Published online Nov 26, 2021. doi: 10.4252/wjsc.v13.i11.1610
Peer-review started: February 25, 2021
First decision: March 29, 2021
Revised: April 6, 2021
Accepted: September 29, 2021
Article in press: September 29, 2021
Published online: November 26, 2021
Processing time: 272 Days and 22.5 Hours
Dental stem cells can differentiate into different types of cells. Dental pulp stem cells, stem cells from human exfoliated deciduous teeth, periodontal ligament stem cells, stem cells from apical papilla, and dental follicle progenitor cells are five different types of dental stem cells that have been identified during different stages of tooth development. The availability of dental stem cells from discarded or removed teeth makes them promising candidates for tissue engineering. In recent years, three-dimensional (3D) tissue scaffolds have been used to reconstruct and restore different anatomical defects. With rapid advances in 3D tissue engineering, dental stem cells have been used in the regeneration of 3D engineered tissue. This review presents an overview of different types of dental stem cells used in 3D tissue regeneration, which are currently the most common type of stem cells used to treat human tissue conditions.
Core Tip: Dental stem cell seeding in three-dimensional (3D) engineered scaffolds that mimic the human tissue microenvironment is an emerging technology for regenerative medicine. Dental pulp stem cells, stem cells from human exfoliated deciduous teeth, periodontal ligament stem cells, stem cells from apical papilla, and dental follicle progenitor cells have been used for tissue regeneration utilizing 3D approaches. The analytical results of this literature review reveal many basic and preclinical studies that support the hypothesis that the application of dental stem cells is a feasible approach for translational medicine and is an applicable method for 3D tissue regeneration.
- Citation: Hsiao HY, Nien CY, Hong HH, Cheng MH, Yen TH. Application of dental stem cells in three-dimensional tissue regeneration. World J Stem Cells 2021; 13(11): 1610-1624
- URL: https://www.wjgnet.com/1948-0210/full/v13/i11/1610.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i11.1610
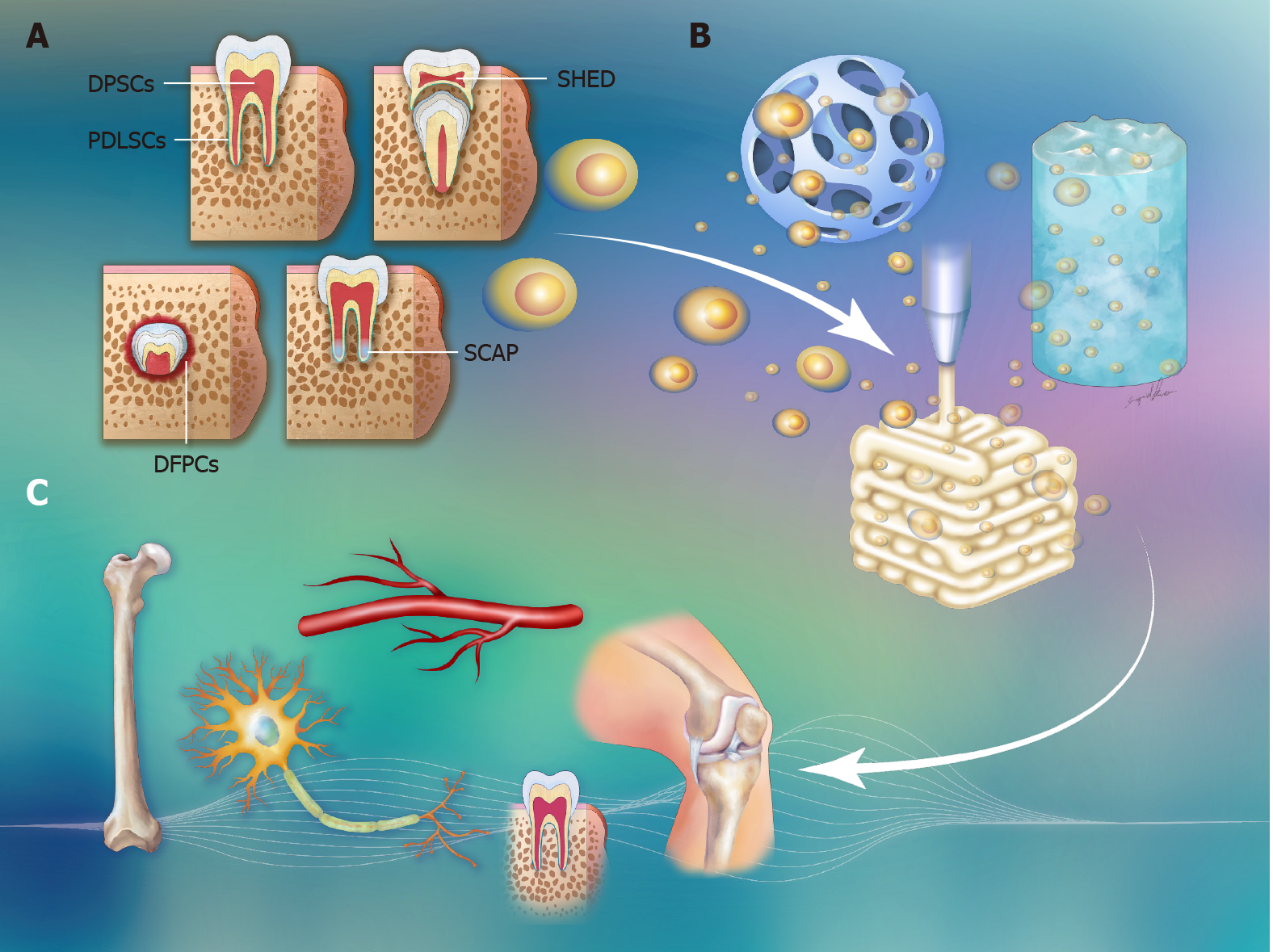
The multipotent properties of stem cells make them excellent sources of material for tissue repair. Five dental-derived cell types have been isolated and characterized as dental stem cells[1]. Dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHEDs), periodontal ligament stem cells (PDLSCs), stem cells from apical papilla (SCAP), and dental follicle progenitor cells (DFPCs) are different types of dental stem cells involved in different stages of tooth development (Figure 1). Considering their differentiation potential, dental stem cells have been introduced to regenerate damaged or lost tissue. Dental stem cells are not restricted to use in dental tissue repair but can also participate in neural, adipose, bone, and cartilage tissue regeneration[2,3]. Recently, three-dimensional (3D) tissue engineering has been applied to therapeutic medicine. Cells are seeded in 3D engineered scaffolds to mimic the human tissue microenvironment during cell differentiation. The cell morphology and gene expression of the cells cultured under 3D conditions are more consistent with those of cells observed in native tissue[4]. The use of customized 3D tooth implants with dental stem cells seeded in suitable scaffolds as replacements for lost teeth is a promising approach in dentistry. In addition to tooth repair, there is growing interest in the concept of 3D tissue regeneration with dental stem cells.
Here, we searched databases to identify the literature on dental stem cells used in 3D tissue regeneration. The literature searches and data mining were performed by customized scripts with the "easyPubMed" and "PubMed.mineR" packages in R for use with the PubMed database[5-7]. The keywords used in the queries included "pulp stem cells", "exfoliated deciduous teeth stem cell", "periodontal ligament stem cell", "apical papilla", "dental follicle cells", "3D", "tissue", "regeneration" and "engineering". The search results were output with the "abstract" format in the "easyPubMed" package and were analyzed by the "PubMed.minR" package. A total of 88 papers were found with the aforementioned criteria. After review, only one-third of the papers articulated original research on dental stem cells in 3D tissue regeneration. In this review, we aim to provide a clear point of view on each type of dental stem cell used in combination with 3D tissue scaffolds, such as microspheres, hydrogels, or 3D printed scaffolds, to regenerate into teeth, neurons, bone, blood vessels and cartilage (Figure 1 and Table 1).
| Dental stem cells | Biomaterials | Addition of materials/growth factors/cells | Type of tissue regeneration | Ref. |
| DPSCs | ||||
| DPSCs | CaP porous granules, NF-gelatin/MgP | No | Odontogenic differentiation | Nam et al[24], and Qu et al[30] |
| SS-PLLA-b-PLYS, pNIPAAm, NF-PLLA | No | Pulp-dentin regeneration | Kuang et al[16], Itoh et al[17], and Soares et al[18] | |
| Coll/HA/PLCL, ABM/ABM-P-15, PVA/PU | No | Bone tissue | Mohanram et al[13], Cooke et al[19], and Akkouch et al[21] | |
| OMMT/PVA | No | Neuro-like cells | Ghasemi Hamidabadi et al[47] | |
| Matrigel | No | Endotheliocytes and pericytes | Luzuriaga et al[51] | |
| Collagen gel | SDF1, bFGF | Dental pulp tissue | Suzuki et al[23] | |
| BMP7 | ||||
| DPSCs with growth factors | Ti6Al4V | Poly-L-lys coating | Osteoblastic differentiation | Galli et al[32] |
| Porous silk fibroin | bFGF | Dental pulp tissue | Yang et al[26] | |
| PCL | VEGF, BMP2 | Vascularized bone tissue | Park et al[39] | |
| HP hydrogel | bFGF | Spinal cord | Luo et al[48] | |
| DPSCs with other cells | Matrigel and collagen gel | Human normal oral epithelial cells | Epithelium invagination-like structure | Xiao and Tsutsui[35] |
| PCL/PLDLA | Endothelial cells | Vascularized bone tissue | Jin and Kim[36] | |
| PLLA/PLGA | Human neonatal dermal fibroblasts | Spinal cord | Guo et al[50] | |
| DPSCs in 3D printed scaffolds | HA/TCP | Apical papilla (SCAP) | Pulp-dentin regeneration | Hilkens et al[40] |
| PCL | Platelet-rich plasma | Calvaria bone | Li et al[27] | |
| Alg-Gel | Bone | Yu et al[38] | ||
| PLAS | Neural differentiation | Hsiao et al[42] | ||
| AMP/ECM | Craniomaxillofacial bone | Dubey et al[41] | ||
| SHED | ||||
| SHED with growth factors | No | EGF, FGF | Spinal cord | Feng et al[58] |
| No | SHED-conditioned medium | Sciatic nerve | Sugimura-Wakayama et al[59] | |
| SHED in 3D formed scaffolds | Polylactoglycolide, SHED aggregated hemisphere | Bone tissue | Laino et al[56], and Vakhrushev et al[57] | |
| PDLSCs | ||||
| PDLSCs | Hydroxyapatite/β-tricalcium phosphate (HA/β-TCP) | Periodontal tissue | Kim et al[66] | |
| GelMA/PEG | PDLSC proliferation | Ma et al[80] | ||
| PDLSCs with growth factors | PLGA | CTGF, BMP-7, BMP-2 | Periodontal tissue | Cho et al[73] |
| Platelet-rich fibrin | Aspirin | Periodontal tissue | Du et al[76] | |
| PDLSCs with other cells | Collagen/Chitosan | Somatic MSCs and DPSCs | Odontogenic differentiation | Ravindran et al[79] |
| No | HUVECs | Periodontal tissue | Kramer[77] | |
| PLGA–PEG–PLGA thermal hydrogel | PDLSCs overexpressing PDGF-BB | Alveolar bone tissue | Pan et al[78] | |
| SCAP | ||||
| SCAP with growth factors | PLLA nanofibrous microspheres (NF-MS) | BMP-2 | Pulp-dentin regeneration | Wang et al[86] |
| No | BMP-2, SDF-1α | Odontoblast differentiation | Xiao et al[87] | |
| Alg-Dent hydrogel | Dentin ECM | Pulp-dentin regeneration | Athirasala et al[95] | |
| DFPCs | ||||
| DFPCs | Coll-nano-HA/OPS | Bone tissue | Salgado et al[102] | |
DPSCs located in the soft connective tissue inside the dental crown were first identified in 2000 (Figure 1)[8]. DPSCs exhibit MSC-like properties, including a high proliferation rate, multilineage potential, and immunomodulatory properties[8,9]. Even though DPSCs exhibit features similar to those of BMSCs, their characteristics of causing little morbidity at the donor site, a higher proliferation rate, and multipotency make DPSCs better stem cell sources for tissue regeneration[10]. DPSCs cocultured with apical bud cells (ABCs) exhibited more active odontogenic differentiation ability than BMSCs cocultured with ABCs[11]. The neural differentiation of IMR-32 cells was significantly enhanced when treated with secretomes derived from DPSCs compared to BMSCs[12]. The assessment of neurogenic potential on the secretome of DPSCs and BMSCs indicated that DPSCs presented better potential for neural differentiation[12]. Most DPSC studies have focused on dental pulp and bone tissue regeneration. Compared to bone marrow stem cells, DPSCs have a higher proliferation rate and better osteogenic capacity when seeded in a scaffold of bone mineral (ABM) coated with a biomimetic collagen peptide (ABM-P-15), even generating a more organized collagenous matrix 8 wk after in vivo implantation[13]. Moreover, different gene expression patterns have been found in the transcriptome profiles of DPSCs compared to those of bone marrow stem cells, indicating unique gene expression patterns within DPSCs[14].
In addition to conventional tissue regeneration approaches with cells loaded on two-dimensional scaffolds, DPSCs have been cultured on 3D biomaterials for the development of tissue constructs. A bioink containing human DPSCs and fibrinogen incorporated with polycaprolactone (PCL) was designed for the production of dentin pulp complex structures[15]. Nanofibrous spongy microspheres made from star-shaped poly(l-lactic acid)-block-poly(l-lysine) (SS-PLLA-b-PLYS) were seeded with DPSCs for dental pulp tissue regeneration[16]. Poly-N-isopropyl acrylamide (pNIPAAm) gel containing DPSCs was made in a rod shape to fill in the root canal for pulp tissue regeneration[17]. Simvastatin and nanofibrous poly(l-lactic acid) (NF-PLLA) scaffolds[18] and a mixture of polyvinyl alcohol (PVA) and polyurethane (PU)[19] were combined with DPSCs to investigate the potential of tissue regeneration. Self-assembling peptides, with structures similar to the extracellular matrix (ECM), are among the smart materials used for 3D culture[20]. A 3D scaffold composed of collagen (Coll), hydroxyapatite (HA), and poly(L-lactide-co"-caprolactone) (PLCL) increased the adhesion and viability of DPSCs and enhanced bone regeneration compared to a PLCL-only scaffold[21]. DPSCs grown in a peptide-based scaffold presented RGD- and vascular endothelial growth factor (VEGF)-mimetic peptide epitopes and exhibited better survival and angiogenic and odontogenic differentiation[22].
With increased knowledge of the function of growth factors, an increasing number of studies have introduced growth factors into different types of tissue regeneration. In 2011, human DPSCs were placed on the surface of 3D collagen cylinders and cultured with the addition of stromal-derived factor-1α, basic fibroblast growth factor (bFGF), and bone morphogenetic protein-7 (BMP-7) for dental pulp regeneration[23]. Seeding DPSCs on 3D calcium phosphate (CaP) porous granules promoted odontogenic differentiation by increasing the gene expression of dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1)[24]. Porous silk fibroin scaffolds fabricated with bFGF, which has been reported to facilitate pulp regeneration[25], were used to fill the root canal space for tooth repair[26]. Platelet-rich plasma (PRP) containing various growth factors along with DPSCs was added to 3D printed PCL mesh for bone regeneration in a rat calvaria defect model[27].
In addition to growth factors, metal ions have also been confirmed to contribute to cell differentiation[28]. Magnesium (Mg) is involved in the process of biomineralization during bone and tooth development[29]. Qu et al[30] incorporated Mg into nanofibrous gelatin biomaterials to develop 3D gelatin/Mg phosphate (NF-gelatin/MgP) scaffolds seeded with DPSCs, and odontogenic proliferation and differentiation were enhanced. The materials used for dental implants, such as titanium-6-aluminum-4-vanadium (Ti6Al4V), are also used as 3D scaffolds for tissue regeneration. Their properties of low corrosion and smooth metal surfaces prevent stem cells from colonizing this biomaterial[31]. Coatings of poly-L-lysine (poly-L-lys), which carries positive charges, induced focal adhesion kinase activation and increased the osteoblastic differentiation of hDPSCs[32]. A coculture system not only provides intercellular factors but also enables communication between two types of cells, which is critical for the development and arrangement of the ECM[33,34]. DPSCs cocultured with human normal oral epithelial cells harvested from gingival tissue were inoculated into 3D Matrigel to form an epithelium invagination-like structure, a key feature of early tooth development[35]. Poly-L/D-lactide (PCL/PLDLA) porous microspheres were loaded with DPSCs and human endothelial cells to promote osteogenesis and angiogenesis for vascularized bone tissue regeneration[36].
3D printing techniques can print cells, growth factors, or biomaterials in the desired location to achieve more complicated multicell tissue structures[37]. In contrast to cultures in 2D alginate/gelatin hydrogel (Alg-Gel) scaffolds, 3D Alg-Gel scaffolds can be printed in a seven-layer coin shape and loaded with DPSCs. These DPSC-loaded 3D printed scaffolds achieved higher cell proliferation, odontoblastic differentiation, and bone mineralization, suggesting that a 3D environment is more suitable for cell proliferation and differentiation[38]. In addition, Park et al[39] designed the printing of DSPCs with VEGF in the central zone and bone morphogenetic protein-2 (BMP-2) in the peripheral area of the 3D-printed construct to fabricate vascularized bone structures. A cone-shaped scaffold was printed with hydroxyapatite/tricalcium phosphate (HA/TCP) powder that was polymerized by an ultraviolet (UV) photoinitiator. DPSCs and SCAP were mixed with collagen gel and loaded into the 3D printed HA/TCP scaffold for dental pulp regeneration[40]. 3D PCL mesh supplemented with PRP containing various growth factors along with DPSCs was custom printed to fit rat calvarial defects for bone regeneration[27]. PRP containing various growth factors, along with DPSCs, was added to 3D printed PCL mesh for bone regeneration in a rat calvaria defect model[27]. A novel DPSC-loaded bioink containing a mixture of amorphous Mg phosphates and ECM increased the bone density during craniomaxillofacial bone regeneration[41]. With the 3D printing technique, the shape, pore size, and gap size can be precisely controlled to study their microenvironmental effects on cell proliferation and differentiation. Polylactic acid scaffolds (PLASs) were printed in different gap sizes, and it was discovered that smaller gaps in 3D PLASs presented with different cellular orientations[42].
In addition to their osteogenic and odontoblastic potential, the chondrogenic potential of DPSCs has been investigated. Zhang et al[43] successfully induced DPSCs to undergo a chondrogenic differentiation process, and their synthesis of sulfated glycosaminoglycans was confirmed. DPSCs formed into 3D pellets were subjected to chondrogenic potential investigation, resulting in the enrichment of collagen I deposition. The content of glycosaminoglycan or collagen type II was not enhanced even with the addition of chondroinductive growth factors, suggesting that the chondrogenic lineage of DPSCs favors differentiation into fibrous cartilage rather than hyaline cartilage[44]. DPSCs, derived from cranial neurons, can differentiate into neuron-like cells for axon regeneration and are potential cell sources for neuron regeneration[45,46]. DPSCs were seeded within chitosan-intercalated montmorillonite/poly(vinyl alcohol) (OMMT/PVA) nanofibrous mesh, and they differentiated into neuron-like cells[47]. A thermosensitive heparin-poloxamer hydrogel with DPSCs and bFGF enhanced motor and sensory functional recovery after spinal cord injury repair[48]. Chitosan scaffolds have been demonstrated to enhance neuronal cell survival and differentiation. Zheng et al[49] incorporated bFGF into chitosan scaffolds and found that it promoted DPSC differentiation into neuronal cells but did not affect cell survival. Human adipose microvascular endothelial cells were coseeded in a PLLA/poly(lactic-co-glycolic acids) (PLGA) scaffold with DPSCs to fabricate a prevascularized scaffold, which promoted revascularization, axon regeneration, myelin deposition, and sensory recovery in a rat complete spinal cord transection model[50]. Moreover, DPSCs seeded in Matrigel were able to differentiate into endotheliocytes and pericytes in serum-free culture media and secrete VEGF[51].
SHED cells, first isolated in 2003, present with positive expression of embryonic stem cell markers, such as OCT4 and NANOG, stage-specific embryonic antigens (SSEA-3 and SSEA-4), and mesenchymal stem cell markers (STRO-1 and CD146)[52-54]. Compared to DPSCs, SHEDs showed higher levels of osteocalcin expression and alkaline phosphatase activity[55]. SHEDs were confirmed to be more immature than DPSCs, allowing them to be “osteoblast-like’’ and ‘‘odontoblast-like’’, expressing osteocalcin and RUNX-2 markers[53]. Moreover, when SHEDs were cultured in medium with dexamethasone, they differentiated into adipocytes. After in vitro culturing for 2 wk in osteogenic medium, extracellular mineralized matrix started to be secreted by the SHEDs. This multilineage potential makes SHEDs alternative sources of dental stem cells[56].
SHEDs cultured in vitro for 7 d were found to aggregate together, and they started to form a 3D ossification hemisphere after 36 d[56]. This mineral matrix was identified by alizarin red staining within the self-formed 3D woven bone tissue. SHEDs can be applied in a 3D polylactoglycolide scaffold fabricated by a surface-selective laser sintering device. The expression of osteocalcin was elevated in SHED-loaded polyla
Periodontitis is a very common oral disease resulting in periodontal tissue destruction and, more seriously, tooth loss[61]. Many periodontal regeneration treatments have been performed to restore the damaged periodontium. PDLSCs were isolated from mature periodontal ligaments and found to express the stem cell markers CD105, CD90, and CD73[62-64]. Seo et al[63] successfully isolated PDLSCs from human third molars, and the expression of the stem cell markers STRO-1 and CD146/MUC18 was found in PDLSCs. In addition to the expression of stem cell markers, the osteogenic and adipogenic potential of PDLSCs was also identified[65], which makes PDLSCs alternative cell sources for tissue regeneration. The regeneration steps of periodontal tissue were demonstrated by PDLSCs incorporated with hydroxyapatite/β-tricalcium phosphate (HA/β-TCP) as carriers[66]. First, the proliferation of PDLSCs was increased, and collagen matrices were formed. Subsequently, the collagen fibers started to assemble, and cemental-like tissue was observed. Mineralization was present in the cemental-like tissue, and along with the presence of Sharpey’s fibers, mature collagen fibers were present. Later, the maturation of cemental-like tissue was identified by the expression of cemental tissue genes, such as α-smooth muscle actin antibody, collagen type XII (ColXII), osteoblast specific factor-2/periostin, and aspirin/PLAP-1[67].
A 3D collagen scaffold was fabricated with precise control of the pore size, pore wall alignment, and percolation diameter to investigate the effect of the scaffold structure on periodontal tissue regeneration. The results suggested that a larger percolation diameter increased PDLSC cell elongation and directionality, whereas the pore size influenced cell invasion and cell distribution[68]. In addition to the manipulation of the scaffold structure, the addition of growth factors also promoted the capacity of tissue regeneration. During cemental tissue formation, connective tissue growth factor (CTGF) was found to promote the differentiation of periodontal ligament fibroblasts during the process of osteogenesis[69]. BMP-7, expressed in the cementum, alveolar bone, and periodontal ligament, induces cementogenic differentiation by acting as a progenitor for cementoblasts[70,71]. The expression of BMP-2, localized only in alveolar bone, was also involved in cementogenic differentiation by increasing the expression of cementum attachment protein (CAP)[72]. Since CTGF, BMP-7, and BMP-2 are beneficial for periodontal ligament formation, Cho et al[73] compared the effect of these three growth factors by incorporating them into 3D printed PLGA microspheres, and the results indicated that BMP-7 triggered thicker cementum-like layers, better integration with the dentin surface and higher expression of cementum protein 1[73]. In addition to supplying growth factors to promote tissue regeneration, inhibition of inflammatory reactions can also improve tissue formation. For instance, Liu et al[74] demonstrated that reductions in tumor necrosis factor-alpha and interferon-gamma levels by the introduction of BMMSCs enhanced bone regeneration. Cao et al[75] demonstrated that aspirin promoted BMMSC-based calvarial bone regeneration. Thus, platelet-rich fibrin-containing PDLSCs were treated with aspirin, a non-steroidal anti-inflammatory drug, which increased periodontal bone formation[76].
Instead of providing a direct supply of factors that are required for tissue regeneration, human umbilical vein endothelial cells (HUVECs) were cocultured with PDLSCs to form 3D cell sheet constructs, which were wrapped around human tooth roots for implantation into the subcutaneous layer of mice. The HUVEC and PDLSC coculture group exhibited the thickest PDL ligament-like arrangement compared to the PDLSC-only group, suggesting that HUVECs contributed to regulating the thickness of the periodontal compartment[77]. Another strategy for improving the supply of vasculature to bone regeneration is the introduction of genetically modified PDLSCs. A lentiviral construct containing platelet-derived growth factor BB (PDGF-BB), an angiogenic gene, was introduced into PDLSCs to overexpress PDGF-BB. A PLGA-PEG-PLGA thermal hydrogel seeded with PDLSCs overexpressing PDGF-BB promoted bone formation in alveolar bone defects[78]. To investigate the possibility of incorporating somatic MSCs in tissue regeneration, a mixture of PDLSCs, somatic MSCs, and DPSCs was cocultured within 3D collagen/chitosan scaffolds for odonto
SCAP is only present at the tip of the developing tooth root before the tooth erupts. Although SCAP shares some similar characteristics with DPSCs, there are still some differences between these two types of stem cells[8]. In contrast to DPSCs, which are the sources of replacement odontoblasts, SCAP is the primary source of odontoblasts involved in the formation of root dentin[81]. Comparing their in vitro osteo/ odontogenic differentiation potential with DPSCs, SCAP presents stem cell markers (STRO-1, CD146, and CD34) similar to those of DPSCs but with a significantly higher proliferation rate and mineralization potential during dental formation[82]. Other MSC markers, CD73, CD90, and CD105, were also identified in SCAP[40]. Liu et al[83] found that CD24 was exclusively expressed in SCAP, not in DPSCs. SCAP are comparatively easy to isolate from the tips of developing roots. They are digested with a cocktail of collagenase to isolate single-cell suspensions, which are grown under routine cell culture conditions[84].
In addition to using residual dental pulp in dentin regeneration, SCAP with osteogenic potential obtained from dental roots have been applied for dentin regeneration[85]. Injectable PLLA nanofibrous microspheres (NF-MS) with the ability to controllably release BMP-2 were encapsulated in SCAP for dentin regeneration[86]. More mineralization and osteodentin formation were observed in NF-MS with controlled BMP-2 release microspheres, suggesting their potential for dental tissue repair. In addition to BMP-2 release, SCAP cotreated with stromal cell-derived factor-1α, which is able to promote odontoblast differentiation of dental pulp cells, were shown to undergo odontogenic differentiation-related gene and protein expression[87]. PDGF-BB is known to promote angiogenesis during tissue regeneration[88,89]. The addition of PDGF-BB promoted the proliferation of SCAP and improved new bone formation and mineralization in a rat calvaria defect model[90].
The growth factor TGBβ3 was shown to be involved in tissue regeneration[91]. Somoza et al[92] observed that TGBβ3 secretion by SCAP was elevated when they were grown in a 3D microenvironment regardless of the materials used for the scaffold. Thus, SCAP were applied and incorporated into a 3D scaffold for tissue regeneration. Considering the secretion properties of SCAP, Na et al[93] developed a 3D scaffold-free stem-cell sheet-derived pellet (CSDP) by culturing a large amount of SCAP on a culture dish to form a cell sheet that enriched the secreted ECM. CSDP exhibited the odontogenic/osteogenic potential to form dental pulp-like and dentine-like tissue after implantation into the subcutaneous layer in immunodeficient mice. Dental ECM was reported to enhance cell proliferation and mineralization[94]. A novel SCAP-loaded bioink was developed by applying dental ECM to printable alginate to form dentin-derived bioink, in which soluble dentin molecules significantly enhanced odontogenic differentiation[95].
The dental follicle is the connective tissue surrounding the enamel organ and dental papilla that forms a vascular fibrous sac. In 2005, Morsczeck et al[96] isolated DFPCs from the dental follicle of human third molar teeth, which were found to express the stem cell markers Notch and Nestin. Their potential for osteogenic, adipogenic, chondrogenic, and neural differentiation was further confirmed[97]. Subsequently, DFPCs were applied for tissue regeneration, such as the regeneration of the salivary glands, dental roots, and bone tissue[98-100].
Among the applications of dental stem cells in tissue regeneration, only a few studies have introduced DFPCs to 3D tissue regeneration. DFPCs cultured in a 3D rotatory culture system displayed many follicle markers, such as CD44, CD90, CD146, CD31, CD34, and CD45Ag[101]. Furthermore, their differentiation potential was increased when DFPCs were cultured in a 3D dynamic culture system. For the generation of 3D tissue constructs, DFPCs were seeded in 3D porous scaffolds of collagen-nanohydroxyapatite/phosphoserine (collagen-nano-HA/OPS) biocomposite cryogels and implanted into the subcutaneous layer of nu mice. These 3D DFPC-loaded collagen-nano-HA/OPS constructs exhibited greater osteogenic differentiation with higher levels of osteopontin secretion[102].
The use of dental stem cells for autologous or allogeneic transplantation has been introduced into clinical practice. The biological safety of dental stem cells requires strict regulation. Standard examinations for viruses, pathogenic microorganisms, or any sources with animal origins are necessary[103-105]. Due to the immune response, a same-species origin of the stem cell culture system is recommended for cell therapy[106]. According to the Clinical Gov website, there are fewer than 10 cases of the use of dental stem cells in clinical applications, implying a gap in the application of dental stem cells between basic research and clinical practice. There is a scarcity of data for the use of decellularized biological membranes for preparing 3D dental regenerative constructs, which is a crucial approach for regenerative dentistry. Indeed, dental stem cells are not the most suitable stem cell choice for tissue regeneration due to harvest contamination, small cell amounts available per patient and invasive harvesting approaches. However, the regenerative potential of dental stem cells is still supported by several clinical results. A clinical study reported that most clinical trials based on the use of DPSCs cells were performed for bone regeneration, periodontitis, and dental pulp regeneration, whereas trials involving the use of periodontal PDLSCs were conducted to study periodontal disease treatment. No clinical trials that used DFPCs were found[107]. Overall, dental stem cells are not commonly used to treat human diseases. Identical to the original issues hindering stem cell therapy, ethical concerns and cell sources are the main obstacles. Moreover, the survival of grafted dental stem cells exhibited different results after long-term follow-up observations. Autologous PDLSCs were detected after 8 wk in an ovine periodontal defect model, whereas donor PDLSCs implanted into recipient mice were untraceable two weeks after implantation[108]. Whether autologous or allogeneic stem cell sources affect the survival rate of transplanted cells remains to be further investigated.
Dental-derived stem cells with mesenchymal stem cell properties are promising cell sources for tissue regeneration. Comparisons among these five types of dental-derived stem cells showed that DPSCs, SHEDs, and PDLSCs present a higher growth potential than BMSCs[109]. Moreover, SCAP and DPSCs showed weaker adipogenic differentiation than BMSCs[84]. Regardless of whether the different types of dental stem cells have osteogenic or odontogenic potential, each cell type presents unique differentiation potentials in the corresponding tissue type. Although dental stem cells present differentiation potential for adipogenesis, chondrogenesis, and neurogenesis, most of their clinical utility lies in the field of regenerative dentistry. With the trend of 3D tissue engineering, the application of dental stem cells to 3D tissue reconstruction has been emphasized. In this review, many basic research and preclinical studies were presented to support the idea that dental stem cells can be applied in a feasible approach to translational medicine and are available resources for 3D tissue regeneration.
The authors thank Miss Kuo I and the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital for creating the illustrations used herein.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kannan T, Khan A, Shawcross SG, Sukumaran A S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Bojic S, Volarevic V, Ljujic B, Stojkovic M. Dental stem cells--characteristics and potential. Histol Histopathol. 2014;29:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 2. | Ercal P, Pekozer GG, Kose GT. Dental Stem Cells in Bone Tissue Engineering: Current Overview and Challenges. Adv Exp Med Biol. 2018;1107:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Yao L, Flynn N. Dental pulp stem cell-derived chondrogenic cells demonstrate differential cell motility in type I and type II collagen hydrogels. Spine J. 2018;18:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 2015;227:746-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 5. | Fantini D. Easypubmed: Search and retrieve scientific publication records from pubmed. R package version 2.13. 2019. [cited 3 April 2021]. In: CRAN.R-project [Internet]. Available from: https://CRAN.R-project.org/package=easyPubMed. |
| 6. | Rani J, Shah AB, Ramachandran S. pubmed.mineR: an R package with text-mining algorithms to analyse PubMed abstracts. J Biosci. 2015;40:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Team RC. R: A language and environment for statistical computing. R foundation for statistical computing, vienna, austria. 2020. [cited 3 April 2021]. In: R-project [Internet]. Available from: https://www.R-project.org/. |
| 8. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3433] [Article Influence: 132.0] [Reference Citation Analysis (1)] |
| 9. | Mortada I, Mortada R. Dental pulp stem cells and osteogenesis: an update. Cytotechnology. 2018;70:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 257] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells vs dental pulp stem cells. Biol Cell. 2007;99:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Kumar A, Kumar V, Rattan V, Jha V, Bhattacharyya S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol Neurobiol. 2017;54:4672-4682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Mohanram Y, Zhang J, Tsiridis E, Yang XB. Comparing bone tissue engineering efficacy of HDPSCs, HBMSCs on 3D biomimetic ABM-P-15 scaffolds in vitro and in vivo. Cytotechnology. 2020;72:715-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Kim SH, Kim YS, Lee SY, Kim KH, Lee YM, Kim WK, Lee YK. Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J Periodontal Implant Sci. 2011;41:192-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Han J, Kim DS, Jang H, Kim HR, Kang HW. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J Tissue Eng. 2019;10:2041731419845849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, Ma PX. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016;33:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res. 2018;97:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Soares DG, Zhang Z, Mohamed F, Eyster TW, de Souza Costa CA, Ma PX. Simvastatin and nanofibrous poly(l-lactic acid) scaffolds to promote the odontogenic potential of dental pulp cells in an inflammatory environment. Acta Biomater. 2018;68:190-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Cooke ME, Ramirez-GarciaLuna JL, Rangel-Berridi K, Park H, Nazhat SN, Weber MH, Henderson JE, Rosenzweig DH. 3D Printed Polyurethane Scaffolds for the Repair of Bone Defects. Front Bioeng Biotechnol. 2020;8:557215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Rubert Pérez CM, Stephanopoulos N, Sur S, Lee SS, Newcomb C, Stupp SI. The powerful functions of peptide-based bioactive matrices for regenerative medicine. Ann Biomed Eng. 2015;43:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Akkouch A, Zhang Z, Rouabhia M. Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly (L-lactide-co-ε-caprolactone) scaffold. J Biomater Appl. 2014;28:922-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Xia K, Chen Z, Chen J, Xu H, Xu Y, Yang T, Zhang Q. RGD- and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int J Nanomedicine. 2020;15:6631-6647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 2011;90:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Nam S, Won JE, Kim CH, Kim HW. Odontogenic differentiation of human dental pulp stem cells stimulated by the calcium phosphate porous granules. J Tissue Eng. 2011;2011:812547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D'Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Yang JW, Zhang YF, Sun ZY, Song GT, Chen Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J Biomater Appl. 2015;30:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Li J, Chen M, Wei X, Hao Y, Wang J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials (Basel). 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Liu YJ, Su WT, Chen PH. Magnesium and zinc borate enhance osteoblastic differentiation of stem cells from human exfoliated deciduous teeth in vitro. J Biomater Appl. 2018;32:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Wiesmann HP, Tkotz T, Joos U, Zierold K, Stratmann U, Szuwart T, Plate U, Höhling HJ. Magnesium in newly formed dentin mineral of rat incisor. J Bone Miner Res. 1997;12:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X. Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng Part A. 2014;20:2422-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Tognarini I, Sorace S, Zonefrati R, Galli G, Gozzini A, Carbonell Sala S, Thyrion GD, Carossino AM, Tanini A, Mavilia C, Azzari C, Sbaiz F, Facchini A, Capanna R, Brandi ML. In vitro differentiation of human mesenchymal stem cells on Ti6Al4V surfaces. Biomaterials. 2008;29:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Galli D, Benedetti L, Bongio M, Maliardi V, Silvani G, Ceccarelli G, Ronzoni F, Conte S, Benazzo F, Graziano A, Papaccio G, Sampaolesi M, De Angelis MG. In vitro osteoblastic differentiation of human mesenchymal stem cells and human dental pulp stem cells on poly-L-lysine-treated titanium-6-aluminium-4-vanadium. J Biomed Mater Res A. 2011;97:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 550] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 34. | Manz BN, Groves JT. Spatial organization and signal transduction at intercellular junctions. Nat Rev Mol Cell Biol. 2010;11:342-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Xiao L, Tsutsui T. Three-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: expression patterns of relevant molecules. J Cell Biochem. 2012;113:1875-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Jin GZ, Kim HW. Co-culture of Human Dental Pulp Stem Cells and Endothelial Cells Using Porous Biopolymer Microcarriers: A Feasibility Study for Bone Tissue Engineering. Tissue Eng Regen Med. 2017;14:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 678] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 38. | Yu H, Zhang X, Song W, Pan T, Wang H, Ning T, Wei Q, Xu HHK, Wu B, Ma D. Effects of 3-dimensional Bioprinting Alginate/Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J Endod. 2019;45:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Park JY, Shim JH, Choi SA, Jang J, Kim M, Lee SH, Cho DW. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J Mater Chem B. 2015;3:5415-5425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Hilkens P, Bronckaers A, Ratajczak J, Gervois P, Wolfs E, Lambrichts I. The Angiogenic Potential of DPSCs and SCAPs in an In Vivo Model of Dental Pulp Regeneration. Stem Cells Int. 2017;2017:2582080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Dubey N, Ferreira JA, Malda J, Bhaduri SB, Bottino MC. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl Mater Interfaces. 2020;12:23752-23763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 42. | Hsiao D, Hsu SH, Chen RS, Chen MH. Characterization of designed directional polylactic acid 3D scaffolds for neural differentiation of human dental pulp stem cells. J Formos Med Assoc. 2020;119:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 44. | Longoni A, Utomo L, van Hooijdonk IE, Bittermann GK, Vetter VC, Kruijt Spanjer EC, Ross J, Rosenberg AJ, Gawlitta D. The chondrogenic differentiation potential of dental pulp stem cells. Eur Cell Mater. 2020;39:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 46. | Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S. Human Dental Pulp Stem Cells Differentiate into Oligodendrocyte Progenitors Using the Expression of Olig2 Transcription Factor. Cells Tissues Organs. 2014;200:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Ghasemi Hamidabadi H, Rezvani Z, Nazm Bojnordi M, Shirinzadeh H, Seifalian AM, Joghataei MT, Razaghpour M, Alibakhshi A, Yazdanpanah A, Salimi M, Mozafari M, Urbanska AM, Reis RL, Kundu SC, Gholipourmalekabadi M. Chitosan-Intercalated Montmorillonite/Poly(vinyl alcohol) Nanofibers as a Platform to Guide Neuronlike Differentiation of Human Dental Pulp Stem Cells. ACS Appl Mater Interfaces. 2017;9:11392-11404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, Xia J, Xu H, Zhao Y, Xiao J, He Y, Ye Q. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int. 2018;2018:2398521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Zheng K, Feng G, Zhang J, Xing J, Huang D, Lian M, Zhang W, Wu W, Hu Y, Lu X, Feng X. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int J Neurosci. 2021;131:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 50. | Guo S, Redenski I, Landau S, Szklanny A, Merdler U, Levenberg S. Prevascularized Scaffolds Bearing Human Dental Pulp Stem Cells for Treating Complete Spinal Cord Injury. Adv Healthc Mater. 2020;9:e2000974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Luzuriaga J, Irurzun J, Irastorza I, Unda F, Ibarretxe G, Pineda JR. Vasculogenesis from Human Dental Pulp Stem Cells Grown in Matrigel with Fully Defined Serum-Free Culture Media. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 53. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 2012] [Article Influence: 87.5] [Reference Citation Analysis (1)] |
| 54. | Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 55. | Koyama N, Okubo Y, Nakao K, Bessho K. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Laino G, Graziano A, d'Aquino R, Pirozzi G, Lanza V, Valiante S, De Rosa A, Naro F, Vivarelli E, Papaccio G. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Vakhrushev IV, Antonov EN, Popova AV, Konstantinova EV, Karalkin PA, Kholodenko IV, Lupatov AY, Popov VK, Bagratashvili VN, Yarygin KN. Design of tissue engineering implants for bone tissue regeneration of the basis of new generation polylactoglycolide scaffolds and multipotent mesenchymal stem cells from human exfoliated deciduous teeth (SHED cells). Bull Exp Biol Med. 2012;153:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Feng X, Xing J, Feng G, Sang A, Shen B, Xu Y, Jiang J, Liu S, Tan W, Gu Z, Li L. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. Cell Mol Neurobiol. 2013;33:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Sugimura-Wakayama Y, Katagiri W, Osugi M, Kawai T, Ogata K, Sakaguchi K, Hibi H. Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cells Dev. 2015;24:2687-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke. 2013;44:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Zhu W, Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cells Int. 2015;2015:972313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 62. | Iwasaki K, Komaki M, Yokoyama N, Tanaka Y, Taki A, Kimura Y, Takeda M, Oda S, Izumi Y, Morita I. Periodontal ligament stem cells possess the characteristics of pericytes. J Periodontol. 2013;84:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2557] [Article Influence: 116.2] [Reference Citation Analysis (1)] |
| 64. | Liu W, Konermann A, Guo T, Jäger A, Zhang L, Jin Y. Canonical Wnt signaling differently modulates osteogenic differentiation of mesenchymal stem cells derived from bone marrow and from periodontal ligament under inflammatory conditions. Biochim Biophys Acta. 2014;1840:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Deng C, Sun Y, Liu H, Wang W, Wang J, Zhang F. Selective adipogenic differentiation of human periodontal ligament stem cells stimulated with high doses of glucose. PLoS One. 2018;13:e0199603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Kim YT, Park JC, Choi SH, Cho KS, Im GI, Kim BS, Kim CS. The dynamic healing profile of human periodontal ligament stem cells: histological and immunohistochemical analysis using an ectopic transplantation model. J Periodontal Res. 2012;47:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 797] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 68. | Ansari S, Diniz IM, Chen C, Sarrion P, Tamayol A, Wu BM, Moshaverinia A. Human Periodontal Ligament- and Gingiva-derived Mesenchymal Stem Cells Promote Nerve Regeneration When Encapsulated in Alginate/Hyaluronic Acid 3D Scaffold. Adv Healthc Mater. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 69. | Duan X, Ji M, Deng F, Sun Z, Lin Z. Effects of connective tissue growth factor on human periodontal ligament fibroblasts. Arch Oral Biol. 2017;84:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Thomadakis G, Ramoshebi LN, Crooks J, Rueger DC, Ripamonti U. Immunolocalization of Bone Morphogenetic Protein-2 and -3 and Osteogenic Protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur J Oral Sci. 1999;107:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, Briand-Mesange F, Gadelorge M, Arzate H, Narayanan AS, Brunel G, Salles JP. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Pitaru S, Pritzki A, Bar-Kana I, Grosskopf A, Savion N, Narayanan AS. Bone morphogenetic protein 2 induces the expression of cementum attachment protein in human periodontal ligament clones. Connect Tissue Res. 2002;43:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Cho H, Tarafder S, Fogge M, Kao K, Lee CH. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect Tissue Res. 2016;57:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Liu Y, Yang R, Shi S. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue Eng Part A. 2015;21:498-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, Liu Y. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Du J, Mei S, Guo L, Su Y, Wang H, Liu Y, Zhao Z, Wang S. Platelet-rich fibrin/aspirin complex promotes alveolar bone regeneration in periodontal defect in rats. J Periodontal Res. 2018;53:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Kramer JL. Decreasing the organ donor shortage. Am J Forensic Med Pathol. 1995;16:257; author reply 259-257; author reply 260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Pan J, Deng J, Luo Y, Yu L, Zhang W, Han X, You Z, Liu Y. Thermosensitive Hydrogel Delivery of Human Periodontal Stem Cells Overexpressing Platelet-Derived Growth Factor-BB Enhances Alveolar Bone Defect Repair. Stem Cells Dev. 2019;28:1620-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Ravindran S, Huang CC, George A. Extracellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSCs. Front Physiol. 2014;4:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Ma Y, Ji Y, Huang G, Ling K, Zhang X, Xu F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication. 2015;7:044105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 82. | Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56:709-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 83. | Liu C, Xiong H, Chen K, Huang Y, Yin X. Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. Int Endod J. 2016;49:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Nada OA, El Backly RM. Stem Cells From the Apical Papilla (SCAP) as a Tool for Endogenous Tissue Regeneration. Front Bioeng Biotechnol. 2018;6:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 85. | Kitamura C, Nishihara T, Terashita M, Tabata Y, Washio A. Local regeneration of dentin-pulp complex using controlled release of fgf-2 and naturally derived sponge-like scaffolds. Int J Dent. 2012;2012:190561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Wang W, Dang M, Zhang Z, Hu J, Eyster TW, Ni L, Ma PX. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016;36:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 87. | Xiao M, Qiu J, Kuang R, Zhang B, Wang W, Yu Q. Synergistic effects of stromal cell-derived factor-1α and bone morphogenetic protein-2 treatment on odontogenic differentiation of human stem cells from apical papilla cultured in the VitroGel 3D system. Cell Tissue Res. 2019;378:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 321] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Lange S, Heger J, Euler G, Wartenberg M, Piper HM, Sauer H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc Res. 2009;81:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Deng J, Pan J, Han X, Yu L, Chen J, Zhang W, Zhu L, Huang W, Liu S, You Z, Liu Y. PDGFBB-modified stem cells from apical papilla and thermosensitive hydrogel scaffolds induced bone regeneration. Chem Biol Interact. 2020;316:108931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18-28. [PubMed] |
| 92. | Somoza RA, Acevedo CA, Albornoz F, Luz-Crawford P, Carrión F, Young ME, Weinstein-Oppenheimer C. TGFβ3 secretion by three-dimensional cultures of human dental apical papilla mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Na S, Zhang H, Huang F, Wang W, Ding Y, Li D, Jin Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J Tissue Eng Regen Med. 2016;10:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Salehi S, Cooper P, Smith A, Ferracane J. Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent Mater. 2016;32:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Athirasala A, Tahayeri A, Thrivikraman G, França CM, Monteiro N, Tran V, Ferracane J, Bertassoni LE. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication. 2018;10:024101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 96. | Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 634] [Article Influence: 30.2] [Reference Citation Analysis (4)] |
| 97. | Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015;33:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 98. | Xu QL, Furuhashi A, Zhang QZ, Jiang CM, Chang TH, Le AD. Induction of Salivary Gland-Like Cells from Dental Follicle Epithelial Cells. J Dent Res. 2017;96:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Chen G, Chen J, Yang B, Li L, Luo X, Zhang X, Feng L, Jiang Z, Yu M, Guo W, Tian W. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials. 2015;52:56-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 100. | Tsuchiya S, Ohshima S, Yamakoshi Y, Simmer JP, Honda MJ. Osteogenic differentiation capacity of porcine dental follicle progenitor cells. Connect Tissue Res. 2010;51:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Steimberg N, Angiero F, Farronato D, Berenzi A, Cossellu G, Ottonello A, Kaigler D, Mazzoleni G. Advanced 3D Models Cultured to Investigate Mesenchymal Stromal Cells of the Human Dental Follicle. Tissue Eng Part C Methods. 2018;24:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Salgado CL, Barrias CC, Monteiro FJM. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front Bioeng Biotechnol. 2020;8:724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Healy L, Hunt C, Young L, Stacey G. The UK Stem Cell Bank: its role as a public research resource centre providing access to well-characterised seed stocks of human stem cell lines. Adv Drug Deliv Rev. 2005;57:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Washio K, Iwata T, Mizutani M, Ando T, Yamato M, Okano T, Ishikawa I. Assessment of cell sheets derived from human periodontal ligament cells: a pre-clinical study. Cell Tissue Res. 2010;341:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Gombold J, Karakasidis S, Niksa P, Podczasy J, Neumann K, Richardson J, Sane N, Johnson-Leva R, Randolph V, Sadoff J, Minor P, Schmidt A, Duncan P, Sheets RL. Systematic evaluation of in vitro and in vivo adventitious virus assays for the detection of viral contamination of cell banks and biological products. Vaccine. 2014;32:2916-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Osathanon T. Transplantation of cryopreserved teeth: a systematic review. Int J Oral Sci. 2010;2:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Yamada Y, Nakamura-Yamada S, Konoki R, Baba S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res Ther. 2020;11:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 108. | Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, Gronthos S. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev. 2014;23:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 109. | Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015;9:1205-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |