Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.1
Peer-review started: June 5, 2019
First decision: August 1,2019
Revised: October 11, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: January 26, 2020
Processing time: 209 Days and 12.1 Hours
This article presents the stem and progenitor cells from subcutaneous adipose tissue, briefly comparing them with their bone marrow counterparts, and discussing their potential for use in regenerative medicine. Subcutaneous adipose tissue differs from other mesenchymal stromal/stem cells (MSCs) sources in that it contains a pre-adipocyte population that dwells in the adventitia of robust blood vessels. Pre-adipocytes are present both in the stromal-vascular fraction (SVF; freshly isolated cells) and in the adherent fraction of adipose stromal/stem cells (ASCs; in vitro expanded cells), and have an active role on the chronic inflammation environment established in obesity, likely due their monocytic-macrophage lineage identity. The SVF and ASCs have been explored in cell therapy protocols with relative success, given their paracrine and immunomodulatory effects. Importantly, the widely explored multipotentiality of ASCs has direct application in bone, cartilage and adipose tissue engineering. The aim of this editorial is to reinforce the peculiarities of the stem and progenitor cells from subcutaneous adipose tissue, revealing the spheroids as a recently described biotechnological tool for cell therapy and tissue engineering. Innovative cell culture techniques, in particular 3D scaffold-free cultures such as spheroids, are now available to increase the potential for regeneration and differentiation of mesenchymal lineages. Spheroids are being explored not only as a model for cell differentiation, but also as powerful 3D cell culture tools to maintain the stemness and expand the regenerative and differentiation capacities of mesenchymal cell lineages.
Core tip: Adipose tissue, notably subcutaneous, has a population of CD34-positive progenitor cells functionally known as pre-adipocytes. The pre-adipocytes have molecular and functional identities with the monocytic-macrophagic lineage and are altered in metabolic diseases such as obesity. To what extent will new 3D tools in cell culture, such as spheroids, be able to overcome the limitations imposed by 2D monolayer culture and unravel dormant capabilities of adipose stromal/stem cells?
- Citation: Baptista LS. Adipose stromal/stem cells in regenerative medicine: Potentials and limitations. World J Stem Cells 2020; 12(1): 1-7
- URL: https://www.wjgnet.com/1948-0210/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i1.1
Mesenchymal stromal/stem cells (MSCs) were first described and isolated from bone marrow as adherent colony-forming units of fibroblasts (CFU-F), and the primary role attributed to MSCs was to form niches for hematopoietic cells, supporting hematopoiesis[1]. In 1999, Pittenger et al[2] first described the in vitro multipotential nature of human bone marrow MSCs, introducing their use in cell therapy approaches, by delivering MSC suspensions to injury sites. The hypothesis was that MSCs were capable of tissue repair through grafting and differentiation into tissue-resident cells[3,4]. A few years later, an adipose tissue MSC population was described that shared some properties with MSCs isolated from the bone marrow, but had important unique characteristics[5,6].
Currently the widely accepted mechanism for tissue repair using bone marrow and adipose tissue sources (based on data from preclinical studies) is that MSCs interact with injured cells, creating tissue microenvironments or temporary niches that facilitate repair[7]. Thus, tissue regeneration by MSC transplantation may not rely exclusively on MSC differentiation, and the potential of MSCs to differentiate into multiple lineages is yet to be confirmed in vivo. In regenerative medicine approaches, the paracrine activity of MSCs fits well with cellular therapy protocols, while there in vitro multilineage potential is beneficial for tissue engineering. Furthermore, the “stemness” of MSCs and their in vitro multilineage potential can be optimized by cell culture conditions. The aim of this editorial is to reinforce the peculiarities of the stem and progenitor cells from subcutaneous adipose tissue, revealing the spheroids as a recently described biotechnological tool for cell therapy and tissue engineering. Spheroids are a 3D cell culture approach where cell clusters are formed in the absence of a scaffold (scaffold-free), optimizing cell-cell and cell-extracellular matrix interactions[8,9]. Recent studies have shown that culture as spheroids can be used to optimize the stemness and multilineage potential of MSCs[10], unraveling unknown characteristics of these cells, as well as opening new avenues for MSC use in regenerative medicine.
The subcutaneous adipose tissue is composed of adipocytes and of a heterogeneous “stromal-vascular fraction” (SVF). These two main cell compartments can be separated by a centrifugation approach that results in the adipocytes floating as a layer, while SVFs sediment to the bottom of the tube. Previously, the SVF was known as a compartment containing cells capable of accumulating intracytoplasmic lipids in vitro[11]. Currently, the SVF is defined as a heterogeneous population containing pre-adipocytes, endothelial mature cells, macrophages and fibroblasts. Furthermore, the SVF contains stem and progenitors cells showing different degrees of differentiation[12]. Due to their cell heterogeneity, the SVF is a major contributor to the unique molecular identity of the different depots of adipose tissue[13].
In 2001, Zuk et al[5] first described an MSC population in human subcutaneous adipose tissue isolated from the SVF. In 2013, the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy established the minimal definitions for stromal cells derived from subcutaneous adipose tissue[14]. The stromal cells within the SVF comprise heterogeneous cell types not amenable to culture in vitro, and a population of adherent, stromal/stem cells that can be culture in vitro. The latter are referred to as “adipose tissue derived stromal/stem cells” (ASCs), and this term will be used throughout this manuscript.
While ASCs were initially described as having the same in vitro multipotential nature, clonogenic potential (CFU-F) and similar surface markers as human bone marrow MSCs[15], differences between bone marrow and adipose MSCs emerged in subsequent publications (Table 1). Importantly, the tissue microenvironment differs significantly between the bone marrow and white adipose tissue. In these tissues, MSCs interacts with different neighboring cells, including an osteoblastic niche in the bone marrow[16] and cells from the more vascularized microenvironment in the white adipose tissue[6]. Consequently, bone marrow MSCs shows an intrinsic capacity to form bone and to support hematopoiesis after in vivo transplantation to ectopic sites[17], while ASCs have a superior angiogenic capacity[18]. Intriguingly, adipose tissue is also capable of supporting hematopoiesis (in a specific form), despite the remarkable differences in tissue microenvironment relative to the bone marrow[21]. In comparison with the MSC population derived from bone marrow after in vitro expansion, MSCs derived from subcutaneous adipose tissue can be distinguished by being positive for CD36 and negative for CD106[14]. Given the differences between bone marrow MSCs and ASCs, different morphogens are required and commonly used to induce the full range of multipotential differentiation of these cells in vitro.
| Tissue | MSC niche | Cell subpopulations | MSC in vitro surface markers | Multipotentiality |
| Bone marrow | Subendosteal and vascular | Osteoblasts, Endothelial progenitor and mature cells, Macrophages, MSCs | Positive: CD44, CD71, CD73, CD90, CD105, CD106, CD120a and | Adipogenic, Chondrogenic, and Osteogenic. Pre-committed into osteogenic lineage. |
| Hematopoietic stem and progenitor cells, lymphocytes, megakaryocytes, erythrocytes, monocytes, neutrophils, basophils, eosinophils | CD124 | |||
| Negative: CD14, CD34 and CD45 | ||||
| Adipose tissue | Vascular | Adipocytes | Positive: CD13, CD29, | Adipogenic, Chondrogenic, and Osteogenic. Pre-committed into adipogenic lineage. |
| CD44, CD73, CD34, CD36, CD90 and CD105 | ||||
| SVF: Pre-adipocytes, endothelial progenitor and mature cells, macrophages, fibroblasts, MSCs | Negative: CD31, CD45, CD235a and CD106 |
Importantly, uncultured SVFs from subcutaneous adipose tissue contain a unique cell population: The pre-adipocytes[14]. These cells dwell in the adventitia of robust blood vessels and are identified as negative for the pan hematopoietic surface marker (CD45), the mesenchymal stem cell surface marker (CD146) and for the mature endothelial cell surface marker (CD31), being positive only for CD34[6]. Pre-adipocytes had already been identified in adipose tissue even before the discovery of the MSC population[11,22]. In mice, pre-adipocytes and macrophages both originate from the monocytic lineage (CD14 positive cells)[23]. In line with this observation, pre-adipocytes share some surface markers with macrophages, as well as having the capacity to acquire certain macrophage properties[24].
Macrophages, especially the tissue resident population (M2), have a crucial role in adipose tissue homeostasis[22]. This role is highlighted in obesity, where chronic inflammation leads to macrophage polarization from an M2 to an M1 phenotype, disrupting adipose tissue homeostasis[25]. This disruption also alters the behavior of pre-adipocyte, as well as increasing their frequency in early stages of obesity[26]. In our hands, the subcutaneous adipose tissue samples from obese individuals do not present alterations in the pre-adipocyte population per se, but have increased frequency of mesenchymal precursors in the SVF, and ASCs with altered behavior in vitro[27].
Our research group first showed that the frequency and size of blood vessels are increased in subcutaneous adipose tissue from ex-obese donors that have been subjected to bariatric surgery[28]. In addition to blood vessels alterations, we described a significant increase in the number of pre-adipocytes cells in the SVF, together with a more heterogeneous population of ASCs, containing pre-adipocytes[27]. The increase in pre-adipocyte frequency can be linked to the increase in the size of blood vessels, since robust vessels have the adventitia layer, where pre-adipocytes dwell[6]. Thus, adipose tissue from ex-obese individuals appears to keep a cellular “memory” of the inflammatory microenvironment of obese tissue, despite relevant clinical improvement in obesity[29].
Both fractions of subcutaneous adipose cells - SVF and ASCs - have been extensively used in clinical trials, mainly due their paracrine and immunomodulatory potentials; however some discrepancies between studies have emerged, mainly due donor-to-donor variability combined with differences between the protocols for cell isolation and expansion in vitro, highlighting the need to better characterize even the ASCs[30]. In spite of their apparent homogeneity in vitro, ASCs contain a population of pre-adipocytes whose true potential has not yet been fully elucidated, especially in obese and ex-obese subcutaneous adipose tissue samples.
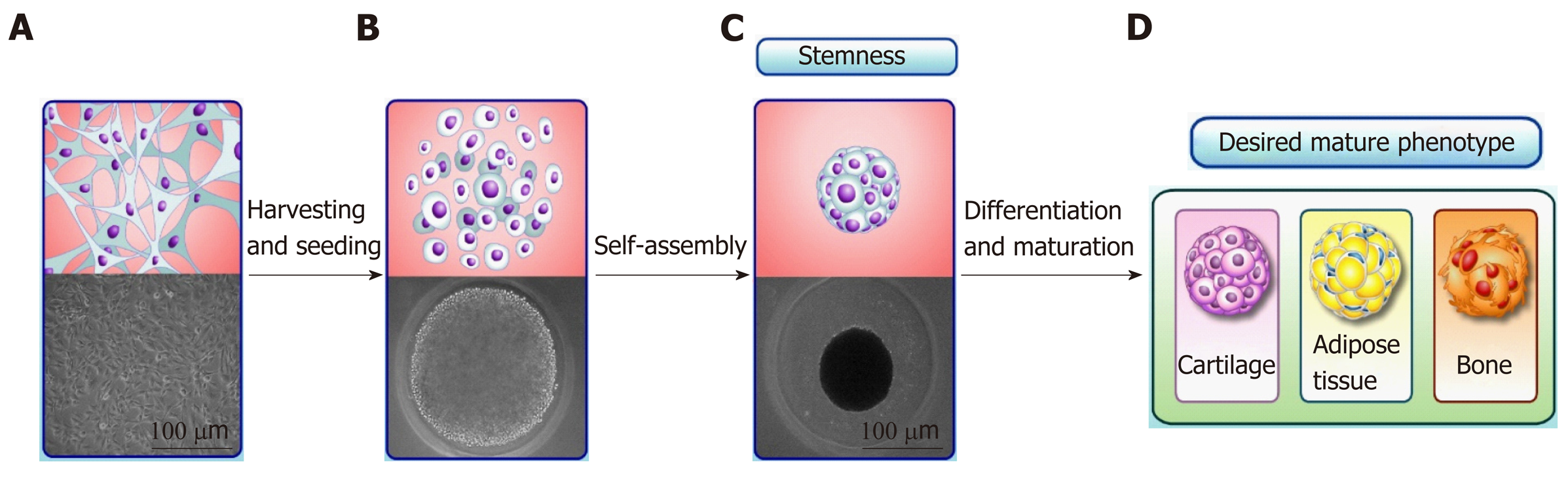
ASCs has been extensively described in the scientific literature as having their embryonic origin in a mesodermal progenitor population[31]. As a consequence, the typical multilineage capacity of ASCs represents their ability to form adipose tissue, bone and cartilage in vitro[14]. The multilineage capacity of ASCs has been extensively explored in tissue engineering, mainly by scaffold-based approaches. Recently, non-classical, scaffold-free approaches to tissue engineering have emerged that often rely on the production of 3D cell clusters called “spheroids”[32]. Spheroids mimic the embryonic stages of tissue development, optimizing the multilineage differentiation capacity of ASCs and MSCs (Figure 1). Moreover, spheroids are currently used not only as 3D culture models of cell differentiation in vitro, but also as a powerful cell culture tool to maintain the stemness and increase the regenerative, anti-inflammatory and angiogenic potentials of ASCs and MSCs[33]. The increase in the stemness of ASC and MSC spheroids (compared with 2D culture) is indicated by their higher multilineage potential, increased expression of pluripotency genes and late senescence[10], which reflect the cytoskeletal reorganization and expressive changes in cell morphology observed in spheroids[34]. However, the limitations of ASC and MSC spheroids comprise the low proliferation rate, causing in vitro cell expansion to still occur by monolayer culture. Furthermore, part of tissue engineering approaches intends to repair tissue critical-sized defects, requiring a scaffold.
Embryonic development in mammals starts with a cluster of epiblast stem cells[35]. Therefore, in vitro culture as spheroid-like cell clusters is used not only for embryonic stem cells, which are isolated from the early blastocyst stage, but also for induced pluripotent stem cells (iPSC) obtained by “reprogramming” adult somatic cells. Recapitulating embryogenesis, iPSCs initially form cell clusters that eventually become 3D cell aggregates representing spheroids.
A population of cells recently isolated from mesenchymal human tissue, named multilineage-differentiating stress enduring cells (Muse), is capable of forming pluripotent spheroid-like cell clusters[36] in the absence of in vivo tumorigenic capacity[37]. The differentiation of Muse cells for non-mesenchymal lineages relies on cell culture as clusters or spheroids, as well as on the use of a lower percentage of serum (or even on serum deprivation) during cell culture[38]. Human ASCs cultured in early passage under a lower percentage of autologous serum formed floating 3D spheroid-like cell clusters spontaneously[39]. Adipose-Muse cells differentiate into mesodermal, ectodermal and endodermal lineages, without teratoma formation[40].
The capacity of mature adipocytes to dedifferentiate and to differentiate into multiple cell lineages was already described before the identification of Adipose-Muse cells[41,42], with some signs of pluripotency[36]. Accordingly, it is not surprising that spheroid-based culture, which is known to increase the stemness capacity of cells, may increase the potential of ASC differentiation beyond that expected for mesenchymal lineage cells. The major advantage in exploring the pluripotency of ASC spheroids will be their safety in regenerative medicine protocols, since some studies show the absence of teratoma formation after Adipose-Muse transplantation[40].
In 2001, ASCs emerged as an accessible source of adult multipotent stem cells, showing high angiogenic and regenerative potential. The in vitro expansion of ASCs as monolayers may mask the multipotency and anti-inflammatory capacities of ASCs from obese and ex-obese donors. Embryonic development is marked by the formation of spheroid-like cell clusters, which can be mimicked in vitro by 3D culture as spheroids. Spheroid culture promises to reveal features of ASCs that were masked by culture in monolayers, including their pluripotency. In conclusion, ASC spheroids can be delivered into the injury site in an undifferentiated state due their regenerative potential or even as a tissue engineered construct, while allowing the use of obese and ex-obese ASCs in regenerative medicine protocols.
The transition from 2D monolayer culture of ASCs to 3D culture as spheroids brought previously unimaginable advantages. The next step is to translate the advantages of spheroid culture into novel therapeutic uses of ASCs in tissue regeneration and tissue engineering.
| 1. | Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83-92. [PubMed] |
| 2. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15369] [Article Influence: 569.2] [Reference Citation Analysis (2)] |
| 3. | Popp FC, Piso P, Schlitt HJ, Dahlke MH. Therapeutic potential of bone marrow stem cells for liver diseases. Curr Stem Cell Res Ther. 2006;1:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ohnishi S, Ohgushi H, Kitamura S, Nagaya N. Mesenchymal stem cells for the treatment of heart failure. Int J Hematol. 2007;86:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5837] [Article Influence: 233.5] [Reference Citation Analysis (1)] |
| 6. | Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 911] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 8. | Musumeci G, Mobasheri A, Trovato FM, Szychlinska MA, Graziano AC, Lo Furno D, Avola R, Mangano S, Giuffrida R, Cardile V. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014;116:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 10. | Cesarz Z, Tamama K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:9176357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 330] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Glick JM, Rothblat GH. Effects of metabolic inhibitors on the synthesis and release of lipoprotein lipase in cultured cells derived from the stromal-vascular fraction of rat adipose tissue. Biochim Biophys Acta. 1980;618:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Casteilla L, Charrière G, Laharrague P, Cousin B, Planat-Benard V, Péricaud L, Chavoin JP. [Adipose tissue, plastic and reconstructive surgery: come back to sources]. Ann Chir Plast Esthet. 2004;49:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Peinado JR, Jimenez-Gomez Y, Pulido MR, Ortega-Bellido M, Diaz-Lopez C, Padillo FJ, Lopez-Miranda J, Vazquez-Martínez R, Malagón MM. The stromal-vascular fraction of adipose tissue contributes to major differences between subcutaneous and visceral fat depots. Proteomics. 2010;10:3356-3366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 14. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1407] [Article Influence: 108.2] [Reference Citation Analysis (10)] |
| 15. | Fraser JK, Schreiber RE, Zuk PA, Hedrick MH. Adult stem cell therapy for the heart. Int J Biochem Cell Biol. 2004;36:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Balduino A, Mello-Coelho V, Wang Z, Taichman RS, Krebsbach PH, Weeraratna AT, Becker KG, de Mello W, Taub DD, Borojevic R. Molecular signature and in vivo behavior of bone marrow endosteal and subendosteal stromal cell populations and their relevance to hematopoiesis. Exp Cell Res. 2012;318:2427-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Charbord P, Livne E, Gross G, Häupl T, Neves NM, Marie P, Bianco P, Jorgensen C. Human bone marrow mesenchymal stem cells: a systematic reappraisal via the genostem experience. Stem Cell Rev Rep. 2011;7:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Casteilla L, Planat-Bénard V, Dehez S, De Barros S, Barreau C, André M. Endothelial and cardiac regeneration from adipose tissues. Methods Mol Biol. 2011;702:269-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lucas D. The Bone Marrow Microenvironment for Hematopoietic Stem Cells. Adv Exp Med Biol. 2017;1041:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Polymeri A, Giannobile WV, Kaigler D. Bone Marrow Stromal Stem Cells in Tissue Engineering and Regenerative Medicine. Horm Metab Res. 2016;48:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Cousin B, Casteilla L, Laharrague P, Luche E, Lorsignol A, Cuminetti V, Paupert J. Immuno-metabolism and adipose tissue: The key role of hematopoietic stem cells. Biochimie. 2016;124:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrague P, Casteilla L, Pénicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 222] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Luche E, Cousin B, Garidou L, Serino M, Waget A, Barreau C, André M, Valet P, Courtney M, Casteilla L, Burcelin R. Metabolic endotoxemia directly increases the proliferation of adipocyte precursors at the onset of metabolic diseases through a CD14-dependent mechanism. Mol Metab. 2013;2:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850-9855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 357] [Article Influence: 15.5] [Reference Citation Analysis (6)] |
| 25. | Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Martyniak K, Masternak MM. Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation. Exp Gerontol. 2017;94:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 27. | Silva KR, Liechocki S, Carneiro JR, Claudio-da-Silva C, Maya-Monteiro CM, Borojevic R, Baptista LS. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell Res Ther. 2015;6:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Baptista LS, da Silva KR, da Pedrosa CS, Claudio-da-Silva C, Carneiro JR, Aniceto M, de Mello-Coelho V, Takiya CM, Rossi MI, Borojevic R. Adipose tissue of control and ex-obese patients exhibit differences in blood vessel content and resident mesenchymal stem cell population. Obes Surg. 2009;19:1304-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Baptista LS, Silva KR, Borojevic R. Obesity and weight loss could alter the properties of adipose stem cells? World J Stem Cells. 2015;7:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Bajek A, Gurtowska N, Olkowska J, Kazmierski L, Maj M, Drewa T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch Immunol Ther Exp (Warsz). 2016;64:443-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Sági B, Maraghechi P, Urbán VS, Hegyi B, Szigeti A, Fajka-Boja R, Kudlik G, Német K, Monostori E, Gócza E, Uher F. Positional identity of murine mesenchymal stem cells resident in different organs is determined in the postsegmentation mesoderm. Stem Cells Dev. 2012;21:814-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Ovsianikov A, Khademhosseini A, Mironov V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018;36:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 33. | Laschke MW, Menger MD. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017;35:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 313] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 34. | Zhou Y, Chen H, Li H, Wu Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J Cell Mol Med. 2017;21:1073-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Shahbazi MN, Scialdone A, Skorupska N, Weberling A, Recher G, Zhu M, Jedrusik A, Devito LG, Noli L, Macaulay IC, Buecker C, Khalaf Y, Ilic D, Voet T, Marioni JC, Zernicka-Goetz M. Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature. 2017;552:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (20)] |
| 36. | Jumabay M, Boström KI. Dedifferentiated fat cells: A cell source for regenerative medicine. World J Stem Cells. 2015;7:1202-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA. 2010;107:8639-8643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 38. | Labusca L, Mashayekhi K. Human adult pluripotency: Facts and questions. World J Stem Cells. 2019;11:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 39. | Bogdanova-Jatniece A, Berzins U, Kozlovska T. Growth Properties and Pluripotency Marker Expression of Spontaneously Formed Three-dimensional Aggregates of Human Adipose-derived Stem Cells. Int J Stem Cells. 2014;7:143-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, Chazenbalk G, Aiba S, Dezawa M. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014;23:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1270] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 42. | Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P-Reviewer: Garg M, Hernanda PY, Musumeci G S-Editor: Dou Y L-Editor: A E-Editor: Zhang YL