Published online Jul 26, 2020. doi: 10.4252/wjsc.v12.i7.562
Peer-review started: February 26, 2020
First decision: April 25, 2020
Revised: May 17, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: July 26, 2020
Processing time: 150 Days and 12.2 Hours
Photodynamic therapy (PDT) is an effective and promising cancer treatment. PDT directly generates reactive oxygen species (ROS) through photochemical reactions. This oxygen-dependent exogenous ROS has anti-cancer stem cell (CSC) effect. In addition, PDT may also increase ROS production by altering metabolism, endoplasmic reticulum stress, or potential of mitochondrial membrane. It is known that the half-life of ROS in PDT is short, with high reactivity and limited diffusion distance. Therefore, the main targeting position of PDT is often the subcellular localization of photosensitizers, which is helpful for us to explain how PDT affects CSC characteristics, including differentiation, self-renewal, apoptosis, autophagy, and immunogenicity. Broadly speaking, excess ROS will damage the redox system and cause oxidative damage to molecules such as DNA, change mitochondrial permeability, activate unfolded protein response, autophagy, and CSC resting state. Therefore, understanding the molecular mechanism by which ROS affect CSCs is beneficial to improve the efficiency of PDT and prevent tumor recurrence and metastasis. In this article, we review the effects of two types of photochemical reactions on PDT, the metabolic processes, and the biological effects of ROS in different subcellular locations on CSCs.
Core tip: Photodynamic therapy (PDT) is an effective and promising cancer treatment. PDT directly produces reactive oxygen species (ROS) through photochemical reactions. In this article, we review the production process of oxygen-dependent exogenous ROS and the possible endogenous ROS generation process after PDT-mediated subcellular organelle stress. The intracellular metabolism of several ROS produced by PDT is analyzed. Given the extremely short half-life and limited diffusion distance of ROS, we explain from the subcellular localization of photosensitizers how PDT affects the characteristics of cancer stem cells through changes in mitochondrial permeability, activation of unfolded protein responses, autophagy and so on.
- Citation: Zhang ZJ, Wang KP, Mo JG, Xiong L, Wen Y. Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species. World J Stem Cells 2020; 12(7): 562-584
- URL: https://www.wjgnet.com/1948-0210/full/v12/i7/562.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i7.562
Photodynamic therapy (PDT) is an effective and promising cancer treatment. By injecting a tumor-targeted photosensitizer (PS) into the patient’s body and directly irradiating the tissue with a laser, a significant tumor ablation effect can be achieved[1]. The space- and time-selective uptake characteristics of the PS protect normal cells, while the laser radiation is directly pointed to the tumor[2]. Thus, PDT is a multiple-targeting method. Since the US Food and Drug Administration listed PDT as a new treatment method for the clinical treatment of cancer patients[3], alone or combined with surgery[4] and/or chemotherapy[5], PDT has been applied in large numbers worldwide. For PDT treatment, some Western countries have established relatively systematic treatment plans[6-8]. The main mechanism of PDT depends on the reactive oxygen species (ROS) components generated by the photochemical reaction, which can oxidize a large number of intracellular active components (such as DNA and lipid compounds) in tumor cells[9,10]. This chemical-dependent treatment is more sensitive than drug treatments and can minimize tumors in the short term.
It is currently recognized that ROS play decisive roles in the biological effects mediated by PDT because PDT is based on a natural cold photochemical reaction[11]. Although PDT can be divided into two types of reactions, depending on the type of PS, the products can both be considered ROS. Essentially, the exogenous ROS induced by this cold photochemical reaction are the most important and first effector molecules of PDT, although they also depend on the intracellular oxygen levels most of the time. In addition to exogenous ROS, PDT-induced ROS can also cause intracellular metabolic changes, induce endoplasmic reticulum stress[12], and/or destroy mitochondrial potential[13] to increase endogenous ROS production. On the one hand, the photochemical reaction directly caused by PDT produces a short ROS duration (< 0.05 μs), with high reactivity and a limited diffusion distance (< 0.02 μm)[14]. Therefore, the main target position of the photochemical reaction in the cell is often near the subcellular components where the PS is localized, which explains the heterogeneity of the effects of different PSs[14]. On the other hand, the metabolic induction of mitochondrial ROS production is much more complicated; for example, it may involve the partial inactivation of respiratory complexes I, II, and III of the mitochondrial electron transport chain[15]. In general, excessive ROS destroy the redox system in cells and cause oxidative damage to biomolecules, including DNA and other molecules[10,11]. In previous studies, DNA was considered an important target of PDT because double-strand DNA breaks are the most lethal form of damage to tumor cells[16]. Recently, more studies have suggested that the activation of the mitochondrial permeability transition increases the levels of reactive nitrogen substances, such as nitric oxide[17,18]. Of course, ROS-induced intracellular metabolism affects mitochondria to an even greater extent. The ROS-related effects on the endoplasmic reticulum, nucleus, and cell membrane are described in detail in a subsequent section.
There is growing evidence that ROS play roles in cell signaling. These signals are transmitted in tissues to coordinate various cellular processes. At physiological doses, ROS maintain cell nutrition and cytokine balance; however, in some specific cases, small changes in ROS level may have a profound impact on the fate of stem cells[19], directly induce cancer stem cell (CSC) differentiation, or induce CSC heterogeneity in tumors[20]. Furthermore, ROS are related to the level of many biological processes, including but not limited to gene expression, protein translation, and protein or nucleic acid interactions[21]. As genomics and proteomics advance, increasing pathway information on ROS, explaining the mechanisms by which they maintain and regulate cellular processes, is being mined. Especially in stem cells, changes in the oxidative state (also known as redox regulation) may indicate the regulatory elements for communication between key organelles such as endoplasmic reticulum-mitochondria and mitochondria-nucleus crosstalk[22,23]. Redox-mediated mitochondrial-nuclear crosstalk can explain the coordination of cell metabolism and chromatin remodeling, gene expression, cell cycle progression, DNA repair, and cell differentiation. The endoplasmic reticulum-mitochondria crosstalk (as well as that of other organelles with mitochondria) can explain endoplasmic reticulum stress, mitochondrial autophagy, stemness induction, apoptosis, and/or survival through ROS.
ROS are also related to immunogens and tolerogenic processes[24]. The increased systemic immunity or enhanced tumor immunity induced by PDT may also be mediated by ROS[25]. Currently, although little is known about whether or how ROS are involved in stem cell immunity, it has been determined that identifying the mechanism by which ROS metabolism affects the fate of stem cells will promote the necessary understanding to apply PDT to inhibit the spread of distant cancer stem cells. Although PDT is used to treat superficial malignancies, its immunogenicity has the potential to eliminate systemic CSCs. But in a counterintuitive outcome, some research found that low-dose PDT promotes tumor cells metastasis[25,26]. The epithelial-mesenchymal transition (EMT) has been shown to be one of the causes of cancer cell migration and invasion[27]. PDT can induce EMT in vitro[28]. EMT may be closely related to the metabolic reprogramming of CSCs and cancer cells[28]. ROS can induce stemness and metabolic changes in cancer cells. Therefore, PDT appears to induce the EMT and promote CSC phenotype acquisition by regulating cellular metabolism. The EMT, stemness, and oncogenic metabolism are known to be associated with resistance to PDT. Therefore, understanding PDT-induced metabolism and the molecular mechanism of the EMT is also conducive to accurately generating the appropriate level of ROS and enhance the efficacy of PDT. Therefore, in this review, we present the differences in ROS produced by the two types of photochemical reactions induced by PDT, the metabolic processes of endogenous ROS, and the similarities and differences in the biological effects of different ROS. We analyze the effects of ROS on cells at different sites and explain how they might affect the fate of stem cells. Finally, in view of some controversial characteristics of CSCs, we propose how to leverage the advantages of PDT to manipulate the fate of CSCs.
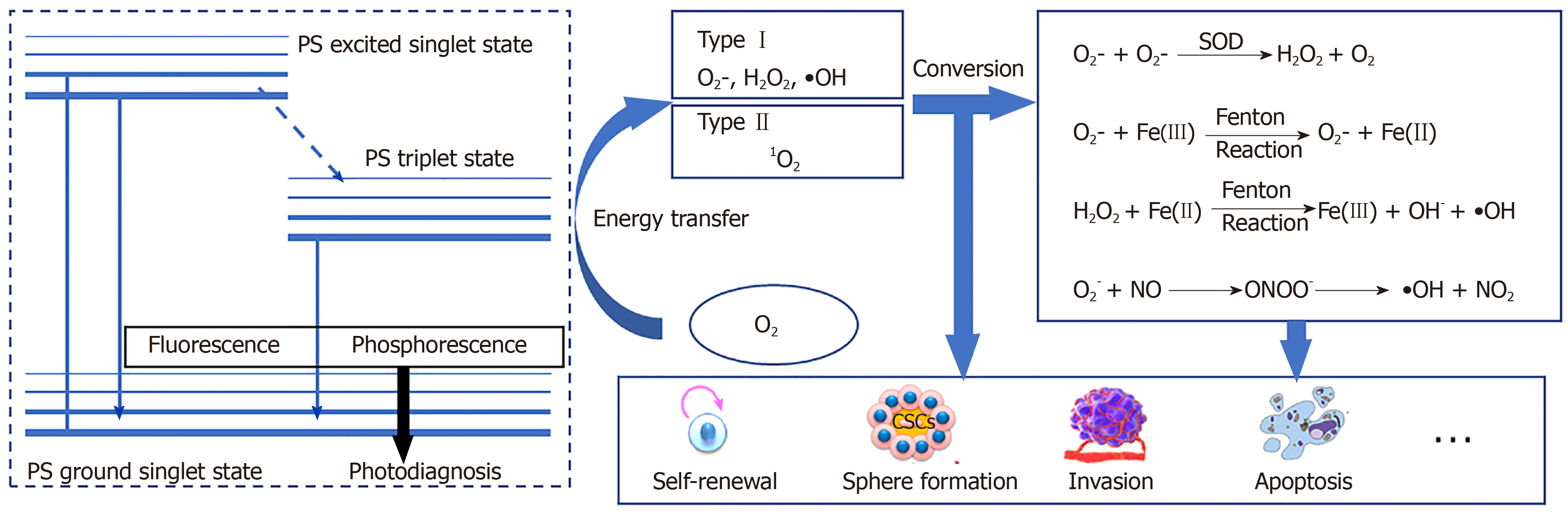
PDT is a chemical reaction between a PS and oxygen under laser energy; when the three are combined, ROS are produced. Under normal circumstances, the PS is in the ground singlet state, where all the electrons rotate in pairs in low-energy orbits. When irradiated with the wavelength of the PS absorption peak, one of the electrons in the highest occupied molecular orbitals of the PS moves to the lowest unoccupied molecular orbital, which places PS into a transient and unstable activated singlet state, leading to a series of events[29,30]. Due to the aromatic nature of many PSs, the energy difference between highest occupied molecular orbitals and lowest unoccupied molecular orbital is quite small. The light emitted at the excitation wavelength is usually in the visible or near-infrared parts of the spectrum. The activated singlet of the PS reverses the rotation of the activated electrons to generate a triplet, which is a process that enables triplets to cross between PS systems. The PS triplet has a lower energy and longer life than the singlet because the activated electrons spin parallel to the previous paired electrons and are not easily separated to create a singlet. To enter a more stable state, the triplet electron excited by the PS either enters the correct rotating orbit, is released to fall to the ground singlet state, and emits fluorescence (slow process) or directly interacts with molecules in the environment to return to the singlet state. The interaction between triplet states is reversible, but the interaction between the triplet state and the singlet state is irreversible. Therefore, the activated PS triplet can only interact with the molecules in the triplet rotation. In the cell, the triplet state of O2 has obvious double radical properties in the ground state; therefore, energy is easily transferred from the triplet PS to oxygen molecules. This process is the basis of the chemical reaction of PDT.
The effectiveness of PDT is due to free radicals and electronically activated oxygen species, and their production depends on whether a PDT type I or II reaction occurs[31](Figure 1). As mentioned earlier, the outermost layer of the ground state triplet has two unpaired orbits that rotate in parallel; therefore, when the triplet state is excited by PS, the reactions of different molecules are quite different[29,32]. In a type I reaction, PS directly removes an electron to generate a superoxide anion (O2−). O2− can quickly form other substances, including hydroxyl radicals and hydrogen peroxide. In the type II reaction, the energy that excites the PS triplet state is transferred to O2. The spin of the outermost electron of O2 thus flips and moves to an orbit that previously contained unpaired (natural) electrons with opposite spins; this describes a singlet oxygen (1O2) in the active state. 1O2 is not a free radical because all the electrons spin in pairs, but they are very active and short lived[33,34].
Under normal circumstances, approximately 90% of the ROS in the body are produced by the mitochondrial electron transfer chain[35]. The yield of ROS produced by the ETC increase during hypoxia, light stimulation, ischemia-reperfusion, aging, and mitochondrial respiratory depression. Over 90% of the oxygen in mitochondria is reduced by cytochrome oxidase to water molecules, while only 0.1%-0.2% of O2 forms ROS through electron flow, mainly through electron transport chain complexes I and III[15,36]. The rate of the ROS produced by mitochondria is mainly affected by mitochondrial membrane potential (MMP) regulation.
In theory, PDT also has the ability to increase endogenous ROS by changing the MMP[37]. Generally, it is difficult for exogenous ROS to directly induce MMP changes to produce more endogenous ROS, but it can be accomplished through endoplasmic reticulum-mitochondria crosstalk. Briefly, PDT induces endoplasmic reticulum stress (ER stress, ERS), which mainly transmits death signals from the endoplasmic reticulum to the mitochondria by the proteinkinase R-like ER kinase (PERK) pathway. In this process, Ca2+ influx from the endoplasmic reticulum to mitochondria induces a decrease in the MMP and promotes ROS production[38,39]. In more detail, the mitochondria-associated ER membrane (MAM) may explain this phenomenon. MAMs whose contacting is increased in CSCs are connected by protein-binding complexes[40]. These two membranes are 10-25 nm apart and communicate through the calcium ion-related pathway[40]. Mitofusion2 (Mfn2) maintains MAM distance and prevents ER and mitochondria from being too close and thus prevent Ca2+-mediated MMP changes and apoptosis. When 5-aminolevulinic acid mediated PDT is performed, Mfn2 expression was reduced, suggesting an production of endogenous ROS[41]. In addition, phosphofurin acidic cluster sorting protein 2 (PACS2) is an essential protein that mediates MAM-Ca2+ overload[42]. PACS2 promotes the cleavage of BCR-associated protein(BAP)-31, which is an important factor in the caspase-8 apoptosis pathway activated by Ca2+. During PDT, BAP31 is cleaved before the mitochondria changes and mediates the release of ERS and Ca2+[43]. From the point of view of enzyme catalysis, PDT regulates flavin adenine dinucleotide, ubiquinones, and cytochrome in mitochondrial respiratory chain complexes I and III to produce ROS[44-46]; mitochondrial triphosphopyridine nucleotide(NADPH) oxidase and xanthine oxidase catalyze the production of O2−[47]; mitochondrial myeloperoxidase myeloperoxidase (MPO) catalyzes the production of OH; and protein kinase C catalyzes the production of H2O2[48,49]. Because the dynamic changes in ROS are rapid, in trace amounts, and complex, it is difficult to accurately detect the process by which ROS are produced, from exogenous to endogenous ROS, by PDT within a short period. Therefore, although we believe that PDT may induce endogenous ROS, very few studies have addressed or clarified this process.
According to the type of PDT photochemical reaction, ROS produced by subsequent metabolism can also be generally divided into two parts. The most active free radical molecule is •OH, which is converted into stable hydroxide ions by receiving electrons and generating water and protons. O2- receives electrons to form peroxide ions (O22-) and then is rapidly protonated to form H2O2. O2- is inert in biological systems because the antioxidant action of superoxide dismutase converts O2- to H2O2 and O2. H2O2 is converted into water and oxygen molecules[50,51]. Nonetheless, H2O2 may react with very low concentrations of electron-deficient substances[52], such as ferrous ions (Fe2+), which cause the oxygen and oxygen bonds of H2O2 to break, producing ferric iron (Fe3+), hydroxide, and •OH (Fenton reaction)[53]. •OH cannot be catabolized by enzymes but can be broken down by antioxidant peptides (such as glutathione) or antioxidant vitamins (such as vitamin C)[54]. PDT type I reaction products can also indirectly cause the formation of reactive nitrogen because O2− reacts with nitric oxide (NO) to generate peroxynitrite anion (ONOO−)[17,55,56]. ONOO- is very active and has a short life span. Rapid homogeneous fission forms •OH and nitrogen dioxide (NO2). ONOO- also reacts with carbon dioxide to form carbonate anion radicals (CO3-) and NO2. All the resulting free radicals are destructive and they continue to move in the cell until they are paired (free-radical pairing). Although not a free radical, 1O2 reacts with macromolecules in several different ways. 1O2 can act as a dienophile in the Diels–Alder cycloaddition reaction, and it can react with the aromatics and the diene of the conjugated system, leading to the degradation of many lipids and proteins. Disulfide bonds and other electron-rich substances may also attack 1O2[57] (Figure 1). In contrast to free radicals, 1O2 cannot be destroyed by enzymes but can be inactivated by antioxidants (such as carotenoids).
The body has a complex antioxidant system. Normally, the production and removal of ROS are maintained in a dynamic balance so that they do not cause damage to the body[58]. Disruption of stem cell metabolism can directly determine whether stem cells are at rest, self-renewing, or differentiating[59-61], and controlling ROS levels is one of the feasible ways in which metabolism is disrupted. After PDT treatment, intracellular ROS accumulation increases, causing excessive oxidation of proteins, DNA, and lipids, which may be a direct means of controlling the fate of stem cells. ROS bind to proteins to generate carbonyl derivatives[62], alter the tertiary structure of the proteins, and promote protein/DNA[63]-protein cross-linking, leading to changes in the protein activity of CSC marker proteins such as octamer binding transcription factor 4 and sex determining region Y box 2 (Sox2)[64]. ROS directly attack DNA bases and easily cause deoxyguanosine modifications to one carbon atom (that is, 8-OHdG)[62], which may be one of the causes of point mutations in proto-oncogenes or tumor suppressor genes, such as Ras and p53[65,66]. Free radicals generate lipid peroxides through oxidation, which can damage cell membranes and promote ferroptosis.
However, whether ROS oxidize lipids, inactivate proteins, or damage DNA in CSCs largely depends on where the ROS are generated (because the half-life of the ROS produced by PDT ranges from 3.5 μs to 5 s, and the 1O2 diffusion distance is approximately 40 nm)[14]. Some people think that the killing effect of a PS in mitochondria is significantly higher than that of the same PS is in other organelles, and the importance of PS positioning is even higher than the ROS yield[67]. Therefore, before describing the effects of PDT and ROS on CSCs in detail, it is necessary to summarize the subcellular localization of commonly, recently used PSs. Of course, in special cases, PDT undergoes different localization and interacts with unique effector sites before activation. For example, in our previous studies, we observed that a mitochondrial PS induced ERS-mediated apoptosis, which suggested that intercellular organelle crosstalk was involved in the ROS-regulated CSCs[68]. In this review, PS mapping for tumor PDT can be roughly divided into three areas: Mitochondria[69-78], endoplasmic reticulum[12,68,79-82], and lysosomes[83-88]. These PSs can be roughly summarized into porphyrin-based photosensitizers, such as porphyrins[89-92], chlorins[80,93,94], phthalocyanines, and naphthalocyanines[95-99], and non-porphyrin-based photosensitizers, such as cyanine, methylene blue, Nile blue, rhodamine, triarylmethane, and acridine[68,100-105]. These PSs and their derivatives are summarized by category in Table 1. Therefore, to more accurately control and eliminate CSCs, the effects of ROS on PDT in different cell structures should be classified and explained.
| Photosensitizer | Location | Cancer (parental cell lines) | Ref. |
| 5-aminolevulinic acid | Mitochondria | Breast cancer; T-cell leukemia | Song et al[89] |
| Protoporphyrin IX | Mitochondria | Colorectal cancer (HT29); liver cancer (Hep3B) | Taba et al[91] |
| Porphyrin-based photosensitizer meso-5-[ρ-diethylene triamine pentaacetic acid- aminophenyl]-10,15,20-triphenyl-porphyrin | Lysosome | Gastric cancer (HGC27, SNU-1) | Chen et al[92] |
| Porphyrin-based amphiphilic block copolymer (PEG-b-PCL-a-porphyrin) | Nucleus; lysosome | Lung cancer (A549) | Tian et al[83] |
| Tetrahydroporphyrin-tetratosylat | Lysosome | Human bronchial smooth muscle cells (HBSMCs) | Berndt-Paetz et al[85] |
| Meso-5-[ρ-diethylene triamine pentaacetic acid- aminophenyl]-10,15,20-triphenyl-porphyrin | Lysosome | Gastric cancer (HGC27, SNU-1) | Chen et al[92] |
| {Carboxymethyl-[2-(carboxymethyl-{[4-(10,15,20-triphenylporphyrin-5-yl)-phenylcarbamoyl]-methyl}-amino)-ethyl]-amino}-acetic acid | Lysosome | Liver cancer (HepG2); gastric cancer (BGC-823) | Chen et al[88] |
| Sinoporphyrin sodium (DVDMS-2) | Endoplasmic reticulum | Cholangiocarcinoma (CX-1) | Kong et al[12] |
| Chlorin e6 | Mitochondria; cytoskeleton; lysosome; endoplasmic reticulum | Gliosarcoma (9L/LacZ); colorectal cancer (SW620) | de Almeida et al[93] Yang et al[80] |
| Meta-tetrahydroxyphenylchlorin | Mitochondria, endoplasmic reticulum | Colorectal cancer (SW620) | Abdulrehman et al[81] |
| β-CD through an amide bond | Mitochondria | Breast cancer (MCF-7) | Tong et al[94] |
| Palmatine hydrochloride | Mitochondria, endoplasmic reticulum | Breast cancer (MCF-7); colorectal cancer (HT29) | Wu et al[77] Wu et al[78] |
| Ce6-loaded branched polyethylenimine-PEGylated ceria nanoparticles | Lysosome; large vesicle | Cervical cancer (Hela) | Yang et al[86] |
| Mannose-conjugated chlorin | Lysosome; endoplasmic reticulum | Tumor-associated macrophages (TAM) | Hayashi et al[82] |
| Glucose-conjugated chlorin compound e6 | Endoplasmic reticulum | Esophageal cancer (OE21,KYSE30); gastric cancer(MKN45); colorectal cancer (HT29) | Ichikawa et al[79] |
| (G-Mito-Pc) silicon (II) phthalocyanine (Pc) | Mitochondria | Cervical cancer (Hela) | Zhao et al[95] |
| Zinc (II) phthalocyanine | Mitochondria; lysosome | Liver cancer (HepG2) | Chen et al[96] |
| Chloroaluminum phthalocyanine | Mitochondria | Prostate cancer (LNCaP) | Leandro et al[97] |
| Pheophorbide a | Mitochondria | Cervical cancer (Hela) | Choi et al[98] |
| Silicon (IV) phthalocyanines | Mitochondria | Liver cancer (HepG2); gastric cancer (BGC-823) | Zheng et al[99] |
| Tamoxifen-zinc (II) phthalocyanine | Lysosome | Breast cancer (MCF-7) | Zhang et al[84] |
| Cyanines (AlPc and Pc green) | Lysosome | Gastric cancer (EPG85-257P) | Zielichowska et al[87] |
| Cyanine-derivative photosensitizer | Mitochondria | Breast cancer (MCF-7) | Zhao et al[69] |
| Ru(ii) polypyridyl complexes | Mitochondria; lysosome | Lung cancer (A549) | Qiu et al[100] |
| Ethyl acetate extract of cichorium | Mitochondria | Colorectal cancer (SW620, HCT116) | Wen et al[68] |
| Hypocrellin A | Mitochondria | Lung cancer (A549) | Qi et al[101] |
| Cyanopyridinium salts | Mitochondria | Cervical cancer (Hela) | Zhao et al[69] |
| Rhodamine organic dye | Mitochondria | Breast cancer (MCF-7) | Liu et al[70] |
| Cis-[Pt(NH)(L)Cl](NO) (1-3) having boron-dipyrromethene (BODIPY) pendants (L) with 1,3,5,7-tetramethyl-8-(4-pyridyl)-4,4'-difluoroboradiazaindacene moieties | Mitochondria | Lung cancer (A549); breast cancer (MCF-7) | Raza et al[72] |
| Hypericin | Mitochondria | Adenoidal thyroid cancer (FRO) | Kim et al[102] |
| Platinum(II) complexes [Pt(L)(R-BODIPY)]Cl | Mitochondria | Lung cancer (A549); breast cancer (MCF-7); cervical cancer (Hela) | Ramu et al[103] |
| Cis-[Pt(NH)(L)Cl](NO), where L is an imidazole base conjugated to 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) | Mitochondria | Breast cancer (MCF-7) | Raza et al[72] |
| Triphenylphosphonium | Mitochondria | Lung cancer (A549) | Rui et al[74] |
| Triphenylphosphonium | Mitochondria | Cervical cancer (Hela) | Choi et al[98] |
| Methyl-functionalized derivatives of the drug carrier triphenylphosphonium | Mitochondria | Cervical cancer (Hela); gastric cancer (FU97) | Hu et al[75] |
| Hypocrellin B | Mitochondria | Breast cancer (MDA-MB-231) | Jia et al[76] |
| Aloe-emodin | Mitochondria; endoplasmic reticulum | Osteosarcoma (MG63) | Li et al[104] |
| BODIPY-Appended 2-(2-Pyridyl) benzimidazole Platinum (II) Catecholates | Mitochondria | Keratinocytes (Hacat) | Mitra et al[105] |
| Acetylatedglucose-conjugated chlorin | Endoplasmic ruticulum | Esophageal cancer (OE21, KYSE30); gastric cancer (MKN45); | Ichikawa et al[79] |
By the way, it is worth mentioning that photochemical internalization is a special CSC targeting strategy though PDT does not play a major role in this approach. In photochemical internalization, PS are modified by CSC biomarkers (such as CD133, CD44, CSPG4, and EpCAM)[106-109] that are first anchored to the cell membrane and then are endocytosed into intracellular vesicles. Finally, the drugs carried into the cell are released through PDT. For more details, please refer to a previous review[110].
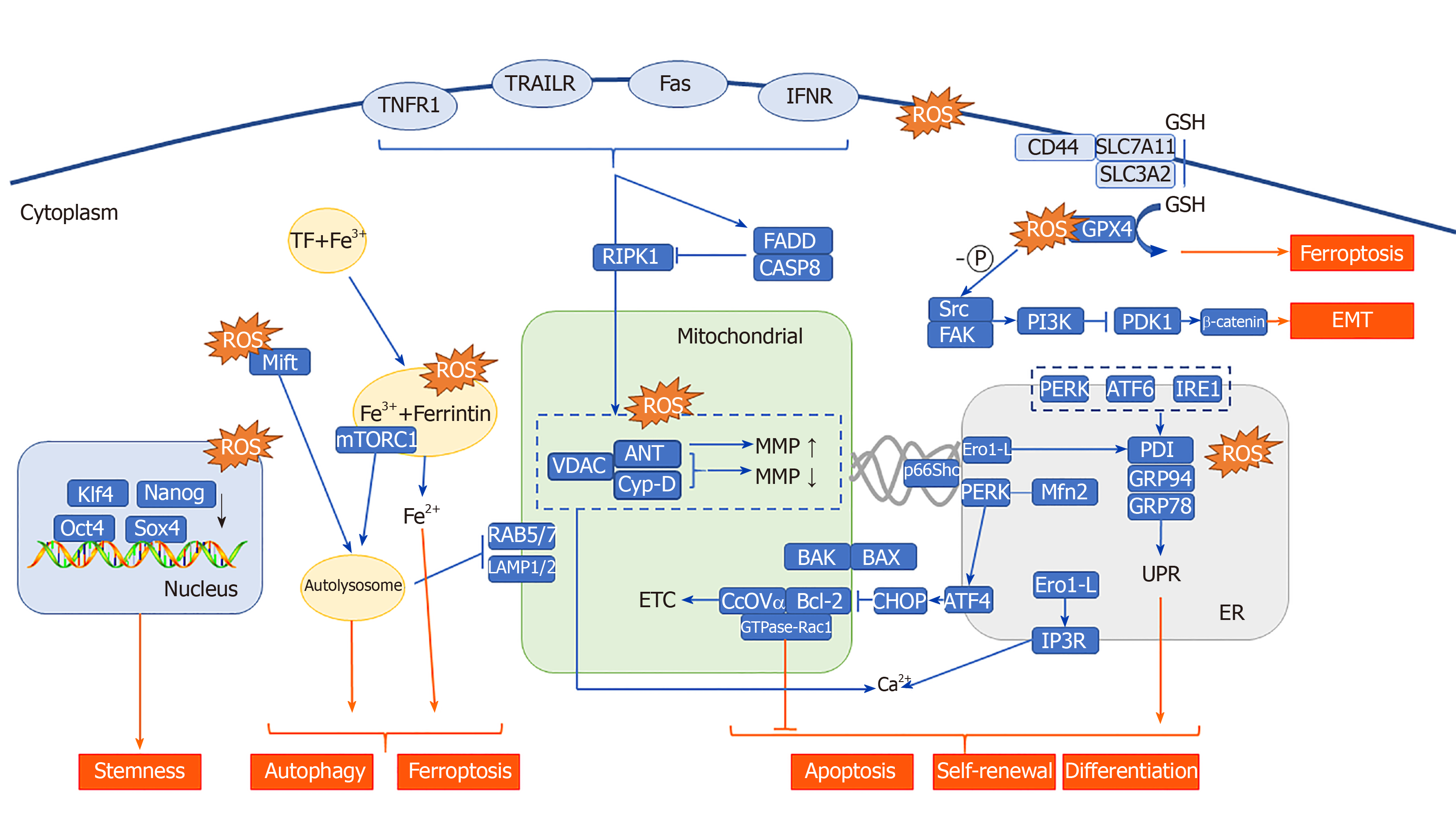
The effect of ROS on the function of mitochondria has always been one of the research focuses of CSCs. ROS produced through PDT can induce apoptosis through increased mitochondrial membrane potential[111]. Studies have shown that mitochondrial photooxidative stress can cause a large number of lipid peroxidation reactions in the mitochondrial membrane, leading to rapid changes in the MMP, which stimulates pressure-dependent anion channels [voltage-dependent anion channels (VDACs)][112] and promotes the opening of the permeability transition pore complex[113,114], thereby releasing cytochrome C (Cyt C)[115,116] into the cytoplasm. Cyt C combines with caspase-1 to form a multimer, which initiates apoptosis in CSCs in a caspase-dependent manner; in the non-caspase-dependent apoptosis pathway, other proteins are activated and released, such as apoptosis inducing factor, Omi/HtrA2, and endonuclease G during PDT[117-119]. The release of these enzymes depends on the cleavage of calpain[120]. These factors all directly lead to the apoptosis of CSCs.
B-cell leukemia 2 (BCL-2) family protein interactions are important in the induction of apoptosis induced by changes to the MMP in CSCs. And they are also important mitoROS modulators. In the canonical pathway, BCL-2 associated X and K proteins are located on the cytoplasmic side and the mitochondrial side of the outer mitochondrial membrane under normal conditions, respectively. When the MMP is increased, BCL-2 associated X protein is transported and inserted into the outer mitochondrial membrane, and the local conformation of BCL-2 associated K protein forms homo-oligomers or hetero-oligomers, which release Cyt C in the membrane space and initiate CSC apoptosis[121]. In the non-canonical pathway, BCL-2 may be involved in the regulation of the cell redox state without antioxidant characteristics. First, there is a physical interaction between BCL-2 and CcOVα (cytochrome c oxidase subunit Vα). Overexpression of BCL-2 causes an increase in mitochondrial localized CcOVα, which is conducive to CcO total enzyme assembly and the ETC process[122,123]. Second, the BCL-2 BH3 domain interacts with glutathione (GSH) in vitro, suggesting that BCL-2 functions in regulating mitochondrial GSH content[124]. Finally, the mitochondrial localization of GTPase-Rac1, which is associated with stem cell deletion, and its interaction with BCL-2 suggest that Rac1 plays an important antioxidant role[125]. These results suggest that BCL-2 may be a bridge connecting mitochondrial apoptosis and ROS in CSCs.
Energy metabolism is one of the main functions of mitochondria, and this process is closely related to the stemness of tumor cells. Research on energy metabolism in CSCs at various stages is rife with controversy. Early studies found that CSCs have more obvious anaerobic glycolytic characteristics than are expressed in differentiated cancer cells; that is, CSCs have increased expression of glycolytic enzymes, increased production of lactic acid, and decreased or resting mitochondrial function[126,127], and their ROS levels are usually lower than those of cancer cells. Recent studies have suggested that mitochondria in CSCs have increased mass and membrane potential, and their mitochondrial function reflects higher mitochondrial ROS levels and enhanced oxygen consumption rates[128]. In any case, mitochondrial function and oxygen concentration are essential for maintaining CSC function[129,130]. It has been inferred that under hypoxic conditions, some CSCs preferentially undertake oxidative phosphorylation for survival and maintenance of stemness and convert to glycolytic metabolism during differentiation[131]. The production of ROS by PDT consumes a large amount of oxygen, which forces CSCs to change from oxidative phosphorylation in the “stem cell” state to anaerobic glycolysis during differentiation, suggesting that PDT can effectively control the differentiation of CSCs. In addition, ROS can reduce the expression of caveolin-1 in cancer-associated fibroblasts, the major component of tumor stroma. The reduction of caveolin-1 stabilizes HIF-1α (which forms a heterodimer), enhances glycolysis to adapt to hypoxic conditions, and leads to a further increase in ROS production[132-134]. These studies indicate that oxygen depletion in mitochondria caused by PDT mediated ROS production inhibits stemness of cancer cells.
Some key genes may mediate both of these effects. PDT inhibits the Wnt pathway[135] which plays an important role in CSCs[136,137], inducing mitochondrial repression and glycolytic conversion by activating Dlx-2 and Snail[138-140]. This mitochondrial suppression is mediated by the inhibition of mitochondrial complex IV[140]. Wnt also directly targets pyruvate dehydrogenase kinase, thereby inhibiting mitochondrial respiration and promoting glycolytic conversion[141]. Therefore, the Wnt pathway may regulate CSCs through the above two functions at the same time. Currently, little is known about the relationship between typical stemness markers and the regulation of CSC metabolism, but researchers have shown that the stem cell marker CD44 may be crucial in the regulation of glycolytic metabolism[142,143]. The direct interaction between CD44 and the GSH transporter--solute carrier family 7 member 11 has been reported in multiple PDT articles[144,145], also suggesting the ability of PDT to manipulate CSCs through redox and energy metabolism.
Mitochondrial autophagy also plays a protective role against ROS in CSCs. Currently, it is widely recognized that mitochondrial autophagy counteracts PDT. For details, please refer to our previous review[146].
The most widespread and common effect of PDT-mediated photooxidative stress is the unfolded protein response, secondary to endoplasmic reticulum stress (UPRER, UPR). Increased endoplasmic reticulum-related ROS in PDT have been shown to cause upregulation of various ER molecular chaperones, such as calcium-binding proteins (GRP78/Bip and GRP94) and protein disulfide isomerase. These key proteins lead to the accumulation of unfolded proteins in the ER cavity, leading to PERK-, IRE1-, and ATF6-mediated UPR[147-149]. In the intestine, activation of UPR by PERK kinase leads to differentiation of intestinal epithelial stem cells and colon CSCs, and the absence of X box binding protein 1 results in increased stemness and adenoma formation. X box binding protein 1 activation results in reduced cell proliferation and intestinal epithelial cell stemness due to cross-activation of the PERK-eIF2α signaling[150]. In pancreatic cancer, GRP78 downregulates stem cell clone formation and self-renewal characteristics, suggesting that the UPR plays a role in inhibiting stemness in pancreatic cancer[151]. The contradictory results mentioned above can be explained by the dual nature of UPRER in both survival and apoptosis signaling. The fate of cells with respect to these two signal cascades depends on the intensity of the photooxidative stress. Severe ER photooxidative stress can stimulate more cascades that are transducing death-promoting signals (such as apoptosis), such as that stimulated by CCAAT/enhancer binding protein homologous protein, which is a key pro-apoptotic transcription factor in ERS[152,153]. A small or low level of ER photooxidative stress can stimulate more promoting survival signaling cascades (such as autophagy, p38 mitogen-activated protein kinase(MAPK) signaling, antioxidant signaling, and amino-terminal kinase JNK signaling)[154,155].
In addition to the UPR mechanism described above, ROS can affect the fate of CSCs through endoplasmic reticulum-mitochondrial crosstalk. PDT treatment causes the release of internal Ca2+ of the endoplasmic reticulum into the cytoplasm and mitochondria, inducing MMP-mediated apoptosis. In the above process, MAM (mitochondria-associated membrane), which is a solid-state connection between mitochondria and the endoplasmic reticulum, overexpress in CSCs. Its state and efficiency of the coupling are among the primary regulatory characteristics by which factors influence Ca2+ concentration in mitochondria[38-40]. Therefore, ROS generated by PDT may affect MAMs by various genes, such as P53, PML, ERO1, and p66Shc. P53 proved to be differentially expressed in PDT is involved in the regulation of Ca2+-mediated apoptosis in a transcription-independent manner. Among the proteins that accumulate in the MAMs, p53 activates the pathway to cell death, while p53 deletion leads to a Ca2+ decrease in the endoplasmic reticulum[156]. PML (promyelocytic leukemia) proved to be another tumor suppressor involved in regulating the endoplasmic reticulum-mitochondrial Ca2+ dialog[157]. The endoplasmic reticulum oxide protein endoplasmic oxidoreductin 1(ERO1)-Lα regulates the release of Ca2+, and the generation of ROS, from the endoplasmic reticulum through inositol 1,4,5-triphosphate receptor type 1 and thioredoxin domain containing 4[158,159]. It is involved in the formation of disulfide bonds with protein disulfide isomerase and therefore plays an important role in protein folding. p66Shc is an ROS-generating protein located in MAMs. When it undergoes oxidative stress, p66Shc is phosphorylated at Ser36 and then is translocated to mitochondria and/or MAMs. It is involved in ROS generation and apoptosis-related signaling pathways[160,161].
By integrating some studies, we also speculate that some factors are involved in the regulation of ERS and MAM at the same time after PDT treatment. First, PERK is highly expressed after PDT and can activate UPR. PERK is found to be localized to MAMs and promotes endoplasmic reticulum-mitochondria coupling[162]. Second, Mfn2, a kind of skeleton in MAMs, can regulate the endoplasmic reticulum associated autophagy and apoptosis by downregulating the activity of PERK[163,164]. PACS2, another important component of MAM, also participates in the autophagy process. In PACS2-knockout and Mfn2-knockout cells, the accumulation of autophagic markers and the translocation of endoplasmic reticulum-related proteins were significantly reduced, indicating that MAMs play specific roles in the formation of autophagosomes and ERS[165]. This shows that there is mutual regulation between ERS and MAM. Based on the research of ER-mitochondria crosstalk, the efficacy of autophagy and ERS inhibitors/activators combined with PDT in the treatment of CSCs has been verified in multiple studies, regardless of whether the photosensitizer is localized to the endoplasmic reticulum or mitochondria.
Lysosome status also directly or indirectly affects CSCs, such as through apoptosis initiation or autophagy flux. However, in addition to apoptosis and autophagy, lysosomes have recently been found to an play important role in the switch of eukaryotic cells to deep quiescence[166]. The dormant state that can be reversed by the stimulation of growth signals is called cell resting. In contrast, it is irreversible in state of senescence. The depth of the resting state of the cells is directly related to the difficulty of maintaining the stemness of CSCs and re-entering the proliferative state. Fujimaki et al[166] found that resting depth-related genes were significantly enriched in the lysosome pathway. By measuring the autophagy flux that characterizes lysosomal function, it was found that, as the resting state of the cells deepened, lysosomal function gradually decreased. The exogenously expressed transcription factor microphthalmia associated transcription factor enhanced lysosomal function in cells, and an increase in lysosomal function reduced the concentration of the ROS in the cell to prevent deepening of the resting state. The “switch” works by regulating the concentration of ROS in cells. The ROS produced by PDT may also regulate the resting state of cells through lysosomes, which may interfere with the stemness of CSCs and play a therapeutic role.
PDT mediates the activation of lysosomal-related PS, which can significantly induce the production of autophagy and mediate the release of cathepsin and lysosomal-mitochondrial crosstalk. The proliferation of colonic CSC spheres depends on the key autophagy related mTORC kinase, which is activated by the ROS produced by the NADPH oxidase (NOX)[167]. NOX1 is colocalized with mTORC1 in vacuolar assembly protein (VPS)41-/VPS39- lysosomes, where mTORC1 binds S100A9 (a member of the S100 calcium-binding protein) in an ROS-dependent manner, and S100A9 can thus be oxidized by ROS. This finding indicates that ROS in VPS41-/VPS39- lysosomes mediate S100A9 oxidation and mTORC1 activation, which are essential processes for colonic CSC proliferation and colon cancer progression[168]. Moreover, NOX1/2 and ROS-containing endosomal compartment co-localize with RAS and associated protein (RAB) 5/7[169,170], which is involved in the lysosomal-mitochondrial crosstalk pathway and plays a key role in maintaining CSC survival[171]. The investigators determined that RAB5/7 overexpression can effectively inhibit CSCs. Further, they found that mefloquine hydrochloride (an autophagy inhibitor) is involved in the endolysosomal pathway by targeting RAB5/7, and this effect is dependent on the mitochondrial autophagy key protein PTEN induced putative kinase 1/parkinson disease protein 2. Although there is no direct evidence, it can be speculated that ROS produced by NOX may mediate lysosomal-mitochondrial crosstalk.
Fe2+ plays an important role in lysosomal-mitochondrial crosstalk. The substance to be degraded in the cytoplasm is entrapped in autophagic vesicles, and then, the contents of autophagic vesicles and lysosomes are degraded under the action of lysosomal enzymes. Autophagy leads to a large amount of redox-active iron (Fe2+) accumulating in the lysosome cavity. These high concentrations of active iron can cause membrane instability when the lysosome is undergoing slight oxidative stress. PDT that targets lysosomes can directly result in the release of a large number of proteases upon photooxidative stress and thus promote activation of endogenous apoptosis-related protein[172,173]. In addition, in the study of the antitumor effect of bafilomycin (an autophagy inhibitor) combined with phthalocyanine 4 (Pc4, photosensitizer), the lysosome was found to be alkalized by bafilomycin, causing the collapse of the pH gradient and the release of Fe2+. Subsequently, mitochondria accumulate a large amount of active iron (Fe2+) through the one-way Ca2+ transport channel, thus generating OH through the Fenton reaction with H2O2. Thus, the apoptosis pathway is initiated. This study shows that autophagy inhibitors combined with Fe2+ can further increase the effect of PDT on CSCs. In fact, the Fenton reaction is very commonly used in the design and synthesis of nano-photosensitizers. Currently, some studies have used the Fenton reaction to increase the concentration of O2[174] or hydroxyl radicals[175] in the tumor microenvironment, and others used it to directly kill CSCs. PDT combined with the Fenton reaction[176] is a feasible treatment method for CSCs showing strong drug resistance. In addition, a new type of programmed cell death, ferroptosis, is also closely related to Fe2+ and the Fenton reaction[177]. Some researchers have found that ferricin chelates lysosomal iron[178]. These compounds induce iron depletion by preventing iron transport, leading to the dissolution of ferritin. Enzymatic degradation, followed by ROS formation and iron release, mediates the lysosomal ferroptosis pathway. In the development of PS, some scientists have induced ferroptosis by artificially reducing Fe2+ or releasing Fe2+ in the lysosome, creating a potential therapeutic mechanism for killing apoptosis-resistant CSCs (Figure 2).
Excessive lipophilic dyes, anionic dyes, and photosensitizers (especially localized on biological membranes) can affect different unsaturated phospholipids and membrane cholesterol, leading to excessive lipid peroxidation[179], which directly leads to a wide range of membrane changes, loss of membrane integrity and fluidity, and inactivation of the membrane protein system, causing accidental cell necrosis. This effect can be induced with all types of PDT under sufficient ROS. The exogenous pathway of apoptosis is mediated by death receptors on the cell membrane. Tumor necrosis factor type-I receptor (TNFR1), Fas/CD95, DR3, and TNF-related apoptosis-inducing ligands R1 and R2 (TRAIL-R1 and TRAIL-R2) were found to be differentially expressed upon PDT, and their death domains are necessary for exogenous apoptosis. Exogenous apoptosis induced by PDT is usually activated by cytokines released by photooxidative stress or dead cells[180,181]. A very low dose of sulfathiophene (2.0 μg/mL) combined with radachlorin-PDT (0.5 μg/mL) showed a synergistic effect of exogenous and endogenous apoptosis[182]. The CSCs in the CD44 (+)/CD24 (-/low) subgroup were more sensitive to Fas- and TRAIL-mediated exogenous apoptotic pathways; therefore, the PDT-induced exogenous receptor apoptosis pathway may be very sensitive in this subgroup[183]. Through methotrexate-mediated epigenetic enhancement of ALA-PDT, Salva and colleagues restored sensitivity to death receptor-related pathways and increased the effect in subgroups of cells with low expression of Fas[184]. Exogenous and endogenous apoptosis induced by PDT generally does not happen separately. In many studies, PDT mediated by phenalenone[181], coralyne[185], ALA[186], hypericin[187], dipyrromethene boron difluoride(BODIPY)[188], and zinc phthalocyanine[189] can cause both endogenous and exogenous apoptosis. This dual apoptosis phenomenon is generated primarily by signaling molecules, including p38 MAPK, Janus kinase (JAK)-2, and signal transducer and activator of transcription(STAT)-1.
PDT is a treatment method that mainly induces apoptosis, and most forms of apoptosis are based on immune silencing or immune tolerance, that is, tolerogenic apoptosis[190]. This immune tolerance to apoptosis is an important host protection mechanism. After photooxidative stress, most apoptotic cells express chemotaxis signals, such as intercellular cell adhesion molecule, phosphatidylethanolamine, phosphatidylinositol, and low-density lipoprotein, and signals that inhibit anti-phagocytosis proteins, such as CD31 and CD47, to promote the phagocytosis of tolerogenic cells by macrophages, prevent immune responses, and exhibit tolerant immunobiological characteristics[191-194].
However, in a significant portion of PDT samples, lysates of necrotic or apoptotic cells enhance immunogenicity[173]. Through this PDT-induced enhanced immunogenicity, CSCs may be cleared by immunocytes. Immunogenic apoptosis has all the biochemical markers of tolerogenic apoptosis, but tolerogenic apoptosis does not have the following two main characteristics: (1) Exposure to or secretion of important immunogenic signaling proteins or damage-associated molecular patterns (DAMPs); and (2) The ability to activate the host's immune system. DAMPs can be secreted or expressed on the cell surface after cell injury or death to promote inflammation[195,196]. It has been found that photooxidative stress, especially mitochondrial photooxidative stress, can cause DAMPs to be expressed on the cell surface (ecto-) or released outside the cell (exo-), such as calreticulin (CRT) and GRP78[197,198]. IFN-1 has recently been recognized as a new type of DAMP that links innate and adaptive immunity, and it has been hypothesized to be the basic requirement for inducing immunogenic cell death, especially through the activation of dendritic cells. Me-ALA-induced PpIX (endogenous PS) was found to upregulate IFN-1 expression in B16-OVA melanoma cells[199]. This upregulation of α/β transcripts coincided with the interferon regulatory factor-3 phosphorylation that activated STAT1 and increased the expression of ligand receptors and interferon- stimulated genes (ISGs, like chemokine (C-X-C motif) ligand 10, interferon-induced GTP-binding protein Mx1, and ubiquitin-like protein ISG15). In this sense, PDT-treated melanoma cells induce IFN-1-dependent phenotype maturation of dendritic cells by enhancing costimulatory signals (CD80 and MHC-II molecules) and tumor-directed chemotaxis. Based on the discovery of enhanced immunogenicity, there have been studies combining PDT and immunotherapy, loading si-PD-L1[200], docetaxel (DTX)[201], PD-L1 monoclonal antibody[202], and anti-PD-L1 peptide[203], among others, to enhance the antitumor effect.
Compared with those of specific drug-induced immunogenicity, PDT-mediated immunogenic activation also has certain targeting advantages. Although mitoxantrone, mitomycin C, 5-fluorouracil, camptothecin, cisplatin, oxaliplatin, ultraviolet radiation, and γ-radiation can induce ROS-mediated ERS[204-207], in most cases, ERS induction is nontargeted and not completely efficient. In the pre-apoptotic stage, ERS-mediated immunogenic apoptosis is accompanied by ecto-CRT and exo-ATP production[197,201]. Another study found that Photofrin-PDT mainly produces mitochondrial photooxidative stress and a small amount of ER photooxidative stress. ER photooxidative stress mediated by hypericin-PDT can induce immunogenic apoptosis through targeted oxidative stress[208]. The expression of pre-apoptotic ecto-CRT in the mitochondria of Hyp-PDT-treated cells is not as high as it is under ERS targeting[197]. This finding suggests that photosensitizers targeting the ER may have a better effect in inducing immunogenicity.
The activation of cancer cell invasion and metastasis is one of the characteristics that changes the normal function of cells to acquire enhanced malignant growth, and it is also the main obstacle for humans to overcome cancer[209]. The EMT refers to the biological process in which epithelial cells are transformed into cells with interstitial phenotypes under special physiological or pathological conditions; that is, the epithelial cells lose their original phenotype connected with the basement membrane and acquire resistance to the phenotype of interstitial cells, such as the ability to undergo apoptosis or degrade the extracellular matrix, and higher migration and invasion[210]. The weakening of tumor cell adhesion and the enhancement of tumor cell movement are the basis of invasion and metastasis. The EMT provides the conditions for invasion and metastasis of tumor cells of epithelial origin. Therefore, the EMT is closely related to tumor invasion and metastasis. The relevant molecular mechanism of the EMT in tumor cells is the loss of cell epithelial morphology and related markers (including E-cadherin, desmosome smoothelin, Muc-1, cytokeratin 18, occludins, claudins, and ZO-1) and the acquisition of mesenchymal markers (including N-cadherin, vimentin, fibronectin, vitronectin, alpha smooth muscle actin [α-SMA], and FSP1)[27]. This process is affected by the regulatory effects of Snail, Slug, ZEB1, and Twist1 in the tumor microenvironment. Among all the factors that promote tumor cell migration, ROS play key roles by activating signals that cause cell migration, such as activating the proto-oncogene tyrosine protein kinase (Src) and focal adhesion kinase (FAK)[211]. The EMT is the initial step of ROS-activated tumor cell migration. During the ROS generation process, the EMT is also affected by the regulation of Src and FAK, resulting in tumor cell migration. Studies have found that para-cresyl sulfate can promote tumor cell migration by activating ROS/Src/FAK signaling in bladder cancer tumor cells[212]. Goulet et al[213,214] found that IL6 can regulate cancer-associated fibroblast-induced EMT through the STAT3/AKT signaling pathway in bladder cancer, induce myeloma cell proliferation through Src, and regulate the Src/STAT3 signaling pathway in lymphatic endothelial cells. Therefore, IL6 may be a regulator of ROS-activated Src/FAK signaling.
The EMT plays an important role in the process of acquiring stemness by tumor stem cells. Transcription factors that regulate EMT, such as Snail, ZEB1, and Twist1, are involved in the process of conferring stemness to CSCs. For example, Snail can induce a CSC phenotype in colorectal cancer cells, in which it enhances stemness properties and resistance to radiotherapy[215]. ZEB1 inhibits the expression of miRNAs, including miR-183, miR-200c, and miR-203, and thus upregulates the stem cell-related factors Sox2 and Klf4. Knocking out ZEB1 prevents not only the EMT and cell invasion and metastasis but also the emergence of stem cell phenotypes[216]. Twist1 can induce the EMT and stem cell properties by increasing Bmi-1 expression and acting synergistically with it. EMT induced by TGF-β1 plays a key role in the generation of CSCs and is involved in maintaining the characteristics of CSCs, such as self-renewal and differentiation[217]. Most cholangiocarcinoma CSCs have epithelial and mesenchymal characteristics and express EMT markers. IL6, which may be involved in ROS-activated Src/FAK signaling, can regulate stem cell self-renewal. In breast cancer, head and neck squamous cell carcinoma, gastric cancer, and glioma, IL-6 promotes stem cell self-renewal through the classic IL-6R/gp130/STAT3 signaling pathway, while IL-6 also increases N-cadherin, E-cadherin, Twist, Snail, and Vimentin expression to accelerate the tumor cell EMT, which leads to cancer metastasis. Studies have shown that ROS may activate the NF-κB pathway and cause gastric cancer cells and cancer-associated fibroblast cells to release IL-6, thereby mediating tumor metastasis and CSC self-renewal and maintenance[218,219]. It can be seen from the above literature that PDT mainly inhibits CSCs through IL-6/SRC/FAK mediated EMT mechanism.
Although PDT is promising in the treatment of CSCs, several pitfalls will have to be overcome in order to take a step forward in clinical application. First, despite the obvious tumor targeting of PS, the heterogeneity of CSCs determines that it is difficult for a single targeted PS to eliminate all CSCs. The single-cell sequencing will be very useful for exploring molecular subtypes common to different CSCs. Second, CSCs account for a small proportion of tumors and are widely distributed. In order to enhance the killing effect against deep CSCs by PDT, it is necessary to design the PS that can absorb longer wavelengths. The dosimetry of PDT is also one of the existing challenges. The ROS yield of PS determines whether the CSCs will metastasize and recur. Developing different PS-PDT treatment criteria from different subcellular localizations can help reduce adverse outcomes. Finally, in view of the immune activating effect of PDT, PDT combined with immunotherapy has shown good experimental results. However, how to safely and effectively use PDT to activate immunity is still a difficult problem in clinical treatment design. Based on these considerations, although PDT exhibits anti-tumor (including CSCs) effects, further research is needed to expand clinical application.
We thank Dr. Xiao-Xue Li for her comments and suggestions.
| 1. | Chen J, Fan T, Xie Z, Zeng Q, Xue P, Zheng T, Chen Y, Luo X, Zhang H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials. 2020;237:119827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 2. | Huis In 't Veld RV, Ritsma L, Kleinovink JW, Que I, Ossendorp F, Cruz LJ. Photodynamic cancer therapy enhances accumulation of nanoparticles in tumor-associated myeloid cells. J Control Release. 2020;320:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Kessel D. Photodynamic Therapy: A Brief History. J Clin Med. 2019;8:pii: E1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Wang X, Ramamurthy G, Shirke AA, Walker E, Mangadlao J, Wang Z, Wang Y, Shan L, Schluchter MD, Dong Z, Brady-Kalnay SM, Walker NK, Gargesha M, MacLennan G, Luo D, Sun R, Scott B, Roy D, Li J, Basilion JP. Photodynamic Therapy Is an Effective Adjuvant Therapy for Image-Guided Surgery in Prostate Cancer. Cancer Res. 2020;80:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. Photosensitizers for Photodynamic Therapy. Adv Healthc Mater. 2019;8:e1900132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 666] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 6. | Ozog DM, Rkein AM, Fabi SG, Gold MH, Goldman MP, Lowe NJ, Martin GM, Munavalli GS. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol Surg. 2016;42:804-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Bichakjian CK, Olencki T, Aasi SZ, Alam M, Andersen JS, Berg D, Bowen GM, Cheney RT, Daniels GA, Glass LF, Grekin RC, Grossman K, Higgins SA, Ho AL, Lewis KD, Lydiatt DD, Nehal KS, Nghiem P, Olsen EA, Schmults CD, Sekulic A, Shaha AR, Thorstad WL, Tuli M, Urist MM, Wang TS, Wong SL, Zic JA, Hoffmann KG, Engh A. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Gilaberte Y, Aguilar M, Almagro M, Correia O, Guillén C, Harto A, Pérez-García B, Pérez-Pérez L, Redondo P, Sánchez-Carpintero I, Serra-Guillén C, Valladares LM. Spanish-Portuguese consensus statement on use of daylight-mediated photodynamic therapy with methyl aminolevulinate in the treatment of actinic keratosis. Actas Dermosifiliogr. 2015;106:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Hashimoto A, Takamura-Enya T, Oda Y. Synthesis and In Vitro Biological Evaluation of Psoralen-Linked Fullerenes. Photochem Photobiol. 2019;95:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Homma T, Kobayashi S, Fujii J. Induction of ferroptosis by singlet oxygen generated from naphthalene endoperoxide. Biochem Biophys Res Commun. 2019;518:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers (Basel). 2017;9:pii: E19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 659] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 12. | Kong F, Zou H, Liu X, He J, Zheng Y, Xiong L, Miao X. miR-7112-3p targets PERK to regulate the endoplasmic reticulum stress pathway and apoptosis induced by photodynamic therapy in colorectal cancer CX-1 cells. Photodiagnosis Photodyn Ther. 2020;29:101663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Lange C, Lehmann C, Mahler M, Bednarski PJ. Comparison of Cellular Death Pathways after mTHPC-mediated Photodynamic Therapy (PDT) in Five Human Cancer Cell Lines. Cancers (Basel). 2019;11:pii: E702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40:340-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1454] [Cited by in RCA: 1529] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 15. | Wang HW, Zhang Y, Tan PP, Jia LS, Chen Y, Zhou BH. Mitochondrial respiratory chain dysfunction mediated by ROS is a primary point of fluoride-induced damage in Hepa1-6 cells. Environ Pollut. 2019;255:113359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Wang XQ, Gao F, Zhang XZ. Initiator-Loaded Gold Nanocages as a Light-Induced Free-Radical Generator for Cancer Therapy. Angew Chem Int Ed Engl. 2017;56:9029-9033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Deng Y, Jia F, Chen S, Shen Z, Jin Q, Fu G, Ji J. Nitric oxide as an all-rounder for enhanced photodynamic therapy: Hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials. 2018;187:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 18. | Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015;22:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 667] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 19. | Lee BWL, Ghode P, Ong DST. Redox regulation of cell state and fate. Redox Biol. 2019;25:101056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Liang R, Ghaffari S. Stem cells, redox signaling, and stem cell aging. Antioxid Redox Signal. 2014;20:1902-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1570] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 22. | Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 1170] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 23. | Rimmelé P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, Ghaffari S. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Reports. 2014;3:44-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Akhtar MJ, Ahamed M, Alhadlaq HA. Challenges facing nanotoxicology and nanomedicine due to cellular diversity. Clin Chim Acta. 2018;487:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Li Z, Wang C, Deng H, Wu J, Huang H, Sun R, Zhang H, Xiong X, Feng M. Robust Photodynamic Therapy Using 5-ALA-Incorporated Nanocomplexes Cures Metastatic Melanoma through Priming of CD4+CD8+ Double Positive T Cells. Adv Sci (Weinh). 2019;6:1802057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Zhou TJ, Xing L, Fan YT, Cui PF, Jiang HL. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J Control Release. 2019;307:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Chakraborty S, Mir KB, Seligson ND, Nayak D, Kumar R, Goswami A. Integration of EMT and cellular survival instincts in reprogramming of programmed cell death to anastasis. Cancer Metastasis Rev. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Della Pietra E, Simonella F, Bonavida B, Xodo LE, Rapozzi V. Repeated sub-optimal photodynamic treatments with pheophorbide a induce an epithelial mesenchymal transition in prostate cancer cells via nitric oxide. Nitric Oxide. 2015;45:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Baptista MS, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente C, Nunez SC, Ribeiro MS, Thomas AH, Vignoni M, Yoshimura TM. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem Photobiol. 2017;93:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 582] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 30. | Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 844] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Rywkin S, Lenny L, Goldstein J, Geacintov NE, Margolis-Nunno H, Horowitz B. Importance of type I and type II mechanisms in the photodynamic inactivation of viruses in blood with aluminum phthalocyanine derivatives. Photochem Photobiol. 1992;56:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic Biol Med. 2007;43:711-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Mehraban N, Freeman HS. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials (Basel). 2015;8:4421-4456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Gemmell NR, McCarthy A, Kim MM, Veilleux I, Zhu TC, Buller GS, Wilson BC, Hadfield RH. A compact fiber-optic probe-based singlet oxygen luminescence detection system. J Biophotonics. 2017;10:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Sarniak A, Lipińska J, Tytman K, Lipińska S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig Med Dosw (Online). 2016;70:1150-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Payen VL, Zampieri LX, Porporato PE, Sonveaux P. Pro- and antitumor effects of mitochondrial reactive oxygen species. Cancer Metastasis Rev. 2019;38:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 796] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 38. | Zhang Y, Sun R, Geng S, Shan Y, Li X, Fang W. Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium. J Virol. 2019;93:pii: e01784-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Farrukh MR, Nissar UA, Afnan Q, Rafiq RA, Sharma L, Amin S, Kaiser P, Sharma PR, Tasduq SA. Oxidative stress mediated Ca (2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J Dermatol Sci. 2014;75:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Matsumoto T, Uchiumi T, Monji K, Yagi M, Setoyama D, Amamoto R, Matsushima Y, Shiota M, Eto M, Kang D. Doxycycline induces apoptosis via ER stress selectively to cells with a cancer stem cell-like properties: importance of stem cell plasticity. Oncogenesis. 2017;6:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Zhao H, Yin R, Wang Y, Lee YH, Luo T, Zhang J, Qiu H, Ambrose S, Wang L, Ren J, Yao J, Chen D, Wang Y, Liang Z, Zhen J, Wu S, Ye Z, Zeng J, Huang N, Gu Y. Modulating mitochondrial morphology enhances antitumor effect of 5-ALA-mediated photodynamic therapy both in vitro and in vivo. J Photochem Photobiol B. 2017;176:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Yu S, Zhang L, Liu C, Yang J, Zhang J, Huang L. PACS2 is required for ox-LDL-induced endothelial cell apoptosis by regulating mitochondria-associated ER membrane formation and mitochondrial Ca2+ elevation. Exp Cell Res. 2019;379:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM, Hunt DW. Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 1998;437:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Kalpage HA, Bazylianska V, Recanati MA, Fite A, Liu J, Wan J, Mantena N, Malek MH, Podgorski I, Heath EI, Vaishnav A, Edwards BF, Grossman LI, Sanderson TH, Lee I, Hüttemann M. Tissue-specific regulation of cytochrome c by post-translational modifications: respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 2019;33:1540-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 45. | Yen HC, Liu CC, Kan CC, Chen CS, Wei HR. Suppression of coenzyme Q10 levels and the induction of multiple PDSS and COQ genes in human cells following oligomycin treatment. Free Radic Res. 2014;48:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Kim WS, Lee KS, Kim JH, Kim CK, Lee G, Choe J, Won MH, Kim TH, Jeoung D, Lee H, Kim JY, Ae Jeong M, Ha KS, Kwon YG, Kim YM. The caspase-8/Bid/cytochrome c axis links signals from death receptors to mitochondrial reactive oxygen species production. Free Radic Biol Med. 2017;112:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Lee SR, An EJ, Kim J, Bae YS. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol Ther (Seoul). 2020;28:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Zhang Q, Wang S, Zheng S, Zhang Z, Xu S. Chlorpyrifos Suppresses Neutrophil Extracellular Traps in Carp by Promoting Necroptosis and Inhibiting Respiratory Burst Caused by the PKC/MAPK Pathway. Oxid Med Cell Longev. 2019;2019:1763589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Boukemara H, Hurtado-Nedelec M, Marzaioli V, Bendjeddou D, El Benna J, Marie JC. Anvillea garcinii extract inhibits the oxidative burst of primary human neutrophils. BMC Complement Altern Med. 2016;16:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Nagano T. [Chemical and biochemical studies on reactivities, formations and toxicities of reactive oxygen species]. Yakugaku Zasshi. 1991;111:103-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Nandi A, Yan LJ, Jana CK, Das N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid Med Cell Longev. 2019;2019:9613090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 660] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 52. | Saita M, Kobayashi K, Yoshino F, Hase H, Nonami T, Kimoto K, Lee MC. ESR investigation of ROS generated by H2O2 bleaching with TiO2 coated HAp. Dent Mater J. 2012;31:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Petronek MS, Spitz DR, Buettner GR, Allen BG. Linking Cancer Metabolic Dysfunction and Genetic Instability through the Lens of Iron Metabolism. Cancers (Basel). 2019;11:pii: E1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Liu T, Jin R, Yuan P, Bai Y, Cai B, Chen X. Intracellular Enzyme-Triggered Assembly of Amino Acid-Modified Gold Nanoparticles for Accurate Cancer Therapy with Multimode. ACS Appl Mater Interfaces. 2019;11:28621-28630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Hu D, Deng Y, Jia F, Jin Q, Ji J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano. 2020;14:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 296] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 56. | Wu M, Ding Y, Li L. Recent progress in the augmentation of reactive species with nanoplatforms for cancer therapy. Nanoscale. 2019;11:19658-19683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Clennan EL, Pace A. Advances in Singlet Oxygen Chemistry. Tetrahedron. 2005;61:6665-6691. [RCA] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 368] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 58. | Masschelin PM, Cox AR, Chernis N, Hartig SM. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front Physiol. 2019;10:1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 59. | Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 317] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 60. | Das B, Pal B, Bhuyan R, Li H, Sarma A, Gayan S, Talukdar J, Sandhya S, Bhuyan S, Gogoi G, Gouw AM, Baishya D, Gotlib JR, Kataki AC, Felsher DW. MYC Regulates the HIF2α Stemness Pathway via Nanog and Sox2 to Maintain Self-Renewal in Cancer Stem Cells versus Non-Stem Cancer Cells. Cancer Res. 2019;79:4015-4025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 61. | Moreira H, Szyjka A, Paliszkiewicz K, Barg E. Prooxidative Activity of Celastrol Induces Apoptosis, DNA Damage, and Cell Cycle Arrest in Drug-Resistant Human Colon Cancer Cells. Oxid Med Cell Longev. 2019;2019:6793957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Sytykiewicz H, Łukasik I, Goławska S, Chrzanowski G. Aphid-Triggered Changes in Oxidative Damage Markers of Nucleic Acids, Proteins, and Lipids in Maize (Zea mays L.) Seedlings. Int J Mol Sci. 2019;20:pii: E3742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Guo L, Zhao Y, Liu D, Liu Z, Chen C, Xu R, Tian M, Wang X, Chen H, Kong MG. Cold atmospheric-pressure plasma induces DNA-protein crosslinks through protein oxidation. Free Radic Res. 2018;52:783-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Kim SL, Choi HS, Kim JH, Jeong DK, Kim KS, Lee DS. Dihydrotanshinone-Induced NOX5 Activation Inhibits Breast Cancer Stem Cell through the ROS/Stat3 Signaling Pathway. Oxid Med Cell Longev. 2019;2019:9296439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Ozsvari B, Sotgia F, Lisanti MP. A new mutation-independent approach to cancer therapy: Inhibiting oncogenic RAS and MYC, by targeting mitochondrial biogenesis. Aging (Albany NY). 2017;9:2098-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, Chen YJ. Resveratrol Impedes the Stemness, Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through p53 Activation. Evid Based Complement Alternat Med. 2013;2013:590393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Chen D, Tao R, Tao K, Chen B, Choi SK, Tian Q, Xu Y, Zhou G, Sun K. Efficacy Dependence of Photodynamic Therapy Mediated by Upconversion Nanoparticles: Subcellular Positioning and Irradiation Productivity. Small. 2017;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Wen Y, Zhang ZJ, Huang YP, Wang KP, Liu K, Zou H, Zhou JJ, Zou ZX, Luo SL, Liu ZT, Wu ZC, Chen W, Xiong L. Application of the Ethyl Acetate Extract of Cichorium as a Potential Photosensitizer in Photodynamic Therapy Induces Apoptosis and Autophagy in Colorectal Cancer Cell Lines via the Protein Kinase R-Like Endoplasmic Reticulum Kinase Pathway. J Biomed Nanotechnol. 2019;15:1867-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Zhao X, Yang Y, Yu Y, Guo S, Wang W, Zhu S. A cyanine-derivative photosensitizer with enhanced photostability for mitochondria-targeted photodynamic therapy. Chem Commun (Camb). 2019;55:13542-13545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Zhao N, Li P, Zhuang J, Liu Y, Xiao Y, Qin R, Li N. Aggregation-Induced Emission Luminogens with the Capability of Wide Color Tuning, Mitochondrial and Bacterial Imaging, and Photodynamic Anticancer and Antibacterial Therapy. ACS Appl Mater Interfaces. 2019;11:11227-11237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Liu C, Zhou L, Wei F, Li L, Zhao S, Gong P, Cai L, Wong KM. Versatile Strategy To Generate a Rhodamine Triplet State as Mitochondria-Targeting Visible-Light Photosensitizers for Efficient Photodynamic Therapy. ACS Appl Mater Interfaces. 2019;11:8797-8806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 72. | Raza MK, Gautam S, Howlader P, Bhattacharyya A, Kondaiah P, Chakravarty AR. Pyriplatin-Boron-Dipyrromethene Conjugates for Imaging and Mitochondria-Targeted Photodynamic Therapy. Inorg Chem. 2018;57:14374-14385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Raza MK, Gautam S, Garai A, Mitra K, Kondaiah P, Chakravarty AR. Monofunctional BODIPY-Appended Imidazoplatin for Cellular Imaging and Mitochondria-Targeted Photocytotoxicity. Inorg Chem. 2017;56:11019-11029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 74. | Rui L, Xue Y, Wang Y, Gao Y, Zhang W. A mitochondria-targeting supramolecular photosensitizer based on pillar[5]arene for photodynamic therapy. Chem Commun (Camb). 2017;53:3126-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 75. | Hu Z, Sim Y, Kon OL, Ng WH, Ribeiro AJ, Ramos MJ, Fernandes PA, Ganguly R, Xing B, García F, Yeow EK. Unique Triphenylphosphonium Derivatives for Enhanced Mitochondrial Uptake and Photodynamic Therapy. Bioconjug Chem. 2017;28:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Jia Y, Wang X, Liu Q, Leung AW, Wang P, Xu C. Sonodynamic action of hypocrellin B triggers cell apoptoisis of breast cancer cells involving caspase pathway. Ultrasonics. 2017;73:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Wu J, Xiao Q, Zhang N, Xue C, Leung AW, Zhang H, Tang QJ, Xu C. Palmatine hydrochloride mediated photodynamic inactivation of breast cancer MCF-7 cells: Effectiveness and mechanism of action. Photodiagnosis Photodyn Ther. 2016;15:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Wu J, Xiao Q, Zhang N, Xue C, Leung AW, Zhang H, Xu C, Tang QJ. Photodynamic action of palmatine hydrochloride on colon adenocarcinoma HT-29 cells. Photodiagnosis Photodyn Ther. 2016;15:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Ichikawa H, Nishie H, Yano S, Komai Y, Yamaguchi H, Nomoto A, Suzuki T, Tanaka M, Shimura T, Mizoshita T, Kubota E, Tanida S, Kataoka H. Antitumor Effect of a Novel Photodynamic Therapy With Acetylated Glucose-conjugated Chlorin for Gastrointestinal Cancers. Anticancer Res. 2019;39:4199-4206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Yang K, Niu T, Luo M, Tang L, Kang L. Enhanced cytotoxicity and apoptosis through inhibiting autophagy in metastatic potential colon cancer SW620 cells treated with Chlorin e6 photodynamic therapy. Photodiagnosis Photodyn Ther. 2018;24:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Abdulrehman G, Xv K, Li Y, Kang L. Effects of meta-tetrahydroxyphenylchlorin photodynamic therapy on isogenic colorectal cancer SW480 and SW620 cells with different metastatic potentials. Lasers Med Sci. 2018;33:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Hayashi N, Kataoka H, Yano S, Tanaka M, Moriwaki K, Akashi H, Suzuki S, Mori Y, Kubota E, Tanida S, Takahashi S, Joh T. A novel photodynamic therapy targeting cancer cells and tumor-associated macrophages. Mol Cancer Ther. 2015;14:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Tian J, Xu L, Xue Y, Jiang X, Zhang W. Enhancing Photochemical Internalization of DOX through a Porphyrin-based Amphiphilic Block Copolymer. Biomacromolecules. 2017;18:3992-4001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Zhang FL, Song MR, Yuan GK, Ye HN, Tian Y, Huang MD, Xue JP, Zhang ZH, Liu JY. A Molecular Combination of Zinc(II) Phthalocyanine and Tamoxifen Derivative for Dual Targeting Photodynamic Therapy and Hormone Therapy. J Med Chem. 2017;60:6693-6703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Berndt-Paetz M, Weimann A, Sieger N, Schastak S, Riyad YM, Griebel J, Arthanareeswaran VKA, Stolzenburg JU, Neuhaus J. Tetrahydroporphyrin-tetratosylat (THPTS): A near-infrared photosensitizer for targeted and efficient photodynamic therapy (PDT) of human bladder carcinoma. An in vitro study. Photodiagnosis Photodyn Ther. 2017;18:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Yang ZY, Li H, Zeng YP, Hao YH, Liu C, Liu J, Wang WD, Li R. Photosensitizer-Loaded Branched Polyethylenimine-PEGylated Ceria Nanoparticles for Imaging-Guided Synchronous Photochemotherapy. ACS Appl Mater Interfaces. 2015;7:24218-24228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Zielichowska A, Saczko J, Garbiec A, Dubińska-Magiera M, Rossowska J, Surowiak P, Choromańska A, Daczewska M, Kulbacka J, Lage H. The photodynamic effect of far-red range phthalocyanines (AlPc and Pc green) supported by electropermeabilization in human gastric adenocarcinoma cells of sensitive and resistant type. Biomed Pharmacother. 2015;69:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Chen JJ, Hong G, Gao LJ, Liu TJ, Cao WJ. In vitro and in vivo antitumor activity of a novel porphyrin-based photosensitizer for photodynamic therapy. J Cancer Res Clin Oncol. 2015;141:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Song L, Huang Y, Hou X, Yang Y, Kala S, Qiu Z, Zhang R, Sun L. PINK1/Parkin-Mediated Mitophagy Promotes Resistance to Sonodynamic Therapy. Cell Physiol Biochem. 2018;49:1825-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Yang Y, Liu Y, Chen X, Gong J, Huang Z, Wang W, Shi Y, Wang Y, Yao J, Shen Z, Tian Z, Jin H, Tian Y. 5-Aminolevulinic Acid-Mediated Sonodynamic Therapy Alleviates Atherosclerosis via Enhancing Efferocytosis and Facilitating a Shift in the Th1/Th2 Balance Toward Th2 Polarization. Cell Physiol Biochem. 2018;47:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Taba F, Onoda A, Hasegawa U, Enoki T, Ooyama Y, Ohshita J, Hayashi T. Mitochondria-Targeting Polyamine-Protoporphyrin Conjugates for Photodynamic Therapy. ChemMedChem. 2018;13:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Chen JJ, Gao LJ, Liu TJ. Photodynamic therapy with a novel porphyrin-based photosensitizer against human gastric cancer. Oncol Lett. 2016;11:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | de Almeida RMS, Fontana LC, Dos Santos Vitorio G, Pereira AHC, Soares CP, Pinto JG, Ferreira-Strixino J. Analysis of the effect of photodynamic therapy with Fotoenticine on gliosarcoma cells. Photodiagnosis Photodyn Ther. 2020;101685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Tong H, Du J, Li H, Jin Q, Wang Y, Ji J. Programmed photosensitizer conjugated supramolecular nanocarriers with dual targeting ability for enhanced photodynamic therapy. Chem Commun (Camb). 2016;52:11935-11938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Zhao X, Huang Y, Yuan G, Zuo K, Huang Y, Chen J, Li J, Xue J. A novel tumor and mitochondria dual-targeted photosensitizer showing ultra-efficient photodynamic anticancer activities. Chem Commun (Camb). 2019;55:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Chen JJ, Huang YZ, Song MR, Zhang ZH, Xue JP. Silicon Phthalocyanines Axially Disubstituted with Erlotinib toward Small-Molecular-Target-Based Photodynamic Therapy. ChemMedChem. 2017;12:1504-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Leandro FZ, Martins J, Fontes AM, Tedesco AC. Evaluation of theranostic nanocarriers for near-infrared imaging and photodynamic therapy on human prostate cancer cells. Colloids Surf B Biointerfaces. 2017;154:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Choi YS, Kwon K, Yoon K, Huh KM, Kang HC. Photosensitizer-mediated mitochondria-targeting nanosized drug carriers: Subcellular targeting, therapeutic, and imaging potentials. Int J Pharm. 2017;520:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Zheng BY, Shen XM, Zhao DM, Cai YB, Ke MR, Huang JD. Silicon (IV) phthalocyanines substituted axially with different nucleoside moieties. Effects of nucleoside type on the photosensitizing efficiencies and in vitro photodynamic activities. J Photochem Photobiol B. 2016;159:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Qiu K, Wen Y, Ouyang C, Liao X, Liu C, Rees TW, Zhang Q, Ji L, Chao H. The stepwise photodamage of organelles by two-photon luminescent ruthenium(ii) photosensitizers. Chem Commun (Camb). 2019;55:11235-11238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 101. | Qi S, Guo L, Yan S, Lee RJ, Yu S, Chen S. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm Sin B. 2019;9:279-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 102. | Kim H, Kim SW, Seok KH, Hwang CW, Ahn JC, Jin JO, Kang HW. Hypericin-assisted photodynamic therapy against anaplastic thyroid cancer. Photodiagnosis Photodyn Ther. 2018;24:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Ramu V, Gautam S, Garai A, Kondaiah P, Chakravarty AR. Glucose-Appended Platinum(II)-BODIPY Conjugates for Targeted Photodynamic Therapy in Red Light. Inorg Chem. 2018;57:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 104. | Li KT, Chen Q, Wang DW, Duan QQ, Tian S, He JW, Ou YS, Bai DQ. Mitochondrial pathway and endoplasmic reticulum stress participate in the photosensitizing effectiveness of AE-PDT in MG63 cells. Cancer Med. 2016;5:3186-3193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Mitra K, Gautam S, Kondaiah P, Chakravarty AR. BODIPY-Appended 2-(2-Pyridyl)benzimidazole Platinum(II) Catecholates for Mitochondria-Targeted Photocytotoxicity. ChemMedChem. 2016;11:1956-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Olsen CE, Cheung LH, Weyergang A, Berg K, Vallera DA, Rosenblum MG, Selbo PK. Design, Characterization, and Evaluation of scFvCD133/rGelonin: A CD133-Targeting Recombinant Immunotoxin for Use in Combination with Photochemical Internalization. J Clin Med. 2019;9:pii: E68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Liu C, Chen Z, Wang Z, Li W, Ju E, Yan Z, Liu Z, Ren J, Qu X. A graphitic hollow carbon nitride nanosphere as a novel photochemical internalization agent for targeted and stimuli-responsive cancer therapy. Nanoscale. 2016;8:12570-12578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 108. | Eng MS, Kaur J, Prasmickaite L, Engesæter BØ, Weyergang A, Skarpen E, Berg K, Rosenblum MG, Mælandsmo GM, Høgset A, Ferrone S, Selbo PK. Enhanced targeting of triple-negative breast carcinoma and malignant melanoma by photochemical internalization of CSPG4-targeting immunotoxins. Photochem Photobiol Sci. 2018;17:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Lund K, Bostad M, Skarpen E, Braunagel M, Kiprijanov S, Krauss S, Duncan A, Høgset A, Selbo PK. The novel EpCAM-targeting monoclonal antibody 3-17I linked to saporin is highly cytotoxic after photochemical internalization in breast, pancreas and colon cancer cell lines. MAbs. 2014;6:1038-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Selbo PK, Bostad M, Olsen CE, Edwards VT, Høgset A, Weyergang A, Berg K. Photochemical internalisation, a minimally invasive strategy for light-controlled endosomal escape of cancer stem cell-targeting therapeutics. Photochem Photobiol Sci. 2015;14:1433-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Yang B, Liu H, Yang H, Chen W, Wu J, Feng X, Tong R, Yu H, Chen Y, Lv Z, Sun W, He B, Wu J, Yu G, Mao Z, Zheng S. Combinatorial photochemotherapy on liver cancer stem cells with organoplatinum(ii) metallacage-based nanoparticles. J Mater Chem B. 2019;7:6476-6487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Killig F, Stark G. Photodynamic activation of ion transport through lipid membranes and its correlation with an increased dielectric constant of the membrane. Biochim Biophys Acta. 2002;1564:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Battogtokh G, Choi YS, Kang DS, Park SJ, Shim MS, Huh KM, Cho YY, Lee JY, Lee HS, Kang HC. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives. Acta Pharm Sin B. 2018;8:862-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 114. | Morris RL, Azizuddin K, Lam M, Berlin J, Nieminen AL, Kenney ME, Samia AC, Burda C, Oleinick NL. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194-5197. [PubMed] |
| 115. | P V, D D, Bala M, N S. Photoactivated [Mn(CO)3Br(μ-bpcpd)]2 induces apoptosis in cancer cells via intrinsic pathway. J Photochem Photobiol B. 2018;188:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Rizzi V, Fini P, Semeraro P, Cosma P. Detailed investigation of ROS arisen from chlorophyll a/Chitosan based-biofilm. Colloids Surf B Biointerfaces. 2016;142:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Faustova M, Nikolskaya E, Sokol M, Zabolotsky A, Mollaev M, Zhunina O, Fomicheva M, Lobanov A, Severin E, Yabbarov N. High-effective reactive oxygen species inducer based on Mn-tetraphenylporphyrin loaded PLGA nanoparticles in binary catalyst therapy. Free Radic Biol Med. 2019;143:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Furre IE, Møller MT, Shahzidi S, Nesland JM, Peng Q. Involvement of both caspase-dependent and -independent pathways in apoptotic induction by hexaminolevulinate-mediated photodynamic therapy in human lymphoma cells. Apoptosis. 2006;11:2031-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Chen CJ, Shih YL, Yeh MY, Liao NC, Chung HY, Liu KL, Lee MH, Chou PY, Hou HY, Chou JS, Chung JG. Ursolic Acid Induces Apoptotic Cell Death Through AIF and Endo G Release Through a Mitochondria-dependent Pathway in NCI-H292 Human Lung Cancer Cells In Vitro. In Vivo. 2019;33:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 120. | Kessel D, Reiners JJ. Effects of Combined Lysosomal and Mitochondrial Photodamage in a Non-small-Cell Lung Cancer Cell Line: The Role of Paraptosis. Photochem Photobiol. 2017;93:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Kalkavan H, Green DR. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018;46-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 516] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 122. | Chen ZX, Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 123. | Chabrier-Roselló Y, Giesselman BR, De Jesús-Andino FJ, Foster TH, Mitra S, Haidaris CG. Inhibition of electron transport chain assembly and function promotes photodynamic killing of Candida. J Photochem Photobiol B. 2010;99:117-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 124. | Lee DH, Nam YJ, Lee CS. Quercetin-3-O-(2″-galloyl)-α-L-rhamnopyranoside attenuates cholesterol oxidation product-induced apoptosis by suppressing NF-κB-mediated cell death process in differentiated PC12 cells. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 125. | Chong SJF, Lai JXH, Qu J, Hirpara J, Kang J, Swaminathan K, Loh T, Kumar A, Vali S, Abbasi T, Pervaiz S. A feedforward relationship between active Rac1 and phosphorylated Bcl-2 is critical for sustaining Bcl-2 phosphorylation and promoting cancer progression. Cancer Lett. 2019;457:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 126. | Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D'Agostino D, Forlì F, D'Aguanno S, Todaro M, Stassi G, Di Ilio C, De Laurenzi V, Urbani A. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 221] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Dando I, Cordani M, Dalla Pozza E, Biondani G, Donadelli M, Palmieri M. Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells. Oxid Med Cell Longev. 2015;2015:425708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | De Luca A, Fiorillo M, Peiris-Pagès M, Ozsvari B, Smith DL, Sanchez-Alvarez R, Martinez-Outschoorn UE, Cappello AR, Pezzi V, Lisanti MP, Sotgia F. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 2015;6:14777-14795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 129. | Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 444] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 130. | Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 1021] [Article Influence: 85.1] [Reference Citation Analysis (14)] |
| 131. | Cipolleschi MG, Marzi I, Santini R, Fredducci D, Vinci MC, D'Amico M, Rovida E, Stivarou T, Torre E, Dello Sbarba P, Stecca B, Olivotto M. Hypoxia-resistant profile implies vulnerability of cancer stem cells to physiological agents, which suggests new therapeutic targets. Cell Cycle. 2014;13:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 132. | Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 133. | Yang J, Wang F, Chen X, Qiu S, Cui L, Hu L. β-Pentagalloyl-Glucose Sabotages Pancreatic Cancer Cells and Ameliorates Cachexia in Tumor-Bearing Mice. Am J Chin Med. 2019;47:675-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Varela-Guruceaga M, Milagro FI, Martínez JA, de Miguel C. Effect of hypoxia on caveolae-related protein expression and insulin signaling in adipocytes. Mol Cell Endocrinol. 2018;473:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Liu A, Zhang W, Chen Y, Zhou D, Wang Z, Kang J, Wei L. EtNBSe-PDT inhibited proliferation and induced autophagy of HNE-1 cells via downregulating the Wnt/β-catenin signaling pathway. Photodiagnosis Photodyn Ther. 2019;26:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Li B, Cao Y, Meng G, Qian L, Xu T, Yan C, Luo O, Wang S, Wei J, Ding Y, Yu D. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. 2019;39:239-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 137. | Fiorillo M, Peiris-Pagès M, Sanchez-Alvarez R, Bartella L, Di Donna L, Dolce V, Sindona G, Sotgia F, Cappello AR, Lisanti MP. Bergamot natural products eradicate cancer stem cells (CSCs) by targeting mevalonate, Rho-GDI-signalling and mitochondrial metabolism. Biochim Biophys Acta Bioenerg. 2018;1859:984-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 138. | Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH, Park HG, Han SI, Kang HS. Oncogenic Metabolism Acts as a Prerequisite Step for Induction of Cancer Metastasis and Cancer Stem Cell Phenotype. Oxid Med Cell Longev. 2018;2018:1027453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 139. | Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 421] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 140. | Lee SY, Jeon HM, Ju MK, Jeong EK, Kim CH, Yoo MA, Park HG, Han SI, Kang HS. Dlx-2 is implicated in TGF-β- and Wnt-induced epithelial-mesenchymal, glycolytic switch, and mitochondrial repression by Snail activation. Int J Oncol. 2015;46:1768-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Cai CF, Ye GD, Shen DY, Zhang W, Chen ML, Chen XX, Han DX, Mi YJ, Luo QC, Cai WY, Yang SY. Chibby suppresses aerobic glycolysis and proliferation of nasopharyngeal carcinoma via the Wnt/β-catenin-Lin28/let7-PDK1 cascade. J Exp Clin Cancer Res. 2018;37:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 142. | Goldman A, Khiste S, Freinkman E, Dhawan A, Majumder B, Mondal J, Pinkerton AB, Eton E, Medhi R, Chandrasekar V, Rahman MM, Ichimura T, Gopinath KS, Majumder P, Kohandel M, Sengupta S. Targeting tumor phenotypic plasticity and metabolic remodeling in adaptive cross-drug tolerance. Sci Signal. 2019;12:pii: eaas8779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 143. | Gao R, Li D, Xun J, Zhou W, Li J, Wang J, Liu C, Li X, Shen W, Qiao H, Stupack DG, Luo N. CD44ICD promotes breast cancer stemness via PFKFB4-mediated glucose metabolism. Theranostics. 2018;8:6248-6262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 79] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 144. | Xu W, Qian J, Hou G, Wang Y, Wang J, Sun T, Ji L, Suo A, Yao Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019;83:400-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 145. | Okazaki S, Umene K, Yamasaki J, Suina K, Otsuki Y, Yoshikawa M, Minami Y, Masuko T, Kawaguchi S, Nakayama H, Banno K, Aoki D, Saya H, Nagano O. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019;110:3453-3463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 146. | Duan X, Chen B, Cui Y, Zhou L, Wu C, Yang Z, Wen Y, Miao X, Li Q, Xiong L, He J. Ready player one? Autophagy shapes resistance to photodynamic therapy in cancers. Apoptosis. 2018;23:587-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 147. | Firczuk M, Gabrysiak M, Barankiewicz J, Domagala A, Nowis D, Kujawa M, Jankowska-Steifer E, Wachowska M, Glodkowska-Mrowka E, Korsak B, Winiarska M, Golab J. GRP78-targeting subtilase cytotoxin sensitizes cancer cells to photodynamic therapy. Cell Death Dis. 2013;4:e741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018-1026. [PubMed] |
| 149. | Tang PM, Bui-Xuan NH, Wong CK, Fong WP, Fung KP. Pheophorbide a-Mediated Photodynamic Therapy Triggers HLA Class I-Restricted Antigen Presentation in Human Hepatocellular Carcinoma. Transl Oncol. 2010;3:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 150. | Spaan CN, Smit WL, van Lidth de Jeude JF, Meijer BJ, Muncan V, van den Brink GR, Heijmans J. Expression of UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell proliferation and stemness by activating PERK signaling. Cell Death Dis. 2019;10:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 151. | Dauer P, Sharma NS, Gupta VK, Durden B, Hadad R, Banerjee S, Dudeja V, Saluja A, Banerjee S. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining "stemness". Cell Death Dis. 2019;10:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 152. | Li H, Chen H, Li R, Xin J, Wu S, Lan J, Xue K, Li X, Zuo C, Jiang W, Zhu L. Cucurbitacin I induces cancer cell death through the endoplasmic reticulum stress pathway. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 153. | Huang Y, Leng TD, Inoue K, Yang T, Liu M, Horgen FD, Fleig A, Li J, Xiong ZG. TRPM7 channels play a role in high glucose-induced endoplasmic reticulum stress and neuronal cell apoptosis. J Biol Chem. 2018;293:14393-14406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 154. | Li R, Yang Y, Hong P, Zhang Z, Li L, Hui J, Zheng X. β-carotene attenuates weaning-induced apoptosis via inhibition of PERK-CHOP and IRE1-JNK/p38 MAPK signalling pathways in piglet jejunum. J Anim Physiol Anim Nutr (Berl). 2020;104:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 155. | Qi Z, Chen L. Endoplasmic Reticulum Stress and Autophagy. Adv Exp Med Biol. 2019;1206:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 156. | Hasna J, Hague F, Rodat-Despoix L, Geerts D, Leroy C, Tulasne D, Ouadid-Ahidouch H, Kischel P. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: the p53 connection. Cell Death Differ. 2018;25:693-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 157. | Tang H, Jin Y, Jin S, Tan Z, Peng Z, Kuang Y. Arsenite inhibits the function of CD133+ CD13+ liver cancer stem cells by reducing PML and Oct4 protein expression. Tumour Biol. 2016;37:14103-14115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 158. | Zhang J, Zhu Q, Wang X, Yu J, Chen X, Wang J, Wang X, Xiao J, Wang CC, Wang L. Secretory kinase Fam20C tunes endoplasmic reticulum redox state via phosphorylation of Ero1α. EMBO J. 2018;37:pii: e98699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 159. | Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R. Ero1α regulates Ca (2+) fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal. 2012;16:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 160. | Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 947] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 161. | Lewis K, Kiepas A, Hudson J, Senecal J, Ha JR, Voorand E, Annis MG, Sabourin V, Ahn R, La Selva R, Tabariès S, Hsu BE, Siegel MJ, Dankner M, Canedo EC, Lajoie M, Watson IR, Brown CM, Siegel PM, Ursini-Siegel J. p66ShcA functions as a contextual promoter of breast cancer metastasis. Breast Cancer Res. 2020;22:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 162. | Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 163. | Xin Y, Wu W, Qu J, Wang X, Lei S, Yuan L, Liu X. Inhibition of Mitofusin-2 Promotes Cardiac Fibroblast Activation via the PERK/ATF4 Pathway and Reactive Oxygen Species. Oxid Med Cell Longev. 2019;2019:3649808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 164. | Chen Y, Lin J, Chen J, Huang C, Zhang Z, Wang J, Wang K, Wang X. Mfn2 is involved in intervertebral disc degeneration through autophagy modulation. Osteoarthritis Cartilage. 2020;28:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 165. | Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1390] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 166. | Fujimaki K, Li R, Chen H, Della Croce K, Zhang HH, Xing J, Bai F, Yao G. Graded regulation of cellular quiescence depth between proliferation and senescence by a lysosomal dimmer switch. Proc Natl Acad Sci USA. 2019;116:22624-22634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 167. | Eid S, Boutary S, Braych K, Sabra R, Massaad C, Hamdy A, Rashid A, Moodad S, Block K, Gorin Y, Abboud HE, Eid AA. mTORC2 Signaling Regulates Nox4-Induced Podocyte Depletion in Diabetes. Antioxid Redox Signal. 2016;25:703-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 168. | Ohata H, Shiokawa D, Obata Y, Sato A, Sakai H, Fukami M, Hara W, Taniguchi H, Ono M, Nakagama H, Okamoto K. NOX1-Dependent mTORC1 Activation via S100A9 Oxidation in Cancer Stem-like Cells Leads to Colon Cancer Progression. Cell Rep. 2019;28:1282-1295.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 169. | Lamb FS, Hook JS, Hilkin BM, Huber JN, Volk AP, Moreland JG. Endotoxin priming of neutrophils requires endocytosis and NADPH oxidase-dependent endosomal reactive oxygen species. J Biol Chem. 2012;287:12395-12404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 170. | Cano-Domínguez N, Bowman B, Peraza-Reyes L, Aguirre J. Neurospora crassa NADPH Oxidase NOX-1 Is Localized in the Vacuolar System and the Plasma Membrane. Front Microbiol. 2019;10:1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 171. | Takeda M, Koseki J, Takahashi H, Miyoshi N, Nishida N, Nishimura J, Hata T, Matsuda C, Mizushima T, Yamamoto H, Ishii H, Doki Y, Mori M, Haraguchi N. Disruption of Endolysosomal RAB5/7 Efficiently Eliminates Colorectal Cancer Stem Cells. Cancer Res. 2019;79:1426-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 172. | Dai Y, He F, Ji H, Zhao X, Misal S, Qi Z. Dual-Functional NIR AIEgens for High-Fidelity Imaging of Lysosomes in Cells and Photodynamic Therapy. ACS Sens. 2020;5:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 173. | Turubanova VD, Balalaeva IV, Mishchenko TA, Catanzaro E, Alzeibak R, Peskova NN, Efimova I, Bachert C, Mitroshina EV, Krysko O, Vedunova MV, Krysko DV. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer. 2019;7:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 174. | Liang JH, Zheng Y, Wu XW, Tan CP, Ji LN, Mao ZW. A Tailored Multifunctional Anticancer Nanodelivery System for Ruthenium-Based Photosensitizers: Tumor Microenvironment Adaption and Remodeling. Adv Sci (Weinh). 2020;7:1901992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 175. | Wang C, Zhao P, Jiang D, Yang G, Xue Y, Tang Z, Zhang M, Wang H, Jiang X, Wu Y, Liu Y, Zhang W, Bu W. In Situ Catalytic Reaction for Solving the Aggregation of Hydrophobic Photosensitizers in Tumor. ACS Appl Mater Interfaces. 2020;12:5624-5632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 176. | Shi L, Hu F, Duan Y, Wu W, Dong J, Meng X, Zhu X, Liu B. Hybrid Nanospheres to Overcome Hypoxia and Intrinsic Oxidative Resistance for Enhanced Photodynamic Therapy. ACS Nano. 2020;14:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 177. | Jasim KA, Gesquiere AJ. Ultrastable and Biofunctionalizable Conjugated Polymer Nanoparticles with Encapsulated Iron for Ferroptosis Assisted Chemodynamic Therapy. Mol Pharm. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 178. | Hamaï A, Cañeque T, Müller S, Mai TT, Hienzsch A, Ginestier C, Charafe-Jauffret E, Codogno P, Mehrpour M, Rodriguez R. An iron hand over cancer stem cells. Autophagy. 2017;13:1465-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 179. | Ortel B, Shea CR, Calzavara-Pinton P. Molecular mechanisms of photodynamic therapy. Front Biosci (Landmark Ed). 2009;14:4157-4172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 180. | Lin X, Wu M, Li M, Cai Z, Sun H, Tan X, Li J, Zeng Y, Liu X, Liu J. Photo-responsive hollow silica nanoparticles for light-triggered genetic and photodynamic synergistic therapy. Acta Biomater. 2018;76:178-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 181. | Salmerón ML, Quintana-Aguiar J, De La Rosa JV, López-Blanco F, Castrillo A, Gallardo G, Tabraue C. Phenalenone-photodynamic therapy induces apoptosis on human tumor cells mediated by caspase-8 and p38-MAPK activation. Mol Carcinog. 2018;57:1525-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 182. | Biswas R, Mondal A, Chatterjee S, Ahn JC. Evaluation of synergistic effects of sulforaphene with photodynamic therapy in human cervical cancer cell line. Lasers Med Sci. 2016;31:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 183. | Li M, Knight DA, Smyth MJ, Stewart TJ. Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways. Cancer Immunol Immunother. 2012;61:1255-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 184. | Salva KA, Wood GS. Epigenetically Enhanced Photodynamic Therapy (ePDT) is Superior to Conventional Photodynamic Therapy for Inducing Apoptosis in Cutaneous T-Cell Lymphoma. Photochem Photobiol. 2015;91:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 185. | Bhattacharyya R, Gupta P, Bandyopadhyay SK, Patro BS, Chattopadhyay S. Coralyne, a protoberberine alkaloid, causes robust photosenstization of cancer cells through ATR-p38 MAPK-BAX and JAK2-STAT1-BAX pathways. Chem Biol Interact. 2018;285:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 186. | Cai J, Zheng Q, Huang H, Li B. 5-aminolevulinic acid mediated photodynamic therapy inhibits survival activity and promotes apoptosis of A375 and A431 cells. Photodiagnosis Photodyn Ther. 2018;21:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 187. | Zhang K, Gao S, Guo J, Ni G, Chen Z, Li F, Zhu X, Wen Y, Guo Y. Hypericin-photodynamic therapy inhibits proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes cell line MH7A. Iran J Basic Med Sci. 2018;21:130-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 188. | Shivran N, Tyagi M, Mula S, Gupta P, Saha B, Patro BS, Chattopadhyay S. Syntheses and photodynamic activity of some glucose-conjugated BODIPY dyes. Eur J Med Chem. 2016;122:352-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 189. | Doustvandi MA, Mohammadnejad F, Mansoori B, Mohammadi A, Navaeipour F, Baradaran B, Tajalli H. The interaction between the light source dose and caspase-dependent and -independent apoptosis in human SK-MEL-3 skin cancer cells following photodynamic therapy with zinc phthalocyanine: A comparative study. J Photochem Photobiol B. 2017;176:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 190. | Akilov OE, Wu MX, Jin Y, Zhou Z, Geskin LJ, Falo LD, Hasan T. Vaccination with photodynamic therapy-treated macrophages induces highly suppressive T-regulatory cells. Photodermatol Photoimmunol Photomed. 2011;27:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 191. | Kawczyk-Krupka A, Czuba ZP, Kwiatek B, Kwiatek S, Krupka M, Sieroń K. The effect of ALA-PDT under normoxia and cobalt chloride (CoCl2)-induced hypoxia on adhesion molecules (ICAM-1, VCAM-1) secretion by colorectal cancer cells. Photodiagnosis Photodyn Ther. 2017;19:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 192. | Kanda T, Sugihara T, Takata T, Mae Y, Kinoshita H, Sakaguchi T, Hasegawa T, Kurumi H, Ikebuchi Y, Murakami T, Isomoto H. Low-density lipoprotein receptor expression is involved in the beneficial effect of photodynamic therapy using talaporfin sodium on gastric cancer cells. Oncol Lett. 2019;17:3261-3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 193. | Yang K, Wang C, Wei X, Ding S, Liu C, Tian F, Li F. Self-Illuminating Photodynamic Therapy with Enhanced Therapeutic Effect by Optimization of the Chemiluminescence Resonance Energy Transfer Step to the Photosensitizer. Bioconjug Chem. 2020;31:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 194. | Zheng Y, Yin G, Le V, Zhang A, Lu Y, Yang M, Fei Z, Liu J. Hypericin-based Photodynamic Therapy Induces a Tumor-Specific Immune Response and an Effective DC-based cancer Immunotherapy. Biochem Pharmacol. 2014;pii:S0006-2952(14)00075-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 195. | Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 440] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 196. | Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 197. | Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, Vandenabeele P, Agostinis P. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 641] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 198. | Fabrizio G, Di Paola S, Stilla A, Giannotta M, Ruggiero C, Menzel S, Koch-Nolte F, Sallese M, Di Girolamo M. ARTC1-mediated ADP-ribosylation of GRP78/BiP: a new player in endoplasmic-reticulum stress responses. Cell Mol Life Sci. 2015;72:1209-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 199. | Lamberti MJ, Mentucci FM, Roselli E, Araya P, Rivarola VA, Rumie Vittar NB, Maccioni M. Photodynamic Modulation of Type 1 Interferon Pathway on Melanoma Cells Promotes Dendritic Cell Activation. Front Immunol. 2019;10:2614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 200. | Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J, Alfranca G, Sun X, Ma L, de la Fuente JM, Song J, Ni J, Cui D. A tumor microenvironment responsive biodegradable CaCO3/MnO2- based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019;9:6867-6884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 201. | Chen L, Zhou L, Wang C, Han Y, Lu Y, Liu J, Hu X, Yao T, Lin Y, Liang S, Shi S, Dong C. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv Mater. 2019;31:e1904997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 202. | Chen CK, Blanco M, Jackson C, Aznauryan E, Ollikainen N, Surka C, Chow A, Cerase A, McDonel P, Guttman M. Xist recruits the X chromosome to the nuclear lamina to enable chromosome-wide silencing. Science. 2016;354:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 203. | Yu W, He X, Yang Z, Yang X, Xiao W, Liu R, Xie R, Qin L, Gao H. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 204. | Yamamura Y, Tsuchikawa T, Miyauchi K, Takeuchi S, Wada M, Kuwatani T, Kyogoku N, Kuroda A, Maki T, Shichinohe T, Hirano S. The key role of calreticulin in immunomodulation induced by chemotherapeutic agents. Int J Clin Oncol. 2015;20:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 205. | Wen Y, Chen X, Zhu X, Gong Y, Yuan G, Qin X, Liu J. Photothermal-Chemotherapy Integrated Nanoparticles with Tumor Microenvironment Response Enhanced the Induction of Immunogenic Cell Death for Colorectal Cancer Efficient Treatment. ACS Appl Mater Interfaces. 2019;11:43393-43408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 206. | Tran JQ, Muench MO, Heitman JW, Jackman RP. Pathogen reduction with riboflavin and ultraviolet light induces a quasi-apoptotic state in blood leukocytes. Transfusion. 2019;59:3501-3510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 207. | Schröter P, Hartmann L, Osen W, Baumann D, Offringa R, Eisel D, Debus J, Eichmüller SB, Rieken S. Radiation-induced alterations in immunogenicity of a murine pancreatic ductal adenocarcinoma cell line. Sci Rep. 2020;10:686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 208. | Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, Agostinis P. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8:328ra27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 209. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48786] [Article Influence: 3252.4] [Reference Citation Analysis (12)] |
| 210. | Dudas J, Ladanyi A, Ingruber J, Steinbichler TB, Riechelmann H. Epithelial to Mesenchymal Transition: A Mechanism that Fuels Cancer Radio/Chemoresistance. Cells. 2020;9:pii: E428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 211. | Lee JJ, van de Ven RAH, Zaganjor E, Ng MR, Barakat A, Demmers JJPG, Finley LWS, Gonzalez Herrera KN, Hung YP, Harris IS, Jeong SM, Danuser G, McAllister SS, Haigis MC. Inhibition of epithelial cell migration and Src/FAK signaling by SIRT3. Proc Natl Acad Sci USA. 2018;115:7057-7062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 212. | Peng YS, Syu JP, Wang SD, Pan PC, Kung HN. BSA-bounded p-cresyl sulfate potentiates the malignancy of bladder carcinoma by triggering cell migration and EMT through the ROS/Src/FAK signaling pathway. Cell Biol Toxicol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 213. | Goulet CR, Champagne A, Bernard G, Vandal D, Chabaud S, Pouliot F, Bolduc S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer. 2019;19:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 214. | Ishikawa H, Tsuyama N, Kawano MM. Interleukin-6-induced proliferation of human myeloma cells associated with CD45 molecules. Int J Hematol. 2003;78:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 215. | Kyjacova L, Hubackova S, Krejcikova K, Strauss R, Hanzlikova H, Dzijak R, Imrichova T, Simova J, Reinis M, Bartek J. Radiotherapy-induced plasticity of prostate cancer mobilizes stem-like non-adherent, Erk signaling-dependent cells. Cell Death Differ 2015; 22: 898-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 216. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1365] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 217. | Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ. Author Correction: Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2019;21:533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 218. | Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol. 2014;89:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 219. | Delgado-Hernández R, Hernández-Balmaseda I, Rodeiro-Guerra I, Cesar Rodriguez Gonzalez J, De Wever O, Logie E, Declerck K, Pérez-Novo C, Vanden Berghe W. Anti-angiogenic effects of mangiferin and mechanism of action in metastatic melanoma. Melanoma Res. 2020;30:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Durán-Alonso MB, Wang QE S-Editor: Wang J L-Editor: Wang TQ E-Editor: Xing YX