Published online Dec 26, 2021. doi: 10.4252/wjsc.v13.i12.1826
Peer-review started: March 16, 2021
First decision: May 5, 2021
Revised: June 29, 2021
Accepted: November 30, 2021
Article in press: November 30, 2021
Published online: December 26, 2021
Processing time: 283 Days and 17.7 Hours
Mesenchymal stem cells (MSCs) represent the most clinically used stem cells in regenerative medicine. However, due to the disadvantages with primary MSCs, such as limited cell proliferative capacity and rarity in the tissues leading to limited MSCs, gradual loss of differentiation during in vitro expansion reducing the efficacy of MSC application, and variation among donors increasing the uncertainty of MSC efficacy, the clinical application of MSCs has been greatly hampered. MSCs derived from human pluripotent stem cells (hPSC-MSCs) can circumvent these problems associated with primary MSCs. Due to the infinite self-renewal of hPSCs and their differentiation potential towards MSCs, hPSC-MSCs are emerging as an attractive alternative for regenerative medicine. This review summarizes the progress on derivation of MSCs from human pluripotent stem cells, disease modelling and drug screening using hPSC-MSCs, and various applications of hPSC-MSCs in regenerative medicine. In the end, the challenges and concerns with hPSC-MSC applications are also discussed.
Core Tip: Mesenchymal stem cells (MSCs) exhibit great potential in regenerative medicine. However, the clinical application of primary MSCs has been greatly hampered by the limitations of primary MSCs. MSCs derived from human pluripotent stem cells (hPSC-MSCs) are an attractive source of cells to overcome such problems with primary MSCs. This review summarizes the various derivation approaches and applications of hPSC-MSCs in regenerative medicine. Lastly, the challenges with the use of hPSC-MSCs are also discussed, which indicate that more efforts are needed for the clinical application of hPSC-MSCs.
- Citation: Liu TM. Application of mesenchymal stem cells derived from human pluripotent stem cells in regenerative medicine. World J Stem Cells 2021; 13(12): 1826-1844
- URL: https://www.wjgnet.com/1948-0210/full/v13/i12/1826.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i12.1826
Mesenchymal stem cells (MSCs) are adult stem cells with fibroblast-like morphology and plastic adherence. They express MSC surface antigens such as CD73, CD90, and CD105 but lack hematopoietic markers such as CD11b, CD19, CD34, and CD45[1]. More importantly, MSCs can give rise to multiple mesenchymal lineages, including bone, cartilage, and fat cells[1-3]. Friedenstain and colleagues first described an adherent subpopulation in bone marrow termed as marrow stromal cells[4-7]. The term of MSCs was later introduced in 1991 to refer to these cells[8]. MSCs reside in nearly all tissues, including bone marrow and adipose tissues, among others. Due to their expandability, multipotency, immunosuppression, and limited ethical concerns as compared to other types of stem cells, human MSCs have emerged as an attractive cell source for regenerative medicine. Moreover, MSCs exhibit low expression of major histocompatibility (MHC) antigens, thereby reducing the need for MHC match between different donors and recipients in allogeneic MSC transplant. Due to these characteristics that MSCs possess, MSC-based allogeneic transplantation is now the forefront of regenerative medicine. As a fast-growing field in regenerative medicine, MSCs represent the most clinically used stem cells with over 1000 registered clinical trials with an established safety record in patients that can efficaciously treat more than 30 diseases. However, there are several limitations of primary MSCs that greatly hamper their clinical application. They include limited cell proliferative capacity, gradual loss of differentiation potential during in vitro expansion, variation across donors, rarity in organs, invasive procedures required for harvesting, etc.
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), represent a promising solution to overcome the issues associated with primary MSCs. Due to the pluripotency of hPSCs, they exhibit unlimited proliferation ability and are able to differentiate into various types of cells, including MSCs. Therefore, hPSCs can provide unlimited and uniform MSCs as an alternative cell source to primary MSCs. This review summarizes the derivation approaches and various applications of hPSC-MSCs, and ultimately the challenges associated with safety and efficacy of hPSC-MSCs are discussed.
Although primary MSCs have been widely used for clinical application, the previously mentioned limitations with the use of primary MSCs significantly hamper their clinical applications. To overcome the problems with primary MSCs, substantial advan
During embryonic development, MSCs develop from neural crest cells (NCCs), lateral plate mesoderm, or paraxial mesoderm, which further develop into craniofacial skeleton, appendicular skeleton, and axial skeleton, respectively. The neural crest is a transient structure formed through epithelial-mesenchymal transition (EMT) with potential to differentiate into a wide range of cell types, including MSCs. It was shown that neural crest cells were derived from hPSCs[9-13], which were able to develop or differentiate into MSCs[14-16]. Morikawa et al[15] showed that MSCs in the adult bone marrow had at least two developmental origins, one of which was the neural crest. By lineage tracing, Takashima et al[16] showed that Sox1+ neuroepithelium gave rise to MSCs in part through a neural crest intermediate stage. The combination of the glycogen synthase kinase 3 beta inhibitor and transforming growth factor-beta (TGFβ) inhibitor very efficiently induced hPSCs towards hNCCs (70%-80%), which further differentiated into MSCs with chemically defined medium[14]. The mesoderm is a major source of MSCs, and we recently reported a stepwise, serum-free, chemically defined and highly efficient protocol to generate hPSC-MSCs via lateral plate mesoderm. The resultant iPSC-MSCs displayed similar MSC surface antigen profile, gene expression profile, and epigenetic profile. iPSC-MSCs had three lineage differentiation. Significantly, hPSC-MSCs were able to repair cartilage defects, similar to bone marrow-MSCs (BM-MSCs)[17]. Upon differentiation, mESCs gave rise to VEGFR-2+PDGFR+ population followed by VEGFR-2-PDGFR+ population via paraxial mesoderm[18]. hESC-derived KDR-PDGFRa+ paraxial mesoderm-like cells showed robust chondrogenic activity and generated a hyaline-like translucent cartilage particle whereas STRO1+ BM-MSCs showed relatively weaker chondrogenesis and formed more fibrotic cartilage particles in vitro[19].
MSCs in the placenta develop from trophoblasts in the extraembryonic tissue chorion[20]. MSCs can also be derived via trophoblasts. hESCs cultured in serum containing medium[21] and serum free medium[22] containing BMP4 and A83-01 were able to differentiate into trophoblasts and then into MSCs. Trophoblast-derived MSCs produced less interleukin 6 (IL-6), C-X-C motif chemokine ligand 10, and C–C motif chemokine ligand 2 but more programmed death-ligand 1 in response to IFN gamma (IFNγ) treatment as compared with MSCs[21]. Compared with MSCs from serum containing medium, serum free approach took longer than serum containing approach to derive MSCs, but serum-free derived MSCs grew faster and produced less IL-6 and interleukin 8[22].
Barberi et al[23] first reported that MSCs were derived from hESCs by coculturing hESCs with monolayer of murine OP9 stromal cells. However, the undefined condition in this approach inevitably led to spontaneous differentiation, giving rise to an undesired type of cells. Besides MSCs, non-MSCs such as CD34 (+) primitive hematopoietic cells, were also present[24]. Vodyanik et al[25] showed that MSCs were derived from a common precursor of mesenchymal and endothelial cells called mesenchymoangioblast by coculturing hESCs with OP9.
Culturing hPSCs in the undefined condition of FBS-containing MSC medium is another way to derive hPSC-MSCs by providing growth factors required for differentiation towards MSCs. When hESCs or iPSCs were cultured in FBS-containing MSC medium for 4 wk to derive hPSC-MSCs, hPSC-MSCs inhibited cell proliferation and cytolytic function of natural killer (NK) cells in the same fashion that BM-MSCs did. However, they were more resistant to preactivated NK cells as compared with adult BM-MSCs[26]. A high density of hESCs on a porcine gelatin-coated dish were cultured in a medium containing 10% FBS for 7 d to outgrow the cells and then enrich hESC-MSCs by 1-2 passages[27]. Functional iPSC-MSCs were also derived on coating with gelatin, and the resultant iPSC-MSCs pre-induced into osteogenesis for 4 d formed bone in the calvaria defects confirmed by human specific nuclear antigen and mitochondrial antibodies[28]. hESC/iPSCs were seeded onto collagen coating and cultured in FBS-containing medium for 10 d to generate hESC/iPSC-MSCs[29]. Spontaneously differentiated cells (raclures) from feeder-free hESCs were cultured in FBS-containing MSC medium for 4 wk, and hESC-MSCs were enriched by following passage[30]. Chen et al[31] reported the derivation of hPSC-MSCs by serum-free medium containing TGFβ inhibitor and EMT inducer (SB431542) for 10 d to induce the mesoderm followed by induction of MSCs in FBS-containing MSC medium. The resultant hPSC-MSCs had robust osteogenesis and chondrogenesis but weaker adipogenesis. This approach does not require EB and feeder cell coculture.
To mimic in vivo development, Brown et al[32] derived hESC-MSCs via EB in MSC medium and enriched them by sorting for CD73 and CD105. EBs from iPSCs were exposed to TGFβ1-containing medium, and two types of MSCs were generated. Although early (aiMSCs) and late (tiMSCs) outgrowing cells were similar in surface antigen profile and three lineage differentiation, aiMSCs were better in osteogenesis than tiMSCs and BM-MSCs. Compared with BM-MSCs, aiMSCs were more of stemness whereas tiMSCs were more osteogenic, and in vivo bone formation was confirmed via ectopic injection[33].
The use of undefined components (such as FBS and feeder) or animal-derived components affects clinical applications of hPSC-MSCs. To overcome the problems from undefined conditions, serum-free and chemically defined protocols are desired to generate clinically compliant hPSC-MSCs. Lian et al[34,35] reported a clinically compliant protocol to generate hESC-MSCs and iPSC-MSCs. After 1 wk of differentiation, MSCs were enriched by FACS for CD24- CD105+ cells. The transplanted iPSC-MSCs were superior to BM-MSCs in attenuating severe hindlimb ischemia, which may result from better in vivo survival and trophic factors of iPSC-MSCs, and higher proliferation of iPSC-MSCs related to increased hEAG1 potasium channel expression[36]. The use of animal products, such as gelatin for coating, compromises the application of hPSC-MSCs. To generate xeno-free MSCs, FBS was replaced with human serum, and porcine gelatin was replaced with human gelatin. Transplanted hESC-MSCs into renal capsule formed cartilage[27]. Human platelet lysate is an alternative to FBS for the generation of hPSC-MSCs. Compared with the FBS-containing medium, the hPL-supplemented medium generated significantly more MSCs[37].
hPSC-MSCs are similar to primary MSCs in morphology, immunophenotype, differentiation potential, gene expression profile, and epigenetic modification[17,22,38-40]. However, there are some differences observed between primary MSCs and hPSC-MSCs. hPSC-MSCs are smaller in size and proliferate faster than BM-MSCs and adipose tissue-MSCs[22,36,39-41]. hPSC-MSCs express higher levels of cell proliferation-related genes whereas BM-MSCs express higher levels of immune-related genes, therefore hPSC-MSCs had a superior proliferative ability to BM-MSCs[39,42,43]. In addition, iPSC-MSCs express higher levels of pluripotent genes and lower levels of mesodermal genes compared with original MSCs, which harbor mtDNA mutations from original MSCs as well as iPSCs. Compared with primary MSCs, iPSC-MSCs express a lower level of VCAM1, leading to lower initiating cell frequency of HSCs after long-term culture with iPSC-MSCs as feeder[44]. Compared with dental tissue-derived MSCs, re-differentiated iPSC-MSCs expressed higher levels of pluripotent genes and lower levels of mesodermal genes, but displayed lower mitochondrial respiration[45]. iPSC-MSCs also express the lowest level of the HLA-II upon stimulation with IFNγ compared with BM-MSCs and fetal-MSCs. Compared with BM-MSCs, more iPSC-MSCs survived, and less inflammatory cell accumulations and better recovery of hind limb ischemia were also observed upon transplant. These suggest that iPSC-MSCs are not sensitive to IFNγ stimulation and have a stronger immune privilege after transplantation[46]. In differentiation potential, hPSC-MSCs differentiated less effectively along the adipogenic, osteogenic, or chondrogenic lineages compared with BM-MSCs[42], especially poorer adipogenesis[31,47,48]. Both hESCs and iPSCs inefficiently formed hyaline cartilage compared with BM-MSCs[43]. In immunosuppression, iPSC-MSCs were impaired in suppressing T cell proliferation compared with primary MSCs but were rejuvenated with regard to age-related DNA methylation, and this suggests that iPSC-MSCs reacquire incomplete immunomodulatory function, and MSC-specific DNA methylation pattern associates with tissue type and aging[38] (Table 1).
| Comparison | Primary MSCs | hPSC-MSCs | Ref. |
| Cell number | Limited | Unlimited | [17,36] |
| Proliferation | Slower | Faster | [36,39,42,43,48,57] |
| Life span | Shorter | Longer | [17] |
| Variation | Higher | Lower | [119] |
| Differentiation potential | Higher | Lower, esp. adipogenesis | [31,43,47,48] |
| Immunosuppression | Higher | Lower | [38,46] |
| Pluripotent genes | Lower | Higher | [45] |
| Mesenchymal genes | Higher | Lower | [45] |
| VCAM1 | Higher | Lower | [44] |
| HLA-II | Higher | Lower | [46] |
The understanding of the pathological mechanism is critical to developing the therapeutic drugs for the treatment of various genetic diseases. In vitro models to mimic in vivo development are very useful to investigate the pathology of human genetic diseases and further develop therapeutic drugs. However, due to inaccessible human tissues and the lack of animal models, research on human genetic diseases and drug screening remains very limited. With the breakthrough in iPSC technology, it makes it possible to model human diseases and develop their therapeutic drugs in vitro. The iPSC-MSC platform can recapitulate the embryonic bone and cartilage development, and therefore provide new insights into pathological progression of human genetic bone and cartilage diseases for disease modelling and further the development of therapeutic drugs.
Hutchinson-Gilford progeria syndrome (HGPS) is a rare but fatal genetic disorder caused by progerin, a truncated and farnesylated form of Lamin A, which causes systemic accelerated aging in children. Zhang et al[49] generated iPSC-MSCs from HGPS patients and showed that HGPS-iPSC-MSCs displayed abnormalities, including increased nuclear dysmorphology, DNA damage, and accumulation of calponin-staining inclusion bodies, leading to their compromised viability under stress, especially to hypoxia. Using HGPS iPSC-MSCs platform, seven compounds were screened from 2800 small molecules, including all-trans retinoic acid and 13-cis-retinoic acid, which decreased ALP activity and progerin expression[50].
Fibrodysplasia ossificans progressiva (FOP) is an inherited disease characterized by heterotopic endochondral ossification in soft tissues after birth and caused by a point mutation in ACVR1. iPSC-MSCs from FOP patients were generated, and it was found that SMAD1/5/8 and SMAD2/3 were activated and chondrogenesis was enhanced via MMP1 and PAI1 in FOP-iMSCs[51-53]. Hino et al[54] screened 6809 small molecule compounds using high-throughput screening, and mTOR signaling was identified to be a critical pathway for aberrant chondrogenesis. Further mechanism study showed that ectonucleotide pyrophosphatase/phosphodiesterase 2 linked FOP-ACVR1 to mTOR signaling, causing FOP pathogenesis.
Due to the multipotency, immunosuppression, and unlimited cell sources, hPSC-MSCs have been used for various applications in regenerative medicine (Table 2).
| hPSC-MSCs | Disease model or application | Animal model or human | Therapeutic effects | Ref. |
| iPSC-MSCs | CKD | Rat | Protect the kidney against CKD injury | [85] |
| iPSC-MSCs | Adriamycin nephropathy | Mouse | Prevent adriamycin nephropathy | [82] |
| iPSC-MSCs | Obesity-associated Kidney injury | Mouse | Ameliorate endoplasmic reticulum stress | [83] |
| hPSC-MSCs | UUO | Mouse | Protect against kidney fibrosis in vivo and in vitro | [84] |
| hESC-MSCs | LN | Mouse | Prevent the progression of LN | [81] |
| iPSC-MSCs | TNBC | Mouse | Significantly decrease the incidence and burdon of metastases | [117] |
| iPSC-MSCs | Breast cancer | Mouse | Decrease EMT, invasion, stemness, and growth of cancer cells | [119] |
| iPSC-MSCs | Skin wounds, pressure ulcers, and osteoarthritis | Mouse | Have therapeutic potential in skin wounds, pressure ulcers, and osteoarthritis | [127] |
| hESC-MSCs | Arthritis | Mouse | Ameliorate collagen-induced arthritis by inducing IDO1 | [72] |
| iPSC-MSCs | Osteonecrosis of the femoral head | Rat | Prevent osteonecrosis of the femoral head | [64] |
| iPSC-MSCs | Vascularized composite allotransplantation | Rat | Induce T cell hyporesponsiveness to prolong hind limb survival | [106] |
| iPSC-MSCs | Limb ischemia | Mouse | Exosomes of iPSC-MSCs attenuate limb ischemia by promoting angiogenesis | [121] |
| iPSC-MSCs | Limb ischemia | Mouse | Insensitivity of iPSC-MSCs to interferon γ potentiates repair efficiency of hind limb ischemia | [46] |
| iPSC-MSCs | Limb ischemia | Mouse | Attenuate limb ischemia | [35] |
| iPSC-MSCs | Periodontal defects | Rat | Aid periodontal regeneration | [68] |
| iPSC-MSCs | Bone defects | Mouse | Regenerate non-union bone defects more efficiently than BM-MSCs upon BMP6 overexpression | [33] |
| iPSC-MSCs | Calvaria defects | Mouse | Repair calvaria defects | [28] |
| iPSC-MSCs | Osteochondral defects | Rat | iPSC-MSCs are able to repair cartilage defects | [17] |
| iPSC-MSCs | FOP | FOP-iPSC-MSCs enhance chondrogenesis via activin A enhanced mTOR signalling | [53,54] | |
| hESC-MSCs | Lupus and uveitis | Mouse | Increase survival of lupus-prone mice and decrease symptoms of uveitis | [40] |
| hESC-MSCs | EAE model of multiple sclerosis | Mouse | Improve EAE symptoms | [101] |
| hESC-MSCs | EAE | Monkey | Attenuate disease progression in a primate EAE model | [41] |
| hESC-MSCs | EAU | Mouse | Slow down the development of EAU | [103] |
| iPSC-MSCs | Inflammatory bowel disease models | Mouse | Promote intestinal repair via TSG-6 | [111] |
| hESC-MSCs | Experimental inflammatory bowel disease | Mouse | Protect against experimental inflammatory bowel disease | [107] |
| iPSC-MSCs | SS | Mouse | Prevent the progression of SS | [112] |
| iPSC-MSCs | Allergic rhinitis | Modulate T-cell phenotypes towards Th2 suppression through inducing Treg expansion | [108] | |
| iPSC-MSCs | Asthma Inflammation | Mouse | Alleviate asthma inflammation by CX43-mediated mitochondrial transfer | [110] |
| iPSC-MSCs | Corneal injury | Mouse | Exert therapeutic effects in the cornea by reducing inflammation | [99] |
| iPSC-MSCs | Skin wound | Rat | iPSC-MSC-Exos improve cutaneous wound healing by promoting collagen synthesis and angiogenesis. | [120] |
| iPSC-MSCs | SR-aGvHD | Human | iPSC-MSCs are safe and well tolerated | [114] |
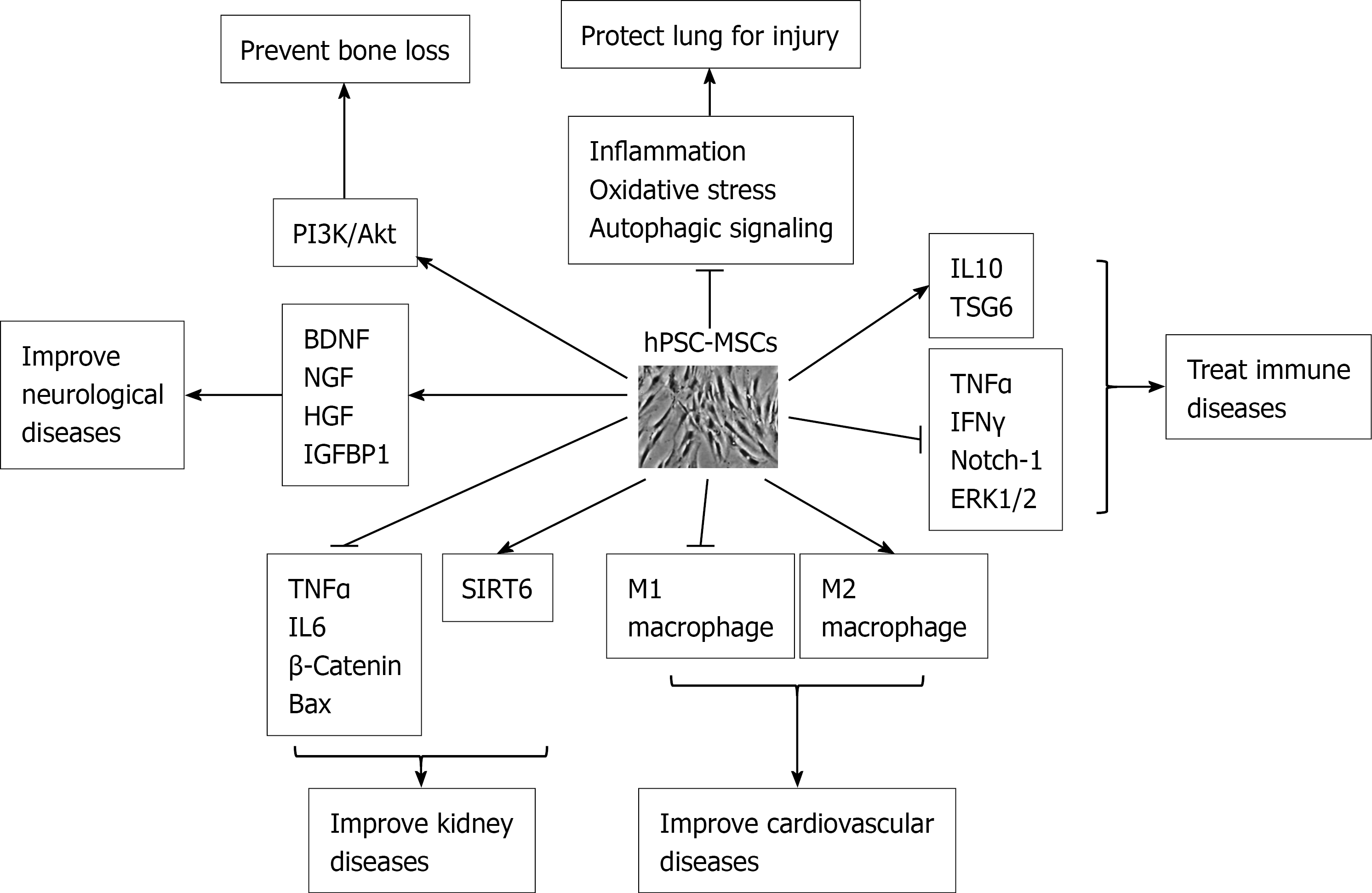
Like BM-MSCs, iPSC-MSCs had osteogenic potential, and therefore they could form typically calcified structure in the scaffolds[55]. iPSC-MSCs had good viability and osteogenic differentiation on the CPC scaffold[56]. iPSC-MSCs were similar to BM-MSCs in preventing bone loss and promoting bone repair for the necrosis region of the femoral head[57]. Engineered non-native peptides increased the attachment of iPSC-MSCs to the scaffolds and enhanced bone and vasculature formation in vivo[58]. Biofunctional agents, such as Arg-Gly-Asp (RGD), improved the proliferation and bone mineralization of iPSC-MSCs[59]. When iPSC-MSCs were treated with metformin, a widely used drug for diabetes, they showed enhanced bone formation and increased osteogenic markers and mineralized nodule formation, suggesting that metformin might be used to improve bone and periodontal regeneration[60]. Recently increasing reports have shown that MSCs exerted their pleiotropic effects by the secretion of soluble paracrine factors rather than their differentiation potential[61]. MSC-derived exosomes contain cytokines, growth factors, mRNAs, and regulatory miRNAs[62]. iPSC-MSC exosomes increased the proliferation, migration, and osteogenesis of BM-MSCs[63], significantly prevented bone loss, and promoted local angiogenesis by activating the PI3K/Akt signalling pathway in endothelial cells in a steroid-induced rat osteonecrosis model[64] (Figure 1).
Genetic modification can improve the bone formation of iPSC-MSCs. Distal-less homeobox 3 (DLX3) overexpression enhanced bone formation of iPSC-MSCs as shown by increased osteogenic genes and mineralized nodules at the expense of decreased proliferation[65]. Bone morphogenetic protein 2 overexpression enhanced bone formation on RGD-grafted calcium phosphate cement (CPC) of iPSC-MSCs[66]. Neural EGFL like 1 (NELL1) overexpression greatly improved osteogenesis of iPSC-MSCs on RGD-CPC[67].
Due to osteogenic differentiation potential, iPSC-MSCs have the capacity for periodontal regeneration. When transplanted into periodontal defects, iPSC-MSCs formed new mineralized tissues and significantly improved regeneration, suggesting that iPSC-MSCs represent a promising stem cell source for clinical application in periodontitis[68].
Articular cartilage has limited intrinsic healing potential, leading to a loss of joint function. Like BM-MSCs, iPSC-MSCs can differentiate into chondrocytes in vitro[69]. In view that autologous chondrocytes and primary MSCs are limited in cell number, iPSC-MSCs are gaining attention as a new cell therapy for cartilage regeneration due to unlimited cells and chondrogenic differentiation potential. Our previous data showed that primary BM-MSCs were able to repair cartilage defects effectively[70]. Multiple injections of hESC-MSCs into knee joint of osteoarthritis (OA) rats induced by anterior cruciate ligament transection repaired cartilage better than the single dose and negative control groups in a rat OA model[71]. hESC-MSCs also ameliorated collagen-induced arthritis by inducing indoleamine 2,3-dioxygenase 1 (IDO1) in mice[72]. In addition, exosomes from hESC-MSCs prevented cartilage destruction by maintaining the chondrocyte function[73]. By our defined, step-wise and chemically defined protocol, we generated iPSC-MSCs via lateral plate mesoderm and have shown that iPSC-MSCs repaired osteochondral defects similar to BM-MSCs[17].
As an attractive candidate for cell-based therapy, MSCs are therapeutically beneficial to improving lung disease or repairing lung damage. iPSC-MSCs protected lung cells against mitochondrial dysfunction and apoptosis induced by oxidative stress to reduce lung injury and inflammation in in vivo models of lung disease[74]. iPSC-MSCs reduced airway inflammation and hyperresponsiveness to protect against lung diseases induced by oxidative stress, such as chronic obstructive pulmonary disease[75]. iPSC-MSCs protected the lung against ischemia-reperfusion injury (IRI) by suppressing the inflammatory, oxidative stress, and autophagic signalling pathways[76]. Treatment with iPSC-MSCs also significantly prevented airway allergic inflammation, decreased Th2 cytokine levels, and changed long non-coding RNAs profiles[77]. iPSC-MSCs ameliorated cigarette smoke (CS)-induced apoptosis and proliferation imbalance of airway cells partly through the paracrine section of stem cell factor (SCF)[78]. Asthma is a chronic disease with inflamed airways. iPSC-MSCs were able to prevent chronic allergic airway inflammation[79]. Compared with BM-MSCs, iPSC-MSCs transferred mitochondria to bronchial epithelial cells more effectively via tunnelling nanotubes. Therefore, iPSC-MSCs were superior to BM-MSCs in attenuating CS-induced airspace enlargement[80].
hPSC-MSCs improved both acute and chronic adriamycin nephropathy (AN) by preventing renal function loss. hESC-MSCs prevented the progression of fatal lupus nephritis in a mouse model by significantly decreasing two inflammatory cytokines associated with systemic lupus erythematosus, tumour necrosis factor α (TNFα) and IL-6[81]. iPSC-MSCs prevented the apoptosis of tubular cells by downregulating B-cell lymphoma 2 associated X (Bax) and Bax/B-cell lymphoma 2 and upregulating survivin in the short-term AN model whereas iPSC-MSCs inhibited fibrosis via hedgehog signalling in the long-term AN model[82]. iPSC-MSCs also ameliorated palmitic acid-induced lipotoxic kidney injury by alleviating endoplasmic reticulum (ER) stress, inflammation, and apoptosis to suppress ER stress and its downstream pro-inflammatory and pro-apoptotic effects via hepatocyte growth factor (HGF)/c-Met signalling[83]. Chronic kidney disease (CKD) is characterized by a gradual loss of kidney function over time due to renal fibrosis[84]. Intravenously administrated iPSC-MSCs effectively protected the kidney against CKD injury in CKD parenchyma[85]. iPSC-MSCs were also able to effectively protect kidney from acute ischemia-reperfusion injury[86]. hPSC-MSC-derived exosomes reduced the renal fibrosis, decreased inflammatory reactions, and improved renal function in unilateral ureteral obstruction mice by increasing SIRT6 and decreasing β-catenin[84] (Figure 1).
MSCs have the potential to improve cardiovascular diseases. Coculture with hESC-MSCs promoted the maturation of hESC-derived cardiomyocyte microtissues[87]. iPSC-MSCs increased the level of M2 macrophages and deceased the level of M1 macrophages after cardiac arrest (Figure 1), suggesting that iPSC-MSCs play a crucial role in immunomodulation during cardiopulmonary resuscitation[88]. iPSC-MSCs improved CS-induced cardiac remodelling and dysfunction better than BM-MSCs as shown by an increase in percentage of left ventricular ejection fraction and fractional shortening. iPSC-MSCs attenuated cardiac pro-inflammatory cytokines and restored anti-inflammatory cytokines[89]. Conditioned medium from iPSC-MSCs alleviated heart failure and reduced cardiomyocyte apoptosis and fibrosis better than that from BM-MSCs, showing that iPSC-MSCs could provide cell-free therapeutic cardio-protection[90]. Extracellular vesicles (EVs) of iPSC-MSCs mitigated arterial ageing by attenuating ageing-associated vascular endothelial dysfunction, arterial stiffness, and hypertension[91]. In addition, overexpression of myocardin in iPSC-MSCs resulted in partial transdifferentiation into cardiomyocyte phenotype[92].
MSCs demonstrate significant neuroprotection and promote functional recoveries of the pathological nervous system. MSCs were shown to secret brain-derived neurotrophic factor and nerve growth factor, which supported neuronal cell survival and induced nerve regeneration (Figure 1). Conditional medium of hESC-MSCs could significantly ameliorate neurological deficits and infarct volume in middle cerebral artery occlusion (MCAO) rats[93]. hESC-MSCs differentiated into neural-like cells in standard neurogenic differentiation medium, and hESC-MSCs in sphere secreted more HGF and IGFBP1 than those in single-cell suspension[94] (Figure 1). hPSC-MSCs expressed higher levels of neural genes than BM-MSCs and rapidly differentiated into neural-like cells when differentiated into neural lineage[95]. Although ESC-MSCs induced autophagy similar to BM-MSCs, ESC-MSCs survived better in amyloid-β (Aβ) -induced cellular models and reduced more intracellular Aβ levels compared with BM-MSCs. ESC-MSCs significantly decreased Aβ-induced cell death and promoted autophagolysosomal clearance of Aβ in a rat model of Alzheimer's disease, leading to higher memory performance. Intra-arterially transplanted ESC-MSCs were safe and free from cerebral ischemia[96]. iPSC-MSCs markedly decreased brain-infarct volume and improved neurological function mainly by inhibiting inflammation[97]. ESC-MSCs had a superior neuroprotective capacity over fetal MSCs in mouse hypoxic-ischemic brains[98].
In addition, hESC-MSC EVs also protected retinal ganglion cells and preserved retinal function in a mouse model of optic nerve injury by improving retinal ganglion cell (RGC) survival and preventing retinal nerve fiber layer degeneration. iPSC-MSCs significantly reduced corneal opacity by reducing inflammation similar to BM-MSCs[99]. Transplanted iPSC-MSCs significantly improved the survival of RGCs by effectively transferring functional mitochondria to RGCs[100].
Multiple sclerosis (MS) is a potentially disabling disease of the central nervous system caused by an attack of the protective sheath by the immune system, leading to communication problems between the brain and the rest of the body. As yet, there is no cure for MS, the most common demyelinating disease. Compared with BM-MSCs, hESC-MSCs improved efficacy in a mouse experimental autoimmune encephalitis (EAE) model of MS due to its lowered IL-6 expression. In addition, hESC-MSCs are less vulnerable than BM-MSCs in therapeutic capacity during in vitro culture[101]. After hESC-MSCs were intrathecally injected into the central nervous system of EAE-induced monkeys, hESC-MSCs greatly decreased the clinical symptoms, brain lesions, and neuronal demyelination in the EAE monkeys. hESC-MSCs could transdifferentiate into neural cells in vivo in the CNS of the treated monkeys as shown by elevated expression of genes for neuronal markers, neurotrophic factors, and neuronal myelination[41].
hPSC-MSCs have a strong immune regulatory effect during anti-inflammation. Microphages serve as a bridge between innate and specific immune responses. hPSC-MSCs altered macrophage polarization by suppressing the Notch-1 signalling pathway[102] (Figure 1). Due to the immunosuppression property of iPSC-MSCs, they have been used for the treatment of various immune diseases. hESC-MSCs slowed down the development of severe experimental autoimmune uveitis through systemic immune modulation[103], whereas iPSC-MSCs inhibited proliferation, shifted the secretome of peripheral blood mononuclear cells, and significantly suppressed CD8 T proliferation, activation, and differentiation[104]. iPSC-MSCs also suppressed T-cell effector cells of Th1/Th2 and increased regulatory T cell (Treg) response[105]. iPSC-MSCs prolonged hind limb survival by reducing mononuclear cell infiltration, lowering TNFα and IFNγ, increasing interleukin 10, and thus protecting against acute rejection in a rat vascularized composite allotransplantation model[106] (Figure 1). iPSC-MSCs disrupted NK cell cytolytic machinery to prevent allograft rejection by decreasing activation markers and ERK1/2 signalling, leading to impaired immunologic synapses and secreted cytotoxic granules. However, iPSC-MSCs were more resistant than BM-MSCs to pro-activate NK cells[26]. hESC-MSCs could protect against an experimental model of inflammatory bowel disease[107]. iPSC-MSCs modulated T-cell phenotypes towards Th2 suppression by inhibiting lymphocyte proliferation and promoting Treg response, suggesting that iPSC-MSCs can treat allergic airway diseases[108]. iPSC-MSCs regulate T cell responses by decreasing secreted soluble factors[109]. iPSC-MSCs also improved asthma inflammation by connexin 43-mediated mitochondrial transfer[110]. iPSC-MSCs accelerated intestinal epithelial cell proliferation to promote intestinal repair in murine colitis through tumor necrosis factor-stimulated gene-6 (TSG-6) via Akt-dependent interaction between the extracellular matrix HA and CD44+ cells[111]. iPSC-MSC EVs prevented the progression of Sjogren’s syndrome (SS), a chronic autoimmune disease, by suppressing activation of immune cells and proinflammation factors essential for SS progression[112]. Due to intrinsic immunosuppression, MSCs significantly prolonged the survival of humanized mouse model of graft vs host disease (GvHD)[113]. The first iPSC-MSC clinical trial was reported in 2020. iPSC-MSCs were produced using an optimized and good manufacturing practice-compliant manufacturing process to treat steroid-resistant acute GvHD. Based on the complete response, overall response, and overall survival of participants, the higher dose level of iPSC-MSC showed better outcomes than the lower dose, and iPSC-MSCs were safe and well tolerated without serious adverse events reported[114].
Like primary MSCs, hPSC-MSCs also have therapeutic potentials in treating cancer or repairing tissue damages caused by cancers. hPSC-MSCs can overcome the limitation of drug delivery. iPSC-MSCs expressing cytosine deaminase limited tumor growth and decreased lung metastases in a mouse xenogeneic model of human breast cancer[115]. EVs from hPSC-MSCs also showed promising results to improve cancer treatment. hESC-MSC microvesicles decreased the proliferation of leukemia cells[116]. Treatment with iPSC-MSC nanovesicles showed no detectable immunogenicity and significantly decreased the incidence of metastases from triple-negative breast cancer in mouse models[117]. iPSC-MSC nanovesicles also significantly decreased tumor growth of metastatic prostate cancer[118]. These suggest that iPSC-MSC nanovesicle is a promising platform to improve the treatment of metastatic cancer. iPSC-MSCs can home to cancers with a similar efficiency as BM-MSCs. As compared with BM-MSCs, iPSC-MSCs expressed lower levels of interleukin-1 and TGFβ receptors, downstream pro-tumor factors, and hyaluronan and its cofactor TSG6, and therefore iPSC-MSCs have much less potential to promote tumours than BM-MSCs by promoting the EMT, invasion, stemness, and growth of cancer cells[119].
hPSC-MSCs are also used for other applications. iPSC-MSC exosome improved cutaneous wound healing by promoting collagen synthesis and angiogenesis[120]. Furthermore, iPSC-MSC exosome via intramuscular injection could enhance micro-vessel density and blood perfusion by activating angiogenesis-related molecule expression and promoting HUVEC migration, proliferation, and tube formation[121]. iPSC-MSCs supported the proliferation of hematopoietic stem and progenitor cells (HPCs), and maintained a primitive immunophenotype and colony forming unit of CD34+ HPCs. Long-term culture initiating cell frequency was lower compared with primary MSCs, suggesting that iPSC-MSCs are less suitable than primary MSCs as feeder cells[44]. iPSC-MSCs also can be used as feeder cells to culture human iPSCs. Human iPSCs cultured on human iPSC-MSC feeder were slightly thinner and flatter than the other feeder system. However, iPSC-MSCs still maintain the proliferation and pluripotency of iPSCs[122]. hESC-MSCs restored the structure of the injured ovarian structure and function in premature ovarian failure via paracrine effect and ovarian cell survival to rescue fertility in mice[123,124]. hESC-MSC secreted trophic factors to support hepatocytes on an acute liver failure model[125]. hESC-MSC EVs ameliorated cirrhosis in thioacetamide-induced chronic liver injury[126].
Primary MSCs have drawbacks due to their limited scalability, interdonor variability, and inconsistent outcomes of clinical trials. iPSC-MSCs have the potential to overcome the fundamental limitations of conventional and donor-derived MSC production processes. The derivation of hPSC-MSCs has made substantial progress with an increasing number of reports on the use of hPSC-MSCs for regenerative medicine over the past years. However, the issues and challenges related to safety and efficacy of hPSC-MSCs remain to be understood and addressed. These include the effects of cell origins and derivation approaches on hPSC-MSCs, the understanding of difference between hPSC-MSCs and primary MSCs, MSC stemness/potency biomarkers, the differentiation potential of hPSC-MSCs, choice of autologous or allogeneic hPSC-MSC source, manufacturing of clinical grade hPSC-MSCs, etc.
The use of MSCs is already in various phases of clinical applications. However, little is known about the difference in features of hPSC-MSCs from different origins, particularly in their differentiation potential, a critical feature to their clinical application. Although hPSC-MSCs derived from various approaches exhibit MSC morphology and express MSC surface antigens, their differentiation potential is not as efficient as BM-MSCs, especially in adipogenesis[31,47]. Due to epigenetic memory or incomplete reprogramming, iPSC variations exist, and iPSC-MSCs exhibit preferential differentiation into their original cell lineage. Eto et al[127] showed that iPSC-MSCs via the mesoderm and neuroepithelium had the capacity for self-renewal and multipotency as well as therapeutic potential in skin wounds, pressure ulcers, and OA in a mouse model. However, different therapeutic effects of iPSC-MSCs from different origins were also observed, suggesting that the therapeutic efficacy of hPSC-MSCs is dependent on cell origins. In addition, hPSC-MSCs derived by differentiation approaches vary extensively in their quality and efficiency. The use of fibroblast growth factor in the differentiation medium[27,47,128] promotes MSC proliferation at the expense of its differentiation potential[129]. Therefore, the effects of cell origins and differentiation approaches on iPSC-MSCs need to be elucidated.
Compared with primary MSCs, hPSC-MSCs have advantages of faster proliferation, longer life span, more reliable and homogeneous cell source, but somehow immature differentiation potential and impaired immunosuppression. What are intrinsic and extrinsic mechanisms underlying the difference between iPSC-MSCs and primary MSCs?
So far, little is known about regulators or biomarkers associated with MSC stemness/ potency, and there is no critical quality attribute available for use to distinguish good MSCs from bad ones before cellular manufacturing. The mechanism underlying MSC stemness or potency remains poorly understood, which greatly hampers the clinical application of hPSC-MSCs. It was shown that kindlin-2 increased the survival, proliferation, stemness, and migration of iPSC-MSCs. Kindlin-2 knockdown increased apoptosis and differentiation response whereas kindlin-2 overexpression increased proliferation, decreased apoptosis, and slowed down trilineage differentiation. More significantly, kindlin-2 overexpression increased the migration of iPSC-MSCs in the wound-scratch assay[130]. In the future, substantial efforts are needed to explore MSC stemness/potency-related regulators or biomarkers for clinical application.
It is well accepted that MSCs have potential to differentiate into multiple mes
MSCs have anti-inflammatory and immune-modulatory properties. However, patient-derived autologous hPSC-MSCs still represent a better option for regenerative medicine as there are lesser concern regarding the immune response compared with allogeneic MSCs.
Although iPSCs are generated by integration-free methods and iPSC-MSCs are derived by a number of approaches, there are few approaches available to regenerate clinical-grade hPSC-MSCs for clinical application. Most protocols have used undefined components, such as FBS, feeder cells, and other animal-derived components, which compromise the clinical application of iPSC-MSCs. To generate clinical grade iPSC-MSCs, reliable, efficient, scalable, and clinically compliant approaches are required throughout the whole manufacturing process of iPSC-MSCs. These processes include generation and expansion of iPSCs, freezing and thawing of iPSCs, differentiation of iPSCs towards MSCs, expansion of iPSC-MSCs, freezing and thawing iPSC-MSCs, etc. In addition, comprehensive assays should be established to evaluate the safety, quality, or potency of hPSC-MSCs during cellular manufacturing for clinical application.
hPSC-MSCs have enormous potential for regenerative medicine, and can be used for disease modelling, drug screening, and treatment of various diseases in regenerative medicine. Although multiple approaches have been reported in deriving MSCs from hPSCs, the use of undefined and animal-derived components greatly compromises the clinical application of hPSC-MSCs. Much effort is needed to derive clinically relevant and sufficient hPSC-MSCs with good quality for clinical application, and criteria need be established to evaluate the safety and efficacy of hPSC-MSCs before clinical application. In addition, many issues or challenges with hPSC-MSCs also need to be addressed.
| 1. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15369] [Article Influence: 569.2] [Reference Citation Analysis (2)] |
| 2. | Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 3. | Pountos I, Jones E, Tzioupis C, McGonagle D, Giannoudis PV. Growing bone and cartilage. The role of mesenchymal stem cells. J Bone Joint Surg Br. 2006;88:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 970] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 5. | Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83-92. [PubMed] |
| 6. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 7. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] |
| 8. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3539] [Cited by in RCA: 3342] [Article Influence: 95.5] [Reference Citation Analysis (1)] |
| 9. | Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3036] [Cited by in RCA: 2800] [Article Influence: 164.7] [Reference Citation Analysis (0)] |
| 10. | Menendez L, Kulik MJ, Page AT, Park SS, Lauderdale JD, Cunningham ML, Dalton S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc. 2013;8:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240-19245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Mica Y, Lee G, Chambers SM, Tomishima MJ, Studer L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013;3:1140-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Milet C, Monsoro-Burq AH. Embryonic stem cell strategies to explore neural crest development in human embryos. Dev Biol. 2012;366:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Fukuta M, Nakai Y, Kirino K, Nakagawa M, Sekiguchi K, Nagata S, Matsumoto Y, Yamamoto T, Umeda K, Heike T, Okumura N, Koizumi N, Sato T, Nakahata T, Saito M, Otsuka T, Kinoshita S, Ueno M, Ikeya M, Toguchida J. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS One. 2014;9:e112291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379:1114-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Liu TM, Yildirim ED, Li P, Fang HT, Denslin V, Kumar V, Loh YH, Lee EH, Cool SM, Teh BT, Hui JH, Lim B, Shyh-Chang N. Ascorbate and Iron Are Required for the Specification and Long-Term Self-Renewal of Human Skeletal Mesenchymal Stromal Cells. Stem Cell Reports. 2020;14:210-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Sakurai H, Era T, Jakt LM, Okada M, Nakai S, Nishikawa S. In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells. 2006;24:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Umeda K, Zhao J, Simmons P, Stanley E, Elefanty A, Nakayama N. Human chondrogenic paraxial mesoderm, directed specification and prospective isolation from pluripotent stem cells. Sci Rep. 2012;2:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1279] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 21. | Wang X, Lazorchak AS, Song L, Li E, Zhang Z, Jiang B, Xu RH. Immune modulatory mesenchymal stem cells derived from human embryonic stem cells through a trophoblast-like stage. Stem Cells. 2016;34:380-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Li E, Zhang Z, Jiang B, Yan L, Park JW, Xu RH. Generation of Mesenchymal Stem Cells from Human Embryonic Stem Cells in a Complete Serum-free Condition. Int J Biol Sci. 2018;14:1901-1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Trivedi P, Hematti P. Simultaneous generation of CD34+ primitive hematopoietic cells and CD73+ mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp Hematol. 2007;35:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, Durrbach A, Bennaceur-Griscelli A. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118:3254-3262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Karlsson C, Emanuelsson K, Wessberg F, Kajic K, Axell MZ, Eriksson PS, Lindahl A, Hyllner J, Strehl R. Human embryonic stem cell-derived mesenchymal progenitors--potential in regenerative medicine. Stem Cell Res. 2009;3:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM, Krebsbach PH. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7:e33225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Sheyn D, Ben-David S, Shapiro G, De Mel S, Bez M, Ornelas L, Sahabian A, Sareen D, Da X, Pelled G, Tawackoli W, Liu Z, Gazit D, Gazit Z. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl Med. 2016;5:1447-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, Lim SK. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (1)] |
| 36. | Zhang J, Chan YC, Ho JC, Siu CW, Lian Q, Tse HF. Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether-à-go-go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol. 2012;303:C115-C125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Luzzani C, Neiman G, Garate X, Questa M, Solari C, Fernandez Espinosa D, García M, Errecalde AL, Guberman A, Scassa ME, Sevlever GE, Romorini L, Miriuka SG. A therapy-grade protocol for differentiation of pluripotent stem cells into mesenchymal stem cells using platelet lysate as supplement. Stem Cell Res Ther. 2015;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Frobel J, Hemeda H, Lenz M, Abagnale G, Joussen S, Denecke B, Sarić T, Zenke M, Wagner W. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports. 2014;3:414-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 39. | Yen ML, Hou CH, Peng KY, Tseng PC, Jiang SS, Shun CT, Chen YC, Kuo ML. Efficient derivation and concise gene expression profiling of human embryonic stem cell-derived mesenchymal progenitors (EMPs). Cell Transplant. 2011;20:1529-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Kimbrel EA, Kouris NA, Yavanian GJ, Chu J, Qin Y, Chan A, Singh RP, McCurdy D, Gordon L, Levinson RD, Lanza R. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Yan L, Jiang B, Niu Y, Wang H, Li E, Yan Y, Sun H, Duan Y, Chang S, Chen G, Ji W, Xu RH, Si W. Intrathecal delivery of human ESC-derived mesenchymal stem cell spheres promotes recovery of a primate multiple sclerosis model. Cell Death Discov. 2018;4:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Brown PT, Squire MW, Li WJ. Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Cell Tissue Res. 2014;358:149-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Sfougataki I, Varela I, Stefanaki K, Karagiannidou A, Roubelakis MG, Kalodimou V, Papathanasiou I, Traeger-Synodinos J, Kitsiou-Tzeli S, Kanavakis E, Kitra V, Tsezou A, Tzetis M, Goussetis E. Proliferative and chondrogenic potential of mesenchymal stromal cells from pluripotent and bone marrow cells. Histol Histopathol. 2020;35:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Vasko T, Frobel J, Lubberich R, Goecke TW, Wagner W. iPSC-derived mesenchymal stromal cells are less supportive than primary MSCs for co-culture of hematopoietic progenitor cells. J Hematol Oncol. 2016;9:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Park J, Lee Y, Shin J, Lee HJ, Son YB, Park BW, Kim D, Rho GJ, Kang E. Mitochondrial genome mutations in mesenchymal stem cells derived from human dental induced pluripotent stem cells. BMB Rep. 2019;52:689-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Sun YQ, Zhang Y, Li X, Deng MX, Gao WX, Yao Y, Chiu SM, Liang X, Gao F, Chan CW, Tse HF, Shi J, Fu QL, Lian Q. Insensitivity of Human iPS Cells-Derived Mesenchymal Stem Cells to Interferon-γ-induced HLA Expression Potentiates Repair Efficiency of Hind Limb Ischemia in Immune Humanized NOD Scid Gamma Mice. Stem Cells. 2015;33:3452-3467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL. Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:1897-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Kang R, Zhou Y, Tan S, Zhou G, Aagaard L, Xie L, Bünger C, Bolund L, Luo Y. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res Ther. 2015;6:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang Y, Tse HF, Stewart CL, Colman A. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 385] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 50. | Lo Cicero A, Jaskowiak AL, Egesipe AL, Tournois J, Brinon B, Pitrez PR, Ferreira L, de Sandre-Giovannoli A, Levy N, Nissan X. A High Throughput Phenotypic Screening reveals compounds that counteract premature osteogenic differentiation of HGPS iPS-derived mesenchymal stem cells. Sci Rep. 2016;6:34798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Matsumoto Y, Hayashi Y, Schlieve CR, Ikeya M, Kim H, Nguyen TD, Sami S, Baba S, Barruet E, Nasu A, Asaka I, Otsuka T, Yamanaka S, Conklin BR, Toguchida J, Hsiao EC. Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation. Orphanet J Rare Dis. 2013;8:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Matsumoto Y, Ikeya M, Hino K, Horigome K, Fukuta M, Watanabe M, Nagata S, Yamamoto T, Otsuka T, Toguchida J. New Protocol to Optimize iPS Cells for Genome Analysis of Fibrodysplasia Ossificans Progressiva. Stem Cells. 2015;33:1730-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Nakajima T, Shibata M, Nishio M, Nagata S, Alev C, Sakurai H, Toguchida J, Ikeya M. Modeling human somite development and fibrodysplasia ossificans progressiva with induced pluripotent stem cells. Development. 2018;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Hino K, Horigome K, Nishio M, Komura S, Nagata S, Zhao C, Jin Y, Kawakami K, Yamada Y, Ohta A, Toguchida J, Ikeya M. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J Clin Invest. 2017;127:3339-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 55. | Zou L, Luo Y, Chen M, Wang G, Ding M, Petersen CC, Kang R, Dagnaes-Hansen F, Zeng Y, Lv N, Ma Q, Le DQ, Besenbacher F, Bolund L, Jensen TG, Kjems J, Pu WT, Bünger C. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci Rep. 2013;3:2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 56. | Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014;20:1295-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Zhou M, Xi J, Cheng Y, Sun D, Shu P, Chi S, Tian S, Ye S. Reprogrammed mesenchymal stem cells derived from iPSCs promote bone repair in steroid-associated osteonecrosis of the femoral head. Stem Cell Res Ther. 2021;12:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 58. | Ramaraju H, Kohn DH. Cell and Material-Specific Phage Display Peptides Increase iPS-MSC Mediated Bone and Vasculature Formation In Vivo. Adv Healthc Mater. 2019;8:e1801356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | TheinHan W, Liu J, Tang M, Chen W, Cheng L, Xu HH. Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res. 2013;4:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Wang P, Ma T, Guo D, Hu K, Shu Y, Xu HHK, Schneider A. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2018;12:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | Warnecke A, Prenzler N, Harre J, Köhl U, Gärtner L, Lenarz T, Laner-Plamberger S, Wietzorrek G, Staecker H, Lassacher T, Hollerweger J, Gimona M, Rohde E. First-in-human intracochlear application of human stromal cell-derived extracellular vesicles. J Extracell Vesicles. 2021;10:e12094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 62. | Sandonà M, Di Pietro L, Esposito F, Ventura A, Silini AR, Parolini O, Saccone V. Mesenchymal Stromal Cells and Their Secretome: New Therapeutic Perspectives for Skeletal Muscle Regeneration. Front Bioeng Biotechnol. 2021;9:652970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 63. | Zhang J, Liu X, Li H, Chen C, Hu B, Niu X, Li Q, Zhao B, Xie Z, Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 64. | Liu X, Li Q, Niu X, Hu B, Chen S, Song W, Ding J, Zhang C, Wang Y. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci. 2017;13:232-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 65. | Li J, Lin Q, Lin Y, Lai R, Zhang W. Effects of DLX3 on the osteogenic differentiation of induced pluripotent stem cellderived mesenchymal stem cells. Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Liu J, Chen W, Zhao Z, Xu HH. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials. 2013;34:7862-7872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Liu J, Chen W, Zhao Z, Xu HHK. Effect of NELL1 gene overexpression in iPSC-MSCs seeded on calcium phosphate cement. Acta Biomater. 2014;10:5128-5138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Hynes K, Menicanin D, Han J, Marino V, Mrozik K, Gronthos S, Bartold PM. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 69. | Guzzo RM, Gibson J, Xu RH, Lee FY, Drissi H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem. 2013;114:480-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Liu TM, Guo XM, Tan HS, Hui JH, Lim B, Lee EH. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011;63:2711-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Xing D, Wang K, Wu J, Zhao Y, Liu W, Li JJ, Gao T, Yan D, Wang L, Hao J, Lin J. Clinical-Grade Human Embryonic Stem Cell-Derived Mesenchymal Stromal Cells Ameliorate the Progression of Osteoarthritis in a Rat Model. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Gonzalo-Gil E, Pérez-Lorenzo MJ, Galindo M, Díaz de la Guardia R, López-Millán B, Bueno C, Menéndez P, Pablos JL, Criado G. Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis Res Ther. 2016;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 74. | Michaeloudes C, Li X, Mak JCW, Bhavsar PK. Study of Mesenchymal Stem Cell-Mediated Mitochondrial Transfer in In Vitro Models of Oxidant-Mediated Airway Epithelial and Smooth Muscle Cell Injury. Methods Mol Biol. 2021;2269:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Li X, Michaeloudes C, Zhang Y, Wiegman CH, Adcock IM, Lian Q, Mak JCW, Bhavsar PK, Chung KF. Mesenchymal stem cells alleviate oxidative stress-induced mitochondrial dysfunction in the airways. J Allergy Clin Immunol. 2018;141:1634-1645.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 76. | Lin KC, Yeh JN, Chen YL, Chiang JY, Sung PH, Lee FY, Guo J, Yip HK. Xenogeneic and Allogeneic Mesenchymal Stem Cells Effectively Protect the Lung Against Ischemia-reperfusion Injury Through Downregulating the Inflammatory, Oxidative Stress, and Autophagic Signaling Pathways in Rat. Cell Transplant. 2020;29:963689720954140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Wang SY, Fan XL, Yu QN, Deng MX, Sun YQ, Gao WX, Li CL, Shi JB, Fu QL. The lncRNAs involved in mouse airway allergic inflammation following induced pluripotent stem cell-mesenchymal stem cell treatment. Stem Cell Res Ther. 2017;8:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Li X, Zhang Y, Liang Y, Cui Y, Yeung SC, Ip MS, Tse HF, Lian Q, Mak JC. iPSC-derived mesenchymal stem cells exert SCF-dependent recovery of cigarette smoke-induced apoptosis/proliferation imbalance in airway cells. J Cell Mol Med. 2017;21:265-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Zhong H, Fan XL, Fang SB, Lin YD, Wen W, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent chronic allergic airway inflammation via TGF-β1-Smad2/Smad3 signaling pathway in mice. Mol Immunol. 2019;109:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, Lian Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 81. | Thiel A, Yavanian G, Nastke MD, Morales P, Kouris NA, Kimbrel EA, Lanza R. Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Sci Rep 2015; 5: 17685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Wu HJ, Yiu WH, Wong DWL, Li RX, Chan LYY, Leung JCK, Zhang Y, Lian Q, Lai KN, Tse HF, Tang SCW. Human induced pluripotent stem cell-derived mesenchymal stem cells prevent adriamycin nephropathy in mice. Oncotarget. 2017;8:103640-103656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Li B, Leung JCK, Chan LYY, Yiu WH, Li Y, Lok SWY, Liu WH, Chan KW, Tse HF, Lai KN, Tang SCW. Amelioration of Endoplasmic Reticulum Stress by Mesenchymal Stem Cells via Hepatocyte Growth Factor/c-Met Signaling in Obesity-Associated Kidney Injury. Stem Cells Transl Med. 2019;8:898-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 84. | Liu L, Wu Y, Wang P, Shi M, Wang J, Ma H, Sun D. PSC-MSC-Derived Exosomes Protect against Kidney Fibrosis In Vivo and In Vitro through the SIRT6/β-Catenin Signaling Pathway. Int J Stem Cells. 2021;14: 310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Sheu JJ, Sung PH, Wallace CG, Yang CC, Chen KH, Shao PL, Chu YC, Huang CR, Chen YL, Ko SF, Lee MS, Yip HK. Intravenous administration of iPS-MSCSPIONs mobilized into CKD parenchyma and effectively preserved residual renal function in CKD rat. J Cell Mol Med. 2020;24:3593-3610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Ko SF, Chen YT, Wallace CG, Chen KH, Sung PH, Cheng BC, Huang TH, Chen YL, Li YC, Chang HW, Lee MS, Yang CC, Yip HK. Inducible pluripotent stem cell-derived mesenchymal stem cell therapy effectively protected kidney from acute ischemia-reperfusion injury. Am J Transl Res. 2018;10:3053-3067. [PubMed] |
| 87. | Varzideh F, Mahmoudi E, Pahlavan S. Coculture with noncardiac cells promoted maturation of human stem cell-derived cardiomyocyte microtissues. J Cell Biochem. 2019;120:16681-16691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Yu Y, Wang D, Li H, Fan J, Liu Y, Zhao X, Wu J, Jing X. Mesenchymal stem cells derived from induced pluripotent stem cells play a key role in immunomodulation during cardiopulmonary resuscitation. Brain Res. 2019;1720:146293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Liang Y, Li X, Zhang Y, Yeung SC, Zhen Z, Ip MSM, Tse HF, Lian Q, Mak JCW. Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells Attenuate Cigarette Smoke-Induced Cardiac Remodeling and Dysfunction. Front Pharmacol. 2017;8:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai YH, He J, Tse HF, Lian Q. Potent Paracrine Effects of human induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells Attenuate Doxorubicin-induced Cardiomyopathy. Sci Rep. 2015;5:11235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 91. | Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J Extracell Vesicles. 2020;9:1783869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 92. | Zhang J, Ho JC, Chan YC, Lian Q, Siu CW, Tse HF. Overexpression of myocardin induces partial transdifferentiation of human-induced pluripotent stem cell-derived mesenchymal stem cells into cardiomyocytes. Physiol Rep. 2014;2:e00237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Asgari Taei A, Nasoohi S, Hassanzadeh G, Kadivar M, Dargahi L, Farahmandfar M. Enhancement of angiogenesis and neurogenesis by intracerebroventricular injection of secretome from human embryonic stem cell-derived mesenchymal stem cells in ischemic stroke model. Biomed Pharmacother. 2021;140:111709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 94. | Lee EJ, Xu L, Kim GH, Kang SK, Lee SW, Park SH, Kim S, Choi TH, Kim HS. Regeneration of peripheral nerves by transplanted sphere of human mesenchymal stem cells derived from embryonic stem cells. Biomaterials. 2012;33:7039-7046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Peng KY, Lee YW, Hsu PJ, Wang HH, Wang Y, Liou JY, Hsu SH, Wu KK, Yen BL. Human pluripotent stem cell (PSC)-derived mesenchymal stem cells (MSCs) show potent neurogenic capacity which is enhanced with cytoskeletal rearrangement. Oncotarget. 2016;7:43949-43959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Kim DY, Choi SH, Lee JS, Kim HJ, Kim HN, Lee JE, Shin JY, Lee PH. Feasibility and Efficacy of Intra-Arterial Administration of Embryonic Stem Cell Derived-Mesenchymal Stem Cells in Animal Model of Alzheimer's Disease. J Alzheimers Dis. 2020;76:1281-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Chen KH, Lin KC, Wallace CG, Li YC, Shao PL, Chiang JY, Sung PH, Yip HK. Human induced pluripotent stem cell-derived mesenchymal stem cell therapy effectively reduced brain infarct volume and preserved neurological function in rat after acute intracranial hemorrhage. Am J Transl Res. 2019;11:6232-6248. [PubMed] |
| 98. | Hawkins KE, Corcelli M, Dowding K, Ranzoni AM, Vlahova F, Hau KL, Hunjan A, Peebles D, Gressens P, Hagberg H, de Coppi P, Hristova M, Guillot PV. Embryonic Stem Cell-Derived Mesenchymal Stem Cells (MSCs) Have a Superior Neuroprotective Capacity Over Fetal MSCs in the Hypoxic-Ischemic Mouse Brain. Stem Cells Transl Med. 2018;7:439-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 99. | Yun YI, Park SY, Lee HJ, Ko JH, Kim MK, Wee WR, Reger RL, Gregory CA, Choi H, Fulcher SF, Prockop DJ, Oh JY. Comparison of the anti-inflammatory effects of induced pluripotent stem cell-derived and bone marrow-derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy. 2017;19:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Jiang D, Xiong G, Feng H, Zhang Z, Chen P, Yan B, Chen L, Gandhervin K, Ma C, Li C, Han S, Zhang Y, Liao C, Lee TL, Tse HF, Fu QL, Chiu K, Lian Q. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics. 2019;9:2395-2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 101. | Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, Xu RH. Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Reports. 2014;3:115-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 102. | Yu Y, Wang D, Li H, Liu Y, Xiang Z, Wu J, Jing X. IPSCMSC inhibition assessment in Raw 264.7 cells following oxygen and glucose deprivation reveals a distinct function for cardiopulmonary resuscitation. Mol Med Rep. 2018;17:8212-8220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 103. | Qin Y, Chan AM, Chang YL, Matynia A, Kouris NA, Kimbrel EA, Ashki N, Parikh S, Gorin MB, Lanza R, Levinson RD, Gordon LK. Human Embryonic Stem Cell-Derived Mesenchymal Stromal Cells Decrease the Development of Severe Experimental Autoimmune Uveitis in B10.RIII Mice. Ocul Immunol Inflamm. 2018;26:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Wang LT, Jiang SS, Ting CH, Hsu PJ, Chang CC, Sytwu HK, Liu KJ, Yen BL. Differentiation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Results in Downregulation of c-Myc and DNA Replication Pathways with Immunomodulation Toward CD4 and CD8 Cells. Stem Cells. 2018;36:903-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 105. | Ng J, Hynes K, White G, Sivanathan KN, Vandyke K, Bartold PM, Gronthos S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J Cell Biochem. 2016;117:2844-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Mitsuzawa S, Ikeguchi R, Aoyama T, Ando M, Takeuchi H, Yurie H, Oda H, Noguchi T, Ohta S, Zhao C, Ikeya M, Matsuda S. Induced pluripotent stem cell-derived mesenchymal stem cells prolong hind limb survival in a rat vascularized composite allotransplantation model. Microsurgery. 2019;39:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Sánchez L, Gutierrez-Aranda I, Ligero G, Rubio R, Muñoz-López M, García-Pérez JL, Ramos V, Real PJ, Bueno C, Rodríguez R, Delgado M, Menendez P. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 108. | Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB, Shi JB, Sun YQ, Wen W, Tse HF, Lian Q, Xu G. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012;67:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 109. | Li CL, Leng Y, Zhao B, Gao C, Du FF, Jin N, Lian QZ, Xu SY, Yan GL, Xia JJ, Zhuang GH, Fu QL, Qi ZQ. Human iPSC-MSC-Derived Xenografts Modulate Immune Responses by Inhibiting the Cleavage of Caspases. Stem Cells. 2017;35:1719-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, Fang SB, Chiu S, Tse HF, Lian Q, Fu QL. Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation. Stem Cell Reports. 2018;11:1120-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 111. | Yang H, Feng R, Fu Q, Xu S, Hao X, Qiu Y, Feng T, Zeng Z, Chen M, Zhang S. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019;10:718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 112. | Hai B, Shigemoto-Kuroda T, Zhao Q, Lee RH, Liu F. Inhibitory Effects of iPSC-MSCs and Their Extracellular Vesicles on the Onset of Sialadenitis in a Mouse Model of Sjögren's Syndrome. Stem Cells Int. 2018;2018:2092315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 113. | Ozay EI, Vijayaraghavan J, Gonzalez-Perez G, Shanthalingam S, Sherman HL, Garrigan DT, Jr. , Chandiran K, Torres JA, Osborne BA, Tew GN, Slukvin, II, Macdonald RA, Kelly K, Minter LM. Cymerus™ iPSC-MSCs significantly prolong survival in a pre-clinical, humanized mouse model of Graft-vs-host disease. Stem Cell Res 2019; 35: 101401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 114. | Bloor AJC, Patel A, Griffin JE, Gilleece MH, Radia R, Yeung DT, Drier D, Larson LS, Uenishi GI, Hei D, Kelly K, Slukvin I, Rasko JEJ. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft vs host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med. 2020;26:1720-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 115. | Ullah M, Kuroda Y, Bartosh TJ, Liu F, Zhao Q, Gregory C, Reger R, Xu J, Lee RH, Prockop DJ. iPS-derived MSCs from an expandable bank to deliver a prodrug-converting enzyme that limits growth and metastases of human breast cancers. Cell Death Discov 2017; 3: 16064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Ji Y, Ma Y, Chen X, Ji X, Gao J, Zhang L, Ye K, Qiao F, Dai Y, Wang H, Wen X, Lin J, Hu J. Microvesicles released from human embryonic stem cell derived-mesenchymal stem cells inhibit proliferation of leukemia cells. Oncol Rep. 2017;38:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Zhao Q, Hai B, Zhang X, Xu J, Koehler B, Liu F. Biomimetic nanovesicles made from iPS cell-derived mesenchymal stem cells for targeted therapy of triple-negative breast cancer. Nanomedicine. 2020;24:102146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 118. | Zhao Q, Hai B, Kelly J, Wu S, Liu F. Extracellular vesicle mimics made from iPS cell-derived mesenchymal stem cells improve the treatment of metastatic prostate cancer. Stem Cell Res Ther. 2021;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 119. | Zhao Q, Gregory CA, Lee RH, Reger RL, Qin L, Hai B, Park MS, Yoon N, Clough B, McNeill E, Prockop DJ, Liu F. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci U S A. 2015;112:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 120. | Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 121. | Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 122. | Havasi P, Nabioni M, Soleimani M, Bakhshandeh B, Parivar K. Mesenchymal stem cells as an appropriate feeder layer for prolonged in vitro culture of human induced pluripotent stem cells. Mol Biol Rep. 2013;40:3023-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 123. | Bahrehbar K, Rezazadeh Valojerdi M, Esfandiari F, Fathi R, Hassani SN, Baharvand H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J Stem Cells. 2020;12:857-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 124. | Yoon SY, Yoon JA, Park M, Shin EY, Jung S, Lee JE, Eum JH, Song H, Lee DR, Lee WS, Lyu SW. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem Cell Res Ther. 2020;11:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 125. | Lotfinia M, Kadivar M, Piryaei A, Pournasr B, Sardari S, Sodeifi N, Sayahpour FA, Baharvand H. Effect of Secreted Molecules of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells on Acute Hepatic Failure Model. Stem Cells Dev. 2016;25:1898-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 126. | Mardpour S, Hassani SN, Mardpour S, Sayahpour F, Vosough M, Ai J, Aghdami N, Hamidieh AA, Baharvand H. Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury. J Cell Physiol. 2018;233:9330-9344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 127. | Eto S, Goto M, Soga M, Kaneko Y, Uehara Y, Mizuta H, Era T. Mesenchymal stem cells derived from human iPS cells via mesoderm and neuroepithelium have different features and therapeutic potentials. PLoS One. 2018;13:e0200790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 128. | Lian Q, Zhang Y, Liang X, Gao F, Tse HF. Directed Differentiation of Human-Induced Pluripotent Stem Cells to Mesenchymal Stem Cells. Methods Mol Biol. 2016;1416:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 129. | Lai WT, Krishnappa V, Phinney DG. Fibroblast growth factor 2 (Fgf2) inhibits differentiation of mesenchymal stem cells by inducing Twist2 and Spry4, blocking extracellular regulated kinase activation, and altering Fgf receptor expression levels. Stem Cells. 2011;29:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 130. | Moslem M, Eggenschwiler R, Wichmann C, Buhmann R, Cantz T, Henschler R. Kindlin-2 Modulates the Survival, Differentiation, and Migration of Induced Pluripotent Cell-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2017;2017:7316354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Szaraz P, Gratch YS, Iqbal F, Librach CL. In Vitro Differentiation of Human Mesenchymal Stem Cells into Functional Cardiomyocyte-like Cells. J Vis Exp 2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 132. | Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 133. | Takeda YS, Xu Q. Neuronal Differentiation of Human Mesenchymal Stem Cells Using Exosomes Derived from Differentiating Neuronal Cells. PLoS One. 2015;10:e0135111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 134. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 207] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell Biology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Liu Y, Mournetas V, Peng XC, Yi X S-Editor: Chang (Online Science Editor) KL L-Editor: Wang TQ P-Editor: Chang KL