Published online Oct 26, 2023. doi: 10.4252/wjsc.v15.i10.960
Peer-review started: July 30, 2023
First decision: September 27, 2023
Revised: October 7, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: October 26, 2023
Processing time: 87 Days and 11.4 Hours
Peripheral nerve injury (PNI) seriously affects people’s quality of life. Stem cell therapy is considered a promising new option for the clinical treatment of PNI. Dental stem cells, particularly dental pulp stem cells (DPSCs), are adult pluripotent stem cells derived from the neuroectoderm. DPSCs have significant potential in the field of neural tissue engineering due to their numerous advantages, such as easy isolation, multidifferentiation potential, low immunogenicity, and low transplant rejection rate. DPSCs are extensively used in tissue engineering and regenerative medicine, including for the treatment of sciatic nerve injury, facial nerve injury, spinal cord injury, and other neurodegenerative diseases. This article reviews research related to DPSCs and their advantages in treating PNI, aiming to summarize the therapeutic potential of DPSCs for PNI and the underlying mechanisms and providing valuable guidance and a foundation for future research.
Core Tip: This article reviews the potential applications of dental pulp stem cells (DPSCs) and their derivatives in the field of nerve regeneration. First, this paper describes the current status of stem cell therapies for peripheral nerve injury (PNI) and discusses the advantages of DPSCs in this field. Then, the status of research on the neuroregenerative ability of DPSCs and their derivatives is reviewed. Finally, the potential of DPSCs in treating PNI and the underlying mechanism are summarized, with an aim to provide valuable guidance and a basis for future research.
- Citation: Xing WB, Wu ST, Wang XX, Li FY, Wang RX, He JH, Fu J, He Y. Potential of dental pulp stem cells and their products in promoting peripheral nerve regeneration and their future applications. World J Stem Cells 2023; 15(10): 960-978
- URL: https://www.wjgnet.com/1948-0210/full/v15/i10/960.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i10.960
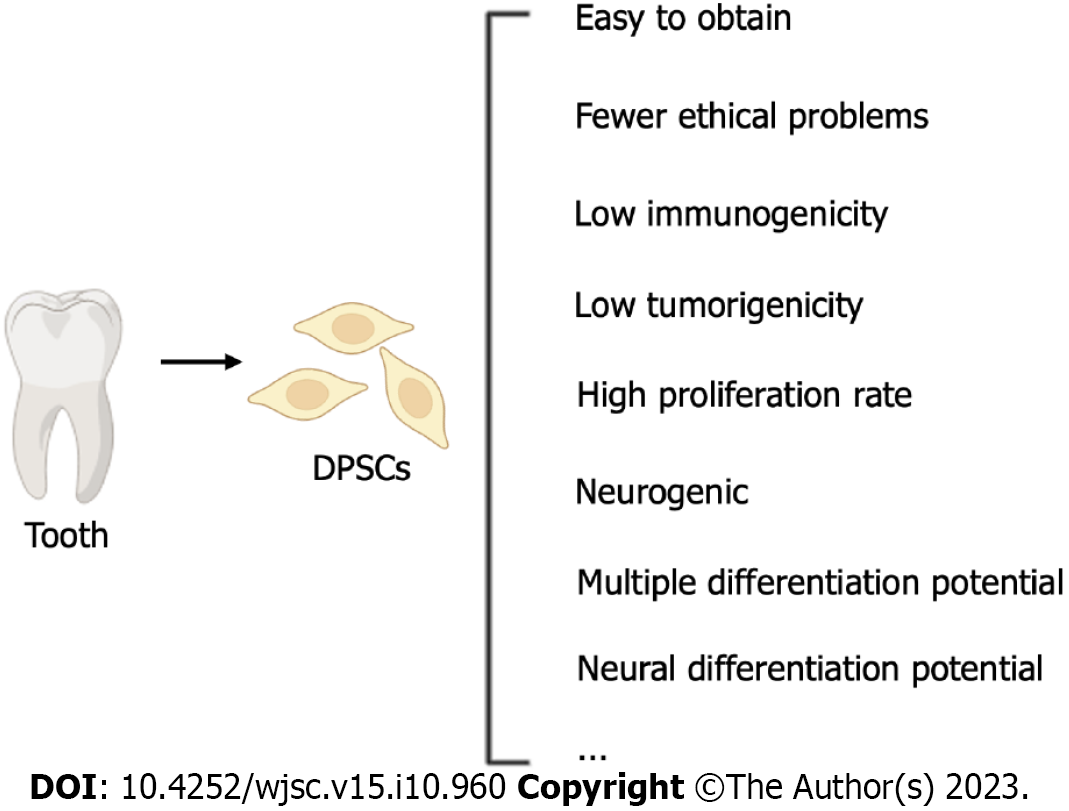
It has been reported that approximately 2.8% of trauma patients suffer from peripheral nerve injury (PNI), resulting in permanent disabilities[1]. PNI can lead to muscle function loss, sensory impairment, and painful neuropathy[2]. While autologous nerve transplantation is a standard treatment option, its drawbacks, such as the difficulty in obtaining donor nerves, the need for surgical procedures to acquire donor nerves, secondary deformities at the donor site, and potential mismatch issues, limit its widespread use[3,4]. With advancements in biomaterials and tissue regeneration, significant progress has been made in nerve regrowth techniques[5]. The restoration of function after injury is of paramount importance, and experiments on stem cells have shown that they can accelerate nerve regeneration[6]. Compared to other types of stem cells, dental pulp stem cells (DPSCs) are more advantageous because they can be obtained via noninvasive operation, can be preserved at low temperature long-term, are simple to use, are associated with few ethical problems, and have low immunogenicity, so they are ideal materials for tissue engineering[7]. Human DPSCs (hDPSCs) exhibit remarkable self-renewal, multilineage differentiation, and cloning capabilities[8]. Schwann cells play a critical role in nerve regeneration, and stem cells with the same embryonic origin as Schwann cells are suitable tools for PNI treatment. DPSCs originate from neural crest cells[9] and share homology with Schwann cells[10]. Therefore, due to DPSCs’ unique neural differentiation and nerve regeneration abilities, DPSCs-derived Schwann cells are viable tools for achieving nerve regeneration after PNI in vitro[11]. To keep abreast of the latest developments in this field, articles published in PubMed between 2010 and 2023 were screened using the following search terms: “peripheral nerve”, “tooth-derived stem cells”, “exosomes”, and “dental stem cells”. The primary aim of this review is to provide information on the recent applications of DPSCs in PNI treatment, with a specific focus on both cellular therapies and noncellular therapies involving DPSCs. Additionally, the review discusses the therapeutic effects of DPSCs and their potential future applications in treating PNI.
PNI is a prevalent clinical condition often resulting in long-term functional impairments. The effectiveness of surgical treatments is frequently unsatisfactory[12], and the restoration of nerve function is often not optimal[13]. Peripheral nerve regeneration is a complex process involving Wallerian degeneration, axon sprouting, and myelin regeneration[14]. While nerves may regenerate over relatively short distances after mild nerve injury, the outcomes of nerve regeneration are often unsatisfactory. In cases of peripheral nerve amputation, a series of molecular and cellular changes occur, known as Waller’s degeneration[15,16]. Subsequently, monocytes and macrophages migrate to the nerve stump to clear axon fragments and myelin sheaths at the damaged end, while the proliferation of Schwann cells results in the formation of longitudinal cell columns, known as Bungner bands[17,18]. According to the literature, various factors, such as scaffolds for axonal migration, supporting cells (including Schwann cells and macrophages), growth factors, and the extracellular matrix, play crucial roles in regeneration after PNI[2].
Schwann cells originate from neural crest cells[19] and play a crucial role in the regeneration of peripheral nerves, influenced by neurotrophic factors (NTFs) such as Krox-20, Oct-6, and Sox-10[18]. These cells play an instrumental role in nerve regeneration by selectively promoting axonal regrowth of both motor and sensory nerves[20-22]. After nerve injury, Schwann cells may undergo dedifferentiation, a process mainly regulated by the negative regulatory factor c-Jun. This dedifferentiation is vital because it can help nerve survival and facilitate axonal regeneration[23]. Researchers have explored the effect of transplanting Schwann cells isolated from peripheral nerves into rat sciatic nerve injury models and found that these cells promote the regeneration of nerve axons, proving the potential of autologous stem cell transplantation in treating PNI[24]. However, such regenerative strategies are associated with challenges, as collecting Schwann cells is difficult and their survival rate after transplantation is low[25]. In recent studies, DPSCs-derived conditioned medium (DPSCs-CM) was shown to promote the proliferation of Schwann cells and increase the production of myelin-associated proteins[26]. This may provide insight for the development of a new method for the treatment of peripheral nerve diseases.
Earlier studies have revealed that allogeneic stem cell transplantation has a favorable effect on nerve regeneration and thus warrants further investigation[27]. Acellular nerve allografts (ANAs) promote axon regeneration through Schwann cell proliferation[28]. However, the therapeutic efficacy of ANAs diminishes over time after nerve injury, likely due to the limitations of Schwann cell function in the host nerve. Therefore, there is an urgent need to explore alternative or more effective therapies for Schwann cell replacement.
An increasing number of studies have demonstrated the remarkable potential of stem cells in promoting neural regeneration. Adipose-derived stem cells have been shown to differentiate into Schwann-like cells and effectively promote nerve regeneration[29-31]. Additionally, Shimizu et al[32] reported that human bone marrow mesenchymal stem cells (MSCs) can serve as Schwann cell substitutes, making them a viable option for nerve regeneration applications. Furthermore, muscle-derived stem/progenitor cells have shown the ability to differentiate into myelinated Schwann cells in vivo, thereby promoting axonal regeneration[33]. Al-Zer and Kalbouneh[11] successfully induced the differentiation of DPSCs into Schwann cells by utilizing retinoic acid, mercaptoethanol, and neuromodulin β1. Among stem cell sources, dental stem cells have garnered significant attention due to their excellent nerve regeneration capabilities and ease of availability.
Recent studies have revealed that during tooth development, glial cells related to the peripheral nervous system produce a significant number of MSCs, including dental pulp cells and odontoblasts[34]. DPSCs, stem cells from human exfoliated deciduous teeth (SHEDs), periodontal ligament stem cells (PDLSCs), stem cells from the apical papilla, and dental follicle progenitor cells all fall under the category of dental stem cells[35]. These dental tissue-derived MSCs exhibit the ability of multidirectional differentiation and hold great potential in immunomodulation and tissue regeneration[36]. Moreover, MSCs derived from hair follicles, the dental pulp, and nipples show similar biological characteristics as those from the teeth of the same donor and display osteogenic, adipogenic, and chondrogenic differentiation capabilities[37].
Apical pulp-derived cells and coronal pulp cells have demonstrated the ability to differentiate into nerve cells in vitro and express markers associated with neural crest cells (p75, Snail, and Slug) as well as neural stem cell markers (Nestin and Musashi1)[38]. SHEDs can differentiate into many types of cells, including nerve cells[39]. Pereira et al[40] utilized polyglycolic acid tubes to induce SHEDs to differentiate into Schwann cells. Human PDLSCs have exhibited the ability to promote axonal regeneration after optic nerve injury, potentially through the secretion of brain-derived neurotrophic factor (BDNF)[41]. The ERK1/2 signaling pathway plays a role in the differentiation of PDLSCs into Schwann cells[42], thus making them a viable alternative source for autologous Schwann cells[43]. Ng et al[44] successfully induced adult PDLSCs to differentiate into retina-like cells with the biological characteristics of nerve cells. Dental embryonic stem cells have the potential to achieve nerve tissue regeneration due to their common origin with the nervous system[5,14], making them a promising source of stem cells for nerve tissue regeneration[45]. The neurological potential of DPSCs makes them a viable candidate cell type for the treatment of peripheral nerve diseases[46]. DPSCs can differentiate into neuron-like cells, and after 5 d of neuron differentiation, Tub3 is activated, accompanied by increased Nestin expression[47]. Insulin-like growth factor binding protein 5 promotes the formation of neurospheres by DPSCs. Angiogenic markers such as vascular endothelial-derived growth factor (VEGF), platelet derived growth factor subunit A, and angiopoietin-1 and neurogenic markers such as neural cell adhesion molecule, Nestin, βIII-tubulin, and tyrosine hydroxylase are upregulated in DPSCs, reflecting the vascular and neurogenic differentiation potential of DPSCs[48]. Additionally, DPSCs can secrete NTFs that support nerve cell function[49].
Compared with other stem cells, DPSCs have stronger multidifferentiation potential, self-renewal ability, and colony formation ability[8]. The Eph/ephrin interaction indicates that unlike that of other stem cells, the formation of DPSCs involves the neural crest[50,51]. Because of their spinal origin, DPSCs have the ability to differentiate into other spinal cord-related cells[52]. Unlike bone marrow MSCs (BM-MSCs), DPSCs are heterogeneous, and they also express markers of endothelial cells (vascular cell adhesion molecule 1 and MUC-18), smooth muscle (a-smooth muscle actin), bone (alkaline phosphatase, type I collagen, osteonectin, osteopontin, and osteocalcin), and fibroblasts (type III collagen and fibroblast growth factor 2)[39,53]. DPSCs express the neural precursor and glial cell markers nestin and glial fibrillary acidic protein (GFAP), which indicates that DPSCs are similar to BM-MSCs and have the potential to differentiate into neural cells[8]. Compared with BM-MSCs, DPSCs can more strongly inhibit the proliferation of PHA-stimulated T cells and exert immunosuppressive effects[54]. Compared with umbilical cord stem cells, the secretion of vascular endothelial growth factor-A and follistatin in DPSCs is more obvious, while umbilical cord stem cells tend to secrete vascular endothelial growth factor-C, which does not produce angiogenic effects. The levels of vascular endothelial growth factor-A and vascular endothelial growth factor-D secreted by DPSCs are higher than those secreted by BM-MSCs[55]. DPSCs can survive for a long time under extreme stress conditions[56]. DPSCs have the same immunomodulatory effect as BM-MSCs in the treatment of nervous system diseases. DPSCs are easier to obtain than other stem cells, have high multidifferentiation potential and a strong proliferation ability, and are not carcinogenic. They are a good substitute for stem cells in the treatment of PNI[5].
Moreover, compared with dental follicle- and papilla-derived stem cells, DPSCs have a stronger Na+ current, indicating that they have a higher potential for neural differentiation[57]. Both DPSCs and BM-MSCs implanted in the vitreous body secrete nerve growth factor (NGF), BDNF, and neurotrophin 3 (NT-3), and the amount of NGF and BDNF secreted by DPSCs is significantly higher than that secreted by BMSCs, which support nerve survival and axonal regeneration[58]. Adipose tissue-derived stem cells also have the potential for nerve regeneration but mainly play an active role in stimulating endogenous stem cells by releasing NTFs[59]. Wang et al[60] successfully induced vascular endothelial cells and BM-MSCs to differentiate into neurons with tricyclodedecane-9-yl xanthate. Cryopreserved DPSCs were also shown to be able to differentiate into cholinergic neurons by tricyclodecane-9-yl xanthate[61]. By comparing stem cells from different sources, Isobe et al[62] found that BM-MSCs and synovial fluid-derived stem cells showed significant osteogenic effects. An increase in alkaline phosphatase and osteocalcin levels suggested that synovial fluid-derived stem cells had the highest cartilage formation ability. Reverse transcription-polymerase chain reaction showed increased expression of class III β-tubulin and microtubule-associated protein 2, suggesting that DPSCs and human deciduous tooth stem cells have the potential for neural regeneration. In addition, studies by Isobe et al[62] showed that DPSCs have stronger neural differentiation potential than pluripotent stem cells isolated from bone marrow and synovial fluid.
In light of these findings, DPSCs have emerged as excellent candidate stem cells for treating PNI, with stronger tissue regeneration potential than other types of stem cells (Figure 1). Consequently, the focus of this article will be on research progress related to the effectiveness of DPSCs in treating PNI.
Teeth originate from the cranial neural crest[63]. Chai et al[64] were the first to confirm the involvement of cranial neural crest cells in the formation of dental pulp cells. In recent years, there has been a growing interest in research on utilizing DPSCs for nerve repair[65]. Janebodin et al[52] demonstrated that DPSCs originate from spinal nerves and possess the potential to differentiate into tissues derived from other neural crest-derived structures. Gronthos et al[53] provided evidence that the dental pulp contains cells with the ability to form clones, proliferate, and regenerate tissue, classifying them as stem cells. The embryonic origin of DPSCs endows them with the potential to serve as stem cells for neural tissue engineering.
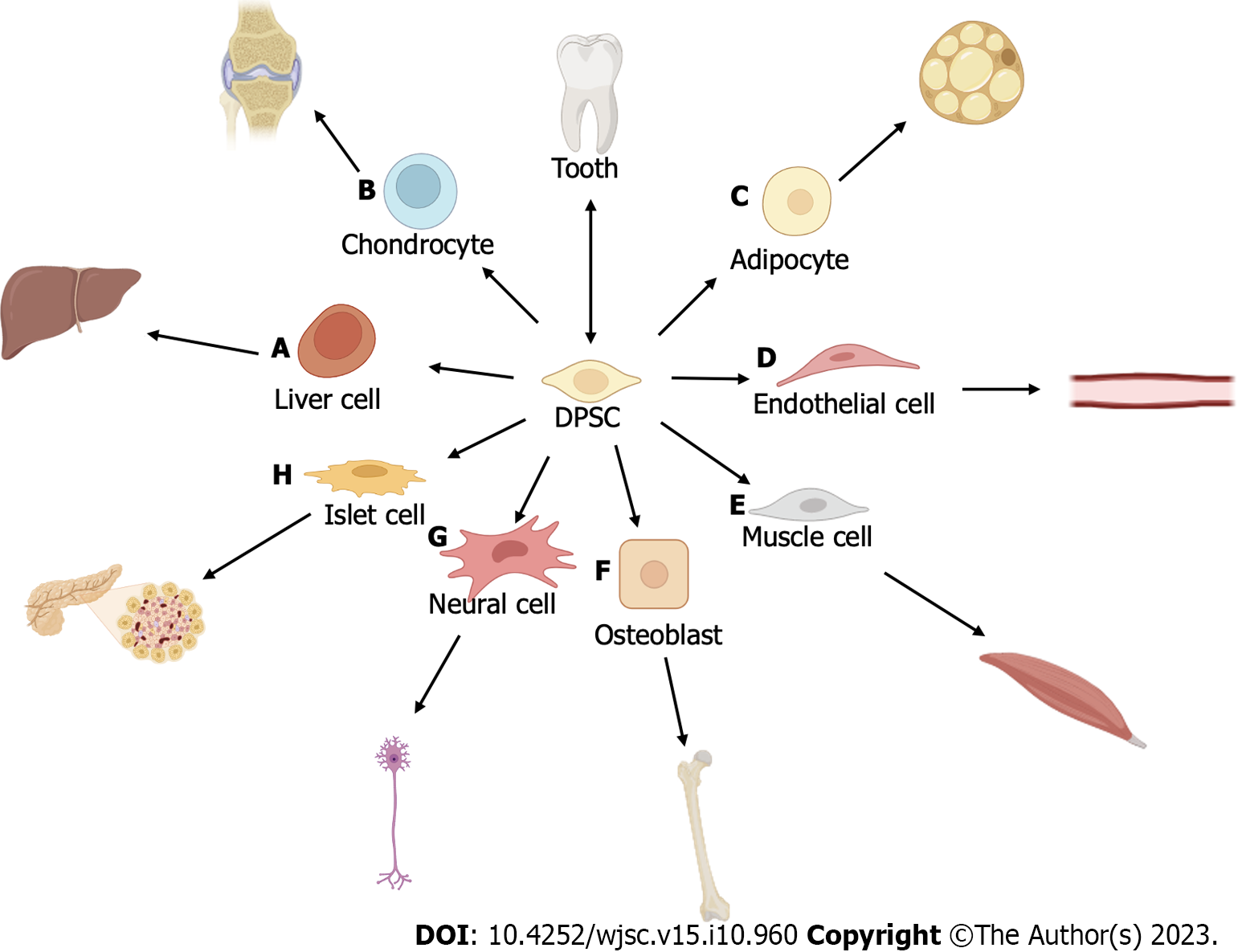
DPSCs have been shown to effectively promote axonal regeneration both in vivo and in vitro[66] (Tables 1 and 2). Typically, DPSCs exhibit several characteristics of pluripotent stem cells and display high proliferation rates, expressing CD44, CD90, and CD166[65]. Moreover, DPSCs express embryonic stem cell markers such as Oct-4, Nanog, SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81, along with several other MSC markers[67]. DPSCs have the remarkable capability to differentiate into several stromal-derived cell types (Figure 2). These cell types include osteoblasts[68-72], neurons[73], adipogenic cells[69] chondrogenic cells[69,70], smooth and skeletal muscle cells[74], dental pulp cells[75], Schwann cells[76], pancreatic cell lineage cells[70,77], blood vessel cells[78], and hepatocytes[79,80]. These findings strongly support the potential of DPSCs to be applied for tissue engineering[7].
| Publication year | Cell source | Induction program | Pretransplantation procedure | Material(s) | Dose/concentration | Disease model | Experiment duration | Treatment effect | Ref. |
| 2022 | Human | DMEM + 10% (FBS + P/S) | 3D culture | Fibrinogen, calcium chloride, and thrombin-like protein | 3 × 105 cells | Avulsion of spinal motor roots in rats | 12 wk | The transcription of TNF-α, IL-1β, IL-6, and IL-17 and the expression of anti-inflammatory cytokines (TGF-β, IL-4, IL-10, and IL-13) were increased; the animals in the reimplantation + 2D group showed the best functional recovery | [82] |
| 2021 | Human | α-MEM + 20% (FBS + P/S) | Collected and resuspended in GelMA-bFGF | 10% GFD in a CSM tube | 1 × 106 cells/mL | 15 mm defect of the sciatic nerve in rats | 12 wk | Cell based therapy repaired large gap defects in peripheral nerves; the differentiation of DPSCs into nerve cells and Schwann-like nerve cells and the formation of myelinated nerve fibers were observed | [98] |
| 2021 | Human | DMEM + 10% (FBS + P/S + NEAA) | NLCs differentiated from DPSCs | - | 1 × 105 NLCs | 10 mm sciatic nerve defect in athymic nude rats | 12 wk | Two weeks after transplantation, approximately 75% of the transplanted cells differentiated into platelet-derived growth factor receptor alpha + OPCs expressing p75NTRd; transplantation promoted axon growth and improved nerve function | [145] |
| 2021 | Human | α-MEM + 15% FBS | Exosome collection | - | 200 μg/100 μL | Mouse model of spinal cord injury | 4 wk | Inhibited the ROS-MAPK-NFκB P65 signaling pathway to reduce M1 macrophage polarization, suppress the inflammatory response, and alleviate neurological damage | [121] |
| 2020 | Human | DMEM + 20% (FBS + P/S) | Preparation of scaffold-free cell sheets by coculture with FGF2 | - | 2 × 106 cells/cell sheet | Rat model of facial nerve crush injury | 3 wk | Cell sheets promoted axonal regeneration and functional recovery through continuous delivery of neurotrophic factors such as BDNF and GDNF | [116] |
| 2020 | Human | α-MEM + 10% (FBS + P/S + NEAA) | Induction of DPSCs differentiation into N-DPSCs; induction of DPSCs differentiation into N-DPSCs | - | - | Rat model of sciatic nerve crush injury | 1 mo | Both DPSCs and N-DPSCs promoted peripheral nerve repair through the expression of neurotrophic factors such as NGF, BDNF, and GDNF; the nerve repair effect of N-DPSCs was longer lasting | [146] |
| 2019 | Human (children) | DMEM + 15% (FBS+P/S) | - | - | 5 × 105 cells in 4 μL DMEM | Unilateral facial nerve crush injury in rats | 6 wk | Immature DPSCs promoted nerve regeneration and the formation of new myelin; the expression of nerve growth factor and anti-inflammatory cytokines (IL-6 and IL-10) increased significantly 7 d after treatment, and there was a decrease in the levels of soluble proinflammatory factors such as IL-2, IL-4, TNF-α, and IFN-γ | [87] |
| 2019 | Human | - | Induction of DPSCs differentiation into nerve cells | A PDO-based cell carrier | 7.5 × 105 cells | 6 mm defect of the sciatic nerve in rats | 12 wk | Multiperforated PDO tubes were effective biomaterial carriers; delivery of DPSCs impacted the inflammatory environment and promoted nerve regeneration and functional recovery | [89] |
| 2018 | Human | - | Isolation of STRO-1+/c-Kit+/CD34+ cells | Collagen scaffolds | 5 × 105 cells/animal | 6 mm defect of the sciatic nerve in rats | 4 wk | Nerve fiber regeneration and myelination and many myelinated axons were observed; DPSCs grafted into the sciatic nerve defect expressed the typical Schwann cell marker S100B and were positive for human NeuN | [12] |
| 2018 | Human | ADMEM + 10% FBS | Differentiated into neuronal cells (DF-DPSCs) | A conduit made from a Lyoplant membrane | - | 7-8 mm defect of the sciatic nerve in rats | 12 wk | DPSCs relieved neuropathic pain and inhibited inflammation in rats earlier than DF-DPSCs; at 12 wk after the operation, the expression of pAMPK/SIRT1 in DF-DPSCs and DPSCs increased, the expression of proinflammatory cytokines decreased, and the expression of NFκB decreased | [81] |
| 2018 | Human | ADMEM + 10% (FBS + P/S) | Differentiation into cholinergic neurons by adding D609 | Biodegradable tubule and fibrin glue | 1 × 106 DF-chNs | 5 mm defect of the sciatic nerve in rats | 8 wk | Transplanted DF-chNs promoted motor nerve regeneration and axon growth and expressed nerve growth factor receptor (p75NGFR) | [61] |
| 2018 | Human | - | - | An absorbable hemostat filled with human DPCs containing 1% atelocollagen, fibronectin, and laminin | 3 × 105 cells | Crush injury of the sciatic nerve in rats | 2 wk | DPCs stimulated Schwann cell differentiation and promoted peripheral nerve regeneration | [114] |
| 2017 | Human | Standard: α-MEM + 10% (FBS + NEAA + P/S). Differentiation: Standard + forskolin + bFGF + PDGF-AA + HRG1-β | Differentiation into Schwann-like cells (d-hDPSCs) | NeuraWrap™ conduits | - | 15 mm defect of the sciatic nerve in rats | 8 wk | Growth of axons, myelinated nerve fibers, and blood vessels; DPSCs still exerted strong angiogenic effects after differentiating into Schwann-like cells | [102] |
| 2017 | Human | α-MEM + 15% (FBS + AA + P/S + NEAA) | - | Fibrin conduits | 2 × 106/20 μL | 10 mm defect of the sciatic nerve in rats | 2 wk | Promoted nerve and axon regeneration; the transplanted cells expressed BDNF near the cell body, and the expression level of caspase-3 decreased | [45] |
| 2017 | Human | ADMEM + 10% FBS | Induction of DPSCs differentiation into nerve cells | Fibrin glue scaffold and collagen tubulation | 1 × 106 cells | 5 mm defect of the sciatic nerve in rats | 12 wk | Both hDPSCs and DF-hDPSCs promoted nerve regeneration and functional recovery; they could directly differentiate into nerve cells or facilitate nerve cell differentiation | [99] |
| 2015 | Human | α-MEM + 10% (FBS + P/S/AmB) | Transfection with Olig2 gene via a tetracycline (Tet) inducible system | - | 2 × 105 cells | Mouse model of local sciatic nerve demyelination | 6 wk | Recovery of sciatic nerve function; DPSCs differentiated into oligodendrocyte progenitors, and specific markers of oligodendrocyte progenitors and oligodendrocytes were expressed | [108] |
| 2015 | Human | DMEM + 10% FBS | G-CSF-induced stem cell mobilization (mobilized DPSCs and MDPSCs) | Collagen conduits | 3.0 × 105 MDPSCs | 5 mm defect of the sciatic nerve in rats | 5 wk | MDPSCs secreted neurogenic/angiogenic factors and promoted peripheral nerve regeneration | [112] |
| 2015 | Human | Culture dishes containing essential medium (alpha modification) + 10% (FBS + P/S + amphotericin B) | Induction of DPSCs differentiation into OPCs by transfection with a plasmid containing the human Olig2 gene | 2 × 105 cells | Sciatic nerve demyelination in mice | 6 wk | DPSCs differentiated into OPCs, and transplantation promoted myelin sheath formation and peripheral nerve function recovery | [107] | |
| 2015 | Human | DMEM + b-ME; DMEM + 10% (FBS + RA); DMEM + 10% (FBS + FSK + b-FGF + PDGF + HRG) | Differentiation of hDPSCs into Schwann-like cells | Cells combined with a pulsed electromagnetic field (PEMF) | 1 × 106 cells/10 mL/rat | Crush injury of the peripheral nerve in rats | 3 wk | Schwann-like cells derived from DPSCs exhibited the characteristics of glial cells, expressing CD104, S100, GFAP, laminin, and p75NTR; application of a PEMF promoted peripheral nerve regeneration after cell transplantation | [147] |
| 2012 | Human | DMEM + 10% FBS | - | - | 1 × 106 cells | Rat spinal cord transection model | 8 wk | DPSCs promoted axonal growth, differentiated into oligodendrocytes to treat spinal cord injury, and protected the nerve by inhibiting apoptosis and paracrine signaling | [105] |
| 2018 | Human | α-MEM + 10% (FBS + NEAA + P/S) | Application of fresh medium containing vitamin C cells reached approximately 80% confluence | - | - | Patients diagnosed with a traumatized permanent incisor | 12 mo | HDPSCs transplantation promoted the regeneration of pulp tissue including neuronal tissue, and the neuron marker NeuN was expressed | [75] |
| Human | PBS + P/S | Collection of hDPSCs aggregates | The root canals of human teeth | - | Immunocompromised mice | 8 wk | Dental pulp tissue containing sensory nerves and blood vessels regenerated after HDPSCs transplantation | ||
| Human | α-MEM + 10% (FBS + NEAA + P/S) | - | - | 3 × 105 cells | Rats injected into the dorsal root ganglion | 2 mo | HDPSCs exhibited the morphology of neurons and expressed TRPV1 and TRPM8 | ||
| Pig | PBS + P/S | Collection of hDPSCs aggregates | - | - | Permanent incisors of young female minipigs | 3 mo | Pig DPSCs resulted in the 3D regeneration of dental pulp with neural function | ||
| 2015 | Pig | Culture medium + 10% (FBS + L-AA -2-P + P/S) | - | Fibrin membrane | - | Porcine intercostal nerve transection model | 6 mo | DPSCs alleviated nerve injury and express NSE; neuroelectrophysiological evaluation showed that neurological function was restored | [88] |
| 2020 | Rat | α-MEM + 20% FBS | - | - | 1 × 106 cells/rat | Diabetic rats | 4 wk | Multiple factors secreted by DPSCs increased the nerve conduction velocity and blood flow to nerves | [118] |
| 2019 | Rat | α-MEM + glucose + 20% FBS | Collection of DPSCs-CM | - | 1 mL/rat | Diabetic rats | 4 wk | DPSCs-CM ameliorated peripheral neuropathy by exerting neuroprotective, angiogenic, and anti-inflammatory effects | [132] |
| 2017 | Rat | α-MEM + 20% FBS | - | - | 1 × 106 cells | Streptozotocin-induced diabetes rat model | 4 wk | Sensory disturbance was alleviated, the thickness and area of the myelin sheath increased, the transplanted DPSCs secreted multiple factors such as angiogenic factors, neurotrophic factors, and immunosuppressive factors | [84] |
| 2015 | Rat | α-MEM + glucose + 20% FBS | - | - | 1 × 106 cells | Diabetic rats | 4 wk | DPSCs transplantation relieved diabetic polyneuropathy by inhibiting inflammation, exerting immunomodulatory effects, and secreting neurotrophic factors | [86] |
| 2015 | Rat | α-MEM + 20% FBS | - | - | 1 × 106 cells/limb | Diabetic rats | 8 wk | DPSCs increased the nerve conduction velocity and blood flow to nerves and promoted an increase in the number of nerve fibers in diabetic rats | [83] |
| 2013 | Rat | DMEM + 10% (FBS + P/S) | - | - | 1.5 × 105 cells | Crush injury of the optic nerve in rats | 3 wk | Transplantation of DPSCs significantly increased the survival rate of retinal ganglion cells in rats and promote axonal regeneration | [58] |
| 2007 | Rat | - | Embedded in 10 mL type I collagen gel | 10-mm silicone tube | 1 × 105 cells | 7 mm defect of the facial nerve in rats | 2 wk | Regeneration of axons, blood vessels, and Schwann cells; Tuj1-positive axons and S100-positive Schwann-like supportive cells were found in regenerated nerves | [91] |
| 2018 | Rabbit | DMEM + 10% FBS | Construction of an acellular nerve graft for nerve regeneration | Xenogenic acellular nerve matrix | 6 × 105 cells per graft | 10 mm defect of the sciatic nerve in rabbits | 3 mo | Regeneration of nerve space showed that acellular nerve grafts containing DPSCs treated with myroilysin had a strong neural induction effect | [148] |
| Publication year | Cell source | Induction protocol | Material(s) applied | Dose/concentration | Neurocyte type | Culture duration | Results | Ref. |
| 2022 | Human | DMEM + 20% (FBS + P/S + AA + bFGF) | Dental pulp cell sheets | 3200 cells/cm2 | SH-SY5Y neuroblastoma cells | 3 d | Dental pulp cell sheets provided neurotrophic support by expressing NTF; the amount of neurotrophic factors produced by dental pulp cell sheets was sufficient to induce nerve regeneration in vitro and promote nerve repair in vivo; dental pulp cell sheets improved axon guidance and reduced axon branching | [113] |
| 2020 | Rat | α-MEM + 10% (FBS + P/S + NEAA) | DPSCs-CM | - | TGNCs from rats | 3 wk | DPSCs-CM was found to contain significant levels of nerve growth factor, brain-derived neurotrophic factor, neurotrophic factor-3, and glial cell line-derived neurotrophic factor; DPSCs-CM increased the survival rate of primary trigeminal ganglion neurons and promoted the growth of neurites | [130] |
| 2020 | Rat | α-MEM + 10% (FBS + P/S + NEAA) | DPSCs-CM | 50% DPSCs-CM | PC12 cells | 8 d | DPSCs-CM was found to contain neurotrophic factors, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor, which increased the viability and differentiation of PC12 cells and played an important role in axonal growth and survival, proving that DPSCs-CM treatment is a potential cell-free therapy for peripheral nerve repair and has a stronger effect on PC12 cells than DPSCs | [115] |
Subsequent studies have revealed that a large number of dental MSCs originate from glial cells associated with peripheral nerves[34]. Long-term cryopreserved DPSCs retain their potential to differentiate into cholinergic nerves, indicating their suitability for prolonged preservation[61], and they have demonstrated beneficial anti-inflammatory effects in nerve injury[81]. Paes et al[82] conducted a pioneering comparison between monolayer and spheroid cultures of hDPSCs, demonstrating that both exhibit promising nerve regeneration potential through different mechanisms. Furthermore, hDPSCs were found to be effective in treating diabetic polyneuropathy[83]. In a groundbreaking study, Omi et al[84] treated neuropathy in diabetic rats through DPSCs transplantation for the first time and observed nerve function recovery that was potentially attributed to basic fibroblast growth factor (bFGF), VEGF, NGF, and NT-3 secreted by DPSCs. As an effective therapeutic tool for treating PNI, DPSCs are anticipated to be widely used in clinical settings in the future. With the deepening of research (Figure 3), it has been confirmed that DPSCs can be used to treat PNI via many mechanisms.
DPSCs can differentiate into nerve-like cells, making them be of great significance in the field of nerve regeneration. Dental pulp MSCs exhibit strong expression of nerve and glial cell markers and can be induced to adopt a nerve-like morphology[5]. DPSCs can be induced to differentiate into cells that exhibit a neuronal morphology and express neuronal-specific markers, such as the immature neuron markers Nestin and PSA-NCAM, by a neural induction regimen in vivo and in vitro[85]. Moreover, DPSCs transplantation has been shown to promote macrophage polarization toward the anti-inflammatory M2 phenotype[86]. Saez et al[87] demonstrated that DPSCs can effectively promote peripheral nerve regeneration when used to treat unilateral nerve crush injury in rats.
The peripheral nerve transection model is widely utilized in the study of peripheral PNI, and DPSCs have demonstrated good nerve regeneration ability in this model[88]. Stocchero et al[89] transplanted dental pulp cells into Wistar rats with sciatic nerve defect and observed significant promotion of nerve regeneration during the first 2 wk. Combining dental pulp cells with biodegradable materials is a promising approach to avoid secondary surgery[90]. Additionally, Sasaki et al[91] embedded dental pulp cells in silicone tubes to repair facial nerve defects in rats, and the results indicated the formation of blood vessels and myelin sheath tissue by dental pulp cells. DPSCs have exhibited a robust capacity to promote nerve regeneration in peripheral nerve transection models, and optimizing material combination schemes to fully unleash DPSCs’ potential in nerve regeneration remains a prominent research focus in this field.
As previously reported, DPSCs, being derived from the neural ridge, possess a strong ability to differentiate into neuronal lineages. Numerous studies have explored various methods to induce the neural differentiation of DPSCs, and these cells have been applied to treat PNI[92]. After neural differentiation induction, DPSCs generate neural progenitor cells, expressing the neural markers Nestin, TuJ-1, and GFAP[93]. Additionally, inner ear neurotrophins, such as BDNF and NT-3, along with glial cell-derived neurotrophic factor (GDNF), have been found to promote the differentiation of DPSCs into spiral ganglion neuron-like cells[94]. Notably, when Schwann cells differentiated from human DPSCs were employed to treat a rat sciatic nerve defect model, significant regeneration of blood vessels and nerve processes was observed, highlighting the crucial role of revascularization in supporting nerve regeneration and survival[95]. Moreover, Zheng et al[96] demonstrated that coculture of chitosan scaffold with bFGF can enhance the neural differentiation of DPSCs. The ERK signaling pathway, a classical MAPK pathway, plays a pivotal role in this process. As a downstream effector of bFGF, DPSCs are actively engaged in neural differentiation. Numerous neural induction protocols exist for DPSCs; however, an optimal scheme that is simple, efficient, and quick is currently lacking.
Although significant progress has been made in the tissue field, finding a nerve conduit that can match the effectiveness of autologous transplantation remains a challenge in the treatment of PNI. Das and Bellare[97] developed a uniform bead-free nanofibrous scaffold primarily composed of polycaprolactone and gelatin A, which has been shown to support DPSCs regeneration and neural differentiation. In our previous studies, we confirmed that combining DPSCs with a third-generation nerve regeneration conduit can be used to effectively repair 15 mm long defects of the sciatic nerve in rats. Remarkably, the effect of this approach is comparable to that of autotransplantation. Furthermore, we observed that nerve tissue at the repair site mainly originated from differentiating DPSCs. This promising finding offers a potential tissue engineering strategy for the treatment of PNI[98].
Ullah et al[99] made an intriguing discovery that there was no significant difference in nerve regeneration ability between hDPSCs and differentiated neuronal cells derived from hDPSCs. More research on this topic is needed to clarify the underlying mechanisms and find a more effective treatment strategy.
Previous studies have demonstrated that autologous adult Schwann cells have a remarkable capacity to support extensive peripheral nerve regeneration and can evade rejection[100]. Currently, Schwann cells remain a focal point of animal studies on PNI. Substitutive cell therapies bring renewed hope for nerve regeneration[20]. Schwann cells play a pivotal role in Wallerian degeneration, myelin regeneration, and supporting axon growth.
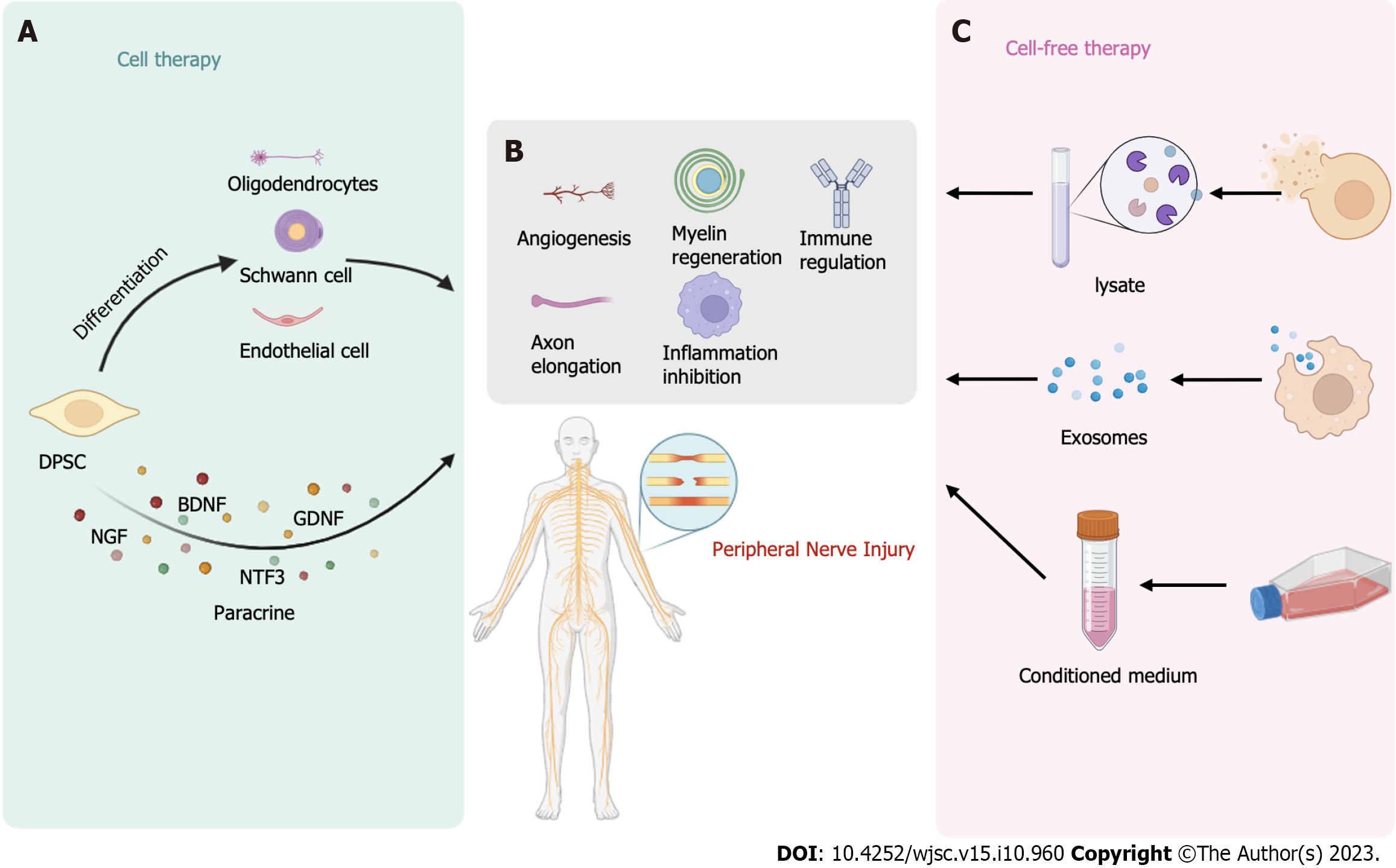
Martens et al[76] were the first to demonstrate that Schwann cells differentiated from hDPSCs can promote nerve regeneration. These differentiated Schwann cells express neural markers such as laminin, p75, GFAP, and CD104 and exhibit an increase in the expression of NTFs such as BDNF, b-NGF, NT-3, and GDNF. Medium conditioned by these differentiated Schwann cells was found to increase the survival of dorsal root ganglion cells and stimulate axon elongation. Subsequently, Al-Zer et al[101] induced the differentiation of DPSCs into Schwann cells by using forskolin, bFGF, platelet-derived growth factor, and recombinant human neuregulin-β1. The results revealed that the differentiated neural stem cells strongly expressed the Schwann cell marker Sox10. Additionally, Sanen et al[102] demonstrated that hDPSCs, after differentiating into Schwann-like cells, have the potential to stimulate endothelial cell migration and tubule formation. Neuronally differentiated DPSCs secrete higher levels of the angiogenic factor VEGF-A, suggesting that DPSCs retain their angiogenic ability even after differentiating into Schwann cells. However, the underlying mechanism by which DPSCs-derived Schwann cells promote endothelial cell proliferation requires further investigation. These findings collectively indicate the ability of DPSCs to differentiate into Schwann cells, making them potential candidates for tissue engineering-based approaches for treating nerve injuries. Lambrichts et al[103] showed that hDPSCs-derived Schwann cells could form myelin sheaths and dorsal root ganglia. Similarly, Carnevale et al[12] discovered that STRO-1+/c-Kit+/CD34+ hDPSCs, which originate from the neural ridge, promote axonal regeneration in an animal model of PNI and express S100b, a typical marker of Schwann cells. The differentiation of DPSCs into Schwann cells thus is a potential strategy for nerve regeneration, and the refinement of differentiation protocols and materials remains an important area of investigation.
The primary function of oligodendrocytes is to form myelin, creating intricate connections between neurons in the nervous system. The transcription factor OLIG-2 and proteoglycan NG2 are markers of oligodendrocyte progenitor cells (OPCs)[104]. In a previous study, Sakai et al[105] transplanted SHEDs into rats with spinal cord transection and found that these cells exerted promising effects in promoting axon regeneration. This effect was achieved through the inhibition of various axon growth inhibitor signals and the differentiation of the transplanted cells into oligodendrocytes to replace damaged cells. Bagheri-Hosseinabadi et al[106] successfully induced DPSCs to differentiate into oligodendrocytes using cerebrospinal fluid and retinoic acid and obtained cells with a fibroblastic morphology and high adherence potential. Moreover, Askari et al[107] successfully induced DPSCs to differentiate into OPCs by transfecting them with a virus carrying the human Orig2 gene. The differentiated oligodendrocytes displayed a typical morphology and expressed neural markers such as GFAP, oligodendrocyte lineage transcription factor 2, and MBP. Subsequently, they applied transplanted oligodendrocytes into sciatic nerve demyelination model mice and demonstrated their effectiveness in nerve repair[108]. The nerve regeneration potential of DPSCs has been substantiated through various studies, offering hope for the treatment of peripheral nerve demyelination.
Adequate blood supply is crucial for the survival of stem cells and nerve regeneration following injury. When MSCs differentiate into Schwann cells, they increase the secretion of angiogenic factors, including angiopoietin-1 and VEGF-A[109]. DPSCs can increase the migration of endothelial cells and promote angiogenesis in vitro and in vivo[110]. Sanen et al[102] also confirmed this phenomenon. Moreover, research has demonstrated that stem cells can directly differentiate into endothelial cells. In a rat facial nerve defect model, it was observed that DPSCs can directly differentiate into RECA1-positive endothelial cells, promoting nerve regeneration by increasing blood supply[91]. DPSCs were found lining the blood vessel wall of newly formed braided bone, indicating that angiogenesis occurred in vitro. Osteoblasts and endothelial cells were found after transplantation, and ultimately, bone-containing blood vessels were produced. Flk-1 is very important for the coupling of osteogenesis and angiogenesis. d'Aquino et al[69] proved that DPSCs can differentiate into Flk-1+/STRO-1+/CD44+/CD54+ endothelial progenitor cells. Sasaki et al[91] found that regenerated nerves contained S100-positive Schwann cells and RECA1-positive endothelial cells derived from dental pulp 14 d after DPSCs transplantation into rats with facial nerve defect. Newborn blood vessels are composed of endothelial cells from both recipient and donor sources. It has been suggested that DPSCs can differentiate into nerve cells and endothelial cells at the same time to promote nerve and vascular regeneration to treat PNI[91]. Subsequently, Maraldi et al[71] also proved that transplanted DPSCs can differentiate into endothelial cells in vivo. The ability of DPSCs to differentiate into endothelial cells further increases their potential for nerve regeneration.
PNI triggers the dedifferentiation of Schwann cells and induces the formation and secretion of protogranules, which play a crucial role in nerve repair and the promotion of axonal growth[111]. Yamamoto et al[112] discovered that mobilized DPSCs (MDPSCs) treated with a granulocyte-colony stimulating factor (G-CSF) gradient express a variety of NTFs. These NTFs not only stimulate Schwann cells but also regulate their apoptosis and proliferation. Additionally, the differentiation of hDPSCs into Schwann cells increases the expression of glial markers and the secretion of NTFs, including BDNF, GDNF, NGF, NTF3, ANGPT1, and VEGFA[45,76]. Furthermore, linearly arranged dental pulp cell slices have been shown to guide and support axonal regeneration, and the abundant NTFs produced by the cells were found to make a significant contribution to this phenomenon[113]. Implantation of DPSCs into the vitreous body can effectively treat retinal ganglion cell injuries in adult rats, with the secretion of NTFs being a critical factor. The neuroprotective effect of DPSCs is weakened when K252a and Trk are blocked, indicating the importance of the NTFs NGF, BDNF, and NT-3 in this process[58]. In the repair of sciatic nerve crush injury in rat models, transplanted dental pulp cells may secrete NTFs[114]. In a rat model of sciatic nerve injury with a 10 mm defect, DPSCs were found to exert their effects on Schwann cells through paracrine signaling, leading to significant promotion of axonal regeneration[45]. The strong paracrine effect of DPSCs, coupled with their potential for nerve regeneration, makes them promising candidates for nerve tissue engineering.
An increasing number of studies have demonstrated that odontogenic stem cells treat nerve injury through paracrine mechanisms[105]. NTFs, such as NGF, BDNF, and GDNF, can stimulate axonal growth, making DPSCs potential candidates for acellular therapy[115,116]. Kumar et al[117] showed that DPSCs secrete high levels of cytokines such as G-CSF, interferon gamma, and transforming growth factor (TGF)-β, which promote nerve differentiation and axonal growth. Unlike some other stem cell types, DPSCs demonstrate strong potential for nerve regeneration, supporting the use of their secreted factors for exocrine therapy for PNI. Kanada et al[118] found that both DPSCs and secreted factors from DPSCs (DPSCs-SFs) exerted therapeutic effects in a rat model of diabetic polyneuropathy. The effects included increases in sciatic nerve motor/sensory nerve conduction velocity and sciatic nerve blood flow. DPSCs-SFs were found to include angiogenic, neurotrophic, and immunomodulatory proteins. However, DPSCs transplantation may provide benefits over a longer duration of time[118].
With the continuous development of research on DPSCs-derived exosomes (DPSCs-Exos), DPSCs-Exos have been demonstrated to exert potential therapeutic effects in various diseases. They have shown promise for the treatment of periodontitis[119], Parkinson’s disease[120], SCI[121], degenerative diseases[122], and cerebral ischemia[123] and in achieving pulp regeneration[124]. DPSCs-Exos have garnered significant attention due to their immunomodulatory properties[125], ability to promote angiogenesis[126], inhibitory effect on inflammation[127], and marked neuroregenerative and neuroprotective effects[128]. Studies have revealed that DPSCs-Exos can increase the formation of SH-SY5Y cell axons, leading to improved neuronal ultrastructure and increased expression of neural markers[129]. Research on acellular therapies involving DPSCs is still relatively limited, and the therapeutic potential of DPSCs-Exos and DPSCs lysates requires further experimentation and validation. Progress has been made in the techniques used to extract exosomes and analyze their composition, but there is still much work to be done before their clinical application can be realized.
CM derived from D-MSCs has been shown to promote axonal growth[45]. Sultan et al[130] conducted a study on the neuroprotective effect of DPSCs and DPSCs-CM on isolated TGNCs for the first time. The results demonstrated that DPSCs-CM can increase neuronal survival and promote axonal growth through the action of various NTFs, including GDNF, BDNF, NT-3, and CNTF[130]. CM from human dental pulp cells was found to contain bone morphogenetic protein 7, FGF7, insulin-like growth factor (IGF)-1, FGF4, growth hormone, and VEGF-D, all of which are related to nerve regeneration and protection, vascular regeneration, and osteogenesis. Furthermore, the addition of B-27 to CM was observed to enhance the promotion of axon growth[131]. DPSCs-CM has shown the ability to alleviate polyneuropathy in diabetic rats and reduce the number of macrophages in diseased peripheral nerves[132]. DPSCs-CM holds significant promise in the treatment of peripheral nerve injuries. However, further research is needed to explore the underlying mechanisms of nerve regeneration.
Neurotrophin, BDNF, GDNF, IGF, NGF, and VEGF were detected near transplanted DPSCs 14 d after transplantation. The secretion of vascular endothelial growth factors supports angiogenesis, thus promoting nerve regeneration[112]. BM-MSCs can also secrete NTFs such as BDNF, b-FGF, and CNTF to promote peripheral nerve regeneration and thus treat peripheral nerve defects[133]. G-CSF-MDPSCs express higher levels of granulocyte-macrophage CSF, matrix metalloproteinase 3, VEGF, and NGF than BM-MSCs. The effects of G-CSF-MDPSCs in angiogenesis, neurite extension, and migration and their anti-apoptotic effects were found to be stronger than those of BM-MSCs in the same environment[134]. Compared with medium conditioned by CD31- cells derived from bone marrow and fat, medium conditioned by CD31- cells from pulp results in higher levels of angiogenesis/NTFs and exerts stronger angiogenic and neurogenic effects[135]. Kumar et al[117] also proved that DPSCs and their secreted factor may exert beneficial effects in treating neurological disorders and injuries. Exosomes derived from DPSCs can inhibit the differentiation of CD4+ T cells into helper T cells 17 (Th17), reduce the secretion of the proinflammatory factors interleukin (IL)-17 and tumor necrosis factor-alpha (TNF-α), promote the polarization of CD4+ T cells into regulatory T cells, and increase the release of the anti-inflammatory factors IL-10 and TGF-β. Compared with BM-derived exosomes, exosomes derived from DPSCs have a stronger immunoregulatory effect. In CD4+ T cells stimulated by exosomes derived from DPSCs, the expression levels of IL-10 and TGF-β mRNA were found to be the highest, while the transcription levels of IL-17 and TNF-α were found to be the lowest. Exosomes derived from DPSCs have stronger anti-inflammatory effects than exosomes derived from BM-MSCs[125]. Exosomes derived from DPSCs/BM-MSCs significantly decrease the activity of caspase3/7 and exert a significant antiapoptotic effect to play a neuroprotective role by upregulating endogenous expression of neuronal survival factors. Specifically, the cell survival-related PI3K-Bcl-2 pathway protects hippocampal neurons from excitotoxicity. Exosomes derived from DPSCs have stronger antiapoptotic and anti-visceral necrosis effects than exosomes derived from bone marrow stem cells[122]. Interestingly, the factors secreted by DPSCs are different in different environments[136]. There is no doubt that the neuroprotective effect of DPSCs-Exos is stronger than that of BM-MSC-derived exosomes. However, for clinical application, it is necessary to better determine the optimal composition, dosage, and culture conditions of exosomes[121].
PNI is a prevalent clinical issue that often leads to long-term pain in patients. Recent research has demonstrated the beneficial effects of Schwann cells on axonal regeneration and functional recovery after injury[137]. However, isolating and cultivating Schwann cells are challenging due to their limited availability and low proliferation rate[138]. Tissue engineering strategies involving nerve grafts consisting of physical scaffolds combined with supporting cells and/or growth factors or other biomolecules are promising approaches for treating PNI[139].
DPSCs may occupy the injury site and exert immunomodulatory effects, participate in paracrine signaling, and directly differentiate into relevant cell types, thus promoting the repair and regeneration of diseased and injured tissues. To promote the application of DPSCs and their products in nerve regeneration in the future, clinical strategies and various administration methods are being studied on a large scale[140]. A study revealed that inducing DPSCs to differentiate into oligoprogenitor cells is a potential treatment strategy for neurodegenerative diseases[141]. DPSCs have the multilineage differentiation potential and can differentiate into neurotrophoblasts. DPSCs-CM and DPSCs-Exos contain rich NTFs, and DPSCs-Exos can also cross the blood-brain barrier. In addition, DPSCs have the ability to reduce inflammation, promote axonal growth, and resist apoptosis. They have great potential in the treatment of PNI[142].
Previous in vivo studies have focused on the sciatic nerve and facial nerve of rats and analyzed the behavioral and pathological manifestations of rats with sciatic and facial nerve injury, but electrophysiological studies are lacking. Moreover, it is also necessary to compare therapeutic effects among different models to identify the optimal application of DPSCs. However, there are still some questions to be answered. Do DPSCs have the same effect on nerve crush injury and amputation injury? Do different treatment methods have different effects on different models. Which is better, cell therapy or acellular therapy? What are the key factors in the neuroprotective and regenerative abilities of acellular therapies? In addition, researchers should pay more attention to the function of differentiated nerve cells in vitro. Furthermore, organoids may become a hot research topic in the future. A DPSCs cell bank is also urgently needed[143]. It is believed that DPSCs will bring hope to patients with PNI soon.
The unique biological characteristics of DPSCs make them significant cell sources for the treatment of PNI. DPSCs exhibit neurogenic potential, are easily accessible, exhibit pluripotency, and can be preserved for extended periods, making them excellent candidates for tissue engineering applications. DPSCs (Figure 4) can be utilized directly or in combination with nerve conduits or hydrogels or used after differentiation into nerve-like cells to treat PNI. Moreover, DPSCs lysates, DPSCs-Exos, and DPSCs-CM all hold promise in the treatment of PNI. However, overcoming safety and ethical problems and avoiding tumor formation are still the keys for translating DPSCs for clinical use[144].
This review focused on the therapeutic potential of DPSCs in treating PNI, elaborating on the neuroprotective and regenerative capabilities of DPSCs through both cellular and acellular approaches. DPSCs hold significant promise for the treatment of PNI, and it is anticipated that they will play a crucial role in the clinical treatment of PNI in the future. As research on the effect of DPSCs on PNI continues to progress, more efficient and expedient treatment protocols are expected to be developed. However, the safety of cell therapies and the efficacy of acellular therapies in treating long-gap injuries require thorough evaluation. The search for convenient and effective treatment strategies involving DPSCs and their products for PNI remains ongoing. Furthermore, the underlying mechanisms and key components of DPSCs in peripheral nerve treatment warrant further investigation. As acellular therapies are novel stem cell therapies, there is a need to refine the purification process of cell materials and establish a standardized and effective cell production plan. Comparative studies assessing the safety, efficacy, and production costs of various therapies are also essential.
We thank Qing-Song Ye for his comments on the manuscript.
| 1. | Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 644] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Evans GR. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19:312-318. [PubMed] [DOI] [Full Text] |
| 3. | Mackinnon SE, Hudson AR. Clinical application of peripheral nerve transplantation. Plast Reconstr Surg. 1992;90:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 147] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Ortigüela ME, Wood MB, Cahill DR. Anatomy of the sural nerve complex. J Hand Surg Am. 1987;12:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Ibarretxe G, Crende O, Aurrekoetxea M, García-Murga V, Etxaniz J, Unda F. Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration. Stem Cells Int. 2012;2012:103503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Khalifian S, Sarhane KA, Tammia M, Ibrahim Z, Mao HQ, Cooney DS, Shores JT, Lee WP, Brandacher G. Stem cell-based approaches to improve nerve regeneration: potential implications for reconstructive transplantation? Arch Immunol Ther Exp (Warsz). 2015;63:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | d'Aquino R, De Rosa A, Laino G, Caruso F, Guida L, Rullo R, Checchi V, Laino L, Tirino V, Papaccio G. Human dental pulp stem cells: from biology to clinical applications. J Exp Zool B Mol Dev Evol. 2009;312B:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1449] [Article Influence: 60.4] [Reference Citation Analysis (10)] |
| 9. | Miletich I, Sharpe PT. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today. 2004;72:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Shi H, Gong Y, Qiang L, Li X, Zhang S, Gao J, Li K, Ji X, Tian L, Gu X, Ding F. Derivation of Schwann cell precursors from neural crest cells resident in bone marrow for cell therapy to improve peripheral nerve regeneration. Biomaterials. 2016;89:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Al-Zer H, Kalbouneh H. Dental pulp stem cells-derived schwann cells for peripheral nerve injury regeneration. Neural Regen Res. 2015;10:1945-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Carnevale G, Pisciotta A, Riccio M, Bertoni L, De Biasi S, Gibellini L, Zordani A, Cavallini GM, La Sala GB, Bruzzesi G, Ferrari A, Cossarizza A, de Pol A. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J Tissue Eng Regen Med. 2018;12:e774-e785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Höke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 15. | Seckel BR. Enhancement of peripheral nerve regeneration. Muscle Nerve. 1990;13:785-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Chaudhry V, Glass JD, Griffin JW. Wallerian degeneration in peripheral nerve disease. Neurol Clin. 1992;10:613-627. [PubMed] |
| 17. | Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989;18:671-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 392] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Le Douarin NM. Cell line segregation during peripheral nervous system ontogeny. Science. 1986;231:1515-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 261] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Guénard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci. 1992;12:3310-3320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 331] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521-3531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 878] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 22. | Johnson EO, Zoubos AB, Soucacos PN. Regeneration and repair of peripheral nerves. Injury. 2005;36 Suppl 4:S24-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 398] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 24. | Levi AD, Guénard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci. 1994;14:1309-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Naruse K. Schwann Cells as Crucial Players in Diabetic Neuropathy. Adv Exp Med Biol. 2019;1190:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol. 2002;173:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 180] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Saheb-Al-Zamani M, Yan Y, Farber SJ, Hunter DA, Newton P, Wood MD, Stewart SA, Johnson PJ, Mackinnon SE. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Razavi S, Ahmadi N, Kazemi M, Mardani M, Esfandiari E. Efficient transdifferentiation of human adipose-derived stem cells into Schwann-like cells: A promise for treatment of demyelinating diseases. Adv Biomed Res. 2012;1:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Saller MM, Huettl RE, Mayer JM, Feuchtinger A, Krug C, Holzbach T, Volkmer E. Validation of a novel animal model for sciatic nerve repair with an adipose-derived stem cell loaded fibrin conduit. Neural Regen Res. 2018;13:854-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Shimizu M, Matsumine H, Osaki H, Ueta Y, Tsunoda S, Kamei W, Hashimoto K, Niimi Y, Watanabe Y, Miyata M, Sakurai H. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen. 2018;26:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007;359:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Lavasani M, Thompson SD, Pollett JB, Usas A, Lu A, Stolz DB, Clark KA, Sun B, Péault B, Huard J. Human muscle-derived stem/progenitor cells promote functional murine peripheral nerve regeneration. J Clin Invest. 2014;124:1745-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (2)] |
| 35. | Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1370] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 36. | Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015;33:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 37. | Patil R, Kumar BM, Lee WJ, Jeon RH, Jang SJ, Lee YM, Park BW, Byun JH, Ahn CS, Kim JW, Rho GJ. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp Cell Res. 2014;320:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 38. | Abe S, Hamada K, Miura M, Yamaguchi S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol Int. 2012;36:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 2021] [Article Influence: 87.9] [Reference Citation Analysis (1)] |
| 40. | Pereira LV, Bento RF, Cruz DB, Marchi C, Salomone R, Oiticicca J, Costa MP, Haddad LA, Mingroni-Netto RC, Costa HJZR. Stem Cells from Human Exfoliated Deciduous Teeth (SHED) Differentiate in vivo and Promote Facial Nerve Regeneration. Cell Transplant. 2019;28:55-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Cen LP, Ng TK, Liang JJ, Zhuang X, Yao X, Yam GH, Chen H, Cheung HS, Zhang M, Pang CP. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells. 2018;36:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Dapeng L, Xiaojie L, Ping G, Yan D, Gang S. Erk1/2 signalling is involved in the differentiation of periodontal ligament stem cells to Schwann cells in dog. Arch Oral Biol. 2014;59:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Li B, Jung HJ, Kim SM, Kim MJ, Jahng JW, Lee JH. Human periodontal ligament stem cells repair mental nerve injury. Neural Regen Res. 2013;8:2827-2837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 44. | Ng TK, Yung JS, Choy KW, Cao D, Leung CK, Cheung HS, Pang CP. Transdifferentiation of periodontal ligament-derived stem cells into retinal ganglion-like cells and its microRNA signature. Sci Rep. 2015;5:16429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7:12605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 46. | Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig. 2013;17:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | Zainal Ariffin SH, Kermani S, Zainol Abidin IZ, Megat Abdul Wahab R, Yamamoto Z, Senafi S, Zainal Ariffin Z, Abdul Razak M. Differentiation of dental pulp stem cells into neuron-like cells in serum-free medium. Stem Cells Int. 2013;2013:250740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Li J, Diao S, Yang H, Cao Y, Du J, Yang D. IGFBP5 promotes angiogenic and neurogenic differentiation potential of dental pulp stem cells. Dev Growth Differ. 2019;61:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Luo L, He Y, Wang X, Key B, Lee BH, Li H, Ye Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018;2018:1731289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | Méndez-Maldonado K, Vega-López GA, Aybar MJ, Velasco I. Neurogenesis From Neural Crest Cells: Molecular Mechanisms in the Formation of Cranial Nerves and Ganglia. Front Cell Dev Biol. 2020;8:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Stokowski A, Shi S, Sun T, Bartold PM, Koblar SA, Gronthos S. EphB/ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem Cells. 2007;25:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Janebodin K, Horst OV, Ieronimakis N, Balasundaram G, Reesukumal K, Pratumvinit B, Reyes M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6:e27526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3448] [Article Influence: 132.6] [Reference Citation Analysis (1)] |
| 54. | Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 55. | Caseiro AR, Santos Pedrosa S, Ivanova G, Vieira Branquinho M, Almeida A, Faria F, Amorim I, Pereira T, Maurício AC. Mesenchymal Stem/ Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/ Stromal Cells secretome. PLoS One. 2019;14:e0221378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Mitsiadis TA, Woloszyk A. Odyssey of human dental pulp stem cells and their remarkable ability to survive in extremely adverse conditions. Front Physiol. 2015;6:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 57. | Ullah I, Subbarao RB, Kim EJ, Bharti D, Jang SJ, Park JS, Shivakumar SB, Lee SL, Kang D, Byun JH, Park BW, Rho GJ. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci. 2016;154:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544-7556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 59. | Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010;63:e811-e817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Wang N, Du CQ, Wang SS, Xie K, Zhang SL, Miao JY. D609 induces vascular endothelial cells and marrow stromal cells differentiation into neuron-like cells. Acta Pharmacol Sin. 2004;25:442-446. [PubMed] |
| 61. | Jang S, Kang YH, Ullah I, Shivakumar SB, Rho GJ, Cho YC, Sung IY, Park BW. Cholinergic Nerve Differentiation of Mesenchymal Stem Cells Derived from Long-Term Cryopreserved Human Dental Pulp In Vitro and Analysis of Their Motor Nerve Regeneration Potential In Vivo. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Isobe Y, Koyama N, Nakao K, Osawa K, Ikeno M, Yamanaka S, Okubo Y, Fujimura K, Bessho K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int J Oral Maxillofac Surg. 2016;45:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 63. | Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103 Suppl:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 401] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 64. | Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 988] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 65. | Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch Oral Biol. 2012;57:1439-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 66. | Armiñán A, Gandía C, Bartual M, García-Verdugo JM, Lledó E, Mirabet V, Llop M, Barea J, Montero JA, Sepúlveda P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 68. | Laino G, Carinci F, Graziano A, d'Aquino R, Lanza V, De Rosa A, Gombos F, Caruso F, Guida L, Rullo R, Menditti D, Papaccio G. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006;17:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | d'Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 70. | Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Ab Aziz ZA, Abdullah M, Musa S, Kasim NH, Bhonde RR. Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res. 2011;90:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 71. | Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F, La Sala GB, Motta A, De Pol A. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther. 2013;4:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 72. | Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 306] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | Király M, Porcsalmy B, Pataki A, Kádár K, Jelitai M, Molnár B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int. 2009;55:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 75. | Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 76. | Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 77. | Carnevale G, Riccio M, Pisciotta A, Beretti F, Maraldi T, Zavatti M, Cavallini GM, La Sala GB, Ferrari A, De Pol A. In vitro differentiation into insulin-producing β-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig Liver Dis. 2013;45:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 427] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 79. | Kumar A, Kumar V, Rattan V, Jha V, Pal A, Bhattacharyya S. Molecular spectrum of secretome regulates the relative hepatogenic potential of mesenchymal stem cells from bone marrow and dental tissue. Sci Rep. 2017;7:15015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Ishkitiev N, Yaegaki K, Calenic B, Nakahara T, Ishikawa H, Mitiev V, Haapasalo M. Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J Endod. 2010;36:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Ullah I, Choe YH, Khan M, Bharti D, Shivakumar SB, Lee HJ, Son YB, Shin Y, Lee SL, Park BW, Ock SA, Rho GJ. Dental pulp-derived stem cells can counterbalance peripheral nerve injury-induced oxidative stress and supraspinal neuro-inflammation in rat brain. Sci Rep. 2018;8:15795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Paes SM, Castro MV, Barbosa RM, Politti Cartarozzi L, Coser LO, Kempe PRG, Decarli MC, Moraes ÂM, Barraviera B, Ferreira Júnior RS, Oliveira ALR. Human dental pulp stem cell monolayer and spheroid therapy after spinal motor root avulsion in adult rats. Brain Res. 2023;1802:148229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 83. | Hata M, Omi M, Kobayashi Y, Nakamura N, Tosaki T, Miyabe M, Kojima N, Kubo K, Ozawa S, Maeda H, Tanaka Y, Matsubara T, Naruse K. Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: therapeutic plausibility of freshly isolated and cryopreserved dental pulp stem cells. Stem Cell Res Ther. 2015;6:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | Omi M, Hata M, Nakamura N, Miyabe M, Ozawa S, Nukada H, Tsukamoto M, Sango K, Himeno T, Kamiya H, Nakamura J, Takebe J, Matsubara T, Naruse K. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res Ther. 2017;8:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 436] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 86. | Omi M, Hata M, Nakamura N, Miyabe M, Kobayashi Y, Kamiya H, Nakamura J, Ozawa S, Tanaka Y, Takebe J, Matsubara T, Naruse K. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J Diabetes Investig. 2016;7:485-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 87. | Saez DM, Sasaki RT, Martins DO, Chacur M, Kerkis I, da Silva MCP. Rat Facial Nerve Regeneration with Human Immature Dental Pulp Stem Cells. Cell Transplant. 2019;28:1573-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Spyridopoulos T, Lambropoulou M, Pagonopoulou O, Birbilis T, Tsaroucha AK, Kouzi-Koliakou K, Botaitis S, Deftereou TE, Gaitanidis A, Pitiakoudis M. Regenerated Nerve Defects with a Nerve Conduit Containing Dental Pulp Stem Cells in Pigs: An Immunohistochemical and Electrophysiological Evaluation. J Reconstr Microsurg. 2015;31:516-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Stocchero IN, Lizier NF, Stelini RF, de Oliveira OCG, de Oliveira PRG, Ayoub CA, Rotta TD, Stocchero GF, Kharmandayan P. A Reliable Stem Cell Carrier: An Experimental Study in Wistar Rats. Aesthetic Plast Surg. 2019;43:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Ogiuchi H, Okano T, Ando T. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med. 2011;5:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Okano T, Ogiuchi H. Tubulation with dental pulp cells promotes facial nerve regeneration in rats. Tissue Eng Part A. 2008;14:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Nuti N, Corallo C, Chan BM, Ferrari M, Gerami-Naini B. Multipotent Differentiation of Human Dental Pulp Stem Cells: a Literature Review. Stem Cell Rev Rep. 2016;12:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 93. | Lopez-Lozano AP, Arevalo-Niño K, Gutierrez-Puente Y, Montiel-Hernandez JL, Urrutia-Baca VH, Del Angel-Mosqueda C, De la Garza-Ramos MA. SSEA-4 positive dental pulp stem cells from deciduous teeth and their induction to neural precursor cells. Head Face Med. 2022;18:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 94. | Gonmanee T, Thonabulsombat C, Vongsavan K, Sritanaudomchai H. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. Arch Oral Biol. 2018;88:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 96. | Zheng K, Feng G, Zhang J, Xing J, Huang D, Lian M, Zhang W, Wu W, Hu Y, Lu X, Feng X. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int J Neurosci. 2021;131:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 97. | Das S, Bellare JR. Dental Pulp Stem Cells in Customized 3D Nanofibrous Scaffolds for Regeneration of Peripheral Nervous System. Methods Mol Biol. 2020;2125:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Luo L, He Y, Jin L, Zhang Y, Guastaldi FP, Albashari AA, Hu F, Wang X, Wang L, Xiao J, Li L, Wang J, Higuchi A, Ye Q. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact Mater. 2021;6:638-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 99. | Ullah I, Park JM, Kang YH, Byun JH, Kim DG, Kim JH, Kang DH, Rho GJ, Park BW. Transplantation of Human Dental Pulp-Derived Stem Cells or Differentiated Neuronal Cells from Human Dental Pulp-Derived Stem Cells Identically Enhances Regeneration of the Injured Peripheral Nerve. Stem Cells Dev. 2017;26:1247-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13072] [Article Influence: 688.0] [Reference Citation Analysis (12)] |
| 101. | Al-Zer H, Apel C, Heiland M, Friedrich RE, Jung O, Kroeger N, Eichhorn W, Smeets R. Enrichment and Schwann Cell Differentiation of Neural Crest-derived Dental Pulp Stem Cells. In Vivo. 2015;29:319-326. [PubMed] |
| 102. | Sanen K, Martens W, Georgiou M, Ameloot M, Lambrichts I, Phillips J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J Tissue Eng Regen Med. 2017;11:3362-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 103. | Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, Wolfs E, Bronckaers A, Hilkens P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J Endod. 2017;43:S12-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 104. | Kettenmann H, Verkhratsky A. [Neuroglia--living nerve glue]. Fortschr Neurol Psychiatr. 2011;79:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 106. | Bagheri-Hosseinabadi Z, Hamidabadi HG, Bojnordi MN. An efficient induction protocol for deriving mature oligodendrocytes from human dental stem cells. Bratisl Lek Listy. 2019;120:86-88. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S. Human Dental Pulp Stem Cells Differentiate into Oligodendrocyte Progenitors Using the Expression of Olig2 Transcription Factor. Cells Tissues Organs. 2014;200:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S. Tetracycline-regulated expression of OLIG2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. Neuroscience. 2015;305:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Saffari S, Saffari TM, Ulrich DJO, Hovius SER, Shin AY. The interaction of stem cells and vascularity in peripheral nerve regeneration. Neural Regen Res. 2021;16:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 110. | Hilkens P, Fanton Y, Martens W, Gervois P, Struys T, Politis C, Lambrichts I, Bronckaers A. Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 2014;12:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 111. | Hyung S, Im SK, Lee BY, Shin J, Park JC, Lee C, Suh JF, Hur EM. Dedifferentiated Schwann cells secrete progranulin that enhances the survival and axon growth of motor neurons. Glia. 2019;67:360-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 112. | Yamamoto T, Osako Y, Ito M, Murakami M, Hayashi Y, Horibe H, Iohara K, Takeuchi N, Okui N, Hirata H, Nakayama H, Kurita K, Nakashima M. Trophic Effects of Dental Pulp Stem Cells on Schwann Cells in Peripheral Nerve Regeneration. Cell Transplant. 2016;25:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 113. | Drewry MD, Dailey MT, Rothermund K, Backman C, Dahl KN, Syed-Picard FN. Promoting and Orienting Axon Extension Using Scaffold-Free Dental Pulp Stem Cell Sheets. ACS Biomater Sci Eng. 2022;8:814-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Okuwa Y, Toriumi T, Nakayama H, Ito T, Otake K, Kurita K, Nakashima M, Honda M. Transplantation effects of dental pulp-derived cells on peripheral nerve regeneration in crushed sciatic nerve injury. J Oral Sci. 2018;60:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Sultan N, Amin LE, Zaher AR, Grawish ME, Scheven BA. Dental pulp stem cells stimulate neuronal differentiation of PC12 cells. Neural Regen Res. 2021;16:1821-1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Ahmed MN, Shi D, Dailey MT, Rothermund K, Drewry MD, Calabrese TC, Cui XT, Syed-Picard FN. Dental Pulp Cell Sheets Enhance Facial Nerve Regeneration via Local Neurotrophic Factor Delivery. Tissue Eng Part A. 2021;27:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 117. | Kumar A, Kumar V, Rattan V, Jha V, Bhattacharyya S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol Neurobiol. 2017;54:4672-4682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Kanada S, Makino E, Nakamura N, Miyabe M, Ito M, Hata M, Yamauchi T, Sawada N, Kondo S, Saiki T, Minato T, Miyazawa K, Goto S, Matsubara T, Naruse K. Direct Comparison of Therapeutic Effects on Diabetic Polyneuropathy between Transplantation of Dental Pulp Stem Cells and Administration of Dental Pulp Stem Cell-Secreted Factors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, Lin Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 120. | Sharma Y, Shobha K, Sundeep M, Pinnelli VB, Parveen S, Dhanushkodi A. Neural Basis of Dental Pulp Stem Cells and its Potential Application in Parkinson's Disease. CNS Neurol Disord Drug Targets. 2022;21:62-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Liu C, Hu F, Jiao G, Guo Y, Zhou P, Zhang Y, Zhang Z, Yi J, You Y, Li Z, Wang H, Zhang X. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J Nanobiotechnology. 2022;20:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 122. | Venugopal C, K S, Rai KS, Pinnelli VB, Kutty BM, Dhanushkodi A. Neuroprotection by Human Dental Pulp Mesenchymal Stem Cells: From Billions to Nano. Curr Gene Ther. 2018;18:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 123. | Li S, Luo L, He Y, Li R, Xiang Y, Xing Z, Li Y, Albashari AA, Liao X, Zhang K, Gao L, Ye Q. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54:e13093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 124. | Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 125. | Ji L, Bao L, Gu Z, Zhou Q, Liang Y, Zheng Y, Xu Y, Zhang X, Feng X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol Res. 2019;67:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 126. | Zhou Z, Zheng J, Lin D, Xu R, Chen Y, Hu X. Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway. Int J Mol Med. 2022;50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 127. | Pivoraitė U, Jarmalavičiūtė A, Tunaitis V, Ramanauskaitė G, Vaitkuvienė A, Kašėta V, Biziulevičienė G, Venalis A, Pivoriūnas A. Exosomes from Human Dental Pulp Stem Cells Suppress Carrageenan-Induced Acute Inflammation in Mice. Inflammation. 2015;38:1933-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 128. | Gugliandolo A, Mazzon E. Dental Mesenchymal Stem Cell Secretome: An Intriguing Approach for Neuroprotection and Neuroregeneration. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 129. | Gervois P, Wolfs E, Dillen Y, Hilkens P, Ratajczak J, Driesen RB, Vangansewinkel T, Bronckaers A, Brône B, Struys T, Lambrichts I. Paracrine Maturation and Migration of SH-SY5Y Cells by Dental Pulp Stem Cells. J Dent Res. 2017;96:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 130. | Sultan N, Amin LE, Zaher AR, Grawish ME, Scheven BA. Neurotrophic effects of dental pulp stem cells on trigeminal neuronal cells. Sci Rep. 2020;10:19694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 131. | Chouaib B, Collart-Dutilleul PY, Blanc-Sylvestre N, Younes R, Gergely C, Raoul C, Scamps F, Cuisinier F, Romieu O. Identification of secreted factors in dental pulp cell-conditioned medium optimized for neuronal growth. Neurochem Int. 2021;144:104961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 132. | Makino E, Nakamura N, Miyabe M, Ito M, Kanada S, Hata M, Saiki T, Sango K, Kamiya H, Nakamura J, Miyazawa K, Goto S, Matsubara T, Naruse K. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig. 2019;10:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 133. | Yang Y, Yuan X, Ding F, Yao D, Gu Y, Liu J, Gu X. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17:2231-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 134. | Murakami M, Hayashi Y, Iohara K, Osako Y, Hirose Y, Nakashima M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal Stem Cells From Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/Dentin Regeneration. Cell Transplant. 2015;24:1753-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 135. | Ishizaka R, Hayashi Y, Iohara K, Sugiyama M, Murakami M, Yamamoto T, Fukuta O, Nakashima M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials. 2013;34:1888-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 136. | Bousnaki M, Bakopoulou A, Pich A, Papachristou E, Kritis A, Koidis P. Mapping the Secretome of Dental Pulp Stem Cells Under Variable Microenvironmental Conditions. Stem Cell Rev Rep. 2022;18:1372-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 137. | Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia. 2001;34:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | Iwashita Y, Fawcett JW, Crang AJ, Franklin RJ, Blakemore WF. Schwann cells transplanted into normal and X-irradiated adult white matter do not migrate extensively and show poor long-term survival. Exp Neurol. 2000;164:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 140. | Yuan SM, Yang XT, Zhang SY, Tian WD, Yang B. Therapeutic potential of dental pulp stem cells and their derivatives: Insights from basic research toward clinical applications. World J Stem Cells. 2022;14:435-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 141. | Moayeri A, Nazm Bojnordi M, Haratizadeh S, Esmaeilnejad-Moghadam A, Alizadeh R, Ghasemi Hamidabadi H. Transdifferentiation of Human Dental Pulp Stem Cells Into Oligoprogenitor Cells. Basic Clin Neurosci. 2017;8:387-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 142. | Wang D, Wang Y, Tian W, Pan J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019;52:e12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 143. | Häfner SJ. Bargain with the tooth fairy - The savings accounts for dental stem cells. Biomed J. 2020;43:99-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 144. | Thalakiriyawa DS, Dissanayaka WL. Advances in Regenerative Dentistry Approaches: An Update. Int Dent J. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 145. | Takaoka S, Uchida F, Ishikawa H, Toyomura J, Ohyama A, Watanabe M, Matsumura H, Marushima A, Iizumi S, Fukuzawa S, Ishibashi-Kanno N, Yamagata K, Yanagawa T, Matsumaru Y, Bukawa H. Transplanted neural lineage cells derived from dental pulp stem cells promote peripheral nerve regeneration. Hum Cell. 2022;35:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 146. | Wang DR, Wang YH, Pan J, Tian WD. Neurotrophic effects of dental pulp stem cells in repair of peripheral nerve after crush injury. World J Stem Cells. 2020;12:1196-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 147. | Hei WH, Kim S, Park JC, Seo YK, Kim SM, Jahng JW, Lee JH. Schwann-like cells differentiated from human dental pulp stem cells combined with a pulsed electromagnetic field can improve peripheral nerve regeneration. Bioelectromagnetics. 2016;37:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 148. | Qiao W, Lu L, Wu G, An X, Li D, Guo J. DPSCs seeded in acellular nerve grafts processed by Myroilysin improve nerve regeneration. J Biomater Appl. 2019;33:819-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haider KH, Saudi Arabia; Sheykhhasan M, Iran S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang XD