Published online Feb 26, 2023. doi: 10.4252/wjsc.v15.i2.16
Peer-review started: October 14, 2022
First decision: November 5, 2022
Revised: November 10, 2022
Accepted: January 18, 2023
Article in press: January 18, 2023
Published online: February 26, 2023
Processing time: 129 Days and 8.4 Hours
Diseases caused by ischemia are one of the leading causes of death in the world. Current therapies for treating acute myocardial infarction, ischemic stroke, and critical limb ischemia do not complete recovery. Regenerative therapies opens new therapeutic strategy in the treatment of ischemic disorders. Mesenchymal stem cells (MSCs) are the most promising option in the field of cell-based therapies, due to their secretory and immunomodulatory abilities, that contribute to ease inflammation and promote the regeneration of damaged tissues. This review presents the current knowledge of the mechanisms of action of MSCs and their therapeutic effects in the treatment of ischemic diseases, described on the basis of data from in vitro experiments and preclinical animal studies, and also summarize the effects of using these cells in clinical trial settings. Since the obtained therapeutic benefits are not always satisfactory, approaches aimed at enhancing the effect of MSCs in regenerative therapies are presented at the end.
Core Tip: Mesenchymal stem cell (MSC) transplantation is an innovative therapy with positive therapeutic effects for many ischemic diseases. Ischemia of an area is defined as insufficient blood supply to specific tissues and various organs or individual parts of the body. The leading cause of tissue ischemia is the narrowing or blockage of the lumen of an artery, most often due to the formation of atherosclerotic plaques, thrombus, or spasms of a specific artery. Here, the potential therapeutic mechanisms of MSCs in ischemic diseases were discussed, along with examples of preclinical and clinical studies.
- Citation: Szydlak R. Mesenchymal stem cells in ischemic tissue regeneration. World J Stem Cells 2023; 15(2): 16-30
- URL: https://www.wjgnet.com/1948-0210/full/v15/i2/16.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i2.16
Ischemia of an area is defined as insufficient blood supply to specific tissues and various organs or individual parts of the body (e.g., limbs). Reduced blood perfusion causes inadequate transport of oxygen and nutrients to tissue-resident cells, leading to ischemia and ultimately damaging tissues and organs. The leading cause of tissue ischemia is the narrowing or blockage of the lumen of an artery, most often due to the formation of atherosclerotic plaques, thrombus, or spasms of a specific artery[1]. Disruption of blood flow to particular organs or parts of the body may be chronic developing over several months or years or acute occurring suddenly (e.g., during exercise) and usually takes a more rapid course often with a worse prognosis. Ischemia resulting from atherosclerotic lesions is most commonly found in the heart muscle, lower extremities, kidney, and brain[1-4]. Examples of ischemic diseases caused by the narrowing of the coronary and cerebral arteries that cause high morbidity and mortality in patients are myocardial infarction and ischemic stroke (Figure 1)[5]. Ischemia may entail several functional changes at the level of individual cells that build tissues, causing their dysfunction and death due to necrosis or apoptosis[6].
Standard treatments for ischemia include invasive and pharmacological control and treatments for the effects of ischemia in the damaged tissues. These methods emphasize improving the quality of and extending the patient’s life, but they cannot fully reverse the effects of tissue ischemia in case of people suffering from congenital heart disease[7], ischemic heart failure[8], acute limb ischemia[9], critical limb ischemia[10], and ischemic stroke[11]. Even with the substantial progress of therapeutic strategies, these approaches do not provide the expected clinical benefits for all patients. Therefore, novel treatment pathways while replacing or supporting classic therapeutic approaches should continue to be investigated[12-14].
Currently, great hopes are placed on regenerative treatment, i.e., therapies based on cellular preparations, including using various progenitor and stem cells (SCs) and their products[8,12,15]. Over the past decade, growing effort has been directed to the regenerative properties of SCs in relation to the biological treatment strategy of substitution of damaged cells in the tissue with new ones[16]. It is also believed that SCs may be involved in the neovascularization of ischemic tissues[17,18].
In 1970, Friedenstein et al[19] discovered an exceptional type of cell that has been extensively researched over the years for its potential use in regenerative medicine to treat ischemic damage[20]. These cells, called mesenchymal SCs (MSCs), reside in both young and adult donors [e.g., umbilical cord (UC), Wharton’s jelly (WJ) amniotic fluid, UC blood (UCB), placenta, adipose tissue (AT), bone marrow (BM), dental pulp (DP), and others], which has been a particular and exciting source of SCs for many years, mainly for autotransplantation and allotransplantation. Regardless of the tissue source, a cell that meets the criteria set out by the International Society for Cell Therapy (Table 1) may be qualified as MSCs[21].
| Morphology, growth conditions | Spindle-shaped (fibroblast-like), adherent |
| Specific surface markers | CD73+, CD90+, CD105+, CD11b-, CD14-, CD19-, CD45-, CD79α-, MHC-II, HLA-DR- |
| Differentiation potential | Adipogenic, chondrogenic, osteogenic |
They now account for the most popular SC population used in clinical trials worldwide (clinicaltrials.gov). MSCs derived from birth-related tissues have more promise due to better proliferative capacity compared with MSCs obtained from adult tissues. They are safe in terms of both sourcing and ethical aspects[22,23]. Due to several specific characteristics, these cells are essential candidates for regenerative therapies for ischemic tissues. Moreover, it is possible to use these cells to manufacture ready-to-use SC-based medicinal products. This review described the potential therapeutic mechanisms of MSCs in the context of ischemic disorder treatment. Exemplary clinical trials and procedures enhancing the therapeutic effect of MSCs were also discussed.
The leading cause of ischemic disorders is the chronic inflammatory disease of the arteries, called “atherosclerosis”. It can be caused by many risk factors, including aging, high blood pressure, diabetes, hypercholesterolemia, and smoking[24,25]. In the course of atherosclerosis, pathological damage and dysregulation of the endothelium lining the blood vessel wall, accumulation of lipids, smooth muscle cells, leukocytes, and foam cells are observed. Also, there is an aggregation of platelets in the lumen of the blood vessels, which leads to the formation of plaques narrowing the lumen of the vessels[26,27]. Moreover, in atherosclerotic lesions, increased expression of matrix metalloproteinases (MMP) and their participation in weakening the vascular wall through the degradation of the extracellular matrix (ECM) have been demonstrated[28].
To compensate for the degradation of the ECM, smooth muscle cells migrate from the outer layers of the artery wall to the inner lining of the sheath to increase the collagen secretion rate[25]. It often causes undesirable remodeling as macrophages secrete cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 to induce smooth muscle cell apoptosis[25]. The unbalanced degradation rate of the ECM caused by increased collagen production results in the formation of atherosclerotic plaques with a thin fibrous collagen cap[29]. Injecting MSCs at this stage can modulate immune cell function, MMP activity, and the secretion of proinflammatory cytokines and restore collagen homeostasis[30]. In addition, due to hemodynamic changes and high shearing stresses in atherosclerotic plaques, ruptures and bleeding may occur, increasing platelet recruitment, coagulation processes, and thrombus formation[31]. Due to the secretion of proangiogenic factors and the ability to differentiate in the endothelium, MSCs can promote angiogenesis to restore blood flow to ischemic tissues for tissue regeneration and organ function restoration[32].
Over the past decade, scientists and clinicians often discuss the regenerative properties of SCs in the context of biological treatment approaches. These strategies involve the replacement of damaged tissue cells with new SCs, including ischemic myocardium[16,33]. SCs are also considered cells that can participate in the neovascularization of ischemic tissues, which may also be associated with the improvement of the function of this organ[18,34,35].
One type of cell studied for years for their potential future use in regenerative medicine to treat myocardial damage or improve perfusion in limb muscle tissue is the mesenchymal/stromal SC (MSC) isolated from the BM, AT, birth-associated tissues, and other sites. These cells were found in both young and adult donors and have been an essential and exciting source of SCs, primarily for autotransplantation. They now account for the most often used SC population in clinical trials worldwide (clinicaltrial.gov)[18,36,37].
The history of studies on MSCs began in the 1970s when Friedenstein et al[19] observed colony-forming unit-fibroblasts (today known as MSCs). These cells constituted a fraction of adherent cells in the BM[19]. Moreover, their previous studies showed that subpopulations of BM cells could differentiate to other cell types, i.e., osteoblasts[38]. These discoveries initiated an increase in interest in MSCs and the search for these cells in other tissues as well.
One of the primary and best-known sources of MSCs is BM[39,40]. Cells with similar morphology and biological characteristics can also be found in other tissues collected from adult donors, such as peripheral blood, AT, DP, and fetal tissues such as UCB and WJ[41].
BM-MSCs show morphological and phenotypic similarities to AT-MSCs and WJ-MSCs. These markers include CD29 +, CD44 +, CD73 +, CD90 +, CD105 +, CD166 +, human leukocyte antigen (HLA)-ABC +, CD11b-, CD14-, CD19a-, CD34-, CD45-, CD79-, and HLA-DR[42,43]. However, the source tissues from which MSCs are isolated differ in the content of these SCs. For example, the number of MSCs in the BM is lower than in AT[43,44]. The BM contains from 0.0017% to 0.02% of MSCs among all mononuclear cells, while in AT, these cells constitute 5.0% to 25.6% of all cells, which is the so-called stromal-vascular fraction obtained from this tissue[44-46]. The number of colony-forming unit-fibroblast colonies isolated from the same number of plated BM or AT cells is several times higher from AT than BM[42]. Also, WJ-MSCs cells have a higher frequency of colony-forming unit-fibroblasts than BM-MSCs cells[47]. Considering the differentiation potential of BM-MSCs, AT-MSCs, and UC-MSCs, these cells show a comparable capacity to differentiate into osteoblasts, chondrocytes, and adipocytes[42,48], confirming their mesodermal origin.
MSCs derived from various tissues can also be differentiated in vitro into phenotypically similar cells, including cardiomyocytes[49-53], vascular endothelial cells (ECs)[54,55], and nerve cells[56]. However, it has been shown that the effectiveness of such differentiation is variable and depends on the tissue origin[49,51,53,56]. Within one culture of MSCs, cells may be more or less predisposed to differentiate to a specific phenotype of the mature cell[57]. Despite the morphological and antigenic similarity between MSCs from different source tissues, the results of world studies showed that BM-MSCs may offer a different expression profile of many genes compared to placenta-derived MSCs[57], which suggests that MSCs obtained from BM, WJ, and AT may differ in terms of their molecular composition and their ability to differentiate.
The International Society for Cellular Therapy, to clarify the nomenclature and define the requirements of human MSCs and facilitate the comparison of test results between laboratories around the world, proposed three minimum criteria for characterizing human MSCs[21].
Due to numerous world studies showing the relatively wide potential of MSCs to differentiate into various types of tissues, these cells constituting a population obtained from mature tissues have for years interested scientists in the context of their potential use in tissue regeneration[58-60].
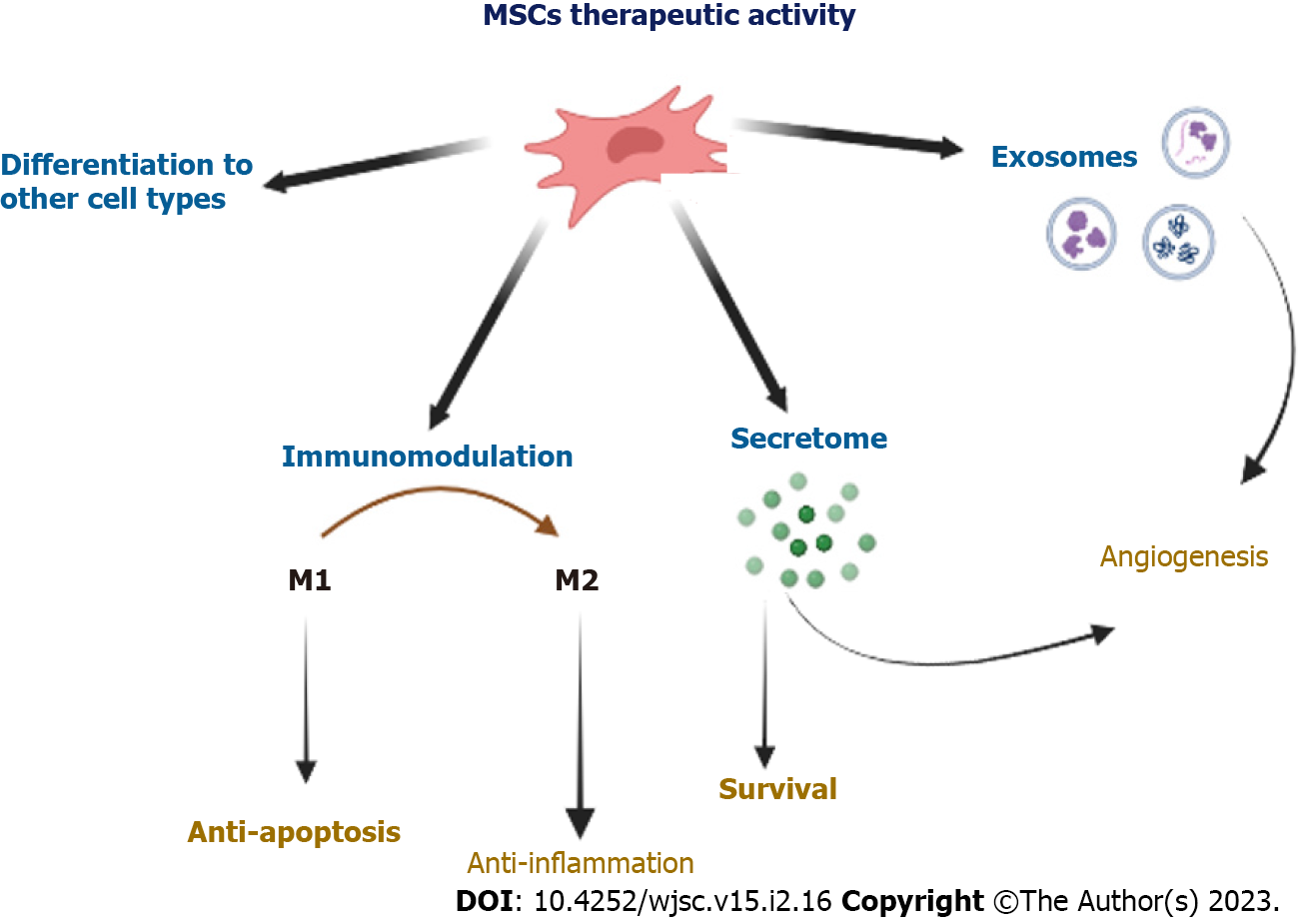
Multipotent MSCs show many features desirable from the point of view of their potential use in the regeneration of damaged tissues, not only in autologous but also in allogeneic transplants. Therefore, over the last decade, many studies have been undertaken to explain the mechanisms of action of these cells replaced by tissue damage. Figure 2 presents several essential mechanisms of action contributing to ischemic tissue regeneration.
It was initially suggested that MSCs administrated into the area of tissue damage are able to differentiate into desired cell types, including muscle-building cardiac mesenchymal cells (CMCs), vascular smooth muscle cells, and ECs[59,61,62]. It was shown in vivo that MSCs injected to the heart muscle had a phenotype similar to differentiating cells, including CMCs and ECs[63-65]. Pochon et al[66] confirmed that WJ-MSCs differentiated into CMCs in vitro, expressing CMC markers and spontaneous throbbing, which might be evidence of their terminal maturation into specialized cells capable of playing their proper functions. Although MSCs show the potential to differentiate to CMCs in vitro, the fundamental issue is to restore damaged tissue in vivo. Effective delivery and retention must be emphasized here because if cells do not reach the target tissue, they cannot exert any therapeutic effect[60]. Even if studies have shown that MSCs can differentiate into various types of myocardial cells, an increasing number of studies show that this is not a main mechanism for their regenerative activity in the cardiovascular system.
Several articles now contain information on the neural differentiation of MSCs from various sources in vitro[67-69]. In the described studies, the prevailing view suggests that MSCs, derived from immature tissues due to their plastic properties, can differentiate more effectively into cells with neural phen
MSCs can communicate with neighboring cells through direct cell-cell interactions, including gap junctions and tunneling nanotubes[75]. Moreover, MSCs are able to transport mitochondria through nanotubes and thus obtain cardiological protection by regaining the respiratory chain in myocytes[76]. To allow damaged tissue to regenerate, MSCs communicate with other cells in the damaged areas to recruit other types of SCs. For example, studies have demonstrated that CMCs can re-enter the cell cycle after supplementation with specific cytokines secreted by MSCs (e.g., transforming growth factor-β)[77]. MSCs can also keep other cells to active migration to the area of tissue damage, as demonstrated by the trafficking of hematopoietic SCs to the damaged myocardium[78].
Despite the reported low rate of retention of MSCs in ischemic heart muscle after their injection, the results of many experiments showed improvement in the functional heart parameters, like inhibition of adverse tissue remodeling myocardium and left ventricle ejection fraction (LVEF)[33]. So, this begs the questions: Is the improvement in injured organ function following the administration of MSCs results only from the implantation and ability of MSCs to differentiate into specific cell types or is another mechanism also involved in this process?
No doubt, that in order to answer this question, other studies, which have focused on the immunomodulatory properties of MSCs, should be mentioned here[79-81]. The remarkable ability of MSCs to produce an enormous number of soluble factors, such as anti-inflammatory cytokines, enables them to modulate the immune system response[82,83]. For example, MSCs secrete the cytokines, IL-4 and IL-10, which inhibit the proliferation of T cells, the growth factor, hepatocyte growth factor, which inhibits the proliferation of CD4+ T cells, and transforming growth factor-β1, which with prostaglandin E2 inhibits the inflammation process[84-86]. Moreover, they encourage the maturation of monocytes towards anti-inflammatory macrophages type M2[79,87].
In addition, the most extraordinary attribute of MSCs is an immunological privilege. MSCs are known to be capable of avoiding and suppressing immune responses[88,89]. Most MSCs show the low expression of HLA class I and a lack of HLA class II markers. Due to this feature, they do not cause an immune conflict between the transplant recipient and the injected cells. Additionally, MSCs possess HLA-G, which is a key factor in the elimination of the fetus rejection by the maternal immune system[90,91]. Because of the high expression of HLA-G, MSCs can modulate the tolerance of the immune system and it has a very beneficial effect on acceptance of the transplant.
Another reported mechanism of therapeutic activity of MSCs can be attributed to the secretion of paracrine factors, including several cytokines, growth factors, and chemokines, that may regulate many regenerative processes at the MSCs implantation site[92]. Proangiogenic molecules produced by MSCs involve, among others, the protein fibroblast growth factor-2[92-94], platelet-derived growth factor[95], and an extremely proangiogenic vascular endothelial growth factor, supporting the proliferation of vascular smooth muscle cells and ECs as well as the migration and the new blood vessel structure formation[96].
On the other hand, molecules that promote ECM remodeling involve MMP1, MMP2, MMP9, a family of enzymes that degrade ECM structure, and TNF-α and the activator plasminogen that leads to ECM protein impairment[78,97]. A distinct category of molecules produced by MSCs are the factors responsible for MSCs survival, proliferation, and migration to the area of tissue injury, which involve fibroblast growth factor-2 supporting the proliferation of vascular smooth muscle cells and ECs, stromal cell-derived factor-1 reducing apoptosis and regulating cell migration, insulin-like growth factor-1 controlling cell differentiation and growth and inhibiting apoptosis, and a secreted frizzled-related protein-2 supporting CMC survival at the conditions of low oxygen availability in vivo[77,98].
Extracellular vesicles are biological nanoparticle structures containing bioactive molecules, including protein, and nucleic acids. They can influence other cells and participate in intercellular communication over long distances[99]. Many studies on tissue regeneration mechanisms demonstrated that extracellular vesicles released by SCs can deliver bioactive molecules to target cells, which may influence the function of those cells, including the process of damaged tissue regeneration[100].
Most of the molecular and cellular mechanisms that affect the therapeutic potential of MSCs in the therapy of ischemic tissues were initially identified in animal models. The capacity of MSCs to survive in the recipient after administration and the ability to differentiate into Ecs and CMCs has been proven in acute myocardial infarction in a mini-swine model[65]. In this paper, Zhang et al[65] also confirmed the migratory activity of MSCs towards inflammation, inhibition of CMC apoptosis, stimulation of cardiac SCs, reduction of fibrosis, myocardium reverse remodeling, and enhancement of LVEF. It has been demonstrated that MSCs derived from UCB (UCB-MSCs) can reduce the acute myocardial infarction size by ≥ 50% and enhance LVEF[101]. The observed therapeutic effect may be due to the ability of MSCs to secrete bioactive factors. In turn, thanks to their immunosuppressive properties and the paracrine effect, MSCs can alleviate inflammation and ischemic heart disorders, contributing to the reduction of infarct size and improving LVEF through a paracrine effect[102].
The therapeutic efficacy of UC-MSCs and heart function improvement has been demonstrated[103]. Intravenous administration of MSCs has improved LVEF contractility, function, perfusion, and reverse remodeling[18]. The transplantation of MSCs in a rat model of acute myocarditis can reduce inflammation by decreasing the infiltration of an inflammatory cell, reducing CMC death, and remodeling adverse myocardium[104,105]. Based on the results of animal studies, hopes are high regarding enhancing many heart functions, such as reduction of scar tissue, myocardium reverse remodeling, increase in cardiac contractility, improvement of ejection fraction, and increase in heart perfusion. However, there is still a need for long-term observation of the effects of injecting MSCs to ensure the safety and efficacy of therapy.
In preclinical studies on transgenic animals of models of neurological diseases, significant functional improvement was observed after MSC cell transplantation[106]. The use of these cells may be related to their direct action, i.e., replacement of damaged cells as a result of neural differentiation or to an indirect influence positively influencing the endogenous regenerative processes of the organism. In addition, preclinical studies on animals have confirmed the neuroprotective properties of MSC transplants, which may be linked to their production of numerous growth, anti-inflammatory, and anti-apoptotic factors important for neurons[107].
Clinical trials testing MSCs in regenerative therapy are growing (ClinicalTrials.gov). Regenerative therapies based on MSCs for several ischemic disorders are now carefully examined. The remedial effects obtained are promising and prove that the transplantation of MSCs may be beneficial in the treatment of many diseases[108,109]. The concept of clinical application of MSCs may improve the health of patients suffering from many cardiovascular diseases[110]. So far, a few dozen studies have been registered in the international database of clinical trials to assess the safety and effectiveness of MSCs administration in the treatment of ischemic diseases.
The most popular source of therapeutic MSCs used in clinical practice is BM. The first MSC-based biological drug, AMI HeartiCellgram®, used in myocardial infarction therapy was also based on BM-MSCs. Lee et al[111] described the manufacturing procedure for this drug, and Kim et al[112] proved its effectiveness in a clinical experiment. The therapeutic benefits included the restoration of normal systolic heart function and the reduction of post-infarction scar tissue[113,114].
The pioneer pilot clinical study applying WJ-MSCs in treating ischemic disorders was performed by Musialek et al[115] in 2015. This group has shown the safety of injected MSCs as an off-the-shelf product, percutaneous allogeneic SC therapy in human acute myocardial infarction. Later observation proved no clinical adverse events in the treated tissue, except for a local rise in body temperature of 1 patient[115]. Currently, Musialek et al[115] are examining the safety and effectiveness of the “CardioCell” drug (WJ-MSC-based biological therapeutic) in a phase II/III randomized, placebo-controlled, double-blind clinical trial in several ischemic disorders [i.e., acute myocardial infarction (EudraCT Number: 2016-004662-25), chronic ischemic heart failure (EudraCT Number: 2016-004683-19), and non-option critical limb ischemia (EudraCT Number: 2016-004684-40)][116,117].
A clinical trial with MSCs has shown the enhancement in heart muscle function in cases of heart failure. For example, Bartolucci et al[118] have shown that intravenous administration of UC-MSCs improved LVEF, functional status, and standard of living. Also, exosomes released by UC-MSCs can alleviate the effect of acute myocardial ischemic injury[119]. Scientists confirmed that the injection of UC-MSCs exosomes can greatly improve contractile heart function and minimize myocardial fibrosis. These bioactive bubbles protected heart cells from death and supported EC migration and angiogenesis. UC-MSCs have also been applied in a clinical study for the treatment of chronic coronary occlusion[17]. Also in this trial, the improvement the heart function and better left ventricular ejection fraction were reported[17].
So far, several clinical trials of ischemic stroke have shown that transplanting MSCs into patients with successful reperfusion therapy reduces the volume of lesions after stroke and promotes the regeneration of neurological function. This success is shown by improvements in human functional, behavioral, and sensorimotor assessments, such as the Barthel Index, Modified Rankin Scale, European Stroke Scale, Fugl-Meyer Scale, and National Institutes of Health Stroke Scale[120,121]. MSCs participate in the regeneration of ischemic tissues and organs with beneficial effects, as outlined above (Table 2). The therapeutic activity is presumed to include immunomodulation, cardioprotective effects, activation of endogenous repair processes, and tissue remodeling.
| Disorder | Ref. | Trial number (acronym) | Type of transplant and stem cell source | Results |
| Myocardial infarction | Hare et al[36], 2009 | NCT00114452 | Allogenic BM-MSCs | No tumorogenicity, no arrythomogenicity |
| Myocardial infarction | Houtgraaf et al[37], 2012 | NCT00442806 (APOLLO) | Autologous AT-MSCs | No adverse effects, decreased: Scar tissue; increased: Perfusion |
| Myocardial infarction | Gao et al[122], 2015 | NCT01291329 | Allogenic WJ-MSCs | Increased: Ejection fraction; decreased: Heart perfusion |
| Chronic ischemic cardiomyopathy | Hare et al[123], 2012 | NCT01087996 (POSEIDON) | Allogenic vs autologous BM-MSCs | Increased: Ejection fraction; decreased: Scar tissue |
| Chronic ischemic cardiomyopathy | Karantalis et al[124], 2014 | NCT00587990 (PROMETHEUS) | Autologous BM-MSCs | Local; increased: Contraction; decreased: Scar tissue |
| Chronic ischemic cardiomyopathy | Perin et al[125], 2014 | NCT00426868 (PRECISE) | Autologous AT-MSCs | Increased: Left ventricular mass; increased: Contractility; increased: Perfusion |
| Chronic ischemic cardiomyopathy | Bartunek et al[126], 2013 | NCT00810238 (C-CURE) | Preconditioned autologous BM-MSCs | Increased: Ejection fraction |
| Chronic ischemic cardiomyopathy | Bartolucci et al[118], 2017 | NCT01739777 (RIMECARD) | Allogenic UC-MSCs | Increased: Ejection fraction |
| Ischemic heart failure | Teerlink et al[127], 2017 | NCT01768702 (CHART-1) | Autologous BM-MSCs | Left ventricular reverse remodelling, increased: Left ventricular volume |
| Critical limb ischemia | Wijnand et al[128], 2018 | NCT03042572 (SAIL) | Allogenic BM-MSCs | Safety, decreased: Pain rest |
| Critical limb ischemia | Norgren et al[129], 2018 | NCT01732822 (EUCLID) | Allogeneic placental-derived MSCs | Increased: Amputation-free survival; decreased: Pain rest; increased: Tissue perfusion |
| Critical limb ischemia | Gupta et al[130], 2013 | NCT00883870 | Allogenic BM-MSCs | Decreased: Pain rest; increased: Ankle systolic pressure; increased: Ulcer healing |
| Ischemic stroke | Steinberg et al[131], 2016 | NCT01287936 | Modified BM-MSCs | Increased: Motor functions (ESS, NIHSS, Fugle-Meyer scales) |
| Ischemic stroke | Levy et al[132], 2019 | NCT01297413 | Allogenic BM-MSCs | Increased: Barthel index |
| Ischemic stroke | Savitz et al[133], 2019 | NCT01273337 (RECOVER-Stroke) | Autologous BM-MSCs | Safety |
| Ischemic stroke | Laskowitz et al[134], 2018 | NCT03004976 | Allogenic UCB-MSCs | Safety and feasibility |
| Ischemic stroke | Jaillard et al[135], 2020 | NCT00875654 | Autologous BM-MSCs | Increased: Motor functions (NIHSS, Fugle-Meyer scales) |
MSCs can supply alternative therapy in the treatment of many disorders, but many studies have demonstrated that depending on the method of isolation, expansion, and delivery we can obtain cells with distinct functional features. The therapeutic benefits of MSC-based therapy involve paracrine activity, immunomodulation, and enhanced function of the damaged organ. However, not all patient responses to treatment are satisfactory; therefore this approach requires a deep understanding of the therapeutic actions of MSCs after injection into the recipient. The therapeutic efficacy of MSCs is affected by many factors, including the method of MSCs cultivation in vitro, the metabolic activity of the MSCs, the number of injected cells, the patient’s genetic sensitivity, and the stage of the disease[136,137].
The selection of the appropriate source of therapeutic MSCs depending on the disease is crucial as more and more data show source-dependent variations in therapeutic activity such as levels of released trophic proteins or different differentiation capacities. There is much disagreement as to the therapeutic efficiency of MSCs derived from different tissues (fetal and adult sources). Therefore, extensive studies are desirable to obtain consistent data about remedial effects.
Another problem that is much debated is the type of transplant, i.e., allogeneic or autologous. The results obtained from clinical trials showed no difference between the therapeutic effects of allogeneic and autologous MSCs in the treatment of ischemic cardiomyopathy[138]. The undoubted advantage of autologous transplants is the lack of burdening the cells with other diseases because in such cases the donors are healthy volunteers. Autologous SCs obtained from the patient do not have this privilege, which may limit the therapeutic effectiveness. In addition, it is an important issue to obtain the right dose of autologous cells. The autologous transplant requires the collection of appropriate tissue from the patient, isolation of SCs, and obtaining the necessary dose of therapeutic cells, which is a challenge as the disease and the patient’s age may contribute to the reduction of the proliferative activity of MSCs[139,140].
To resolve these issues, scientists introduced allogeneic sources of SCs that can be used to produce ready-to-use biological products. The use of allogeneic SCs shortens the waiting time for a transplant. Allogeneic cells from young and healthy donors are used to produce biological drugs of quality that can be stored frozen. The medicinal product prepared in this way, if necessary for clinical intervention, can be thawed at any time and administrated to the patient.
MSCs applied in the clinic as therapeutic agents must be carefully prepared, according to the good manufacturing practice and good clinical practice standards, with the established quality control system. The manufacturing process should be properly optimized in terms of therapy requirements to reach a sufficient remedial effect. MSCs applied in the clinic as therapeutic agents must be carefully prepared, according to the good manufacturing practice and good clinical practice standards, with the established quality control system. The manufacturing process should be properly optimized in terms of therapy requirements to reach a sufficient remedial effect. Here it should be emphasized that we can prepare therapeutic cells to treat different disorders, such as graft vs host disease, myocardial infarction, Crohn’s disease, and others, however, the therapeutic benefits obtained may not be satisfactory. Therefore, there is still a need for a more sophisticated approach to obtaining highly effective biological medicines. The use of an appropriate approach to the production of therapeutic MSCs for the treatment of a particular disease should contribute to the achievement of satisfactory results.
Additionally, to adopt therapeutic MSCs for demanding conditions in the host body, preconditioning methods can be applied. This approach requires the presence of additional adverse factors (such as low oxygen availability or proinflammatory cytokines) during in vitro culture. Bernardo and Fibbe[141], during the production of therapeutic MSCs, added proinflammatory cytokines to the culture medium, the concentration of which increased after acute myocardial infarction. In this study, an anti-inflammatory response of MSCs was observed within 24-48 h based on the analysis of the composition of the culture medium. The use of selected cytokines in MSCs cultures for the treatment of acute myocardial infarction enhanced anti-inflammatory secretory activity and therapeutic efficacy.
The remarkable ability of MSCs to regenerate damaged body parts to regain lost function is promising in many disorders, including ischemic diseases. Three properties of MSCs render them optimal for ischemic tissue repair and regeneration: (1) Immunomodulatory and immunoregulatory capacity beneficial to ameliorate abnormal immune responses; (2) Soluble and insoluble paracrine factor-generating potential; and (3) Endothelial differentiation.
While MSCs have several advantages, there are still many challenges to overcome. The unique immunomodulatory properties of MSCs are essential to their function, but the mechanisms of the immune regulation of MSCs have not been elucidated. Many factors can influence the therapeutic potential of MSCs, such as donor age, isolation and culture method, induction factors, oxygen concentrations, mechanical stimuli, and others. Hence, optimizing the culture conditions of MSCs may be an effective way to improve the therapeutic potential of MSCs for successful tissue repair.
| 1. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2622] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 2. | Novo S, Coppola G, Milio G. Critical limb ischemia: definition and natural history. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Mendelson SJ, Prabhakaran S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA. 2021;325:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 524] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 4. | Saad A, Herrmann SM, Textor SC. Chronic renal ischemia in humans: can cell therapy repair the kidney in occlusive renovascular disease? Physiology (Bethesda). 2015;30:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5775] [Article Influence: 962.5] [Reference Citation Analysis (2)] |
| 6. | Hofstra L, Liem IH, Dumont EA, Boersma HH, van Heerde WL, Doevendans PA, De Muinck E, Wellens HJ, Kemerink GJ, Reutelingsperger CP, Heidendal GA. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet. 2000;356:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 289] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Ghafarzadeh M, Namdari M, Eatemadi A. Stem cell therapies for congenital heart disease. Biomed Pharmacother. 2016;84:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A, Mirzaei H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J Cell Biochem. 2018;119:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Callum K, Bradbury A. ABC of arterial and venous disease: Acute limb ischaemia. BMJ. 2000;320:764-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kinlay S. Management of Critical Limb Ischemia. Circ Cardiovasc Interv. 2016;9:e001946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Lyden J, Grant S, Ma T. Altered metabolism for neuroprotection provided by mesenchymal stem cells. Brain Circ. 2019;5:140-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Drozdz T, Debicka-Dabrowska D, Styczkiewicz K, Czarnecka D, Kawecka-Jaszcz K. [New non-pharmacological treatment methods in heart failure]. Przegl Lek. 2014;71:441-446. [PubMed] |
| 13. | Gornicka-Pawlak el B, Janowski M, Habich A, Jablonska A, Drela K, Kozlowska H, Lukomska B, Sypecka J, Domanska-Janik K. Systemic treatment of focal brain injury in the rat by human umbilical cord blood cells being at different level of neural commitment. Acta Neurobiol Exp (Wars). 2011;71:46-64. [PubMed] |
| 14. | Sarnowska A, Jablonska A, Jurga M, Dainiak M, Strojek L, Drela K, Wright K, Tripathi A, Kumar A, Jungvid H, Lukomska B, Forraz N, McGuckin C, Domanska-Janik K. Encapsulation of mesenchymal stem cells by bioscaffolds protects cell survival and attenuates neuroinflammatory reaction in injured brain tissue after transplantation. Cell Transplant. 2013;22 Suppl 1:S67-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yu H, Lu K, Zhu J, Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. 2017;121:135-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1049] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 17. | Li X, Hu YD, Guo Y, Chen Y, Guo DX, Zhou HL, Zhang FL, Zhao QN. Safety and efficacy of intracoronary human umbilical cord-derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr Pharm Des. 2015;21:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz-Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez-Aviles F, Jimenez-Quevedo P, Bayes-Genis A, Hernandez-Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W; CHART Program. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38:648-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 969] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 20. | Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1396] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 22. | Marino L, Castaldi MA, Rosamilio R, Ragni E, Vitolo R, Fulgione C, Castaldi SG, Serio B, Bianco R, Guida M, Selleri C. Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int J Stem Cells. 2019;12:218-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Szydlak R, Majka M, Lekka M, Kot M, Laidler P. AFM-based Analysis of Wharton's Jelly Mesenchymal Stem Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Poredos P, Jezovnik MK. The Role of Inflammatory Biomarkers in the Detection and Therapy of Atherosclerotic Disease. Curr Vasc Pharmacol. 2016;14:534-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 2157] [Article Influence: 308.1] [Reference Citation Analysis (0)] |
| 26. | Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 2013;70:3847-3869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Watanabe N, Ikeda U. Matrix metalloproteinases and atherosclerosis. Curr Atheroscler Rep. 2004;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | von Wnuck Lipinski K, Keul P, Lucke S, Heusch G, Wohlschlaeger J, Baba HA, Levkau B. Degraded collagen induces calpain-mediated apoptosis and destruction of the X-chromosome-linked inhibitor of apoptosis (xIAP) in human vascular smooth muscle cells. Cardiovasc Res. 2006;69:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Li F, Guo X, Chen SY. Function and Therapeutic Potential of Mesenchymal Stem Cells in Atherosclerosis. Front Cardiovasc Med. 2017;4:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 685] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 32. | Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang X, Yang J, Shen Z. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Gupta S, Sharma A, S A, Verma RS. Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery. Stem Cell Rev Rep. 2021;17:1666-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Wu Y, Chen A, Zhao Q. Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cell Physiol Biochem. 2015;35:1219-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Yan B, Abdelli LS, Singla DK. Transplanted induced pluripotent stem cells improve cardiac function and induce neovascularization in the infarcted hearts of db/db mice. Mol Pharm. 2011;8:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 1013] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 37. | Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, Duckers HJ. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 38. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] |
| 39. | Islam A, Bielat KL, Glomski C, Henderson ES. In vitro growth and hemopoietic differentiation of mouse bone marrow-derived adherent stromal cells in long-term culture: formation of spheroidal bodies mimicking hemopoietic anlagen. J Med. 1989;20:193-207. [PubMed] |
| 40. | Kadner A, Hoerstrup SP, Zund G, Eid K, Maurus C, Melnitchouk S, Grunenfelder J, Turina MI. A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur J Cardiothorac Surg. 2002;21:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 42. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2385] [Article Influence: 119.3] [Reference Citation Analysis (1)] |
| 43. | Barberini DJ, Freitas NP, Magnoni MS, Maia L, Listoni AJ, Heckler MC, Sudano MJ, Golim MA, da Cruz Landim-Alvarenga F, Amorim RM. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res Ther. 2014;5:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, Otero-Hernandez J. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc. 2013;45:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Minonzio G, Corazza M, Mariotta L, Gola M, Zanzi M, Gandolfi E, De Fazio D, Soldati G. Frozen adipose-derived mesenchymal stem cells maintain high capability to grow and differentiate. Cryobiology. 2014;69:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Raposio E, Simonacci F, Perrotta RE. Adipose-derived stem cells: Comparison between two methods of isolation for clinical applications. Ann Med Surg (Lond). 2017;20:87-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 48. | Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 49. | Shen X, Pan B, Zhou H, Liu L, Lv T, Zhu J, Huang X, Tian J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J Biomed Sci. 2017;24:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Li H, Yu B, Zhang Y, Pan Z, Xu W, Li H. Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;341:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Song K, Wang Z, Li W, Zhang C, Lim M, Liu T. In vitro culture, determination, and directed differentiation of adult adipose-derived stem cells towards cardiomyocyte-like cells induced by angiotensin II. Appl Biochem Biotechnol. 2013;170:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 53. | Nagata H, Ii M, Kohbayashi E, Hoshiga M, Hanafusa T, Asahi M. Cardiac Adipose-Derived Stem Cells Exhibit High Differentiation Potential to Cardiovascular Cells in C57BL/6 Mice. Stem Cells Transl Med. 2016;5:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 959] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 55. | Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 56. | Chen S, Zhang W, Wang JM, Duan HT, Kong JH, Wang YX, Dong M, Bi X, Song J. Differentiation of isolated human umbilical cord mesenchymal stem cells into neural stem cells. Int J Ophthalmol. 2016;9:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, Aicher WK. Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2016;2016:5646384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 58. | Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919-25; discussion 1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 614] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 59. | Haghani K, Bakhtiyari S, Nouri AM. In vitro study of the differentiation of bone marrow stromal cells into cardiomyocyte-like cells. Mol Cell Biochem. 2012;361:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 61. | Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1555] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 62. | Bagheri-Hosseinabadi Z, Salehinejad P, Mesbah-Namin SA. Differentiation of human adipose-derived stem cells into cardiomyocyte-like cells in fibrin scaffold by a histone deacetylase inhibitor. Biomed Eng Online. 2017;16:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Suss PH, Capriglione LG, Barchiki F, Miyague L, Jackowski D, Fracaro L, Schittini AV, Senegaglia AC, Rebelatto CL, Olandoski M, Correa A, Brofman PR. Direct intracardiac injection of umbilical cord-derived stromal cells and umbilical cord blood-derived endothelial cells for the treatment of ischemic cardiomyopathy. Exp Biol Med (Maywood). 2015;240:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Balbi C, Bollini S. Fetal and perinatal stem cells in cardiac regeneration: Moving forward to the paracrine era. Placenta. 2017;59:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Zhang W, Liu XC, Yang L, Zhu DL, Zhang YD, Chen Y, Zhang HY. Wharton's jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coron Artery Dis. 2013;24:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Pochon C, Notarantonio AB, Laroye C, Reppel L, Bensoussan D, Bertrand A, Rubio MT, D'Aveni M. Wharton's jelly-derived stromal cells and their cell therapy applications in allogeneic haematopoietic stem cell transplantation. J Cell Mol Med. 2022;26:1339-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Taran R, Mamidi MK, Singh G, Dutta S, Parhar IS, John JP, Bhonde R, Pal R, Das AK. In vitro and in vivo neurogenic potential of mesenchymal stem cells isolated from different sources. J Biosci. 2014;39:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25:2797-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 69. | Tatard VM, D'Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, Bouckenooghe T, Menei P, Montero-Menei CN, Schiller PC. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone. 2007;40:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Balasubramanian S, Thej C, Venugopal P, Priya N, Zakaria Z, Sundarraj S, Majumdar AS. Higher propensity of Wharton's jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol Int. 2013;37:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Fu L, Zhu L, Huang Y, Lee TD, Forman SJ, Shih CC. Derivation of neural stem cells from mesenchymal stemcells: evidence for a bipotential stem cell population. Stem Cells Dev. 2008;17:1109-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Yang E, Liu N, Tang Y, Hu Y, Zhang P, Pan C, Dong S, Zhang Y, Tang Z. Generation of neurospheres from human adipose-derived stem cells. Biomed Res Int. 2015;2015:743714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Lo Furno D, Mannino G, Giuffrida R, Gili E, Vancheri C, Tarico MS, Perrotta RE, Pellitteri R. Neural differentiation of human adipose-derived mesenchymal stem cells induced by glial cell conditioned media. J Cell Physiol. 2018;233:7091-7100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Sotthibundhu A, Muangchan P, Phonchai R, Promjantuek W, Chaicharoenaudomrung N, Kunhorm P, Noisa P. Autophagy Promoted Neural Differentiation of Human Placenta-derived Mesenchymal Stem Cells. In Vivo. 2021;35:2609-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 75. | Soundara Rajan T, Gugliandolo A, Bramanti P, Mazzon E. Tunneling Nanotubes-Mediated Protection of Mesenchymal Stem Cells: An Update from Preclinical Studies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 829] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 77. | Sobolewski K, Małkowski A, Bańkowski E, Jaworski S. Wharton's jelly as a reservoir of peptide growth factors. Placenta. 2005;26:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells. 2021;13:619-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Huang Y, Wu Q, Tam PKH. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 140] [Reference Citation Analysis (0)] |
| 80. | Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 873] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 81. | Kim YJ, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev Oncol Hematol. 2011;79:112-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (4)] |
| 83. | Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 872] [Article Influence: 124.6] [Reference Citation Analysis (0)] |
| 84. | Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 826] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 85. | Li M, Sun X, Kuang X, Liao Y, Li H, Luo D. Mesenchymal stem cells suppress CD8+ T cell-mediated activation by suppressing natural killer group 2, member D protein receptor expression and secretion of prostaglandin E2, indoleamine 2, 3-dioxygenase and transforming growth factor-β. Clin Exp Immunol. 2014;178:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Mannino G, Longo A, Gennuso F, Anfuso CD, Lupo G, Giurdanella G, Giuffrida R, Lo Furno D. Effects of High Glucose Concentration on Pericyte-Like Differentiated Human Adipose-Derived Mesenchymal Stem Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, Zhang Q, Yang Y. Umbilical Cord-derived Mesenchymal Stem Cells Instruct Monocytes Towards an IL10-producing Phenotype by Secreting IL6 and HGF. Sci Rep. 2016;6:37566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 88. | Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 89. | Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy. 2017;19:1351-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 90. | Fanchin R, Gallot V, Rouas-Freiss N, Frydman R, Carosella ED. Implication of HLA-G in human embryo implantation. Hum Immunol. 2007;68:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The Importance of HLA Assessment in "Off-the-Shelf" Allogeneic Mesenchymal Stem Cells Based-Therapies. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 92. | Zhang LL, Xiong YY, Yang YJ. The Vital Roles of Mesenchymal Stem Cells and the Derived Extracellular Vesicles in Promoting Angiogenesis After Acute Myocardial Infarction. Stem Cells Dev. 2021;30:561-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 660] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 94. | Tesarova L, Jaresova K, Simara P, Koutna I. Umbilical Cord-Derived Mesenchymal Stem Cells Are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Storms R, Lillich M, Parrott R, Noldner P, Meadows N, Cheatham L, Kurtzberg J. Characterization of msc derived from umbilical cord tissues. Cytotherapy 2017; 19: S193-S194. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Chinnici CM, Iannolo G, Cittadini E, Carreca AP, Nascari D, Timoneri F, Bella MD, Cuscino N, Amico G, Carcione C, Conaldi PG. Extracellular Vesicle-Derived microRNAs of Human Wharton's Jelly Mesenchymal Stromal Cells May Activate Endogenous VEGF-A to Promote Angiogenesis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Sen A, Ta M. Altered Adhesion and Migration of Human Mesenchymal Stromal Cells under Febrile Temperature Stress Involves NF-κβ Pathway. Sci Rep. 2020;10:4473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 99. | Lelek J, Zuba-Surma EK. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 100. | Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 101. | Henning RJ, Burgos JD, Ondrovic L, Sanberg P, Balis J, Morgan MB. Human umbilical cord blood progenitor cells are attracted to infarcted myocardium and significantly reduce myocardial infarction size. Cell Transplant. 2006;15:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Henning RJ, Shariff M, Eadula U, Alvarado F, Vasko M, Sanberg PR, Sanberg CD, Delostia V. Human cord blood mononuclear cells decrease cytokines and inflammatory cells in acute myocardial infarction. Stem Cells Dev. 2008;17:1207-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Martinez EC, Vu DT, Wang J, Lilyanna S, Ling LH, Gan SU, Tan AL, Phan TT, Lee CN, Kofidis T. Grafts enriched with subamnion-cord-lining mesenchymal stem cell angiogenic spheroids induce post-ischemic myocardial revascularization and preserve cardiac function in failing rat hearts. Stem Cells Dev. 2013;22:3087-3099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 105. | Zhang C, Zhou G, Cai C, Li J, Chen F, Xie L, Wang W, Zhang Y, Lai X, Ma L. Human umbilical cord mesenchymal stem cells alleviate acute myocarditis by modulating endoplasmic reticulum stress and extracellular signal regulated 1/2-mediated apoptosis. Mol Med Rep. 2017;15:3515-3520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 107. | Badyra B, Sułkowski M, Milczarek O, Majka M. Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl Med. 2020;9:1174-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 108. | Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights From Randomized Controlled Trials. Circ Res. 2015;117:558-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 109. | Liu B, Duan CY, Luo CF, Ou CW, Sun K, Wu ZY, Huang H, Cheng CF, Li YP, Chen MS. Effectiveness and safety of selected bone marrow stem cells on left ventricular function in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Int J Cardiol. 2014;177:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Brychtova M, Thiele JA, Lysak D, Holubova M, Kralickova M, Vistejnova L. Mesenchymal stem cells as the near future of cardiology medicine - truth or wish? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019;163:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K, Kwan J, Park KS, Choi D, Jang YS, Hong MK. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 112. | Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, Lee MG, Kang WY, Lee KS, Ahn YK, Jeong MH, Kim HS. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther. 2018;32:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 113. | Lalu MM, Mazzarello S, Zlepnig J, Dong YYR, Montroy J, McIntyre L, Devereaux PJ, Stewart DJ, David Mazer C, Barron CC, McIsaac DI, Fergusson DA. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2018;7:857-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 114. | Wang Z, Wang L, Su X, Pu J, Jiang M, He B. Rational transplant timing and dose of mesenchymal stromal cells in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2017;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 115. | Musialek P, Mazurek A, Jarocha D, Tekieli L, Szot W, Kostkiewicz M, Banys RP, Urbanczyk M, Kadzielski A, Trystula M, Kijowski J, Zmudka K, Podolec P, Majka M. Myocardial regeneration strategy using Wharton's jelly mesenchymal stem cells as an off-the-shelf 'unlimited' therapeutic agent: results from the Acute Myocardial Infarction First-in-Man Study. Postepy Kardiol Interwencyjnej. 2015;11:100-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Musialek P, Mazurek A, Kwiecien E, Drabik L, Tekieli L, Szot W, Kostkiewicz M, Jarocha D, Banys RP, Urbanczyk M, Plazak W, Olszowska M, Zmudka K, Podolec P, Majka M. P4027Safety and high-grade myocardial uptake of Whartons Jelly Plurioptent Stem Cells transcoronary transfer in acute myocardial infarction in man. Eur Heart J. 2017;38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 117. | Kwiecien E, Drabik L, Mazurek A, Urbanczyk M, Szot W, Kostkiewicz M, Banys RP, Brzyszczyk-Marzec M, Czyz L, Kozynacka A, Plazak W, Olszowska M, Majka M, Podolec P, Musialek P. P4604Insights into left ventricular remodelling and clinical outcomes after Wharton’s jelly multipotent stem cells transcoronary administration in a pilot cohort of CIRCULATE-AMI Trial (NCT03404063). Eur Heart J. 2019;41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 118. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 119. | Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, Zhu W, Han Z, Yu H. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther. 2020;11:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 120. | Toyoshima A, Yasuhara T, Date I. Mesenchymal Stem Cell Therapy for Ischemic Stroke. Acta Med Okayama. 2017;71:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 121. | He JQ, Sussman ES, Steinberg GK. Revisiting Stem Cell-Based Clinical Trials for Ischemic Stroke. Front Aging Neurosci. 2020;12:575990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, Yan XY, Wang Y, Zhu ZM, Li TC, Wang LH, Chen HY, Chen YD, Huang CL, Qu P, Yao C, Wang B, Chen GH, Wang ZM, Xu ZY, Bai J, Lu D, Shen YH, Guo F, Liu MY, Yang Y, Ding YC, Tian HT, Ding QA, Li LN, Yang XC, Hu X. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 123. | Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 924] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 124. | Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 125. | Perin EC, Sanz-Ruiz R, Sánchez PL, Lasso J, Pérez-Cano R, Alonso-Farto JC, Pérez-David E, Fernández-Santos ME, Serruys PW, Duckers HJ, Kastrup J, Chamuleau S, Zheng Y, Silva GV, Willerson JT, Fernández-Avilés F. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J. 2014;168:88-95.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 126. | Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 127. | Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G; CHART Investigators. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017;19:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 128. | Wijnand JGJ, Teraa M, Gremmels H, van Rhijn-Brouwer FCC, de Borst GJ, Verhaar MC; SAIL Study Group. Rationale and design of the SAIL trial for intramuscular injection of allogeneic mesenchymal stromal cells in no-option critical limb ischemia. J Vasc Surg. 2018;67:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Norgren L, Patel MR, Hiatt WR, Wojdyla DM, Fowkes FGR, Baumgartner I, Mahaffey KW, Berger JS, Jones WS, Katona BG, Held P, Blomster JI, Rockhold FW, Björck M; EUCLID Steering Committee and Investigators. Outcomes of Patients with Critical Limb Ischaemia in the EUCLID Trial. Eur J Vasc Endovasc Surg. 2018;55:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 130. | Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 131. | Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke. 2016;47:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 132. | Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke. 2019;50:2835-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 133. | Savitz SI, Yavagal D, Rappard G, Likosky W, Rutledge N, Graffagnino C, Alderazi Y, Elder JA, Chen PR, Budzik RF Jr, Tarrel R, Huang DY, Hinson JM Jr. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation. 2019;139:192-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 134. | Laskowitz DT, Bennett ER, Durham RJ, Volpi JJ, Wiese JR, Frankel M, Shpall E, Wilson JM, Troy J, Kurtzberg J. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Transl Med. 2018;7:521-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 135. | Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, Vadot W, Marcel S, Lamalle L, Grand S, Detante O; (for the ISIS-HERMES Study Group). Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl Stroke Res. 2020;11:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 136. | Caplan AI. Cell-Based Therapies: The Nonresponder. Stem Cells Transl Med. 2018;7:762-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 137. | Moll G, Ankrum JA, Olson SD, Nolta JA. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Transl Med. 2022;11:2-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 138. | Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, Heldman AW, Hare JM. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Transl Res. 2014;7:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 139. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 140. | Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020;28:3827-3842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 141. | Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1093] [Article Influence: 91.1] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lo Furno D, Italy; Prasetyo EP, Indonesia; Shalaby MN, Egypt S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ