Published online Aug 26, 2019. doi: 10.4252/wjsc.v11.i8.506
Peer-review started: February 15, 2019
First decision: March 26, 2019
Revised: May 31, 2019
Accepted: July 29, 2019
Article in press: July 29, 2019
Published online: August 26, 2019
Processing time: 194 Days and 9.6 Hours
Located near the oropharynx, the tonsils are the primary mucosal immune organ. Tonsil tissue is a promising alternative source for the high-yield isolation of adult stem cells, and recent studies have reported the identification and isolation of tonsil-derived stem cells (T-SCs) from waste surgical tissue following tonsillectomies in relatively young donors (i.e., under 10 years old). As such, T-SCs offer several advantages, including superior proliferation and a shorter doubling time compared to bone marrow-derived mesenchymal stem cells (MSCs). T-SCs also exhibit multi-lineage differentiation, including mesodermal, endodermal (e.g., hepatocytes and parathyroid-like cells), and even ectodermal cells (e.g., Schwann cells). To this end, numbers of researchers have evaluated the practical use of T-SCs as an alternative source of autologous or allogenic MSCs. In this review, we summarize the details of T-SC isolation and identification and provide an overview of their application in cell therapy and regenerative medicine.
Core tip: The use of adult stem cells is often limited by the lack of differentiation among stem cells isolated from certain germ layers. However, tonsil-derived stem cells (T-SCs) were able to differentiate into various tissue types from the three germ layers, which is the most advantageous feature of this new stem cell source. T-SCs can also be used as native cells in the treatment of various immune-related diseases. As a result, it can be concluded that T-SCs have great potential for clinical applications in cell therapy and regenerative medicine.
- Citation: Cho KA, Lee HJ, Jeong H, Kim M, Jung SY, Park HS, Ryu KH, Lee SJ, Jeong B, Lee H, Kim HS. Tonsil-derived stem cells as a new source of adult stem cells. World J Stem Cells 2019; 11(8): 506-518
- URL: https://www.wjgnet.com/1948-0210/full/v11/i8/506.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i8.506
Recent achievements in the identification, isolation, in vitro culture, and differenti-ation of various adult stem cells are indicative of the unprecedented potential of these cells in treating various degenerative diseases[1]. Mesenchymal stem cells (MSCs) in particular, have been used clinically for more than 10 years. From animal studies to clinical trials, MSCs have demonstrated great promise in treating numerous diseases, particularly tissue injury and immune disorders[2]. To obtain the large volumes of cells required for testing and treatment, various tissue sources have been investigated for the isolation of MSCs, including bone marrow, adipose tissue, umbilical cord blood, amniotic fluid, the placenta, dental pulp, and urine[3]. However, the isolation yields of MSCs from different tissue sources vary greatly, and the differentiation potential, yield, and maximal lifespan of isolated MSCs decrease significantly with donor age. Therefore, it is important to locate new adult stem cell sources to overcome these limitations.
The human tonsils are located near the oropharynx (palatine tonsils) and nasopharynx (adenoid), which are part of the respiratory and digestive system. Tonsil tissue is one of the primary sensitization systems for the generation of B cells, and tonsil tissue is easily obtained from tonsillectomies, a minimally invasive surgery conducted most often on patients aged between 5 and 19. Tonsil-derived stem cells (T-SCs) were first introduced by Janjanin et al[4]. Due to the younger donors, the isolation yields of T-SCs are much higher than those from other tissue types. Therefore, T-SCs have received much attention as alternative allogeneic or autologous cell sources for clinical use. In this review, we highlight recent research on the isolation and development of T-SCs, which provides strong evidence of their superior characteristics. In addition to their high proliferation and expansion capacity, T-SCs can undergo differentiation into cells from all three germ layers (i.e., ectoderm, mesoderm, and endoderm). This unique differentiation potential is described in detail. Finally, we provide an in-depth discussion of the use of T-SCs in cell therapy and regenerative medicine.
Isolating T-SCs consists of two major steps: Enzymatic disaggregation and density gradient centrifugation[5]. Briefly, small pieces of tonsillar tissues were exposed to enzymes, including collagenase type I and DNase for 30 min at 37 °C under stirring. This solution was then filtered through a wire mesh and 70-µm cell strainer to collect single-cell suspensions. The mononuclear cell (MNC) fraction was obtained using Ficoll-Paque (GE Healthcare, Little Chalfont, United Kingdom) density gradient centrifugation. The MNCs were plated at the density of 108 cells in a T-150 culture flask with Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG; Invitrogen) supplemented with fetal bovine serum and antibiotics. The primary culture (passage 0; P0) was cultivated until the adherent cells reached confluence and were passaged by trypsinization (Trypsin, Life Technologies GmbH, Vienna, Austria).
Immunophenotype characterization, which is based on the expression of cell surface markers, is the most common method for distinguishing different cell clusters. To date, extensive research has identified various cell surface markers that characterize the MSCs derived from different sources. In order to identify T-SCs as a cellular source for new adult MSCs, T-SC surface makers were investigated[5,6]. As with other MSCs, T-SCs expressed the standard positive markers for MSCs, CD73, CD90, CD105, CD29, CD44, CD166, CD58, and CD49e. Most of these markers represent cellular adhesion molecules which possibly render MSCs to act on other cell types via direct interaction. On the other hand, T-SCs were negative for the hematopoietic markers CD14, CD34, CD45, and CD133, the endothelial marker CD31, and co-stimulatory proteins such as the antigens CD40, CD80, and CD86. In addition, class II MHC antigens are entirely absent on T-SCs[5,6]. Because tonsil tissue is part of the mucosal immune system and contains large numbers of follicular dendritic cells (FDCs), additional research has been carried out to verify the lack of FDC markers CD11b, CD21, CD23, CD35, and CD54 in T-SCs[5,7] to confirm no-contamination with FDC. FDCs are known to originate from tonsillar stromata and proliferate on and adhere to plastic in vitro. Therefore, the lack of these markers is an important indicator that can be used to distinguish T-SCs from FDCs.
Although MSCs can be isolated from various tissue types, they were initially harvested from bone marrow (BM), which requires a highly invasive procedure[4]. Here, we highlight the significant benefits of using T-SCs in terms of isolation and clinical use compared with BM-MSCs.
Isolating BM-MSCs has several limitations, including donor morbidity, and they are challenging to harvest, thus requiring a high degree of skill. Bone marrow extraction takes approximately two hours under general anesthesia and requires the hospitalization and recovery of the donor. Therefore, it is always difficult to find a sufficient number of donors. In contrast, T-SCs are easily obtained from discarded tissue; more than 530000 tonsillectomies are performed annually in children younger than 15 years in the United States[8], meaning that tonsils are one of the most abundant tissue sources for stem cell isolation.
The age of the donor affects the isolation yield of MSCs, with the number of MSCs harvested from bone marrow decreasing with donor age. For example, infants have one colony forming units-fibroblast (CFU-f) per 10000 cells in bone marrow, but this falls to 1 per 400000 in donors in their 50 s[9]. In contrast, approximately 8-10 × 108 MSCs are isolated from one-third of one tonsil (2 cm x 1.5 cm x 1.5 cm) from donors under 10 years old[5].
When compared with BM-MSCs, T-SCs offer superior stem cell properties, such as high self-renewal and proliferation. For example, T-SCs show a doubling time of 37.1 ± 3.4 h for an initial population, compared to 58.2 ± 2.3 h for BM-MSCs[4]. Other research has also confirmed the more rapid proliferation of T-SCs compared with MSCs derived from adipose tissue[10].
The proliferation of BM-MSCs gradually decreases with passage number, whereas T-SCs retain their physiological properties for much longer. In general, most cells become more prominent, longer, less defined, and less proliferative during long-term in vitro culture as they experience senescence. T-SCs also exhibit the signs of senescence from passage 7, but the cells proliferate up to passage 15 with no change in the MSC markers. Tonsil tissue contains as many B cells and T cells as immune organs, and these cells affect the immune modulation of stem cells. Pro-inflammatory cytokines may also affect the positive differentiation and proliferation of T-SCs[4,11,12], and this has been supported by research on tissue obtained from tonsillectomies in response to chronic bacterial infections and chronic tonsillitis[13-15].
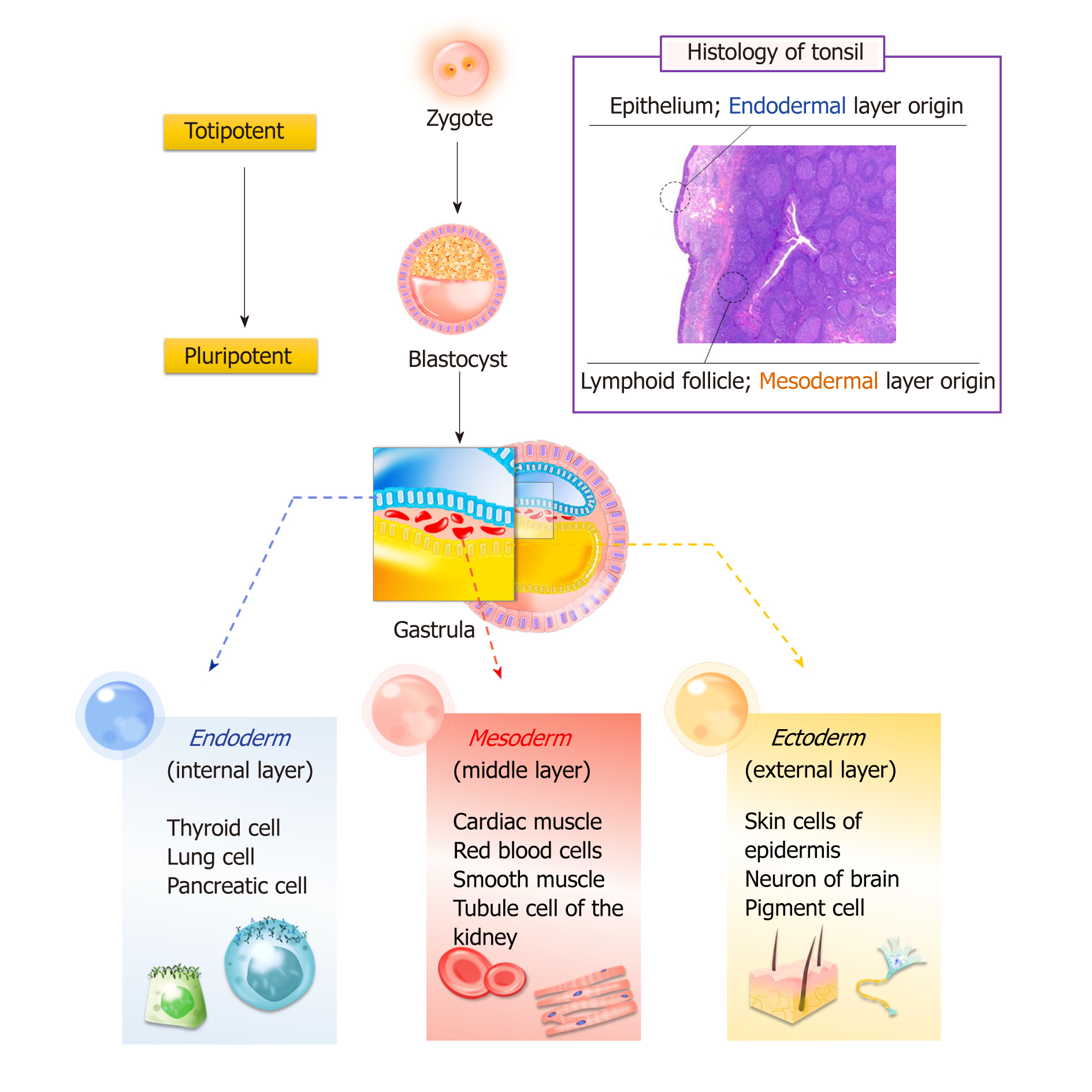
Bone marrow and adipose tissue originate from the mesoderm layer, whereas tonsil tissue has two origins: The epithelial cells derive from the second pharyngeal pouch in the endoderm layer, and lymphoid tissue comes from the mesoderm layer, which invades during fetal development. Research has confirmed that T-SCs can be easily differentiated into endodermal, ectodermal, and mesodermal cells (Figure 1).
Cell therapy and tissue engineering have been investigated to regenerate lost or malfunctioning organs. These approaches utilize biomaterial scaffolds and MSCs to facilitate initial cell adhesion and retention while promoting cell growth for tissue regeneration[16,17]. In particular, the differentiation properties of MSCs are of great importance for tissue regeneration. It is generally known that isolated MSCs are often limited to germ-layer specific differentiation. As mentioned earlier, T-SCs offer multipotent differentiation potential that can be applied in regenerating various tissue types without concern for their germ layer origin.
Ectodermal differentiation is often difficult to achieve with MSCs isolated from bone marrow and adipose tissue. However, under the right conditions, T-SCs can be differentiated into non-mesenchymal lineages, including ectodermal differentiation into neurons, astrocytes, and Schwann-like cells to support nerve regeneration.
The neuronal differentiation of T-SCs was investigated in a three dimensional (3D) hybrid scaffold system by Patel et al[18]. This scaffold was fabricated by increasing the temperature of an aqueous solution of poly (ethylene glycol)-poly(L-alanine) to 37 °C, thus instigating the heat-induced sol-to-gel transition, in which T-SCs and growth factor-releasing microspheres were suspended. The gel exhibited a modulus of 800 Pa at 37 °C, similarly to that of brain tissue, and was robust enough to hold the microspheres and cells within the 3D cell culture. Neuronal growth factors were released over 12–18 d, and the encapsulated T-SCs gradually exhibited morphological changes from spherical to multipolar elongation. Significantly higher expression levels of neuronal biomarkers such as nuclear receptor-related protein, neuron-specific enolase, microtubule-associated protein-2, neurofilament-M, and glial fibrillary acidic protein were observed at both the mRNA and protein level in the hybrid system. This study clearly demonstrates the advantages of 3D hybrid scaffolds and highlights the importance of the sustained release of growth factors from hybrid systems to support the neuronal differentiation of T-SCs.
Schwann cells are the glial cells of peripheral nerves that wrap around the axons to form myelin in the peripheral nervous system. Schwann cells promote nerve regeneration by secreting trophic support molecules and establishing a supportive growth matrix[19]. Jung et al[20] demonstrated that T-SCs could be differentiated into Schwann-like cells over several steps. Briefly, T-SCs were induced to form neurospheres under stimulation with EGF, bFGF, and B27 for 7 d. These neurospheres were then triturated and re-plated onto laminin-coated dishes with Schwann cell differentiation medium. After 10 d of culturing, the cells exhibited morphological changes, including the formation of elongated bipolar and tripolar spindle shapes. Schwann-like cells differentiated from T-SCs highly express the Schwann cell markers GFAP, NGFR, S100B, KROX20, and KROX24. Notably, Schwann cells differentiated from T-SCs were able to produce myelinate axons in vitro when co-cultured with mouse dorsal root ganglion neurons. In a mouse model with a sciatic nerve injury, a marked improvement in gait and increased nerve regeneration were observed with Schwann-cell treatment. Therefore, T-SCs can be a useful source for Schwann cell-based cell therapy to treat neuropathic diseases.
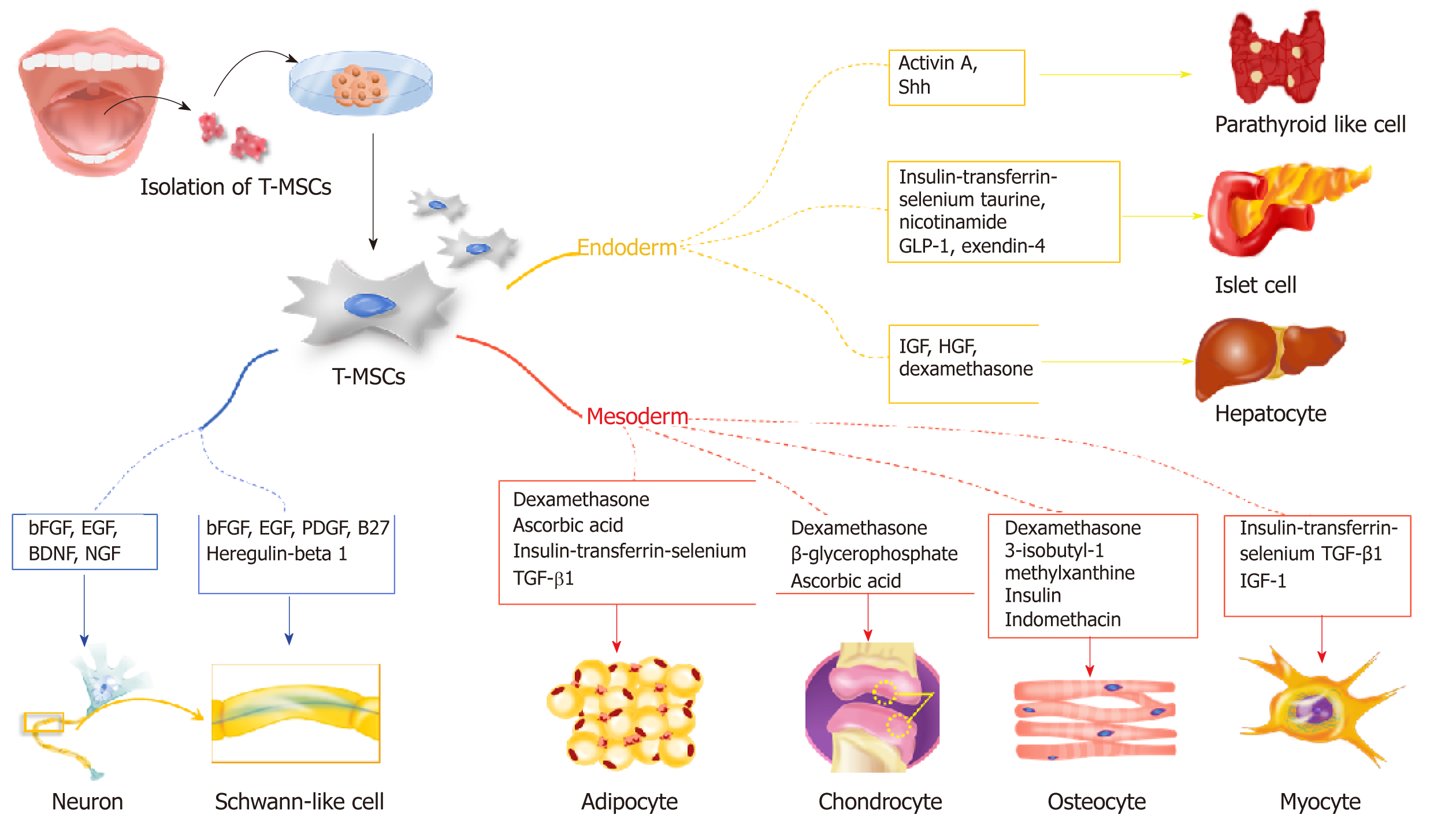
Mesodermal differentiation is mainly achieved with adult MSCs from the mesodermal germ layer. Previously, a variety of cell sources from bone marrow and adipose tissue was utilized for mesodermal differentiation to treat bone, cartilage, and fat disorders. In this section, we highlight the potential use of T-SCs as an alternative cell source for mesodermal differentiation (Figure 2), and we provide a comparative study that illustrates the advantages of T-SCs.
For osteogenic differentiation, Choi et al[21] cultured T-SCs in commercially available osteogenic media (α MEM supplemented with 10% FBS, 0.1 mmol/L dexamethasone, 10 μmol/L β glycerophosphate, and 50 μg/mL ascorbic acid) for three weeks. Alizarin Red S staining confirmed the successful deposition of extracellular calcium in culture. During osteogenic differentiation, mRNA expression of osteocalcin decreased 0.28-fold after cryopreservation, whereas ALP expression showed no difference. This profile remained stable even after passage 15 (P15). Interestingly, the osteogenic differentiation of T-SCs increased with the number of passages, with the peak osteogenic potential observed for passage 10 (P10), which exhibited a 1.4-fold increase over P3[6]. The expression of CCN1, a gene that is closely related to the osteogenic differentiation of MSCs, increased at P10. This finding is consistent with previous studies that have reported that CCN1 expression modulates the osteogenic potential of MSCs by regulating the Wnt3A pathway[6,22].
Various scaffolds have been employed to enhance skeletal regeneration to replace damaged bone. Because bone tissue is highly vascularized, integration with the host tissue followed by subsequent angiogenesis is critical for successful treatment. As an example, Park et al[23] encapsulated T-SCs within highly water-swollen hydrogel through the sol-gel transition of the thermoresponsive polymer poly(ethylene glycol)-poly(L-alanine-co-L-phenyl alanine) (PEG-PAF). The encapsulated T-SCs were cultured in vitro in the presence of an osteogenic-induction medium. The osteogenic differentiation of T-SCs was investigated by evaluating the expression of osteogenic genes, including Runx 2, ALP, and OCN. With support from the hydrogel, osteogenic gene expression was two times higher than that of conventional tissue cultures without soluble factor supplements.
Incorporating specific functional groups into scaffold substrates is also known to affect the osteogenic differentiation of MSCs[24,25]. T-SCs were encapsulated in poly (ethylene glycol)-poly(L-alanine) diblock copolymer (PEG-L-PA) thermogel that was modified with the phosphate functional groups of polystyrene microspheres to facilitate the osteogenic differentiation of encapsulated MSCs. The osteogenic differentiation of tonsil-MSCs (T-MSCs) was analyzed, and all osteogenic biomarker expressions were significantly higher for the modified thermogel than for the native thermogel. Immunofluorescence staining also confirmed high OCN expression, and Kye et al[24] also found that, compared with thermogel modified with carboxylate group microspheres, the phosphate functional group (-PO43-) more readily induced the osteogenic differentiation of T-SCs.
The osteogenic differentiation of T-SCs can be further improved by the over-expression of BMP-2 through genetic material transfer. In Jeong et al[26], BMP-2 minicircle DNA vectors were employed to form nano-sized polyplexes with the CBA-106 polymer. CBA-106 is a bioreducible cationic poly (amido amine) that facilitates the intracellular delivery of genetic material. The osteogenic differentiation of T-SCs was investigated by evaluating the gene expression of osteogenic markers such as osteocalcin, Runx2, and Col 1. In vitro calcium deposition was also confirmed using Alizarin Red S staining on day 7 and day 14 of the cell culture. In addition, in vivo bone regeneration was attempted using T-SCs transfected with the BMP-2 gene. The T-SCs were 3D cultured using PLLA/PLGA scaffolds for one week and then transplanted into the skulls of immunodeficient mice. It was shown that bone regeneration increased 1.96-fold compared to the control group five weeks after treatment.
To mimic a cartilage-like microenvironment, T-SCs were encapsulated in thermogel consisting of the PEG-PAF block copolymer by Park et al[23]. Under specific medium conditions (25 μL chondrogenic supplement/2.5 mL basal medium), the much higher expression of Col II and sulfated glycosaminoglycan in T-SCs was achieved using the thermogel than using a monolayer culture. Moreover, unique branching was observed among the encapsulated cells within the hydrogel. These changes in cellular morphology may influence the chondrogenic differentiation of T-SCs during 3D culturing. In vivo studies also confirmed that T-SCs successfully undergo chondrogenic differentiation with high expression levels of biomarkers such as Col II, AGG, and Col X.
Kye et al[24] also investigated the chondrogenic differentiation of T-SCs within PEG-L-PA thermogel. To enhance the cellular attachment in the 3D hydrogel micro-environment, various polystyrene microspheres with thiol (-SH), phosphate (-PO3¬), carboxylate (-COO) and amino (-NH2) functional groups were incorporated into the thermogel. Of the incorporated microparticles, the PS-S, PS-P, and PS-C microspheres exhibited significantly higher COL II expression. COL II mRNA expression was much higher in the PS-S thermogel than in the PS-N thermogel even though the PS-S and the PS-N microspheres had similar sizes, clearly indicating that surface functional groups play an important role in stem cell differentiation.
Similarly, graphene oxide (GO) and reduced graphene oxide (rGO) were incorporated into PEG-L-PA thermogel by Park et al[27] to enhance surface functionality and thus provide good cellular adhesion. GO, or rGO (1 wt%) was suspended in a PEG- L-PA solution. T-SCs in GO/PEG-L-PA or rGO/PEG-L-PA were cultured in DMEM, and the spherical cellular morphology of the T-SCs was observed. When chondrogenic culture media enriched with TGF-β3 was utilized, T-SCs in hybrid systems aggregated extensively, and the expression levels of chondrogenic biomarkers such as SOX 9, COL II A1, COL II, and COL X increased. In particularly, COL II mRNA expression was 13 times higher in the GO/PEG-L-PA hybrid system than in the PEG-L-PA 3D system. Immunofluorescence analysis also revealed a significant increase in COL II expression and cell aggregation in the GO/PEG-L-PA hybrid system. In particular, the GO/PEG-L-PA 2D/3D hybrid system demonstrated the most significant increase in chondrogenic biomarker expression. These results indicate that the cooperative interaction among TGF-β3, COL II, and GO may be closely related to signaling cascades for chondrogenic differentiation.
Ryu et al[5] investigated T-SCs as a source for adipogenic differentiation due to their multi-lineage differentiation potential and self-renewal capacity by culturing them using commercially available adipogenic media (Gibco StemPro™ Adipogenesis Differentiation Kit, Thermo Fisher Scientific, Waltham, MA, USA) for three weeks. The adipogenic potential of the T-SCs was evaluated for P3, P7, P10, and P15. Interestingly, unlike other types of T-SC differentiation, the adipogenesis of T-SCs decreased continuously with passage number, with P10 approximately two-thirds that of P3.
Adipogenic differentiation of T-SCs in thermogel was also investigated using PEG-L-PA (molecular weight of each block: 1000−1080 Da) by Kye et al[24]. The differentiation potential of T-SCs was investigated by incorporating polystyrene microspheres with different functional groups into the hydrogel. mRNA expression and immune histochemical assays indicated that T-SCs preferentially underwent adipogenesis in ammonium (−NH3+)- or thiol (−SH)-functionalized thermogels, whereas chondrogenesis occurred predominantly in phosphate (PO32−)- or carboxylate (−COO−)-functionalized thermogels. This study thus suggests that the surface functional groups of microspheres can control the preferential differentiation of stem cells into specific cell types in 3D cultures.
In addition to functionalized hydrogels, Patel et al[28] reported that a composite system of GO and polypeptide thermogel (GO/P), prepared using the temperature-sensitive sol-to-gel transition of GO-suspended poly(ethylene glycol)-poly(L-alanine) (PEG-PA), significantly enhanced the expression of adipogenic biomarkers, including PPAR-γ, CEBP-α, LPL, AP2, ELOVL3, and HSL when compared with a native hydrogel system. It appears that insulin, an adipogenic differentiation factor, can preferentially adhere to the surface of the GO incorporated into thermogels; insulin is then slowly released into the environment in a sustained manner during the cell culturing period. In contrast, more hydrophobic graphene may interfere with insulin, causing partial denaturation, reducing the adipogenic differentiation of T-SCs.
Various MSCs and progenitor cells have been evaluated their differentiation capacity into the myogenic cells for skeletal muscle regeneration. MSCs derived from BM, adipose, and umbilical cord tissue, and their use in cell therapy to augment the skeletal muscle injury response, have also been reported[29-31]. An alternative cellular source for MSCs, T-SCs have been shown to differentiate into myogenic cells in vitro, and transplanting the myoblasts and myocytes generated from T-SCs mediates the recovery of muscle function following injury in vivo[32]. For myogenic differentiation, T-SCs are treated in three sequential steps: Sphere formation on a petri dish in low-glucose DMEM, rosette-like spread formation on a collagen-coated dish, and two weeks of myogenic induction. In this final step, the cells express myogenic markers, including desmin, dystrophin, MHC, skeletal markers, α-Actinin, TNNI1, and myogenin. Furthermore, the intramuscular injection of T-MSC-derived myogenic cells into myectomized C57BL/6 mice enhances muscle function as demonstrated by gait assessment and the restoration of the skeletal muscle structure.
The endodermal differentiation of MSCs is important because many degenerative diseases are related to organs that originate from the endoderm, including the liver, pancreas, and parathyroid. In this section, we highlight recent research that employs T-SCs to produce functional hepatocytes, pancreatic beta cells, and parathyroid cells (Figure 2).
Many clinical studies have indicated that BM-MSCs are safe and effective in the treatment of liver disease[33]. They can alleviate end-stage liver disease and improve symptoms and liver function[34,35]. However, some studies have indicated that BM-MSCs have the potential to aggravate fibrosis[36-38]. Thus, employing BM-MSCs as a therapy for liver fibrosis remains controversial.
As a novel cell source for treating liver disease, Park et al[39] demonstrated that T-SCs differentiate into hepatocyte-like cells and ameliorate live fibrosis via the activation of autophagy and the downregulation of TGF-β. A three-week culture in a differentiation medium containing IGF, HGF, dexamethasone, and oncostatin M led to the development of hepatocyte-like cells from T-SCs, as revealed by the expression of albumin and HNF-4α. In addition, transplanting T-SCs into a carbon tetrachloride (CCl4)-induced liver injury mouse model confirmed that T-SCs have a regenerative effect by migrating to the site of the liver injury and differentiating into hepatocyte-like cells. These results prove that T-SCs was able to differentiate into hepatocyte-like cells both in vitro and in vivo.
In addition to the direct differentiation of T-SCs into liver hepatocytes, T-SCs have also been investigated in terms of hepatogenic differentiation using PEG-L-PA thermogel[40,41]. The thermogel exhibited a physical modulus of 1000 Pa, which is similar to that of decellularized liver tissue. Three different 3D culture systems were compared in relation to the use of soluble factors such as hepatogenic growth factors. The spherical morphology and size of the encapsulated cells were maintained in the native 3D culture system during a culture period of 28 d, whereas the cells changed their morphology and aggregated significantly in 3D systems with growth factors. Hepatocyte-specific biomarker expression and metabolic functions were negligible in the native culture system. However, the expression levels of the hepatogenic genes of albumin and cytokeratin 18 and hepatocyte nuclear factor 4α were high in the two systems supplemented with growth factors. In addition, albumin and α-fetoprotein production were also significant[40]. PEG-L-PA thermogel thus provides a biocompatible microenvironment for the hepatogenic differentiation of T-SCs. In particular, the successful results of the growth factor encapsulated hydrogel system suggest that PEG-L-PA thermogel is a promising injectable tissue engineering system for liver tissue regeneration[41].
Metabolic disturbances associated with diabetes lead to a number of complications ranging from cardiovascular and cerebrovascular disease to neuropathy, retinopathy, nephropathy, and the poor healing of wounds[42]. The only curative therapy available is pancreatic islet cell replacement, for which suitable donors are rare and which requires immunosuppressant therapy to reduce rejection. Recently, stem cell therapy has been proposed for the treatment of diabetes. Transplanting insulin-secreting cells produced from various stem cells, including embryonic and induced pluripotent stem cells and MSCs has shown therapeutic effects in diabetic animals[43]. In addition, differentiating various MSCs, including BM-MSCs and adipose MSCs, into insulin-producing cells has been suggested[44]. Kim et al[10] investigated the efficiency of differentiating T-SCs into insulin-producing cells by comparing two different methods and found that T-SCs differentiated more efficiently with insulin-transferrin-selenium (ITS) than with β-mercaptoethanol. The ITS method is composed of three steps: Two days of culturing in high-glucose α-MEM with 1% fatty acid-free bovine serum albumin (BSA) and 1 × ITS on a nonadherent dish; four days of culturing in high glucose α-MEM with 1% fatty acid-free BSA, ITS, 3 mM taurine, and 10 mM nicotinamide; and four days of culturing in high-glucose α-MEM with 1% fatty acid-free BSA, ITS, 3 mmol/L taurine, 10 mmol/L nicotinamide, 100 nmol/L glucagon-like peptide, and 10 Nm exendin-4. Notably, T-SCs exhibited a differentiation capability that was superior to that of adipose cell-derived MSCs. Further, implanting T-MSC-derived insulin-producing cells significantly alleviated streptozotocin-induced glucose intolerance in mice. These results suggest that T-SCs have the potential to be reprogrammed into pancreatic β-cells and applied to the clinical treatment of diabetes in the future.
Hypoparathyroidism is a rare endocrine disorder, resulting in low serum calcium and increased serum phosphorus[45]. Hypoparathyroidism is the only hormonal insufficiency state that does not have a hormone-replacement-therapy approved. Current managements include supplementation with oral calcium and active vitamin D, which cause various life-long adverse effects[45].
Stem cells have shown some promise in treating hypoparathyroidism in clinical applications. It has been reported that human embryonic stem cells (hESCs) and differentiated thymic stromal cells can be used in the in vitro regeneration of parathyroid-like cells[46-48]. However, the use of hESCs have critical ethical limitations, and it takes over 10 weeks for thymic stromal cells to differentiate and secrete PTH. Because of this, T-SCs have been considered as an alternative cell source for cell therapy. Park et al[49] demonstrated that T-SCs differentiate into parathyroid-like cells that release intact PTH using the modified Bingham protocol. Briefly, T-SCs at 90% confluence were cultured in a differentiation medium containing activin A and soluble sonic hedgehog for 7-21 d. Surprisingly, the T-SC-derived parathyroid-like cells differentially secreted PTH in response to extracellular calcium levels. Further, the therapeutic effects of T-SC-derived parathyroid-like cells embedded in Matrigel in rats that have undergone a parathyroidectomy suggest that embedding differentiated T-SCs in hydrogel scaffolds is a promising strategy for restoring parathyroid function.
In addition to their multipotent differentiation potential, stem cells hold great promise for the treatment of numbers of diseases, especially those related to tissue damage involving immune reactions. The therapeutic effects of MSCs depend largely on their capacity to regulate inflammation and tissue homeostasis via an array of immunosuppressive factors, cytokines, growth factors, and differentiation factors[50]. Interestingly, depending on their type and intensity, inflammatory stimuli can lead MSCs to suppress the immune response in some cases or to enhance it in others. This plasticity of MSCs in immunomodulation leads them to act as suppressors or enhancers in response to the microenvironment[51]. In particular, the palatine tonsil is secondary lymphoid tissue that continuously encounters antigens and subsequently drives efficient immune response[52]. This tissue specificity may account for the intrinsic property of T-SCs in terms of immune regulatory plasticity.
Previously, T-SCs have shown excellent immunomodulatory properties in targeting muscular fibrosis[53], skin inflammation[54], B-cell-mediated immune response[55], and autoimmune-mediated colitis[56]. In addition, T-SCs have been shown to improve the immune system by facilitating myelopoiesis in an allogeneic BMT mouse model[57]. These studies report that non-differentiated, non-stimulated native T-SCs constitutively secrete anti-inflammatory cytokines such as IL-1Ra, PD-L1, and EBI3 protein. Conditioned media from T-SCs that contain high levels of IL-1Ra efficiently regulate the mediation of the pro-fibrogenic process of myotubes by altering their IL-1β activity. Similarly, PD-L1 is a well-known immune-suppressive protein that targets numbers of immune and nonimmune cells. Notably, T-SCs express both soluble and membrane-bound forms of PD-L1 at higher levels compared with BM-MSCs and AT-MSCs. Indeed, T-MSC-derived PD-L1 has been demonstrated to attenuate Th17cell-mediated skin inflammation in psoriatic skin dermatitis in mice.
Recent studies have reported that IL-35 is a regulatory protein that acts on B cells[58] and that T-SCs constitutively produce EBI3, which is a critical component of IL-35[55]. Of note, T-SCs significantly ameliorate the estrogen-induced B-cell response both in vitro and in vivo in an IL-35-dependent manner.
Allogeneic hematopoietic stem cell transplantation is a routine treatment for intractable hematologic malignancies. The co-transplantation of BM-derived MSCs and donor HSCs promotes hematopoietic cell engraftment and prevents graft-versus-host disease with accelerated marrow stromal regeneration[59,60]. Research has also found enhanced myelocytic or megakaryocytic engraftment in the co-transplantation of MSCs and HSCs. Ryu et al[57] reported the supporting role of T-SCs in BM reconstitution and in supplementing hematopoiesis in a BMT mouse model. Considering that hematopoietic cells give rise to all of the mature blood cell types, including immune cells, normalizing hematopoiesis may eventually reverse immune deficiency induced by Bu/Cy preconditioning. Park et al[39] reported another study that demonstrated immune activation by T-SCs. In CCl4-induced liver fibrosis in mice, T-SCs migrated directly to injured tissue in the liver and promoted the restoration of liver function. Furthermore, T-SCs have been shown to promote the activation of autophagy, which ultimately resolved fibrotic processes[39]. These results indicate that T-SCs can have a dual function on immunity by either activating or inhibiting the immune system.
The mass production of stem cells is vital for their widespread use, but, unfortunately, this process is expensive and time-consuming. Therefore, the regular use of stem cells as therapeutic agents lies in the distant future. However, T-SCs present new possibilities for the clinical application of stem cells. Obtaining T-SCs is more cost-effective than obtaining other types of stem cell; for example, tonsil tissue is readily obtained from tonsillectomies without the need for additional procedures. The yields and doubling times of T-SCs are also better than those for other stem cell types, and T-SCs from multiple donors can be used together[5]. The differential potential of T-SCs is also very cost-effective for clinical applications. For example, it takes only 14 d for tonsil tissue to differentiate and secrete PTH, whereas it takes over 10 weeks when thymic cells are employed[32]. T-SCs can also differentiate into various tissue types from all three germ layers. These features suggest that T-SCs can be a new cell source for regenerative medicine.
T-SCs have yet to be fully characterized. Tonsils are composed of various tissue types and cells (e.g., connective tissue, endothelium, epithelium, and lymphocytes), which presents both advantages and disadvantages. As summarized above, T-SCs show promise for differentiation, and this is thought to be due to the various components of T-SCs and to the generally younger age of the donors. However, the histological diversity of T-SCs can be an obstacle to clinical applications because of the risk of tumorigenesis. Further, it possibly made variations of the T-SCs capacities, e.g., differentiation into certain cell types. Therefore, the thorough characterization, including molecular mechanisms that facilitating differentiation of T-SCs should precede any clinical trials.
Tonsil tissue is a promising alternative source for the high-yield isolation of adult stem cells. Although T-SCs exhibit a cellular morphology and surface markers that are similar to those of bone marrow-derived MSCs, T-SCs possess superior stem cell properties that are very useful for various applications in regenerative medicine. Unlike other adult stem cell sources, T-SCs are typically isolated from young donors under age of 10. This is particularly beneficial in that tonsillectomies provide not only with a source of abundant tissue but also with good proliferation and differentiation potential of isolated T-SCs. In particular, isolated T-SCs exhibit multi-lineage differentiation, which is not often observed in other sources. As a result, it is clearly that T-SCs hold great promise for clinical applications in cell therapy and regenerative medicine.
| 1. | Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1009] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 2. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1140] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 3. | Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248-269. [PubMed] |
| 4. | Janjanin S, Djouad F, Shanti RM, Baksh D, Gollapudi K, Prgomet D, Rackwitz L, Joshi AS, Tuan RS. Human palatine tonsil: a new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res Ther. 2008;10:R83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Ryu KH, Cho KA, Park HS, Kim JY, Woo SY, Jo I, Choi YH, Park YM, Jung SC, Chung SM, Choi BO, Kim HS. Tonsil-derived mesenchymal stromal cells: evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy. 2012;14:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Yu Y, Park YS, Kim HS, Kim HY, Jin YM, Jung SC, Ryu KH, Jo I. Characterization of long-term in vitro culture-related alterations of human tonsil-derived mesenchymal stem cells: role for CCN1 in replicative senescence-associated increase in osteogenic differentiation. J Anat. 2014;225:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Cho KA, Kim JY, Kim HS, Ryu KH, Woo SY. Tonsil-derived mesenchymal progenitor cells acquire a follicular dendritic cell phenotype under cytokine stimulation. Cytokine. 2012;59:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, Darrow DH, Giordano T, Litman RS, Li KK, Mannix ME, Schwartz RH, Setzen G, Wald ER, Wall E, Sandberg G, Patel MM; American Academy of Otolaryngology-Head and Neck Surgery Foundation. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144:S1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 9. | Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1405] [Cited by in RCA: 1361] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 10. | Kim SY, Kim YR, Park WJ, Kim HS, Jung SC, Woo SY, Jo I, Ryu KH, Park JW. Characterisation of insulin-producing cells differentiated from tonsil derived mesenchymal stem cells. Differentiation. 2015;90:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Passàli D, Damiani V, Passàli GC, Passàli FM, Boccazzi A, Bellussi L. Structural and immunological characteristics of chronically inflamed adenotonsillar tissue in childhood. Clin Diagn Lab Immunol. 2004;11:1154-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Tsukahara S, Ikeda R, Goto S, Yoshida K, Mitsumori R, Sakamoto Y, Tajima A, Yokoyama T, Toh S, Furukawa K, Inoue I. Tumour necrosis factor alpha-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. Biochem J. 2006;398:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 14. | Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 870] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 15. | Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 380] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1596] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 18. | Patel M, Moon HJ, Jung BK, Jeong B. Microsphere-Incorporated Hybrid Thermogel for Neuronal Differentiation of Tonsil Derived Mesenchymal Stem Cells. Adv Healthc Mater. 2015;4:1565-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Jung N, Park S, Choi Y, Park JW, Hong YB, Park HH, Yu Y, Kwak G, Kim HS, Ryu KH, Kim JK, Jo I, Choi BO, Jung SC. Tonsil-Derived Mesenchymal Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Peripheral Nerve Regeneration. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Choi JS, Lee BJ, Park HY, Song JS, Shin SC, Lee JC, Wang SG, Jung JS. Effects of donor age, long-term passage culture, and cryopreservation on tonsil-derived mesenchymal stem cells. Cell Physiol Biochem. 2015;36:85-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Park MH, Yu Y, Moon HJ, Ko du Y, Kim HS, Lee H, Ryu KH, Jeong B. 3D culture of tonsil-derived mesenchymal stem cells in poly(ethylene glycol)-poly(L-alanine-co-L-phenyl alanine) thermogel. Adv Healthc Mater. 2014;3:1782-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Kye EJ, Kim SJ, Park MH, Moon HJ, Ryu KH, Jeong B. Differentiation of tonsil-tissue-derived mesenchymal stem cells controlled by surface-functionalized microspheres in PEG-polypeptide thermogels. Biomacromolecules. 2014;15:2180-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Moon HJ, Patel M, Chung H, Jeong B. Nanocomposite versus Mesocomposite for Osteogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Adv Healthc Mater. 2016;5:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Jeong H, Lee ES, Jung G, Park J, Jeong B, Ryu KH, Hwang NS, Lee H. Bioreducible-Cationic Poly(amido amine)s for Enhanced Gene Delivery and Osteogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. J Biomed Nanotechnol. 2016;12:1023-1034. [PubMed] |
| 27. | Park J, Kim, IY, Patel M, Moon HJ, Hwang SJ, Jeong B. 2D and 3D hybrid systems for enhancement of chondrogenic differentiation of tonsil-derived mesenchymal stem cells. Adv Funct Mater. 2015;2573-2582. [DOI] [Full Text] |
| 28. | Patel M, Moon HJ, Ko du Y, Jeong B. Composite System of Graphene Oxide and Polypeptide Thermogel As an Injectable 3D Scaffold for Adipogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2016;8:5160-5169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 30. | Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528-1530. [PubMed] |
| 31. | Hosoyama T, McGivern JV, Van Dyke JM, Ebert AD, Suzuki M. Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Transl Med. 2014;3:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Park S, Choi Y, Jung N, Yu Y, Ryu KH, Kim HS, Jo I, Choi BO, Jung SC. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int J Mol Med. 2016;37:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, Kim HS, Jang JY, Lee CH, Kim BS, Jang YO, Cho MY, Jung ES, Kim YM, Bae SH, Baik SK. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 34. | Liang J, Zhang H, Zhao C, Wang D, Ma X, Zhao S, Wang S, Niu L, Sun L. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int J Rheum Dis. 2017;20:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Margini C, Vukotic R, Brodosi L, Bernardi M, Andreone P. Bone marrow derived stem cells for the treatment of end-stage liver disease. World J Gastroenterol. 2014;20:9098-9105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 36. | Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH, Gonelle-Gispert C. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4:e6657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L, Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Yang L, Chang N, Liu X, Han Z, Zhu T, Li C, Yang L, Li L. Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-β1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J Pathol. 2012;181:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim HS, Park YS, Jo I, Park JW, Jung SC, Lee H, Jeong B, Ryu KH. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci Rep. 2015;5:8616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Kim SJ, Park MH, Moon HJ, Park JH, Ko du Y, Jeong B. Polypeptide thermogels as a three dimensional culture scaffold for hepatogenic differentiation of human tonsil-derived mesenchymal stem cells. ACS Appl Mater Interfaces. 2014;6:17034-17043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Hong JH, Lee HJ, Jeong B. Injectable Polypeptide Thermogel as a Tissue Engineering System for Hepatogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2017;9:11568-11576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Khamaisi M, Balanson SE. Stem Cells for Diabetes Complications: A Future Potential Cure. Rambam Maimonides Med J. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Lilly MA, Davis MF, Fabie JE, Terhune EB, Gallicano GI. Current stem cell based therapies in diabetes. Am J Stem Cells. 2016;5:87-98. [PubMed] |
| 44. | Gabr MM, Zakaria MM, Refaie AF, Abdel-Rahman EA, Reda AM, Ali SS, Khater SM, Ashamallah SA, Ismail AM, Ismail HEA, El-Badri N, Ghoneim MA. From Human Mesenchymal Stem Cells to Insulin-Producing Cells: Comparison between Bone Marrow- and Adipose Tissue-Derived Cells. Biomed Res Int. 2017;2017:3854232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Abate EG, Clarke BL. Review of Hypoparathyroidism. Front Endocrinol (Lausanne). 2017;7:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Bingham EL, Cheng SP, Woods Ignatoski KM, Doherty GM. Differentiation of human embryonic stem cells to a parathyroid-like phenotype. Stem Cells Dev. 2009;18:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Sulzbacher S, Schroeder IS, Truong TT, Wobus AM. Activin A-induced differentiation of embryonic stem cells into endoderm and pancreatic progenitors-the influence of differentiation factors and culture conditions. Stem Cell Rev. 2009;5:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Woods Ignatoski KM, Bingham EL, Frome LK, Doherty GM. Directed trans-differentiation of thymus cells into parathyroid-like cells without genetic manipulation. Tissue Eng Part C Methods. 2011;17:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Park YS, Kim HS, Jin YM, Yu Y, Kim HY, Park HS, Jung SC, Han KH, Park YJ, Ryu KH, Jo I. Differentiated tonsil-derived mesenchymal stem cells embedded in Matrigel restore parathyroid cell functions in rats with parathyroidectomy. Biomaterials. 2015;65:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (4)] |
| 51. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1107] [Article Influence: 100.6] [Reference Citation Analysis (1)] |
| 52. | Sada-Ovalle I, Talayero A, Chavéz-Galán L, Barrera L, Castorena-Maldonado A, Soda-Merhy A, Torre-Bouscoulet L. Functionality of CD4+ and CD8+ T cells from tonsillar tissue. Clin Exp Immunol. 2012;168:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Cho KA, Park M, Kim YH, Woo SY, Ryu KH. Conditioned media from human palatine tonsil mesenchymal stem cells regulates the interaction between myotubes and fibroblasts by IL-1Ra activity. J Cell Mol Med. 2017;21:130-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Kim JY, Park M, Kim YH, Ryu KH, Lee KH, Cho KA, Woo SY. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J Tissue Eng Regen Med. 2018;12:e1022-e1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Cho KA, Lee JK, Kim YH, Park M, Woo SY, Ryu KH. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Yu Y, Song EM, Lee KE, Joo YH, Kim SE, Moon CM, Kim HY, Jung SA, Jo I. Therapeutic potential of tonsil-derived mesenchymal stem cells in dextran sulfate sodium-induced experimental murine colitis. PLoS One. 2017;12:e0183141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Ryu JH, Park M, Kim BK, Kim YH, Woo SY, Ryu KH. Human tonsilderived mesenchymal stromal cells enhanced myelopoiesis in a mouse model of allogeneic bone marrow transplantation. Mol Med Rep. 2016;14:3045-3051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 605] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 59. | Battiwalla M, Barrett AJ. Bone marrow mesenchymal stromal cells to treat complications following allogeneic stem cell transplantation. Tissue Eng Part B Rev. 2014;20:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Open-Access: This is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited Manuscript
Specialty type: Cell and tissue engineering
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, Li SC, Saeki K, Tanabe S, Wakao H S-Editor: Cui LJ L-Editor: A E-Editor: Xing YX