Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.146
Peer-review started: March 16, 2021
First decision: May 5, 2021
Revised: May 19, 2021
Accepted: February 14, 2022
Article in press: February 14, 2022
Published online: February 26, 2022
Processing time: 346 Days and 0.7 Hours
Cancer stem cells (CSCs) comprise a subpopulation of cancer cells with stem cell properties, which exhibit the characteristics of high tumorigenicity, self-renewal, and tumor initiation and are associated with the occurrence, metastasis, therapy resistance, and relapse of cancer. Compared with differentiated cells, CSCs have unique metabolic characteristics, and metabolic reprogramming contributes to the self-renewal and maintenance of stem cells. It has been reported that CSCs are highly dependent on lipid metabolism to maintain stemness and satisfy the requirements of biosynthesis and energy metabolism. In this review, we demonstrate that lipid anabolism alterations promote the survival of CSCs, including de novo lipogenesis, lipid desaturation, and cholesterol synthesis. In addition, we also emphasize the molecular mechanism underlying the relationship between lipid synthesis and stem cell survival, the signal trans-duction pathways involved, and the application prospect of lipid synthesis reprogramming in CSC therapy. It is demonstrated that the dependence on lipid synthesis makes targeting of lipid synthesis metabolism a promising therapeutic strategy for eliminating CSCs. Targeting key molecules in lipid synthesis will play an important role in anti-CSC therapy.
Core Tip: Cancer stem cells (CSCs) are associated with the occurrence, metastasis, therapy resistance, and relapse of cancer. CSCs are highly dependent on lipid metabolism to maintain stemness and satisfy the requirements of biosynthesis and energy metabolism. Here, we review the molecular mechanism underlying the relationship between lipid synthesis and stem cell survival, the signal transduction pathways involved, and the application prospect of lipid synthesis reprogramming in CSC therapy. We demonstrate that lipid anabolism alterations promote the survival of CSCs.
- Citation: Wang SY, Hu QC, Wu T, Xia J, Tao XA, Cheng B. Abnormal lipid synthesis as a therapeutic target for cancer stem cells. World J Stem Cells 2022; 14(2): 146-162
- URL: https://www.wjgnet.com/1948-0210/full/v14/i2/146.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i2.146
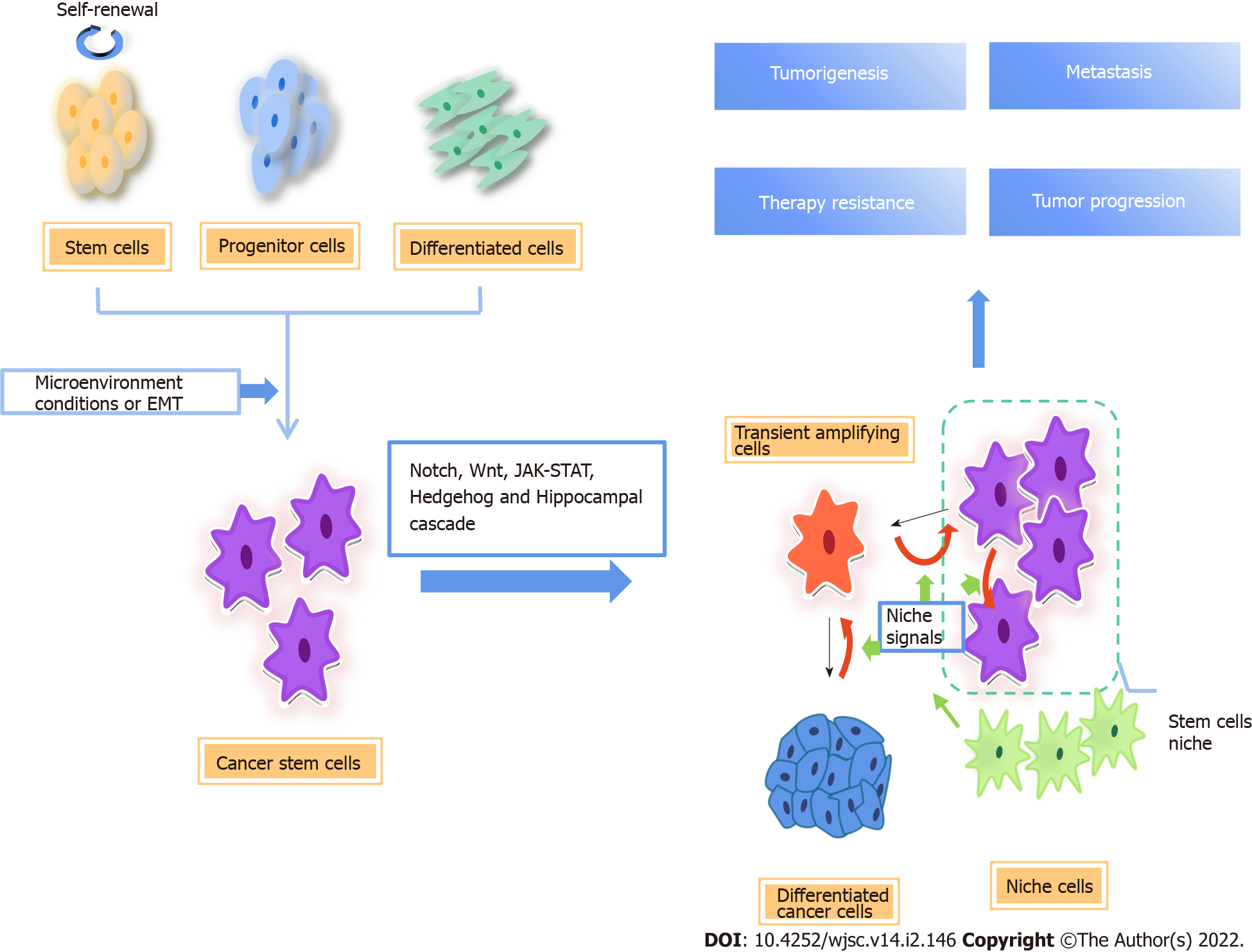
Cancer stem cells (CSCs) comprise a subpopulation of cancer cells with stem cell properties, which exhibit the characteristics of high tumorigenicity, self-renewal, and tumor initiation. They may be responsible for cancer occurrence, metastasis, therapy resistance, and relapse of cancer[1,2]. CSCs are able to differentiate into diverse cancer cell progenies to maintain the hierarchical organization of a tumor[3].
In solid tumors, the expression of the CSC markers, including CD133, CD44, and aldehyde dehydrogenase (ALDH)1, is similar to that in normal human embryonic stem cells, thus transformed adult stem cells are one possible source of CSCs. Another possibility is differentiated cells under long-term stress conditions, which transform into CSCs through reprogramming due to genetic instability and epigenetic abnormalities[4-6] (Figure 1).
Various studies have shown that both CSC and non-CSC are plastic, and the interconversion between them may be a common phenomenon. Epithelial-to-mesenchymal transition (EMT) is the process by which epithelial cancer cells acquire a mesenchymal gene program that promotes migration and invasion. Many studies suggest that EMT promotes the transition from non-CSCs to CSC[7]. During EMT, cancer cells obtain stem cell-like properties to migrate and grow into distant tissues[8-10]. In a human model, the EMT major transcription factor Snail was elevated in cancer cells that displayed enhanced oncogenic capability and metastatic potential and was tightly associated with a CSC phenotype[11]. The plasticity of CSCs is also closely related to microenvironment. Angiogenesis, the hypoxic niche, and extracellular matrix are essential for maintaining the stemness of glioblastoma stem cells[12]. In addition, there is evidence that, in colon cancer, myofibroblasts enhance Wnt signaling through secreted factors, establishing a CSC niche and restoring the stemness of highly differentiated cancer cells[13]. In non-CSCs, the promoter of zinc-finger E-box-binding (ZEB)1, the key regulator of EMT, maintains the bivalent chromatin configuration, making non-CSCs respond readily to microenvironmental signals. When the promoter converts to active chromatin configuration, ZEB1 transcription increases and non-CSCs convert to the CSC state.
Independent of the origin, CSCs are important cancer cell subsets. The existence of CSCs is clearly demonstrated in different types of cancer, including leukemia[14,15], tongue squamous cell carcinoma[16], breast cancer[17], glioblastoma[18], lung cancer[19,20], and osteosarcoma[21]. They actuate tumorigenesis and progression, and promote therapy resistance, metastasis, and recurrence of cancers. A growing number of studies have shown that metabolic reprogramming of cancer cells caused by changes in the microenvironment exerts a marked effect on the properties of stem cells.
The interaction between CSCs and the tumor microenvironment (TME) is related to tumorigenesis and disease progression[22]. Due to the rapid proliferation of tumor cells and insufficient angiogenesis, the TME has the characteristics of hypoxic, acidic, and nutrient-poor conditions; therefore, tumor cells must adjust energy metabolism to deal with this adverse microenvironment, and maintain the rapid growth and proliferation of tumor cells[23-25], a process called metabolic reprogramming. The metabolic phenotype of CSCs may depend on the microenvironment to a great extent.
Several studies have been conducted on a variety of cancer types, such as nasopharyngeal carcinoma[26], leukemia[27], osteosarcoma[28], breast cancer[29], and ovarian cancer[30], which suggest that CSCs show a greater reliance on glycolysis for energy supply compared with other differentiated cancer cells in vitro and in vivo. Evidence suggests that paracrine hepatocyte growth factor/c-MET enhances the expression of hexokinase 2 and promotes glycolysis by activating Yes-associated protein (YAP)/ hypoxia-inducible factor-1α in pancreatic cancer, which may facilitate CSC-like properties[31].
However, there is also growing evidence that mitochondrial oxidative metabolism is the preferred form of energy production in CSCs, including CD133+ colon cancer cells[32], CD44+ and CD117+ ovarian cancer cells[33], cholangiocarcinoma cells[34], brain tumor cells[35], and leukemia cells[36]. In addition, it is found that pancreatic CSCs (PaCSCs) are enriched in the oxidative phosphorylation (OXPHOS) promotion system using galactose instead of glucose as carbon source in vitro. And significant CSC features are present, such as the expression of multiple CSC biomarkers, the overexpression of stem-related pathways, the enhancement of self-renewal ability, and the significant improvement of tumorigenicity in vivo. Meanwhile, OXPHOS promoted the immune escape properties of PaCSCs[37].
A large number of the above studies have shown that CSC metabolism is highly heterogeneous. CSCs exhibit a metabolic phenotype dependent on glycolysis or OXPHOS, which mainly depends on the heterogeneity of tumor origin and surrounding microenvironmental conditions.
In addition to glucose metabolism, alterations in lipid metabolism also modulate tumor development and progression. Lipid metabolism is related to the stem cell properties in cancers. A growing body of evidence suggests that alterations in metabolic pathways associated with lipids, including fatty acids (FA) and cholesterol, are crucial for maintaining the stemness of CSCs. Lipid synthesis and catabolism are strictly regulated by CSCs to maintain self-renewal, proliferation, and chemotherapy resistance of the CSCs. Increased de novo lipid biosynthesis and lipid storage, as well as enhanced lipid oxidation, are unique features of many CSCs. It has been reported that fatty acid oxidation (FAO) can support self-renewal and drug resistance of breast CSCs. The Leptin-LEPR-JAK-STAT3-dependent FAO pathway plays an important role in the self-renewal of breast cancer stem cell (BCSC) associated with chemotherapy resistance in breast cancer. Blocking FAO and/or Leptin re-sensitize them to chemotherapy and inhibit breast CSCs in vivo[38]. Furthermore, targeting FAO enhances the chemotherapy efficacy of cytarabine (AraC) in AraC-resistant acute myeloid leukemia enriched in leukemic stem cells[39]. Mesenchymal stem cells promoted stemness and chemoresistance in gastric cancer cells through FAO in vitro and in vivo[40]. Lipid droplets (LDs), organelles that store neutral lipids, are accumulated in CSCs in numerous types of cancer[41,42]. LDs are more abundant in pancreatic and colorectal CSCs than in isogenic non-CSCs[43].
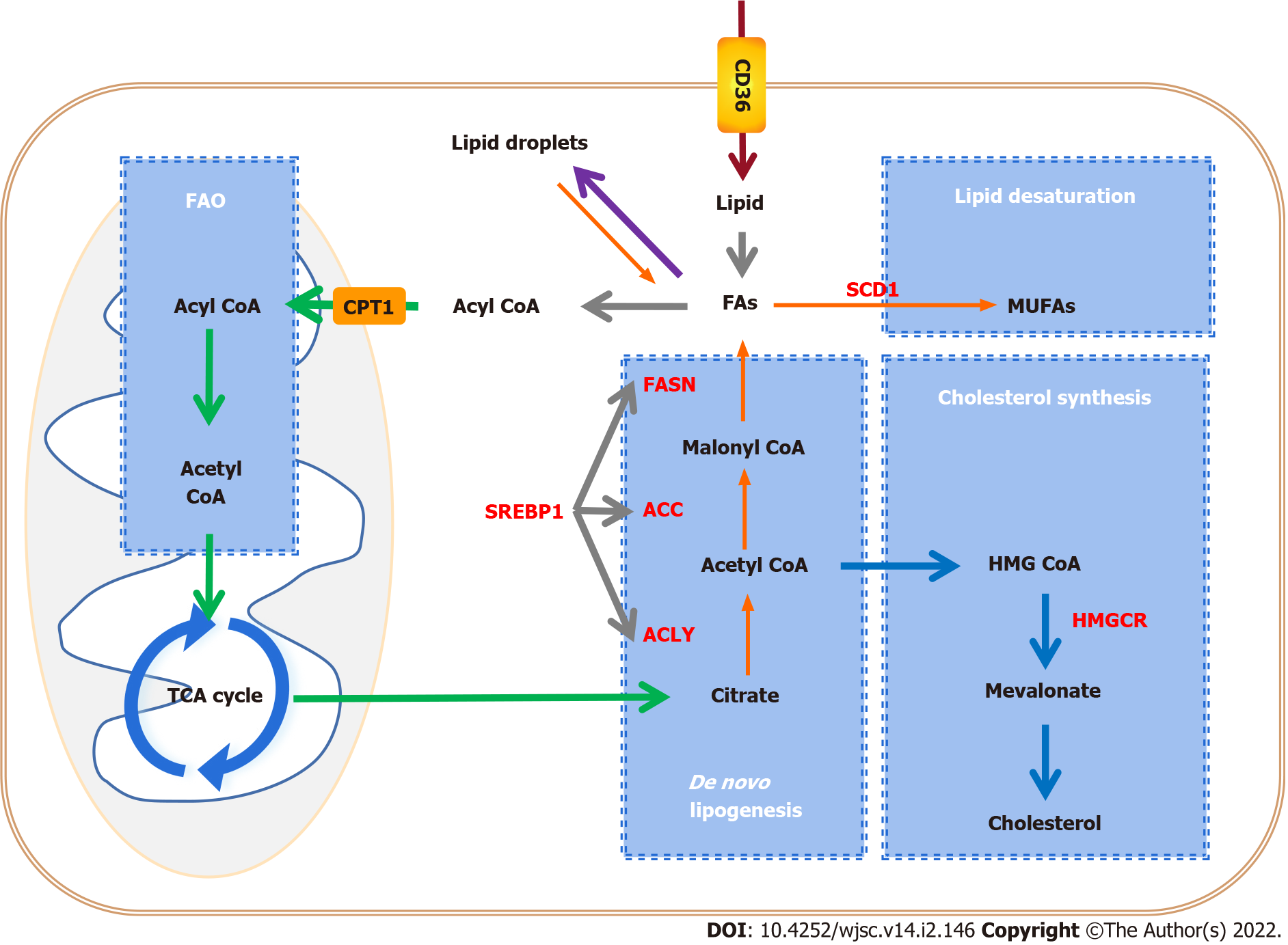
Lipid synthesis has been shown to play a significant role in maintaining the characteristics of CSCs during tumorigenesis. De novo lipid biosynthesis is one of the most targetable features of CSCs[44]. We will highlight the important role of lipid synthesis in CSCs, including the pathways involved and promising therapeutic targets (Figure 2).
Lipid synthesis includes de novo lipid biosynthesis, lipid desaturation, and cholesterol synthesis. Metabonomic analysis demonstrated that FA and cholesterol synthesis displays high activity in triple-negative breast CSCs (TNBCSCs). Cholesterol synthesis is essential for the survival and migration of CSCs, and inhibition of cholesterol synthesis induces cytotoxic effects on CSCs. For instance, pyridine pamoate (PP) can induce a cell killing effect on CSCs and prevent tumor metastasis by inhibiting cholesterol anabolic flux. By supplementing cholesterol to restore the level of free and bound cholesterol, the cytotoxicity induced by PP is effectively limited[45]. Compared with non-CSCs, the rates of lipid unsaturation in the CSCs were further increased[46,47]. In addition, in various cancers such as ovarian cancer, glioblastoma multiforme, and colon cancer, more monounsaturated FAs (MUFAs) are demanded by CSCs, which indicates that MUFAs may be involved in mediating various signaling pathways in CSCs and associated with stemness, and lipid desaturation may be an ideal and specific therapeutic target for CSCs[48,49].
Experimental investigation indicated that de novo FA synthesis is more active in CSCs than in differentiated cells, suggesting that it is essential for CSCs to maintain stemness. In CSCs, the key rate-limiting enzymes of de novo FA synthesis, including ATP-citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), and fatty acid synthase (FASN), as well as sterol regulatory element-binding proteins (SREBPs), which regulate the expression level of lipid synthesis genes, are highly expressed.
ACLY is principally located in the cytoplasm, which catalyzes the conversion of citrate to acetyl-CoA. Acetyl-CoA is not only an important substrate for the synthesis of FAs and cholesterol, but it is also necessary for protein acetylation reactions. Therefore, ACLY is a key enzyme of lipid synthesis that links catabolic pathways to biosynthesis. In many types of cancer, ACLY is upregulated or activated[50-52]. ACLY upregulation contributes to stemness maintenance and tumorigenesis[53,54]. ACLY overexpression increased the expression of Snail, which is known to promote EMT and stemness[55]. ACLY inhibition decreased the invasiveness of breast cancer cells, and targeting ACLY attenuated the proliferation potential and cisplatin resistance in ovarian cancer[56,57].
ACC catalyzes the ATP-dependent carboxylation of acetyl CoA to generate malonyl-CoA, which is a rate-limiting step in de novo FA synthesis. In pancreatic cancer cells, inhibition of ACC inhibits Wnt and Hedgehog (HH) signal transduction by inhibiting palmitoylation of their ligands, and inhibits the growth of pancreatic tumors in vivo and in vitro. ACC inhibitors can restore tumor cells to histological epithelial phenotype in vitro[58]. Moreover, ACC is highly expressed in induced pluripotent stem cells (iPSCs). Pharmacological inhibition of ACC significantly reduced reprogramming efficiency in iPSCs[59]. Research reveals that inhibiting the activation of ACC can effectively restore intracellular lipid levels, reduce EMT, and inhibit the features of CSCs[60].
FASN, the key enzyme of de novo lipogenesis, is highly expressed in human pluripotent stem cells (hPSCs) compared with that in hPSC-derived cardiomyocytes (hPSC-CMs)[61]. In addition, it is highly active in adult neural stem and progenitor cells, which require FASN-dependent lipogenesis for proliferation[62]. Data suggest that de novo lipogenesis is higher and FASN expression is upregulated in glioma stem cells (GCSs). Pharmacological inhibition of FASN dramatically decreases the expression of GSC stemness markers, including Sox2, Nestin, CD133, and FABP7, and thus inhibits cell proliferation and invasiveness of GSCs[63]. Moreover, downregulation of FASN suppresses CSCs in breast cancer[64] and pancreatic cancer[65].
SREBPs are a class of transcription factors that regulate lipid homeostasis by controlling the expression of a series of key enzymes required for cholesterol and FA synthesis. Three SREBP subtypes have distinctive roles in lipid synthesis: SREBP1a regulates FA and cholesterol synthesis, and cholesterol absorption, SREBP1c regulates FA synthesis, and SREBP2 specifically regulates cholesterol synthesis and uptake. SREBPs are downstream molecules of the PI3K/AKT/mTOR signaling pathway. Regulation of SREBPs through the PI3K/AKT/mTOR pathway can regulate glucose production and FA synthesis, and affect the proliferation and invasion of cancer cells[66,67]. Downregulation of SREBP inhibited the growth of non-small-cell lung cancer cells and liver cancer cells[67,68]. SREBP1 targets key enzymes of FA synthesis, such as ACLY, ACC, FASN, and stearyl coenzyme A desaturase 1 (SCD1), to regulate lipid metabolism[69], and is highly expressed in various cancers[69-71]. Compared to differentiating melanosphere-derived cells, the expression of SREBP1 is enhanced in melanosphere-derived CSCs[42]. Gemcitabine is a standard treatment for advanced pancreatic cancer patients but can cause chemoresistance during treatment. The chemoresistant cells have features of CSCs. Gemcitabine is widely used in chemotherapy for advanced pancreatic cancer, but chemotherapy in turn promotes the stemness of CSCs. Resveratrol inhibits SREBP1, resulting in the inhibition of lipid synthesis and the stemness induced by gemcitabine, and enhances the sensitivity of gemcitabine[72].
MUFAs, such as palmitoleic acid and oleic acid, are key substrates in the formation of complex lipids such as phospholipids, triglycerides, and cholesterol esters, and maintain optimal fluidity of cellular membranes. Moreover, MUFAs have a protective function against the lipotoxicity caused by excess saturated FAs and other cellular stresses[73,74]. SCD catalyzes the committed step in the biosynthesis of MUFAs from saturated FAs[75,76]. There are two isoforms in humans, SCD1 and SCD5. The expression of SCD5 is high in the brain and pancreas, while SCD1 is the main subtype, and is highly expressed in adipose tissue, the brain, liver, heart, and lung[77]. SCD1 is overexpressed in a variety of tumors, including ovarian cancer[78], breast cancer[79], prostate cancer[80], and colon cancer[81]. The upregulation of SCD1, which increases lipid desaturation and relieves endoplasmic reticulum stress, promotes ovarian cancer progression and metastasis[82]. Inhibition of SCD1 can inhibit the growth of leukemic cells in the central nervous system[83]. A growing number of studies on SCD1 have indicated that it plays a key role in tumorigenesis and maintenance of stemness[84-86]. SCD1 promotes the activation of NF-κB by increasing the synthesis of polyunsaturated FA (PUFAs) to promote CSC characteristics. In turn, the NF-κB pathway regulates the expression of lipid desaturase by regulating transcription. This supports a positive feedback loop involving the NF-κB pathway and lipid desaturase in ovarian CSCs[46]. Furthermore, SCD1 controls the fate of breast CSCs by regulating Wnt/β-catenin signaling[87].
Cholesterol is an important component of cell membranes and lipid rafts. Highly proliferating cancer cells require increased cholesterol synthesis to meet the need for rapid production of cell membranes. At the same time, metabolically active cancer cells need lipid rafts to form signal complexes for multiple complex signal transduction[88,89]. Cholesterol is produced by a variety of biosynthetic processes or obtained from the diet. Cholesterol synthesis occurs in most tissues and cells. The synthetic pathway involves the conversion of acetyl-CoA to cholesterol through a series of enzymatic reactions, including the biosynthesis of mevalonate (MVA) and squalene[90,91]. There are three crucial players in the cholesterol synthesis pathway, namely, SREBP2 and the two key rate-limiting enzymes, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and squalene epoxidase (SQLE). Of these, SREBP2 is the master transcriptional regulator of cholesterol biosynthesis. HMGCR and SQLE reduce HMG-CoA to MVA and catalyze the oxidation of squalene to 2,3-epoxy-quinenone, respectively[92]. Increased cholesterol synthesis is considered to be a unique hallmark of many cancers[93]. Pharmacological inhibition of cholesterol biosynthesis dramatically suppressed crypt growth in vivo and ex vivo, which demonstrates that cholesterol itself acts as a mitogen for intestinal stem cells (ISCs). Cholesterol biosynthesis can drive ISC proliferation and tumorigenesis[94]. Proteomic analysis of tumor tissues, patient-derived xenograft, and mammospheres known to be enriched in CSCs revealed that the expression of proteins involved in the cholesterol synthesis pathway in CSCs increased. Simvastatin or siRNA blocking cholesterol biosynthesis reduced the formation of mammospheres. These results confirm that CSCs are highly dependent on metabolic processes associated with cholesterol biosynthesis, suggesting that the cholesterol biosynthesis pathway is a potential therapeutic target for the elimination of CSCs[95].
SREBP2 specifically regulates cholesterol synthesis and uptake to maintain intracellular cholesterol homeostasis. Evidence indicates that apoA-I binding protein-mediated cholesterol efflux activates endothelial SREBP2 which in turn transactivates Notch and promotes hematopoietic stem and progenitor cell (HSPC) emergence. SREBP2 inhibition impairs hypercholesterolemia-induced HSPC expansion[96]. Biofunctional analyses demonstrated that SREBP2 promotes stem cell-like characteristics and metastasis of prostate cancer cells. The overexpression of SREBP2 increases the population of prostate CSCs and promotes the tumorigenicity of prostate cancer cells in vivo, while gene silencing of SREBP2 inhibits the growth, metastasis, and stemness of prostate cancer cells[97]. In colon cancer, inhibition of SREBP2 blocked the proliferation of cancer cells and reduced CSC properties. Knockdown of SREBP inhibits the growth of xenograft tumor in vivo[98].
The MVA pathway produces isoprenoids, such as cholesterol and vitamin D, which are essential for a variety of cellular functions from cholesterol synthesis to cell survival and growth[91]. Many studies have shown that numerous enzymes (HMGCR, FDPS, squalene synthase, and SQLE) required for cholesterol synthesis in the MVA pathway are overexpressed and overactivated in several cancers, including multiple myeloma, as well as breast, gastric, lung, colon, and prostate cancers. Targeting MVA can effectively inhibit the survival and proliferation ability of cancer cells and reduce the tumorigenic potential[99-104]. Overactivation of key enzymes in cholesterol synthesis in the MVA pathway is usually associated with a poor prognosis with shorter disease-free survival and reduced overall survival[105-107]. Statins inhibit HMGCR, the rate-limiting enzyme of the MVA pathway. Genetic variants associated with low HMG-CoA reductase function significantly reduced the risk of epithelial ovarian cancer[108]. Lovastatin inhibited SOX2 promoter transactivation and reduced the efficiency of mammosphere formation and the percentage of ALDH+ cells in vitro. Gene set enrichment analysis indicated that lovastatin downregulates genes that are involved in stemness and invasiveness of breast CSCs[109]. Atorvastatin has a stronger anti-proliferative effect on CSCs by inhibiting the MVA pathway[110]. Cholesterol and MVA increase the proliferation of breast CSCs and promote breast cancer progression, invasion, and chemotherapy resistance through activation of the estrogen-related receptor α pathway[111]. Long non-coding RNA (lncRNA)/mRNA microarray assays showed that a novel lncRNA (named lnc030) cooperates with poly (rC) binding protein 2 (PCBP2) to stabilize SQLE mRNA, resulting in increased cholesterol which activates PI3K/Akt signaling in governing BCSC stemness[112].
In addition, the MVA pathway is the only source of intracellular isopentenyl- diphosphate, which produces farnesyl-diphosphate and geranylgeranyl-diphosphate (GGPP) for the prenylation of proteins. For example, different types of preacylation enable the RasGTPase superfamily, including Ras and Ral/Rho, to be correctly directed to specific subcellular membranes to function. The RasGTPase superfamily affects a variety of cellular processes in cancer progression and participates in EMT, tumor progression, metastasis, and chemotherapy resistance. Inhibition of the MVA pathway can reduce GTPases prenylation and can induce the death of cancer cells, suggesting that these MVA pathway metabolites are essential for cancer cell viability[91,110]. In addition, inhibiting the MVA pathway with small-molecule inhibitors such as statins has been shown to cause inhibition of YAP/transcriptional co-activator with PDZ-binding motif (TAZ) activity. Studies have shown that the activation of RhoGTPases requires GGPP, and the Rho-dependent YAP/TAZ regulatory pathway inhibits YAP/TAZ phos-phorylation and promotes their nuclear accumulation to play a role[113-115]. Decreasing the activation of Rho-GTPases and Hippo-YAP/TAZ represses the expression of genes associated with breast cancer stemness[116]. YAP/TAZ nuclear accumulation and transcriptional activity are attenuated by Rho-GTPase/F-actin signaling to increase the sensitivity to chemotherapeutic drugs and suppress breast cancer chemoresistance[117].
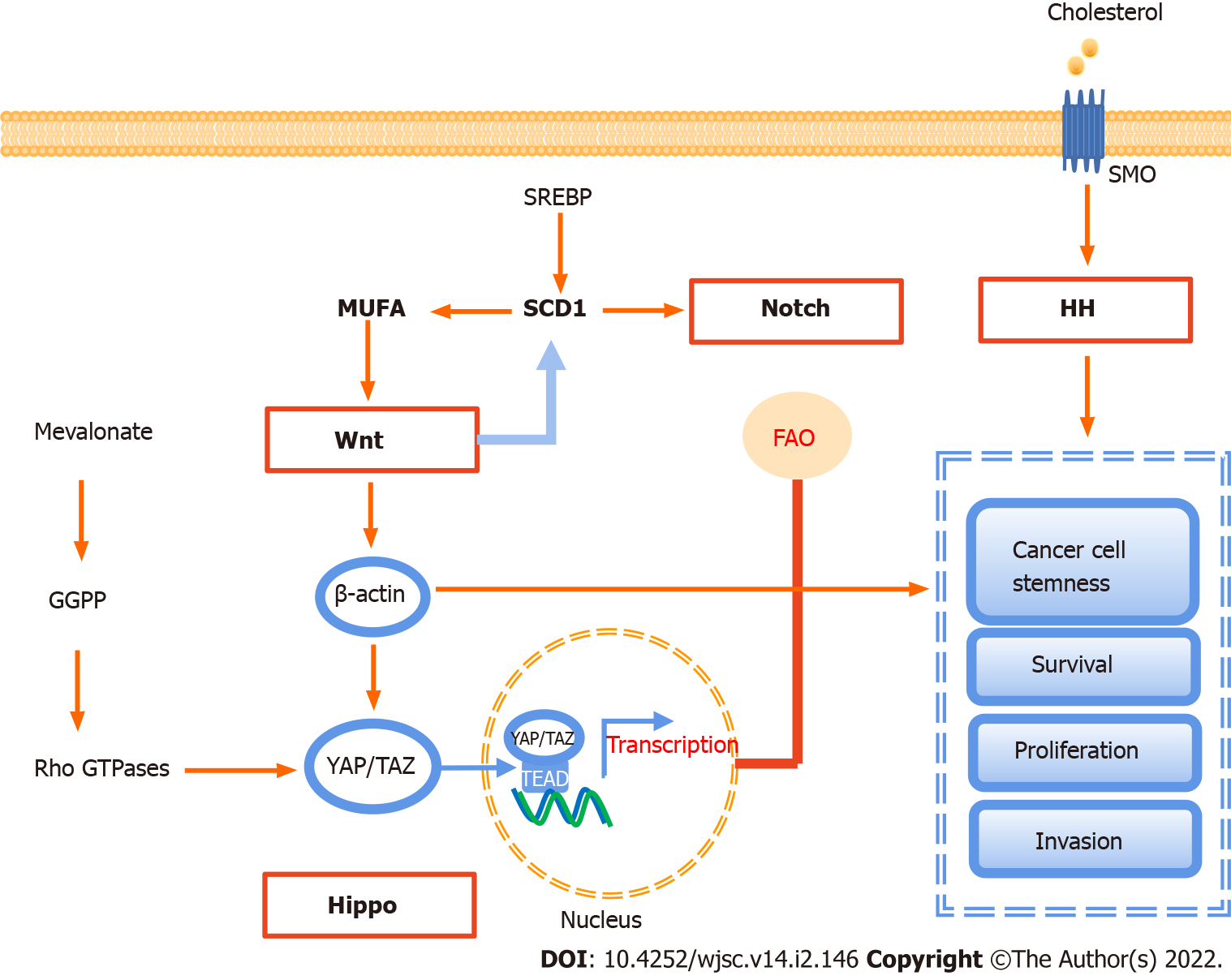
In CSCs, there are a series of pathways involved in lipid metabolism to maintain cell stemness, and sustain their survival, proliferation, and invasion, including Notch, hippocampal cascade, HH, and Wnt signaling (Figure 3).
Notch signaling is a highly conservative signal transduction pathway, which is closely related to various biological behaviors such as tumor metastasis and immune escape[22,118,119]. In terms of lipid metabolism, the Notch signaling pathway can regulate the expression of peroxisome proliferator-activated receptor α and lipid oxidation genes to achieve lipid homeostasis and redox homeostasis[120]. In colon cancer, targeting SCD1-dependent lipid desaturation selectively eliminates colon CSCs by inhibiting Notch signaling[49,121].
The Wnt signal cascade includes three main pathways: The canonical Wnt pathway, which leads to the accumulation of β-catenin, activates the transactivation complex, and participates in tumorigenesis, the non-canonical planar cellular polarity pathway, and the non-canonical Wnt-calcium pathway[119]. At least 19 Wnt family members have been identified in humans, all of which are lipid-modified secretory glycoproteins. They are the ligands of ten Frizzled family receptors[22,122].
Wnt signaling plays a key role in regulating CSCs[13,123,124]. The canonical Wnt signaling pathway, activated by ligands such as Wnt2β and Wnt3, promotes the proliferation of CSC by up-regulating β-catenin and terminating target β-catenin and STOP-target proteins, such as FOXM1, MYC, and YAP/TAZ, while the non-canonical Wnt signaling pathway in CSCs is activated by non-canonical Wnt ligands such as Wnt5A and Wnt11, thus activating the PI3K/AKT signal and inducing YAP/TAZ-dependent transcriptional activation to promote survival and therapeutic resistance of CSCs[125]. In contrast, tumor invasion and metastasis are driven by both the canonical and non-canonical Wnt signaling cascades. Canonical Wnt/β-catenin and Wnt/STOP signaling cascades cooperatively upregulate SNAI1 to initiate EMT of CSCs[126].
Wnt signaling has also been associated with lipid synthesis in CSCs. The canonical Wnt/β-catenin pathway regulates de novo lipogenesis and fatty acid monounsaturation[127]. SCD could be a key regulator between the Wnt signaling pathway and lipid metabolism. In mouse liver CSCs, the expression of SCD is regulated by the Wnt-β-catenin signaling pathway, while MUFAs produced by SCD provide a positive feedback loop to amplify Wnt signaling by promoting the stability and expression of Lrp5/6 mRNA[128]. Another study suggests that MUFAs are crucial in the production and secretion of Wnt ligands[129]. Finally, FA metabolism, especially SCD1 activity, in YAP/TAZ signaling depends on the activity of the β-catenin pathway in CSCs[130].
The core of the Hippo signaling pathway is the kinase cascade involving mammalian STE20-like (MST)1/2 and LATS1/2. MST1/2 activates LATS1/2 by promoting autosphosphorylation of LATS1/2 or by phosphorylation of MOB1, resulting in degradation of the downstream transcriptional coactivators YAP1 and TAZ, thereby limiting YAP activity[22,131]. YAP/TAZ activation leads to the induction of CSC properties, including self-renewal, tumorigenic potential, anoikis resistance, EMT, drug resistance, and metastasis, in a wide range of human cancers[132,133]. As mentioned earlier, in lung CSCs, SCD1 regulates lung cancer stemness by stabilizing YAP/TAZ and nuclear localization[130]. The positive feedback loops of LATS2 and p53 inhibit cholesterol synthesis, and LATS2 binds to the endoplasmic reticulum tethered precursor (P-SREBP) of SREBP1 and SREBP2, and inhibits the transcription of SREBP mRNA, thus inhibiting the activity of cellular SREBP[134]. Recent studies have revealed that the cancer-promoting properties of YAP/TAZ depend on cholesterol biosynthesis activity and MVA-dependent nuclear localization and activity of YAP/TAZ[114]. YAP/TAZ-mediated lipid synthesis may be an important factor affecting the metabolic changes of CSCs[135].
The HH signaling pathway, which is responsible for the signal transmission from the cell membrane to the nucleus, is a highly conservative pathway. HH ligands mainly include Sonic hedgehog (SHH), Indian HH, and Desert HH. The HH signal pathway is activated by the binding of HH ligands to the transmembrane proteins Patched (PTCH)1/2, which release the inhibition of smoothened (SMO), leading to the activation of glioma transcription factors, thus inducing target gene transcription[22]. HH ligands have been found to be activated in CSCs. High fibrillar collagen content resulting from HH pathway activation promotes breast cancer cell stemness. In cholangiocarcinoma, hypoxia promoted SHH pathway activation. Inhibition of the SHH pathway by cyclopamine significantly attenuated the expression of CSC transcription factors, leading to the abrogation of CD133 expression and EMT[136].
Previous evidence suggested that lipids are key regulators of HH signaling. The cholesterol covalent modification of SMO is regulated by the HH signaling pathway and is very important for the signal transduction and cell biological function of HH. PTCH1 inhibits the cholesterol modification of SMO, while the overexpression of SHH increases the cholesterol modification of SMO[137]. In addition, SMO activates adenosine monophosphate kinase via the non-canonical pathway, directly or indirectly inhibiting FA and cholesterol synthesis[138].
CSCs can adapt easily to changes in the nearby environment and are more resistant to conventional therapies than other cancer cells. However, their proliferation and survival are highly dependent on lipid synthesis, which provides a point of penetration for the establishment of efficient targeting strategies to eliminate CSCs. Targeted clearance of CSCs can be achieved by interfering with different aspects of lipid synthesis, such as FA synthesis, lipid desaturation, and cholesterol synthesis (Table 1).
| Metabolism type | Targeting enzyme | Drug | Cancer type | Metabolic processes or signaling pathways involved | Study type |
| Lipogenesis | FASN | Cerulenin | Glioma stem cells[63], pancreatic CSCs[65] | FASN | Preclinical trial |
| FASN | TVB-2640 | NSCLC and breast cancer[139] | FASN | Clinical trial | |
| ACC | Soraphen A | Breast CSCs[140] | FASN | Preclinical trial | |
| ACC | ND-646 | Non-small-cell lung CSCs[142] | FASN | Preclinical trial | |
| ACC | Leptin | Breast CSCs[141] | TAK1-AMPK signaling | Preclinical trial | |
| Lipid desaturation | SCD1 | CAY10566 | Ovarian CSCs[46], glioblastoma CSCs[84] | NF-κB pathway, ER stress | Preclinical trial |
| SCD1 | A939572 | Liver cancer[146], etc. | MUFA synthesis | Preclinical trial | |
| SCD1 | MF-438 | Colon CSCs[121], lung CSCs[85] | Wnt, Notch, and YAP/TAZ signaling | Preclinical trial | |
| SCD1 | PluriSIn#1 | Colon CSCs[121], liver CSCs[150] | Wnt/β-catenin and Notch signaling | Preclinical trial | |
| Delta 6 desaturase | SC-26196 | Ovarian CSCs[46] | Polyunsaturated fatty acid synthesis | Preclinical trial | |
| Cholesterol synthesis | SREBPs | 25-HC or fatostatin | Colon CSCs[98] | Fatty acid synthesis and cholesterol synthesis | Preclinical trial |
| Pyrvinium pamoate | TNBC CSCs[45] | Cholesterol biosynthesis | Preclinical trial | ||
| HMGCR | Simvastatin | Breast CSCs[95] | Cholesterol biosynthesis | FDA-approved cardiovascular system drug |
FASN is the most targetable among the lipogenesis genes. Some FASN inhibitors have shown anti-CSC and anti-tumor activities. Both inhibitor and RNA silencing of FASN decreased invasiveness, sphere formation, and expression of stemness markers to kill various CSCs[63,65]. A new generation of FASN inhibitors is being developed, and data from early clinical trials on TVB-2640, a FASN inhibitor, show a partial tumor response in patients with non-small-cell lung cancer and breast cancer when TVB-2640 was used in combination with paclitaxel[139]. Similarly, Soraphen A, an ACC inhibitor, suppressed mammosphere formation. Sorafen A treatment inhibited the self-renewal and growth of CSC-like cells by blocking FA synthesis and eliminated the promoting effect of human epidermal growth factor receptor 2 on CSC proliferation[140]. Moreover, inhibition of ACC suppresses tumor growth, metastasis, and recurrence in non-small-cell lung cancer and breast cancer[141,142], indicating that ACC has great significance and potential in inhibiting CSCs and cancer.
However, in addition to being produced through the ACLY pathway, acetyl-CoA can also be produced by glucose or acetate metabolism to enter the process of fatty acid synthesis[143,144]. In cancer cells, ACLY silencing increases the expression of ACC2, which maintains lipid synthesis in an acetate-dependent manner[145]. Despite the knockdown of ACLY diminishing the number of breast CSCs, the effect of ACLY deficiency remains to be studied in CSCs.
Targeting SCD1, which converts fully saturated fatty acids to MUFAs, can selectively kill CSCs. It is reported that SCD1 inhibitors, such as CAY10566 and A939572, suppress cancer stemness and prevent tumorigenesis, and can counteract cancer cell chemoresistance[46,146]. Significantly, MF-438 and PluriSIn #1, as SCD1 inhibitors, selectively eliminate colon CSCs but not the bulk cancer cells[121]. Furthermore, inhibition of SCD1 increased the sensitivity of CSCs to cisplatin and reduced drug resistance[85]. Therefore, combining SCD1 inhibitors with chemotherapy may be a more effective treatment strategy. Other studies have shown that miR-600 targeting SCD1 regulates Wnt/β-catenin signaling, thereby inhibiting the self-renewal and differentiation of mammary CSCs. Therefore, in addition to SCD1 inhibitors, nanovectorized miR-600 agonists (promiRNAs) may serve as a targeted tumor stem cell therapy[87]. Delta 6-desaturase inhibitors block the globular formation and tumor-initiating ability of ovarian CSCs by inhibiting the synthesis of PUFAs[46].
Activation of cholesterol synthesis could be relevant to the aggressive and metastatic potential in CSCs. Inhibition of SREBP activation by 25-HC or fatostatin inhibits lipogenesis, including FA and cholesterol, and decreases the expression of genes associated with CSCs[98]. PP significantly inhibits lipid anabolism in CSCs. In triple-negative breast cancer, PP exerts cytotoxic effects on TNBCSCs by inhibiting cholesterol synthesis[45]. Simvastatin significantly reduced mammosphere formation and growth through inhibition of cholesterol biosynthesis[96]. In addition, statins target CSCs by inhibiting the signaling associated with protein farnesylation, and protein geranylgeranylation in the MVA pathway[147,148]. Similarly, metformin suppresses CSCs through inhibiting protein prenylation of the MVA pathway in colorectal cancer[149].
In the past few years, many studies have shown that CSCs are responsible for tumor occurrence and development, distant metastasis, and therapy resistance. Metabolic alterations are the main pathways for cancer cells and CSCs to escape from adverse environmental effects. Among the reprogrammed metabolic pathways, alterations in lipid synthesis such as de novo lipogenesis, lipid desaturation, and cholesterol synthesis are closely related to CSC generation and stemness maintenance. Furthermore, lipid synthesis is also involved in the activation of several important oncogenic signaling pathways, including Notch, Wnt/β-catenin, Hippo, and HH signaling. Taking the key molecules of lipid synthesis as the target shows promising application potential in the elimination of CSCs. Therefore, we believe that altered lipid synthesis metabolism is a promising target for CSC elimination and tumor therapy.
| 1. | Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1469] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 2. | Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 317] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 3. | Vlashi E, Pajonk F. Cancer stem cells, cancer cell plasticity and radiation therapy. Semin Cancer Biol. 2015;31:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Feinberg AP, Koldobskiy MA, Göndör A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet. 2016;17:284-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 647] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 5. | Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 1228] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 6. | Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 2018] [Article Influence: 224.2] [Reference Citation Analysis (0)] |
| 7. | Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 3570] [Article Influence: 396.7] [Reference Citation Analysis (0)] |
| 8. | Eun K, Ham SW, Kim H. Cancer stem cell heterogeneity: origin and new perspectives on CSC targeting. BMB Rep. 2017;50:117-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Najafi M, Mortezaee K, Ahadi R. Cancer stem cell (a)symmetry & plasticity: Tumorigenesis and therapy relevance. Life Sci. 2019;231:116520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu Y, Yao Y, Li D. The epithelial to mesenchymal transition (EMT) and cancer stem cells: implication for treatment resistance in pancreatic cancer. Mol Cancer. 2017;16:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 11. | Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 583] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 12. | Sattiraju A, Sai KKS, Mintz A. Glioblastoma Stem Cells and Their Microenvironment. Adv Exp Med Biol. 2017;1041:119-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1469] [Article Influence: 91.8] [Reference Citation Analysis (1)] |
| 14. | Zhou H, Xu R. Leukemia stem cells: the root of chronic myeloid leukemia. Protein Cell. 2015;6:403-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Chopra M, Bohlander SK. The cell of origin and the leukemia stem cell in acute myeloid leukemia. Genes Chromosomes Cancer. 2019;58:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Xie SL, Fan S, Zhang SY, Chen WX, Li QX, Pan GK, Zhang HQ, Wang WW, Weng B, Zhang Z, Li JS, Lin ZY. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/β-catenin pathway. Int J Cancer. 2018;142:1252-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Yang F, Xu J, Tang L, Guan X. Breast cancer stem cell: the roles and therapeutic implications. Cell Mol Life Sci. 2017;74:951-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Janiszewska M, Suvà ML, Riggi N, Houtkooper RH, Auwerx J, Clément-Schatlo V, Radovanovic I, Rheinbay E, Provero P, Stamenkovic I. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 19. | Heng WS, Gosens R, Kruyt FAE. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol. 2019;160:121-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol Ther. 2016;158:71-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Brown HK, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 22. | Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020;17:204-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 546] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 23. | Li L, Bi Z, Wadgaonkar P, Lu Y, Zhang Q, Fu Y, Thakur C, Wang L, Chen F. Metabolic and epigenetic reprogramming in the arsenic-induced cancer stem cells. Semin Cancer Biol. 2019;57:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 1021] [Article Influence: 85.1] [Reference Citation Analysis (14)] |
| 25. | Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 4291] [Article Influence: 429.1] [Reference Citation Analysis (0)] |
| 26. | Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Qing Y, Dong L, Gao L, Li C, Li Y, Han L, Prince E, Tan B, Deng X, Wetzel C, Shen C, Gao M, Chen Z, Li W, Zhang B, Braas D, Ten Hoeve J, Sanchez GJ, Chen H, Chan LN, Chen CW, Ann D, Jiang L, Müschen M, Marcucci G, Plas DR, Li Z, Su R, Chen J. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m6A/PFKP/LDHB axis. Mol Cell. 2021;81:922-939.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 28. | Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem. 2014;115:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Peng F, Wang JH, Fan WJ, Meng YT, Li MM, Li TT, Cui B, Wang HF, Zhao Y, An F, Guo T, Liu XF, Zhang L, Lv L, Lv DK, Xu LZ, Xie JJ, Lin WX, Lam EW, Xu J, Liu Q. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene. 2018;37:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 198] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 31. | Yan B, Jiang Z, Cheng L, Chen K, Zhou C, Sun L, Qian W, Li J, Cao J, Xu Q, Ma Q, Lei J. Paracrine HGF/c-MET enhances the stem cell-like potential and glycolysis of pancreatic cancer cells via activation of YAP/HIF-1α. Exp Cell Res. 2018;371:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Song IS, Jeong YJ, Han J. Mitochondrial metabolism in cancer stem cells: a therapeutic target for colon cancer. BMB Rep. 2015;48:539-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Foo BJ, Eu JQ, Hirpara JL, Pervaiz S. Interplay between Mitochondrial Metabolism and Cellular Redox State Dictates Cancer Cell Survival. Oxid Med Cell Longev. 2021;2021:1341604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Raggi C, Taddei ML, Sacco E, Navari N, Correnti M, Piombanti B, Pastore M, Campani C, Pranzini E, Iorio J, Lori G, Lottini T, Peano C, Cibella J, Lewinska M, Andersen JB, di Tommaso L, Viganò L, Di Maira G, Madiai S, Ramazzotti M, Orlandi I, Arcangeli A, Chiarugi P, Marra F. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J Hepatol. 2021;74:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 35. | Strzyz P. Immortalizing switch to OXPHOS. Nat Rev Mol Cell Biol. 2020;21:658-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Long NA, Golla U, Sharma A, Claxton DF. Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications. Stem Cell Rev Rep. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Valle S, Alcalá S, Martin-Hijano L, Cabezas-Sáinz P, Navarro D, Muñoz ER, Yuste L, Tiwary K, Walter K, Ruiz-Cañas L, Alonso-Nocelo M, Rubiolo JA, González-Arnay E, Heeschen C, Garcia-Bermejo L, Hermann PC, Sánchez L, Sancho P, Fernández-Moreno MÁ, Sainz B Jr. Exploiting oxidative phosphorylation to promote the stem and immunoevasive properties of pancreatic cancer stem cells. Nat Commun. 2020;11:5265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 38. | Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:136-150.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 592] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 39. | Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R, Bosc C, Sugita M, Stuani L, Fraisse M, Scotland S, Larrue C, Boutzen H, Féliu V, Nicolau-Travers ML, Cassant-Sourdy S, Broin N, David M, Serhan N, Sarry A, Tavitian S, Kaoma T, Vallar L, Iacovoni J, Linares LK, Montersino C, Castellano R, Griessinger E, Collette Y, Duchamp O, Barreira Y, Hirsch P, Palama T, Gales L, Delhommeau F, Garmy-Susini BH, Portais JC, Vergez F, Selak M, Danet-Desnoyers G, Carroll M, Récher C, Sarry JE. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017;7:716-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 40. | He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, Liu Z, Yao Z, Wu Q, Liao W, Zhang S, Liu Y, Xiang Y, Liu J, Shi M. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38:4637-4654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 41. | Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, Potze L, Marotta R, Ruffilli R, Rajamanickam VP, Malerba M, De Angelis F, Falqui A, Carbone E, Todaro M, Medema JP, Stassi G, Di Fabrizio E. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells. 2015;33:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 42. | Giampietri C, Petrungaro S, Cordella M, Tabolacci C, Tomaipitinca L, Facchiano A, Eramo A, Filippini A, Facchiano F, Ziparo E. Lipid Storage and Autophagy in Melanoma Cancer Cells. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Kuramoto K, Yamamoto M, Suzuki S, Togashi K, Sanomachi T, Kitanaka C, Okada M. Inhibition of the Lipid Droplet-Peroxisome Proliferator-Activated Receptor α Axis Suppresses Cancer Stem Cell Properties. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Yi M, Li J, Chen S, Cai J, Ban Y, Peng Q, Zhou Y, Zeng Z, Peng S, Li X, Xiong W, Li G, Xiang B. Emerging role of lipid metabolism alterations in Cancer stem cells. J Exp Clin Cancer Res. 2018;37:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 45. | Dattilo R, Mottini C, Camera E, Lamolinara A, Auslander N, Doglioni G, Muscolini M, Tang W, Planque M, Ercolani C, Buglioni S, Manni I, Trisciuoglio D, Boe A, Grande S, Luciani AM, Iezzi M, Ciliberto G, Ambs S, De Maria R, Fendt SM, Ruppin E, Cardone L. Pyrvinium Pamoate Induces Death of Triple-Negative Breast Cancer Stem-Like Cells and Reduces Metastases through Effects on Lipid Anabolism. Cancer Res. 2020;80:4087-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017;20:303-314.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 47. | Song M, Lee H, Nam MH, Jeong E, Kim S, Hong Y, Kim N, Yim HY, Yoo YJ, Kim JS, Cho YY, Mills GB, Kim WY, Yoon S. Loss-of-function screens of druggable targetome against cancer stem-like cells. FASEB J. 2017;31:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Lo Re O, Douet J, Buschbeck M, Fusilli C, Pazienza V, Panebianco C, Castracani CC, Mazza T, Li Volti G, Vinciguerra M. Histone variant macroH2A1 rewires carbohydrate and lipid metabolism of hepatocellular carcinoma cells towards cancer stem cells. Epigenetics. 2018;13:829-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Choi S, Yoo YJ, Kim H, Lee H, Chung H, Nam MH, Moon JY, Lee HS, Yoon S, Kim WY. Clinical and biochemical relevance of monounsaturated fatty acid metabolism targeting strategy for cancer stem cell elimination in colon cancer. Biochem Biophys Res Commun. 2019;519:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Khwairakpam AD, Shyamananda MS, Sailo BL, Rathnakaram SR, Padmavathi G, Kotoky J, Kunnumakkara AB. ATP citrate lyase (ACLY): a promising target for cancer prevention and treatment. Curr Drug Targets. 2015;16:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 51. | Granchi C. ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem. 2018;157:1276-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 52. | Icard P, Wu Z, Fournel L, Coquerel A, Lincet H, Alifano M. ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Lett. 2020;471:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 53. | Clementino M, Xie J, Yang P, Li Y, Lin HP, Fenske WK, Tao H, Kondo K, Yang C, Wang Z. A Positive Feedback Loop Between c-Myc Upregulation, Glycolytic Shift, and Histone Acetylation Enhances Cancer Stem Cell-like Property and Tumorigenicity of Cr(VI)-transformed Cells. Toxicol Sci. 2020;177:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Yang D, Peng M, Hou Y, Qin Y, Wan X, Zhu P, Liu S, Yang L, Zeng H, Jin T, Qiu Y, Li Q, Liu M. Oxidized ATM promotes breast cancer stem cell enrichment through energy metabolism reprogram-mediated acetyl-CoA accumulation. Cell Death Dis. 2020;11:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Hanai JI, Doro N, Seth P, Sukhatme VP. ATP citrate lyase knockdown impacts cancer stem cells in vitro. Cell Death Dis. 2013;4:e696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Lucenay KS, Doostan I, Karakas C, Bui T, Ding Z, Mills GB, Hunt KK, Keyomarsi K. Cyclin E Associates with the Lipogenic Enzyme ATP-Citrate Lyase to Enable Malignant Growth of Breast Cancer Cells. Cancer Res. 2016;76:2406-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Wei X, Shi J, Lin Q, Ma X, Pang Y, Mao H, Li R, Lu W, Wang Y, Liu P. Targeting ACLY Attenuates Tumor Growth and Acquired Cisplatin Resistance in Ovarian Cancer by Inhibiting the PI3K-AKT Pathway and Activating the AMPK-ROS Pathway. Front Oncol. 2021;11:642229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Petrova E, Scholz A, Paul J, Sturz A, Haike K, Siegel F, Mumberg D, Liu N. Acetyl-CoA carboxylase inhibitors attenuate WNT and Hedgehog signaling and suppress pancreatic tumor growth. Oncotarget. 2017;8:48660-48670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Vazquez-Martin A, Corominas-Faja B, Cufi S, Vellon L, Oliveras-Ferraros C, Menendez OJ, Joven J, Lupu R, Menendez JA. The mitochondrial H(+)-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle. 2013;12:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Bort A, Sánchez BG, de Miguel I, Mateos-Gómez PA, Diaz-Laviada I. Dysregulated lipid metabolism in hepatocellular carcinoma cancer stem cells. Mol Biol Rep. 2020;47:2635-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Tanosaki S, Tohyama S, Fujita J, Someya S, Hishiki T, Matsuura T, Nakanishi H, Ohto-Nakanishi T, Akiyama T, Morita Y, Kishino Y, Okada M, Tani H, Soma Y, Nakajima K, Kanazawa H, Sugimoto M, Ko MSH, Suematsu M, Fukuda K. Fatty Acid Synthesis Is Indispensable for Survival of Human Pluripotent Stem Cells. iScience. 2020;23:101535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 62. | Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Araúzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 63. | Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, Owada Y. Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS One. 2016;11:e0147717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 64. | Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, Wakabayashi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Fujioka T, Tanji S, Mo YY, Cao D, Wilber AC, Watabe K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E, Scarpa A, Cecconi D, Palmieri M. Proteomic analysis of pancreatic cancer stem cells: Functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 66. | Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 883] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 67. | Zhang B, Wu J, Guo P, Wang Y, Fang Z, Tian J, Yu Y, Teng W, Luo Y, Li Y. Down-Regulation of SREBP via PI3K/AKT/mTOR Pathway Inhibits the Proliferation and Invasion of Non-Small-Cell Lung Cancer Cells. Onco Targets Ther. 2020;13:8951-8961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Guo D, Wang Y, Wang J, Song L, Wang Z, Mao B, Tan N. RA-XII Suppresses the Development and Growth of Liver Cancer by Inhibition of Lipogenesis via SCAP-dependent SREBP Supression. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Sun Q, Yu X, Peng C, Liu N, Chen W, Xu H, Wei H, Fang K, Dong Z, Fu C, Xu Y, Lu W. Activation of SREBP-1c alters lipogenesis and promotes tumor growth and metastasis in gastric cancer. Biomed Pharmacother. 2020;128:110274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, Liu XS, Lee YR, Fung J, Katon JM, Menon AV, Webster KA, Ng C, Palumbieri MD, Diolombi MS, Breitkopf SB, Teruya-Feldstein J, Signoretti S, Bronson RT, Asara JM, Castillo-Martin M, Cordon-Cardo C, Pandolfi PP. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet. 2018;50:206-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 71. | Zhang Y, Li C, Hu C, Wu Q, Cai Y, Xing S, Lu H, Wang L, Huang, Sun L, Li T, He X, Zhong X, Wang J, Gao P, Smith ZJ, Jia W, Zhang H. Lin28 enhances de novo fatty acid synthesis to promote cancer progression via SREBP-1. EMBO Rep. 2019;20:e48115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Zhou C, Qian W, Ma J, Cheng L, Jiang Z, Yan B, Li J, Duan W, Sun L, Cao J, Wang F, Wu E, Wu Z, Ma Q, Li X. Resveratrol enhances the chemotherapeutic response and reverses the stemness induced by gemcitabine in pancreatic cancer cells via targeting SREBP1. Cell Prolif. 2019;52:e12514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 73. | Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 344] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 74. | Liu G, Kuang S, Cao R, Wang J, Peng Q, Sun C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019;33:10089-10103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 75. | Peck B, Schulze A. Lipid desaturation - the next step in targeting lipogenesis in cancer? FEBS J. 2016;283:2767-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 76. | Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem J. 1999;340 ( Pt 1):255-264. [PubMed] |
| 77. | ALJohani AM, Syed DN, Ntambi JM. Insights into Stearoyl-CoA Desaturase-1 Regulation of Systemic Metabolism. Trends Endocrinol Metab. 2017;28:831-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 78. | Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, Furdui CM, Hegde P, Torti FM, Torti SV. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019;79:5355-5366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 79. | Angelucci C, Maulucci G, Colabianchi A, Iacopino F, D'Alessio A, Maiorana A, Palmieri V, Papi M, De Spirito M, Di Leone A, Masetti R, Sica G. Stearoyl-CoA desaturase 1 and paracrine diffusible signals have a major role in the promotion of breast cancer cell migration induced by cancer-associated fibroblasts. Br J Cancer. 2015;112:1675-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Galbraith L, Leung HY, Ahmad I. Lipid pathway deregulation in advanced prostate cancer. Pharmacol Res. 2018;131:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Chen L, Ren J, Yang L, Li Y, Fu J, Tian Y, Qiu F, Liu Z, Qiu Y. Stearoyl-CoA desaturase-1 mediated cell apoptosis in colorectal cancer by promoting ceramide synthesis. Sci Rep. 2016;6:19665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 82. | Zhang Q, Yu S, Lam MMT, Poon TCW, Sun L, Jiao Y, Wong AST, Lee LTO. Angiotensin II promotes ovarian cancer spheroid formation and metastasis by upregulation of lipid desaturation and suppression of endoplasmic reticulum stress. J Exp Clin Cancer Res. 2019;38:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 83. | Savino AM, Fernandes SI, Olivares O, Zemlyansky A, Cousins A, Markert EK, Barel S, Geron I, Frishman L, Birger Y, Eckert C, Tumanov S, MacKay G, Kamphorst JJ, Herzyk P, Fernández-García J, Abramovich I, Mor I, Bardini M, Barin E, Janaki-Raman S, Cross JR, Kharas MG, Gottlieb E, Izraeli S, Halsey C. Metabolic adaptation of acute lymphoblastic leukemia to the central nervous system microenvironment is dependent on Stearoyl CoA desaturase. Nat Cancer. 2020;1:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Pinkham K, Park DJ, Hashemiaghdam A, Kirov AB, Adam I, Rosiak K, da Hora CC, Teng J, Cheah PS, Carvalho L, Ganguli-Indra G, Kelly A, Indra AK, Badr CE. Stearoyl CoA Desaturase Is Essential for Regulation of Endoplasmic Reticulum Homeostasis and Tumor Growth in Glioblastoma Cancer Stem Cells. Stem Cell Reports. 2019;12:712-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 85. | Pisanu ME, Noto A, De Vitis C, Morrone S, Scognamiglio G, Botti G, Venuta F, Diso D, Jakopin Z, Padula F, Ricci A, Mariotta S, Giovagnoli MR, Giarnieri E, Amelio I, Agostini M, Melino G, Ciliberto G, Mancini R. Blockade of Stearoyl-CoA-desaturase 1 activity reverts resistance to cisplatin in lung cancer stem cells. Cancer Lett. 2017;406:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 86. | Bruschini S, di Martino S, Pisanu ME, Fattore L, De Vitis C, Laquintana V, Buglioni S, Tabbì E, Cerri A, Visca P, Alessandrini G, Facciolo F, Napoli C, Trombetta M, Santoro A, Crescenzi A, Ciliberto G, Mancini R. CytoMatrix for a reliable and simple characterization of lung cancer stem cells from malignant pleural effusions. J Cell Physiol. 2020;235:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | El Helou R, Pinna G, Cabaud O, Wicinski J, Bhajun R, Guyon L, Rioualen C, Finetti P, Gros A, Mari B, Barbry P, Bertucci F, Bidaut G, Harel-Bellan A, Birnbaum D, Charafe-Jauffret E, Ginestier C. miR-600 Acts as a Bimodal Switch that Regulates Breast Cancer Stem Cell Fate through WNT Signaling. Cell Rep. 2017;18:2256-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 88. | Ray S, Kassan A, Busija AR, Rangamani P, Patel HH. The plasma membrane as a capacitor for energy and metabolism. Am J Physiol Cell Physiol. 2016;310:C181-C192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50 Suppl:S323-S328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 90. | Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 91. | Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 521] [Article Influence: 52.1] [Reference Citation Analysis (2)] |
| 92. | Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 1304] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 93. | Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 657] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 94. | Wang B, Rong X, Palladino END, Wang J, Fogelman AM, Martín MG, Alrefai WA, Ford DA, Tontonoz P. Phospholipid Remodeling and Cholesterol Availability Regulate Intestinal Stemness and Tumorigenesis. Cell Stem Cell. 2018;22:206-220.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 95. | Ehmsen S, Pedersen MH, Wang G, Terp MG, Arslanagic A, Hood BL, Conrads TP, Leth-Larsen R, Ditzel HJ. Increased Cholesterol Biosynthesis Is a Key Characteristic of Breast Cancer Stem Cells Influencing Patient Outcome. Cell Rep. 2019;27:3927-3938.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 96. | Gu Q, Yang X, Lv J, Zhang J, Xia B, Kim JD, Wang R, Xiong F, Meng S, Clements TP, Tandon B, Wagner DS, Diaz MF, Wenzel PL, Miller YI, Traver D, Cooke JP, Li W, Zon LI, Chen K, Bai Y, Fang L. AIBP-mediated cholesterol efflux instructs hematopoietic stem and progenitor cell fate. Science. 2019;363:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 97. | Li X, Wu JB, Li Q, Shigemura K, Chung LW, Huang WC. SREBP-2 promotes stem cell-like properties and metastasis by transcriptional activation of c-Myc in prostate cancer. Oncotarget. 2016;7:12869-12884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 98. | Wen YA, Xiong X, Zaytseva YY, Napier DL, Vallee E, Li AT, Wang C, Weiss HL, Evers BM, Gao T. Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer. Cell Death Dis. 2018;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 99. | Longo J, Smirnov P, Li Z, Branchard E, van Leeuwen JE, Licht JD, Haibe-Kains B, Andrews DW, Keats JJ, Pugh TJ, Trudel S, Penn LZ. The mevalonate pathway is an actionable vulnerability of t(4;14)-positive multiple myeloma. Leukemia. 2021;35:796-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 100. | Sethunath V, Hu H, De Angelis C, Veeraraghavan J, Qin L, Wang N, Simon LM, Wang T, Fu X, Nardone A, Pereira R, Nanda S, Griffith OL, Tsimelzon A, Shaw C, Chamness GC, Reis-Filho JS, Weigelt B, Heiser LM, Hilsenbeck SG, Huang S, Rimawi MF, Gray JW, Osborne CK, Schiff R. Targeting the Mevalonate Pathway to Overcome Acquired Anti-HER2 Treatment Resistance in Breast Cancer. Mol Cancer Res. 2019;17:2318-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 101. | Chushi L, Wei W, Kangkang X, Yongzeng F, Ning X, Xiaolei C. HMGCR is up-regulated in gastric cancer and promotes the growth and migration of the cancer cells. Gene. 2016;587:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 102. | Fatehi Hassanabad A, Mina F. Targeting the Mevalonate Pathway for Treating Lung Cancer. Am J Clin Oncol. 2020;43:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Longo J, Mullen PJ, Yu R, van Leeuwen JE, Masoomian M, Woon DTS, Wang Y, Chen EX, Hamilton RJ, Sweet JM, van der Kwast TH, Fleshner NE, Penn LZ. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Mol Metab. 2019;25:119-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 104. | Zhou S, Xu H, Tang Q, Xia H, Bi F. Dipyridamole Enhances the Cytotoxicities of Trametinib against Colon Cancer Cells through Combined Targeting of HMGCS1 and MEK Pathway. Mol Cancer Ther. 2020;19:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Brown DN, Caffa I, Cirmena G, Piras D, Garuti A, Gallo M, Alberti S, Nencioni A, Ballestrero A, Zoppoli G. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci Rep. 2016;6:19435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 106. | Wang X, Xu W, Zhan P, Xu T, Jin J, Miu Y, Zhou Z, Zhu Q, Wan B, Xi G, Ye L, Liu Y, Gao J, Li H, Lv T, Song Y. Overexpression of geranylgeranyl diphosphate synthase contributes to tumour metastasis and correlates with poor prognosis of lung adenocarcinoma. J Cell Mol Med. 2018;22:2177-2189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Yang YF, Jan YH, Liu YP, Yang CJ, Su CY, Chang YC, Lai TC, Chiou J, Tsai HY, Lu J, Shen CN, Shew JY, Lu PJ, Lin YF, Huang MS, Hsiao M. Squalene synthase induces tumor necrosis factor receptor 1 enrichment in lipid rafts to promote lung cancer metastasis. Am J Respir Crit Care Med. 2014;190:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 108. | Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Smith GD, Lewis SJ, Relton CL, Martin RM. Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer. JAMA. 2020;323:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 109. | Vásquez-Bochm LX, Velázquez-Paniagua M, Castro-Vázquez SS, Guerrero-Rodríguez SL, Mondragon-Peralta A, De La Fuente-Granada M, Pérez-Tapia SM, González-Arenas A, Velasco-Velázquez MA. Transcriptome-based identification of lovastatin as a breast cancer stem cell-targeting drug. Pharmacol Rep. 2019;71:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Göbel A, Rauner M, Hofbauer LC, Rachner TD. Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochim Biophys Acta Rev Cancer. 2020;1873:188351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 111. | Brindisi M, Fiorillo M, Frattaruolo L, Sotgia F, Lisanti MP, Cappello AR. Cholesterol and Mevalonate: Two Metabolites Involved in Breast Cancer Progression and Drug Resistance through the ERRα Pathway. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Qin Y, Hou Y, Liu S, Zhu P, Wan X, Zhao M, Peng M, Zeng H, Li Q, Jin T, Cui X, Liu M. A Novel Long Non-Coding RNA lnc030 Maintains Breast Cancer Stem Cell Stemness by Stabilizing SQLE mRNA and Increasing Cholesterol Synthesis. Adv Sci (Weinh). 2021;8:2002232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 113. | Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. 2018;20:888-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 782] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 114. | Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 636] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 115. | Reggiani F, Gobbi G, Ciarrocchi A, Ambrosetti DC, Sancisi V. Multiple roles and context-specific mechanisms underlying YAP and TAZ-mediated resistance to anti-cancer therapy. Biochim Biophys Acta Rev Cancer. 2020;1873:188341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A. 2019;116:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 117. | Zheng L, Xiang C, Li X, Guo Q, Gao L, Ni H, Xia Y, Xi T. STARD13-correlated ceRNA network-directed inhibition on YAP/TAZ activity suppresses stemness of breast cancer via co-regulating Hippo and Rho-GTPase/F-actin signaling. J Hematol Oncol. 2018;11:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 118. | Bocci F, Gearhart-Serna L, Boareto M, Ribeiro M, Ben-Jacob E, Devi GR, Levine H, Onuchic JN, Jolly MK. Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc Natl Acad Sci U S A. 2019;116:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (8)] |
| 119. | Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1040] [Article Influence: 94.5] [Reference Citation Analysis (2)] |
| 120. | Song NJ, Yun UJ, Yang S, Wu C, Seo CR, Gwon AR, Baik SH, Choi Y, Choi BY, Bahn G, Kim S, Kwon SM, Park JS, Baek SH, Park TJ, Yoon K, Kim BJ, Mattson MP, Lee SJ, Jo DG, Park KW. Notch1 deficiency decreases hepatic lipid accumulation by induction of fatty acid oxidation. Sci Rep. 2016;6:19377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Yu Y, Kim H, Choi S, Yu J, Lee JY, Lee H, Yoon S, Kim WY. Targeting a Lipid Desaturation Enzyme, SCD1, Selectively Eliminates Colon Cancer Stem Cells through the Suppression of Wnt and NOTCH Signaling. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 122. | Takebe N, Ivy SP. Controversies in cancer stem cells: targeting embryonic signaling pathways. Clin Cancer Res. 2010;16:3106-3112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Fendler A, Bauer D, Busch J, Jung K, Wulf-Goldenberg A, Kunz S, Song K, Myszczyszyn A, Elezkurtaj S, Erguen B, Jung S, Chen W, Birchmeier W. Inhibiting WNT and NOTCH in renal cancer stem cells and the implications for human patients. Nat Commun. 2020;11:929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 124. | Patel S, Alam A, Pant R, Chattopadhyay S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front Immunol. 2019;10:2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 125. | Cho YH, Ro EJ, Yoon JS, Mizutani T, Kang DW, Park JC, Il Kim T, Clevers H, Choi KY. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat Commun. 2020;11:5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 126. | Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51:1357-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 370] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 127. | Bagchi DP, Nishii A, Li Z, DelProposto JB, Corsa CA, Mori H, Hardij J, Learman BS, Lumeng CN, MacDougald OA. Wnt/β-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol Metab. 2020;42:101078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 128. | Lai KKY, Kweon SM, Chi F, Hwang E, Kabe Y, Higashiyama R, Qin L, Yan R, Wu RP, Lai K, Fujii N, French S, Xu J, Wang JY, Murali R, Mishra L, Lee JS, Ntambi JM, Tsukamoto H. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology. 2017;152:1477-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 129. | Rios-Esteves J, Resh MD. Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins. Cell Rep. 2013;4:1072-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 130. | Noto A, De Vitis C, Pisanu ME, Roscilli G, Ricci G, Catizone A, Sorrentino G, Chianese G, Taglialatela-Scafati O, Trisciuoglio D, Del Bufalo D, Di Martile M, Di Napoli A, Ruco L, Costantini S, Jakopin Z, Budillon A, Melino G, Del Sal G, Ciliberto G, Mancini R. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene. 2017;36:4573-4584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 131. | Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1795] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 132. | Park JH, Shin JE, Park HW. The Role of Hippo Pathway in Cancer Stem Cell Biology. Mol Cells. 2018;41:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 109] [Reference Citation Analysis (0)] |
| 133. | Maugeri-Saccà M, De Maria R. Hippo pathway and breast cancer stem cells. Crit Rev Oncol Hematol. 2016;99:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 134. | Aylon Y, Oren M. The Hippo pathway, p53 and cholesterol. Cell Cycle. 2016;15:2248-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 135. | Koo JH, Guan KL. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018;28:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 340] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 136. | Bhuria V, Xing J, Scholta T, Bui KC, Nguyen MLT, Malek NP, Bozko P, Plentz RR. Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma. Exp Cell Res. 2019;385:111671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 137. | Hu A, Song BL. The interplay of Patched, Smoothened and cholesterol in Hedgehog signaling. Curr Opin Cell Biol. 2019;61:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 138. | Blassberg R, Jacob J. Lipid metabolism fattens up hedgehog signaling. BMC Biol. 2017;15:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 139. | Jones SF, Infante JR. Molecular Pathways: Fatty Acid Synthase. Clin Cancer Res. 2015;21:5434-5438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 140. | Corominas-Faja B, Cuyàs E, Gumuzio J, Bosch-Barrera J, Leis O, Martin ÁG, Menendez JA. Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget. 2014;5:8306-8316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 141. | Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, McGarry L, James D, Shanks E, Kalna G, Saunders RE, Jiang M, Howell M, Lassailly F, Thin MZ, Spencer-Dene B, Stamp G, van den Broek NJ, Mackay G, Bulusu V, Kamphorst JJ, Tardito S, Strachan D, Harris AL, Aboagye EO, Critchlow SE, Wakelam MJ, Schulze A, Gottlieb E. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 662] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 142. | Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, Lombardo PS, Van Nostrand JL, Hutchins A, Vera L, Gerken L, Greenwood J, Bhat S, Harriman G, Westlin WF, Harwood HJ Jr, Saghatelian A, Kapeller R, Metallo CM, Shaw RJ. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 143. | Diaz-Moralli S, Aguilar E, Marin S, Coy JF, Dewerchin M, Antoniewicz MR, Meca-Cortés O, Notebaert L, Ghesquière B, Eelen G, Thomson TM, Carmeliet P, Cascante M. A key role for transketolase-like 1 in tumor metabolic reprogramming. Oncotarget. 2016;7:51875-51897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 145. | Zaidi N, Royaux I, Swinnen JV, Smans K. ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol Cancer Ther. 2012;11:1925-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 146. | Tracz-Gaszewska Z, Dobrzyn P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 147. | Ginestier C, Monville F, Wicinski J, Cabaud O, Cervera N, Josselin E, Finetti P, Guille A, Larderet G, Viens P, Sebti S, Bertucci F, Birnbaum D, Charafe-Jauffret E. Mevalonate metabolism regulates Basal breast cancer stem cells and is a potential therapeutic target. Stem Cells. 2012;30:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 148. | Iannelli F, Roca MS, Lombardi R, Ciardiello C, Grumetti L, De Rienzo S, Moccia T, Vitagliano C, Sorice A, Costantini S, Milone MR, Pucci B, Leone A, Di Gennaro E, Mancini R, Ciliberto G, Bruzzese F, Budillon A. Synergistic antitumor interaction of valproic acid and simvastatin sensitizes prostate cancer to docetaxel by targeting CSCs compartment via YAP inhibition. J Exp Clin Cancer Res. 2020;39:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 149. | Seo Y, Kim J, Park SJ, Park JJ, Cheon JH, Kim WH, Kim TI. Metformin Suppresses Cancer Stem Cells through AMPK Activation and Inhibition of Protein Prenylation of the Mevalonate Pathway in Colorectal Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 150. | Ma XL, Sun YF, Wang BL, Shen MN, Zhou Y, Chen JW, Hu B, Gong ZJ, Zhang X, Cao Y, Pan BS, Zhou J, Fan J, Guo W, Yang XR. Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer. 2019;19:760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li WJ, Li SC, Madamsetty VS S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ