Published online Apr 26, 2020. doi: 10.4252/wjsc.v12.i4.241
Peer-review started: December 25, 2019
First decision: January 19, 2020
Revised: February 7, 2020
Accepted: March 22, 2020
Article in press: March 22, 2020
Published online: April 26, 2020
Processing time: 123 Days and 19.4 Hours
Degenerative musculoskeletal disorders are one of the top causes of pain and disability in the adult population. Current available alternatives to mitigate symptoms include conservative treatments such as the administration of pharmacological agents and an educative approach towards lifestyle modification. The use of certain analgesics, such as opiates and corticosteroids, delivers short term results but do not address the etiological source of pain and disability. Also, prolonged use of such medications may cause additional complications. Therefore, the demand for musculoskeletal tissue regeneration has led to an alternative approach referred to as “orthobiologics”. This alternative is based on cellular and molecular components capable of inducing and promoting tissue repair. Bone marrow (BM) aspirate (BMA) and concentrate are well-known orthobiologics used to treat musculoskeletal conditions. Orthobiologics derived from the BM have been discussed in the literature; however, the lack of standardization regarding collection and processing protocols presents a challenge for generalization of study outcomes and determination of efficacy. Since BM-derived orthobiologics have not yet been classified, to our knowledge, this manuscript proposes the ACH classification system, which speaks to BMA (A), BMA and concentrate (C) and hybrid (H), which combines A and C. This classification proposes and describes 8 parameters that are relevant for the quality of biological products. The more parameters used would imply greater characterization and complexity of the evaluation of the biological product used. The ACH classification envisages a necessary contribution to the comprehension of both clinical procedures and research outcomes, ultimately ushering in a standardization of best practice.
Core tip: Degenerative musculoskeletal disorders are one of the top causes of pain and disability in the adult population. The use of certain analgesics delivers short term results but do not address the etiological source of pain and disability. The demand for musculoskeletal tissue regeneration has led to an alternative approach referred to as orthobiologics, which is based on cellular and molecular components capable of promoting tissue repair. Bone marrow aspirate and concentrate are well-known orthobiologics used to treat musculoskeletal conditions. Since bone marrow-derived orthobiologics have not yet been classified, to our knowledge, this manuscript proposes the ACH classification system.
- Citation: Purita J, Lana JFSD, Kolber M, Rodrigues BL, Mosaner T, Santos GS, Caliari-Oliveira C, Huber SC. Bone marrow-derived products: A classification proposal – bone marrow aspirate, bone marrow aspirate concentrate or hybrid? World J Stem Cells 2020; 12(4): 241-250
- URL: https://www.wjgnet.com/1948-0210/full/v12/i4/241.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i4.241
The increasing incidence of degenerative diseases affecting the musculoskeletal system is the main cause of pain and disability among adults. Current options for the management of these conditions mainly focus on conservative care such as activity modification and pharmacological therapies. While pharmacological therapies such as opiates and non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids offer short term efficacy, they are associated with well-known side effects if used on a long-term basis[1,2]. Moreover, few options exist outside of surgical solutions for those individuals recalcitrant to conservative care.
The need for musculoskeletal tissue regeneration has led to an alternative approach referred to as orthobiologics, which is based on cellular and molecular components responsible for inducing and promoting tissue repair[3]. Orthobiologics, which comprise platelet rich plasma (PRP), bone marrow (BM) aspirate (BMA) and concentrate (BMAC), fat grafting (Bio fat), and expanded mesenchymal stem cells (MSCs), have shown promising results for the care of musculoskeletal disorders[4-7].
Orthobiologics have been discussed in the literature with promising results, however, the lack of standardization regarding the methods of obtaining and processing the cells and associated components, have led to uncertain conclusions in terms of efficacy and ability to generalize outcomes[8]. Specifically, the main components of orthobiologics (platelet concentrations, growth factors, and cytokines) may vary based on the processing method, which might affect anabolic and anti-inflammatory properties, and consequently lead to inconsistent outcomes[8]. Thus, the need for standardization and classification of orthobiologics is imperative for understanding procedures and dissemination of research outcomes. A classification system has been developed for PRP[9]; however, no such classification exists for BM-derived orthobiologics. Thus, the purpose of this paper is to present a proposal for a classification system for BM derived orthobiologics.
The main function of BM is to provide circulating blood with an optimal supply of erythrocytes, leukocytes, and platelets. In addition to this, BM supplies hematopoietic stem cells (HSCs), endothelial cells, MSCs and other precursor cells. The human skeleton possesses red BM which is hematopoietically active, and yellow, which is hematopoietically inactive[10].
Red and yellow BMs have different cellular and molecular content: Yellow BM comprises 95% fat cells, whereas the red BM comprises 60% hematopoietic cells. The whole skeleton is filled with red BM at birth, however, during childhood a physiological conversion of red BM into yellow BM occurs. The conversion of red to yellow marrow and progresses to the axial skeleton, and this entire process may be completed by the age of 25 years[10].
BM is a potent source of stem and progenitor cells, and this characteristic has gained attention for cell-based therapies in orthopedics[11-13]. Given the diversity in stem cell lineages and phenotypes in the marrow, BM represents a functional organ in which distinct types of cells function cooperatively. Specifically, HSCs play a critical role in the formation of the hematopoietic microenvironment, whereas MSCs support hematopoiesis and both MSCs and/or skeletal stem cells are responsible for the development and maintenance of skeletal tissues[14,15].
MSCs are non-hematopoietic stromal cells that are composed of a small fraction (0.001%–0.01%) of the stem cell content in BM[16]. MSCs are found in other tissues, such as adipose tissue, placenta, and umbilical cord, and although they differ in their differentiation potential, they possess common features associated with those from the BM, which might imply that MSC-like populations share a similar ontogeny[17,18].
MSCs exhibit the potential ability to differentiate into mesodermal linage cells (e.g., cartilage, bone, fat, muscle, meniscus and tendon)[19], which is fundamental for the regeneration process. Moreover, these cells have paracrine effects, thus are able to alter their local microenvironment[20].
Given the varying MSC markers that laboratories may use to characterize these cells, there is a lack in standard phenotypic criteria. This heterogeneity is also due to the fact that MSCs are able to express a range of cell-lineage specific antigens that may differ depending on the culture preparation, culture duration, or plating density[21,22]. However, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy have proposed minimal criteria to characterize MSCs, which comprise the following attributes: Must be plastic-adherent when maintained in culture; must be able to differentiate in vitro into chondroblasts, adipocytes and osteoblasts; and must express CD105, CD73 and CD90, and lack expression of CD45, CD11b, CD34 or CD14, CD79α or CD19 and HLA-DR surface molecules[23].
MSCs lack significant immunogenicity and can be easily isolated, which allows allogenic transplantation. In allogenic circumstances these cells should be considered immune evasive. However, the effects of MSCs in cellular-based therapies depends on the ability of these cells to home and engraft (long-term) into the target tissue[24]. One theory suggests that MSCs have a rather short life span and are phagocytized by monocytes and subsequently stimulate the production of T-reg cells which may very well contribute to the overall clinical improvement[25].
Cells from injured tissue release chemokines responsible for MSC recruitment. Once in the target tissue, MSCs are able to modulate wound-healing responses by reducing apoptosis and fibrosis, attenuate the inflammatory process and stimulate cell proliferation and differentiation via paracrine and autocrine pathways[26]. These properties are attributed to the ability of MSCs to release key agents, such as vasculoendothelial growth factor, transforming growth factor beta (TGF-β), stromal-derived factor 1, and stem cell factor, among others. Also, they induce a downregulation of pro-inflammatory cytokines, including interleukin 1 (IL-1), IL-6, interferon-γ, and tumor necrosis factor α[16,27,28]. MSCs also possess immunomo-dulatory properties as they are able to inhibit the activation of type 1 macrophages, natural killer cells, and both B and T lymphocytes[29].
HSCs also known for expressing CD34+, are located at the top of the hematopoietic hierarchy. They are responsible for the daily supply of more than 100 billion mature blood cells, including erythrocytes, leukocytes, and platelets[30]. This process, called hematopoiesis, is of extreme importance in the maintenance and regulation of the immune system, especially for the cells from myeloid lineage, such as granulocytes, monocytes and dendritic cells, due to their short half-life[31,32].
Past studies have reported that the hematopoietic and stromal environments are related and overlapped. For example, Simons et al[33] observed generations of fibroblasts colony-forming unit (CFU-F) from CD34+ human BM cells. Also, it has been reported that the number of osteoblast progenitor cells is higher in sorted CD34+ cells (1/5000 approximately) than in CD34 - populations (1/33000), and when these sorted cells were cultured in a long-term marrow system, the generation of a heterogeneous population that included smooth muscle cells, adipocytes, fibroblast and macrophages was observed[34]. This possible relation was then supported by Mehrotra et al[35] who reported that HSC give rise to osteocytes and chondrocytes in an experimental study.
Immune cells – Leukocytes have a common origin from the hematopoietic stem cell and develop along distinct differentiation pathways in response to external and internal stimuli. In order to promote regeneration, leukocytes circulate through the blood and lymphatic system and are recruited to specific regions of the body when damage occurs[32].
The mononuclear phagocyte system represents a subset of leukocytes that was originally described as BM-derived myeloid cells[32]. Monocytes are immune effector cells that, although they circulate in the blood, BM and spleen, they do not proliferate in a steady state[36,37]. They are equipped with chemokine receptors that mediate migration from blood to the injured sites and produce inflammatory cytokines. During inflammation, the monocytes differentiate into dendritic cells (DC) or macrophages, and this process is likely determined by the inflammatory environment and pathogen-associated pattern-recognition receptors[38].
Macrophages are phagocytic cells that reside in lymphoid and nonlymphoid tissues[32]. Given the broad range of pathogen-recognition receptors that macrophages possess, they are known as an efficient tool at maintaining tissue homeostasis as they provide clearance of apoptotic cells and remodeling of the extracellular matrix[39,40]. Macrophages play a key role in recruiting and inducing the proliferation of osteoblasts, stem and progenitor cells as they secrete bone morphogenetic proteins, IL-1β, TGF-β, platelet derived growth factor and insulin-like growth factors, in areas of infection or injury in different tissues in the body[41]. Extrinsic stimuli that induce an inflammatory process, such as infection or injury, promote changes in gene transcription that classify macrophages as type 1 (M-1) and type 2 (M-2). The M-2 type offers a healing function, while M-1 promotes the host defense. After injury, M2 can switch into M1, and this change is modulated by the cytokines such as interferon-γ, and M2 type by IL-4[42].
Neutrophils belong to a polymorphonuclear family and are known for being the main cell type response to bacterial infections. It was reported that neutrophils are highly plastic cells influenced by environmental cues that result in a site-specific neutrophil transcriptome as they migrate from BM to sites of inflammation[43]. As a granulocyte, which includes eosinophils and basophils, neutrophils are able to secrete a variety of cytokines, such as TGF-β, vasculoendothelial growth factor and platelet derived growth factor, playing an important role in angiogenesis and vasculogenesis[44]. Neutrophils undergo spontaneous apoptosis to regulate the resolution of inflammation[45].
The main goal in treating orthopedic injuries, especially joint disease, is cartilage regeneration. One approach to achieve this outcome is by using BM-derived MSC (BM-MSC), which has been supported in the literature[46,47]. However, its clinical utility is limited by complexity, such as the need for a specialized laboratory and procedural cost. In this sense, the use of BMA has emerged as a novel regenerative tool for degenerative joint diseases as a non-fractioned product that retains potentially supportive chondrogenic components[48].
Even though different harvest sources for BM have been described in the literature the main harvest site (either for BMA or BMAC use) is the posterior iliac crest, which allows a considerable amount of BM and about 1.6-fold more osteoblastic connective tissue progenitor cells than other sites[49,50]. However, evidence suggests the quality of the product is technique-dependent[51].
There are a few studies that have used this approach in the literature; however, most of them are related to nonunion fractures. The first to describe the use of unprocessed marrow was Lindholm and Urist[52] that reported the replacement of bone matrix by new bone in composite grafts in vivo (non-human study). Almost a decade later, Connolly et al[53] observed callus formation sufficient to unite tibial nonunions in humans after injection of autologous BMA.
In 2013, Hauser and Orlofsky published a case series describing their experience with BMA in combination with hyperosmotic dextrose, also known as prolotherapy, in the treatment of knee, hip, and ankle osteoarthritis. After two to seven treatments over twelve months, all patients reported improvement in pain, joint function, and quality of life. Also, three out of seven patients had achieved complete symptomatic relief[48].
Butala et al[54] reported the efficacy of BMA in bone union as they injected unprocessed BM at fracture sites in 10 patients with tibia, humerus, femur, and forearm delayed union fractures. After 12 wk, nine of these patients had signs of union, such as decreased tenderness at fracture site, pain-free joint mobilization and ability to ambulate without assistance[54].
A study performed in 2017 by Lal[55], evaluated the use of percutaneous autologous BM injections in 56 patients with delayed and 37 patients with nonunion of long bone. Twelve weeks after the injections, it was observed that all fractures were united, and the minimum period for union was 8-weeks. Although a significant correlation (P = 0.081) was not present, it was reported that the time to observe bone union after the injection of autologous BM was longer in patients who were smokers. Women, however, were observed to have a reduced time for bone union than the male patients (P = 0.041)[55].
Although the number of studies with BMA are limited and of lower quality, they show a promising efficacy and safety profile with regards to adverse events.
In an attempt to increase the proportion of MSCs, the aspirate of BM may be processed to produce BMAC, which has been widely investigated in orthopedics, especially for nonunions, surgical augmentation, osteonecrosis, as well as osseous and cartilage defects[11-13].
Although the exact mechanism of action has not been fully elucidated, the effects of BMAC may rely on the recovery of nucleated cells from BM, which possesses a paracrine effect by delivering cytokines into the injured site in order to stimulate endogenous tissue repair[56]. In vitro studies have shown that the platelets present in BMAC release growth factors that induce stem cells migration to the injured area. Moreover, a concentrated number of HSCs may provide vascular support and drive MSC into osteogenic differentiation pathways[57].
Current clinical studies have reported the efficacy and safety of BMAC for the treatment of small lesions. Centeno et al[6] studied the effects of BMAC on 115 shoulders of 102 patients who had rotated cuff injuries and shoulder osteoarthritis. In the aforementioned study, a 52.6% improvement in joint function and disability and 44.2% decrease in pain was reported with both outcomes reaching statistical significance (P = 0.001). The mean improvement reported by the patients was 48.8%. The reduction of disability and pain was observed from the first month after treatment and was maintained for up to 2 years after the treatment, based on this time being the terminal point of data collection. No side effects or adverse events were reported with BMAC in these 2 years of study[6].
BMAC has also been studied with various surgical scaffolds. Gobbi et al[58] evaluated 15 patients with grade IV cartilage lesions who underwent injections of BMAC on a collagen matrix. Two years after the injections improvements in pain, joint functionality and quality of life were identified. Biopsy of these lesions showed hyaline-like tissue at repeat arthroscopy 2- years later[58]. Enea et al[59] evaluated patients who underwent microfracture covered with a resorbable composite of natural hyaluronan matrix and synthetic polyglycolic acid with BMAC. It was observed that, 12 mo after the injection, the lesions were macroscopically normal, presenting production of hyaline-like tissue. The defect filling was confirmed by magnetic resonance imaging[59].
The use of BMAC has also been studied in combination with other regenerative medicine approaches. Sampson et al[60] evaluated the injection of BMAC followed by PRP in 125 patients who presented moderate/severe ankle, knee, spine and/or shoulder osteoarthritis, eight weeks after the injection, The authors observed a median of 5 points in pain relief, based on a visual analogic scale (VAS), and the patients reported 9.0/10 satisfaction with the treatment. Kim et al[61] studied the association of BMAC with adipose tissue (fat graft) in 75 osteoarthritic knees (41 patients). Twelve months after the injections, a decrease in pain, improved joint function, and an increase in quality of life was reported. The authors also suggest that BMAC would present a more effective result in early to moderate phases of osteoarthritis.
Some studies evaluated the optimal volume of BM needed to achieve clinical response: The quality of the product decreases with higher volume of BM withdrawn, and it was observed that small volume of marrow aspirated in a 10 mL syringe would be an ideal volume to concentrate MSC and progenitor cells. Larger volume syringes may cause blood dilution[62,63]. The components of BM aspirated are concentrated following centrifugation steps. Although there are some protocols of BMAC preparation in the literature[64,65] there is no study regarding the optimal centrifuge force and time to achieve an increased cellular concentration.
Although BMAC presents a well-established cellular and molecular content, only few studies evaluating its efficacy and safety have performed quantitative and qualitative assessment[8].
The lack of standardization of the BM-derived products for regenerative medicine has emerged, thus the need to classify the processing methods according to quality and procedural details has been established[11]. Classification of such factors would allow for procedural standardization and interpretation of both clinical results and research findings.
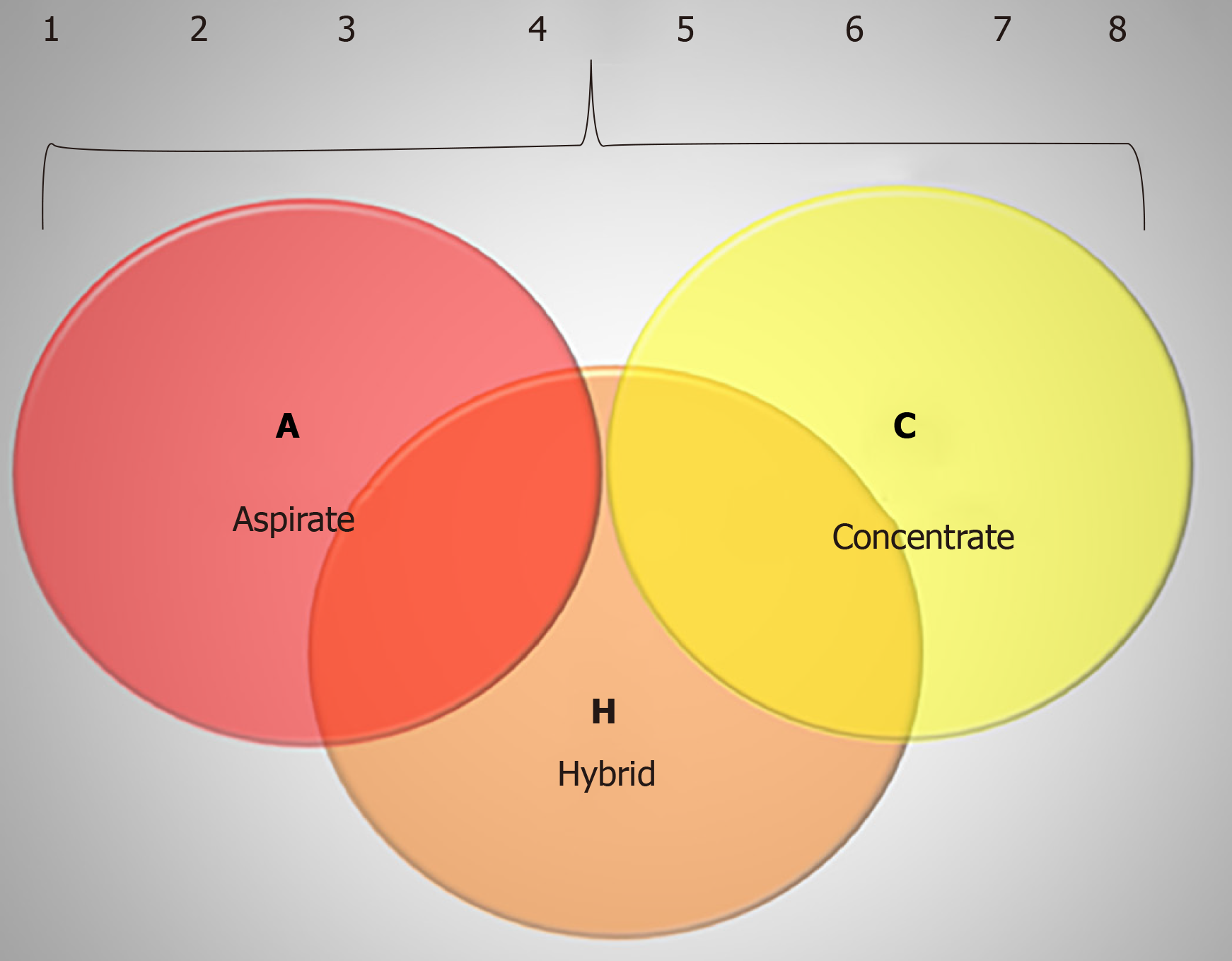
The ACH (aspirate, concentrate, hybrid) classification system comprises the two main techniques involving bone marrow-derived products: BMA, which represents the letter A (for aspirate), BMAC, which represents the letter C (for concentrated), and the letter H (for hybrid) is used when BMA is combined with BMAC.
The ACH classification is focused on whether the cellular and molecular content present in the product was evaluated and described increasing the complexity of description/characterization. For each classification (A, C and H) sub grouping would occur as follows: (1) Product would only be collected and injected with no additional analysis; (2) Description of harvesting – BM site of harvesting (posterior/anterior iliac crest, axial skeleton), type of needle, multiple insertions, single insertion, type of syringe, type of anticoagulant, volume harvested; (3) The cellular content would be assessed by a cell count machine, which would enable to quantify mono- and polymorphonuclear cells, giving the number of total nucleated cells; (4) Dosage of molecular content, such as interleukins and/or growth factors is made by multiplex platform or ELISA technique; (5) Indirect quantification of MSC number measured through CFU in culture; (6) Phenotyping of MSC and HSC for characterization through flow cytometry – it is wise to use a full panel for the clusters of differen-tiation, especially of the MSC since there are a lot of markers for positive and negative evaluation; (7) For the complete characterization of MSC the differentiation in three cell types in culture is necessary, including the induction of chondrocytes, adipocytes and osteocytes; and (8) To finalize, the most complex level of evaluation of MSC is the evaluation of its function, which includes assays like wound healing (proliferation and migration), lymphocytes proliferation (immunossupressor potential), and population doubling time. The representation of the ACH classification is shown in Table 1.
| Letter | Relates to | Classification |
| A | BMA | 1 – Collection and injection |
| 2 – Description of harvesting | ||
| 3 – Cell count | ||
| 4 – Dosage of cytokines (GF and/or IL) | ||
| 5 – CFU | ||
| 6 - MSC and HSC phenotyping | ||
| 7- Differentiation evaluation | ||
| 8 – Functional assays | ||
| C | BMAC | 1 – Collection and injection |
| 2 – Description of harvesting | ||
| 3 – Cell count | ||
| 4 – Dosage of cytokines (GF and/or IL) | ||
| 5 - CFU | ||
| 6 – HSC and/or MSC phenotyping | ||
| 7 – Differentiation evaluation | ||
| 8 – Functional Assays | ||
| H | BMA + BMAC used together | 1 – Collection and injection |
| 2 – Description of harvesting | ||
| 3 – Cell count | ||
| 4 – Dosage of cytokines (GF and/or IL) | ||
| 5 - CFU | ||
| 6 – HSC and/or MSC phenotyping | ||
| 7 – Differentiation evaluation | ||
| 8 – Functional assays |
The idea of this classification is that for each type of BM used (BMA, BMAC or hybrid) the increase of the number indicates an improvement in the characterization and complexity of the evaluation of this biological product. When a study or procedure with BMAC reports that only BM was collected and injected, it would be classified as C1, according to the ACH classification. On the other hand, if the BMAC presents the description of harvesting procedure (site, syringe, volume, and anticoagulant use) it will be classified as C1-2. If the total nuclear cells were counted by a cell counter, which would include leukocytes, MSC and HSC, using the description of technique for harvesting it would be classified as C1-3. In this BMAC if the harvesting technique was described, cell count was made and evaluation of molecular content, it will be classified as C1-4. If the HSC and/or MSC are quantified and characterized by flow cytometry in the same BMAC, has the description of harvesting, dosage of cytokines and CFU it would be classified as C1-6 product. The last level of description is the C1-8 which encompass the description of harvesting, cell count, evaluation of cytokines and growth factors, MSC and HSC phenotyping, CFU, evaluation of differentiation and functional assays, being classified as C1-8.
In the case where this is not a progression of steps in numeric order the omitted step number would not be used. For example, if a procedure with BMA harvesting had a description, cell count, and CFU, without the quantification of the molecular content, this study will be classified as A1-3;5, as demonstrated in Table 2.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Example |
| X | X | X | X | X | X | 1-3; 5-7 | ||
| X | X | X | X | X | 1; 3-6 | |||
| X | X | X | X | X | 2-4; 7-8 | |||
| X | X | X | X | 1-3; 8 |
For a general view of ACH, we described a schematic illustration of the ACH classification exemplified by Figure 1.
Although studies using both BMA and BMAC for the treatment of various musculoskeletal disorders have shown promising clinical results, inconsistent preparation methods with deficient reporting has led to questionable outcomes with respect to generalization and reproducibility. In order to optimize the efficacy and safety of BM-derived products, and to allow validation and standardization of such products, studies should report stepwise descriptions of the preparation protocol and additional information to further classify the product used. The ACH classification focuses on describing parameters that are relevant for the quality of biological products, such as the collection technique, cell count and its nature (whether stromal or hematopoietic), and molecular content dose. The ACH classification would contribute to a greater understanding of both clinical procedures and research outcomes and, over time, lead to a standardization of best practice. Together, we believe that the ACH Classification proposal is an easily recalled and useful method for the classification of BM-derived products in order to provide a comparative between product composition and clinical outcomes.
It should also be emphasized that this classification is pertaining only to BMA products. There are other aspects of bone marrow preparation such as photobiomodulation of the aspirate or the concentrate that have not been discussed. Unfortunately, there is not much literature supporting this concept. Thus, this is mentioned as a matter of anecdotal interest.
| 1. | Solomon DH, Husni ME, Libby PA, Yeomans ND, Lincoff AM, Lϋscher TF, Menon V, Brennan DM, Wisniewski LM, Nissen SE, Borer JS. The Risk of Major NSAID Toxicity with Celecoxib, Ibuprofen, or Naproxen: A Secondary Analysis of the PRECISION Trial. Am J Med. 2017;130:1415-1422.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Lee T, Lu N, Felson DT, Choi HK, Dalal DS, Zhang Y, Dubreuil M. Use of non-steroidal anti-inflammatory drugs correlates with the risk of venous thromboembolism in knee osteoarthritis patients: a UK population-based case-control study. Rheumatology (Oxford). 2016;55:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Sampson S, Msiv HV, Aufiero D, Botto-Van Bemden A. Orthobiologics: A New Frontier for Musculoskeletal Disease. J Stem Cell Res Transpl. 2014;1:3. |
| 4. | Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, Ambach MA, Vincent H, Urban-Paffaro A, Onodera CM, Annichino-Bizzacchi JM, Santana MH, Belangero WD. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12:69-78. [PubMed] |
| 5. | Dallari D, Stagni C, Rani N, Sabbioni G, Pelotti P, Torricelli P, Tschon M, Giavaresi G. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am J Sports Med. 2016;44:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 666] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 8. | Piuzzi NS, Hussain ZB, Chahla J, Cinque ME, Moatshe G, Mantripragada VP, Muschler GF, LaPrade RF. Variability in the Preparation, Reporting, and Use of Bone Marrow Aspirate Concentrate in Musculoskeletal Disorders: A Systematic Review of the Clinical Orthopaedic Literature. J Bone Joint Surg Am. 2018;100:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Lana JFSD, Purita J, Paulus C, Huber SC, Rodrigues BL, Rodrigues AA, Santana MH, Madureira JL Jr, Malheiros Luzo ÂC, Belangero WD, Annichino-Bizzacchi JM. Contributions for classification of platelet rich plasma - proposal of a new classification: MARSPILL. Regen Med. 2017;12:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 10. | Małkiewicz A, Dziedzic M. Bone marrow reconversion - imaging of physiological changes in bone marrow. Pol J Radiol. 2012;77:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Piuzzi NS, Chahla J, Schrock JB, LaPrade RF, Pascual-Garrido C, Mont MA, Muschler GF. Evidence for the Use of Cell-Based Therapy for the Treatment of Osteonecrosis of the Femoral Head: A Systematic Review of the Literature. J Arthroplasty. 2017;32:1698-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Salamanna F, Contartese D, Nicoli Aldini N, Barbanti Brodano G, Griffoni C, Gasbarrini A, Fini M. Bone marrow aspirate clot: A technical complication or a smart approach for musculoskeletal tissue regeneration? J Cell Physiol. 2018;233:2723-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Imam MA, Holton J, Ernstbrunner L, Pepke W, Grubhofer F, Narvani A, Snow M. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2017;41:2213-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1352] [Article Influence: 54.1] [Reference Citation Analysis (7)] |
| 15. | Ambrosi TH, Longaker MT, Chan CKF. A Revised Perspective of Skeletal Stem Cell Biology. Front Cell Dev Biol. 2019;7:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Madry H, Gao L, Eichler H, Orth P, Cucchiarini M. Bone Marrow Aspirate Concentrate-Enhanced Marrow Stimulation of Chondral Defects. Stem Cells Int. 2017;2017:1609685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 925] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 18. | Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3539] [Cited by in RCA: 3337] [Article Influence: 95.3] [Reference Citation Analysis (1)] |
| 20. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1062] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 21. | Papathanasopoulos A, Giannoudis PV. Biological considerations of mesenchymal stem cells and endothelial progenitor cells. Injury. 2008;39 Suppl 2:S21-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl. 2002;38:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13033] [Article Influence: 685.9] [Reference Citation Analysis (12)] |
| 24. | Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, Shankar AS, O'Flynn L, Elliman SJ, Roy D, Betjes MGH, Newsome PN, Baan CC, Hoogduijn MJ. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018;36:602-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 383] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 26. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2250] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 27. | Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 28. | Li H, Fu X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012;348:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 334] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 30. | Sawai CM, Babovic S, Upadhaya S, Knapp DJHF, Lavin Y, Lau CM, Goloborodko A, Feng J, Fujisaki J, Ding L, Mirny LA, Merad M, Eaves CJ, Reizis B. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity. 2016;45:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 31. | Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34:398-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 32. | Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2322] [Cited by in RCA: 2292] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 33. | Simmons PJ, Torok-Storb B. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991;78:2848-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES. Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells. 1997;15:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Mehrotra M, Williams CR, Ogawa M, LaRue AC. Hematopoietic stem cells give rise to osteo-chondrogenic cells. Blood Cells Mol Dis. 2013;50:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 1773] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 37. | Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1210] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 38. | Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 863] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 39. | Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 517] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 40. | Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 822] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 41. | Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:565187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3128] [Cited by in RCA: 3481] [Article Influence: 290.1] [Reference Citation Analysis (0)] |
| 43. | Lakschevitz FS, Visser MB, Sun C, Glogauer M. Neutrophil transcriptional profile changes during transit from bone marrow to sites of inflammation. Cell Mol Immunol. 2015;12:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol. 2012;13:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 46. | Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 47. | Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 48. | Hauser RA, Orlofsky A. Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: a case series. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Pierini M, Di Bella C, Dozza B, Frisoni T, Martella E, Bellotti C, Remondini D, Lucarelli E, Giannini S, Donati D. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Malempati S, Joshi S, Lai S, Braner DA, Tegtmeyer K. Videos in clinical medicine. Bone marrow aspiration and biopsy. N Engl J Med. 2009;361:e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Watson JT. Overview of biologics. J Orthop Trauma. 2005;19:S14-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Lindholm TS, Urist MR. A quantitative analysis of new bone formation by induction in compositive grafts of bone marrow and bone matrix. Clin Orthop Relat Res. 1980;288-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 94] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Butala R, Agarwal A, Khedekar RG, Gohain N, Grover A, Garg A. Outcome Of Autologous Bone Marrow Injection At Fracture Site In Delayed Union Of Long Bone: A Series Of 10 Cases. J Evolution Med Dent Sci. 2016;5:3841-3843. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Sahu RL. Percutaneous autogenous bone marrow injection for delayed union or non-union of long bone fractures after internal fixation. Rev Bras Ortop. 2018;53:668-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Jäger M, Jelinek EM, Wess KM, Scharfstädt A, Jacobson M, Kevy SV, Krauspe R. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Holton J, Imam MA, Snow M. Bone Marrow Aspirate in the Treatment of Chondral Injuries. Front Surg. 2016;3:1-6. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-up. Cartilage. 2011;2:286-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Sampson S, Smith J, Vincent H, Aufiero D, Zall M, Botto-van-Bemden A. Intra-articular bone marrow concentrate injection protocol: short-term efficacy in osteoarthritis. Regen Med. 2016;11:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Kim JD, Lee GW, Jung GH, Kim CK, Kim T, Park JH, Cha SS, You YB. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24:1505-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 62. | Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy. 2012;14:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Hernigou P, Homma Y, Flouzat Lachaniette CH, Poignard A, Allain J, Chevallier N, Rouard H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37:2279-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop J Sports Med. 2016;4:2325967115625481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 65. | Nishimoto S, Oyama T, Matsuda K. Simultaneous concentration of platelets and marrow cells: a simple and useful technique to obtain source cells and growth factors for regenerative medicine. Wound Repair Regen. 2007;15:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited Manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): C
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labusca L S-Editor: Ma YJ L-Editor: A E-Editor: Xing YX