Published online Sep 26, 2020. doi: 10.4252/wjsc.v12.i9.897
Peer-review started: May 4, 2020
First decision: May 24, 2020
Revised: June 5, 2020
Accepted: August 16, 2020
Article in press: August 16, 2020
Published online: September 26, 2020
Processing time: 140 Days and 19.9 Hours
Dental stem cells (DSCs) are self-renewable cells that can be obtained easily from dental tissues, and are a desirable source of autologous stem cells. The use of DSCs for stem cell transplantation therapeutic approaches is attractive due to their simple isolation, high plasticity, immunomodulatory properties, and multipotential abilities. Using appropriate scaffolds loaded with favorable biomolecules, such as growth factors, and cytokines, can improve the proliferation, differentiation, migration, and functional capacity of DSCs and can optimize the cellular morphology to build tissue constructs for specific purposes. An enormous variety of scaffolds have been used for tissue engineering with DSCs. Of these, the scaffolds that particularly mimic tissue-specific micromilieu and loaded with biomolecules favorably regulate angiogenesis, cell-matrix interactions, degradation of extracellular matrix, organized matrix formation, and the mineralization abilities of DSCs in both in vitro and in vivo conditions. DSCs represent a promising cell source for tissue engineering, especially for tooth, bone, and neural tissue restoration. The purpose of the present review is to summarize the current developments in the major scaffolding approaches as crucial guidelines for tissue engineering using DSCs and compare their effects in tissue and organ regeneration.
Core Tip: Dental stem cells have been used for different types of cell transplantation therapies, including teeth, bone, and neural tissue regeneration. In planning for successful tissue engineering toward organ-specific regeneration, choosing an appropriate scaffold that mimics the extracellular matrix in native tissue and loaded with suitable biomolecules to boost dental stem cell functions is of utmost importance.
- Citation: Granz CL, Gorji A. Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies. World J Stem Cells 2020; 12(9): 897-921
- URL: https://www.wjgnet.com/1948-0210/full/v12/i9/897.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i9.897
Stem cells are undifferentiated cells with self-renewing and clonogenic capabilities, which can differentiate into various cell lineages. According to the basis of their origin, stem cells are categorized as embryonic, induced pluripotent stem cells (iPS), and adult (tissue-specific) stem cells[1-3]. Based on their differentiation potential, stem cells can be classified as totipotent (the ability to give rise to all types of cells), pluripotent (the potential of the cells to produce any type of cells in the organism), multipotent (the potential to give rise to cells of their tissue of origin), oligopotent (the potential to differentiate into only a few cell types), and unipotent (the ability to produce one cell type)[4]. Embryonic stem cells are pluripotent, whereas adult stem cells are limited to differentiating into various cell types of their original tissue (multipotent). iPS are pluripotent cells that originated from somatic differentiated cells after transduction. Adult stem cells exist in different tissues and organs, such as the bone marrow, blood vessels, peripheral blood, and skeletal muscles as well as the brain, heart, skin, intestine, liver, gonads, and teeth[5-7].
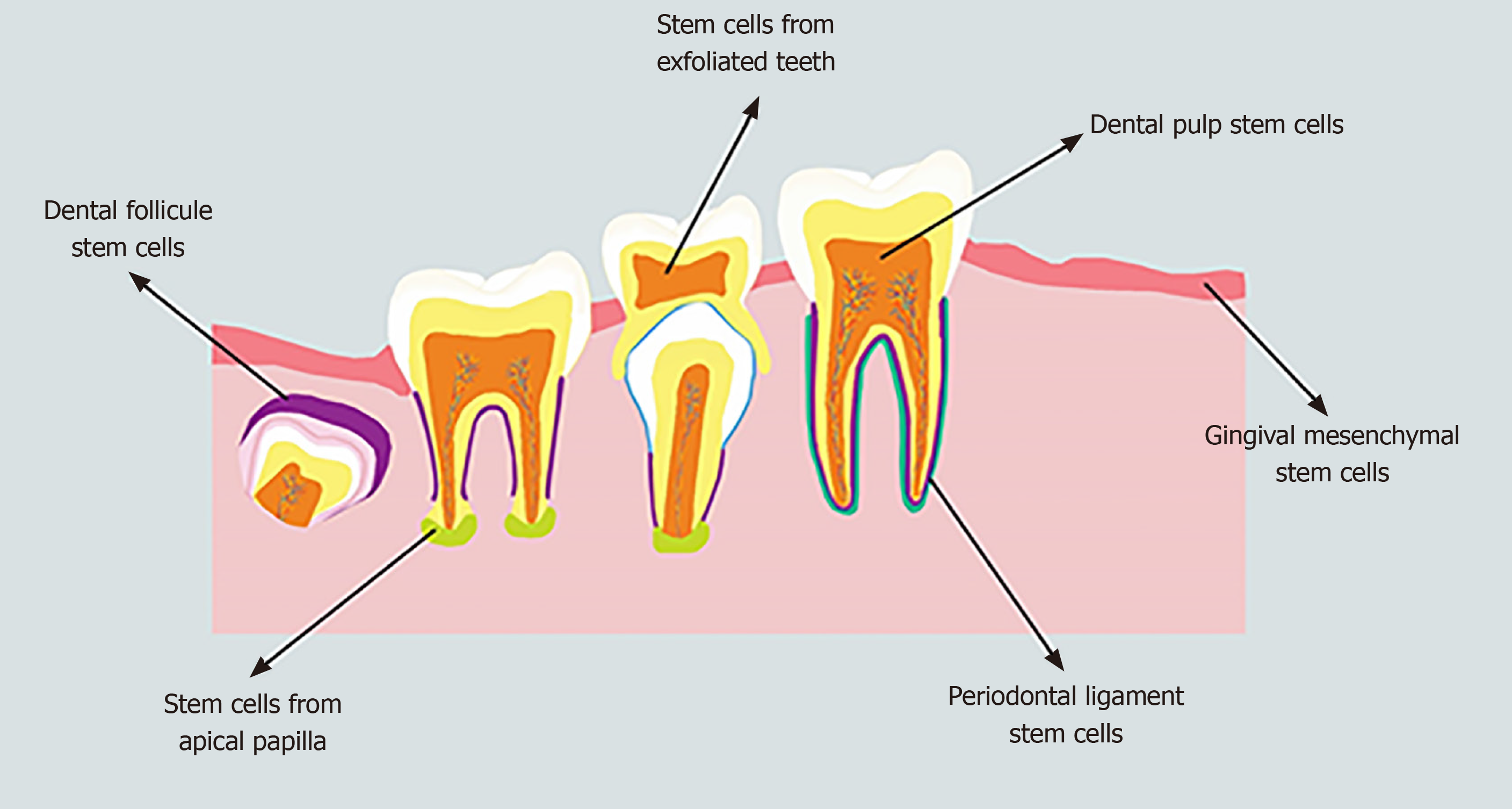
Human mesenchymal stem cells (MSCs), which are multipotent non-hematopoietic progenitor cells, have been isolated from both adult and fetal tissues, such as the bone marrow, adipose tissue, endometrium, bone, muscle, umbilical cord, blood, Wharton's jelly, and amniotic fluid as well as nervous and dental tissues[8]. Human MSCs have the potential to differentiate into both mesodermal (osteocytes, adipocytes, and chondrocytes) and non-mesodermal (endodermal and ectodermal) lineages (hepatocytes and neuronal cells)[9] with both anti- and pro-tumorigenic properties[10] as well as a limited risk of inflammatory reactions and uncontrolled growth[11]. The source of MSCs has a crucial role in the outcomes of stem cell-based tissue engineering[12]. Dental stem cells (DSCs) are neural crest-derived cells that can be obtained easily from dental tissues of both adults and children; therefore, they are a reliable, accessible source of autologous stem cells[13,14]. DSCs are undifferentiated cells that have non-limited self-renewal, multipotent differentiation potential, and colony-forming capacity[15]. DSCs can be isolated from the dental pulp of deciduous, natal, and permanent teeth, the periodontal ligament, the apical papilla, the dental follicle, and gingival tissue (Figure 1)[16,17]. One of the unique characteristics of DSCs is their ability to differentiate into mesodermal, ectodermal, and endodermal cell lineages[18]. DSCs from each source are capable of specifically differentiating into various distinct cells, including epithelial cells, odontoblasts, osteoblasts, chondroblasts, adipocytes, vascular cells, endotheliocytes, neuronal cells, glial cells, photoreceptor cells, and muscle cells[19,20]. Although all stem cells obtained from various sources are named DSCs in this study, their phenotype, differentiation potential (both in in vitro and in vivo conditions) and functional properties (such as biological response during differentiation and tissue repair) are different[21]. For instance, stem cells obtained from the apical papilla possess greater proliferation ability, express a higher variety of neural markers, and induce more uniform dentine-like tissues compared to dental pulp stem cells[22-24]. Furthermore, DSCs isolated from exfoliated deciduous teeth exert a higher capacity for osteogenic regeneration and a greater proliferation rate compared to dental pulp stem cells[25]. DSCs isolated from pulp tissues are the first and most frequent cells evaluated for their odontogenic, osteogenic, and neurogenic differentiation potentials[26]. This heterogeneity of DSCs is effectively modulated by the function of their microenvironment[27]. DSCs obtained from different sources exhibit various patterns of cell surface markers (Table 1)[28-31].
| Markers | DPSCs | SCEDT | PLSCs | DFSCs | SCAP |
| Nestin | + | + | + | + | |
| Vimentin | + | + | + | ||
| SOX2 | + | + | + | + | |
| SOX10 | + | + | |||
| Stro-1 | + | + | + | + | + |
| Oct-4 | + | + | + | + | + |
| EphB | + | ||||
| Nanog | + | + | + | + | |
| CD10 | + | + | |||
| CD13 | + | + | + | + | + |
| CD14 | + | + | + | ||
| CD19 | + | ||||
| CD24 | + | + | |||
| CD25 | + | ||||
| CD29 | + | + | + | + | + |
| CD34 | + | + | + | + | + |
| CD44 | + | + | + | + | + |
| CD45 | + | + | + | + | + |
| CD49 | + | ||||
| CD53 | + | ||||
| CD59 | + | + | + | ||
| CD73 | + | + | + | + | + |
| CD90 | + | + | + | + | + |
| CD105 | + | + | + | + | + |
| CD106 | + | ||||
| CD117 | + | ||||
| CD146 | + | + | + | + | |
| CD150 | + | ||||
| CD166 | + | + | |||
| CD271 | + | + | |||
| SSEA-3 | + | ||||
| SSEA-4 | + | ||||
| TWIST-1 | + | ||||
| c-myc | + | + | |||
| Notch | + | + | |||
| 3G5 | + | ||||
| Klf-4 | + | ||||
| FlK1 | + | + |
DSCs secrete numerous immunomodulatory mediators, such as interleukin (IL)-6, IL-10, IL-1β, interferon-γ, and tumor necrosis factor-α as well as transforming growth factor-beta (TGF-β), hepatocyte growth factor, and vascular endothelial growth factor (VEGF)[32], and do not express the major histocompatibility complex class II antigen[33], which suggests their potential in the regulation of immune responses to promote tissue regeneration[34]. DSCs from different sources may exert their immuno-modulatory properties through the suppression of T-cell proliferation and lymphocyte activity as well as the activation of T-cell apoptosis[34,35].
The fate of the stem cells (proliferation and differentiation) is regulated via a combination of intrinsic and extrinsic mechanisms. Intrinsic mechanisms consist of various transcription factors expressed by the cells. Extrinsic mechanisms are signals provided by the dynamic microenvironment (or “niche”), including the extracellular matrix (ECM), signaling molecules (such as growth factors and hormones), and neighboring cells[36,37]. The microenvironment, which is a three-dimensional (3D) structure surrounded by specific cells and ECM, protects stem cells from inappropriate differentiation, cell damage, and apoptosis and governs tissue maintenance, regeneration, and repair[38,39]. In addition to providing a physical microenvironment for cells, the ECM gives the tissue its mechanical properties (elasticity and rigidity), provides bioactive molecules and cues to residing cells, and establishes an environment to facilitate tissue remodeling in response to dynamic processes, such as wound healing[40]. Furthermore, the ECM is produced and arranged by tissue-resident cells and secreted into the surrounding environment to provide support to the stem cells with its bioactive compounds[41]. Stem cell behaviors are reciprocally regulated by the ECM and signals from the surrounding cells and molecules. Furthermore, inorganic ions, such as calcium and magnesium, as well as metabolic products, such as oxygen drive metabolites, and maintain stem cell fate[42].
The nature of the stem cell microenvironment differs in various tissues. In teeth, a particular microenvironment exists at specific anatomic sites that regulates the behavior of DSCs[39]. Two different stem cell microenvironments have been identified in teeth; (1) the pulp cell-rich zone; and (2) the perivascular and perineurium of the dental pulp. The pulp is composed of four distinct zones; an outermost layer containing the odontoblasts, a cell-free zone (zone of Weil) with no cells and rich in the ECM, the cell-rich zone contains stem/progenitor cells, and the pulp core. Dental pulp tissue is populated by odontoblasts, fibroblasts, dendritic cells, macrophages, and progenitor cells, whereas the pulp core contains dental pulp cells, vessels, nerves, and ECM[43]. The induction of odontoblasts, the biological cells of neural crest origin that survive throughout life, occurs during tooth development. However, under appropriate conditions, DSCs can differentiate into pre-odontoblasts and later secretory odontoblasts, which actively participate in reactionary dentinogenesis[44]. Odontoblasts produce the main part of the ECM components of dentin and are involved in dentin mineralization[45]. The dentin ECM consists of collagen (approximately 90%; Type I, III, and V), proteoglycans (such as chondroitin sulfate and heparan sulfate), growth factors [such as TGF-β and bone morphogenetic protein (BMP)], and enzymes (such as matrix metallopeptidase 1, 2, 3, 9, and 20)[46]. In the dental pulp, DSCs also reside in perivascular and perineurium regions[47], which can be identified by aldehyde dehydrogenase-1 expression[48]. The EphB/ephrin-B signaling pathway reciprocally modulates the attachment and migration of DSCs originated from the perivascular niche via the mitogen-activated protein kinase pathway and phosphorylation of Src family tyrosine kinases[49].
Pointing to the importance of ECM in maintaining homeostasis for proliferation and differentiation of DSCs, several studies have indicated that reconstruction of the appropriate microenvironment and boosting its interaction with stem cells are essential steps to successful cell therapy[50]. The application of DSCs in stem cell therapeutic approaches is attractive due to their simple isolation and efficient administration[51]. The multi-lineage capacity of DSCs differentiation to various tissues and organs suggests their greater ability than other adult stem cell populations for the treatment of different diseases[52]. There is an enormous amount of evidence to indicate that DSCs have great potential for therapeutic cell approaches in various diseases, including liver disease, diabetes, myocardial infarction, ophthalmologic diseases, muscular dystrophy, Alzheimer’s disease, Parkinson’s disease, cerebral ischemia, and spinal cord injury[53]. Furthermore, several studies have explored the potential of DSCs in the treatment of caries, periodontal disease, oral and maxillofacial defects, and alveolar bone atrophy[54,55]. DSCs possess strong immunomodulatory abilities, which suggest that they are a favorable cell source for cell transplantation therapy in inflammatory disorders[33]. It has been shown that DSCs are more beneficial for axonal regeneration than bone marrow stem cells due to their greater release of neurotrophic factors[56]. Despite these extensive efforts, several essential parameters still need to be optimized for the clinical use of DSCs in cell transplantation therapy. One of the key challenges is the lack of an appropriate stem cell microenvironment, which leads to short-term survival of DSCs after implantation. To increase cell viability, transplanted cells require particular 3D structures with specific ECM components that protect DSCs from cell damage, maintain the stem cell homeostasis, and promote mutual biological information transfer between stem cells and the ECM[50,57,58]. A large number of investigations have been carried out to reconstruct the stem cell microenvironment to strengthen the viability, proliferation, and appropriate differentiation of the transplanted cells for successful cell therapy[59]. In this context, ECM scaffolds can form a desirable microenvironment for DSCs, which serve as a more favorable template for tissue repair and reconstruction[60,61]. In this review, we provide a critical overview of the role of different biomaterials used to deliver DSCs to damaged tissue and their applications to improve, restore, and maintain tissue or organ reconstruction.
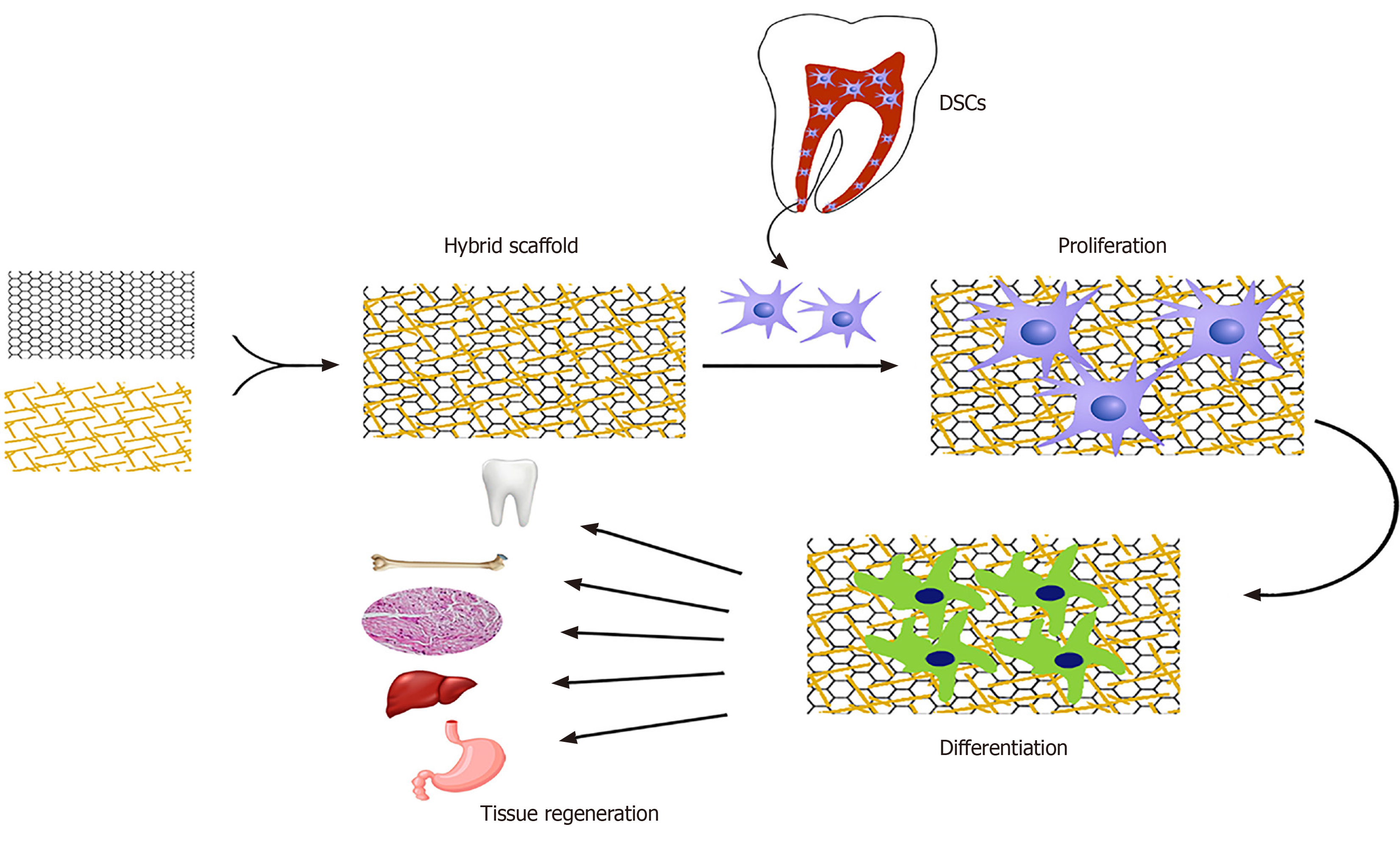
The general concept of tissue bioengineering involves three essential components; identification of suitable stem cells, development of appropriate scaffolds, and induction of potent signals to repair or regenerate human cells, tissues, or organs[62]. Biomaterials are essential components for the construction of scaffolds. Tissue bioengineering combines scaffolds with various types of stem cells to reconstruct damaged tissues (Figure 2). The application of appropriate scaffolds could improve DSCs proliferation, differentiation, adhesion, and migration, which may promote their ability to repair the injured tissues and regenerate functional organs[63]. Acellular tissues, as well as natural and synthetic biomaterials, can be used as the primary source for generating scaffolds[64]. Acellular tissue matrices, such as an acellular adipose matrix, are derived from animal or human tissues with all cells eliminated during manufacture[65]. In addition, the amniotic membrane has been suggested as a suitable biological scaffold for the proliferation and transplantation of DSCs[66]. Natural biomaterials consist of proteins (collagen, gelatin, fibrin, and silk) and polysaccharides (agarose, alginate, hyaluronan, polylactic acid, and chitosan) which tend to be biocompatible due to their cellular adhesion sites, such as Arg-Gly-Asp binding sequences, and the ability to degrade without releasing toxic substances. However, due to the variability of materials, limited mechanical properties, the risk of transmitting pathogens, and provoking immune reactions, their formulations need to be promoted for stem cell culture[67,68]. Synthetic biomaterials, including polymer-based biomaterials (such as polycaprolactone, polylactic acid, poly-lactic-co-glycolic acid, polyglycolide, poly-e-caprolactone, and poly-ethylene glycol) and ceramic-based biomaterials (such as hydroxyapatite, bioactive glass, and calcium phosphate) display a better mechanical property, reproducibility, and electrical conductivity as well as a lower degradation rate[68-70]. Furthermore, synthetic biomaterials possess the possibility of optimizing the chemical and physical properties of a scaffold for a particular application[71]. Hydrogels, which are networks of hydrophilic polymers, can be manufactured from natural biomaterials (such as collagen, fibrin, proteoglycans, and hyaluronic acid) or synthetic polymers (such as self-assembly peptide molecules or poly-ethylene glycol)[37,72,73]. Hydrogels provide tissue-like microenvironments with particular cellular signals, desirable biocompatibility, semi-permeable membranes, and cell delivery vehicles[74]. Furthermore, a wide range of nanocomposite biomaterials has been assembled by merging nanomaterials within the polymeric matrix to promote the efficiency of bioactive scaffolds[75].
The desirable properties of a scaffold for stem cell transplantation are biocompatibility, biodegradability, mimic the 3D biological microenvironment, incorporation of different ECM, pore size, stability, electrical conductivity, porosity, non-immunogenicity, interconnectivity, safety (low or non-toxic) and alignment[70,76,77]. Various fabrication techniques have been developed to produce different scaffolds, such as emulsion freeze-drying, electrospinning, thermally-induced phase separation, solvent casting/particular leaching, computer-aided design/computer-aided manufacturing, melt molding, rapid prototyping (3D printing, selective laser sintering, stereolithography, and fused deposition modeling), nanofiber self-assembly, and photolithography[78,79]. The basic tissue engineering procedures consist of appropriate scaffold manufacture, hydrogel matrix support, and patterning design. The combination of these approaches promotes the development of the desired complex, both in tissue structure and function[20,80].
Scaffold-based cultures are conventionally applied in two-dimensional (2D) systems. Although 2D systems are a valuable medium for the investigation of basic cell biology and preclinical drug testing, data generated in these systems are insufficient to translate into in vivo experimental studies[81]. Furthermore, inappropriate cell-to-cell and cell-to-ECM contacts, reduction of polarization, and alteration of key signaling pathways modulate stem cell differentiation ability[81,82]. Thus, several 3D scaffold-based cultures have been developed. Although existing 3D cultures are not without limitations, they enhance cell viability, growth, differentiation, and migration and improve cellular communications[82]. Combining DSCs with suitable scaffolds offers a promising strategy for cell delivery and transplantation. Two main approaches for this combination are cell-based and cell-free tissue engineering. In the cell-based approach, stem cells are seeded and cultured onto the scaffold in vitro to produce the desired tissue before transplantation[83]. In the cell-free approach, a bioactive scaffold with growth and differentiation factors is embedded in the respective tissues, induces the homing of resident stem cells, and promotes their proliferation and differentiation[84].
Furthermore, the environmental cues, such as various growth factors/morphogens, markedly affect the behavior of DSCs seeded in scaffolds and are vital to the success of regenerative therapies[85,86]. Several proteins, such as BMP, sialoprotein, fibronectin, and osteopontin, are able to coat various types of biomaterials and promote the behaviors of DSCs[87]. Pre-treatment of biomaterials with the abovementioned proteins could enhance adhesion, differentiation, proliferation, migration, and function of DSCs and improve the formation of new tissues[87,88].
DSCs represent an auspicious cell source for tissue engineering, particularly for tooth, bone, and neural tissue reconstruction. A vast number of these investigations point to the importance of various scaffolds to design effective tissue engineering approaches (Table 2).
| Scaffold | Growth factors/bioactive molecules | Experimental model | Target tissue | Ref. |
| Silicon | - | In vitro | Teeth | [168] |
| Collagen sponge | SCF | In vitro and in vivo (mice) | Teeth | [126] |
| Collagen type I and type III | SDF-1 | In vivo (dogs) | Teeth | [91] |
| Collagen type-I and N-acetic acid | SDF-1, bFGF, BMP-7 | In vitro and in vivo (rats) | Teeth | [120] |
| Collagen/chitsosan | - | In vitro | - | [129] |
| Collagen-polyvinylpyrrolidone sponge | - | Case report | Teeth | [144] |
| Silk fibroin | SDF-1 | In vitro and in vivo (mice) | Teeth | [123] |
| Acellular dental pulp ECM | - | In vivo (mice) | Teeth | [108] |
| Intrafibrillar-silicified collagen | - | In vitro and in vivo (mice) | Teeth | [169] |
| Matrigel | bFGF-2, TGF-β1 | In vitro | - | [121] |
| Peptide hydrogel | VEGF, TGF-β1, FGF-1 | In vivo (mice) | Teeth | [118] |
| Gelatin methacrylate hydrogel | - | In vivo (rats) | Teeth | [133] |
| PuraMatrix™ | VEGF | In vivo (mice) | Teeth | [134] |
| Poly-ε-caprolactone and hydroxyapatite | SDF-1, BMP-7 | In vitro and in vivo (rats) | Teeth | [124] |
| Polycaprolactone-poly-glycolic acid | BMP-7 | In vitro and in vivo (mice) | Teeth | [97] |
| Thermoresponsive hydrogel | - | In vitro and in vivo (mice) | Teeth | [111] |
| DL-lactide/co-polymer of L-lactide /DL-lactide, and hydroxyapatite tricalcium phosphate | BMP-2 | In vitro and in vivo (mice) | Teeth | [132] |
| 3D hydroxyapatite scaffolds containing peptide hydrogels | - | In vivo (mice) | Teeth | [112] |
| Poly-lactic-co-glycolic acid | - | In vitro and in vivo (mice) | Teeth | [104] |
| Beta-tricalcium phosphate scaffold | BMP-2 | In vitro and in vivo (mice) | Teeth | [131] |
| Collagen sponge | - | Clinical trial | Bone | [153] |
| Collagen sponge | - | Clinical trial | Bone | [151] |
| Collagen sponge | - | Clinical trial | Bone | [152] |
| Chitosan/gelatin | BMP-2 | In vitro and in vivo (mice) | Bone | [92] |
| Arginine-glycine-aspartic acid | - | In vitro and in vivo (mice) | Bone | [141] |
| Granular 3D chitosan | - | In vitro | Neural tissue | [193] |
| Matrigel | BMP-9 | In vitro and in vivo (mice) | Bone | [180] |
| 3D nano-fibrous gelatin/silica bioactive glass hybrid | - | In vitro | Teeth | [107] |
| 3D gel collagen matrix | BMP-2 | In vitro and in vivo (rats) | Bone | [177] |
| Poly-ε-caprolactone biphasic calcium phosphate | - | In vitro and in vivo (rabbit) | Bone | [160] |
| Glass nanoparticles/chitosan-gelatin | - | In vitro and in vivo (rats) | Bone | [159] |
| 3D poly-lactide | Extracellular vesicles | In vitro and in vivo (rats) | Bone | [179] |
| 2D monolayer culture/3D poly lactic-co-glycolic | - | In vitro | Bone | [174] |
| Dense collagen gel or acellular | - | In vivo (rats) | Bone | [150] |
| Calcium phosphate cement functionalized with iron oxide nanoparticles | - | In vitro | Bone | [166] |
| Poly-lactic-co-glycolic acid | - | In vitro and in vivo (rats) | Bone | [164] |
| Hydroxyapatite-collagen sponge | - | Clinical trial | Bone | [146] |
| 3D porous chitosan | bFGF | In vitro | Neural tissue | [201] |
| Fibrin and collagen | - | In vivo (rats) | Sciatic nerves | [9] |
| Collagen | Tetracycline | In vivo (mice) | Sciatic nerves | [206] |
| Collagen | - | In vitro and in vivo (rats) | Sciatic nerves | [210] |
| 3D alginate/hyaluronic acid | NGF | In vitro and in vivo (mice) | Peripheral nerves | [207] |
| Collagen sponge (DSCs condition medium) | - | In vitro and in vivo (rats) | Facial nerves | [211] |
| 3D bio-printing of scaffold-free nervous tissue | - | In vitro and in vivo (rats) | Facial nerves | [208] |
| Chitosan | - | In vitro and in vivo (rats) | Spinal cord | [198] |
| Aligned electrospun poly-ε-caprolactone/ poly-lactide-co-glycolic acid | - | In vitro and in vivo (rats) | Spinal cord | [202] |
The therapeutic role of DSCs in combination with various scaffolds has been extensively investigated in restoring tooth damage or loss due to caries, periodontal disease, trauma, or genetic disorders[89]. The procedure of dentin formation consists of odontoblastic deposition, vascularization, and neuron formation[20]. Among multiple approaches to promote dentin formation and teeth tissue regeneration, the application of DSCs with a synthetic pre-designed and optimized scaffold is the most accepted technique for tooth regeneration[90]. The appropriate scaffold can be implemented with DSCs and growth factors to induce the generation of dental tissues, which can integrate with the adjacent tissues[16]. Scaffolds developed from either synthetic or natural biomaterials have been used for tooth reconstruction. Natural materials, such as collagen[91], chitosan/gelatin[92], silk protein[93], alginate[94], hyaluronic acid[95] as well as synthetic polymers, such as polyglycolate/poly-l-lactate[96], polycaprolactone-poly glycolic acid[97], polylactic acid-co-polyglycolic acid[98], polycaprolactone /gelatin/ nano- hydroxyapatite[99], nano-hydroxyapatite/collagen/poly-l-lactide[100] and poly-ethyl methacrylate-co-hydroxyethyl acrylate[101] were used as scaffold materials for dental restoration and regeneration. Several investigations have indicated the regeneration of vascularized pulp-like tissue after subcutaneous implantation of tooth slices containing DSCs accompanied by an appropriate scaffold, particularly in the presence of growth factors such as dentin matrix protein[102,103]. In several experiments, a combination of the abovementioned scaffolds was used to promote cell differentiation, vascularization, and safety as well as to reduce immunological and ectopic complications. The development of a vascularized dentin/pulp tissue in a subcutaneously transplanted human root canal containing a poly-lactic-co-glycolic acid scaffold seeded with DSCs has been reported[104]. A scaffold consists of a pulp-specific ECM (an acellular ECM within the hydrogel) and an endothelial ECM (collagen-chitosan hydrogels) to promote odontogenic differentiation of DSCs and induce extensive vascularization in an in vivo model of a tooth root slice[105]. Comparing collagen and gelatin with chitosan, it has been stated that chitosan exerts weaker support for human DSCs growth and differentiation[106]. In addition, 3D nano-fibrous gelatin/silica bioactive glass hybrid scaffolds provide a suitable microenvironment that mimics the architecture and composition of a natural dental micromilieu and enhances the growth and differentiation of human DSCs[107]. Furthermore, the administration of human DSCs associated with acellular dental pulp resulted in pulp-like tissue structures and the maintenance of ECM[108]. DSCs seeded in 3D scaffold-free stem-cell sheet-derived pellets promote odontogenic differentiation[109].
The application of combined DSCs with the ECM scaffold can be used for root canal therapy. It has been shown that DSCs are able to differentiate into functional odontoblasts with angiogenic potential[110]. Implantation of a 3D scaffold by shaping sheet-like aggregates of DSCs with a thermos-responsive hydrogel into the human tooth root canal generates pulp-like tissues with rich neovascularization without adding growth factors[111]. Furthermore, transplantation of human DSCs with 3D hydroxyapatite scaffolds containing peptide hydrogels resulted in vascular ingrowth, osteodentin deposition, and pulp tissue formation in immunocompromised mice[112]. Using bioengineered methods, it has been shown that it is possible to achieve functional teeth with entire roots[113]. The nanofiber hydrogel PuraMatrix is a synthetic matrix that is used to create a biocompatible, biodegradable, and non-toxic 3D environment for a variety of cells[114]. DSCs injected with PuraMatrix into full-length human root canals differentiate into functional odontoblasts; pointing to a novel strategy to facilitate root formation in damaged teeth[115]. Several in vitro and in vivo studies revealed that the addition of various signaling molecules and growth factors [such as granulocyte colony-stimulating factor (G-CSF), stromal cell-derived factor (SDF), basic fibroblast growth factor (bFGF), and VEGF] to different scaffolds (both natural and synthetic) enhances the regeneration of intra-canal pulp-like tissues via the promotion of dentine formation, mineralization, neovascularization, and innervation[116]. DSCs transplanted with SDF-1 or G-CSF on a collagen scaffold promote pulp reconstruction in an animal pulpitis model[91,117]. Autologous DSCs transplanted into a root canal with collagen types I and III associated with SDF-1 after pulpectomy in dogs significantly increased the expression of angiogenic and neurotrophic factors, indicating the potent trophic effects of the combined scaffold and chemokine on neo-vascularization during pulp regeneration[91]. In addition, DSCs seeded into peptide hydrogel loaded with FGF-1, TGF-β1, and VEGF differentiated into odontoblasts-like cells and formed a vascularized dental pulp-like tissue inside the dentin cylinder[118]. Moreover, TGF-β2 increased the odontogenic differentiation of DSCs isolated from the apical papilla[119]. DSCs isolated from adult human tooth pulp and seeded on the surfaces of 3D collagen gel cylinders exhibited significantly increased cellular recruitment when applied with SDF-1α, bFGF, or BMP-7[120]. Encapsulating TGF-β1 and FGF-2 in a biodegradable polymer of lactide and glycolide microspheres provides the controlled release of growth factors to human pulp cells[121]. Furthermore, scaffold composition plays a key role in determining whether the application of signaling molecules or growth factors is needed. Various growth factors, such as SDF, FGF, TGF-β1, VEGF, and BMP were loaded on different scaffolds, such as peptide hydrogel, collagen, gelatin hydrogel, and alginate hydrogel, to enhance endodontic regeneration of DSCs[122]. In addition, a silk fibroin scaffold loaded with bFGF has been described as a promising scaffold for the proliferation and differentiation of DSCs in vitro[123]. Implantation of DSCs with poly-ε-caprolactone and hydroxyapatite in association with SDF-1 and BMP-7 generated tooth-like structures (putative periodontal ligament and new bone formation) in the mandibular incisor extraction socket[124]. In addition to SDF-1, stem cell factor (SCF), a potent chemokine, enhances the mobilization and trafficking of stem cells[125]. SCF promoted neovascularization and new collagen fiber formation after subcutaneous implantation of DSCs with a collagen sponge scaffold in mice. Furthermore, SCF improved DSCs migration, proliferation, and chemotaxis in vitro, possibly via the upregulation of ERK and AKT phosphorylation[126].
Sialoprotein is a dominant non-collagenous protein in dentin, which plays a role in the induction of dental pulp cell differentiation into odontoblast-like cells and is essential for dental pulp stem cell identity and fate[127,128]. Subcutaneous implantation of DSCs seeded on a 3D scaffold containing an acellular ECM embedded in a collagen/chitosan scaffold led to the production of dental pulp-like tissue and the expression of dentin sialoprotein in nude mice[129]. The application of DCSs combined with treated dentin matrix, a biological scaffold, has been suggested as a suitable therapeutic approach for the reconstruction of the tooth root[130]. Immortalized DSCs exhibited potent odontogenic differentiation ability and secreted dentin sialophosphoprotein when seeded in a beta-tricalcium phosphate scaffold and BMP-2 in nude mice[131]. Among three different scaffolds (DL-lactide, co-polymer of L-lactide and DL-lactide, and hydroxyapatite tricalcium phosphate), a copolymer of L-lactide and DL-lactide showed the highest odontogenic regenerative capacity after the addition of DSCs and BMP-2[132].
A few studies have indicated that using a co-culture of DSCs with other stem cells improves neovascularization. The co-culture of DSCs and human umbilical vein endothelial cells with gelatin methacrylate xenogeneic hydrogel resulted in the neovascularization of mouse dental pulp[133]. Transplantation of DSCs and human umbilical vein endothelial cells with VEGF seeded into PuraMatrix significantly enhanced vascularization and mineralization of mouse vascularized pulp-like tissue and osteodentin[134]. In addition, using silk fibroin scaffolds promoted the ability of human DSCs in attracting vessels, which leads to the improvement of healing and regeneration of damaged tissues[135]. Transplantation of DSCs with a tooth fragment/silk fibroin scaffold loaded with SDF-1 resulted in the generation of pulp-like tissues with vascularity, organized fibrous matrix formation, and dentin formation in nude mice[136].
An enormous number of studies have been carried out to investigate the role of various scaffolds on the bone regeneration capacity of DSCs[137]. The osteogenic differentiation ability of DSCs, mostly isolated from dental pulp or periodontal ligament, has been well demonstrated in both in vivo and in vitro studies[138]. DSCs originating from dental pulp, dental follicle, gingival tissue, and periodontal ligament exert different osteogenic capacity[139], which can be modulated by various types of biomaterial scaffolds[140]. For instance, an in vivo investigation has shown that DSCs from the periodontal ligament encapsulated in an arginine-glycine-aspartic acid tripeptide scaffold exhibit a greater ability to repair bone defects by promoting the formation of mineralized tissue compared to gingival MSCs[141]. In addition, DSCs derived from the dental pulp exhibit great neovascularization potential while differentiating into osteoblasts, which subsequently promote bone restoration[142].
The most common scaffolds used to seed DSCs (particularly isolated from human dental pulp or exfoliated deciduous teeth) for bone tissue engineering in both experimental studies and clinical trials are collagen sponge membrane and hydroxyapatite/tri-calcium phosphate granules ceramic[143]. DSCs seeded in collagen sponge scaffolds exhibit strong restoration ability in human mandible bone defects[144]. The application of DSCs seeded onto a collagen-polyvinylpyrrolidone sponge scaffold in the left lower premolar region of a patient with periodontal disease increased bone density and decreased tooth mobility, periodontal pocket depth, and the bone defect area[145]. Using DSCs with a hydroxyapatite-collagen sponge scaffold to fill the alveolar defect in 6 patients with cleft lip and palate resulted in satisfactory bone regeneration[146]. A three-year clinical study revealed that the bone tissue regenerated following the application of human DSCs seeded on collagen scaffolds was uniformly vascularized and compact[147]. However, this study revealed that the new bone developed at the implantation sites was compact and different from the normal spongy alveolar bone in the mandibles[147]. In contrast, no ectopic bone formation was observed when DSCs were seeded on hydroxyapatite–tri-calcium phosphate scaffolds[148]. Furthermore, it should be noted that for any successful cell trans-plantation approach, the optimal number of DSCs is essential. It has been demonstrated that dense culture conditions improve the mineralized nodule formation of DSCs and promote osteogenic-lineage commitment, possibly via the integrin signaling pathway[149]. DSCs seeded in dense collagen gel scaffolds exert a higher beneficial effect on the craniofacial bone healing process compared to acellular scaffolds[150].
DSCs isolated from the dental follicle and the periapical papilla have been considered for the regeneration of alveolar bone and were successfully assessed in a few preclinical pilot studies. The application of dissociative dental pulp with a collagen sponge scaffold in patients with deep intrabony defects due to chronic periodontitis led to the effective restoration of defects with significant stability of the gingival margin[151]. In addition, the application of DSCs seeded onto collagen sponge in the deep intrabony defects of 29 patients suffering from chronic periodontitis significantly improved clinical outcomes of the periodontal regeneration process[152]. Another clinical trial has shown that using DSCs in combination with the collagen sponge scaffold in 6 patients resulted in a well-differentiated bone with Haversian system formation in the tooth extraction site[153].
Scaffold composition and surface properties play a key role in the osteogenic differentiation of DSCs and the process of bone tissue regeneration[142,154]. Significantly greater mineralization occurred when DSCs were seeded into a collagen type I matrix[155]. Furthermore, DSCs seeded on hyaluronic acid, fibrin, and polyesteramide type-C exhibit higher mineralization compared to standard tissue culture polystyrene[156].
Ceramic scaffolds, such as tri-calcium phosphate, hydroxyapatite, bioactive glass biphasic calcium phosphate, and calcium silicate, have chemical and structural similarities to the native bone and are commonly used as scaffolds to enhance bone regeneration and restoration of DSCs[157]. The addition of tricalcium phosphate to the composition of the other scaffolds enhances the differentiation of DSCs into osteoblast-like cells[158]. Chitosan/gelatin scaffolds significantly increased DSCs viability and differentiation as well as the formation of hydroxyapatite-rich nanocrystalline calcium phosphate in immunocompromised mice, particularly when cells were pre-treated with recombinant human BMP-2[92]. Potent bone formation was observed in the defect area of rat femoral bone after application of DSCs seeded in bioactive glass nanoparticles/chitosan-gelatin bionanocomposite compared to mesoporous bioactive glass nanospheres[159]. A combination of poly-ε-caprolactone biphasic calcium phosphate with DSCs increased the newly formed bone regeneration of calvarial defects in rabbit models[160]. Furthermore, a combination of poly-lactic-co-glycolic acid with ceramics is usually used to enhance biomimetic potential and promote bone regeneration[161]. An in vitro study has revealed that human dental pulp SCs adhesion and proliferation, as well as their differentiation toward the osteogenic lineage, are significantly improved when seeded in hydroxyapatite (a member of the calcium phosphate-based bioceramics) and poly-lactide-co-glycolide[162]. Implantation of human DSCs seeded in beta-tri-calcium phosphate scaffolds exerted an anti-inflammatory effect and restored periodontal hard tissue defects[163]. Greater bone regeneration was also reported when human DSCs were seeded on poly-lactic-co-glycolic acid[164] and α- calcium sulfate hemihydrate/amorphous calcium phosphate[165] scaffolds. Calcium phosphate cement functionalized with iron oxide nanoparticles also exhibits a potent effect on the spreading, osteogenic differentiation, and bone mineral synthesis of DSCs, possibly via activation of the extracellular signal-related kinases WNT/β-catenin pathway[166].
Different forms of silicon, particularly the orthosilicic acid form, promote osteoblast proliferation and differentiation, the mineralization process, and collagen production through enhancement of the precipitation of apatite from calcium and phosphate-containing solutions[167]. Semicarbazide-treated porous silicon exerted an appropriate scaffold for DSCs adhesion and in vivo cell therapy, whereas silanization with aminopropyltriethoxysilane-treated porous silicon has been suggested as a favorable scaffold for a long-term in vitro culture system for DSCs proliferation and differentiation[168]. Intrafibrillar-silicified collagen scaffolds markedly improved the proliferation, osteogenic differentiation, and mineralization capacity of human DSCs compared to non-silicified collagen scaffolds[169]. A novel biocompatible nano-engineered osteoinductive and elastomeric scaffold fabricated from a porous nanocomposite of poly-glycerol sebacate and nanosilicates enhanced the physical integrity and mechanical strength of the cellular microenvironment for in vitro osteogenic differentiation and bone regeneration without persistent scaffold-related inflammation in vivo[170].
Hybrid composites are also used as promising biomaterials for bone regeneration. It has been suggested that four different scaffold materials, including porous hydroxyapatite alone or combined with three polymers polylactic-co-glycolic acid, alginate, and ethylene vinylacetate/ethylene vinylversatate, are suitable for DSCs osteogenic differentiation[171]. Electrospun nano-ECM nanofibers with fluorapatite scaffolds enhance the growth, differentiation, and mineralization of DSCs[172], possibly mediated via modulation of the FGF and VEGF signaling pathways[173]. Comparing the behavior of DSCs seeded on a 2D monolayer culture or 3D poly lactic-co-glycolic scaffold, it has been shown that DSCs exerted proper adherence and enhanced osteogenic differentiation on the 3D scaffold cultures[174]. Furthermore, it has been suggested that DSCs seeded in hydrogel scaffolds have greater potential for odontogenic differentiation than cells embedded in collagen-I hydrogel scaffolds[175]. Various layer-by-layer-modified gelatin sponge scaffolds increased the adhesion and proliferation of DSCs and enhanced their potential for bone tissue regeneration[176].
Several differentiation factors, such as BMP, were used to potentiate DSCs bone formation capacity. A 3D gel-based heparin-conjugated collagen matrix combined with recombinant human BMP-2 improved DSCs differentiation and seeding efficiency in vitro and promoted the osteogenic differentiation of these stem cells to form ectopic bone formation in a rat model[177]. Exfoliated human DSCs significantly increased the expression of BMP-2 and 7, bone and cartilage formation markers, when seeded in carbonate apatite scaffold in an in vivo alveolar bone remodeling model in rats[178]. DSCs isolated from human gingival tissues seeded onto 3D poly-lactide scaffolds enriched with extracellular vesicles, small membrane vesicles containing various bioactive molecules, exhibited potent osteogenic inductivity in vitro and showed a marked improvement in bone healing of rat calvaria bone tissue in vivo[179]. On the other hand, some biomaterial scaffolds may facilitate biomolecule-induced tissue formation. For instance, 3D matrigel scaffold enriched with DSCs led to enhanced BMP-9-induced osteogenesis and mineralization in ectopic bones in nude mice[180].
Although the majority of studies rely on the application of DSCs alone, several studies have employed co-culture systems (DSCs in combination with other cells) intending to promote bone regeneration, particularly in 3D scaffolds[181]. Human DSCs and amniotic fluid stem cells seeded onto fibroin scaffolds resulted in pronounced bone repair associated with neovascularization in critical-size rat cranial bone defects[182]. The co-cultured constructs of DSCs and endothelial cells seeded in 3D polycaprolactone blended with poly-L/D-lactide revealed a significantly higher up-regulation of genes related to osteogenesis and angiogenesis[183].
DSCs derived from dental pulp and oral mucosa display high expression of various neural crest-related and developmental genes[184]. DSCs can be differentiated into the neuron-, Schwann-, glia-, and oligodendrocyte-like cells[185]. Due to the high proliferative capacity and propensity to differentiate into neural stem cells, DSCs are attractive candidates for developing a human neuronal lineage for the treatment of various disorders[186]. The role of DSCs in cell transplantation therapy of traumatic and hypoxic-ischemic injuries of the central or peripheral nervous system as well as neurodegenerative diseases has been extensively investigated[187]. DSCs are promising sources for cellular transplantation-based therapeutic strategies for neurological disorders[188]. The seeding of DCSs into different scaffolds promotes cell viability and differentiation towards neuronal-like cells[189,190]. Scaffolds can be designed to provide biological growth factors for neuronal tissues and to accurately adjust the diffusion rate of these essential biomolecules and enzymes[187].
The application of combined DSCs with various scaffolds promotes the function of injured neural tissues and reduces the inflammatory responses. The most common scaffolds applied for neural tissue regeneration and repair include chitosan, heparin-poloxamer, silicone tubes, poly-ε-caprolactone/poly-lactide-co-glycolic acid, and electrospun neuro-supportive scaffolds[191]. Different scaffolds were used to enhance neural differentiation and promote their neuronal characteristics. DSCs can be seeded in the biodegradable electrospun neuro-supportive scaffold, which is amended by different 3D coatings, for enhanced in vitro and in vivo recovery of neuronal damage[192]. The granular 3D chitosan scaffolds provide an appropriate microenvironment for attachment, proliferation, and neural differentiation of DSCs[193]. Furthermore, a 3D floating sphere culture system has been shown to provide a suitable micromilieu for human DSCs to retain their neuronal characteristics compared to myogenic and osteogenic properties[194]. Using an acellular ECM scaffold has been shown to promote DSCs to obtain a neuronal-like organization, including a central body associated with long cytoplasmic extensions that follow the underlying fibers, with high cell-matrix adhesion properties[195]. Some scaffolds can support the neurotrophic release of DSCs for the subsequent survival and differentiation of neural stem cells as well as neural cells. For instance, DSCs promoted the survival and differentiation of adult murine neural stem cells on ethyl acrylate and hydroxyethyl acrylate copolymer scaffold through the enhancement of neurotrophic factor secretion[196].
Transplantation of DSCs with a chitosan scaffold markedly enhanced the recovery of motor function and suppressed inflammatory responses, possibly via the secretion of neurotrophic factors, such as glial cell-derived neurotrophic factor and brain-derived neurotrophic factor, in experimental models of spinal cord injury. Furthermore, the combination of DSCs with scaffolds inhibited cell injury and death through the reduction of caspase activity[197]. A significant functional recovery of hind-limb locomotor activities has also been observed following the transplantation of DSCs seeded in chitosan scaffolds in a spinal cord injury animal model[198]. Solubilized forms of acellular ECM from dentine, bone, and spinal cord have discrete structural, mechanical, and functional properties. Human DSCs exhibited a strong positive response to spinal cord ECM hydrogels by the greater expression of neural lineage markers. This ECM scaffold markedly enhanced the differentiation of DSCs to a neural lineage; indicating the importance of site-specific tissues in the promotion of stem cell behavior for constructive spinal cord regeneration[24]. A combination of DSCs with heparin-poloxamer, a desirable thermosensitive hydrogel for in vivo applications, loaded with various growth factors, such as bFGF and nerve growth factor (NGF), markedly promoted functional recovery, cellular regeneration, and tissue repair in a rat model of spinal cord injury[187], possibly via modulation of the MAPK/ERK, PI3K/Akt and JAK/STAT3 signaling pathways[199]. Indeed, both bFGF and NGF play an essential role in the neural differentiation of DSCs[200]. Chitosan scaffolds in combination with bFGF exerted a synergistic facilitating effect on DSCs differentiation to neural cells, possibly via activation of the ERK signaling pathway[201]. It has been shown that DSCs can proliferate efficiently on an aligned electrospun poly ε-caprolactone/poly lactide-co-glycolic acid scaffold and restore defects in rat spinal cord. Furthermore, these cells contribute to remyelination by the expression of oligodendrogenic lineage markers[202].
Multiple studies have assessed the effects of DSCs with various scaffolds on various experimental models of peripheral nerve injury. DSCs seeded into a polylactic-glycolic acid scaffold significantly improved the regeneration of injured facial nerve and promoted functional recovery compared to autografts[203]. Schwann-like cells derived from DSCs and grown in collagen scaffolds facilitated axonal outgrowth and myelination in both 2D and 3D in vitro models of peripheral nerve injury[204,205]. Furthermore, oligodendrocyte progenitor cells induced by differentiation of human DSCs via gene transfection in combination with collagen or collagen and fibrin scaffolds improved axonal outgrowth and myelination in an animal sciatic nerve injury model[9,206]. Human DSCs isolated from the periodontal ligament and gingival tissues and encapsulated in 3D alginate/hyaluronic acid scaffolds in the presence of NGF improved the proliferation and differentiation of DSCs toward the formation of neural tissues[207]. DSCs seeded on poly-lactic-co-glycolic acid collagen enhanced the interconnections of injured axons in a model of facial nerve injury[203]. When cultured under either 2D- or 3D-collagen scaffolds, human DSCs originating from gingival tissue have shown a greater capability of differentiating into neurons and Schwann-like cells in a 3D collagen scaffold compared to the 2D culture system. Furthermore, these cells with a 3D scaffold improved regeneration and functional recovery of neural tissues in rat facial nerve defects[208]. It has been shown that collagen scaffolds in the presence of different growth factors, such as bFGF, exhibited favorable mechanical properties and improved facial nerve regeneration[209]. Human DSCs expressing STRO-1, c-Kit, and CD34 markers and seeded in a collagen scaffold engrafted into rat sciatic nerve defects improved axonal regeneration from proximal to distal stumps[210]. Interestingly, the administration of serum-free conditioned medium from DSCs plunged in a collagen sponge into the gap caused by rat facial nerve transection, induced axonal regrowth and restored the neurological deficits[211].
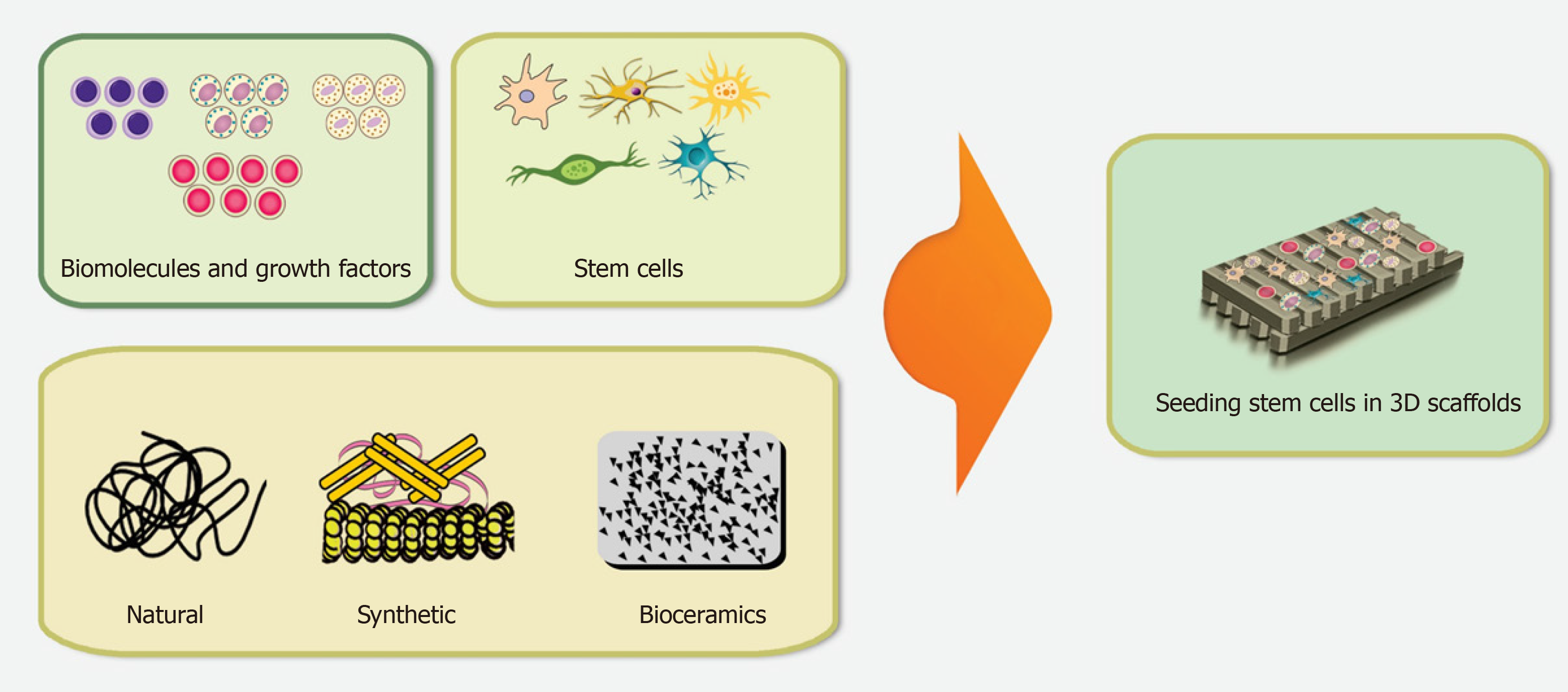
Using an appropriate scaffold can promote the proliferation, differentiation, migration, and functional capacity of DSCs and can optimize and preserve the cellular morphology to build tissue constructs for a specific purpose[63]. Although the application of DSCs alone could yield promising outcomes in cell replacement therapy in particular conditions[212], an appropriate scaffold provides a viable microenvironment to boost the development of DSCs towards new tissue formation, especially in tissues or organs with extensive defects[213]. The optimal number of DSCs is essential to develop tissue and organ substitutes and to restore organ function[149]. In addition to establishing definitive protocols for DSCs preparation, appropriate carrier scaffolds play a crucial role to increase the number of cells for implantation (Figure 3)[214].
A crucial and challenging demand for an appropriate scaffold design is recapitulating the dynamic nature of the native tissue[215]. Although each polymer scaffold has its pros and cons and favorable tissue engineering applications, collagen and fibrin, alone or by forming hybrid scaffolds, provide an adequate pulp connective tissue formation associated with marked vascularization, particularly when loaded with active biomolecules[117,118,216]. Collagen is the main component of the ECM and is expressed widely in bone, teeth, and the brain. A collagen scaffold provides excellent biocompatibility and controllable biodegradability, particularly for bone tissue engineering[217,218]. However, collagen has poor mechanical, chemical, and thermal stability and degrades fast at an uncontrolled rate. Fibrin is a non-toxic biomaterial scaffold that can attach various biological surfaces to regenerate tissues, such as bone and nervous tissues, with a low inflammatory response[219]. However, low mechanical stiffness of fibrin scaffolds limits tissue diffusion and direct implantation of cells to the damaged tissues[220,221]. Different bioceramic scaffolds exhibit excellent biocompatibility and osteoconductivity due to their chemical and structural similarity to native bone, which is characterized by high mechanical stiffness and low elasticity[158,222]. Furthermore, bioceramic scaffolds improve stem cell differentiation and osteogenesis[222]. The main disadvantages of bioceramic scaffolds are brittleness and slow biodegradation in the crystalline phase[218]. Soft polymers with highly aqueous hydrogels, such as collagen, share a resemblance to neural tissues, play an important role as a possible internal filler for neural conduits and increase the quality of peripheral nerve regeneration[223].
Furthermore, the scaffold should be porous and spongy to be able to deliver sufficient DSCs to injured tissues and to allow the stream of ECM and the formation of neovascularization[165,166]. However, some of the currently available biomaterials do not fully imitate the essential functions of natural ECM and fail to provide an appropriate scaffold[224]. Among the different biomaterials, the self-assembly of monodisperse cells into 2D or 3D complex structures that produce more extracellular matrix and promote intercellular communication possess the characteristics of the ideal approach[225]. Although both 2D and 3D cell culture systems provide appropriate methods for stem cell replacement transplantation, 3D systems seem to be more effective at mimicking the ECM in native tissues[226,227]. In general, 3D culture systems have been shown to be more beneficial in providing a template for the reconstruction of defects and cell-to-cell interactions as well as for improving cell adhesion, proliferation, ECM generation, maintenance of cell polarity, and restoration of various tissues[228,229]. In addition, 3D scaffolds enhance the sensitivity of stem cells towards drugs and biomolecules[230]. The optimization of 3D scaffold pore sizes may lead to better tissue regeneration through the enhancement of mechanical strength[231]. The dimension of the defect is a key factor in selecting a scaffold for tissue or organ regeneration[232]. For instance, in the reconstruction of cleft lip and palate, the amount of bone formation may not be enough to fill the bone defect[233], a problem that may be solved by the application of 3D cell culture systems[234]. In this regard, higher osteogenic differentiation of DSCs and MSCs has been observed in 3D than in 2D cell culture[174,235].
In addition to an appropriate scaffold, using bioactive molecules, such as growth and angiogenic factors, has been suggested as a promising strategy for the improvement of DSCs transplantation. Bioactive molecules, such as VEGF, have a short half-life and need to be encapsulated in degradable materials to regulate their release and promote their effects[236]. Scaffolds provide a purposeful approach for better incorporation between stem cells and biomolecules to improve tissue regeneration[26]. The interpolation of active biomolecules with the scaffold is essential for their transport into the injured tissues and for their efficacy to promote the colonization of DSCs and their matrix deposition[123]. DSCs and various scaffolds transplanted together with bioactive molecules, such as G-CSF, BMP, and bFGF, can fill the entire pulp or bone defect as well as develop new dentin or bone formation[92,93,118]. Biomolecules, such as SDF-1, SCF, and G-CSF, help to summon DSCs and enhance the number of cells in the implantation site, and other factors, such as VEGF, can enhance the formation of new blood vessels in regenerative tissues[26,237].
On the contrary, a few investigations have suggested that transplantation of DSCs without scaffold may have more beneficial effects on tissue regeneration. To prevent the inflammatory response, immune rejection, or infections, a few studies have indicated that transplantation of stem cells without scaffolds (such as 3D stem cell spheroids) may be an alternative option for DSCs transplantation[238]. Transplantation of DSCs without a scaffold for injured tooth tissues in 26 patients led to the regeneration of 3D pulp tissue which contained blood vessels and sensory nerves 12 months after therapy[239]. Despite these studies, it seems that the simple injection of competent DSCs inside organ defects is poorly regenerative[123].
A few decades of intense basic studies and clinical trials on DSCs are essential to translate knowledge gained on these cells into the implementation of defined and reproducible therapeutic approaches to cure or alleviate diseases. In addition to the application of an ideal scaffold, the success of cell transplantation therapy using DSCs also relied greatly on designing methodologies for isolation and purification, a sufficient number of stem cells, and effective and safe differentiation into different lineages[240,241]. The development of an accurate immunomodulatory strategy for injectable and implantable biomaterials is of particular importance to facilitate the grafting of DSCs at inflamed sites[242,243].
This study describes the main scaffolds, both natural and synthetic, used in DSCs transplantation and evaluated the advantages and disadvantages of various types of scaffolds. Most of the existing studies concerning the development of novel therapeutic approaches for restoration of damaged tissues have been limited to in vitro and in vivo DSCs testing, with a small number of clinical trials. Although the co-application of biomolecules with an appropriate scaffold seems to be crucial for effective cell transplantation therapy with DSCs, there is still much to learn about the dynamics of these molecules as well as their interactions with the ECM and DSCs to allow planning of appropriate therapeutic approaches. Further advances in tissue engineering need to focus on innovative combinations of biopolymers and biomolecules to promote the capability of DSCs for novel and effective therapeutic approaches (Figure 3).
| 1. | Caplan AI. Are All Adult Stem Cells The Same? Regen Eng Transl Med. 2015;1:4-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Khorraminejad-Shirazi M, Dorvash M, Estedlal A, Hoveidaei AH, Mazloomrezaei M, Mosaddeghi P. Aging: A cell source limiting factor in tissue engineering. World J Stem Cells. 2019;11:787-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Liu G, David BT, Trawczynski M, Fessler RG. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev Rep. 2020;16:3-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 338] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 4. | Xu M, He J, Zhang C, Xu J, Wang Y. Strategies for derivation of endothelial lineages from human stem cells. Stem Cell Res Ther. 2019;10:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:6940283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 6. | Morsczeck C, Reichert TE. Dental stem cells in tooth regeneration and repair in the future. Expert Opin Biol Ther. 2018;18:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Ghasemi S, Aligholi H, Koulivand PH, Jafarian M, Hosseini Ravandi H, Khaleghi Ghadiri M, Gorji A. Generation of motor neurons from human amygdala-derived neural stem-like cells. Iran J Basic Med Sci. 2018;21:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012;2012:461718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 943] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 10. | Lee MW, Ryu S, Kim DS, Lee JW, Sung KW, Koo HH, Yoo KH. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia. 2019;33:597-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 12. | Brown PT, Handorf AM, Jeon WB, Li WJ. Stem cell-based tissue engineering approaches for musculoskeletal regeneration. Curr Pharm Des. 2013;19:3429-3445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Sharpe PT. Dental mesenchymal stem cells. Development. 2016;143:2273-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1140] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 16. | Volponi AA, Sharpe PT. The tooth -- a treasure chest of stem cells. Br Dent J. 2013;215:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Grawish ME. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J Stem Cells. 2018;10:116-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1368] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 20. | Yang X, Li L, Xiao L, Zhang D. Recycle the dental fairy's package: overview of dental pulp stem cells. Stem Cell Res Ther. 2018;9:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig. 2013;17:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 868] [Cited by in RCA: 819] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 23. | Wang W, Dang M, Zhang Z, Hu J, Eyster TW, Ni L, Ma PX. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016;36:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Viswanath A, Vanacker J, Germain L, Leprince JG, Diogenes A, Shakesheff KM, White LJ, des Rieux A. Extracellular matrix-derived hydrogels for dental stem cell delivery. J Biomed Mater Res A. 2017;105:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen LY, Liu SY, Chen L, Ding Y, Xuan K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012;57:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 26. | Bakhtiar H, Mazidi S A, Mohammadi Asl S, Ellini MR, Moshiri A, Nekoofar MH, Dummer PMH. The role of stem cell therapy in regeneration of dentine-pulp complex: a systematic review. Prog Biomater. 2018;7:249-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 722] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 28. | Rodríguez-Lozano FJ, Bueno C, Insausti CL, Meseguer L, Ramírez MC, Blanquer M, Marín N, Martínez S, Moraleda JM. Mesenchymal stem cells derived from dental tissues. Int Endod J. 2011;44:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Pisciotta A, Bertoni L, Vallarola A, Bertani G, Mecugni D, Carnevale G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen Res. 2020;15:373-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 918] [Article Influence: 45.9] [Reference Citation Analysis (1)] |
| 31. | Jeon BG, Kang EJ, Kumar BM, Maeng GH, Ock SA, Kwack DO, Park BW, Rho GJ. Comparative analysis of telomere length, telomerase and reverse transcriptase activity in human dental stem cells. Cell Transplant. 2011;20:1693-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy. 2011;13:1205-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Li Z, Jiang CM, An S, Cheng Q, Huang YF, Wang YT, Gou YC, Xiao L, Yu WJ, Wang J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Andrukhov O, Behm C, Blufstein A, Rausch-Fan X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J Stem Cells. 2019;11:604-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (5)] |
| 35. | Shin C, Kim M, Han JA, Choi B, Hwang D, Do Y, Yun JH. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J Periodontal Res. 2017;52:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 633] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 37. | Sahab-Negah S, Hajali V, Moradi HR, Gorji A. The Impact of Estradiol on Neurogenesis and Cognitive Functions in Alzheimer's Disease. Cell Mol Neurobiol. 2020;40:283-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1071] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 39. | Mitsiadis TA, Feki A, Papaccio G, Catón J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv Dent Res. 2011;23:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17 Suppl 4:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 954] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 41. | Yi S, Ding F, Gong L, Gu X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr Stem Cell Res Ther. 2017;12:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2770] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 43. | Marrelli M, Codispoti B, Shelton RM, Scheven BA, Cooper PR, Tatullo M, Paduano F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front Physiol. 2018;9:1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 44. | Larmas M. Pre-odontoblasts, odontoblasts, or "odontocytes". J Dent Res. 2008;87:198; author reply 199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Goldberg M, Six N, Chaussain C, DenBesten P, Veis A, Poliard A. Dentin extracellular matrix molecules implanted into exposed pulps generate reparative dentin: a novel strategy in regenerative dentistry. J Dent Res. 2009;88:396-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Kawashima N, Okiji T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom (Kyoto). 2016;56:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 47. | Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1043] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 48. | Machado CV, Passos ST, Campos TM, Bernardi L, Vilas-Bôas DS, Nör JE, Telles PD, Nascimento IL. The dental pulp stem cell niche based on aldehyde dehydrogenase 1 expression. Int Endod J. 2016;49:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Stokowski A, Shi S, Sun T, Bartold PM, Koblar SA, Gronthos S. EphB/ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem Cells. 2007;25:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Wan PX, Wang BW, Wang ZC. Importance of the stem cell microenvironment for ophthalmological cell-based therapy. World J Stem Cells. 2015;7:448-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Bonaventura G, Incontro S, Iemmolo R, La Cognata V, Barbagallo I, Costanzo E, Barcellona ML, Pellitteri R, Cavallaro S. Dental mesenchymal stem cells and neuro-regeneration: a focus on spinal cord injury. Cell Tissue Res. 2020;379:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. Methods Mol Biol. 2014;1210:91-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 54. | Onizuka S, Iwata T. Application of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets for Periodontal Regeneration. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Spagnuolo G, Codispoti B, Marrelli M, Rengo C, Rengo S, Tatullo M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent J (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544-7556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell-niche engineering. J Clin Invest. 2010;120:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 58. | Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 59. | Wilems T, Vardhan S, Wu S, Sakiyama-Elbert S. The influence of microenvironment and extracellular matrix molecules in driving neural stem cell fate within biomaterials. Brain Res Bull. 2019;148:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Conde MC, Chisini LA, Demarco FF, Nör JE, Casagrande L, Tarquinio SB. Stem cell-based pulp tissue engineering: variables enrolled in translation from the bench to the bedside, a systematic review of literature. Int Endod J. 2016;49:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Kanjevac T, Gustafson C, Ivanovska A, Ravanetti F, Cacchioli A, Bosnakovski D. Inflammatory Cytokines and Biodegradable Scaffolds in Dental Mesenchymal Stem Cells Priming. Curr Stem Cell Res Ther. 2019;14:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | La Noce M, Paino F, Spina A, Naddeo P, Montella R, Desiderio V, De Rosa A, Papaccio G, Tirino V, Laino L. Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. J Dent. 2014;42:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 63. | Tsutsui TW. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning. 2020;13:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 64. | He Y, Lu F. Development of Synthetic and Natural Materials for Tissue Engineering Applications Using Adipose Stem Cells. Stem Cells Int. 2016;2016:5786257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Sano H, Orbay H, Terashi H, Hyakusoku H, Ogawa R. Acellular adipose matrix as a natural scaffold for tissue engineering. J Plast Reconstr Aesthet Surg. 2014;67:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Monteiro BG, Loureiro RR, Cristovam PC, Covre JL, Gomes JÁP, Kerkis I. Amniotic membrane as a biological scaffold for dental pulp stem cell transplantation in ocular surface reconstruction. Arq Bras Oftalmol. 2019;82:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 67. | Edgar L, McNamara K, Wong T, Tamburrini R, Katari R, Orlando G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials (Basel). 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Willerth SM, Sakiyama-Elbert SE. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. Stem J. 2008;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Alaribe FN, Manoto SL, Motaung SCKM. Scaffolds from biomaterials: advantages and limitations in bone and tissue engineering. Biologia. 2016;71:353-366. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Zarrintaj P, Bakhshandeh B, Saeb MR, Sefat F, Rezaeian I, Ganjali MR, Ramakrishna S, Mozafari M. Oligoaniline-based conductive biomaterials for tissue engineering. Acta Biomater. 2018;72:16-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | FitzGerald JF, Kumar AS. Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons? Clin Colon Rectal Surg. 2014;27:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 72. | Wang C, Feng N, Chang F, Wang J, Yuan B, Cheng Y, Liu H, Yu J, Zou J, Ding J, Chen X. Injectable Cholesterol-Enhanced Stereocomplex Polylactide Thermogel Loading Chondrocytes for Optimized Cartilage Regeneration. Adv Healthc Mater. 2019;8:e1900312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 73. | Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1140] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 74. | Choe G, Park J, Park H, Lee JY. Hydrogel Biomaterials for Stem Cell Microencapsulation. Polymers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 75. | Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111:441-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 732] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 76. | Sahab Negah S, Oliazadeh P, Jahanbazi Jahan-Abad A, Eshaghabadi A, Samini F, Ghasemi S, Asghari A, Gorji A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019;92:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | Sahab Negah S, Khooei A, Samini F, Gorji A. Laminin-derived Ile-Lys-Val-ala-Val: a promising bioactive peptide in neural tissue engineering in traumatic brain injury. Cell Tissue Res. 2018;371:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Janik H, Marzec M. A review: fabrication of porous polyurethane scaffolds. Mater Sci Eng C Mater Biol Appl. 2015;48:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 79. | Raeisdasteh Hokmabad V, Davaran S, Ramazani A, Salehi R. Design and fabrication of porous biodegradable scaffolds: a strategy for tissue engineering. J Biomater Sci Polym Ed. 2017;28:1797-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 80. | Mantha S, Pillai S, Khayambashi P, Upadhyay A, Zhang Y, Tao O, Pham HM, Tran SD. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 81. | Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 659] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 82. | Meng X, Leslie P, Zhang Y, Dong J. Stem cells in a three-dimensional scaffold environment. Springerplus. 2014;3:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 84. | Galler KM, Eidt A, Schmalz G. Cell-free approaches for dental pulp tissue engineering. J Endod. 2014;40:S41-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Alsberg E, von Recum HA, Mahoney MJ. Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin Biol Ther. 2006;6:847-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Demarco FF, Casagrande L, Zhang Z, Dong Z, Tarquinio SB, Zeitlin BD, Shi S, Smith AJ, Nör JE. Effects of morphogen and scaffold porogen on the differentiation of dental pulp stem cells. J Endod. 2010;36:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Chatakun P, Núñez-Toldrà R, Díaz López EJ, Gil-Recio C, Martínez-Sarrà E, Hernández-Alfaro F, Ferrés-Padró E, Giner-Tarrida L, Atari M. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: a current review of the literature. Cell Mol Life Sci. 2014;71:113-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Zhang J, Lu X, Feng G, Gu Z, Sun Y, Bao G, Xu G, Lu Y, Chen J, Xu L, Feng X, Cui Z. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell Tissue Res. 2016;366:129-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 89. | Jazayeri HE, Lee SM, Kuhn L, Fahimipour F, Tahriri M, Tayebi L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent Mater. 2020;36:e47-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 90. | Zhang L, Morsi Y, Wang Y, Li Y, Ramakrishna S. Review scaffold design and stem cells for tooth regeneration. Jpn Dent Sci Rev. 2013;49:14-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 92. | Bakopoulou A, Georgopoulou Grivas I, Bekiari C, Prymak O, Loza Î, Epple M, Papadopoulos GC, Koidis P, Chatzinikolaidou Î. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent Mater. 2019;35:310-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Xu WP, Zhang W, Asrican R, Kim HJ, Kaplan DL, Yelick PC. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng Part A. 2008;14:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 94. | Hori Y, Winans AM, Irvine DJ. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009;5:969-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 95. | Ahmadian E, Eftekhari A, Dizaj SM, Sharifi S, Mokhtarpour M, Nasibova AN, Khalilov R, Samiei M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int J Biol Macromol. 2019;140:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 96. | Wang X, Li G, Liu Y, Yu W, Sun Q. Biocompatibility of biological material polylactic acid with stem cells from human exfoliated deciduous teeth. Biomed Rep. 2017;6:519-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, Giannobile WV. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31:5945-5952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 98. | Maspero FA, Ruffieux K, Müller B, Wintermantel E. Resorbable defect analog PLGA scaffolds using CO2 as solvent: structural characterization. J Biomed Mater Res. 2002;62:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2010;93:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Liu HC, E LL, Wang DS, Su F, Wu X, Shi ZP, Lv Y, Wang JZ. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Tissue Eng Part A. 2011;17:2417-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 101. | Lluch AV, Fernández AC, Ferrer GG, Pradas MM. Bioactive scaffolds mimicking natural dentin structure. J Biomed Mater Res B Appl Biomater. 2009;90:182-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 103. | Bento LW, Zhang Z, Imai A, Nör F, Dong Z, Shi S, Araujo FB, Nör JE. Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res. 2013;92:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Huang W, Shi X, Ren L, Du C, Wang Y. PHBV microspheres--PLGA matrix composite scaffold for bone tissue engineering. Biomaterials. 2010;31:4278-4285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Huang CC, Narayanan R, Warshawsky N, Ravindran S. Dual ECM Biomimetic Scaffolds for Dental Pulp Regenerative Applications. Front Physiol. 2018;9:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Kim NR, Lee DH, Chung PH, Yang HC. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Qu T, Liu X. Nano-Structured Gelatin/Bioactive Glass Hybrid Scaffolds for the Enhancement of Odontogenic Differentiation of Human Dental Pulp Stem Cells. J Mater Chem B. 2013;1:4764-4772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 108. | Hu L, Gao Z, Xu J, Zhu Z, Fan Z, Zhang C, Wang J, Wang S. Decellularized Swine Dental Pulp as a Bioscaffold for Pulp Regeneration. Biomed Res Int. 2017;2017:9342714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 109. | Na S, Zhang H, Huang F, Wang W, Ding Y, Li D, Jin Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J Tissue Eng Regen Med. 2016;10:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 110. | Liu M, Zhao L, Hu J, Wang L, Li N, Wu D, Shi X, Yuan M, Hu W, Wang X. Endothelial cells and endothelin-1 promote the odontogenic differentiation of dental pulp stem cells. Mol Med Rep. 2018;18:893-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 111. | Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res. 2018;97:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 112. | Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, Wolfs E, Bronckaers A, Hilkens P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J Endod. 2017;43:S12-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 113. | Oshima M, Tsuji T. Functional tooth regenerative therapy: tooth tissue regeneration and whole-tooth replacement. Odontology. 2014;102:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Jahanbazi Jahan-Abad A, Sahab Negah S, Hosseini Ravandi H, Ghasemi S, Borhani-Haghighi M, Stummer W, Gorji A, Khaleghi Ghadiri M. Human Neural Stem/Progenitor Cells Derived From Epileptic Human Brain in a Self-Assembling Peptide Nanoscaffold Improve Traumatic Brain Injury in Rats. Mol Neurobiol. 2018;55:9122-9138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 115. | Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013;92:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 116. | Eramo S, Natali A, Pinna R, Milia E. Dental pulp regeneration via cell homing. Int Endod J. 2018;51:405-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 117. | Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, Utunomiya S, Nakamura H, Matsushita K, Nakashima M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med. 2013;2:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 118. | Galler KM, D'Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, Schmalz G. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 119. | Yu S, Li J, Zhao Y, Li X, Ge L. Comparative Secretome Analysis of Mesenchymal Stem Cells From Dental Apical Papilla and Bone Marrow During Early Odonto/Osteogenic Differentiation: Potential Role of Transforming Growth Factor-β2. Front Physiol. 2020;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Suzuki T, Lee CH, Chen M, Zhao W, Fu SY, Qi JJ, Chotkowski G, Eisig SB, Wong A, Mao JJ. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J Dent Res. 2011;90:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 121. | Mathieu S, Jeanneau C, Sheibat-Othman N, Kalaji N, Fessi H, About I. Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J Endod. 2013;39:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Zein N, Harmouch E, Lutz JC, Fernandez De Grado G, Kuchler-Bopp S, Clauss F, Offner D, Hua G, Benkirane-Jessel N, Fioretti F. Polymer-Based Instructive Scaffolds for Endodontic Regeneration. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 123. | Yang JW, Zhang YF, Sun ZY, Song GT, Chen Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J Biomater Appl. 2015;30:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 125. | Swenson ES, Kuwahara R, Krause DS, Theise ND. Physiological variations of stem cell factor and stromal-derived factor-1 in murine models of liver injury and regeneration. Liver Int. 2008;28:308-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 126. | Pan S, Dangaria S, Gopinathan G, Yan X, Lu X, Kolokythas A, Niu Y, Luan X. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling - potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev Rep. 2013;9:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Guo S, Lim D, Dong Z, Saunders TL, Ma PX, Marcelo CL, Ritchie HH. Dentin sialophosphoprotein: a regulatory protein for dental pulp stem cell identity and fate. Stem Cells Dev. 2014;23:2883-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Ritchie H. The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein. Int J Oral Sci. 2018;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 129. | Ravindran S, Zhang Y, Huang CC, George A. Odontogenic induction of dental stem cells by extracellular matrix-inspired three-dimensional scaffold. Tissue Eng Part A. 2014;20:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 130. | Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, Wang H, Zheng X, Long J, Tang W, Guo W, Tian W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix - based scaffold. Biomaterials. 2012;33:2449-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 131. | Li X, Wang L, Su Q, Ye L, Zhou X, Song D, Huang D. Highly Proliferative Immortalized Human Dental Pulp Cells Retain the Odontogenic Phenotype when Combined with a Beta-Tricalcium Phosphate Scaffold and BMP2. Stem Cells Int. 2020;2020:4534128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Atalayin C, Tezel H, Dagci T, Karabay Yavasoglu NU, Oktem G, Kose T. In vivo performance of different scaffolds for dental pulp stem cells induced for odontogenic differentiation. Braz Oral Res. 2016;30:e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 133. | Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A, Yelick PC. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J Dent Res. 2017;96:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 134. | Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A. 2015;21:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 135. | Woloszyk A, Buschmann J, Waschkies C, Stadlinger B, Mitsiadis TA. Human Dental Pulp Stem Cells and Gingival Fibroblasts Seeded into Silk Fibroin Scaffolds Have the Same Ability in Attracting Vessels. Front Physiol. 2016;7:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 136. | Yang JW, Zhang YF, Wan CY, Sun ZY, Nie S, Jian SJ, Zhang L, Song GT, Chen Z. Autophagy in SDF-1α-mediated DPSC migration and pulp regeneration. Biomaterials. 2015;44:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 137. | Ercal P, Pekozer GG. A Current Overview of Scaffold-Based Bone Regeneration Strategies with Dental Stem Cells. Adv Exp Med Biol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Monterubbianesi R, Bencun M, Pagella P, Woloszyk A, Orsini G, Mitsiadis TA. A comparative in vitro study of the osteogenic and adipogenic potential of human dental pulp stem cells, gingival fibroblasts and foreskin fibroblasts. Sci Rep. 2019;9:1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 139. | Zhang Y, Xing Y, Jia L, Ji Y, Zhao B, Wen Y, Xu X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018;27:1634-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 140. | Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, Shi S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101:3285-3294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 141. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 779] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 142. | Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, Shi S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A. 2014;20:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 143. | Leyendecker Junior A, Gomes Pinheiro CC, Lazzaretti Fernandes T, Franco Bueno D. The use of human dental pulp stem cells for in vivo bone tissue engineering: A systematic review. J Tissue Eng. 2018;9:2041731417752766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 144. | d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 145. | Hernández-Monjaraz B, Santiago-Osorio E, Ledesma-Martínez E, Alcauter-Zavala A, Mendoza-Núñez VM. Retrieval of a periodontally compromised tooth by allogeneic grafting of mesenchymal stem cells from dental pulp: A case report. J Int Med Res. 2018;46:2983-2993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | Tanikawa DYS, Pinheiro CCG, Almeida MCA, Oliveira CRGCM, Coudry RA, Rocha DL, Bueno DF. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients. Stem Cells Int. 2020;2020:6234167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 147. | Giuliani A, Manescu A, Langer M, Rustichelli F, Desiderio V, Paino F, De Rosa A, Laino L, d'Aquino R, Tirino V, Papaccio G. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med. 2013;2:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 148. | Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A. 2008;14:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 149. | Noda S, Kawashima N, Yamamoto M, Hashimoto K, Nara K, Sekiya I, Okiji T. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci Rep. 2019;9:5430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 150. | Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, Llorens A, Nicoletti A, Letourneur D, Colombier ML, Nazhat SN, Bouchard P, Chaussain C, Rochefort GY. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6:38814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (2)] |
| 151. | Aimetti M, Ferrarotti F, Gamba MN, Giraudi M, Romano F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int J Periodontics Restorative Dent. 2018;38:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 152. | Ferrarotti F, Romano F, Gamba MN, Quirico A, Giraudi M, Audagna M, Aimetti M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J Clin Periodontol. 2018;45:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 153. | Monti M, Graziano A, Rizzo S, Perotti C, Del Fante C, d'Aquino R, Redi CA, Rodriguez Y Baena R. In Vitro and In Vivo Differentiation of Progenitor Stem Cells Obtained After Mechanical Digestion of Human Dental Pulp. J Cell Physiol. 2017;232:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 154. | Graziano A, d'Aquino R, Laino G, Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 155. | Fang TJ, Wang DH, Wang CY, Poongodi R, Liou NH, Liu JC, Hsu ML, Hong PD, Yang SF, Liu ML. Osteogenic prospective of deriving human dental stem cells in collagen matrix boost. J Mater Sci Mater Med. 2017;28:192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 156. | Apel C, Buttler P, Salber J, Dhanasingh A, Neuss S. Differential mineralization of human dental pulp stem cells on diverse polymers. Biomed Tech (Berl). 2018;63:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 157. | Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017;78:1246-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 758] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 158. | Liao SS, Cui FZ. In vitro and in vivo degradation of mineralized collagen-based composite scaffold: nanohydroxyapatite/collagen/poly(L-lactide). Tissue Eng. 2004;10:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Covarrubias C, Cádiz M, Maureira M, Celhay I, Cuadra F, von Marttens A. Bionanocomposite scaffolds based on chitosan-gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J Biomater Appl. 2018;32:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 160. | Wongsupa N, Nuntanaranont T, Kamolmattayakul S, Thuaksuban N. Assessment of bone regeneration of a tissue-engineered bone complex using human dental pulp stem cells/poly(ε-caprolactone)-biphasic calcium phosphate scaffold constructs in rabbit calvarial defects. J Mater Sci Mater Med. 2017;28:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 161. | Pan Z, Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 162. | Jiménez NT, Carlos Munévar J, González JM, Infante C, Lara SJP. In vitro response of dental pulp stem cells in 3D scaffolds: A regenerative bone material. Heliyon. 2018;4:e00775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 163. | Li Y, Zhao S, Nan X, Wei H, Shi J, Li A, Gou J. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res Ther. 2016;7:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 164. | Xavier Acasigua GA, Bernardi L, Braghirolli DI, Filho MS, Pranke P, Medeiros Fossati AC. Nanofiber scaffolds support bone regeneration associated with pulp stem cells. Curr Stem Cell Res Ther. 2014;9:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Kuo TF, Lee SY, Wu HD, Poma M, Wu YW, Yang JC. An in vivo swine study for xeno-grafts of calcium sulfate-based bone grafts with human dental pulp stem cells (hDPSCs). Mater Sci Eng C Mater Biol Appl. 2015;50:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 166. | Xia Y, Guo Y, Yang Z, Chen H, Ren K, Weir MD, Chow LC, Reynolds MA, Zhang F, Gu N, Xu HHK. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater Sci Eng C Mater Biol Appl. 2019;104:109955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 167. | Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 552] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 168. | Collart-Dutilleul PY, Secret E, Panayotov I, Deville de Périère D, Martín-Palma RJ, Torres-Costa V, Martin M, Gergely C, Durand JO, Cunin F, Cuisinier FJ. Adhesion and proliferation of human mesenchymal stem cells from dental pulp on porous silicon scaffolds. ACS Appl Mater Interfaces. 2014;6:1719-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 169. | Niu LN, Sun JQ, Li QH, Jiao K, Shen LJ, Wu D, Tay F, Chen JH. Intrafibrillar-silicified collagen scaffolds enhance the osteogenic capacity of human dental pulp stem cells. J Dent. 2014;42:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Kerativitayanan P, Tatullo M, Khariton M, Joshi P, Perniconi B, Gaharwar AK. Nanoengineered osteoinductive and elastomeric scaffolds for bone tissue engineering. ACS Biomater Sci Eng. 2017;3:590-600. [RCA] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 171. | Karadzic I, Vucic V, Jokanovic V, Debeljak-Martacic J, Markovic D, Petrovic S, Glibetic M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2015;103:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | Guo T, Li Y, Cao G, Zhang Z, Chang S, Czajka-Jakubowska A, Nör JE, Clarkson BH, Liu J. Fluorapatite-modified scaffold on dental pulp stem cell mineralization. J Dent Res. 2014;93:1290-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 173. | Clark D, Wang X, Chang S, Czajka-Jakubowska A, Clarkson BH, Liu J. VEGF promotes osteogenic differentiation of ASCs on ordered fluorapatite surfaces. J Biomed Mater Res A. 2015;103:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 174. | Jafar H, Abuarqoub D, Ababneh N, Hasan M, Al-Sotari S, Aslam N, Kailani M, Ammoush M, Shraideh Z, Awidi A. hPL promotes osteogenic differentiation of stem cells in 3D scaffolds. PLoS One. 2019;14:e0215667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 175. | Paduano F, Marrelli M, White LJ, Shakesheff KM, Tatullo M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type I. PLoS One. 2016;11:e0148225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 176. | Fu Q, Ren H, Zheng C, Zhuang C, Wu T, Qin J, Wang Z, Chen Y, Qi N. Improved osteogenic differentiation of human dental pulp stem cells in a layer-by-layer-modified gelatin scaffold. J Biomater Appl. 2018;33:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 177. | Fahimipour F, Dashtimoghadam E, Mahdi Hasani-Sadrabadi M, Vargas J, Vashaee D, Lobner DC, Jafarzadeh Kashi TS, Ghasemzadeh B, Tayebi L. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent Mater. 2019;35:990-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 178. | Prahasanti C, Nugraha AP, Saskianti T, Suardita K, Riawan W, Ernawati DS. Exfoliated Human Deciduous Tooth Stem Cells Incorporating Carbonate Apatite Scaffold Enhance BMP-2, BMP-7 and Attenuate MMP-8 Expression During Initial Alveolar Bone Remodeling in Wistar Rats (Rattus norvegicus). Clin Cosmet Investig Dent. 2020;12:79-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 179. | Diomede F, D'Aurora M, Gugliandolo A, Merciaro I, Ettorre V, Bramanti A, Piattelli A, Gatta V, Mazzon E, Fontana A, Trubiani O. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int J Nanomedicine. 2018;13:3805-3825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 180. | Fu T, Liang P, Song J, Wang J, Zhou P, Tang Y, Li J, Huang E. Matrigel Scaffolding Enhances BMP9-induced Bone Formation in Dental Follicle Stem/Precursor Cells. Int J Med Sci. 2019;16:567-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 181. | Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14:15-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 557] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 182. | Riccio M, Maraldi T, Pisciotta A, La Sala GB, Ferrari A, Bruzzesi G, Motta A, Migliaresi C, De Pol A. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng Part A. 2012;18:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 183. | Jin GZ, Kim HW. Co-culture of Human Dental Pulp Stem Cells and Endothelial Cells Using Porous Biopolymer Microcarriers: A Feasibility Study for Bone Tissue Engineering. Tissue Eng Regen Med. 2017;14:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 184. | Abe S, Yamaguchi S, Sato Y, Harada K. Sphere-Derived Multipotent Progenitor Cells Obtained From Human Oral Mucosa Are Enriched in Neural Crest Cells. Stem Cells Transl Med. 2016;5:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 185. | Anitua E, Troya M, Zalduendo M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy. 2018;20:479-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 186. | Tatullo M, Codispoti B, Pacifici A, Palmieri F, Marrelli M, Pacifici L, Paduano F. Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications. Front Cell Dev Biol. 2017;5:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 187. | Luo L, He Y, Wang X, Key B, Lee BH, Li H, Ye Q. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int. 2018;2018:1731289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 188. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 189. | Chang CC, Chang KC, Tsai SJ, Chang HH, Lin CP. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formos Med Assoc. 2014;113:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 190. | Mayo V, Sawatari Y, Huang CY, Garcia-Godoy F. Neural crest-derived dental stem cells--where we are and where we are going. J Dent. 2014;42:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 191. | Wang D, Wang Y, Tian W, Pan J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019;52:e12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 192. | Das S, Bellare JR. Dental Pulp Stem Cells in Customized 3D Nanofibrous Scaffolds for Regeneration of Peripheral Nervous System. Methods Mol Biol. 2020;2125:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 193. | Feng X, Lu X, Huang D, Xing J, Feng G, Jin G, Yi X, Li L, Lu Y, Nie D, Chen X, Zhang L, Gu Z, Zhang X. 3D porous chitosan scaffolds suit survival and neural differentiation of dental pulp stem cells. Cell Mol Neurobiol. 2014;34:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 194. | Pisciotta A, Bertoni L, Riccio M, Mapelli J, Bigiani A, La Noce M, Orciani M, de Pol A, Carnevale G. Use of a 3D Floating Sphere Culture System to Maintain the Neural Crest-Related Properties of Human Dental Pulp Stem Cells. Front Physiol. 2018;9:547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 195. | Laudani S, La Cognata V, Iemmolo R, Bonaventura G, Villaggio G, Saccone S, Barcellona ML, Cavallaro S, Sinatra F. Effect of a Bone Marrow-Derived Extracellular Matrix on Cell Adhesion and Neural Induction of Dental Pulp Stem Cells. Front Cell Dev Biol. 2020;8:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 196. | Soria JM, Sancho-Tello M, Esparza MA, Mirabet V, Bagan JV, Monleón M, Carda C. Biomaterials coated by dental pulp cells as substrate for neural stem cell differentiation. J Biomed Mater Res A. 2011;97:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 197. | Zhang F, Song J, Zhang H, Huang E, Song D, Tollemar V, Wang J, Wang J, Mohammed M, Wei Q, Fan J, Liao J, Zou Y, Liu F, Hu X, Qu X, Chen L, Yu X, Luu HH, Lee MJ, He TC, Ji P. Wnt and BMP Signaling Crosstalk in Regulating Dental Stem Cells: Implications in Dental Tissue Engineering. Genes Dis. 2016;3:263-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 198. | Jung J, Kim JW, Moon HJ, Hong JY, Hyun JK. Characterization of Neurogenic Potential of Dental Pulp Stem Cells Cultured in Xeno/Serum-Free Condition: In Vitro and In Vivo Assessment. Stem Cells Int. 2016;2016:6921097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 199. | Li R, Li Y, Wu Y, Zhao Y, Chen H, Yuan Y, Xu K, Zhang H, Lu Y, Wang J, Li X, Jia X, Xiao J. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials. 2018;168:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 200. | Zhang J, Lian M, Cao P, Bao G, Xu G, Sun Y, Wang L, Chen J, Wang Y, Feng G, Cui Z. Effects of Nerve Growth Factor and Basic Fibroblast Growth Factor Promote Human Dental Pulp Stem Cells to Neural Differentiation. Neurochem Res. 2017;42:1015-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 201. | Zheng K, Feng G, Zhang J, Xing J, Huang D, Lian M, Zhang W, Wu W, Hu Y, Lu X, Feng X. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int J Neurosci. 2020;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 202. | Li X, Yang C, Li L, Xiong J, Xie L, Yang B, Yu M, Feng L, Jiang Z, Guo W, Tian W. A therapeutic strategy for spinal cord defect: human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed Res Int. 2015;2015:197183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 203. | Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Ogiuchi H, Okano T, Ando T. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med. 2011;5:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 204. | Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 205. | Sanen K, Martens W, Georgiou M, Ameloot M, Lambrichts I, Phillips J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? J Tissue Eng Regen Med. 2017;11:3362-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 206. | Askari N, Yaghoobi MM, Shamsara M, Esmaeili-Mahani S. Tetracycline-regulated expression of OLIG2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. Neuroscience. 2015;305:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 207. | Ansari S, Diniz IM, Chen C, Sarrion P, Tamayol A, Wu BM, Moshaverinia A. Human Periodontal Ligament- and Gingiva-derived Mesenchymal Stem Cells Promote Nerve Regeneration When Encapsulated in Alginate/Hyaluronic Acid 3D Scaffold. Adv Healthc Mater. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 208. | Zhang Q, Nguyen PD, Shi S, Burrell JC, Cullen DK, Le AD. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep. 2018;8:6634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 209. | Cui Y, Lu C, Meng D, Xiao Z, Hou X, Ding W, Kou D, Yao Y, Chen B, Zhang Z, Li J, Pan J, Dai J. Collagen scaffolds modified with CNTF and bFGF promote facial nerve regeneration in minipigs. Biomaterials. 2014;35:7819-7827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 210. | Carnevale G, Pisciotta A, Riccio M, Bertoni L, De Biasi S, Gibellini L, Zordani A, Cavallini GM, La Sala GB, Bruzzesi G, Ferrari A, Cossarizza A, de Pol A. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J Tissue Eng Regen Med. 2018;12:e774-e785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 211. | Kano F, Matsubara K, Ueda M, Hibi H, Yamamoto A. Secreted Ectodomain of Sialic Acid-Binding Ig-Like Lectin-9 and Monocyte Chemoattractant Protein-1 Synergistically Regenerate Transected Rat Peripheral Nerves by Altering Macrophage Polarity. Stem Cells. 2017;35:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 212. | Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6268] [Cited by in RCA: 5914] [Article Influence: 369.6] [Reference Citation Analysis (1)] |
| 213. | Amghar-Maach S, Gay-Escoda C, Sánchez-Garcés MÁ. Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J Clin Exp Dent. 2019;11:e373-e381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 214. | Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry--Part II: Clinical applications. J Prosthodont Res. 2012;56:229-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 215. | Ahadian S, Khademhosseini A. Smart scaffolds in tissue regeneration. Regen Biomater. 2018;5:125-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 216. | Khan R, Khan MH. Use of collagen as a biomaterial: An update. J Indian Soc Periodontol. 2013;17:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 217. | Thavornyutikarn B, Chantarapanich N, Sitthiseripratip K, Thouas GA, Chen Q. Bone tissue engineering scaffolding: computer-aided scaffolding techniques. Prog Biomater. 2014;3:61-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 218. | Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 641] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 219. | Jockenhoevel S, Zund G, Hoerstrup SP, Chalabi K, Sachweh JS, Demircan L, Messmer BJ, Turina M. Fibrin gel -- advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 220. | Aper T, Teebken OE, Steinhoff G, Haverich A. Use of a fibrin preparation in the engineering of a vascular graft model. Eur J Vasc Endovasc Surg. 2004;28:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 221. | Chocholata P, Kulda V, Babuska V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 337] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 222. | Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4029] [Cited by in RCA: 3484] [Article Influence: 139.4] [Reference Citation Analysis (0)] |
| 223. | Boni R, Ali A, Shavandi A, Clarkson AN. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci. 2018;25:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 224. | Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 225. | Geckil H, Xu F, Zhang X, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond). 2010;5:469-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 673] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 226. | Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1262] [Article Influence: 84.1] [Reference Citation Analysis (1)] |
| 227. | Ito Y, Tanabe Y, Sugawara K, Koshino M, Takahashi T, Tanigaki K, Aoki H, Chen M. Three-dimensional porous graphene networks expand graphene-based electronic device applications. Phys Chem Chem Phys. 2018;20:6024-6033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 228. | Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: A review. Bioact Mater. 2019;4:271-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 500] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 229. | Jensen C, Teng Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front Mol Biosci. 2020;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 1083] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 230. | Bachmann A, Moll M, Gottwald E, Nies C, Zantl R, Wagner H, Burkhardt B, Sánchez JJ, Ladurner R, Thasler W, Damm G, Nussler AK. 3D Cultivation Techniques for Primary Human Hepatocytes. Microarrays (Basel). 2015;4:64-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 231. | Liu H, Cheng Y, Chen J, Chang F, Wang J, Ding J, Chen X. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 232. | Awadeen MA, Al-Belasy FA, Ameen LE, Helal ME, Grawish ME. Early therapeutic effect of platelet-rich fibrin combined with allogeneic bone marrow-derived stem cells on rats' critical-sized mandibular defects. World J Stem Cells. 2020;12:55-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 233. | Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14:428-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 234. | Rosales-Ibanez R, Mateo NC, Rodriguez-Lorenzo L, Amairany RN, María FS. Potential Benefits from 3D Printing and Dental pulp stem cells in cleft palate treatments: an in vivo model study. Biomed J Sci Tech Res. 2019;16:11950-11953. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 235. | Sankar S, Sharma CS, Rath SN. Enhanced osteodifferentiation of MSC spheroids on patterned electrospun fiber mats - An advanced 3D double strategy for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2019;94:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 236. | Mahon E, Salvati A, Baldelli Bombelli F, Lynch I, Dawson KA. Designing the nanoparticle-biomolecule interface for "targeting and therapeutic delivery". J Control Release. 2012;161:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 237. | Ruangsawasdi N, Zehnder M, Patcas R, Ghayor C, Siegenthaler B, Gjoksi B, Weber FE. Effects of Stem Cell Factor on Cell Homing During Functional Pulp Regeneration in Human Immature Teeth. Tissue Eng Part A. 2017;23:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 238. | Bu NU, Lee HS, Lee BN, Hwang YC, Kim SY, Chang SW, Choi KK, Kim DS, Jang JH. In Vitro Characterization of Dental Pulp Stem Cells Cultured in Two Microsphere-Forming Culture Plates. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 239. | Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 240. | De Luca M, Aiuti A, Cossu G, Parmar M, Pellegrini G, Robey PG. Advances in stem cell research and therapeutic development. Nat Cell Biol. 2019;21:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 241. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1050] [Article Influence: 150.0] [Reference Citation Analysis (35)] |
| 242. | Yu Y, Wu RX, Yin Y, Chen FM. Directing immunomodulation using biomaterials for endogenous regeneration. J Mater Chem B. 2016;4:569-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 243. | Leach DG, Young S, Hartgerink JD. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019;88:15-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding JX, Wakao H S-Editor: Yan JP L-Editor: Webster JR P-Editor: Xing YX