Published online Dec 26, 2023. doi: 10.4252/wjsc.v15.i12.1093
Peer-review started: September 28, 2023
First decision: October 23, 2023
Revised: November 11, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 26, 2023
Processing time: 88 Days and 19.3 Hours
Mesenchymal stem cells (MSCs) are a type of stem cells that possess relevant regenerative abilities and can be used to treat many chronic diseases. Diabetes mellitus (DM) is a frequently diagnosed chronic disease characterized by hyperglycemia which initiates many multisystem complications in the long-run. DM patients can benefit from MSCs transplantation to curb down the pathological consequences associated with hyperglycemia persistence and restore the function of damaged tissues. MSCs therapeutic outcomes are found to last for short period of time and ultimately these regenerative cells are eradicated and died in DM disease model.
To investigate the impact of high glucose or hyperglycemia on the cellular and molecular characteristics of MSCs.
Human adipose tissue-derived MSCs (hAD-MSCs) were seeded in low (5.6 mmol/L of glucose) and high glucose (25 mmol/L of glucose) for 7 d. Cytotoxicity, viability, mitochondrial dynamics, and apoptosis were deplored using specific kits. Western blotting was performed to measure the protein expression of phosphatidylinositol 3-kinase (PI3K), TSC1, and mammalian target of rapamycin (mTOR) in these cells.
hAD-MSCs cultured in high glucose for 7 d demonstrated marked decrease in their viability, as shown by a significant increase in lactate dehydrogenase (P < 0.01) and a significant decrease in Trypan blue (P < 0.05) in these cells compared to low glucose control. Mitochondrial membrane potential, indicated by tetramethylrhodamine ethyl ester (TMRE) fluorescence intensity, and nicotinamide adenine dinucleotide (NAD+)/NADH ratio were significantly dropped (P < 0.05 for TMRE and P < 0.01 for NAD+/NADH) in high glucose exposed hAD-MSCs, indicating disturbed mitochondrial function. PI3K protein expression significantly decreased in high glucose culture MSCs (P < 0.05 compared to low glucose) and it was coupled with significant upregulation in TSC1 (P < 0.05) and downregulation in mTOR protein expression (P < 0.05). Mitochondrial complexes I, IV, and V were downregulated profoundly in high glucose (P < 0.05 compared to low glucose). Apoptosis was induced as a result of mitochondrial impairment and explained the poor survival of MSCs in high glucose.
High glucose impaired the mitochondrial dynamics and regulatory proteins in hAD-MSCs ensuing their poor survival and high apoptosis rate in hyperglycemic microenvironment.
Core Tip: Mesenchymal stem cells (MSCs) have significant regenerative properties that make them a potential treatment for many chronic diseases. Among these, diabetes mellitus (DM) was found to benefit from MSCs transplantation in which they restored the damaged tissues and prevented hyperglycemia-related complications. However, these therapeutic are short-lived hindering the clinical use of MSCs in the treatment of DM. This study aims to elucidate the mechanisms of hyperglycemia-induced effects on MSCs which will help in improving the therapeutic functions of these cells in this stress environment.
- Citation: Abu-El-Rub E, Almahasneh F, Khasawneh RR, Alzu'bi A, Ghorab D, Almazari R, Magableh H, Sanajleh A, Shlool H, Mazari M, Bader NS, Al-Momani J. Human mesenchymal stem cells exhibit altered mitochondrial dynamics and poor survival in high glucose microenvironment. World J Stem Cells 2023; 15(12): 1093-1103
- URL: https://www.wjgnet.com/1948-0210/full/v15/i12/1093.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i12.1093
Mesenchymal stem cells (MSCs) are the most commonly studied forms of stem cells as they possess powerful regenerative and immunomodulatory abilities[1]. MSCs have unique molecular machinery that empowers them to modulate many cellular signaling pathways and restore the functionality of damaged tissues rendering them suitable to treat many acute and chronic diseases[1]. Despite their unprecedented abilities to adapt to many stress conditions, they can still behave unexpectedly under certain stress microenvironments. Many preclinical and clinical studies not only demonstrated the positive outcomes after transplanting MSCs to injured sites[2,3], but also reported the short-lived therapeutic results in hostile microenvironments which need to be figured out[4]. When MSCs are undifferentiated, they rely mainly on glycolysis to support their proliferation, expansion, and immunomodulation[5]. When MSCs are programmed to differentiate, they switch to oxidative phosphorylation (OxPhos)[6], thereby, intact mitochondria are also important to maintain their multi-lineage differentiation capacities[6].
In the last decades, diabetes mellitus (DM) incidence has increased rapidly, and it is deemed among top five chronic diseases[7]. DM is associated with serious multisystem complications that impair the life quality of many patients[8]. DM is characterized mainly by chronic hyperglycemic state that causes many devastating, long-term complications[8]. DM patients can benefit remarkably from MSCs therapy to repair and regenerate damaged tissues, restore their functionality, and reduce the severity of DM-related pathological complications[7]. Several studies reported a significant improvement in glycemic parameters and cardiovascular complications after transplanting MSCs in DM patients[7,9,10]; however, other studies reported that DM can accelerate the decline in MSCs quantity and therapeutic quality, and impair their regenerative capabilities[11]. When MSCs turn into a dysfunctioning cells, they may increase the severity of DM-related complications[11]. Although previous studies had addressed the effects of hyperglycemia on the molecular and cellular characteristics of transplanted MSCs, many aspects still need to be elucidated. Thus, it is important to investigate the effect of high glucose on MSCs biological dynamics, which are essential to understand their fate and performance in diabetic microenvironment, and provide new avenues on possible molecular targets to modulate their survival and therapeutic outcomes in DM patients.
Human adipose tissue-derived MSCs (hAD-MSCs) were commercially purchased from Lonza (Cat# PT5006, Lot# 21TL138912) and expanded using Dulbecco’s Modified Eagle’s Medium Low Glucose (DMEM-Low Glucose, Euroclone) which contained 5.6 mmol/L glucose and were supplemented with 10% fetal bovine serum (FBS, Gibco)[12], 0.1 mg/mL streptomycin and 100 units/mL penicillin G in standard cell culture incubators (5% CO2/95% air; 37 °C). Medium was changed every 72 h, and cells were sub-cultured when confluence exceeded 60%[13]. For high glucose conditions, cells were cultured in Dulbecco’s Modified Eagle’s Medium High Glucose (DMEM-High Glucose, Euroclone) which contained 25 mmol/L glucose[14] and were supplemented with 10% FBS, 0.1 mg/mL streptomycin and 100 units/mL penicillin G for 3, 7, and 14 d in standard cell culture incubators (5% CO2/95% air; 37 °C). High glucose complete medium was changed every 72 h. MSCs that were cultured in low glucose were considered as our study control. MSCs of passage 5 and 6 were used to perform the experiments. AD-MSCs characterization markers can be found in (Table 1).
| Positive markers | Negative markers |
| CD13 | CD14 |
| CD29 | CD31 |
| CD44 | CD45 |
| CD73 | HLA-DR |
| CD90 | |
| CD105 | |
| CD166 |
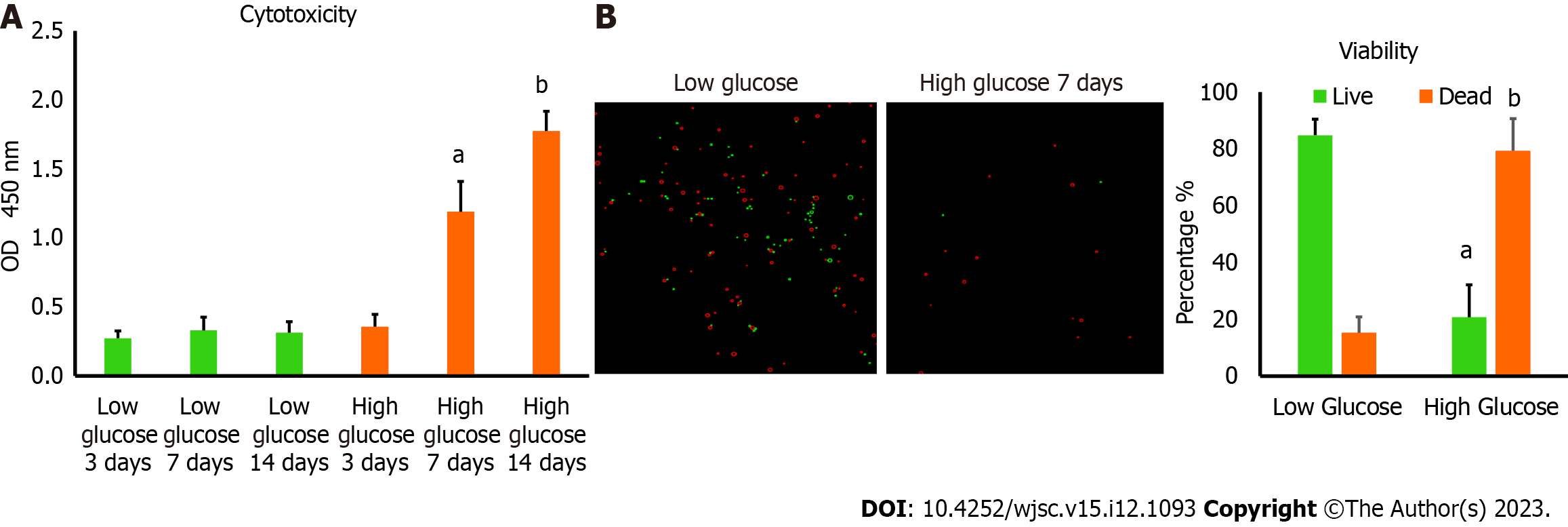
To measure the level of cytotoxicity in human MSCs after being cultured in low and high glucose, we seeded 5 × 104 cells/well in 96 well plate and we measured the lactate dehydrogenase (LDH) which was released from the damaged MSCs (LDH Assay Kit, Abcam, Cat# ab102526) and the absorbance values were obtained using Cytation 5 (BioTek, United States). The viability of MSCs in low and high glucose were also assessed using 0.4% Trypan blue and the percentage of viable cells as well as representative fluorescent images were obtained using Corning® CytoSmart Cell Counter.
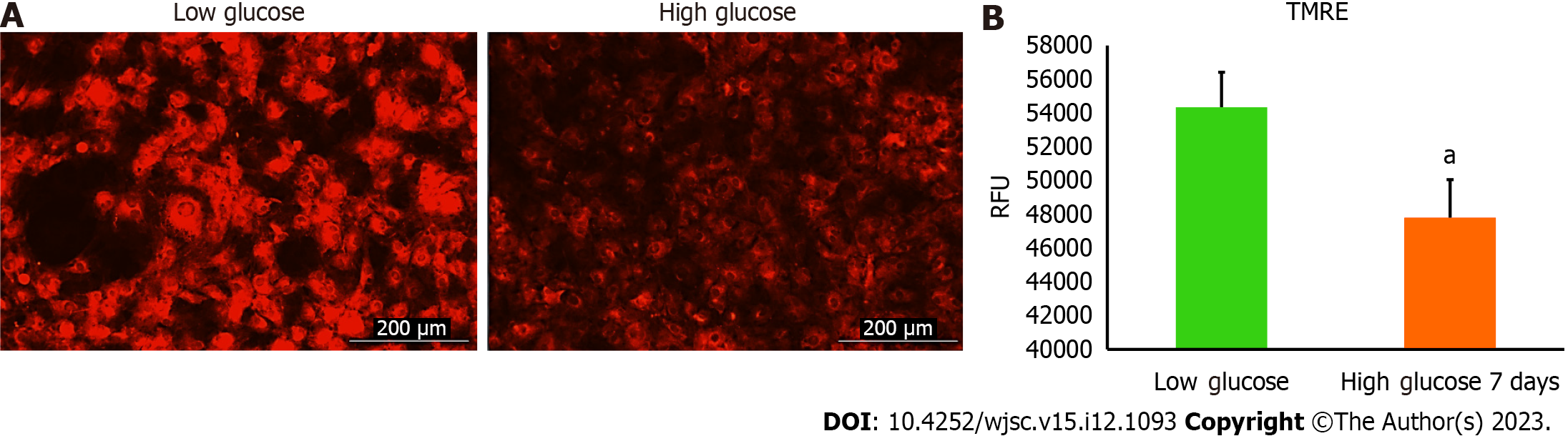
Mitochondrial membrane potential was assessed using tetramethylrhodamine ethyl ester (TMRE)-Mitochondrial Membrane Potential Assay Kit (Abcam, Cat # ab113852). Briefly, human AD-MSCs were placed in 24-well plate at 1 × 104 cells per well and allowed to adhere overnight. After that, cells were cultured either in low glucose or high glucose for 7 d. Then, media were aspirated, and cells were stained using 400 nM TMRE in culture media for 30 min in the incubator, and then media were replaced with 200 μL phosphate buffered saline (PBS) per well. Fluorescence intensity was detected using Cytation 5 (BioTek, United States). (λex = 549 nm, λem = 575 nm), and TMRE fluorescent images were captured at Texas red filter using Cytation 5 (BioTek, United States).
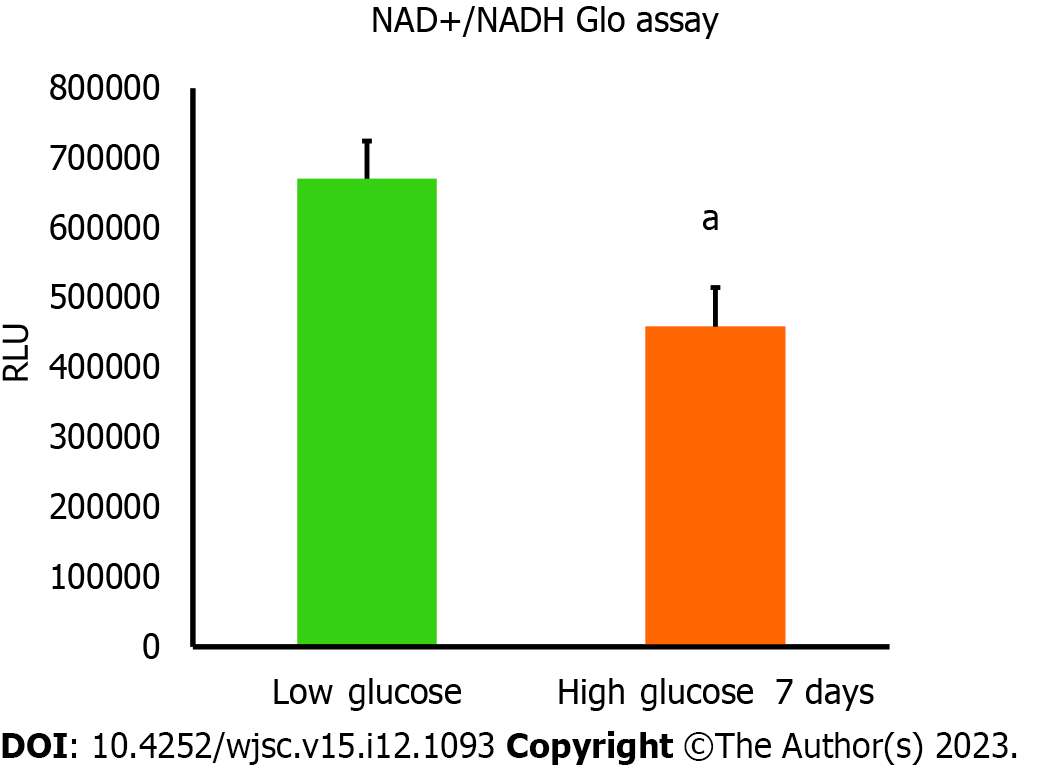
Nicotinamide adenine dinucleotide (NAD+)/NADH ratio in AD-MSCs that were cultured in low and high glucose media was assessed using NAD/NADH-Glo™ assay kit (Promega, Cat# G9071). Briefly, cells were cultured in low and high glucose media for 7 d. After that, cells were detached and placed in 96 well plate at 5 × 105 in 50 μL of either low or high glucose media and were allowed to adhere overnight. 50 μL of NAD/NADH-Glo™ Detection Reagent was added to each well and the plate was gently shaken to mix and lyse the cells and was incubated with the added reagent for 60 min at room temperature. The luminescence values were recorded using Cytation 5 (BioTek, United States) which were corresponding to the total amount of NAD+ produced from NADH.
The protein levels for phosphatidylinositol 3-kinase (PI3K) (Santa Cruz Biotechnology Cat # sc-1637), TSC1 (Santa Cruz Biotechnology Cat # sc-377386), and mammalian target of rapamycin (mTOR) (Santa Cruz Biotechnology Cat # sc-293133), NDUFB8 (Abcam, Cat # ab192878), SDHB (Santa Cruz Biotechnology Cat # sc-271548), UQCRC2 (Santa Cruz Biotechnology Cat # sc-390378), COX5a (Santa Cruz Biotechnology Cat # sc-376907), ATP5A (Santa Cruz Biotechnology Cat # sc-136178), and β-actin (Santa Cruz Biotechnology Cat # sc-47778 HRP) were measured by western blot. Briefly, total protein levels were measured using NanoDrop™ Lite Spectrophotometer, and 40 μg of protein was loaded onto sodium-dodecyl sulfate gel electrophoresis. Following electrophoresis, proteins were transferred to polyvinylidene fluoride membrane and were incubated with appropriate primary and secondary antibodies. The membranes were visualized using VILBER FUSION Gel Documentation System, and bands were quantified using ImageJ for densitometry.
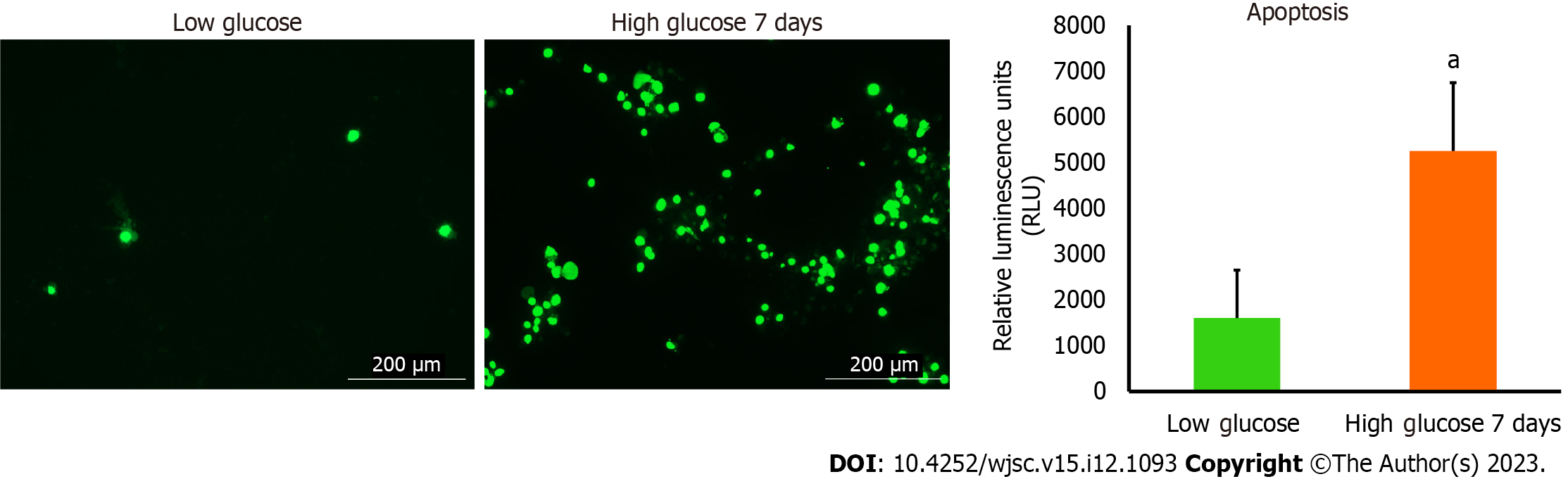
To detect apoptosis in hAD-MSCs after being cultured in low and high glucose, we used RealTime-Glo™ Annexin V Apoptosis live assay (Promega, Cat# JA1011, Lot# 0000400486 following the manufacturer’s guidelines. Briefly, cells were seeded 1 × 104 cells/well in 24 well plate and then cultured in low and high glucose for 7 d. The media were aspirated and each well was washed with PBS followed by the addition of 100 μL of fresh medium having Annexin V-LgBiT, Annexin V-SmBiT, CaCl2, and Annexin V NanoBiT Substrate to each well. After 1 h incubation, the fluorescent images of green color which represented cells undergoing apoptosis were detected at GFP filter using Cytation 5 (BioTek, United States).
Data were reported as mean ± SD. Comparison of data between multiple groups was performed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc multiple comparison test, and analysis between two groups was made using Student’s t-test (two-tailed). Statistical significance is determined as P < 0.05. Each figure represents one of at least three independent quantifiable experiments.
MSCs are known to survive well in low glucose medium which is a physiological requirement to preserve their stemness features. We want to determine the viability of these cells in high glucose microenvironment. hAD-MSCs were cultured in high glucose medium containing 25 mmol/L of glucose for 3, 7, and 14 d. The cytotoxicity was measured by determining the amount of LDH released and was found to be significantly greater (P < 0.01) in hAD-MSCs cultured in high glucose for 7 and 14 d compared with hAD-MSCs cultured in normal low glucose medium containing 5.6 mmol/L of glucose (Figure 1A). To confirm these findings, the viability of hAD-MSCs was measured using 0.4% Trypan blue and found to be remarkably low (P < 0.05) in hAD-MSCs that were cultured in high glucose for 7 d compared to hAD-MSCs cultured in a low glucose medium (Figure 1B).
Mitochondria are integral intracellular organelles promoting cells survival and viability. The variance of mitochondrial dynamics depends on the type of microenvironment where cells exist. Previous reports demonstrated that high glucose can induce the fission and fragmentation of mitochondria in glomeruli podocytes[15], but the knowledge surrounding the impact of high glucose on the mitochondrial dynamics of MSCs is still limited. The mitochondrial membrane potential (ΔΨm) generated by proton pumps (complexes I, III and IV) is imperative to ensure proper ATP production by mitochondrial OxPhos. Abnormalities in the mitochondrial membrane potential (ΔΨm) can significantly elicit a decline in cells viability and trigger unwanted signaling pathways. We measured the mitochondrial membrane potential (ΔΨm) in hAD-MSCs after being grown in a high glucose medium for 7 d using TMRE which is sequestered by active mitochondria. Our results illustrated that the fluorescent intensity of TMRE was significantly low (P < 0.05) in hAD-MSCs cultured in a high glucose medium compared to hAD-MSCs cultured in a normal low glucose medium indicating an impairment in the mitochondrial membrane potential in these cells (Figures 2A and B). NAD+ and its reduced form (NADH) regulate many metabolic pathways, including mitochondrial OxPhos. Maintaining NAD+/NADH pool is required to ensure that the influx of electrons is coupled with the translocation of protons to the intermembrane space to generate the required membrane potential (ΔΨm) across the inner mitochondrial membrane to be harnessed by complex V to produce ATP[16]. To investigate if the decrease in the mitochondrial membrane potential (ΔΨm) is correlated with abnormalities in NAD+/NADH pool, we measure the NAD+/NADH ratio in hAD-MSCs cultured in low and high glucose media (Figure 3). The findings revealed a considerable drop (P < 0.01) in NAD+/NADH ratio when hAD-MSCs were cultured in high glucose. This may explain the disturbance in the inner mitochondrial potential detected by TMRE assay (Figures 2A and B).
Apoptosis is a programmed cell death that can be physiological or pathological depending on the triggering factors that enkindle it. Mitochondrial dysfunction is a notable inciting factor which is able to fire up debilitating cellular apoptosis and impact the survival of numerous cells. The noticeable deterioration in the mitochondrial parameters detected in hAD-MSCs after being cultured in high glucose prompted us to examine the level of apoptosis in these cells. To achieve that, we used RealTime-Glo™ Annexin V Apoptosis kit to unveil the presence of apoptosis or not. The luminescence signal which is proportionally linked to apoptosis intensity was considerably higher in hAD-MSCs that were cultured in high glucose (Figure 4). Fluorescent microscopic images showed that apoptosis (marked by green stained cells) was more in high glucose cultured hAD-MSCs (Figure 4).
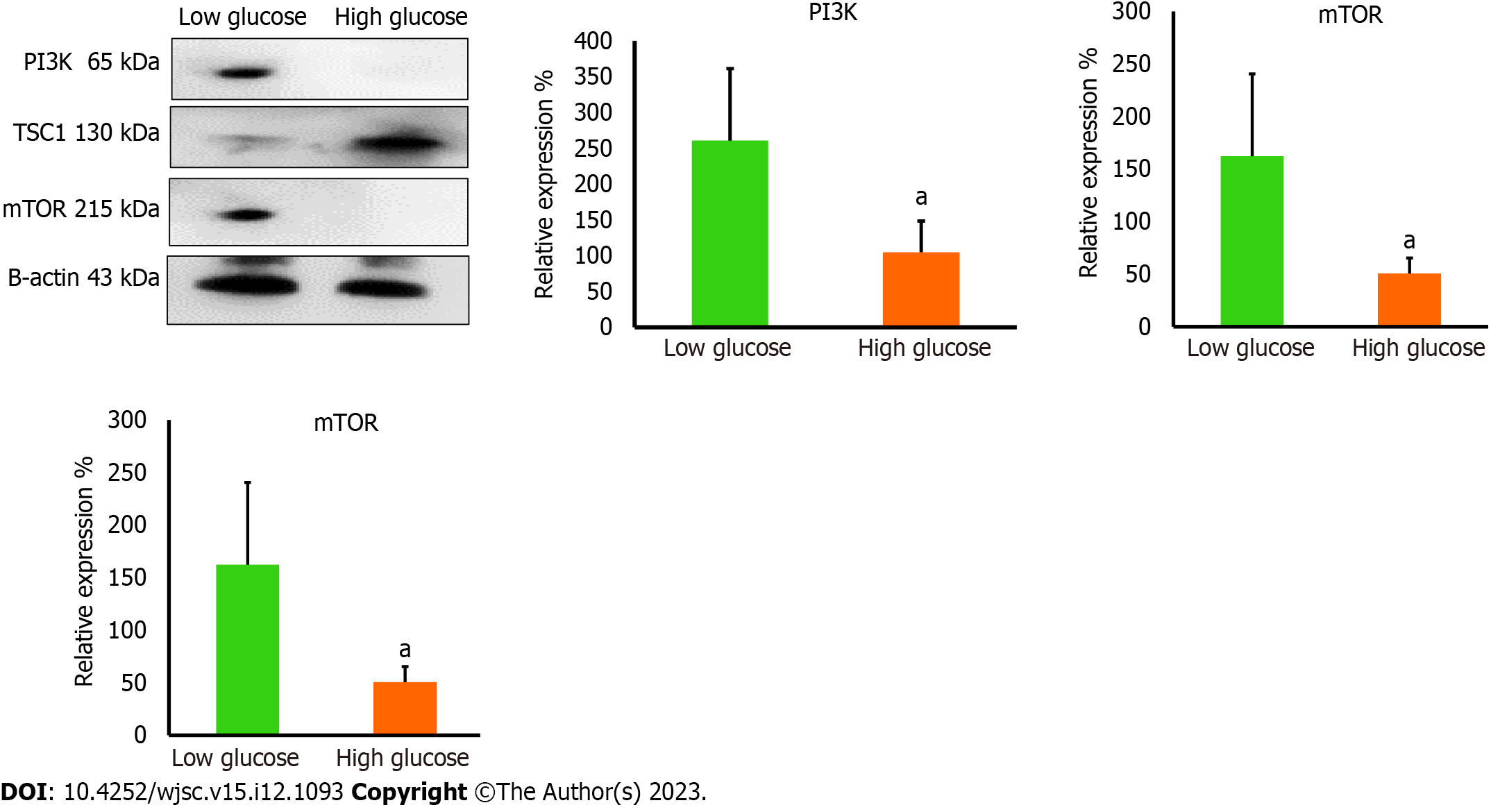
PI3K orchestrates many vital signaling pathways and targets downstream factors that are required to maintain the vivacity of cells. PI3K controls integral proteins that are necessary to preserve mitochondrial dynamics[17,18]. PI3K is a known activator of mTOR which is a central regulator of cell survival and metabolism. It has been reported that mTOR is important to preserve mitochondrial dynamics and generate the required mitochondrial potential to produce ATP. PI3K is required to remove the inhibitory effect of tuberous sclerosis tumor suppressor (TSC1) which binds mTOR and inactivates it[19]. We performed western blotting analysis to measure the expression levels of PI3K, TSC1, and mTOR in high glucose treated hAD-MSCs. Western blotting results demonstrated the loss of PI3K and mTOR in high glucose treated hAD-MSCs (P < 0.05 compared to low glucose), while TSC1 is significantly increased in these cells (P < 0.05 compared to low glucose) (Figure 5). Those findings indicated impaired PI3K/mTOR axis which may explain the disruption in mitochondrial parameters in high glucose cultured MSCs. Taking together, our findings highlight a new mechanism that can be released by high glucose causing the poor survival of MSCs in diabetic microenvironment.
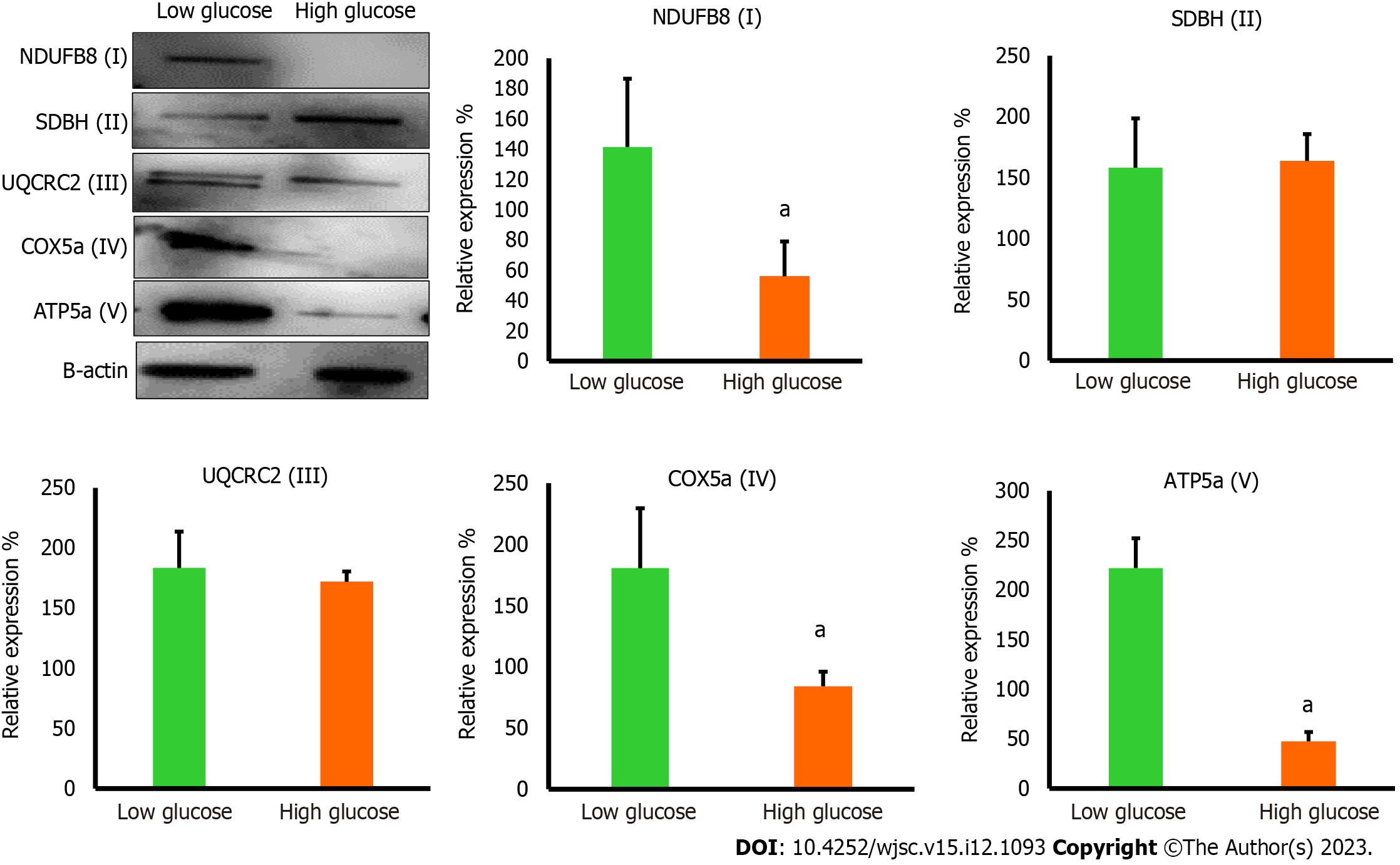
The reduction in mTOR which is essential for inducing the biogenesis of mitochondrial complexes, most importantly complex I and V[20] prompted us to investigate the expression of mitochondrial complexes in low and high glucose-cultured hAD-MSCs. The results demonstrated that hAD-MSCs that were cultured in high glucose exhibited significant downregulation (P < 0.05) in the level of complex I, IV, and V comparing to hAD-MSCs that were cultured in low glucose (Figure 6), while the expression of complex II and III showed no significant changes (P = 0.85) in both culturing conditions (Figure 6). The disturbance in the level of these major complexes can impact the mitochondrial OxPhos and trigger the mitophagy of mitochondria[21].
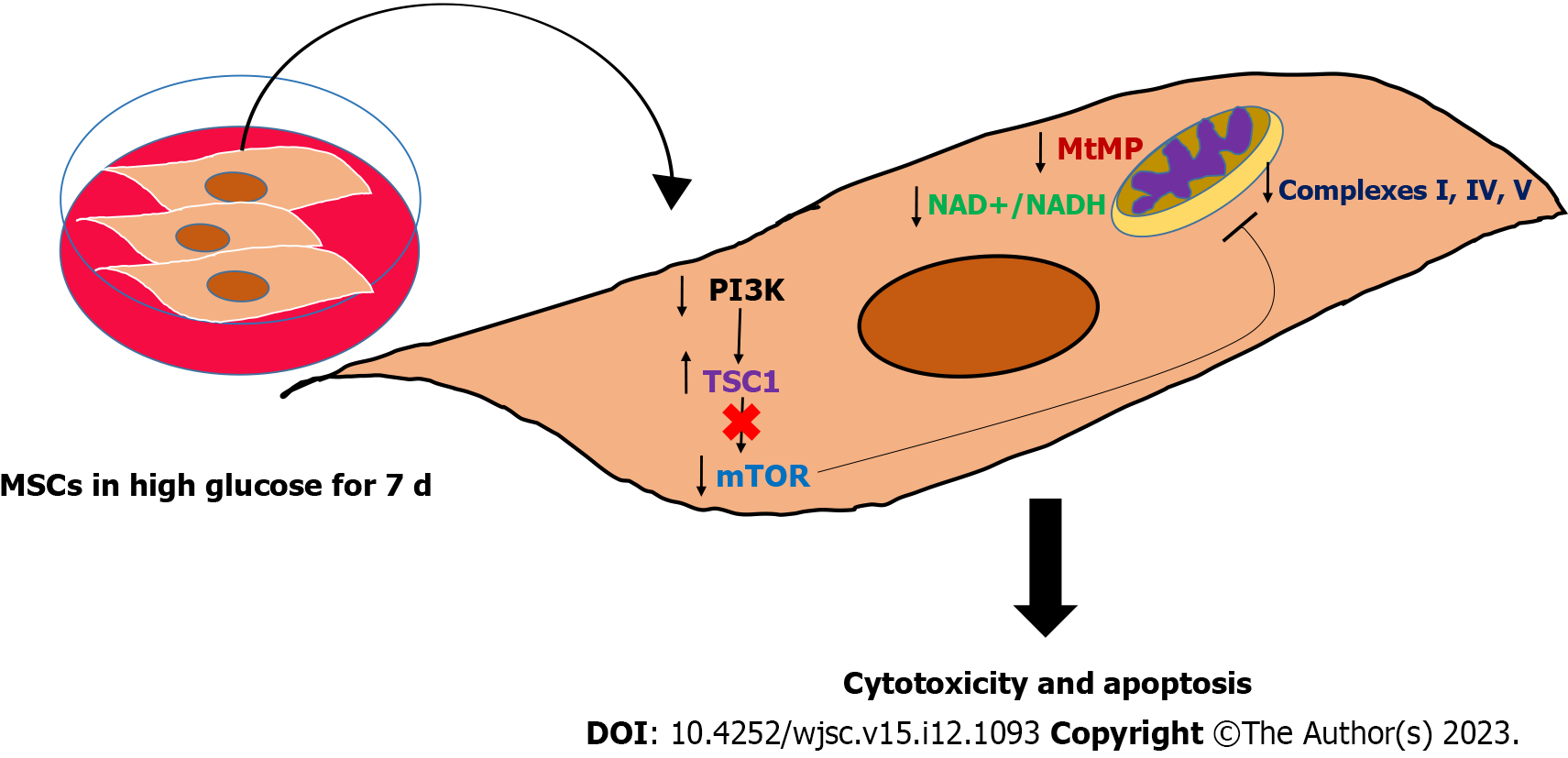
MSCs are the most promising type of stem cells for regenerating damaged tissues and restoring their normal functions which can improve the quality of life of many patients who suffer from debilitating disorders[1]. However, the remarkable improvements in pathological markers reported after transplanting MSCs[22,23] were hindered by the short-lived outcomes and poor survival rate, which need extensive investigation[24,25]. Many pathologies are characterized by stress microenvironments, including hypoxia, inflammation, and nutritional imbalances, which can impact the expected outcomes of transplanting MSCs[26-29], by either inhibiting or activating cellular signaling pathways. DM is a frequent chronic disease that leads to significant multi-system complications[8]. The major hallmark of DM is glucotoxicity, which is featured by the presence of high glucose levels and impaired insulin secretion and function[8]. This glucotoxicity produces massive molecular changes in the residing cells and cause the appearance of DM-related complications, such as cardiovascular and neurological complications[8]. Both animal and clinical studies have demonstrated the great therapeutic efficacy of MSCs transplantation in alleviating chronic hyperglycemia by reversing insulin resistance, improving insulin sensitivity, controlling inflammation, relieving metabolic syndrome symptoms, and ameliorating β-cell destructive abilities[10,30]. Nevertheless, studies also revealed that MSCs exhibited short-lived outcomes due to their poor survival after being transplanted in DM disease model[4]. Several studies have asserted the importance of investigating the mechanisms leading to the poor survival of MSCs in hyperglycemia which is the major insult to DM patients[24,31]. Up to date little is known about the molecular and biological changes in MSCs under hyperglycemia. MSCs are capable of switching their metabolism on to support their regenerative and immunomodulation abilities[32]. MSCs rely primarily on glycolysis when they are undifferentiated as it is important to preserve their stemness and proliferation[33]. Several reports illustrated that glycolysis is required to preserve the immunosuppressive characteristics of MSCs[34]. Upregulation in glycolysis has been found to increase the immunomodulatory abilities of MSCs and promote their capabilities to reprogram immune cells[35]. MSCs use mitochondrial OxPhos when they are induced to differentiate, thus the multi-lineage differentiation capacities of MSCs require intact mitochondria[6]. Maintaining intact mitochondrial dynamics is not only required to support the differentiation capacities of MSCs, but it is also crucial for redox regulation and prevention of excessive production of reactive oxygen species[36]. Recently, it has been proposed by many studies that MSCs are capable of transferring mitochondrial DNA to recipient cells and restoring the normal mitochondrial functions in these cells[37,38]. MSCs-mediated mitochondrial transfer further supports the necessity to conserve normal mitochondrial parameters[38]. Previous reports revealed that the MSCs proliferation decreased in high glucose medium[13,14]. Furthermore, other studies showed that MSCs differentiation capacities might vary when they are cultured in high glucose conditions[39,40]. Our study illustrated that high glucose can reduce the survival rate of MSCs. The reduction in MSCs viability reported in our study is correlated with an impairment in the inner mitochondrial membrane potential (ΔΨm) which is an important parameter and the energy generated from this potential gradient will be utilized by complex V to produce ATP molecules. The maintenance of mitochondrial membrane potential depends on the presence of a plentiful amount of NAD+ molecules[41]. NAD+ serves as an oxidoreductase cofactor that controls many vital energy production pathways. Maintaining normal NAD+/NADH ratio is integral to providing the required electron flux that will be used to generate proper mitochondrial membrane potential (ΔΨm)[41]. High glucose insult causing a substantial drop in NAD+/NADH ratio in hAD-MSCs indicates a needed disturbance in the oxidization power to produce NAD+ and donating electrons that will keep the functionality of mitochondrial complexes. Another important complex that is required to preserve the mitochondrial dynamics is mTOR complex[42]. mTOR is a known regulator of many cellular processes, including the biogenesis of mitochondrial complexes, most importantly complex I and V and physiological mitophagy[42]. The downregulation in the level of mTOR in MSCs cultured in high glucose was associated with significant disturbances in mitochondrial complexes I, IV, and V which can induce mitochondrial dysfunction, mitophagy and massive oxidative stress. The effect of high glucose on modulating mitochondrial functions by targeting mTOR is barely investigated and with controversial results[43]. While other studies found that hyperglycemia activate mTOR pathway in cardiomyocytes[44,45], other evidence showed that insulin can activate mTOR pathway in DM patients and protect cells from developing mitochondrial dysfunction[46], indicating that high glucose can deactivate mTOR and cause pathological consequences[46]. These discrepancies regarding the effect of high glucose on mTOR level and activity seem to differ depending on the type of cells and the upstream molecular regulators. Based on that, the role of high glucose in modulating the level of mTOR needs further investigation in different cell types. In our study, the upstream regulatory protein PI3K decreased remarkably in hAD-MSCs exposed to high glucose. A plentiful number of reports corroborated that PI3K-AKT pathway is required to activate mTOR by removing the inhibitory effect produced by TSC1 or Hamartin protein. We reported in this study that the reduction in PI3K protein level was coupled with marked elevation in TSC1 and downregulation in mTOR in hAD-MSCs cultured in high glucose. Our findings provide a mechanistic explanation of poor survival of MSCs in hyperglycemic microenvironment and suggests new targets that can be modified to enhance the survival rate of MSCs in DM patients. Moreover, our results highlighted the importance of maintaining the desirable pre-transplantation culturing conditions that preserves the salutary properties of MSCs by controlling the level of glucose in the culture media. Schematic summary of the study findings was illustrated in (Figure 7) which highlighted that high glucose microenvironment reduced the level of PI3K and removed its inhibitory effect on TSC1 and caused its upregulation. The increase in the level of TSC1 caused a reduction in mTOR which disturbed many mitochondrial dynamics, mainly MtMB, NAD+/NADH ratio, and mitochondrial complexes that triggered the apoptosis of hAD-MSCs.
MSCs have the potential to regenerate damaged tissues and restore their functions. DM patients can hugely benefit from MSCs transplantation to abrogate the pathological consequences and improve their quality of life. The poor survival and short-lived positive outcomes following MSCs transplantation in DM patients are considered stumbling blocks. High glucose impaired mitochondrial function in MSCs by perturbing mitochondrial regulatory factors, particularly mitochondrial membrane potential, NAD+/NADH pool, and mTOR protein. This mitochondrial dysfunction is associated with triggering apoptosis in MSCs. Preserving these factors may help in improving the survival rate of MSCs in diabetic microenvironment and initiate long-lasting therapeutic outcomes. Future studies should focus on providing new strategies to overcome the poor survival of MSCs in high glucose using genetic modification or biomaterials and nanoparticles. Moreover, the impact of other stressors existed in DM patients other than hyperglycemia should be studied.
Mesenchymal stem cells (MSCs) have the ability to cure many chronic diseases, including diabetes mellitus (DM) and its related multisystem complications.
The therapeutic outcomes of MSCs transplantation in DM are short-lived which require thorough investigation.
This study aimed to determine the effects of hyperglycemia microenvironment on various mitochondrial-related parameters in MSCs to better understand their fate in DM patients.
Adipose tissue-derived MSCs were exposed to low and high glucose media and mitochondrial dynamics and regulators were measured and analyzed.
High glucose induces the apoptosis of adipose tissue-derived MSCs by disturbing many mitochondrial parameters, including mitochondrial membrane potential, nicotinamide adenine dinucleotide (NAD+)/NADH pool, mammalian target of rapamycin, and mitochondrial complexes I, IV, and V.
Hyperglycemia decreases the survival of MSCs by triggering mitochondrial dysfunction in these cells causing their short-lived therapeutic outcomes.
New strategies to improve the survival rate of MSCs in hyperglycemia should be the focus of future studies which can help in increasing the chances of using these cells clinically for the treatment of DM.
This work was conducted in the Applied and Basic Medical Research Lab in the Faculty of Medicine at Yarmouk University. We would like to thank Mr. Muhammad Abu-El-Rub for his efforts in proof-editing and correcting the manuscript.
| 1. | Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, Marofi F, Shamlou S, Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 3. | Mukai T, Tojo A, Nagamura-Inoue T. Mesenchymal stromal cells as a potential therapeutic for neurological disorders. Regen Ther. 2018;9:32-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 5. | Rigaud VOC, Hoy R, Mohsin S, Khan M. Stem Cell Metabolism: Powering Cell-Based Therapeutics. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev. 2017;31:336-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 7. | Arroyave F, Montaño D, Lizcano F. Diabetes Mellitus Is a Chronic Disease that Can Benefit from Therapy with Induced Pluripotent Stem Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1648] [Article Influence: 274.7] [Reference Citation Analysis (0)] |
| 9. | El-Badri N, Ghoneim MA. Mesenchymal stem cell therapy in diabetes mellitus: progress and challenges. J Nucleic Acids. 2013;2013:194858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, Yang X, Liu Y, Shu L, Wang J, He Z. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther. 2021;12:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 11. | Xu J, Zuo C. The Fate Status of Stem Cells in Diabetes and its Role in the Occurrence of Diabetic Complications. Front Mol Biosci. 2021;8:745035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Khasawneh RR, Al Sharie AH, Abu-El Rub E, Serhan AO, Obeidat HN. Addressing the impact of different fetal bovine serum percentages on mesenchymal stem cells biological performance. Mol Biol Rep. 2019;46:4437-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Al-Qarakhli AMA, Yusop N, Waddington RJ, Moseley R. Effects of high glucose conditions on the expansion and differentiation capabilities of mesenchymal stromal cells derived from rat endosteal niche. BMC Mol Cell Biol. 2019;20:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One. 2015;10:e0126537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Ma Y, Chen Z, Tao Y, Zhu J, Yang H, Liang W, Ding G. Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr Connect. 2019;8:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 414] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 17. | Trotta AP, Chipuk JE. Mitochondrial dynamics as regulators of cancer biology. Cell Mol Life Sci. 2017;74:1999-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Iershov A, Nemazanyy I, Alkhoury C, Girard M, Barth E, Cagnard N, Montagner A, Chretien D, Rugarli EI, Guillou H, Pende M, Panasyuk G. The class 3 PI3K coordinates autophagy and mitochondrial lipid catabolism by controlling nuclear receptor PPARα. Nat Commun. 2019;10:1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 407] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, Topisirovic I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 21. | Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 530] [Reference Citation Analysis (0)] |
| 22. | Ocansey DKW, Pei B, Yan Y, Qian H, Zhang X, Xu W, Mao F. Improved therapeutics of modified mesenchymal stem cells: an update. J Transl Med. 2020;18:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Tan L, Liu X, Dou H, Hou Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment - specific factors involved in the regulation of MSC plasticity. Genes Dis. 2022;9:296-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M, Logeart-Avramoglou D, Petite H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15:1505-1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 26. | Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 27. | Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 477] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 28. | Planat-Benard V, Varin A, Casteilla L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front Immunol. 2021;12:626755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 29. | Kornicka K, Houston J, Marycz K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev Rep. 2018;14:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 30. | Izadi M, Sadr Hashemi Nejad A, Moazenchi M, Masoumi S, Rabbani A, Kompani F, Hedayati Asl AA, Abbasi Kakroodi F, Jaroughi N, Mohseni Meybodi MA, Setoodeh A, Abbasi F, Hosseini SE, Moeini Nia F, Salman Yazdi R, Navabi R, Hajizadeh-Saffar E, Baharvand H. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022;13:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Saki N, Jalalifar MA, Soleimani M, Hajizamani S, Rahim F. Adverse effect of high glucose concentration on stem cell therapy. Int J Hematol Oncol Stem Cell Res. 2013;7:34-40. [PubMed] |
| 32. | Dabrowska S, Del Fattore A, Karnas E, Frontczak-Baniewicz M, Kozlowska H, Muraca M, Janowski M, Lukomska B. Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int J Nanomedicine. 2018;13:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 812] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 34. | Burnham AJ, Foppiani EM, Horwitz EM. Key Metabolic Pathways in MSC-Mediated Immunomodulation: Implications for the Prophylaxis and Treatment of Graft Versus Host Disease. Front Immunol. 2020;11:609277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Zegallai HM, Abu-El-Rub E, Cole LK, Field J, Mejia EM, Gordon JW, Marshall AJ, Hatch GM. Tafazzin deficiency impairs mitochondrial metabolism and function of lipopolysaccharide activated B lymphocytes in mice. FASEB J. 2021;35:e22023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Tan YL, Eng SP, Hafez P, Abdul Karim N, Law JX, Ng MH. Mesenchymal Stromal Cell Mitochondrial Transfer as a Cell Rescue Strategy in Regenerative Medicine: A Review of Evidence in Preclinical Models. Stem Cells Transl Med. 2022;11:814-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 37. | Mohammadalipour A, Dumbali SP, Wenzel PL. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front Cell Dev Biol. 2020;8:603292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 38. | Zhang L, Liu Q, Hu H, Zhao L, Zhu K. Progress in mesenchymal stem cell mitochondria transfer for the repair of tissue injury and treatment of disease. Biomed Pharmacother. 2022;153:113482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 39. | Dhanasekaran M, Negi S, Sugiura Y. Designer zinc finger proteins: tools for creating artificial DNA-binding functional proteins. Acc Chem Res. 2006;39:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, Schneider D, Limbert C, Seufert J, Kassem M, Schütze N, Jakob F, Ebert R. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun. 2007;363:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB. Mitochondrial membrane potential. Anal Biochem. 2018;552:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1476] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 42. | de la Cruz López KG, Toledo Guzmán ME, Sánchez EO, García Carrancá A. mTORC1 as a Regulator of Mitochondrial Functions and a Therapeutic Target in Cancer. Front Oncol. 2019;9:1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 43. | Mao Z, Zhang W. Role of mTOR in Glucose and Lipid Metabolism. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 44. | Pandey S, Madreiter-Sokolowski CT, Mangmool S, Parichatikanond W. High Glucose-Induced Cardiomyocyte Damage Involves Interplay between Endothelin ET-1/ET(A)/ET(B) Receptor and mTOR Pathway. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc. 2013;2:e004796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Galizzi G, Di Carlo M. Insulin and Its Key Role for Mitochondrial Function/Dysfunction and Quality Control: A Shared Link between Dysmetabolism and Neurodegeneration. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Jordan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo ZW, China; Shen Y, China; Tanabe S, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S