Published online Sep 26, 2019. doi: 10.4252/wjsc.v11.i9.693
Peer-review started: April 12, 2019
First decision: June 5, 2019
Revised: July 8, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 26, 2019
Processing time: 169 Days and 23.1 Hours
Tumours are known to be a heterogeneous group of cells, which is why they are difficult to eradicate. One possible cause for this is the existence of slow-cycling cancer stem cells (CSCs) endowed with stem cell-like properties of self-renewal, which are responsible for resistance to chemotherapy and radiotherapy. In recent years, the role of lipid metabolism has garnered increasing attention in cancer. Specifically, the key roles of enzymes such as stearoyl-CoA desaturase-1 and 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase in CSCs, have gained particular interest. However, despite accumulating evidence on the role of proteins in controlling lipid metabolism, very little is known about the specific role played by lipid products in CSCs. This review highlights recent findings on the role of lipid metabolism in CSCs, focusing on the specific mechanism by which bioactive lipids regulate the fate of CSCs and their involvement in signal transduction pathways.
Core tip: Cancer stem cells (CSCs) are a minute portion of highly aggressive cells that survive conventional and targeted therapies and ultimately re-populate the tumour. Recent studies have elucidated that stearoyl-CoA desaturase-1 and 3-hydroxy-3-methyl-glutaryl-coenzyme A metabolic pathways involved in lipid metabolism are hyperactive in CSCs. However, the purpose of this enhanced activity is unclear. Here, we review the current literature and discuss the possible pathways and mechanisms that link the enhanced CSC lipid metabolism to bioactivity, specifically, with regard to structural lipids and active bio-molecules involved in cell signalling.
- Citation: Begicevic RR, Arfuso F, Falasca M. Bioactive lipids in cancer stem cells. World J Stem Cells 2019; 11(9): 693-704
- URL: https://www.wjgnet.com/1948-0210/full/v11/i9/693.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i9.693
Cancer progression is characterised by a continuous changeable state generating a very complex and heterogeneous multitude of cells with different morphology, genotype, and phenotype. This heterogeneity is explained by two main models: The clonal evolution model and the cancer stem cell (CSC) model. According to the CSC model, cancers are a heterogeneous combination of genetically different subclones that are arranged in an organised hierarchy, with CSCs at the apex[1,2]. According to the stem cell theory for cancer, only a subset of cancer cells are accountable for tumour initiation and propagation[3]. The primary functional characteristics of CSCs are similar to those of normal stem cells, such as the capacity to self-renew and the ability to differentiate into different cell types. CSCs present an elevated tumorigenic potential and an increased resistance to conventional and targeted therapy[3-8]. Functional recognition of CSCs from the mass of the tumour population involves the demonstration that they are indeed able to self-renew and differentiate[9-13]. These cells must possess the ability to initiate a novel tumour, often in small numbers. There is much dispute on the specificity of markers to be used to identify CSCs. However, the most reliable are functional markers such as ABC transporter activity, namely ABCG2 and ABCB1, which are able to transport the fluorescent dyes Hoechst 33342 and rhodamine 123, respectively[14]. Aldehyde dehydrogenase activity and the ability to cycle slowly are among other characteristics commonly accepted as defining features of CSCs[5,15-18]. The concept that suggests CSCs rely on oxidative phosphorylation (OXPHOS) is becoming more accepted as the metabolic signature of CSCs, making metabolic targeting a rewarding opportunity within the CSC field[5,6,19-27]. Recent studies have highlighted the link between CSCs and enhanced activity in lipid metabolism, particularly for monounsaturated fatty acids and cholesterol. Recent reviews have brilliantly described the role of lipid metabolism alterations in CSCs[28-30]. However, the purpose behind this enhanced activity is not understood. In this review we discuss the latest advances in CSC lipid metabolism and describe how this enhanced lipid metabolism in CSCs can lead to the production of active biolipids as signalling molecules.
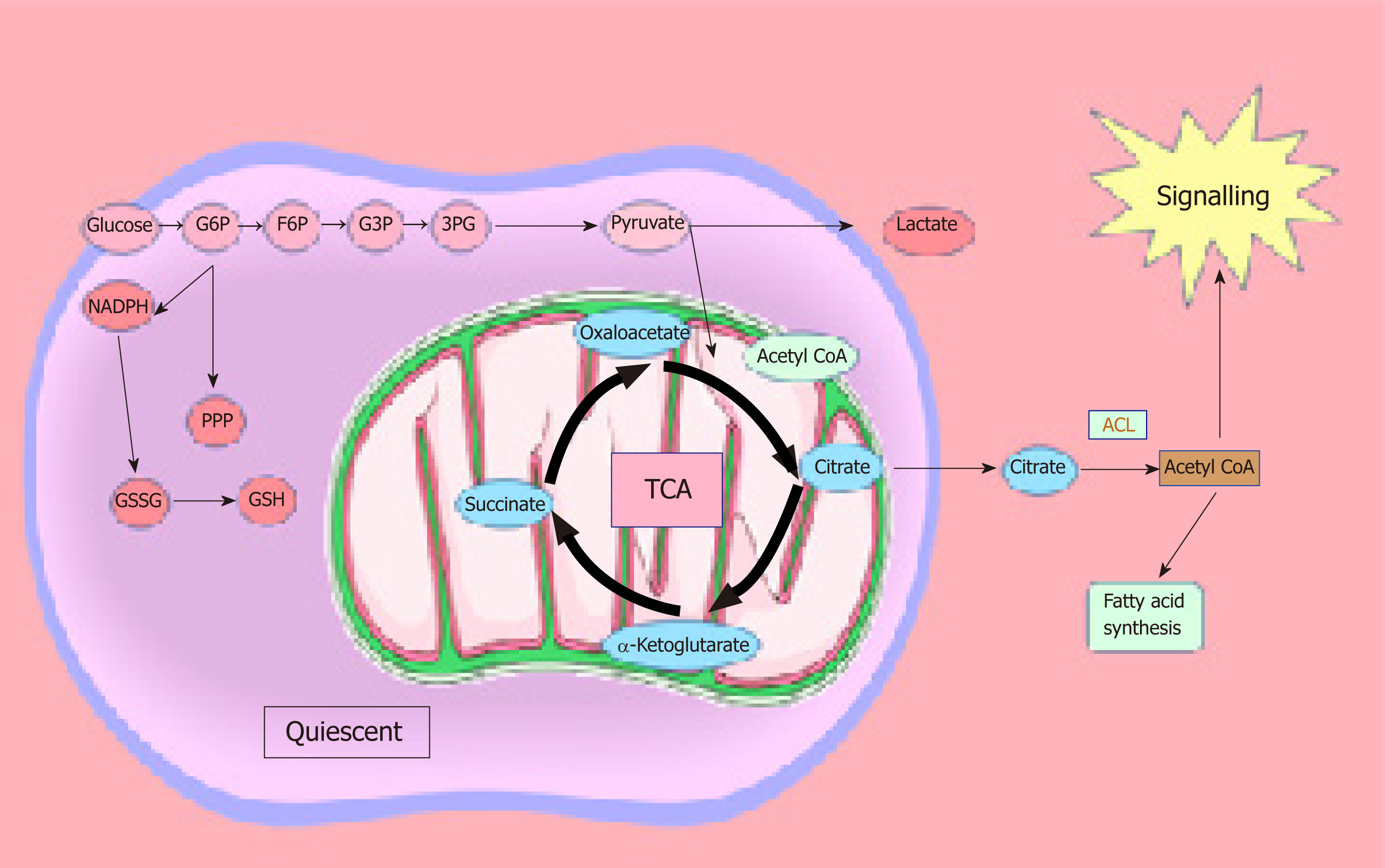
Similar to normal cells, CSCs use energy from mitochondrial OXPHOS, which produces more adenosine triphosphate (ATP) compared to glycolysis and produces tricarboxylic acid cycle intermediates utilised for macromolecule synthesis. CSC functions are regulated by a number of specific signalling pathways[31,32]. These pathways change in response to environmental stresses such as fluctuating oxygen and nutrient levels, pH, inflammation, and anticancer therapies[33]. While cancers rely on angiogenesis, the fast proliferation of cancer cells outstrips the blood supply, which is often leaky and lacks a normal hierarchical structure. Consequently, hypoxia and poor perfusion are common in tumours, so that there is a poor supply of nutrients and clearance of waste products. However, mitochondrial respiration is not impaired until the oxygen concentration drops below 1.0 μM[34]. Furthermore, it has been shown that even at oxygen levels of 0.5%, the electron transport chain is still capable of normal functioning[35]. It has also been reported that hypoxia is necessary for the preservation of embryonic stem cells in an undifferentiated state[36] and that it is accountable for the creation and maintenance of the stem cell niche[37-40,41,42]. These studies exemplify that hypoxia is a necessary condition for ensuring a balance between stem cell phenotypes and metabolism. In addition, it has been demonstrated that tumorigenesis is dependent on functioning mitochondria[5,43], since mitochondrial respiration results in the production of metabolites such as citrate, that can be utilised by ATP citrate lyase, to produce oxaloacetate and acetyl-CoA. In conditions where there are high levels of ATP, it has been shown that acetyl-CoA can be utilised for the regulation of protein acetylation and the synthesis of fatty acids[44]. These findings suggest a role for signalling molecules in the maintenance of the stem cell niche. A recent study demonstrated that glycosylation (specifically O-GlcNAc modification) of pluripotency markers sex-determining region Y-box 2 and octamer-binding transcription factor 4 takes place in undifferentiated mouse embryonic stem cells and this is absent following differentiation[45]. Emerging evidence suggests that the metabolic phenotype of CSCs is dependent on their location, oxygen supply, and metastatic sites. There are studies suggesting that CSCs from lung, breast, glioblastoma, osteosarcoma, ovarian, nasopharyngeal, hepatocellular carcinoma, and colorectal cancers favour glycolysis compared to other differentiated cells in vitro and in vivo[46-52]. This variation may be due to differential location, availability of nutrients, oxygen, stage of lineage specification, tumour heterogeneity, and isolation techniques. It is possible to speculate that metabolic profiles of CSCs change as they migrate from the original site to the metastatic site and that this change is largely attributed to the tumour microenvironment in which they reside. While both glycolytic and mitochondrial metabolism are utilised by cancer cells, due to the heterogeneity among cancer cells within a tumour, some cells are reliant on glucose[53], while others have a strong dependence on aerobic glycolysis[54,55] due to an impaired TCA cycle or electron transport chain. However, due to the plasticity of cancer, some cells can alter their metabolic profile following therapeutic intervention by undertaking therapy-induced senescence[56]. Another impediment to cancer eradication is that slow-cycling CSCs demonstrate dependence on OXPHOS[5,7,57].
It was recently found that the prominent CSC marker aldehyde dehydrogenase (ALDH)1A1 modulates energy metabolism in adipocytes from several species[58]. In this study, retinoic acid deficiency in knock-out ALDH1A1 adipocytes inhibited adipogenesis and increased thermogenesis. Functional CSC markers such as ALDH1A1 activity are increasingly highlighted as a reliable marker in the literature. ALDH1A1 activity requires the involvement of metabolic and signalling pathways. Retinoids play an important role in energy metabolism, and their role in maintaining normal embryonic development is well understood. In retinoid metabolism, retinaldehyde can be oxidised to retinoic acid by ALDH1a1-3. Retinoic acid is a potent transcriptional regulator and controls more than 500 genes. The receptors for retinoic acid (RAR-α, RAR-β, and RAR-γ) are members of the nuclear hormone receptor superfamily, which includes receptors for steroid and thyroid hormones. Upon activation, these receptors initiate cell responses related to proliferation, apoptosis, and differentiation. There is also some evidence that retinoic acid can regulate signalling pathways inside the cell and that all-trans-retinoic acid can bind peroxisome proliferator-activated receptor beta-gamma (PPAR β-γ). The enzymes are involved in several biological functions and their functional role is likely related to cellular detoxification and maintenance of low reactive oxygen species[15].
Lipid dysfunction has been observed as a trait of more aggressive cancers that have adverse survival outcomes. Research is highlighting the specific alterations occurring in pathways involving lipids and cholesterol. An emerging concept is that CSCs are highly dependent on enzymes associated with lipid metabolism, even though the precise reason for this reliance is not completely understood. Hyperactive metabolic routes that produce lipids and cholesterol, together with the increased uptake of exogenous lipids, are required by the tumour to enable proliferation. Lipids are not only substrates but can either provide structural scaffolds for proteins or be incorporated into the protein structure[59], which acts to stabilise signalling proteins to facilitate effective coupling between cellular receptors and signals[59,60]. Lipid metabolism may also be a crucial component in maintaining the cell membrane and protecting against peroxidation by chemotherapeutic agents or the hypoxic niche. It has been shown that the lipid bilayer leaflets have a non-symmetric distribution of lipids[61], and that this is dependent on several factors such as head group, chain length, and degree of saturation, all of which can affect the cell membrane’s flexibility and construction[62,63]. Lipids such as steroid hormones or phosphoinositides can leave the cell and act as active signalling biomolecules in the tumour microenvironment. These molecules can act in an autocrine manner to initiate a signalling cascade that induces proliferation in neighbouring cancer cells[64,65].
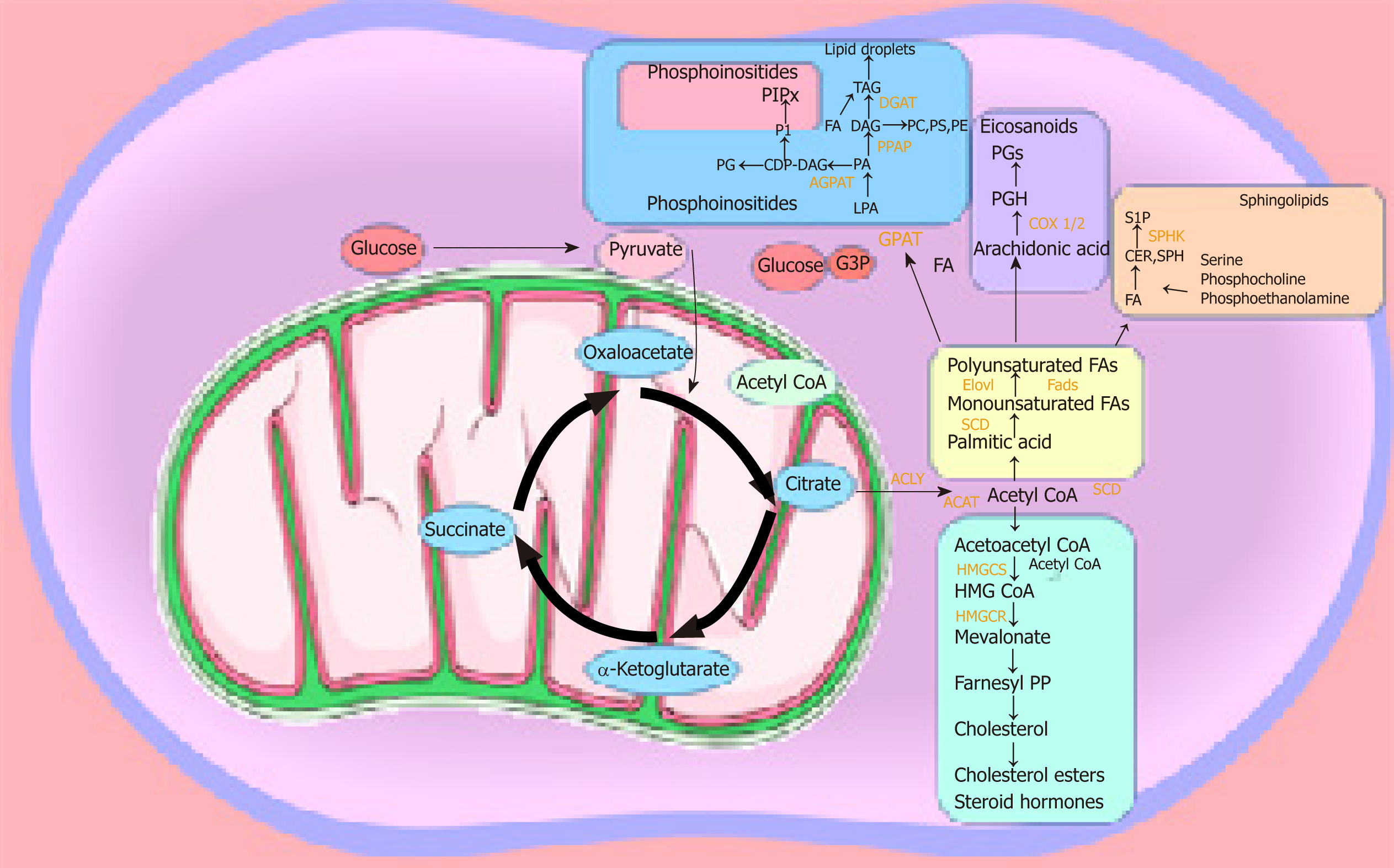
Fatty acid synthesis and oxidation are indispensable components in the maintenance of the adult stem cell and CSC populations from various organs (Figure 1). Both anabolic and catabolic pathways are closely controlled in CSCs and are essential for self-renewal activity. Peroxisome proliferator-activated receptor (PPAR-δ) is crucial for lipid metabolism and is implicated in the control of energy homeostasis. The loss of PPAR-δ results in defects to haematopoietic stem cells but its agonist restores the defect. Similarly, inhibition of mitochondrial fatty acid oxidation generates the disappearance of haematopoietic stem cells[66]. These results suggest that the PPAR fatty acid oxidation axis may be essential for stem cell conservation. Several investigations have linked lipogenesis to CSCs. De novo lipogenesis is more active in glioblastoma multiforme CSCs compared to the bulk tumour population and is needed for stem cell renewal in breast cancer[67,68]. Blockage of fatty acid synthase (FASN) has been shown to diminish breast CSC growth in vivo and maintain breast cancer cells through the PPARγpathway by upregulating de novo lipogenesis[69]. FASN is overexpressed in patient-derived glioblastoma stem cells, and its inhibition significantly reduces the expression of stemness markers SOX2, NESTIN, CD133, and FABP7, as well as reducing the CSCs’ invasiveness and sphere forming ability[67]. Pancreatic CSCs also have higher de novo lipogenesis activity where FASN is overexpressed, and the CSCs are more sensitive to inhibition by FASN specific inhibitors[70]. Breast CSCs have shown elevated levels of lipogenic genes compared to non-CSCs, such as ATP citrate lyase, acetyl CoA carboxylase 1 (ACC1), and FASN. Furthermore, ectopic expression of master regulator of lipogenesis sterol-regulatory binding protein-1 upregulates downstream lipogenic genes (ATP citrate lyase, ACC1, and FASN), resulting in enhanced lipogenesis and mammosphere formation[68]. Inhibition of ACC notably impairs mammosphere forming ability and the number of ALDH1A1+ cells in culture[71].
The co-culture of adipocytes with bone marrow-derived prostate cancer cells has demonstrated the ability of cancer cells to use lipids from adipocytes in their microenvironment in order to promote cancer growth[72]. When looking at stem cell components, both haematopoietic and leukemic-initiating cells depend on fatty acid oxidation. Elevated levels of lipid droplets have been observed in circulating tumour cells and are associated with more aggressive tumour types and poor survival outcomes. Increased extracellular lipid uptake contributes to lipid droplet accumulation and the tumour-initiating capacity in CSCs[73]. These lipid droplets can act as reservoirs inside the cell since they are filled with energy from various fatty acids, cholesterols, and triacylglycerol. An elevated content of lipid droplets is a distinctive feature of colorectal CSCs. There was a direct correlation between CD133+ cells and lipid droplet amounts, and cells with an elevated level of lipid droplets have enhanced clonogenic potential in vitro and in vivo[74]. Lipophagy, a process that involves the fusion of lipid droplets with autophagosomes, confers resistance to pancreatic cancer cells through an increase in fatty acid β-oxidation[5]. The latest progresses in proteomics and metabolomics have highlighted the link between fatty acid oxidation and CSC fate[70,75,76]. For example, the homeobox protein NANOG stimulates hepatocellular carcinoma stem-like cells by reprogramming the metabolic state of cells from OXPHOS to fatty acid oxidation[52]. During lipophagy, free fatty acids are mobilised to the mitochondria, which confer survival to cancer cells when metabolic restrictions are induced[77,78]. Although lipid oxidation, lipid synthesis, and glucose metabolism are closely linked, the exact mechanisms underlying these interactions are not well understood. It is plausible to speculate that the lipid content of lipid droplets such as fatty acids, cholesterol, and triacylglycerol can be used to synthesise the cell membrane. These molecules can also be used to synthesise active signalling biomolecules or be exported out of the cell via exosomes to prepare the pre-metastatic niche.
Lipid desaturation is important in maintaining stemness, tumour formation, and metastasis in breast, colon, and prostate cancers[79,80]. SCD1 is an enzymatic node central to the conversion of saturated fatty acids to mono-unsaturated fatty acids[81]. Monounsaturated fatty acids are precursors to a number of fundamental plasma membrane lipids such as triglycerides, cholesterol esters, and diacylglycerols[82]. More importantly, they can have signalling properties and act as direct effectors of SCD1 activity. In particular, palmitoleic acid has been found to mediate several processes such as enhanced oxygen consumption, fatty acid oxidation, and ATP content in adipocytes. As previously mentioned, lipids act as essential components of the cell wall, which contributes to signal transduction, migration, and metastatic potential[83,84]. Overexpression of SCDs promotes cancer cell proliferation and inhibits cell death[79,80,85]. Lipid unsaturation has been recognised as a biomarker for ovarian CSCs, and its blockage decreases tumour-forming abilities in vivo[76,85]. The same has also been observed in breast CSCs[85]. SCD1 inhibition hindered sphere-forming ability, along with a reduction in markers ALDH1A1, NANOG, and OCT4, and reverted chemoresistance in lung CSCs, while more differentiated cells were unaffected[86]. The presence of carbon-to-carbon single or double bonds can have both physical and chemical properties that are essential in the constitution of cell membranes and signal transduction. As previously mentioned, monounsaturated fatty acids are used as progenitors to a number of molecules, which can act as signalling molecules themselves or as substrates for other signalling molecules. For example, cholesterol esters can enter the mevalonate pathway to synthesise steroid hormones. Phosphoinositides can be converted into lysophosphoinositides. Both of these molecules are powerful bioactive lipids. Similarly, the cell membrane and all of its components such as lipid rafts, in which signalling receptors are embedded, cannot function properly without the proper distribution of triacylglycerides and diacylglycerides. Since CSCs are known for their metastatic potential and chemo-therapy evasion, it is important to note that these lipid by-products can be involved in signal transduction for both migration and physical protection from peroxidation. These findings suggest that lipid desaturases may be the optimal targets for tumour prevention in a variety of cancers. Interestingly, recent data has shown that SCD-dependent fatty acid desaturation is not the only source of monoun-saturated fatty acids in cancer cells[87]. Indeed, it has identified a novel desaturation pathway, the sapienate biosynthesis, as an alternative source of monounsaturated fatty acids.
The mevalonate pathway is the metabolic pathway responsible for the formation of steroid hormones and cholesterol. This is a highly conserved pathway that involves a series of reactions including the rate-limiting step, catalysed by 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, which converts HMG-CoA to mevalonate[88]. Mevalonate downstream products comprise cholesterol, gera-nylgeranyl pyrophosphate, farnesyl diphosphate synthase, and ubiquinone. The mevalonate metabolic route is important in protein prenylation, a post-translational modification that tethers the Ras and Rho family of GTPases to the membrane, which is required for the correct functioning of G protein-coupled receptors, and inhibition of the mevalonate pathway decreased sphere-forming ability in ALDH1A1+ breast CSCs[89]. There is some controversy whether or not increased blood cholesterol is correlated with tumour incidence and mortality. The use of blood cholesterol-lowering statins is correlated with a reduced cancer incidence[90]. However, some reports have shown no correlation[91]. While pre-clinical and mechanistic studies generally support the use of statins for anticancer therapy, conflicting reports may be attributable to compensatory upregulation of HMG-CoA reductase by statins and the resulting dose-limiting toxicities[92]. Nevertheless, total cholesterol is a poor prognostic factor in several different cancers[93], and statin use is associated with reduced cancer-related mortality in cancer patients[94]. Recent studies have found that either blocking cholesterol synthesis or the HMG-CoA pathway exclusively eliminates stem cells of glioblastoma multiforme, colorectal, and lung cancers[95,96]. Further, a high-fat diet enhances in vivo tumour growth, which is supressed by statin treatment[97]. These results strongly suggest that there exists an important and positive role of cholesterol in the biology of CSC functions. Pathways involved in both cholesterol biosynthesis and the synthesis of unsaturated fatty acids have been recently identified as the only selective druggable target in CSCs[98]. Interestingly, a recent study revealed that cholesterol biosynthesis is a key characteristic of breast CSCs and has a clear impact on patient outcome[99]. The findings of the latter study clearly identified the cholesterol biosynthesis pathway as crucial for CSC propagation and a therapeutic target. In addition, this study provides a mechanistic explanation for the beneficial therapeutic effect of the use of statins in breast cancer. Similarly, cholesterol biosynthesis has been found to be a crucial player in the tumorigenicity of human neuroblastoma cell lines and corresponding sphere-forming cells[100].
The majority of studies on lipid metabolism in CSCs have elucidated the enzymes and metabolic pathways involved in lipid synthesis. However, the precise functional role played by the different lipid molecules in CSCs remains unclear. Lipids play a central role in the cell-cell signalling process by maintaining the integrity of the cell membrane and by making lipid rafts, which act as platforms for signal receptors[62,63,101,102]. We can speculate that the hyperactive metabolic activity is used to synthesise lipids, which not only have a structural function by making up the cell membrane, but also have a more active role as bioactive-lipid signalling molecules. These active biomolecules can be released into the extracellular space and activate downstream pathways involved in proliferation, migration/invasion, and differentiation in an autocrine and/or paracrine manner. The latest studies have shown that the metabolism required to produce ATP is tightly regulated in CSCs, and this metabolic profile differs in the bulk of the tumour population[27,103]. CSCs are plastic in nature and change their metabolism as they are migrating from their origin to the metastatic site. They seem to have a preference for OXPHOS and show reduced metabolic plasticity when stressed. As soon as ATP levels reach a certain level, ATP-citrate lyase catalyses the transformation of citrate and CoA to acetyl-CoA and oxaloacetate, respectively. Acetyl-CoA can be converted to malonyl-CoA, which can enter the fatty acid synthesis route. Malonyl-CoA is utilised by AMP-activated kinase in order to regulate the synthesis of fatty acids, which in turn are utilised for the production of phosphoinositides, eicosanoids, lysophospholipids, and sphingolipids[44] (Figure 2).
Lysophospholipids, such as lysophosphatidic acid and sphingosine 1-phosphate, have a key role in stem cell biology[104] and tumour progression[105]. The plasma membrane contains lipid rafts enriched with sphingolipids, which are important participants in signal transmission[106-114]. A recent study of the pancreas highlighted the role of sphingosine-1-phosphate in promoting the survival of progenitor cells and determining acinar and endocrine cell specification[107]. The bioactive lysophospholipid lysophosphatidylinositol can be secreted into the extracellular milieu, initiating a signalling cascade that stimulates the proliferation of surrounding cancer cells[65]. The conversion of acetyl-CoA into acetoacetyl-CoA allows its entry into the mevalonate pathway[44], which is integral for the production of cholesterol esters and steroid hormones that are crucial participants in prostate stem cell maintenance and lineage specification[107-108]. Haematopoietic cells are reliant on phospholipids and essential fatty acids during differentiation[109]. Arachidonic acid is involved in the synthesis of leukotriene, prostacyclin, and thromboxane from phospholipids[109]. Eicosanoids’ primary physiological activity is related to inflammation and modulation of cardiovascular function and tone. Leukotrienes and prostaglandins can create a leaky vascular endothelium, which is a requirement for metastatic spread[110]. Interleukin 1B was found to maintain malignant melanoma initiating cells[111,112]. CSCs are known for their increased ABC transporter activity, which requires ATP for its function. We recently proposed that, apart from their role in chemoresistance, ABC transporter hyperactivity is possibly due to their exportation of signalling molecules, including lipids[113]. Several studies have shown that at least one-third of all 48 mammalian ABC transporters are involved in lipid transport[59,63]. Transporters such as ABCA1, ABCG1, ABCG4, ABCG5, and ABCG8 have been identified as sterol transporters[114]. ABC transporters of the C family transport bioactive lysophospholipids such as lysophosphatidylinositol and sphingosine 1-phosphate[64,115,116]. Of particular interest are ABCG2 and ABCB1, the most well studied members in CSCs. We hypothesise that they may play a specific role in CSCs to maintain stemness and sustain cell survival; specifically, by exporting bioactive-lipid signalling molecules such as steroid hormones, cholesterol, and metabolites, which are the result of enhanced lipid uptake and lipid metabolic pathways observed in CSCs[63,64,113,117,118]. Another emerging processthrough which CSCs can also signal is through the release of exosomes. Exosomes are lipid vesicles released from the cell, which carry important messages including bioactive lipids or enzymes and are able to release signalling lipids. Exosomes are thought to be involved in specific cancer functions such as creating the pre-metastatic niche in the specific secondary site[119]. It is likely that enhanced lipid metabolism in CSCs is used to both synthesise exosomes and their content[120-122]. It would be interesting to analyse the lipidomic profile of CSC-derived exosomes to enhance our understanding of the specific role that exosomes play in cancer progression. Exploring these pathways could elucidate a vulnerability that might be beneficial in targeting these highly aggressive cells. However, first an understanding is needed of the mechanisms behind these metabolic pathways and what purpose they fulfil.
In conclusion, lipid metabolism is emerging as a viable target in CSCs. In particular, the enhanced pathways involved in lipid metabolism, such as SCD1 and HMG-CoA activity. However, some questions still need further investigation, such as the purpose for this enhanced activity. We propose that lipid signalling molecules are synthesised as a result of enhanced metabolic activity and that CSCs use those signals for their survival advantage. Lipid metabolism represents an intriguing target for cancer therapy and we further suggest that to target CSCs, these pathways must be understood. The identification of the deregulated pathways is a good starting point to eradicate CSCs. However, increased knowledge of the role played by bioactive lipids will provide a novel opportunity to eliminate these highly aggressive cells.
The authors acknowledge the infrastructure and staff support provided by the School of Pharmacy and Biomedical Sciences and CHIRI, Faculty of Health Sciences Curtin University. This Project is made possible by an Avner Pancreatic Cancer Foundation Grant (http://www.avnersfoundation.org.au). RRB is supported by the “Australian Government Research Training Program Scholarship”.
| 1. | Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1688] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 2. | Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1549] [Cited by in RCA: 1868] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 3. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6980] [Article Influence: 279.2] [Reference Citation Analysis (0)] |
| 4. | Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8:e76518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 1020] [Article Influence: 85.0] [Reference Citation Analysis (14)] |
| 6. | Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Graña O, Viera CR, Yuneva M, Sainz B, Heeschen C. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015;22:590-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 588] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 7. | Skrtić M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, Hurren R, Jitkova Y, Gronda M, Maclean N, Lai CK, Eberhard Y, Bartoszko J, Spagnuolo P, Rutledge AC, Datti A, Ketela T, Moffat J, Robinson BH, Cameron JH, Wrana J, Eaves CJ, Minden MD, Wang JC, Dick JE, Humphries K, Nislow C, Giaever G, Schimmer AD. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2011;20:674-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 553] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 8. | Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2806] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 9. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2455] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 10. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3068] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 11. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2952] [Cited by in RCA: 3053] [Article Influence: 152.7] [Reference Citation Analysis (0)] |
| 12. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5627] [Article Influence: 255.8] [Reference Citation Analysis (0)] |
| 13. | Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Moitra K. Overcoming Multidrug Resistance in Cancer Stem Cells. Biomed Res Int. 2015;2015:635745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 15. | Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018-11032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 453] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 16. | Clarkson B, Fried J, Strife A, Sakai Y, Ota K, Okita T. Studies of cellular proliferation in human leukemia. 3. Behavior of leukemic cells in three adults with acute leukemia given continuous infusions of 3H-thymidine for 8 or 10 days. Cancer. 1970;25:1237-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Clarkson B, Ohkita T, Ota K, Fried J. Studies of cellular proliferation in human leukemia. I. Estimation of growth rates of leukemic and normal hematopoietic cells in two adults with acute leukemia given single injections of tritiated thymidine. J Clin Invest. 1967;46:506-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Franco J, Balaji U, Freinkman E, Witkiewicz AK, Knudsen ES. Metabolic Reprogramming of Pancreatic Cancer Mediated by CDK4/6 Inhibition Elicits Unique Vulnerabilities. Cell Rep. 2016;14:979-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, Reue K, Christofk H, Mischel PS, Pajonk F. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci U S A. 2011;108:16062-16067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 442] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 20. | Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW, Yu SC, Qian GS. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Janiszewska M, Suvà ML, Riggi N, Houtkooper RH, Auwerx J, Clément-Schatlo V, Radovanovic I, Rheinbay E, Provero P, Stamenkovic I. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 22. | Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, Chen YJ. Resveratrol Impedes the Stemness, Epithelial-Mesenchymal Transition, and Metabolic Reprogramming of Cancer Stem Cells in Nasopharyngeal Carcinoma through p53 Activation. Evid Based Complement Alternat Med. 2013;2013:590393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Pastò A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, Nicoletto MO, Manicone M, Indraccolo S, Amadori A. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5:4305-4319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | De Luca A, Fiorillo M, Peiris-Pagès M, Ozsvari B, Smith DL, Sanchez-Alvarez R, Martinez-Outschoorn UE, Cappello AR, Pezzi V, Lisanti MP, Sotgia F. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 2015;6:14777-14795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 25. | Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 1087] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 26. | Lamb R, Bonuccelli G, Ozsvári B, Peiris-Pagès M, Fiorillo M, Smith DL, Bevilacqua G, Mazzanti CM, McDonnell LA, Naccarato AG, Chiu M, Wynne L, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Mitochondrial mass, a new metabolic biomarker for stem-like cancer cells: Understanding WNT/FGF-driven anabolic signaling. Oncotarget. 2015;6:30453-30471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Domenichini A, Edmands JS, Adamska A, Begicevic RR, Paternoster S, Falasca M. Pancreatic cancer tumorspheres are cancer stem-like cells with increased chemoresistance and reduced metabolic potential. Adv Biol Regul. 2019;72:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Kuo CY, Ann DK. When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun (Lond). 2018;38:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Mancini R, Noto A, Pisanu ME, De Vitis C, Maugeri-Saccà M, Ciliberto G. Metabolic features of cancer stem cells: the emerging role of lipid metabolism. Oncogene. 2018;37:2367-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Yi M, Li J, Chen S, Cai J, Ban Y, Peng Q, Zhou Y, Zeng Z, Peng S, Li X, Xiong W, Li G, Xiang B. Emerging role of lipid metabolism alterations in Cancer stem cells. J Exp Clin Cancer Res. 2018;37:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 31. | Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 556] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 32. | Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 426] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 33. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48671] [Article Influence: 3244.7] [Reference Citation Analysis (12)] |
| 34. | Scandurra FM, Gnaiger E. Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. Adv Exp Med Biol. 2010;662:7-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem. 1990;265:15392-15402. [PubMed] |
| 36. | Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783-4788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 633] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 37. | Huang WJ, Chen WW, Zhang X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol Lett. 2016;12:2283-2288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang YJ, Zhao L, Chen FH, Wang XT, You QD, Guo QL. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012;19:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 39. | Rampazzo E, Persano L, Pistollato F, Moro E, Frasson C, Porazzi P, Della Puppa A, Bresolin S, Battilana G, Indraccolo S, Te Kronnie G, Argenton F, Tiso N, Basso G. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis. 2013;4:e500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Li P, Zhou C, Xu L, Xiao H. Hypoxia enhances stemness of cancer stem cells in glioblastoma: an in vitro study. Int J Med Sci. 2013;10:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Kolenda J, Jensen SS, Aaberg-Jessen C, Christensen K, Andersen C, Brünner N, Kristensen BW. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J Neurooncol. 2011;103:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Garrido W, Rocha JD, Jaramillo C, Fernandez K, Oyarzun C, San Martin R, Quezada C. Chemoresistance in high-grade gliomas: relevance of adenosine signalling in stem-like cells of glioblastoma multiforme. Curr Drug Targets. 2014;15:931-942. [PubMed] |
| 43. | Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788-8793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1412] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 44. | Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 604] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 45. | Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 2012;11:62-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 46. | Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D'Agostino D, Forlì F, D'Aguanno S, Todaro M, Stassi G, Di Ilio C, De Laurenzi V, Urbani A. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 221] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 48. | Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem. 2014;115:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, Keating MJ, Kondo S, Huang P. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843-32853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 50. | Chen KY, Liu X, Bu P, Lin CS, Rakhilin N, Locasale JW, Shen X. A metabolic signature of colon cancer initiating cells. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4759-4762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 52. | Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, Hess S, Machida K. NANOG Metabolically Reprograms Tumor-Initiating Stem-like Cells through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell Metab. 2016;23:206-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 53. | Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8:519-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2361] [Cited by in RCA: 3186] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 54. | Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2574] [Cited by in RCA: 2466] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 55. | Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652-3658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 56. | Wolf DA. Is reliance on mitochondrial respiration a "chink in the armor" of therapy-resistant cancer? Cancer Cell. 2014;26:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE, Krause E, Pätzold S, Villanueva J, Krepler C, Fukunaga-Kalabis M, Hoth M, Bastian BC, Vogt T, Herlyn M. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 568] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 58. | Yang K, Adin C, Shen Q, Lee LJ, Yu L, Fadda P, Samogyi A, Ham K, Xu L, Gilor C, Ziouzenkova O. Aldehyde dehydrogenase 1 a1 regulates energy metabolism in adipocytes from different species. Xenotransplantation. 2017;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Neumann J, Rose-Sperling D, Hellmich UA. Diverse relations between ABC transporters and lipids: An overview. Biochim Biophys Acta Biomembr. 2017;1859:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 60. | Callaghan R, Stafford A, Epand RM. Increased accumulation of drugs in a multidrug resistant cell line by alteration of membrane biophysical properties. Biochim Biophys Acta. 1993;1175:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5841] [Cited by in RCA: 5536] [Article Influence: 307.6] [Reference Citation Analysis (0)] |
| 62. | Pomorski T, Hrafnsdóttir S, Devaux PF, van Meer G. Lipid distribution and transport across cellular membranes. Semin Cell Dev Biol. 2001;12:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Tarling EJ, de Aguiar Vallim TQ, Edwards PA. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol Metab. 2013;24:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 64. | Piñeiro R, Maffucci T, Falasca M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene. 2011;30:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 65. | Ruban EL, Ferro R, Arifin SA, Falasca M. Lysophosphatidylinositol: a novel link between ABC transporters and G-protein-coupled receptors. Biochem Soc Trans. 2014;42:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 67. | Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, Owada Y. Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS One. 2016;11:e0147717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY, Watabe K. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene. 2013;32:5111-5122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Wang X, Sun Y, Wong J, Conklin DS. PPARγ maintains ERBB2-positive breast cancer stem cells. Oncogene. 2013;32:5512-5521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E, Scarpa A, Cecconi D, Palmieri M. Proteomic analysis of pancreatic cancer stem cells: Functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 71. | Corominas-Faja B, Cuyàs E, Gumuzio J, Bosch-Barrera J, Leis O, Martin ÁG, Menendez JA. Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget. 2014;5:8306-8316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1811] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 73. | Menard JA, Christianson HC, Kucharzewska P, Bourseau-Guilmain E, Svensson KJ, Lindqvist E, Indira Chandran V, Kjellén L, Welinder C, Bengzon J, Johansson MC, Belting M. Metastasis Stimulation by Hypoxia and Acidosis-Induced Extracellular Lipid Uptake Is Mediated by Proteoglycan-Dependent Endocytosis. Cancer Res. 2016;76:4828-4840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 74. | Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, Potze L, Marotta R, Ruffilli R, Rajamanickam VP, Malerba M, De Angelis F, Falqui A, Carbone E, Todaro M, Medema JP, Stassi G, Di Fabrizio E. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells. 2015;33:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 75. | Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:136-150.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 590] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 76. | Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017;20:303-314.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 77. | Lue HW, Podolak J, Kolahi K, Cheng L, Rao S, Garg D, Xue CH, Rantala JK, Tyner JW, Thornburg KL, Martinez-Acevedo A, Liu JJ, Amling CL, Truillet C, Louie SM, Anderson KE, Evans MJ, O'Donnell VB, Nomura DK, Drake JM, Ritz A, Thomas GV. Metabolic reprogramming ensures cancer cell survival despite oncogenic signaling blockade. Genes Dev. 2017;31:2067-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Maan M, Peters JM, Dutta M, Patterson AD. Lipid metabolism and lipophagy in cancer. Biochem Biophys Res Commun. 2018;504:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 79. | Peck B, Schug ZT, Zhang Q, Dankworth B, Jones DT, Smethurst E, Patel R, Mason S, Jiang M, Saunders R, Howell M, Mitter R, Spencer-Dene B, Stamp G, McGarry L, James D, Shanks E, Aboagye EO, Critchlow SE, Leung HY, Harris AL, Wakelam MJO, Gottlieb E, Schulze A. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016;4:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 80. | Mason P, Liang B, Li L, Fremgen T, Murphy E, Quinn A, Madden SL, Biemann HP, Wang B, Cohen A, Komarnitsky S, Jancsics K, Hirth B, Cooper CG, Lee E, Wilson S, Krumbholz R, Schmid S, Xiang Y, Booker M, Lillie J, Carter K. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS One. 2012;7:e33823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 81. | Kim YC, Ntambi JM. Regulation of stearoyl-CoA desaturase genes: role in cellular metabolism and preadipocyte differentiation. Biochem Biophys Res Commun. 1999;266:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Castro LF, Wilson JM, Gonçalves O, Galante-Oliveira S, Rocha E, Cunha I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol Biol. 2011;11:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Taraboletti G, Perin L, Bottazzi B, Mantovani A, Giavazzi R, Salmona M. Membrane fluidity affects tumor-cell motility, invasion and lung-colonizing potential. Int J Cancer. 1989;44:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L, Tan Z, Chen X, Mani SA, Chang JT. Candidate Antimetastasis Drugs Suppress the Metastatic Capacity of Breast Cancer Cells by Reducing Membrane Fluidity. Cancer Res. 2016;76:2037-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 85. | Colacino JA, McDermott SP, Sartor MA, Wicha MS, Rozek LS. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res Treat. 2016;158:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 86. | Noto A, Raffa S, De Vitis C, Roscilli G, Malpicci D, Coluccia P, Di Napoli A, Ricci A, Giovagnoli MR, Aurisicchio L, Torrisi MR, Ciliberto G, Mancini R. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. 2013;4:e947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Vriens K, Christen S, Parik S, Broekaert D, Yoshinaga K, Talebi A, Dehairs J, Escalona-Noguero C, Schmieder R, Cornfield T, Charlton C, Romero-Pérez L, Rossi M, Rinaldi G, Orth MF, Boon R, Kerstens A, Kwan SY, Faubert B, Méndez-Lucas A, Kopitz CC, Chen T, Fernandez-Garcia J, Duarte JAG, Schmitz AA, Steigemann P, Najimi M, Hägebarth A, Van Ginderachter JA, Sokal E, Gotoh N, Wong KK, Verfaillie C, Derua R, Munck S, Yuneva M, Beretta L, DeBerardinis RJ, Swinnen JV, Hodson L, Cassiman D, Verslype C, Christian S, Grünewald S, Grünewald TGP, Fendt SM. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. 2019;566:403-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 388] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 88. | Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 461] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 89. | Ginestier C, Monville F, Wicinski J, Cabaud O, Cervera N, Josselin E, Finetti P, Guille A, Larderet G, Viens P, Sebti S, Bertucci F, Birnbaum D, Charafe-Jauffret E. Mevalonate metabolism regulates Basal breast cancer stem cells and is a potential therapeutic target. Stem Cells. 2012;30:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 90. | Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41:554-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 91. | Krens LL, Simkens LH, Baas JM, Koomen ER, Gelderblom H, Punt CJ, Guchelaar HJ. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS One. 2014;9:e112201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Mo H, Jeter R, Bachmann A, Yount ST, Shen CL, Yeganehjoo H. The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins. Front Pharmacol. 2019;9:1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 314] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 94. | Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 806] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 95. | Kim WY. Therapeutic targeting of lipid synthesis metabolism for selective elimination of cancer stem cells. Arch Pharm Res. 2019;42:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Chimento A, Casaburi I, Avena P, Trotta F, De Luca A, Rago V, Pezzi V, Sirianni R. Cholesterol and Its Metabolites in Tumor Growth: Therapeutic Potential of Statins in Cancer Treatment. Front Endocrinol (Lausanne). 2019;9:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 97. | Pelton K, Coticchia CM, Curatolo AS, Schaffner CP, Zurakowski D, Solomon KR, Moses MA. Hypercholesterolemia induces angiogenesis and accelerates growth of breast tumors in vivo. Am J Pathol. 2014;184:2099-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 98. | Song M, Lee H, Nam MH, Jeong E, Kim S, Hong Y, Kim N, Yim HY, Yoo YJ, Kim JS, Kim JS, Cho YY, Mills GB, Kim WY, Yoon S. Loss-of-function screens of druggable targetome against cancer stem-like cells. FASEB J. 2017;31:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Ehmsen S, Pedersen MH, Wang G, Terp MG, Arslanagic A, Hood BL, Conrads TP, Leth-Larsen R, Ditzel HJ. Increased Cholesterol Biosynthesis Is a Key Characteristic of Breast Cancer Stem Cells Influencing Patient Outcome. Cell Rep. 2019;27:3927-3938.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 100. | Liu M, Xia Y, Ding J, Ye B, Zhao E, Choi JH, Alptekin A, Yan C, Dong Z, Huang S, Yang L, Cui H, Zha Y, Ding HF. Transcriptional Profiling Reveals a Common Metabolic Program in High-Risk Human Neuroblastoma and Mouse Neuroblastoma Sphere-Forming Cells. Cell Rep. 2016;17:609-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol. 2001;13:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 102. | Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW, Zhang J. PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A. 2011;108:14509-14514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 103. | Alptekin A, Ye B, Ding HF. Transcriptional Regulation of Stem Cell and Cancer Stem Cell Metabolism. Curr Stem Cell Rep. 2017;3:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Lidgerwood GE, Pitson SM, Bonder C, Pébay A. Roles of lysophosphatidic acid and sphingosine-1-phosphate in stem cell biology. Prog Lipid Res. 2018;72:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Hisano Y, Hla T. Bioactive lysolipids in cancer and angiogenesis. Pharmacol Ther. 2019;193:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 106. | Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T, Kondo S, Katoh H. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 107. | Serafimidis I, Rodriguez-Aznar E, Lesche M, Yoshioka K, Takuwa Y, Dahl A, Pan D, Gavalas A. Pancreas lineage allocation and specification are regulated by sphingosine-1-phosphate signalling. PLoS Biol. 2017;15:e2000949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Hedditch EL, Gao B, Russell AJ, Lu Y, Emmanuel C, Beesley J, Johnatty SE, Chen X, Harnett P, George J; Australian Ovarian Cancer Study Group, Williams RT, Flemming C, Lambrechts D, Despierre E, Lambrechts S, Vergote I, Karlan B, Lester J, Orsulic S, Walsh C, Fasching P, Beckmann MW, Ekici AB, Hein A, Matsuo K, Hosono S, Nakanishi T, Yatabe Y, Pejovic T, Bean Y, Heitz F, Harter P, du Bois A, Schwaab I, Hogdall E, Kjaer SK, Jensen A, Hogdall C, Lundvall L, Engelholm SA, Brown B, Flanagan J, Metcalf MD, Siddiqui N, Sellers T, Fridley B, Cunningham J, Schildkraut J, Iversen E, Weber RP, Berchuck A, Goode E, Bowtell DD, Chenevix-Trench G, deFazio A, Norris MD, MacGregor S, Haber M, Henderson MJ. ABCA transporter gene expression and poor outcome in epithelial ovarian cancer. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 109. | Rizzo MT. The role of arachidonic acid in normal and malignant hematopoiesis. Prostaglandins Leukot Essent Fatty Acids. 2002;66:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 110. | Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 321] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 111. | Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156-47165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Wilson BJ, Saab KR, Ma J, Schatton T, Pütz P, Zhan Q, Murphy GF, Gasser M, Waaga-Gasser AM, Frank NY, Frank MH. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014;74:4196-4207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 113. | Begicevic RR, Falasca M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 114. | Pfeiffer L, Wahl S, Pilling LC, Reischl E, Sandling JK, Kunze S, Holdt LM, Kretschmer A, Schramm K, Adamski J, Klopp N, Illig T, Hedman ÅK, Roden M, Hernandez DG, Singleton AB, Thasler WE, Grallert H, Gieger C, Herder C, Teupser D, Meisinger C, Spector TD, Kronenberg F, Prokisch H, Melzer D, Peters A, Deloukas P, Ferrucci L, Waldenberger M. DNA methylation of lipid-related genes affects blood lipid levels. Circ Cardiovasc Genet. 2015;8:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 115. | Adamska A, Ferro R, Lattanzio R, Capone E, Domenichini A, Damiani V, Chiorino G, Akkaya BG, Linton KJ, De Laurenzi V, Sala G, Falasca M. ABCC3 is a novel target for the treatment of pancreatic cancer. Adv Biol Regul. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Yamada A, Nagahashi M, Aoyagi T, Huang WC, Lima S, Hait NC, Maiti A, Kida K, Terracina KP, Miyazaki H, Ishikawa T, Endo I, Waters MR, Qi Q, Yan L, Milstien S, Spiegel S, Takabe K. ABCC1-Exported Sphingosine-1-phosphate, Produced by Sphingosine Kinase 1, Shortens Survival of Mice and Patients with Breast Cancer. Mol Cancer Res. 2018;16:1059-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 117. | Ghosh S. Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization. Expert Rev Cardiovasc Ther. 2011;9:329-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 118. | Sabnis NG, Miller A, Titus MA, Huss WJ. The Efflux Transporter ABCG2 Maintains Prostate Stem Cells. Mol Cancer Res. 2017;15:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 1122] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 120. | Wang Z, Sun H, Provaznik J, Hackert T, Zöller M. Pancreatic cancer-initiating cell exosome message transfer into noncancer-initiating cells: the importance of CD44v6 in reprogramming. J Exp Clin Cancer Res. 2019;38:132. [PubMed] |
| 121. | Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 617] [Article Influence: 77.1] [Reference Citation Analysis (1)] |
| 122. | Li W, Li C, Zhou T, Liu X, Liu X, Li X, Chen D. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Australia
Peer -review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wakao H, Kiselev SL, Liu L S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX