Published online Apr 26, 2021. doi: 10.4252/wjsc.v13.i4.317
Peer-review started: February 1, 2021
First decision: February 28, 2021
Revised: March 11, 2021
Accepted: March 29, 2021
Article in press: March 29, 2021
Published online: April 26, 2021
Processing time: 80 Days and 6.7 Hours
As human placenta-derived mesenchymal stem cells (hP-MSCs) exist in a physiologically hypoxic microenvironment, various studies have focused on the influence of hypoxia. However, the underlying mechanisms remain to be further explored.
The aim was to reveal the possible mechanisms by which hypoxia enhances the proliferation of hP-MSCs.
A hypoxic cell incubator (2.5% O2) was used to mimic a hypoxic micro
Hypoxia enhanced hP-MSC proliferation, increased the expression of cyclin E1, cyclin-dependent kinase 2, and cyclin A2, and decreased the expression of p21. Under hypoxia, CD99 expression was increased by HIF-1α. CD99-specific small interfering RNA or the ERK1/2 signaling inhibitor PD98059 abrogated the hypoxia-induced increase in cell proliferation.
Hypoxia promoted hP-MSCs proliferation in a manner dependent on CD99 regulation of the MAPK/ERK signaling pathway in vitro.
Core Tip: This study demonstrated the potential of hypoxic conditions to enhance the proliferation capacity of human placenta-derived mesenchymal stem cells (hP-MSCs). RNA sequencing assays and molecular biology experiments found that a novel CD99 gene was involved in hypoxia-mediated promotion of hP-MSC proliferation. Furthermore, CD99 activated pathways that transported ERK1/2 to the nucleus, increasing the activity of cell cycle-associated proteins. Hypoxia-inducible factor 1α-mediated CD99 played a role in proliferation by regulating the MAPK/ERK signaling pathway.
- Citation: Feng XD, Zhu JQ, Zhou JH, Lin FY, Feng B, Shi XW, Pan QL, Yu J, Li LJ, Cao HC. Hypoxia-inducible factor-1α–mediated upregulation of CD99 promotes the proliferation of placental mesenchymal stem cells by regulating ERK1/2. World J Stem Cells 2021; 13(4): 317-330
- URL: https://www.wjgnet.com/1948-0210/full/v13/i4/317.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i4.317
Mesenchymal stem cells (MSCs) are a heterogeneous subpopulation of stromal stem cells that can be isolated from bone marrow, adipose tissue, umbilical cord, and placenta[1-4]. The pluripotency and ease of extraction of MSCs makes them promising therapeutic agents in regenerative medicine[5]. In recent decades, many studies have focused on the biological properties of MSCs for the purpose of exploiting their therapeutic potential. A variety of animal and clinical studies of the therapeutic potential of MSCs for Parkinson disease, diabetes, and liver, kidney, lung, cardiovascular, bone, and cartilage conditions are underway. MSCs alleviate inflammation by releasing cytokines, repair tissue by expressing growth factors, regulate the immune response by secreting immunomodulatory proteins, enhance endogenous repair, and serve as mature functional cells in bone[6]. The biological characteristics and effects of MSCs can be improved by numerous factors[7], including hypoxia, which is a hot research topic.
Hypoxia is generally defined as an O2 level of 0.5% to 10% in the head space, whereas normoxia is defined as a 20% to 21% O2 level during cell culture. There are various types of MSCs in physiological microenvironments with low oxygen tension of 1% to 5% O2) in vivo, but MSCs are typically expanded in vitro in the presence of 20% to 21% O2[8]. Efforts have been made to mimic the physiological conditions for in vitro culture of MSCs. However, the effect of hypoxia on MSCs is controversial. For example, hypoxia has been reported to enhance cell survival, proliferation, and metabolism, and low oxygen tension needed for self-renewal and maintaining the multipotency of human MSCs[9-12]. Further, growth under pro-oxidative conditions (20% to 21% oxygen) can lead to oxidative stress and genetic instability[13]. However, other studies have reached the opposite conclusion[14,15].
Here, we found that human placenta-derived MSCs (hP-MSCs) cultured under hypoxia possessed a greater proliferation capacity than those cultured under normoxia. RNA sequencing (RNA-seq) was used to evaluate the role of CD99 in the hypoxia-mediated promotion of hP-MSCs proliferation. Hypoxia indirectly upregulated CD99 via hypoxia-inducible factor-1α (HIF-1α), leading to a significant increase in the proliferation of hP-MSCs. In addition, the regulation of hP-MSC proliferation under hypoxia was associated with the MAPK/ERK signaling pathway, particularly the expression of phosphorylated ERK in the nucleus.
Placentas were harvested from donors at The First Affiliated Hospital, School of Medicine, Zhejiang University. All protocols for handling human tissues and cells were approved by the Ethics Committee of The First Affiliated Hospital of Zhejiang University (No. 2013-272). The isolation and culture of hP-MSCs was performed as described previously[16,17]. The cells were stabilized in a standard humidified incubator (HERAcell150, Thermo Fisher Scientific Inc., Waltham, MA, United States) with a 21% O2 and 5% CO2 atmosphere. The hypoxic groups were placed in a humidified, water-jacketed CO2 incubator with oxygen control (Forma™ Series II, Thermo Fisher Scientific Inc.) in an atmosphere containing 2.5% O2 and 5% CO2. The normoxia group continued to be incubated in the standard humidified incubator.
Immunophenotyping of hP-MSCs was performed by flow cytometry as previously described [17]. Briefly, the cells were collected and washed with phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA). Next, a single-cell suspension (1 × 106 cells/mL) was stained for 30 min on ice with allophycocyanin-tagged human CD105, CD11b, CD73, CD34, CD90, CD45, and HLA-DR (1:20; eBioscience Inc., San Diego, CA, United States) antibodies. Isotype-matched antibodies (1:20; eBioscience Inc.) were used as controls to exclude nonspecific binding. Finally, flow cytometry data were acquired on a BeamCyte-1026 flow cytometry (BeamDiag Inc., Changzhou, China).
MSCs at passages two to five were seeded on cell culture plates (NunclonTM Delta Surface; Nunc A/S, Roskilde, Denmark) and cultured in adipogenic and osteogenic medium (OriCellTM hMSC Adipogenic and Osteogenic Differentiation Medium; Cyagen Biosciences, Guangzhou, China) per the manufacturer’s protocol. Three or 4 wk later, adipogenesis was evaluated by staining with Oil Red O and osteogenic differentiation was assayed by Alizarin red staining (both Cyagen Biosciences).
Western blotting was conducted as previously described [17] using anti-HIF-1α (1:1000; Cell Signaling Technology, Danvers, MA, United States), anti-cyclin E1 (1:1000; Abcam, Cambridge, United Kingdom), anti-cyclin A2 (1:1000; Abcam), anti-CDK2 (1:1000; Abcam), anti-p21 (1:1000; Abcam), anti-CD99 (1:1000; Abcam), anti-ERK1+ERK2 (1:5000; Abcam), anti-ERK1 (phospho T202)+ERK2 (phospho T185) (1:1000; Abcam) and anti-GAPDH (1:1000; Sangon Biotech Corp., Shanghai, China) primary antibodies. The membranes were then incubated with secondary anti-rabbit antibodies conjugated to horseradish peroxidase (1:4,000; Abcam). Proteins were detected using PierceTM enhanced chemiluminescence (ECL) western blot analysis substrate (Thermo Fisher Scientific Inc.) and analyzed with ImageJ software (NIH, Bethesda, MD, United States).
Cells were seeded in 96-well plates at 1-2 × 103 per well. At 24, 48, 72, and 96 h, the cells were incubated with a CCK-8 reagent (10 µL/well; Dojindo, Kumamoto, Japan) for 2 h at 37 °C. The cell growth rate was determined by measuring the optical density at 450 nm using an Epoch 2 Microplate Reader (BioTek Instruments Inc., Winooski, VT, United States).
5-Ethynyl-20-deoxyuridine (EdU) incorporation assays were performed using a Cell-Light EdU Apollo488 In Vitro Kit (RiboBio, Guangzhou, China). Cells were exposed to 50 μmol/L EdU for 2 h at 37 °C and then processed according to the manufacturer’s protocol. After washing with PBS, cells were reacted with 300 μL of 1 × Apollo reaction cocktail for 30 min and analyzed by flow cytometry (BeamCyte-1026).
Cells were collected and fixed in 66% ethanol at 4 °C for at least 2 h. After fixation, cells were transferred from 4 °C to the bench top and equilibrated to room temperature. The cells were washed with cold PBS and incubated in the dark with 400 µL staining buffer containing RNase A and propidium iodide at 37 °C for 30 min. A flow cytometer (BeamCyte-1026) was used to analyze the cell cycle distribution with ModFit LT ver. 5.0 (Verity Software House).
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) as described in the manufacturer’s instructions. RNA integrity was examined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, United States). Samples with RNA integrity numbers ≥ 7 were subjected to downstream analysis. Libraries were constructed using a TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, United States) and sequenced on the Illumina HiSeqTM 2500 platform to generate 125/150 bp paired-end reads. Transcriptome sequencing and raw data analysis were conducted by OE biotech Co., Ltd. (Shanghai, China). Notably, P < 0.05 and fold change > 2 or < 0.5 were the criteria for identifying significant differentially expressed genes (DEGs). A hierarchical cluster analysis of the DEGs was performed to explore gene expression patterns.
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. The purity and concentration of total RNA were evaluated using a NanoDrop 2000 (Thermo Fisher Scientific, Inc.). Complementary DNA (cDNA) was synthesized from 0.5 μg of total RNA using HiScript II Q RT SuperMix for real-time quantitative polymerase chain reaction (qPCR) (+gDNA wiper) (Vazyme Biotech Corp., Nanjing, China). cDNA was mixed with the primer and SYBR Premix Ex TaqTM II (TaKaRa Bio Inc., Shiga, Japan) according to the manufacturer’s instructions and subjected to qPCR on an ABI 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.). Oligonucleotide primers for human CD99 (forward 5′-GGTGG
Cells were grown on plates to about 70% confluence. After washing with PBS, the cells were fixed in 4% paraformaldehyde for 30 min in darkness and permeabilized with 0.3% Triton X-100 (Sangon Biotech Corp.) in PBS. Next, cells were blocked with 5% BSA and incubated with anti-CD99 (1:100; Abcam) and anti-ERK1 (phospho T202) +ERK2 (phospho T185) (1:100; Abcam) antibodies at 4 °C overnight. Goat anti-rabbit immunoglobulin G (Alexa Fluor® 647, 1:200; Abcam) was incubated with the fixed cells at room temperature for 1 h and protected from light. 4′,6-Diamidino-2-phenylindole was used to counterstain nuclei. Stained slides were observed with a Zeiss LSM710 confocal laser-scanning microscope (Carl Zeiss AG, Germany).
Transfection of small interfering RNAs (siRNAs) (Genomeditech, Shanghai, China) was performed with LipofectamineTM 3000 Transfection Reagent following the manufacturer’s protocols. Three short (50 nmol/L) siRNAs targeting specific gene sequences (Supplementary Table 1) were used to transfect cells. siRNAs that caused significant knockdown effects were used in subsequent analyses.
Firefly luciferase was used as a reporter of CD99 promoter activity by cloning the CD99 promoter upstream of the firefly luciferase-encoding gene. HEK293 cells were co-transfected with the constructed reporter vectors and HIF-1α overexpression vectors using HG Transgene Reagent (Genomeditech, Shanghai, China). Forty-eight h after transfection, cells were harvested in cell lysis buffer and luciferase activity was measured using a dual luciferase reporter system (Promega) according to the manufacturer’s instructions. Renilla luciferase was used as the internal control and activity is expressed as a ratio to Renilla luciferase activity.
The ERK inhibitor PD98059 (50 μmol/L; MedChemExpress, Monmouth Junction, NJ, United States) was added to the medium. Inhibition of ERK1/2 activation was evaluated after 48 h by Western blotting, and the proliferation rate was assessed with a CCK-8 assay kit.
Nuclear and cytoplasmic proteins were extracted using Nuclear and Cytoplasmic Protein Extraction Kits (Beyotime Biotechnology, Shanghai, China). Briefly, cells were harvested in agent A to extract cytoplasmic protein and then with agent B to collect the supernatant. The nuclear pellet was resuspended in nuclear protein extraction buffer and harvested by high-speed centrifugation.
Statistical analysis was performed with Prism 7 (GraphPad Inc., San Diego, CA, United States). Numerical data were reported as means ± SD of three independent assays. Between-group differences were compared by two-tailed Student’s t-test. Multiple comparisons were tested for significance by one-way analysis of variance followed by Dunnett’s test when comparing each group to the control group. A two-tailed P value of < 0.05 was deemed to indicate statistical significance.
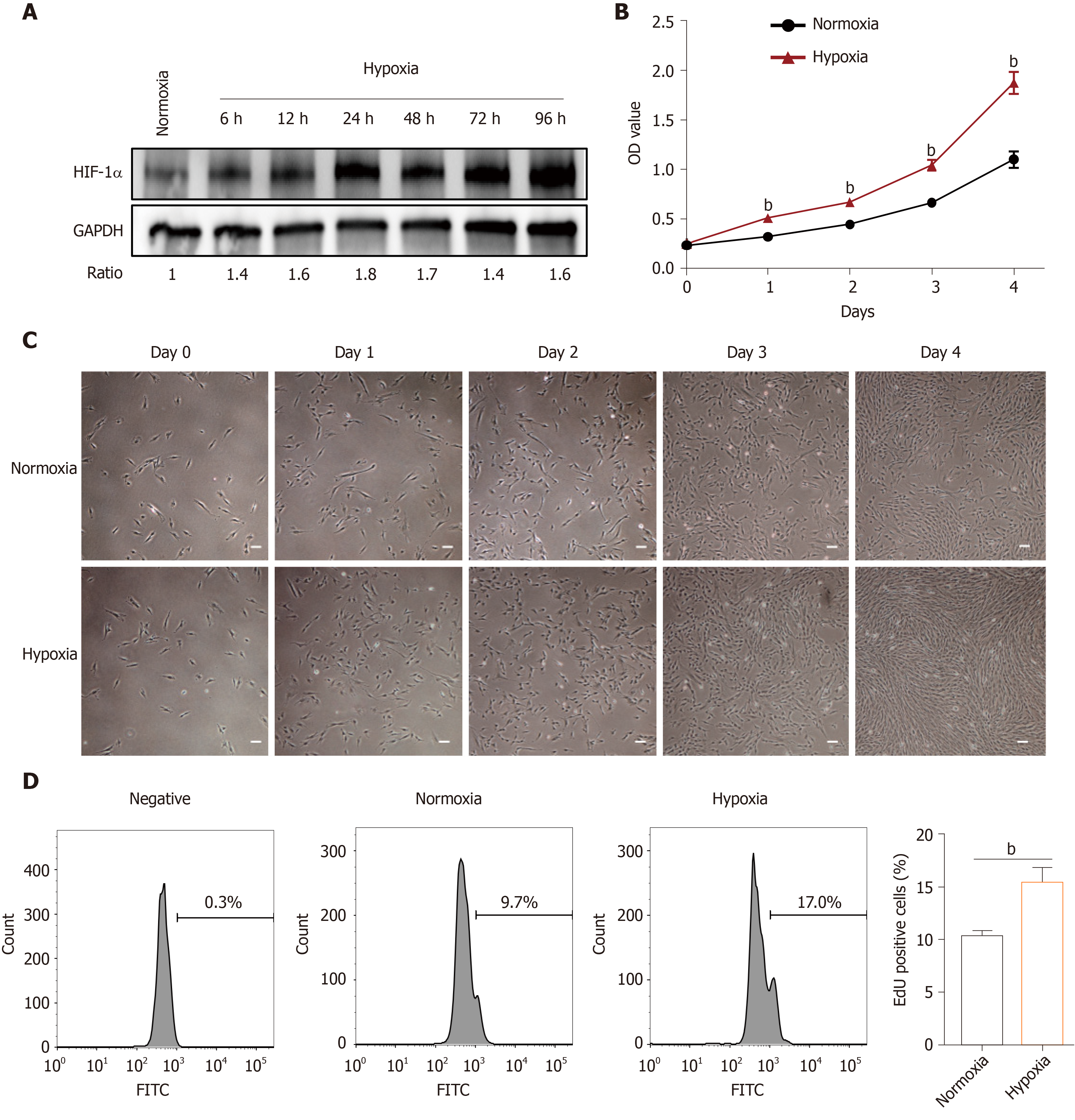
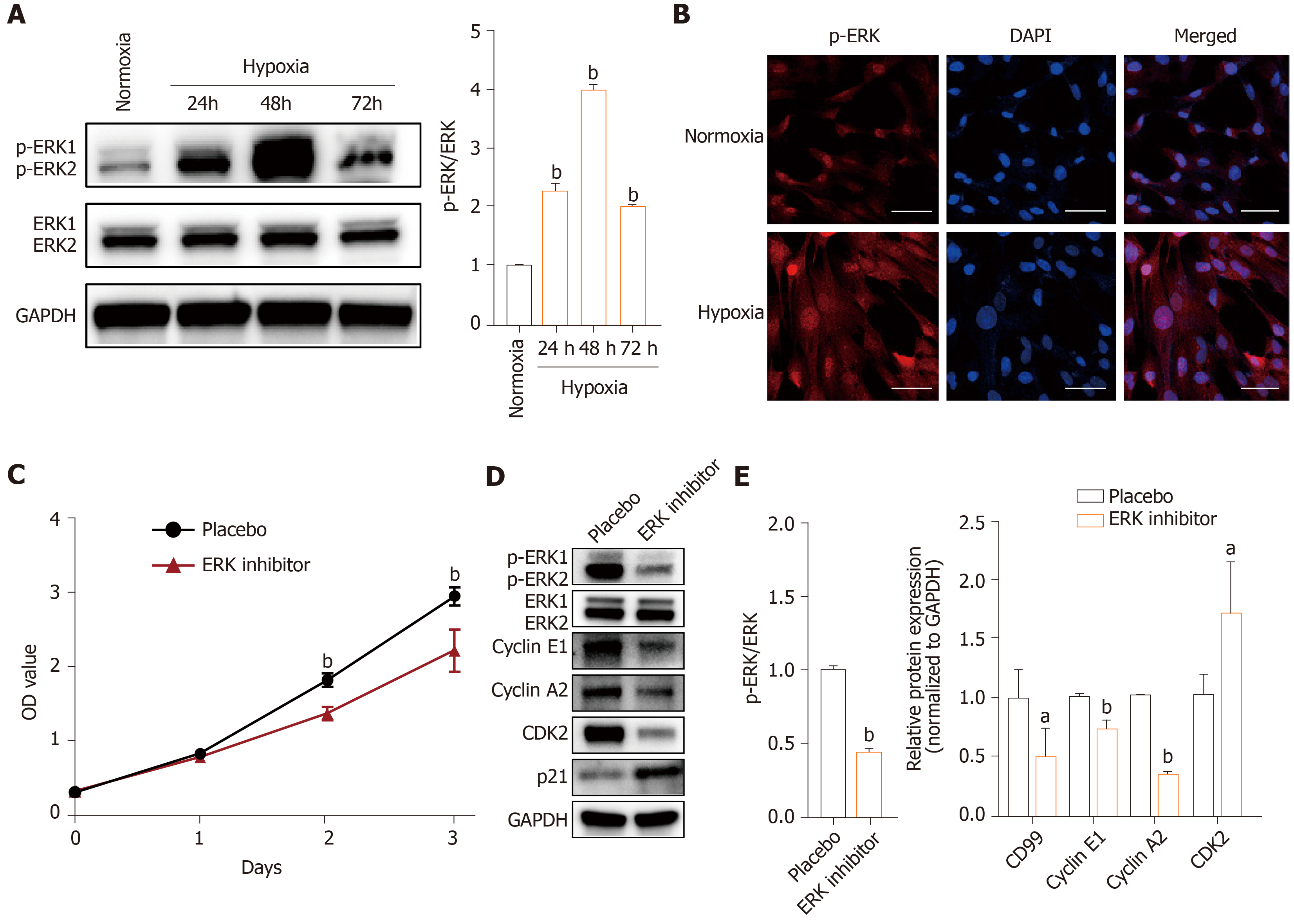
The effects of hypoxia were investigated in hP-MSCs incubated in normoxic and hypoxic conditions. The immunophenotype and differentiation characteristics are shown in Supplementary Figures 1 and 2. We firstly examined the expression of HIF-1α protein, a key transcription factor in hypoxic environments, by immunoblotting. As expected, culture under hypoxic conditions for 6 to 96 h significantly induced HIF-1α (Figure 1A). CCK8 assays showed that hypoxia significantly increased the proliferation rate of hP-MSCs (Figure 1B), which was confirmed by serial microscopic observation (Figure 1C). EdU incorporation assays found that 9.7% of hP-MSCs were EdU-positive under normoxia, compared with 17% under hypoxia, a 1.8-fold increase (Figure 1D). Taken together, the results indicate that a hypoxic environment was conducive to an increase in the proliferation of hP-MSCs.
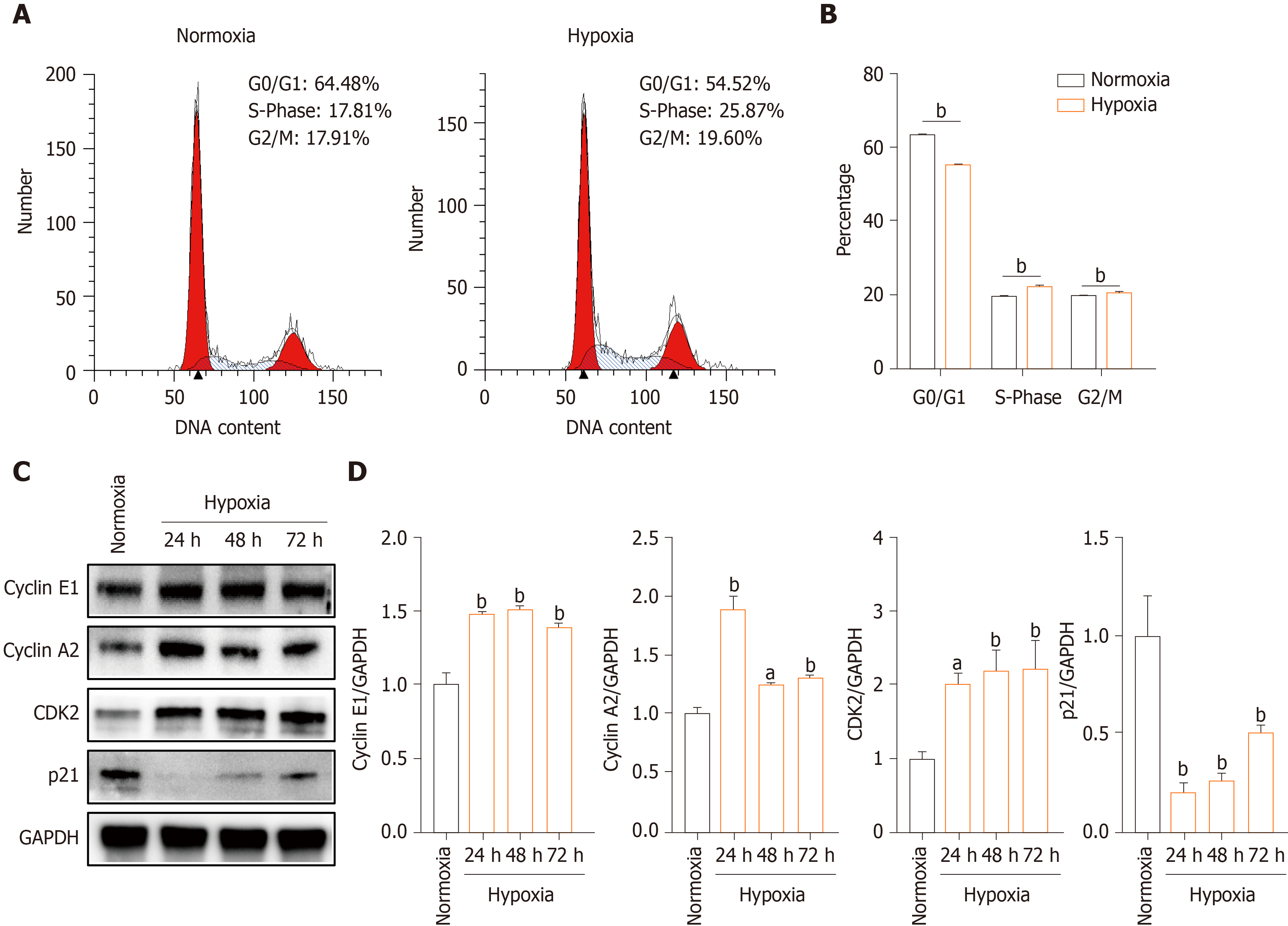
To evaluate the mechanism underlying the effect of hypoxia on hP-MSCs proliferation, we performed cell cycle analysis by flow cytometry. The proportion of G0/G1-phase cells was smaller in the hypoxia than in the normoxia group, and the proportions of both S- and G2/M-phase cells were larger (Figure 2A and B). Next, we quantified the levels of the cell cycle-associated proteins cyclin E1, cyclin A2, CDK2, and p21 by Western blotting. Hypoxia increased the levels of cyclin E1, cyclin A2, and CDK2 and decreased that of p21, a key regulator of G0/G1 proliferative arrest (Figure 2C and D). These findings suggest that hypoxia promoted hP-MSCs proliferation by modulating cell cycle progression.
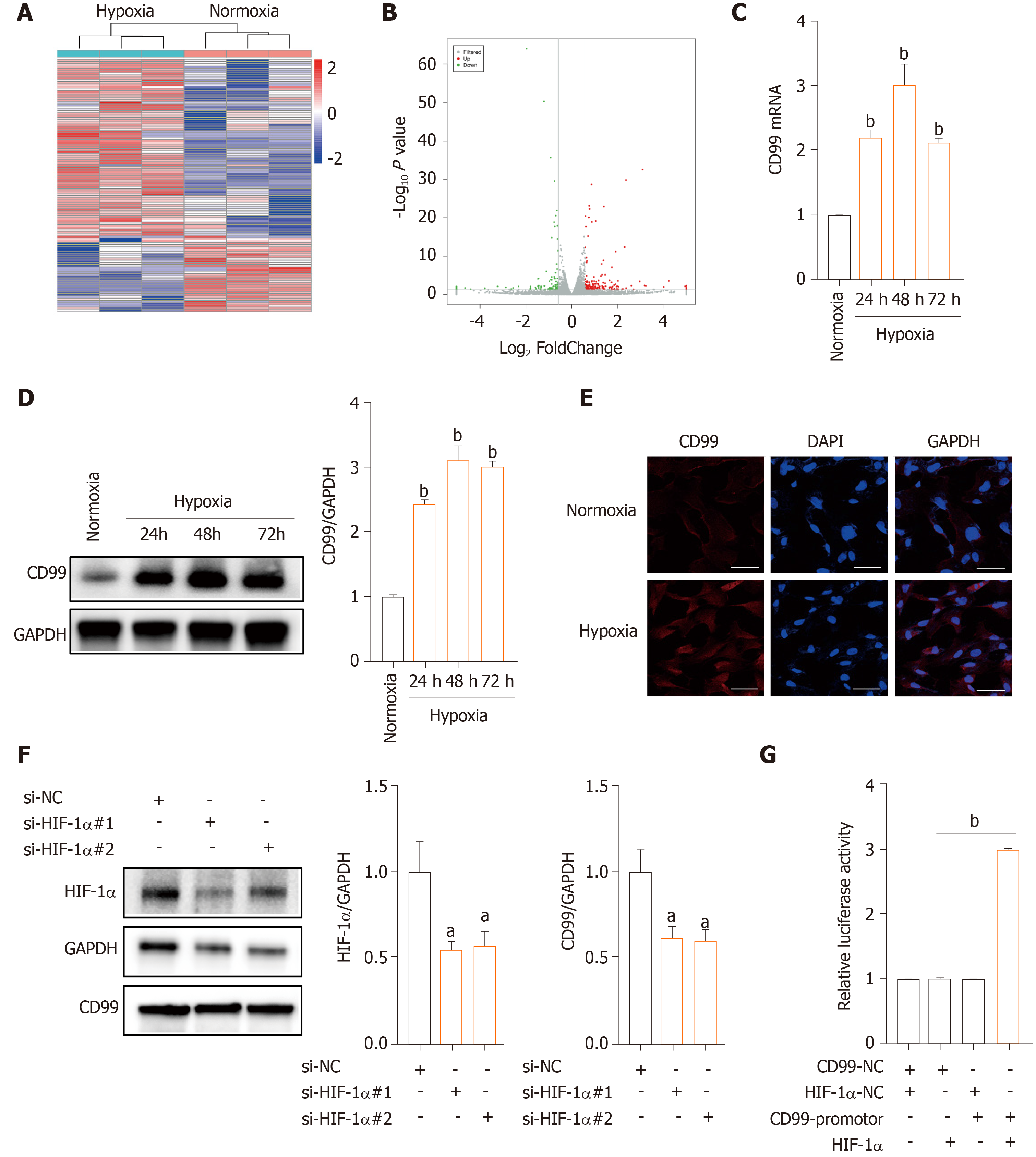
Subsequently, we analyzed the mRNA profile of hP-MSCs under hypoxia by RNA-seq. A total of 113 DEGs was found in hP-MSCs grown in hypoxic and normoxic culture (Supplementary Table 2). Eighty DEGs were upregulated and 33 were downregulated (Figure 3A and B). We next verified the expression of CD99 mRNA by qPCR, which was consistent with the RNA-seq results (Figure 3C). Furthermore, CD99 protein expression was upregulated by hypoxia, a finding that was confirmed by immunostaining (Figure 3D and E). However, the hypoxia-induced expression of CD99 was reduced by an HIF-1α-specific siRNA (Figure 3F). Furthermore, the luciferase reporter assay showed that CD99 was transcriptionally regulated by HIF-1α (Figure 3G and Supplementary Figure 3). Therefore, hypoxia upregulated CD99 by modulating HIF-1α expression.
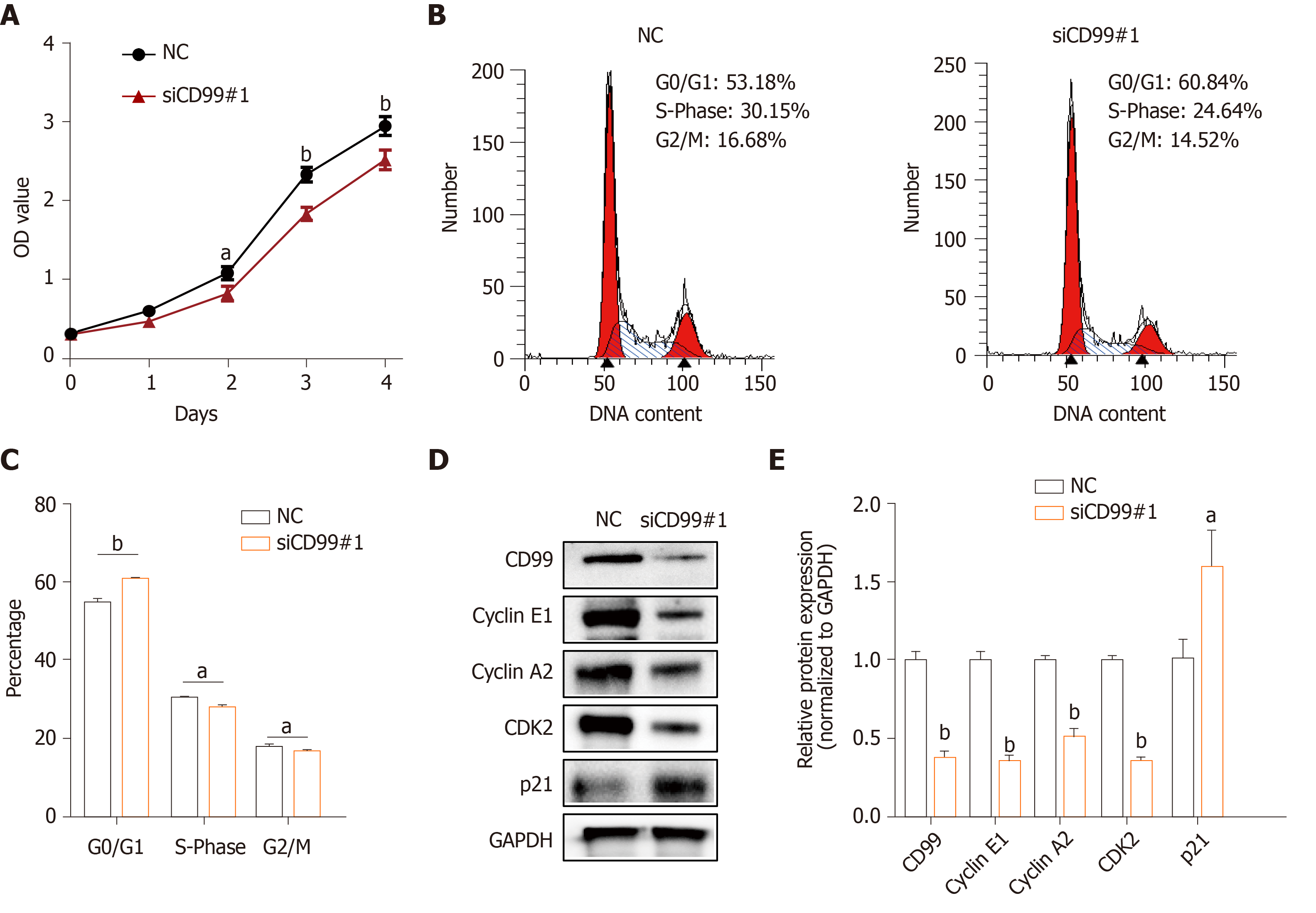
Next, to explore whether hypoxia-induced CD99 had an effect on hP-MSCs proliferation, growth curves of hP-MSCs under hypoxia with or without CD99-specific siRNA were constructed. The results showed that the proliferation of hP-MSCs was reduced by the silencing of CD99 (Figure 4A). Moreover, the proportions of cells in the S- and G2/M-phases were significantly smaller in the CD99-knockdown group than the control group (Figure 4B and C). Cyclin E1, cyclin A2, and CDK2 expression in the CD99-knockdown group decreased significantly under hypoxia, but that of p21 increased (Figure 4D and E). Overall, these data suggest that hypoxia-induced CD99 expression promoted MSCs proliferation.
The MAPK/ERK signaling pathway is important for cell proliferation, transformation, differentiation, and apoptosis. The levels of downstream effectors of the MAPK/ERK pathway, phosphorylated ERK1 and ERK2 (p-ERK) were higher in the hypoxia group than in the normoxia group (Figure 5A and B). Also, addition of PD98059 decreased the proliferation of hP-MSCs compared with placebo (Figure 5C). Western blotting showed that the ERK inhibitor reduced cyclin E1, cyclin A2, and CDK2 expression and increased that of p21 (Figure 5D and E). These findings demonstrate that hypoxia promoted hP-MSC proliferation by modulating the MAPK/ERK signaling pathway (Figure 5).
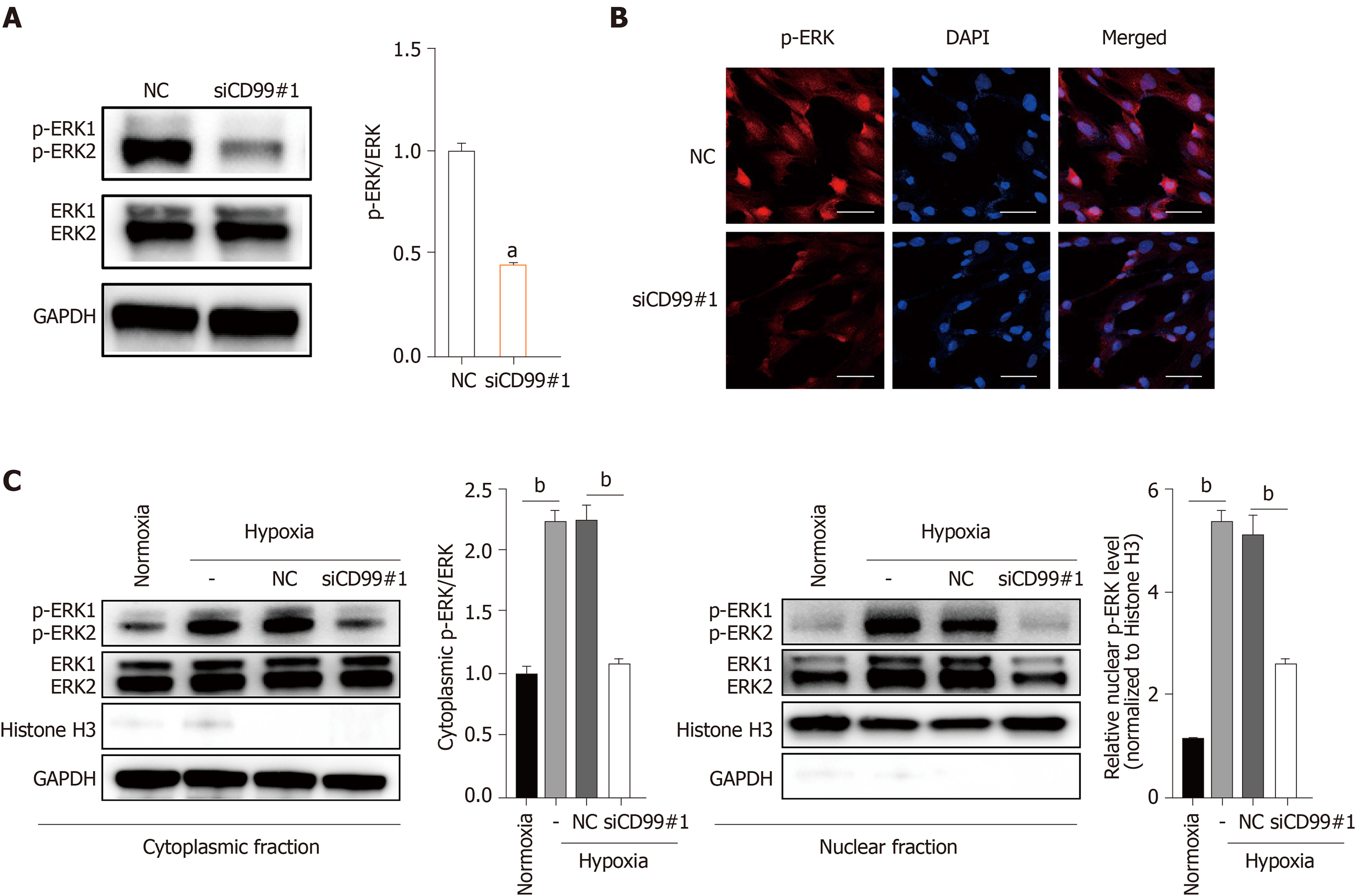
Because both CD99 and the MAPK/ERK signaling pathway promoted hP-MSC proliferation under hypoxia, we evaluated their functional connection. Western blotting and immunofluorescence revealed that hypoxia-induced phosphorylation of ERK1 and ERK2 was decreased by CD99-specific siRNA (siCD99#1, Figure 6A and B). Also, hypoxia increased the p-ERK level in cell lysates and in the cytoplasmic and nuclear fractions (Figure 6C). Moreover, the increase in phosphorylation in the nucleus, where p-ERK activates transcription factors and nuclear phosphatases, was significantly greater than that in the cytoplasm. Conversely, the reduction in p-ERK in the nucleus was greater than that in the cytoplasm (Figure 6C). Therefore, the effect of CD99 on hP-MSC proliferation under hypoxia was associated with nuclear p-ERK (Figure 7).
Oxygen concentration is a key regulator of cell survival, proliferation, migration, differentiation, and metabolism[18-20]. However, the mechanisms underlying the effect of hypoxia on MSCs are unclear. hP-MSCs are exposed to a low oxygen concentration in vivo. We found that hP-MSCs cultured under hypoxia (2.5% O2) exhibited greater proliferation ability. This finding created a rationale to explore the underlying mechanisms of the increased proliferation of MSCs under hypoxia. RNA-seq revealed several significantly upregulated DEGs in hP-MSCs cultured under hypoxia, and those genes may play a role in enhancing cell proliferation.
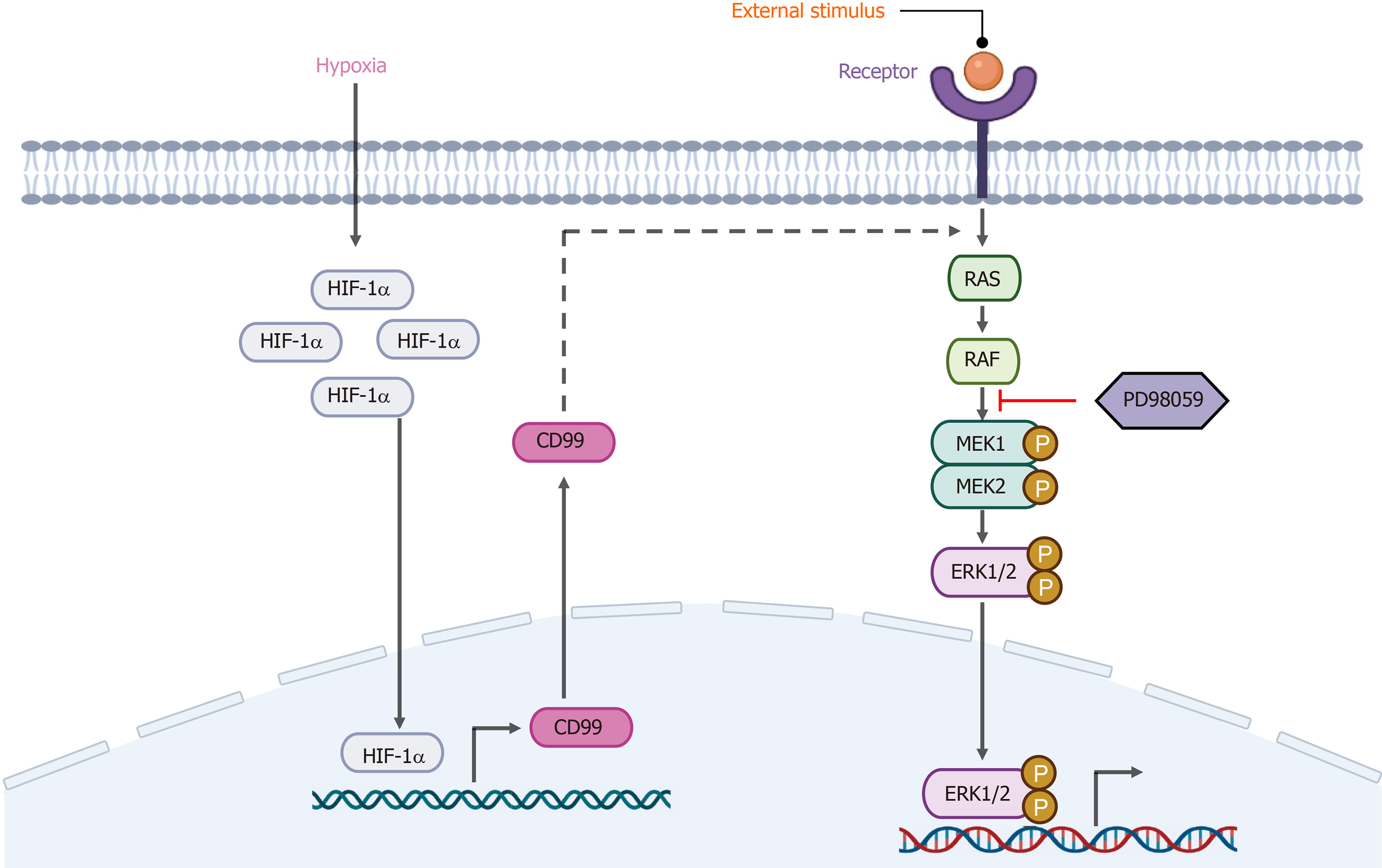
CD99 is a cell-surface glycoprotein with extracellular, transmembrane, and short intracytoplasmic domains. MSCs are reportedly negative or weakly positive for CD99, a multifunctional surface protein[21]. Husak and Dworzak[22] discovered a connection between CD99 and Hsp70, which is a major constituent of the stress response, implicating CD99 in stress-induced signaling. Moreover, Husak and Dworzak[23] subsequently reported that CD99 is involved in the prosurvival adaptation of bone marrow-derived MSCs (BM-MSCs), and that CD99 overexpression promoted their proliferation. Because CD99 was upregulated under hypoxia, we hypothesized its involvement in the promotion of hP-MSC proliferation by hypoxia. CD99 level is reportedly modulated by the HIF system, and by HIF-1a in particular, under hypoxia[24,25]. Indeed, the hypoxia-induced upregulation of CD99 was suppressed by HIF-1α-specific siRNAs. On this basis, we concluded that HIF-1α directly promoted the transcriptional activity of CD99. Also, the increased CD99 expression under hypoxia was, for unknown reasons, concentrated in the cytoplasm rather than on the membrane. In addition, the proliferation of hP-MSCs was decreased by CD99 knockdown, suggesting that it has an important role in promoting hP-MSC proliferation.
Numerous signal transduction pathways are implicated in the responses of cells to changes in the external environment. The MAPK/ERK signaling pathway, which is activated by some stress conditions, modulates stem cell growth, proliferation, and differentiation[26,27]. For example, the MAPK/ERK signaling pathway drives MSC differentiation into the osteogenic lineage by activating osteogenesis-related transcription factors such as runt-related transcription factor 2 (RUNX2) and osterix[28]. In addition, integrin β1 promotes chondrogenic differentiation of human adipose-derived MSCs by activating the ERK signaling pathway[29]. We reported previously that HIF-2α activates the MAPK/ERK pathway and interacts with its main signaling factors to promote hP-MSC proliferation[30]. Here we showed that the ERK signaling pathway was activated by hypoxia. Moreover, PD98059, an ERK1/2 signaling inhibitor decreased the proliferation of hP-MSCs cultured under hypoxia.
Because of their similar effects on the proliferation of hP-MSCs, we explored the connection between CD99 and the MAPK/ERK signaling pathway. Hypoxia-induced phosphorylation of ERK1 and ERK2 was suppressed by CD99 knockdown. Notably, the reduction in the p-ERK level in the nucleus was greater than that in the cytoplasm, implicating that factor in the CD99-mediated regulation of hP-MSC proliferation under hypoxia. Sciandra et al[31] reported a correlation between CD99 and the MAPK/ERK signaling pathway, and concluded that CD99 promoted the activity of the major osteogenic transcription factors AP1 and RUNX2 by regulating cytoplasmic ERK.
Hypoxia enhanced hP-MSCs proliferation in a manner dependent on HIF-1α-mediated CD99 expression and the MAPK/ERK signaling pathway. CD99 activated pathways that transport ERK1/2 to the nucleus, resulting in increased activity of cell cycle-associated proteins.
Mesenchymal stem cells (MSCs) are in a physiologically hypoxic microenvironment in the body, while the expansion of MSCs before cell therapy is typically performed at a 20%-21% oxygen level. Thus, investigators have become increasingly aware of the effects of oxygen levels on MSCs biology and are investigating the natural niche of those cells in vitro for more detailed results.
Although some researches have focused on the effect of hypoxia on MSCs, the underlying mechanisms remain unclear. Thus, this study concentrated on the mechanism of MSC proliferation.
In this study, we aimed to explore the effect of hypoxia on the proliferation capacity of human placenta-derived MSCs (hP-MSCs) and to elucidate underlying mechanisms.
Hypoxic (2.5% oxygen) incubation was used to simulate the hypoxic microen
Hypoxic culture enhanced the proliferation capacity of hP-MSCs by modulating cell cycle progression. Western blotting assay results further confirmed that hP-MSCs cultured under hypoxia exhibited increased cyclin E1, cyclin-dependent kinase 2, and cyclin A2 expression and decreased p21 expression. In addition, CD99 expression was directly regulated by HIF-1α under hypoxia. Also, CD99-specific small interfering RNAs or the ERK1/2 signaling inhibitor PD98059 abrogated the hypoxia-induced increase of cell proliferation, which further confirmed the influence of HIF-1α/CD99/ERK axis on hP-MSC proliferation.
Hypoxia promoted hP-MSC proliferation in vitro in a manner dependent on the HIF-1α/CD99/ERK axis. In detail, CD99 activated pathways that transport ERK1/2 to the nucleus, resulting in increased activity of cell cycle-associated proteins.
This study contributes to the understanding of MSC biology, especially the effect of hypoxia on MSC proliferation capacity.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Asian Pacific Association for the Study of the Liver, No. MN-2162.
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Khan I, Miloso M, Ramasamy T S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1717] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 2. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 616] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 4. | Xie PY, Hu XJ, Guo RM, Meng XC, Pang PF, Zhou ZY, Li D, Shan H. Generation of functional hepatocyte-like cells from human bone marrow mesenchymal stem cells by overexpression of transcription factor HNF4α and FOXA2. Hepatobiliary Pancreat Dis Int. 2019;18:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 497] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 6. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1318] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 7. | Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 8. | Davy P, Allsopp R. Hypoxia: are stem cells in it for the long run? Cell Cycle. 2011;10:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Lee CW, Kang D, Kim AK, Kim DY, Kim DI. Improvement of Cell Cycle Lifespan and Genetic Damage Susceptibility of Human Mesenchymal Stem Cells by Hypoxic Priming. Int J Stem Cells. 2018;11:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33:1818-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res. 2012;2:148-159. [PubMed] |
| 12. | Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H, Levchenko A, Guerrero-Cazares H, Quiñones-Hinojosa A. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis. 2014;5:e1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Estrada JC, Albo C, Benguría A, Dopazo A, López-Romero P, Carrera-Quintanar L, Roche E, Clemente EP, Enríquez JA, Bernad A, Samper E. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19:743-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Kumar S, Vaidya M. Hypoxia inhibits mesenchymal stem cell proliferation through HIF1α-dependent regulation of P27. Mol Cell Biochem. 2016;415:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Chung DJ, Hayashi K, Toupadakis CA, Wong A, Yellowley CE. Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res Vet Sci. 2012;92:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, Li Y, Pan X, Wang Y, Li L. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012;10:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Feng X, Liu J, Xu Y, Zhu J, Chen W, Feng B, Pan Q, Yu J, Shi X, Yang J, Li Y, Li L, Cao H. Molecular mechanism underlying the difference in proliferation between placenta-derived and umbilical cord-derived mesenchymal stem cells. J Cell Physiol. 2020;235:6779-6793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Szablowska-Gadomska I, Zayat V, Buzanska L. Influence of low oxygen tensions on expression of pluripotency genes in stem cells. Acta Neurobiol Exp (Wars). 2011;71:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 20. | Fujisawa K, Takami T, Okada S, Hara K, Matsumoto T, Yamamoto N, Yamasaki T, Sakaida I. Analysis of Metabolomic Changes in Mesenchymal Stem Cells on Treatment with Desferrioxamine as a Hypoxia Mimetic Compared with Hypoxic Conditions. Stem Cells. 2018;36:1226-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Lin PP, Wang Y, Lozano G. Mesenchymal Stem Cells and the Origin of Ewing's Sarcoma. Sarcoma. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Husak Z, Dworzak MN. CD99 Ligation upregulates HSP70 on acute lymphoblastic leukemia cells and concomitantly increases NK cytotoxicity. Cell Death Dis. 2012;3:e425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Husak Z, Dworzak MN. Chronic stress induces CD99, suppresses autophagy, and affects spontaneous adipogenesis in human bone marrow stromal cells. Stem Cell Res Ther. 2017;8:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 25. | Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling. Oncogene. 2000;19:6297-6305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Chan LH, Zhou L, Ng KY, Wong TL, Lee TK, Sharma R, Loong JH, Ching YP, Yuan YF, Xie D, Lo CM, Man K, Artegiani B, Clevers H, Yan HH, Leung SY, Richard S, Guan XY, Huen MSY, Ma S. PRMT6 Regulates RAS/RAF Binding and MEK/ERK-Mediated Cancer Stemness Activities in Hepatocellular Carcinoma through CRAF Methylation. Cell Rep 2018; 25: 690-701. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Baumgartner C, Toifl S, Farlik M, Halbritter F, Scheicher R, Fischer I, Sexl V, Bock C, Baccarini M. An ERK-Dependent Feedback Mechanism Prevents Hematopoietic Stem Cell Exhaustion. Cell Stem Cell 2018; 22: 879-892. e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Lu N, Malemud CJ. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (11)] |
| 29. | Luo S, Shi Q, Li W, Wu W, Zha Z. ITGB1 promotes the chondrogenic differentiation of human adipose-derived mesenchymal stem cells by activating the ERK signaling. J Mol Histol. 2020;51:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Zhu C, Yu J, Pan Q, Yang J, Hao G, Wang Y, Li L, Cao H. Hypoxia-inducible factor-2 alpha promotes the proliferation of human placenta-derived mesenchymal stem cells through the MAPK/ERK signaling pathway. Sci Rep. 2016;6:35489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Sciandra M, Marino MT, Manara MC, Guerzoni C, Grano M, Oranger A, Lucarelli E, Lollini PL, Dozza B, Pratelli L, Renzo MF, Colombo MP, Picci P, Scotlandi K. CD99 drives terminal differentiation of osteosarcoma cells by acting as a spatial regulator of ERK 1/2. J Bone Miner Res. 2014;29:1295-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |