Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.814
Peer-review started: March 15, 2020
First decision: April 7, 2020
Revised: April 23, 2020
Accepted: June 27, 2020
Article in press: June 27, 2020
Published online: August 26, 2020
Processing time: 163 Days and 20 Hours
Mesenchymal stem cells (MSCs) are multipotent stem cells with marked potential for regenerative medicine because of their strong immunosuppressive and regenerative abilities. The therapeutic effects of MSCs are based in part on their secretion of biologically active factors in extracellular vesicles known as exosomes. Exosomes have a diameter of 30-100 nm and mediate intercellular communication and material exchange. MSC-derived exosomes (MSC-Exos) have potential for cell-free therapy for diseases of, for instance, the kidney, liver, heart, nervous system, and musculoskeletal system. Hence, MSC-Exos are an alternative to MSC-based therapy for regenerative medicine. We review MSC-Exos and their therapeutic potential for a variety of diseases and injuries.
Core tip: Mesenchymal stem cell-derived exosomes (MSC-Exos) contain a variety of functional proteins, mRNAs, microRNAs, and signaling lipids. MSC-Exos are more stable than their parent cells and do not have the safety issues of living cells, such as tumorigenesis and occlusion of the microvasculature. MSC-Exos represent an alternative to MSC-based therapies for regenerative medicine. In this review, we summarize the characteristics of MSC-Exos and highlight their functions and therapeutic potential for tissue/organ regeneration and for kidney, liver, cardiovascular, neurological, and musculoskeletal diseases, as well as cutaneous wound healing.
- Citation: Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells 2020; 12(8): 814-840
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/814.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.814
Regenerative medicine, aimed at promoting the repair and regeneration of tissues and organs, is multidisciplinary. It can be understood as the use of biology and tissue engineering to find effective and feasible treatments that promote self-repair and regeneration or the generation of new tissues or organs to maintain, improve, and repair damaged bodies. Stem cell transplantation is the main method for tissue regeneration. Stem cells are immature tissue precursor cells that are capable of self-renewal to form a cloned cell population and, thus, differentiate into multiple cell lineages[1,2]. Stem cells can be classified as (1) Embryonic stem cells derived from early embryos; (2) Induced pluripotent stem cells; and (3) Adult stem cells, including hematopoietic stem cells, neural stem cells, and mesenchymal stem cells (MSCs). The therapeutic potential of stem cells can be attributed to three key mechanisms[3]. The first is homing, the migration of stem cells to the site of injury; the mechanism is thought to be similar to that of leukocyte migration and to involve cell-surface receptors, such as chemotactic receptors. Integrins, vascular cell adhesion molecule 1, and G protein receptor signals are also likely to play important roles in this process. The second is differentiation into diverse cell types, enabling supplementation or replacement of damaged cells[4]. The third is secretion of biologically active factors that affect surrounding tissues. Adult stem cells promote the maintenance and repair of adult tissues and organs[5]. MSCs are one of the most important types of adult stem cell and have been used for cell-based therapy of diverse diseases[6].
MSCs were discovered in 1968 by Friedenstein et al[7], who described them as fibroblasts capable of secreting hematopoietic growth factors and cytokines. Later studies showed that MSCs are ubiquitous and can be isolated from a variety of tissues, including bone marrow, adipose tissue, dental pulp, umbilical cord, umbilical cord blood, placenta, amniotic fluid, Wharton’s jelly, the brain, spleen, liver, kidney, lung, thymus, and pancreas. Moreover, MSCs have self-renewal ability and can differentiate into multiple cell types[8,9]. MSCs can be isolated and expanded from the stroma of many tissues, e.g., bone marrow and subcutaneous adipose tissue[10]. MSCs show promise for cell therapy because of the their ease of isolation, self-renewal and in vitro expansion ability, low immunogenicity, multidirectional differentiation, and release of trophic materials that promote tissue renovation or direct cell replacement[11]. However, the disadvantages of MSCs include the difficulty in producing cells with a stable phenotype, the deleterious effect of the presence of large cells in the pulmonary microvasculature, host cell rejection, ectopic tissue formation, and tumor formation. These disadvantages have restricted their clinical use[12-15]. Thus, alternative MSC-based and complication-free therapeutic strategies are needed. The therapeutic potential of MSCs is determined by their paracrine secretion of a range of growth factors, chemokines, and cytokines[16-18]. Therefore, finding a cell-free therapeutic strategy with the same output and efficacy seems to be necessary.
Research has focused on extracellular vesicles (EVs) secreted by MSCs as a possible non-cellular therapy[19]. MSCs release numerous EVs, including microvesicles (MVs), exosomes, and apoptotic bodies, which may act as paracrine mediators between MSCs and target cells[20]. MVs and exosomes exert a pro-regenerative effect, which is mediated by their protein, mRNA, and regulatory non-coding RNA (e.g., microRNA [miRNA]) contents. Exosomes are the most prominent type of EV and have potential for cell-free therapy because of their biological activities and ability to mediate intercellular communication[21,22]. MSC-derived exosomes (MSC-Exos) replicate the biological activity of MSCs and are thus an alternative to whole-cell therapy[23,24]. In addition, the surface of exosomes can be modified to enable targeting of specific cell types, suggesting their promise for cell-free therapy.
MSC-Exos have potential for tissue engineering and regenerative therapy. In this review, we summarize the characteristics of MSC-Exos and highlight their functions and potential as a novel cell-free strategy for regenerative medicine.
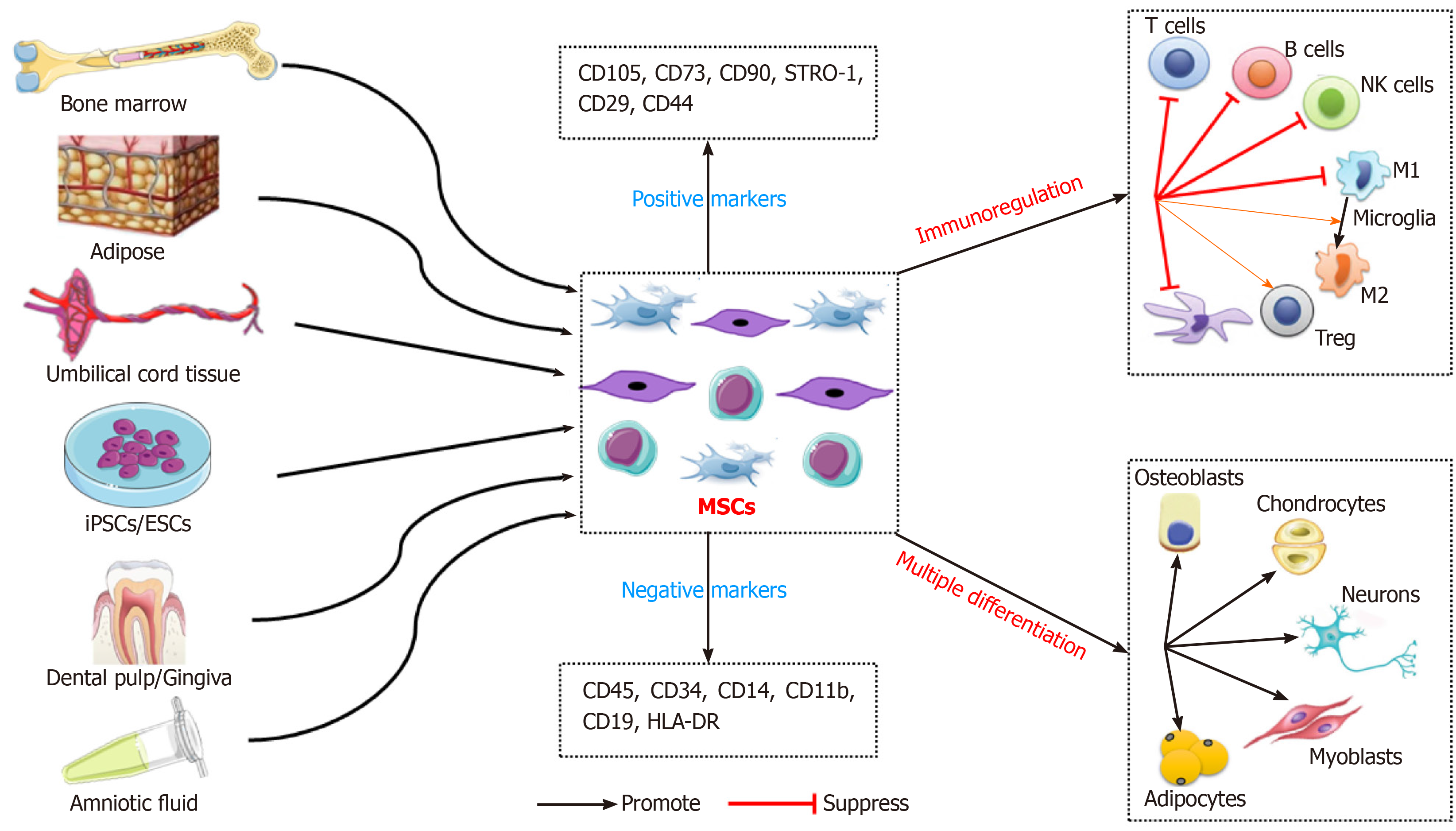
MSCs are an undifferentiated adult stem cell population with self-renewal ability, low immunogenicity, and multilineage differentiation potential. MSCs have plastic adhesion properties and can be easily isolated from a variety of tissues, such as adipose tissue, umbilical cord blood, liver, amniotic fluid, placenta, and dental pulp[8,9]. The International Therapeutic Association of MSCs established the recognition characteristics of human MSCs in 2006. These include maintenance of adherence under standard culture conditions; expression of CD105, CD73, CD90, STRO-1, CD29, and CD44; no expression of CD45, CD34, CD14, CD11b, CD79a, CD19, or HLA-DR; and the ability to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro[25]. The ease of isolation and biological functions of MSCs make them suitable in preclinical and clinical trials of cell therapy (Figure 1).
Multilineage differentiation potential: MSCs can differentiate into multiple mesenchymal (such as osteoblasts, chondrocytes, adipocytes, endothelial cells, and cardiomyocytes) and non-mesenchymal (such as neurons, glial cells, and hepatocytes) lineages. These characteristics make MSCs good seed cells for tissue engineering and regenerative medicine (e.g., bone and cartilage reconstruction, nerve regeneration, and vascular tissue repair)[26].
Promotion of tissue repair: After systemic adoptive transfer, MSCs may occur with lodging in non-specific tissues, homing to natural walls or migration into damaged and/or diseased tissues[27]. MSCs can migrate to injured tissue and release cytokines, inflammatory mediators, extracellular matrix (ECM) components, and antibacterial proteins, thereby generating a suitable microenvironment for tissue repair. MSCs are suitable for repair of tissue injury and treatment of, for instance, diabetes, graft-vs-host, cardiovascular, inflammatory, liver, lung, kidney, nerve, autoimmune, and bone and cartilage diseases[28,29]. In a rat model of lipopolysaccharide (LPS)-induced acute lung injury, allogeneic MSCs transplantation ameliorated the redox environment by upregulating heme oxygenase 1 and protected against lung injury[30]. In a clinical trial, autologous bone-marrow-derived MSCs (BMSCs) ameliorated the motor disability and cognitive impairment in stroke patients[31].
Immunosuppression: The therapeutic effect of MSCs is mainly attributed to their immunoregulatory activity. MSCs exert immunomodulatory and anti-inflammatory effects by regulating lymphocytes associated with the innate and adaptive immune system[32]. MSCs modulate the immune response by inhibiting a wide range of immune cells, including T, B, and natural killer (NK) lymphocytes, and affecting the function of myeloid cells such as monocytes, dendritic cells (DCs), and macrophages[11,33]. Specifically, MSCs inhibit T-cell proliferation, activation, and secretion of inflammatory factors [such as interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon-γ], reduce the Th1/Th2 ratio, and decrease the number of Th17 cells[33]. Also, MSCs induce the generation of regulatory T cells (Tregs), including classic CD4+ CD25+ FoxP3+ Tregs and non-classical Tregs (such as CD8+ CD28- regulatory T cells), and IL-10+ Tr1 cells[34,35]. In addition, MSCs suppress the differentiation of B lymphocytes into plasma cells and their secretion of immunoglobulins[36]. Furthermore, MSCs inhibit the cytotoxicity potential of NK lymphocytes, and promote the transformation of M1 macrophages (pro-inflammatory) to M2 macrophages (anti-inflammatory)[37,38]. MSCs modulate antigen presentation by antigen-presenting cells by downregulating MHC and co-stimulatory molecules (CD40, CD86, and CD80) and suppressing the maturation of DCs[38]. The immunomodulatory properties of MSCs suggest their therapeutic potential for a variety of diseases.
Neuroprotective effect: MSCs transdifferentiate into neural cells and secrete neurotrophic and anti-inflammatory factors following transplantation, thus exerting strong trophic and neuroprotective effects. The therapeutic role of MSCs has been evaluated in preclinical models of neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), Huntington disease, multiple sclerosis (MS), Parkinson disease, and spinal cord injury (SCI)[39]. The neuroprotective effect of MSCs is mediated by production of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor, glial cell line-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 (NT-3)[39,40]. The BDNF and NT-3 released by MSCs act on neural progenitor cells in the lesion, improving neurogenesis[40,41].
MSCs have diverse functions but the underlying mechanisms are unclear. The therapeutic potential of MSCs is based mainly on their immunoregulatory activity and replacement of damaged tissue by differentiating into various cell lineages. It has long been thought that the effect of MSCs on damaged or diseased tissue is based on their immunoregulatory effect[11]. However, the therapeutic benefit of MSCs is attributable not only to their differentiation capacity but also their secretion of soluble factors that exert immunoregulatory, angiogenetic, and ECM remodeling, and anti-apoptotic, anti-fibrotic, and antioxidant effects[8,16]. In this way, MSCs directly or in a paracrine manner rescue damaged cells, reduce tissue damage and, ultimately, accelerate organ repair[42].
Haynesworth et al[42] in 1996 first reported the paracrine effect of MSCs. MSC-derived paracrine factors have been shown to promote angiogenesis, protect against acute renal, liver, and tissue injury, promote neovascularization, and enhance arteriogenesis[5,18,43]. MSCs secrete mediators that directly activate target cells or stimulate neighboring cells to secrete active factors[43]. Interestingly, MSC-derived EVs, including exosomes, exert other paracrine effects on tissue regeneration by transferring information to damaged cells or tissue and have biological activity similar to that of MSCs[19]. Moreover, compared with MSCs, MSC-Exos can cross biological barriers, can be modified to load molecular drugs, have fewer side effects and less immunogenicity, and remain active during storage[44]. Therefore, the regenerative potential of MSC-Exos as cell-free therapy has been evaluated.
Exosomes are one of the main subclasses of EVs, which were discovered in sheep reticulocytes in 1983[45]; the term “exosome” was coined in 1987[46]. Exosomes are small lipid membrane vesicles, which are formed by endocytosis, integration, and efflux. Exosomes are secreted by a wide range of mammalian cell types, including MSCs, B cells, cytotoxic T cells, neurons, cancer cells, oligodendrocytes, platelets, epithelial cells, DCs, and mast cells[47]. Exosomes are present in body fluids such as saliva, blood, bile, urine, semen, cerebrospinal fluid, ascites fluid, amniotic fluid, and colostrum[48]. Morphologically, exosomes are described as cup-shaped or saucer-like when observed by transmission electron microscopy[48,49]. Similar to other lipid vesicles, exosomes float in a sucrose gradient and have a density of 1.13 g/mL (B-cell-derived exosomes) to 1.19 g/mL (intestinal cell-derived exosomes)[49,50]. B-cell exosomes are the most homogeneous in terms of size (60-80 nm)[50].
EVs are classified as exosomes, MVs, or apoptotic bodies, depending on their origin. Exosomes are 30-100 nm in diameter, MVs are 100-1000 nm in diameter, and apoptotic bodies are 1-5 μm in diameter[49,51,52]. There are overlaps in the sizes of EVs, and the lack of standardization is an issue. The major EV subtypes currently recognized, together with their basic characteristics, are summarized in Table 1.
| Feature | Exosomes | Microvesicles | Apoptotic bodies |
| Diameter and shape | 30-100 nm, cup shape | 100-1000 nm, irregular shape | 1-5 μm, heterogeneous shape |
| Sucrose gradient | 1.13-1.19 g/mL | 1.04-1.07 g/mL | 1.18-1.28 g/mL |
| Sedimentation | 100000 g | 10000 g | 16000 g |
| Protein markers | CD63, CD81, CD9, Alix, Tsg101, annexins, heat-shock proteins | Integrins, selectins, CD40, flotillins, CD40, ARF6, VCAMP3 | TSP, C3b, histones |
| Origin | Fusion of multivesicular bodies with cell membrane | Outward budding of cell membrane | Outward budding of apoptotic cell membrane |
| Lipid content | Ceramide | Phosphatidylserine | Phosphatidylserine |
| Nucleic acids | DNA, mRNA, miRNA, non-coding RNA | DNA, mRNA, miRNA, non-coding RNA | Fragmented DNA, mRNA, miRNA, non-coding RNA |
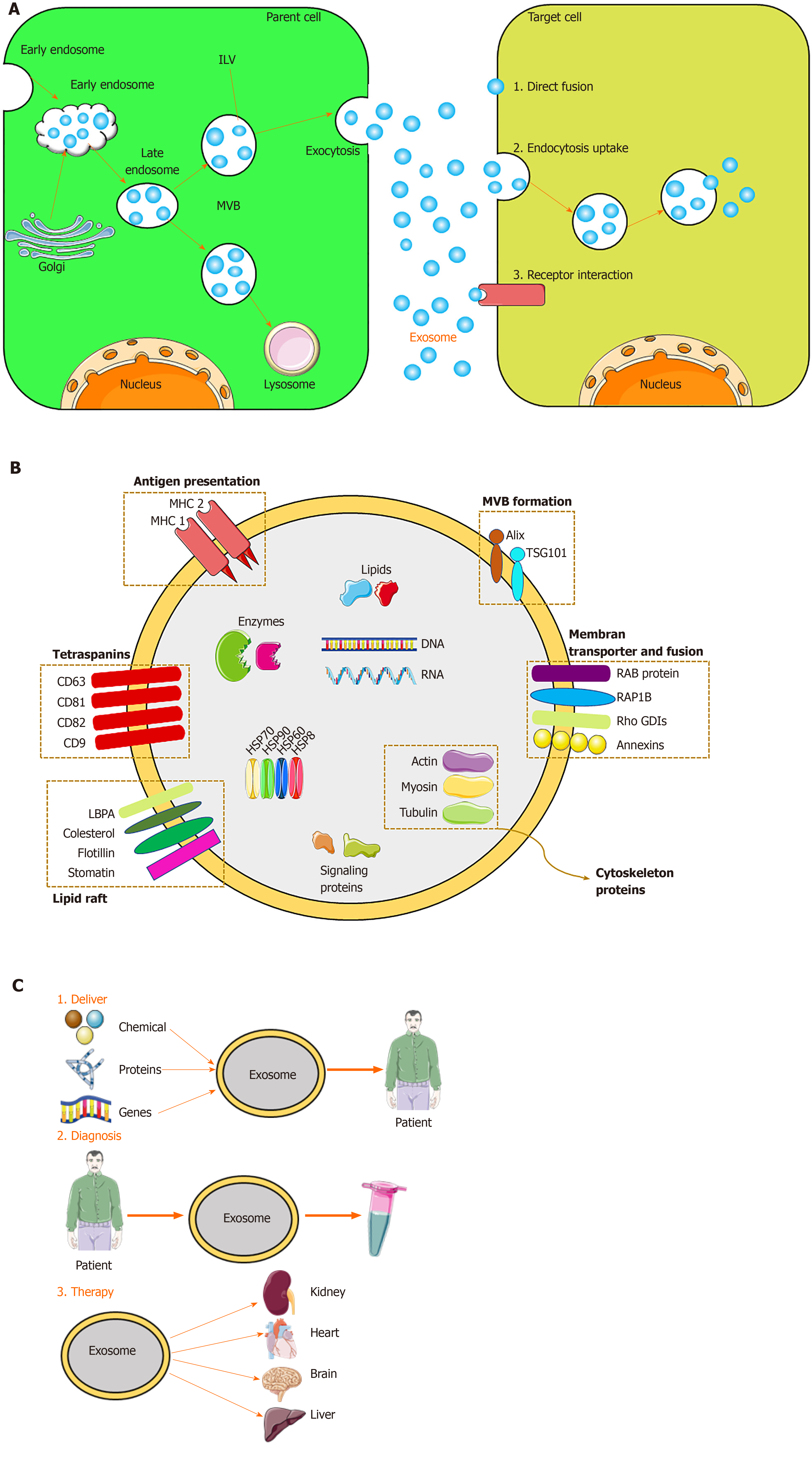
Exosomes originate from the endocytosis-exogenous pathway, while other EVs are derived directly from the plasma membrane. Exosome biogenesis occurs via the endocytosis-ectopic pathway when cells absorb a small amount of intracellular fluid in specific membrane regions and form early endosomes. Those early endosomes begin to mature and expand into late endosomes, which undergo inward germination to form intraluminal vesicles (ILVs) with a diameter of 30 nm to 100 nm. Late endosomes, often referred to as multivesicular bodies (MVBs) due to their inclusion of ILVs, fuse with lysosomes, resulting in degradation of their contents, or fuse with the cell membrane and are released into the extracellular environment – these are defined as exosomes[48,52]. The exosomes are subsequently taken up by recipient cells. Exosomes can be endocytosed or interact with recipient cells through ligand-receptor or direct binding[53] (Figure 2). Although the endosomal-dependent pathway is the main route of exosome biogenesis, direct budding of the plasma membrane can also produce exosomes. Two major MVB and ILV biogenesis pathways have been identified: The endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent pathways (Figure 2). The ESCRT comprises four complexes and their associated proteins, ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, which are involved in identifying ubiquitinated proteins in the endosomal membrane, and budding and separating of the endosomal membrane then modulate the integration process that ultimately produces ILVs[54]. In contrast, the ESCRT-independent pathway integrates cellular content into exosomes via budding of ceramide-induced ILVs[55]. The classification of other proteins is mediated by variations in the normative ESCRT-dependent pathway[56]. In addition, there are other mechanisms in exosome biogenesis, and this finding suggests that ILV formation requires sphingolipid ceramide. Moreover, neutral sphingomyelinase enhances ILV formation by promoting MVB budding[48].
Various exosome separation techniques, including ultracentrifugation-based separation technology, size-based technology, precipitation technology, and immunoaffinity capture, as well as novel combinations of these, are available or under development (Table 2).
| Methods | Mechanism | Advantages | Disadvantages |
| Ultracentrifugation | Physical method | A golden standard; low cost; a wide range of volumes | Low yield; low purity; time-consuming |
| Membrane filtration | Physical method using filters | Simple; fast; high yield; keeps exosomes intact | Low purity; deformation of exosomes |
| Precipitation | Physical/chemical method | High yield; easy; high recoveries | Low purity; contaminants |
| Size exclusion chromatography | Use columns packed with pore beads | High yield; reduces exosome aggregation; keeps exosomes intact | A small number of bands; time-consuming |
| Immunoaffinity capture technology | Magnetic beads bound to specific antibodies | High yield; high purity; specialty | Time-consuming; high cost |
Ultracentrifugation: The method most commonly used to isolate exosomes is ultracentrifugation, frequently in combination with a sucrose density gradient or a sucrose cushion[57]. Cells and larger particles are removed by increasing the centrifugal force, and exosomes are pelleted by centrifugation at ≥ 100000 × g for > 2 h. This method is simple and cost-effective but requires specialized equipment and lacks specificity, so exosomes may be contaminated with other EVs of similar diameter[58].
Membrane filtration: Exosomes can be isolated by membrane filtration[58]. After removing cell debris and macromolecules, the sample is ultrafiltered to remove contaminants. Membrane filtration is rapid and easy to perform. However, it can be difficult to separate exosomes from contaminants, such as apoptotic bodies or vesicles of similar diameter, depending on the pore size of the filter[59].
Precipitation: Polyethylene glycols (PEGs) can be used for precipitation[60]. ExtraPEG was adapted from a PEG-based virus isolation method and can be applied to various vesicle types and biological fluids[61]. PEG-mediated exosome isolation involves low-speed centrifugation followed by a single small-volume filtration purification step. This method is rapid and inexpensive[58], but the exosomes produced are of low purity and the technique is costly[57].
Size exclusion chromatography: Exosome isolation by size exclusion chromatography (SEC) involves a column packed with porous polymeric beads. SEC involves removal of cells and larger particles by low-speed centrifugation, followed by two filtration steps using 0.2 μm pore filters with a 100 kDa molecular weight cut-off and purification by SEC[62]. High yield and no need for specialized equipment are the main advantages of this approach but the exosomes produced have low purity and clogging, vesicle capture, and exosome loss due to membrane attachment can occur[57,58].
Immunoaffinity capture: Immunoaffinity capture of exosomes involves antibodies against exosome markers (including CD81, CD63, or CD9) and specialized lectins targeting mannose[62,63]. This method enables production of exosomes with high purity but is costly, has a low yield, and requires cell-free samples[57].
In addition, exosome isolation kits and precipitation solutions can be used to isolate exosomes. However, there is no one-size-fits-all technique, and it is impractical to separate exosomes completely from other components. Therefore, the most appropriate technique for isolating exosomes should be selected. After isolation, exosomes can be stored at -80 °C.
Exosomes are identified based on their morphology, size, and marker proteins. Methods for identifying exosomes include transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), nanoparticle tracking analysis (NTA), flow cytometry analysis, Western blot, and enzyme-linked immunosorbent assay (ELISA)[47,62]. TEM, SEM, and AFM are used to determine the size and morphology of exosomes; of these, TEM is the most frequently used[64]. NTA is frequently applied to evaluate the size distribution and concentration of exosomes. Flow cytometry and Western blot can be used to identify exosome surface marker proteins[65], for example, CD9, CD63, CD81, and CD82. The proteins in exosomes can be quantified by Bradford or bicinchoninic acid assay or ELISA[65]. Two or three of these methods are often used in combination to analyze exosomes.
Because exosomes are formed by budding from early endosomes, they have a lipid bilayer membrane, which protects the resident genetic material (DNA, mRNA, miRNA, pre-miRNA, and other non-coding RNAs), lipids, and proteins during transportation to target cells[66]. The most common exosomal surface proteins are members of the tetraspanin family, a group of scaffold membrane proteins including CD63, CD81, and CD9, which serve as markers. Other common proteins include membrane transporters and fusion proteins (such as GTPases and annexins), heat shock proteins (such as HSP60, 70, and 90), MVB biogenic proteins (such as ESCRT complex, Alix, and TSG101), lipid-related proteins, and phospholipases[21,67]. The exosome membrane also contains cholesterol, sphingomyelin, and ceramide in a large number of lipid rafts[68]. Exosomes also contain mRNA and miRNA, which, upon endocytosis by the recipient cell, modulate protein synthesis and cell function[68]. The protein, lipid, and nucleic acid contents of exosomes vary according to the identity and physiological condition of the source cell and the extracellular environment. Therefore, the content of exosomes serves as an indicator of their source cell. Unique exosomes containing different proteins and RNAs determine their various subpopulations and therefore exert different effects on recipient cells.
Exosomes have various functions. Depending on their characteristics, exosomes can be used for disease diagnosis, drug delivery, and as therapeutic agents (Figure 2). Exosomes engage in specific interactions with the recipient cells, promoting information and material exchange between widely separated anatomic sites[69]. Because they can cross the blood–brain barrier, exosomes have potential for drug delivery to the brain[70]. The nanometer-scale size and stability of exosomes suggest their diagnostic potential. Finally, exosomes are a cell-free alternative to cellular therapy. Following injection, exosomes are safer and easier to control than live cells, which can undergo uncontrolled growth and tumor formation[24]. The roles of exosomes in immunology and cancer biology have been established[71].
Unlike cells, exosomes do not undergo malignant transformation, do not replicate, and do not induce metastasis. In this review, we focus on the potential of MSC-Exos in regenerative medicine.
MSCs have been isolated from a variety of sources. Because of their ease of isolation, no ethical considerations, and low immunogenicity, MSCs have therapeutic potential for various diseases. However, injection of MSCs may cause malignant transformation and spread of tumors. Also, differentiation of MSCs induces tissue ossification or calcification in animal models. MSC-Exos have the same functions as MSCs, without the above complications.
MSC-Exos were first isolated by Lai et al[72] in 2010, and the purified exosomes reduced the infarct area in a mouse model of myocardial ischemia/reperfusion (I/R) injury. Therefore, exosomes represent novel biological agents for promoting tissue repair. MSCs are the most prolific exosome producers[73], and the exosomes produced by MSCs have similar morphological characteristics, isolation methods, and preservation conditions to those from other cell types. The composition of exosomes depends on their cellular origin. MSC-Exos harbor membrane-bound proteins such as CD44, CD73, and CD29; the surface protein profile of exosomes is dependent on the medium used to culture the source MSCs. mRNAs and miRNAs are encapsulated in MSC-Exos; the miRNAs participate in the exchange of information between cells and modulate the function and fate of the recipient cell[73].
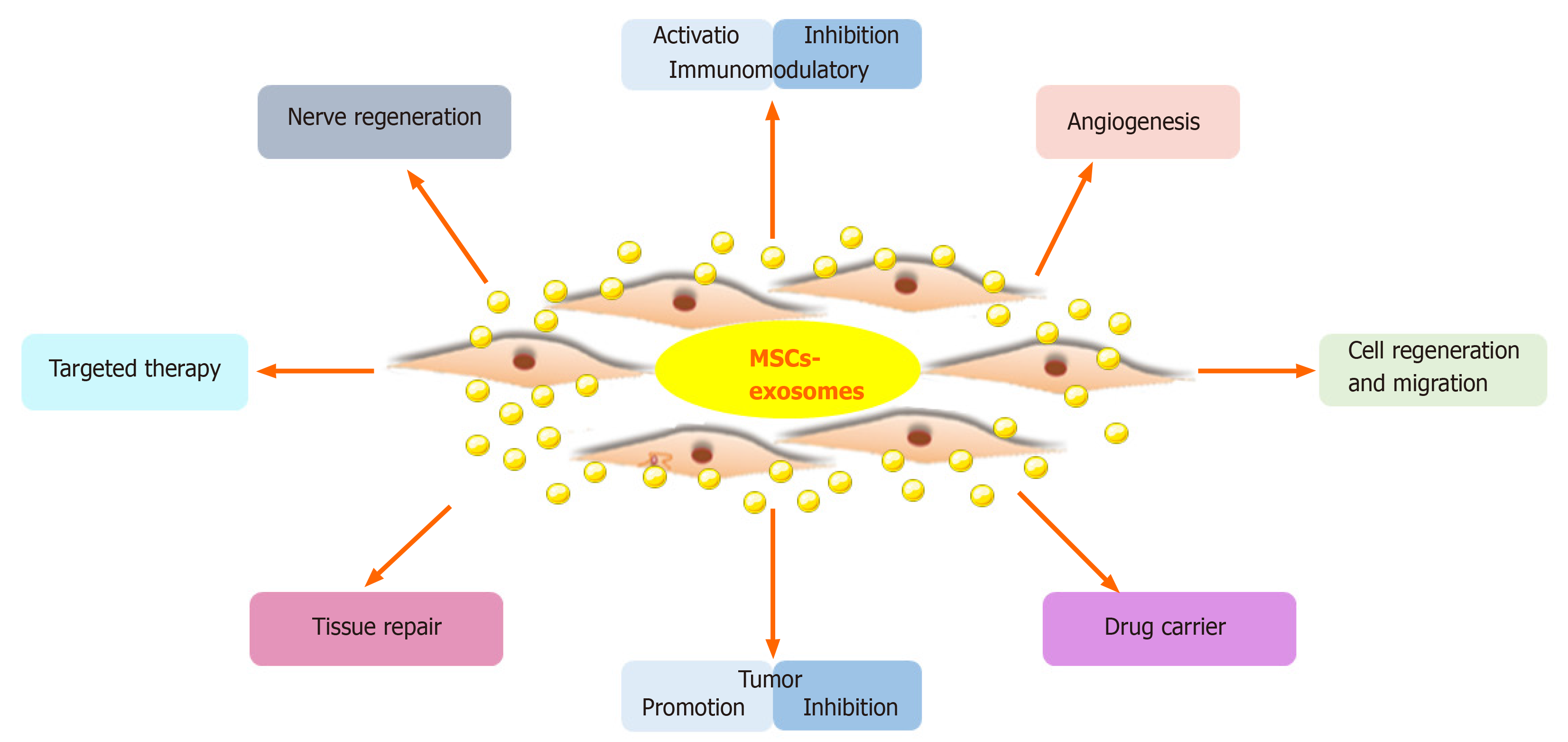
MSC-Exos carry proteins, lipids, DNA, and RNA from MSCs, which is the basis for their therapeutic effect. MSC-Exos have biological functions similar to MSCs, but have a smaller volume, can penetrate biofilm, have low immunogenicity, and can be stored. The lipid bilayer of exosomes protects the contents and protects nucleic acids from RNases[74]. Furthermore, exosomes transport a variety of biologically active components and reflect the physiological and pathological state of the source cell; they transmit information, remove intracellular components, and can transport drugs[73]. Also, MSC-Exos can suppress apoptosis; promote cell regeneration and migration; regulate the immune and inflammatory responses; and promote angiogenesis, nerve regeneration, and tissue repair and regeneration (Figure 3). MSC-Exos have potential for regenerative medicine, as shown in animal models of disease and injury[75].
Ease of collection: Various types of MSCs secrete exosomes, and each produces 1000 to 10000 exosomes. Exosomes can be extracted from culture medium by, for example, ultracentrifugation. Exosomes can be produced on a large scale using specialized cell lines[76]. Compared with MSCs, the production of MSC-Exos is simpler and less costly and time-consuming.
Stability for long-term storage: The volume of exosomes is about one millionth that of MSCs, and they are less complex, have a stable structure, and are easy to produce and store. Exosomes are unaffected by storage at -20 °C for 1 wk, and their activity is maintained during long-term storage at -80 °C[73].
Safety: MSC-based therapies have issues with cell survival, regenerative ability, immune rejection, and differentiation to tumors. These problems can be avoided by using exosomes as cell-free therapy. Due to the low content of exosome membrane-bound proteins, the possibility of immune rejection is very low even after allogeneic administration. In addition, exosomes do not proliferate, so there is no possibility of tumor formation[77]. Therefore, MSC-Exos have better safety than MSCs for clinical applications.
Exosomes as ideal carriers: Exosomes can transfer active substances into recipient cells for cell-to-cell information exchange. Therefore, exosomes can be used as carriers for drugs and biological macromolecules. Sun et al[78] in 2010 reported that curcumin transported in exosomes had a stable structure, improved dissolution ability, a higher blood concentration, and greater anti-inflammatory activity. In animal models, curcumin in exosomes protected mice from LPS-induced septic shock. Furthermore, unlike non-host vehicles, exosomes have low immunogenicity and so do not induce immune rejection or other complications.
Targeting: The exosome membrane harbors proteins with binding affinity for the target cell membrane or a ligand in the ECM. These membrane-bound molecules facilitate the targeting of exosomes to specific tissues or microenvironments. The bilayer membrane of exosomes can be modified with specific factors to enable targeting of cells and tissues[48].
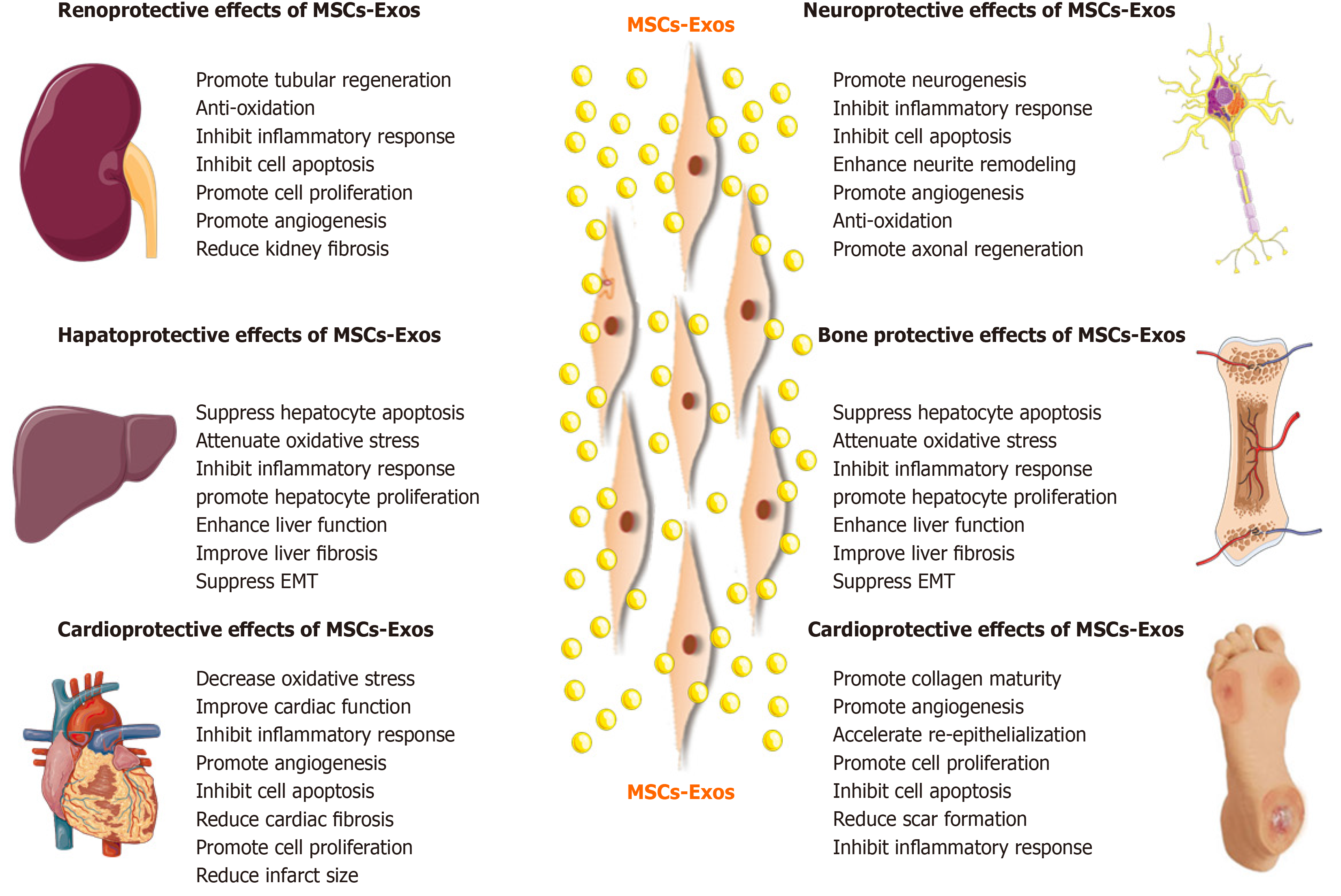
Based on the advantages of MSC-Exos as cell-free therapy, their regenerative and therapeutic potential has been explored in vitro and in vivo. Below we will summarize recent studies on the effects of MSC-Exos on conditions of the kidney, liver, cardiovascular system, nervous system, skin, bone, and muscle (Figure 4).
Acute kidney injury: Renal I/R injury (I/RI) is one of the causes of acute kidney injury (AKI) and is caused by sudden obstruction of blood flow to the kidneys. It is associated with morbidity and mortality in patients with AKI[79]. In addition, AKI is a potential risk factor for progressive chronic kidney disease (CKD), and there is no effective treatment[79]. Wang et al[80] assessed the effect of human bone marrow-derived MSC-Exos in rats with I/R-induced AKI. MSC-Exos improved renal I/RI and renal function by reducing the urea and creatinine levels and inhibiting inflammation and apoptosis. In a mouse model of I/RI, C-C motif chemokine receptor-2 (CCR2)-enriched mouse bone marrow-derived MSC-Exos strongly bound extracellular CCL2 and reduced its concentration, inhibiting the recruitment and activation of peripheral monocytes/macrophages. Importantly, CCR2 knockdown MSC-Exos failed to bind CCL2 and did not protect against renal I/RI[81]. Moreover, miRNAs in MSC-Exos also exert a reno-protective effect. Zhu et al[82] studied the effect of human bone marrow-derived MSC-Exos containing miR 199a 3p on renal I/RI in a mouse model. Injection of MSC-Exos into mice with I/R injury induced recovery of renal function and histologic protection and reduced the cleaved caspase 3 and semaphorin 3A levels. An in vitro study by the same group showed that MSC-Exos increased the expression of the anti-apoptotic protein Bcl-2 and decreased that of the pro-apoptotic proteins Bax and caspase 8 by activating the AKT and ERK pathways in oxygen-glucose deprivation (OGD)-induced HK-2 cells. Co-culture with miR 199a 3p knockdown MSC-Exos reversed these effects[82]. Therefore, exosomal miR 199a 3p plays a crucial role in MSC-Exos mediated suppression of I/R induced apoptosis.
Drug-induced nephrotoxicity is a common cause of AKI, with an incidence as high as 60%. The anticancer drug cisplatin can induce kidney disease and elevate the levels of BUN and creatinine, inducing oxidative stress and apoptosis[79]. Zhou et al[83] showed that injection of human umbilical cord-derived MSC-Exos repaired cisplatin-induced AKI in rats and NRK-52E cell injury by ameliorating oxidative stress and apoptosis and promoting cell proliferation in vivo and in vitro. Human umbilical cord MSC-Exos promoted autophagy of renal tubule epithelial cells and in kidney tissue by inhibiting mTOR, thus alleviating apoptosis and inflammation[84]. Jia et al[85] reported that human umbilical cord-derived MSC-Exos-mediated delivery of 14-3-3ζ enhanced autophagy by modulating ATG16L, thus preventing cisplatin-induced AKI.
CKD: CKD is a progressive disease with complex symptoms and multiple causes. Several factors influence the severity and rate of progression of CKD. AKI is associated with an increased risk of development of CKD, and no effective treatment is available. Zhu et al[86] investigated the effect of human adipose tissue-derived MSC-Exos on the AKI-CKD transition. MSCs upregulated the expression of Sox9 in the renal tubules, promoted the regeneration of renal tubules, ameliorated AKI, and reduced renal fibrosis. These effects were reversed by an inhibitor of MSC-Exos release. Further, the MSCs activated tubular Sox9 and prevented TGF-β1-induced transformation of tubular epithelial cells (TECs) into a pro-fibrotic phenotype via exosome shuttling in vitro. Therefore, MSC exosomes suppressed the AKI-CKD transition by TEC-dependent activation of Sox9[86]. Furthermore, MSC-Exos-mediated delivery of miR-let7c to injured kidneys improved kidney architecture and reduced collagen accumulation in unilateral ureteral obstruction-injured mice, ultimately ameliorating renal fibrosis[87].
Diabetic nephropathy: Diabetic nephropathy (DN) is a serious complication of diabetes and a common cause of end-stage renal disease. Nagaishi et al[88] reported that BMSCs ameliorated DN via the paracrine effect of renal trophic factors, including exosomes. Also, bone marrow-derived MSC-Exos markedly improved renal function, promoted histological restoration of renal tissue, significantly increased LC3 and Beclin-1 expression, and significantly decreased mTOR and fibrotic marker expression in renal tissue in a rat model of DN. These effects were in part abolished by the autophagy inhibitors chloroquine and 3-MA[89]. These findings suggest the therapeutic potential of MSC-Exos for DN.
Liver injury: MSC-Exos can be used for treatment of liver injury. The liver injury caused by I/R affects liver function and increases mortality after liver transplantation and liver resection[90]. Nong et al[91] evaluated the effect of human-induced pluripotent stem cell (hiPSC)-derived MSC-Exos on a rat model of hepatic I/R injury. MSC-Exos markedly suppressed hepatocyte necrosis, sinusoidal congestion, and the levels of markers of hepatocyte injury [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], inflammation (TNF-α and IL-6), apoptosis (caspase-3 and Bax), and oxidative stress (GSH, GSH-Px, and SOD). Therefore, the hiPSC-MSC-Exos alleviated hepatic I/R injury, possibly by inhibiting inflammation, oxidative stress, and apoptosis. Additionally, hiPSC-MSC-Exos alleviated hepatic I/R injury by activating the sphingosine kinase and sphingosine-1-phosphate pathway in hepatocytes and promoting cell proliferation in vitro and in vivo[92]. MiRNAs associated with MSC-Exos also exert a hepatoprotective effect. Zhang et al[90] reported that umbilical cord-derived MSC-Exos containing miR-20a alleviated liver I/R injury. Furthermore, MSC-Exos containing miR-20a stimulated the expression of miR-20a target genes, such as Beclin 1 and FAS, in LO-2 cells. These target genes are involved in apoptosis and autophagy, which are implicated in the pathogenesis of liver I/RI.
Drug-induced liver injury accounts for more than 50% of acute liver failure (ALF) cases in the United States and has become a major clinical problem[93]. Tan et al[93] found that human embryonic (HuES9.E1)-derived MSC-Exos exert a hepatoprotective effect in in vitro models of acetaminophen and H2O2-induced hepatocyte injury and in a mouse model of carbon tetrachloride (CCl4)-induced acute liver injury. The effect was mediated by increasing hepatocyte proliferation, as demonstrated by upregulation of two proliferation factors (PCNA and cyclin D1) and an anti-apoptotic factor (Bcl-xL). Also, the antioxidant activity of human umbilical cord-derived MSC-Exos reportedly suppresses CCl4-induced liver injury[96]. Importantly, MSC-Exos exerted a hepatoprotective effect via antioxidant defenses in the progression from initial liver injury to fibrosis and liver tumor[94]. Additionally, in a CCl4-induced liver injury mouse model, miR-455-3p-enriched exosomes from human umbilical cord MSCs attenuated macrophage infiltration and local liver damage and reduced the serum levels of inflammatory factors, thereby improving liver histology and ameliorating liver injury[95].
Tamura et al[96] evaluated the effect of MSC-Exos on concanavalin-A-induced liver injury as a model of immune-induced liver injury. Bone marrow derived-MSC-Exos reduced the serum ALT level, decreased the hepatic necrotic area, apoptosis, and the production of proinflammatory cytokines, and increased the levels of anti-inflammatory cytokines and regulatory T cells, suggesting an anti-inflammatory effect.
Liver fibrosis: Liver fibrosis is a common outcome of severe chronic liver injury and is characterized by excessive accumulation of the ECM or scar tissue in the liver. If liver fibrosis is not well controlled, it can progress to cirrhosis but, in principle, it is reversible[97]. In a CCl4-induced liver injury model, transplantation of human umbilical cord derived-MSC-Exos reduced the surface fibrous capsules and softened their texture, alleviated hepatic inflammation and collagen production, and inhibited the epithelial-to-mesenchymal transition in the CCl4-induced fibrotic liver[98]. MSC-Exos significantly restored serum AST activity and inactivated the TGF-β1/Smad signaling pathway by decreasing collagen type I/III and TGF-β1 and the phosphorylation of Smad2[100]. Moreover, in vivo administration of human bone marrow derived-MSC-Exos alleviated liver fibrosis by reducing collagen accumulation, enhancing liver functionality, inhibiting inflammation, and increasing hepatocyte regeneration, by inhibiting hepatic stellate cell (HSC) activation through the Wnt/β-catenin pathway[99]. Also, exosomes containing miR-181-5p increased autophagy and ameliorated TGF-β1-induced liver fibrosis by inhibiting the STAT3/Bcl-2/Beclin 1 pathway in HST cells and a CCl4-induced liver fibrosis mouse model[100]. Moreover, adipose tissue-derived MSC-Exos expressing miR-122 decreased the proliferation and activation of HSCs in a liver fibrosis model. Furthermore, MSC-Exos containing miR-122 stimulated the expression of miR-122 target genes such as insulin-like growth factor receptor 1, cyclin G (1), and prolyl-4-hydroxylase α1 in HSCs. These genes are involved in cell proliferation and collagen maturation[101].
Liver failure: ALF is a clinical syndrome caused by inflammation-induced hepatocyte injury, apoptosis, and necrosis, and is a major challenge worldwide. The LPS/D-GalN-induced mouse is generally used as a model of ALF and reflects human liver failure precisely; also, LPS and D-GalN are used to create an animal model of ALF[102]. Jiang et al[102] showed that human umbilical cord derived-MSC-Exos repaired damaged liver tissue and decreased the levels of ALT and AST and the expression of the NLRP3 inflammasome and downstream inflammatory factors in a LPS/D-GalN-induced mouse model of ALF. Moreover, pretreatment with exosomes from human umbilical cord derived-MSCs plus TNF-α alleviated ALF by inhibiting the activation of the NLRP3-related inflammatory pathway, at least in part, by increasing the expression of miRNA-299-3p[103]. In a model of hepatocyte injury and apoptosis induced by LPS/D-GalN, bone marrow derived-MSC-Exos increased the expression of the autophagy marker proteins LC3 and Beclin-1 and promoted the formation of autophagosomes. MSC-Exos significantly decreased the expression levels of the proapoptotic proteins Bax and cleaved caspase-3 and increased that of the anti-apoptotic protein Bcl-2. However, the autophagy inhibitor 3MA significantly reversed the inhibition of apoptosis by MSC-Exos. Therefore, MSC-Exos reduced hepatocyte apoptosis by promoting autophagy after ALF[104].
Myocardial ischemia-reperfusion injury: Myocardial ischemia-reperfusion (MI/R) can induce apoptosis and necrosis of myocardial cells, and even cause cardiac arrest, thereby affecting the outcome of heart disease treatment. Practical and effective therapeutic modalities for MI/R injury are urgently needed. Lai et al[72] reported that human embryonic stem cell-derived MSC-Exos reduced infarct size in a mouse model of MI/R injury. A subsequent study by the same group showed that MSC-Exos increased the levels of ATP and nicotinamide adenine dinucleotide (NADH), decreased oxidative stress, increased phosphorylated-Akt and phosphorylated-GSK-3β (anti-apoptotic factors), and reduced phosphorylated-c-JNK (proapoptotic factor) in I/R hearts, ultimately preventing left ventricular dilatation and improving cardiac performance. MSC-Exos also reduced neutrophil and macrophage infiltration[105]. Hence, MSC-Exos are a potential adjuvant to reperfusion therapy for myocardial infarction (MI). Liu et al[106] found that rat bone marrow-derived MSC-Exos significantly reduced apoptosis and the myocardial infarct size, upregulated myocardial LC3B expression, and improved cardiac function in rats with I/R injury. Also, in vitro, MSC-Exos reduced H2O2-induced ROS production and apoptosis and enhanced autophagy via the AMPK/mTOR and Akt/mTOR pathways in rat H9C2 cardiomyocytes. Moreover, rat bone marrow-derived MSC-Exos reduced MI/R injury, possibly by inhibiting apoptosis and promoting autophagy[107]. Cui et al[108] reported that adipose-derived MSC-Exos significantly attenuated I/R-induced MI, decreased the serum levels of creatine kinase-myocardial band, lactate dehydrogenase, and cardiac troponin I in a rat model of MI/R. MSC-Exos antagonized I/R-induced myocardial apoptosis, upregulated Bcl-2, and downregulated Bax and caspase-3 activity in rat myocardium. Furthermore, MSC-Exos activated Wnt/β-catenin signaling by attenuating the I/R-induced inhibition of Wnt3a, p-GSK-3β (Ser9), and β-catenin expression.
MiRNAs associated with MSC-Exos are also important in protecting against MI/R injury. In an MI/R injury model in which H9C2 cells are subjected to hypoxia/ reoxygenation, Sun et al[109] found that miR-486-5p carried by bone marrow-derived MSC-Exos suppressed PTEN expression, activated the PI3K/AKT signaling pathway, and inhibited the apoptosis of injured cardiomyocytes. MiR-125b reduced the myocardial infarct area and thus ameliorated MI/R. Chen et al[110] loaded miR-125 into bone marrow-derived MSC-Exos, and the resulting MSC-Exos-miR-125b significantly increased cell viability; decreased apoptosis; downregulated Bax and caspase-3; upregulated Bcl-2; decreased the levels of IL-1β, IL-6, and TNF-α in cardiomyocytes; and restored the cardiac function of I/R rats by regulating SIRT7. Also, miRNA-181a delivery by human umbilical cord blood-derived MSC-Exos suppressed inflammation and increased the Treg ratio by inhibiting c-Fos, thus exerting a therapeutic effect on MI/R injury[111]. Therefore, MSC-Exos facilitate the targeted delivery of small RNAs to treat MI/R injury.
Myocardial infarction: MI typically results in irreversible loss of myocardial cells and heart failure due to limited blood supply and is a leading cause of death worldwide. Zhao et al[112] used human umbilical cord derived-MSC-Exos in an acute MI (AMI) rat model. Administration of MSC-Exos significantly improved cardiac systolic function and reduced cardiac fibrosis. Moreover, MSC-Exos protected myocardial cells from apoptosis and promoted tube formation by, and migration of, human umbilical vein endothelial cells (EA.hy926 cells). Therefore, MSC-Exos improved cardiac systolic function by protecting myocardial cells from apoptosis and promoting angiogenesis. Subsequently, the same group reported that human umbilical cord derived-MSC-Exos promote Smad7 expression by suppressing miR-125b-5p to improve myocardial repair[113]. In a follow-up study, adipose-derived MSC-Exos alleviated MI-induced cardiac damage by inhibiting cardiac dysfunction, apoptosis, fibrosis, and inflammation in vitro and in vivo by activating the S1P/SK1/S1PR1 signaling pathway and promoting M2 macrophage polarization[114]. Xu et al[115] reported that exosomes from adipose tissue, bone marrow, and umbilical cord blood derived-MSCs inhibited cardiomyocyte apoptosis and promoted angiogenesis, thereby improving cardiac function and protecting the myocardium in rats with MI. Notably, adipose tissue derived MSC-Exos stimulated the production of cardioprotective factors[115].
MSC-Exos have been genetically modified to enhance their protective effect against MI. Kang et al[116] produced CXCR4-enriched exosomes from rat bone marrow derived MSCs overexpressing CXCR4, which promoted cardiac functional recovery by increasing angiogenesis and cell survival, reducing infarct size, and improving cardiac remodeling by activating the PI3K/Akt signaling pathway following MI. Tissue matrix metalloproteinase inhibitor 2 (TIMP2) is a member of the tissue inhibitor family of metalloproteinases. Because TIMP2-mediated inhibition of matrix metalloproteinases is an important determinant of post-MI remodeling, Ni et al[117] analyzed the therapeutic effects of exosomes from TIMP2-overexpressing human umbilical cord derived-MSCs (MSC-ExosTIMP2) in a rat model of MI. MSC-ExosTIMP2 improved cardiac function by alleviating MI-induced oxidative stress and cardiomyocyte apoptosis, and promoting angiogenesis and ECM remodeling, in part via the Akt/Sfrp2 pathway. Macrophage migration inhibitory factor (MIF), a proinflammatory cytokine, plays a key role in regulating cell homeostasis. Liu et al[118] found that bone marrow derived-MSC-ExosMIF are superior to MSC-Exos for ameliorating MI injury; the effect was mediated by enhancing cardiac function and reducing cardiac remodeling, cardiomyocyte mitochondrial fragmentation, reactive oxygen species generation, and apoptosis.
MiRNAs associated with MSC-Exos also protect against MI. Luther et al[119] demonstrated that bone marrow-derived MSC-Exos expressing miR-21a-5p downregulated the expression of the pro-apoptotic gene products PDCD4, PTEN, Peli1, and FasL, and reduced cardiomyocyte death in an AMI model. Moreover, miR-301 in exosomes secreted by bone marrow derived MSCs protected against MI by inhibiting myocardial autophagy. In a follow-up study, exosomes from human MSCs transfected with the lncRNA KLF3-AS1 were injected into rats with MI. The overexpression of KLF3-AS1 in exosomes reduced the MI area, apoptosis, and pyroptosis, and attenuated MI progression by acting as a competing endogenous RNA (ceRNA) to sponge miR-138-5p that can regulate Sirt1 so as to suppress cell pyroptosis and attenuate MI progression[120]. Moreover, human umbilical cord derived MSC-Exos protected cardiomyocytes from AMI injury by transferring miR-19a, targeting SOX6, activating AKT, and inhibiting JNK3/caspase-3 activation[121]. Also, bone marrow derived exosomal miR-185 suppressed ventricular remolding, myocardial injury, and cardiomyocyte apoptosis, and improved the cardiac function of MI mice by inhibiting SOCS2[122].
In summary, MSC-Exos improve cardiac function and myocardial remodeling by transporting specific factors with anti-apoptotic, anti-inflammatory, antioxidant, and pro-survival effects.
Traumatic brain injury: Traumatic brain injury (TBI) is characterized by functional and structural impairment. There is a need for modalities that improve the recovery rate. Zhang et al[123] found that rat bone marrow derived MSC-Exos improved functional recovery by promoting neurovascular remodeling (angiogenesis and neurogenesis) and by reducing inflammation in rats with TBI. Thus, MSC-Exos may be beneficial for TBI and possibly other neurological diseases. Subsequently, Ni et al[124] investigated the neuroprotective role of rat bone marrow derived MSC-Exos on early-stage controlled cortical impact (CCI)-induced TBI. Administration of MSC-Exos reduced the lesion size and improved neurobehavioral performance; they also inhibited the expression of a pro-apoptotic protein (Bax) and proinflammatory cytokines (TNF-α and IL-1β) and increased the expression of an anti-apoptotic protein (Bcl-2). MSC-Exos also decreased the activation of microglia/M1 macrophages and increased that of M2 macrophages after TBI. Therefore, MSC-Exos exert a neuroprotective effect by inhibiting early neuroinflammation in mice with CCI-induced TBI via modulating microglia/macrophage M2 phenotype polarization[124]. Furthermore, human bone marrow derived MSC-Exos exerted a neuroprotective effect and improved the long-term neurologic outcomes in a porcine model of TBI[125].
Stroke: Stroke is one of the leading causes of death and disability worldwide. Stroke can cause highly dynamic changes in neurovascular units and promote the development of brain injury. MSC-Exos play an important role in neurological and function recovery from stroke. Xin et al[126-128] investigated the effect of rat bone marrow derived MSC-Exos on stroke. The intravenous administration of MSC-Exos improved functional recovery and enhanced neurite remodeling, neurogenesis, and angiogenesis[126]. Also, miR-133b in the exosomes released from MSCs is transferred to neural cells, leading to regulation of gene expression, promotion of neurite remodeling, and improvement of functional recovery in a rat model of stroke[127]. Also, Xin et al[128] reported that MSC-Exos enriched with the miR-17-92 cluster increased neural plasticity and functional recovery from stroke in rats, possibly by inhibiting GSK-3β activity and targeting PTEN to activate the PI3K/AKT/mTOR signaling pathway. In a follow-up study, adipose-derived MSC-Exos promoted angiogenesis by brain microvascular endothelial cells after OGD via the miR-181b-5p/TRPM7 axis, suggesting their therapeutic potential for stroke[129]. Similarly, in the OGD induced rat oligodendrocyte (OL) injury model, miR 134 in rat bone marrow-derived MSC-Exos prevented OL apoptosis by negatively regulating the caspase 8 dependent apoptosis pathway and so have therapeutic potential for ischemic stroke[130]. Moreover, mouse BMSC-Exos promoted the proliferation, and inhibited the apoptosis of, astrocytes injured by OGD, accompanied by inhibition of the expression of inflammatory factors by downregulating lipocalin 2. More importantly, MSC-derived exosomal miR-138-5p reduced neuronal injury following stroke in mice[131].
Spinal cord injury: SCI is a severe central nervous system (CNS) injury for which few efficacious drugs are available. Huang et al[132] reported that systemic administration of rat bone marrow-derived MSC-Exos significantly attenuated lesion size, apoptosis, and inflammation, and promoted angiogenesis, thus enhancing functional recovery from SCI in rats. Therefore, MSC-Exos show potential as a cell-free therapeutic strategy for SCI. Sun et al[133] reported that human umbilical cord-derived MSC-Exos significantly promoted locomotor functional recovery and reduced inflammation after SCI, possibly inducing macrophage polarization from the M1 (proinflammatory) to the M2 (anti-inflammatory) phenotype. In a rat model of traumatic SCI, Liu et al[134] showed that injection of rat bone marrow-derived MSC-Exos attenuated neuron apoptosis, suppressed glial scar formation and inflammation, and promoted axonal regeneration and angiogenesis, ultimately enhancing functional behavioral recovery after traumatic SCI. Administration of MSC-Exos suppressed the activation of A1 neurotoxic reactive astrocytes. Furthermore, rat bone marrow-derived MSC-Exos ameliorated SCI by inhibiting complement mRNA synthesis and release and inhibiting activation of NF-κB signaling by binding to microglial cells[135]. Additionally, rat bone marrow-derived MSC-Exos reduced tissue damage, promoted recovery of motor function, and inhibited neural cell apoptosis after SCI by activating the Wnt/β-catenin signaling pathway[136]. Accordingly, bone marrow-derived MSC-Exos show therapeutic potential for acute SCI.
MSC-Exos have been genetically modified (mainly by miRNAs) to enhance the protective effect against SCI. Li et al[137] found that systemic injection of exosomes from miR-133b-modified rat bone marrow-derived MSCs (MSC-ExosmiR-133b) resulted in transfer of miR-133b into the injured spinal cord, promoting functional recovery after SCI. Also, tail vein injection of MSC-ExosmiR-133b significantly improved the recovery of hindlimb function, reduced lesion volume, preserved neurons, and promoted axon regeneration after SCI, which was attributed in part to the activation of ERK1/2, STAT3, and CREB, and the inhibition of RhoA expression. Moreover, exosomes secreted from miRNA-29b-modified rat bone marrow-derived MSCs relieved SCI in rats, possibly by regulating proteins involved in neuronal regeneration, such as NF200, GAP-43, and GFAP[138]. Two consecutive studies assessed the effect of exosomes secreted from miRNA-126-modified rat bone marrow-derived MSCs on SCI in rat[139,140]. Huang et al[139] indicated that MSC-ExosmiR-126 induce angiogenesis and neurogenesis, inhibit apoptosis, and promote functional recovery after SCI. Yuan et al[140] indicated that MSC-ExosmiR-126 protect the neurons of rats with SCI, stimulate axon regeneration, and improve the recovery of limb motor function after SCI, in part by activating ERK1/2, STAT3, and CREB and inhibiting RhoA expression. Therefore, exosomes from miRNA-modified MSCs is a novel therapeutic approach for SCI.
Neurodegenerative diseases: MSC-Exos play a pivotal role in neuroprotection and neuroregeneration in diverse neurodegenerative diseases. Alzheimer disease (AD) is one of the most common neurodegenerative diseases and causes cognitive and memory disorders. Amyloid-β (Aβ) peptide induces neuroinflammatory processes in the CNS of AD patients, leading to excessive Aβ accumulation. Lee et al[141] reported that human adipose-derived MSC-Exos reduced β-amyloid pathology, and reduced apoptosis of AD neurons. In an AD mouse model, human umbilical cord-derived MSC-Exos reversed cognitive impairment and cleared Aβ deposits. Also, MSC-Exos modulated microglial activation, alleviating neuroinflammation[142]. Moreover, MSC-Exos stimulated neurogenesis in the subventricular zone and alleviated beta amyloid 1-42-induced cognitive impairment in a mouse model of AD[143]. Taken together, these findings demonstrate the therapeutic potential of MSC-Exos for AD. Huntington’s disease (HD) is a hereditary neurodegenerative disease caused by the aggregation of mutant Huntingtin (mHtt). Lee et al[144] investigated the therapeutic role of exosomes from human adipose-derived MSC-Exos in an in vitro model of HD. MSC-Exos significantly decreased mHtt aggregates and reduced mitochondrial dysfunction and apoptosis in R6/2 mouse-derived neurons. ALS is a fatal neurodegenerative disease characterized by selective degeneration and death of upper and lower motor neurons. Treatment of neurons from G93A ALS mice with human adipose-derived MSC-Exos alleviated aggregation of superoxide dismutase 1, and normalized the cellular phenotype, restoring to normal the levels of mitochondrial proteins including p-CREB and PGC-1α[145]. Subsequently, two studies by the same group showed that mouse adipose tissue-derived MSC-Exos exerted an anti-apoptotic effect and rescued the function of mitochondria in an in vitro model of ALS[146,147]. MS is a chronic demyelinating disease caused by CNS inflammation and immune dysfunction, which can result in severe physical disability. Li et al[148] reported that in a model of immune-induced demyelination, rat bone marrow-derived MSC-Exos improved motor function and reduced demyelination and neuroinflammation in rats by regulating M2 polarization of microglia. Thus, bone marrow-derived MSC-Exos have therapeutic potential for MS.
Osteoarthritis: Osteoarthritis (OA) is the most common chronic degenerative OA disease. Because of the limited self-healing ability of cartilage, there is no cure for OA. Exosomes secreted by MSCs show therapeutic potential for OA. Zhu et al[149] compared the effect of exosomes secreted by induced pluripotent stem cell-derived MSCs (iMSC-Exos) and those secreted by synovial membrane MSCs (SMMSC-Exos) on OA. Injection of iMSC-Exos and SMMSC-Exos attenuated OA in the collagenase-induced mouse model – iMSC-Exos had a superior therapeutic effect. Wang et al[150] examined the therapeutic potential for OA of exosomes from human embryonic stem cell-induced MSCs. In vitro, MSC-Exos maintained the phenotype of IL-1β-induced primary mouse chondrocytes by increasing collagen type II synthesis and reducing ADAMTS5 expression. In a mouse model of destabilization of the medial meniscus induced-knee joints, MSC-Exos prevented cartilage destruction[150]. Also, mouse bone marrow-derived MSC-Exos re-established chondrocyte homeostasis, prevented chondrocyte apoptosis, and stimulated macrophage polarization toward an anti-inflammatory phenotype in vivo. Moreover, MSC-Exos protected against cartilage and bone degradation in vivo[151]. Zhang et al[152] demonstrated that human embryonic stem cell-derived MSC-Exos alleviated subchondral bone deterioration, suppressed inflammation, and restored matrix homeostasis in a model of temporomandibular joint OA and, ultimately, promoted temporomandibular joint repair and regeneration. Lumbar facet joint OA (LFJ-OA) is a common cause of lower-back pain (LBP). Li et al[153] evaluated the effect of mouse bone marrow-derived MSC-Exos in an LFJ-OA mouse model. MSC-Exos relieved pain by abrogating aberrant CGRP positive nerves and abnormal H type vessel formation in the subchondral bone. Also, MSC-Exos attenuated cartilage degeneration and suppressed tartrate resistant acid phosphatase expression and RANKL RANK TRAF6 signaling activation to facilitate subchondral bone remodeling. Therefore, bone marrow-derived MSC-Exos can ameliorate LBP and LFJ-OA.
MiRNAs and long noncoding RNAs (lncRNAs) associated with MSC-Exos also protect against OA. Tao et al[154] overexpressed miR-140-5p in human synovial MSCs, and the resulting MSC-ExosmiR-140-5p promoted chondrocyte proliferation and migration and restored ECM secretion by rescuing SOX9, by inhibiting RalA. In an OA rat model, MSC-ExosmiR-140-5p prevented OA and the severe damage to knee articular cartilage caused by instability of the knee joint. Also, human bone marrow-derived MSC-Exos increased the expression of the chondrogenic genes type II collagen alpha 1 and aggrecan and decreased that of the chondrocyte hypertrophy markers matrix metalloproteinase-13 and Runx2 (runt-related transcription factor 2) in chondrocytes from mice with OA. Furthermore, MSC-Exos attenuated the IL-1β-induced inhibition of chondrocyte proliferation and apoptosis via the lncRNA-KLF3-AS1/miR-206/GIT1 axis[155]. Liu et al[156] investigated the effect of human MSC-Exos on IL-1β-induced OA chondrocytes in vitro and in a collagenase-induced rat model of OA in vivo. The lncRNA KLF3-AS1 was markedly enriched in MSC-Exos, which ameliorated IL-1β-induced cartilage injury and suppressed IL-1β-induced apoptosis of chondrocytes in vitro. Also, the exosomal lncRNA KLF3-AS1 promoted cartilage repair and chondrocyte proliferation in a rat model of OA in vivo. Infrapatellar fat pad (IPFP)-derived MSC-Exos ameliorated OA in vivo and inhibited apoptosis, enhanced matrix synthesis, and reduced the expression of catabolic factors in vitro[157]. In addition, IPFP-MSC-Exos partially inhibited mTOR and significantly enhanced autophagy in chondrocytes. However, intra-articular injection of miR-100-5p antagonists significantly suppressed the IPFP-MSC-Exos-mediated protection of articular cartilage in vivo. In summary, IPFP-MSC-Exos improve OA by maintaining cartilage homeostasis, which is likely to be mediated by inhibiting miR-100-5p-regulated mTOR-dependent autophagy[157]. Moreover, human bone marrow-derived MSC-Exos carrying miR-26a-5p inhibited inflammation, proliferation, and migration and promoted apoptosis, thus attenuating OA progression[158].
Osteoporosis: Osteoporosis is an age-related disease that results from an imbalance between bone formation and resorption and is characterized by systemic damage to bone mass and microstructure, ultimately increasing the risk of fragile fractures. Osteoporosis is particularly associated with postmenopausal estrogen deficiency. Qi et al[159] reported that in vitro, human induced pluripotent stem cell-derived MSC-Exos enhanced cell proliferation and alkaline phosphatase activity and upregulated the mRNA and protein levels of osteoblast-related factors in bone marrow MSCs from ovariectomized rats. In vivo, MSC-Exos stimulated bone regeneration and angiogenesis in critical-sized calvarial defects in ovariectomized rats. Zhao et al[160] investigated the effect of rat bone marrow-derived MSC-Exos on osteoblasts in vitro. Co-culture with MSC-Exos promoted the proliferation of hFOB 1.19 osteoblasts cells via the MAPK signaling pathway, alleviating the progression of osteoporosis. Rescue experiments indicated that MSC-Exos promoted the growth and cell cycle of hFOB 1.19 cells; these effects were reversed by p-JNK knockdown. Yang et al[161] showed that the human bone marrow-derived MSC-derived exosomal lncRNA MALAT1 enhanced osteogenic activity and alleviated symptoms of osteoporosis in a mouse model by acting as a miR-34c sponge to upregulate SATB2 expression. Radiotherapy for cancer causes damage to normal tissue, including bone. Radiation-induced bone marrow-derived MSC damage is the main cause of radiation-induced bone loss. Zuo et al[162] investigated the ability of bone marrow-derived MSC-Exos to restore the function of recipient bone marrow-derived MSCs and alleviate radiation-induced bone loss. MSC-Exos attenuated radiation-induced bone loss in a rat model by reducing oxidative stress, accelerating DNA damage repair, promoting proliferation, and increasing the levels of senescence-associated proteins.
Skin wound healing is a complex pathophysiological process involving multiple cells and cytokines. MSC-Exos can accelerate skin healing and reduce excessive scar formation. Zhang et al[163] investigated the use of human induced pluripotent stem cell-derived MSC-Exos in cutaneous wound healing. Transplanting MSC-Exos to wound sites accelerated re-epithelialization, reduced scar width, and promoted collagen maturity. In addition, MSC-Exos not only promoted the formation of new blood vessels but also accelerated the maturation of the skin wound in a rat model. Also, MSC-Exos stimulated the proliferation and migration of human dermal fibroblasts and human umbilical vein endothelial cells and promoted the secretion of types I and III collagen and elastin[163]. Moreover, human umbilical cord-derived MSC-Exos significantly accelerated re-epithelialization, and increased expression of CK19, PCNA, and collagen I (compared to collagen III) in vivo in a rat model of skin burn. In vivo studies confirmed that MSC-Exos-mediated activation of Wnt/β-catenin promotes wound re-epithelialization and cell proliferation. Disruption of Wnt4 expression in MSC-Exos reduced the therapeutic effect in vivo[164]. Hu et al[165] investigated the roles of human adipose-derived MSC-Exos in cutaneous wound healing. MSC-Exos were taken up and internalized by fibroblasts and stimulated their migration, proliferation, and collagen synthesis in a dose-dependent manner; also, the expression of N-cadherin, cyclin-1, PCNA, and collagens I and III was increased. Systemic administration of MSC-Exos increased collagens I and III production during the early stages of wound healing, while MSC-Exos inhibited collagen expression to reduce scar formation in the later stages. Therefore, MSC-Exos promote cutaneous wound healing by optimizing the characteristics of fibroblasts[167]. Ma et al[166] exposed HaCaT keratinocytes to H2O2 to establish a skin lesion model. Human adipose-derived MSC-Exos promoted the proliferation and migration of HaCaT cells and inhibited their apoptosis. In addition, activation of Wnt/β-catenin signaling was confirmed by an increased β-catenin protein level. Therefore, MSC-Exos promote cutaneous wound healing by modulating Wnt/β catenin signaling[168]. He et al[167] found that human bone marrow-derived MSC-Exos accelerated cutaneous wound healing by inducing M2 polarization of macrophages in part by transferring donor exosome-derived miRNAs. Therefore, the miRNAs in MSC-Exos could be applied to enhance the healing of cutaneous wounds.
Diabetic foot ulcer (DFU) is a catastrophic medical problem caused by diabetes, which affects 15% of people with diabetes and increases the risk of amputation. MSC-Exos reportedly accelerate cutaneous wound healing in DFU. In the study of Dalirfardouei et al[168], a full-thickness excisional wound was established on the dorsal skin of streptozotocin induced diabetic mice. Menstrual blood derived MSC-Exos enhanced neoangiogenesis by upregulating vascular endothelial growth factor A, inhibited inflammation by inducing M1-M2 macrophage polarization, accelerated re epithelialization, and reduced scar formation by decreasing the Col1:Col3 ratio. Therefore, menstrual blood derived MSC-Exos ameliorated cutaneous nonhealing DFUs. Moreover, Li et al[169] showed that bone marrow-derived MSC-Exos carrying lncRNA H19 promoted wound healing in mice with DFU by promoting fibroblast proliferation and migration and suppressing apoptosis and inflammation by inhibiting miR-152-3p and promoting PTEN expression.
Although MSC-Exos had shown good clinical application prospects in preclinical studies, a limited number of human clinical studies are already available on the use of MSC-Exos products according to the ClinicalTrials.gov (Table 3). Among them, determining the optimal dose, the appropriate time window for MSC-Exos administration, and the route of administration to achieve maximum efficacy without side effects are the most important issues[170]. A preliminary study demonstrated that increasing dosage of MSC-Exos in a patient with severe treatment-refractory graft-vs-host grade IV disease, affecting the skin and intestinal tract, was well tolerated and showed a significant and sustainable improvement of symptoms, which remained stable for 5 mo[171]. Another clinical trial applied umbilical cord-blood-derived MSC-Exos for improving β-cell mass in type 1 diabetes mellitus patients (NCT02138331). Many more studies are expected to be initiated shortly (Table 3).
| Study title | Disease | Intervention | Phase | NCT |
| Allogenic mesenchymal stem cell derived exosome in patients with acute ischemic stroke | Cerebrovascular disorders | Biological: Exosome | Completed | NCT03384433 |
| A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia | Coronavirus | Biological: MSCs-derived exosomes | Phase 1 | NCT04276987 |
| Effect of microvesicles and exosomes therapy on β-cell mass in type I diabetes mellitus (T1DM) | Diabetes mellitus type 1 | Biological: MSC Exosomes | Phase 2, Phase 3 | NCT02138331 |
| iExosomes in treating participants with metastatic pancreas cancer with KrasG12D mutation | Metastatic pancreatic adenocarcinoma | Drug: Mesenchymal stromal cells-derived exosomes with KRAS G12D siRNA | Phase 1 | NCT03608631 |
| Effect of UMSCs Derived exosomes on dry eye in patients with cGVHD | Dry eye | Drug: Umbilical mesenchymal stem cells derived exosomes | Phase 1, Phase 2 | NCT04213248 |
| Evaluation of adipose derived stem cells exo.in treatment of periodontitis | Periodontitis | Biological: Adipose derived stem cells exosomes | Early phase 1 | NCT04270006 |
| A tolerance clinical study on aerosol inhalation of mesenchymal stem cells exosomes in healthy volunteers | Healthy | Biological: Low level of MSCs-Exo Biological: High level of MSCs-Exo | Phase 1 | NCT04313647 |
| MSC-Exos promote healing of MHs | Macular holes | Biological: Exosomes derived from mesenchymal stem cells (MSC-Exo) | Early Phase 1 | NCT03437759 |
MSC-Exos are effective and safe for regenerative medicine. However, MSC-Exos have several limitations that should be mentioned[44,172]. First, ultracentrifugation can damage or destroy exosomes and the product is typically of low purity. There is no standardized technique for the isolation, quantification, and purification of MSC-Exos. The difficulty of extracting and purifying exosomes increases the cost of their application. Second, the potential of MSC-Exos in tissue repair and regeneration is unclear, as are the components/properties of MSC-Exos that promote tissue regeneration. Also, how to determine the amount of MSC-Exos needed for treatment is unknown, as is whether excess MSC-Exos cause irreversible tissue damage. Third, there is no guidance, supervision, or safety assessment of MSC-Exos. Fourth, it is worth exploring whether MSC exosomes are effective when administered systemically by the intravenous, subcutaneous, or intramuscular route[173]. As an emerging therapeutic agent, the safety, challenges, and risks of MSC-Exos need to be evaluated for their use in tissue repair and regenerative medicine.
MSC-based therapies are widely used worldwide, and the mechanisms underlying their effects may include induced differentiation, immune regulation, cell fusion, paracrine effects, carriage of mRNA or miRNA, and mitochondrial metastasis. MSC-Exos have therapeutic potential because they have most of the therapeutic effects of MSCs themselves. Exosomes can cross biological barriers, can be modified to load molecular drugs, have few side effects and are relatively non-immunogenic, and maintain their activity during storage. MSC-Exos have received attention because of their ability to, for example, promote tissue regeneration, suppress inflammation, and regulate the immune system. In addition, MSC-Exos do not have the safety implications of injecting live cells. The therapeutic efficacy of MSC-Exos against diseases of the kidney, liver, heart, brain, muscle, and skin has been demonstrated, and further research will enable their large-scale production.
The following issues related to MSC-Exos need to be overcome: (1) Lack of standardization of molecular characteristics, comparability, and reproducibility, and difficulty in obtaining high-purity exosomes of defined size; (2) Biodistribution, toxicity, and clearance of MSC-Exos after injection, and verification of their safety; and (3) Most studies of MSC-Exos are short-term, and so their long-term therapeutic effect is unknown, as is the safe dose for humans.
To promote the clinical application of exosomes, we suggest that: (1) Guidelines and standards for use of MSC-Exos are needed; (2) The pathways and recognition signals of MSC-Exos for target cells and organs need to be identified. Exosomal surface molecules can be modified to target MSC-Exos to particular cell types; (3) A standard method of collecting and enriching exosomes is needed, and the purity of MSC-Exos needs to be increased to enhance their therapeutic efficacy; and (4) Because they can pass biological barriers, loading of genes or drugs in MSC-Exos facilitates their targeted delivery.
In summary, MSC-Exos are theoretically superior to intact MSCs for regenerative medicine. However, a series of challenges and difficulties need to be addressed so that the therapeutic potential of exosomes can be realized.
| 1. | Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089-3101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1172] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 3. | Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Zhao X, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Transpl Int. 2006;19:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1645] [Cited by in RCA: 1568] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 6. | Abu-Rmeileh NM, Husseini A, Capewell S, O'Flaherty M; MEDCHAMPS project. Preventing type 2 diabetes among Palestinians: comparing five future policy scenarios. BMJ Open. 2013;3:e003558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 969] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 8. | Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871-3882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 9. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1062] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 10. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2900] [Cited by in RCA: 2914] [Article Influence: 161.9] [Reference Citation Analysis (0)] |
| 11. | Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Wang S, Guo L, Ge J, Yu L, Cai T, Tian R, Jiang Y, Zhao RCh, Wu Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells. 2015;33:3315-3326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Fennema EM, Tchang LAH, Yuan H, van Blitterswijk CA, Martin I, Scherberich A, de Boer J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12:e150-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Kusuma GD, Menicanin D, Gronthos S, Manuelpillai U, Abumaree MH, Pertile MD, Brennecke SP, Kalionis B. Ectopic Bone Formation by Mesenchymal Stem Cells Derived from Human Term Placenta and the Decidua. PLoS One. 2015;10:e0141246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (2)] |
| 16. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2250] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 17. | Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 640] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 18. | Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 19. | Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 917] [Article Influence: 83.4] [Reference Citation Analysis (1)] |
| 20. | Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum Gene Ther. 2015;26:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 623] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 22. | Zhang W, Wang Y, Kong J, Dong M, Duan H, Chen S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci Rep. 2017;7:408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 24. | Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol Ther. 2018;26:1610-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 25. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13033] [Article Influence: 685.9] [Reference Citation Analysis (12)] |
| 26. | Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 262] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 27. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1107] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 28. | Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 29. | Wang J, Liao L, Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther. 2011;11:893-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm (Lond). 2012;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Tsang KS, Ng CPS, Zhu XL, Wong GKC, Lu G, Ahuja AT, Wong KSL, Ng HK, Poon WS. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9:133-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 32. | Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, Cao W, Wang R, Wang W. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 488] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 35. | Chen X, Liu Q, Xiang AP. CD8+CD28- T cells: not only age-related cells but a subset of regulatory T cells. Cell Mol Immunol. 2018;15:734-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1277] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 37. | Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 826] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 38. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 992] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 39. | Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 40. | Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171-18176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 41. | Pollock K, Dahlenburg H, Nelson H, Fink KD, Cary W, Hendrix K, Annett G, Torrest A, Deng P, Gutierrez J, Nacey C, Pepper K, Kalomoiris S, D Anderson J, McGee J, Gruenloh W, Fury B, Bauer G, Duffy A, Tempkin T, Wheelock V, Nolta JA. Human Mesenchymal Stem Cells Genetically Engineered to Overexpress Brain-derived Neurotrophic Factor Improve Outcomes in Huntington's Disease Mouse Models. Mol Ther. 2016;24:965-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 42. | Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 710] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 44. | Sun B, Peng J, Wang S, Liu X, Zhang K, Zhang Z, Wang C, Jing X, Zhou C, Wang Y. Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev Neurosci. 2018;29:531-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1527] [Article Influence: 35.5] [Reference Citation Analysis (5)] |
| 46. | Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420. [PubMed] |
| 47. | Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 48. | Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5188] [Cited by in RCA: 4777] [Article Influence: 398.1] [Reference Citation Analysis (0)] |
| 49. | Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2443] [Cited by in RCA: 3844] [Article Influence: 192.2] [Reference Citation Analysis (0)] |
| 50. | Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2293] [Cited by in RCA: 2756] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 51. | Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791-3799. [PubMed] |
| 52. | Casado S, Lobo MDVT, Paíno CL. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci Rep. 2017;7:6767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Kahroba H, Hejazi MS, Samadi N. Exosomes: from carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell Mol Life Sci. 2019;76:1747-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 54. | Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 55. | Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849-10859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 635] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 56. | Hurley JH, Odorizzi G. Get on the exosome bus with ALIX. Nat Cell Biol. 2012;14:654-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 57. | Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 934] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 58. | Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 870] [Cited by in RCA: 1469] [Article Influence: 163.2] [Reference Citation Analysis (1)] |
| 59. | Yu LL, Zhu J, Liu JX, Jiang F, Ni WK, Qu LS, Ni RZ, Lu CH, Xiao MB. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed Res Int. 2018;2018:3634563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 60. | Yamamoto KR, Alberts BM, Benzinger R, Lawhorne L, Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970;40:734-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1208] [Article Influence: 21.6] [Reference Citation Analysis (9)] |
| 61. | Rider MA, Hurwitz SN, Meckes DG. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci Rep. 2016;6:23978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 541] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 62. | Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 645] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 63. | Xu Y, Shen L, Li F, Yang J, Wan X, Ouyang M. microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J Cell Physiol. 2019;234:21380-21394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 64. | Liu Y, Song B, Wei Y, Chen F, Chi Y, Fan H, Liu N, Li Z, Han Z, Ma F. Exosomes from mesenchymal stromal cells enhance imatinib-induced apoptosis in human leukemia cells via activation of caspase signaling pathway. Cytotherapy. 2018;20:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 66. | Fu M, Gu J, Jiang P, Qian H, Xu W, Zhang X. Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer. 2019;18:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 67. | Ohno S, Ishikawa A, Kuroda M. Roles of exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv Rev. 2013;65:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1433] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 69. | Choi JY, Kim S, Kwak HB, Park DH, Park JH, Ryu JS, Park CS, Kang JH. Extracellular Vesicles as a Source of Urological Biomarkers: Lessons Learned From Advances and Challenges in Clinical Applications to Major Diseases. Int Neurourol J. 2017;21:83-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Stremersch S, De Smedt SC, Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016;244:167-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 71. | Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 920] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 72. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1735] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 73. | Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 615] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 74. | Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 75. | Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 76. | Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 618] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 77. | Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front Pharmacol. 2016;7:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 78. | Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1239] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 79. | Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care. 2011;17:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Wang R, Lin M, Li L, Li L, Qi G, Rong R, Xu M, Zhu T. [Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats]. Zhonghua Yi Xue Za Zhi. 2014;94:3298-3303. [PubMed] |
| 81. | Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, Qiu J, Fan Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016;2016:1240301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 82. | Zhu G, Pei L, Lin F, Yin H, Li X, He W, Liu N, Gou X. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol. 2019;234:23736-23749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 83. | Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 84. | Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H, Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 85. | Jia H, Liu W, Zhang B, Wang J, Wu P, Tandra N, Liang Z, Ji C, Yin L, Hu X, Yan Y, Mao F, Zhang X, Yu J, Xu W, Qian H. HucMSC exosomes-delivered 14-3-3ζ enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am J Transl Res. 2018;10:101-113. [PubMed] |
| 86. | Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, Yang Q, Zhao Z, Xu H, Gao H, Yao W, Luo X, Liao W, Xu G, Zeng R, Yao Y. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8:70707-70726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 87. | Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF, Ricardo SD. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol Ther. 2016;24:1290-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 88. | Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:34842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 89. | Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 90. | Zhang L, Song Y, Chen L, Li D, Feng H, Lu Z, Fan T, Chen Z, Livingston MJ, Geng Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235:3698-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 91. | Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, Wu B, Wang Y, Ai K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 92. | Du Y, Li D, Han C, Wu H, Xu L, Zhang M, Zhang J, Chen X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem. 2017;43:611-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 93. | Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 431] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 94. | Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018;2018:6079642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 95. | Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, Chen M, Zhang B, Zhou Y, Yao R, Shi Y, Bu H. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 96. | Tamura R, Uemoto S, Tabata Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm Regen. 2016;36:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 97. | Guo Y, Chen B, Chen LJ, Zhang CF, Xiang C. Current status and future prospects of mesenchymal stem cell therapy for liver fibrosis. J Zhejiang Univ Sci B. 2016;17:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 99. | Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 254] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 100. | Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491-2502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 337] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 101. | Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng M, Liu Y. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21:2963-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 102. | Jiang L, Zhang S, Hu H, Yang J, Wang X, Ma Y, Jiang J, Wang J, Zhong L, Chen M, Wang H, Hou Y, Zhu R, Zhang Q. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun. 2019;508:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 103. | Zhang S, Jiang L, Hu H, Wang H, Wang X, Jiang J, Ma Y, Yang J, Hou Y, Xie D, Zhang Q. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020;246:117401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 104. | Zhao S, Liu Y, Pu Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. 2019;13:2887-2897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 105. | Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 882] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 106. | Liu L, Jin X, Hu CF, Li R, Zhou Z, Shen CX. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell Physiol Biochem. 2017;43:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 107. | Zou L, Ma X, Lin S, Wu B, Chen Y, Peng C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp Ther Med. 2019;18:2574-2582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 108. | Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes From Adipose-derived Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Wnt/β-Catenin Signaling Pathway. J Cardiovasc Pharmacol. 2017;70:225-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 109. | Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 110. | Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang M, Shen Z, Zheng H, Fu G. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465:103-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 111. | Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z, Dai Q, Kang L, Xu B. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019;232:116632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 112. | Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015;2015:761643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 113. | Wang XL, Zhao YY, Sun L, Shi Y, Li ZQ, Zhao XD, Xu CG, Ji HG, Wang M, Xu WR, Zhu W. Exosomes derived from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. Int J Mol Med. 2018;41:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, Zhang D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 115. | Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121:2089-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 116. | Kang K, Ma R, Cai W, Huang W, Paul C, Liang J, Wang Y, Zhao T, Kim HW, Xu M, Millard RW, Wen Z, Wang Y. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells Int. 2015;2015:659890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 117. | Ni J, Liu X, Yin Y, Zhang P, Xu YW, Liu Z. Exosomes Derived from TIMP2-Modified Human Umbilical Cord Mesenchymal Stem Cells Enhance the Repair Effect in Rat Model with Myocardial Infarction Possibly by the Akt/Sfrp2 Pathway. Oxid Med Cell Longev. 2019;2019:1958941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 118. | Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, Fan B, Zhao H, He H, Zhang F. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 119. | Luther KM, Haar L, McGuinness M, Wang Y, Lynch Iv TL, Phan A, Song Y, Shen Z, Gardner G, Kuffel G, Ren X, Zilliox MJ, Jones WK. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol. 2018;119:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 120. | Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 121. | Huang L, Yang L, Ding Y, Jiang X, Xia Z, You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 2020;19:339-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 122. | Li Y, Zhou J, Zhang O, Wu X, Guan X, Xue Y, Li S, Zhuang X, Zhou B, Miao G, Zhang L. Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. Int Immunopharmacol. 2020;80:106156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 557] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 124. | Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z, Yang J, Pan S, Lin X, Ye H, Xu Z, Wang F, Jin K, Zhuge Q, Huang L. Exosomes Derived From Bone Mesenchymal Stem Cells Ameliorate Early Inflammatory Responses Following Traumatic Brain Injury. Front Neurosci. 2019;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 125. | Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P, Nikolian VC, Chtraklin K, Brown J, Zhang Y, Zhang ZG, Chopp M, Buller B, Alam HB. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J Neurotrauma. 2019;36:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 126. | Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 838] [Cited by in RCA: 780] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 127. | Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 611] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 128. | Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke. 2017;48:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 129. | Yang Y, Cai Y, Zhang Y, Liu J, Xu Z. Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen-Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. J Mol Neurosci. 2018;65:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 130. | Xiao Y, Geng F, Wang G, Li X, Zhu J, Zhu W. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 131. | Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 132. | Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB, Cao Y, Lin FY. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J Neurotrauma. 2017;34:3388-3396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 133. | Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 134. | Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T, Yang S, Yin G, Chen J, Fan J, Cai W. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J Neurotrauma. 2019;36:469-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 135. | Zhao C, Zhou X, Qiu J, Xin D, Li T, Chu X, Yuan H, Wang H, Wang Z, Wang D. Exosomes Derived From Bone Marrow Mesenchymal Stem Cells Inhibit Complement Activation In Rats With Spinal Cord Injury. Drug Des Devel Ther. 2019;13:3693-3704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 136. | Li C, Jiao G, Wu W, Wang H, Ren S, Zhang L, Zhou H, Liu H, Chen Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-catenin Signaling Pathway. Cell Transplant. 2019;28:1373-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 137. | Li D, Zhang P, Yao X, Li H, Shen H, Li X, Wu J, Lu X. Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front Neurosci. 2018;12:845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 138. | Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res. 2019;52:e8735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 139. | Huang JH, Xu Y, Yin XM, Lin FY. Exosomes Derived from miR-126-modified MSCs Promote Angiogenesis and Neurogenesis and Attenuate Apoptosis after Spinal Cord Injury in Rats. Neuroscience. 2020;424:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 140. | Yuan B, Pan S, Dong YQ, Zhang WW, He XD. Effect of exosomes derived from mir-126-modified mesenchymal stem cells on the repair process of spinal cord injury in rats. Eur Rev Med Pharmacol Sci. 2020;24:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 141. | Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer's disease. Brain Res. 2018;1691:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 142. | Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer's Disease. Neurochem Res. 2018;43:2165-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 143. | Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 144. | Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington's disease in vitro model. Eur J Neurosci. 2016;44:2114-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 145. | Lee M, Ban JJ, Kim KY, Jeon GS, Im W, Sung JJ, Kim M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem Biophys Res Commun. 2016;479:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 146. | Bonafede R, Brandi J, Manfredi M, Scambi I, Schiaffino L, Merigo F, Turano E, Bonetti B, Marengo E, Cecconi D, Mariotti R. The Anti-Apoptotic Effect of ASC-Exosomes in an In Vitro ALS Model and Their Proteomic Analysis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 147. | Calabria E, Scambi I, Bonafede R, Schiaffino L, Peroni D, Potrich V, Capelli C, Schena F, Mariotti R. ASCs-Exosomes Recover Coupling Efficiency and Mitochondrial Membrane Potential in an in vitro Model of ALS. Front Neurosci. 2019;13:1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 148. | Li Z, Liu F, He X, Yang X, Shan F, Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol. 2019;67:268-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 149. | Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 337] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 150. | Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 151. | Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 152. | Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 441] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 153. | Li J, Ding Z, Li Y, Wang W, Wang J, Yu H, Liu A, Miao J, Chen S, Wu T, Cao Y. BMSCs-Derived Exosomes Ameliorate Pain Via Abrogation of Aberrant Nerve Invasion in Subchondral Bone in Lumbar Facet Joint Osteoarthritis. J Orthop Res. 2020;38:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 154. | Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 585] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 155. | Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 156. | Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475:3629-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 157. | Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, Chen H, Huang S, Fu Z, Li J, Liu R, Ni Z, Chen L, Yang L. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 158. | Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int Immunopharmacol. 2020;78:105946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 159. | Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int J Biol Sci. 2016;12:836-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 160. | Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3962-3970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 161. | Yang X, Yang J, Lei P, Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY). 2019;11:8777-8791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 162. | Zuo R, Liu M, Wang Y, Li J, Wang W, Wu J, Sun C, Li B, Wang Z, Lan W, Zhang C, Shi C, Zhou Y. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res Ther. 2019;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 163. | Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 590] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 164. | Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 165. | Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 517] [Cited by in RCA: 482] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 166. | Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120:10847-10854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 167. | He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 168. | Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 169. | Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, Fu Y, Zhai A, Bi C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 170. | Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 171. | Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 872] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 172. | Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, Gale BK. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem. 2014;406:7855-7866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 173. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 957] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Goebel WS, Ramasamy T, Tanabe S S-Editor: Dou Y L-Editor: Wang TQ P-Editor: Xing YX