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Hu J, Zeng L, Huang J, Wang G, Lu H. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 113. | Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, Yao Y, Duan R, Jia Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front Neurosci. 2019;13:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 114. | Maldonado-Lasunción I, Haggerty AE, Okuda A, Mihara T, de la Oliva N, Verhaagen J, Oudega M. The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Gruber R, Kandler B, Holzmann P, Vögele-Kadletz M, Losert U, Fischer MB, Watzek G. Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 2005;11:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Ribeiro CA, Fraga JS, Grãos M, Neves NM, Reis RL, Gimble JM, Sousa N, Salgado AJ. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther. 2012;3:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 117. | Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noël A, Brook G, Schoenen J, Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8:e69515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 118. | Liu S, Zhang H, Wang H, Huang J, Yang Y, Li G, Yu K, Yang L. A Comparative Study of Different Stem Cell Transplantation for Spinal Cord Injury: A Systematic Review and Network Meta-Analysis. World Neurosurg. 2022;159:e232-e243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Tang QR, Xue H, Zhang Q, Guo Y, Xu H, Liu Y, Liu JM. Evaluation of the Clinical Efficacy of Stem Cell Transplantation in the Treatment of Spinal Cord Injury: A Systematic Review and Meta-analysis. Cell Transplant. 2021;30:9636897211067804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Shang Z, Wang M, Zhang B, Wang X, Wanyan P. Clinical translation of stem cell therapy for spinal cord injury still premature: results from a single-arm meta-analysis based on 62 clinical trials. BMC Med. 2022;20:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 121. | Xu P, Yang X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2019;28:36-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 122. | Muthu S, Jeyaraman M, Gulati A, Arora A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: systematic review and meta-analysis. Cytotherapy. 2021;23:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 123. | Steeves JD, Kramer JK, Fawcett JW, Cragg J, Lammertse DP, Blight AR, Marino RJ, Ditunno JF Jr, Coleman WP, Geisler FH, Guest J, Jones L, Burns S, Schubert M, van Hedel HJ, Curt A; EMSCI Study Group. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord. 2011;49:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 124. | Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 125. | Chhabra HS, Sarda K, Arora M, Sharawat R, Singh V, Nanda A, Sangodimath GM, Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury--an Indian pilot study. Spinal Cord. 2016;54:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 126. | Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H, Wafaie A, Bilal D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 127. | Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, Loladze G, Shatirishvili G, Chakhunashvili D, Chutkerashvili K. Phase 1 Trial of Autologous Bone Marrow Stem Cell Transplantation in Patients with Spinal Cord Injury. Stem Cells Int. 2016;2016:6768274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, Pádr R, Neuwirth J, Komrska V, Vávra V, Stulík J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeyaraman M, India; Kabdesh I, Russia; Ramadurai R, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ