Published online May 26, 2019. doi: 10.4252/wjsc.v11.i5.236
Peer-review started: January 16, 2019
First decision: January 29, 2019
Revised: February 22, 2018
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: May 26, 2019
Processing time: 130 Days and 23.1 Hours
Alzheimer’s disease (AD) is the most common form of dementia. To date, only five pharmacological agents have been approved by the Food and Drug Administration for clinical use in AD, all of which target the symptoms of the disease rather than the cause. Increasing our understanding of the underlying pathophysiology of AD will facilitate the development of new therapeutic strategies. Over the years, the major hypotheses of AD etiology have focused on deposition of amyloid beta and mitochondrial dysfunction. In this review we highlight the potential of experimental model systems based on human induced pluripotent stem cells (iPSCs) to provide novel insights into the cellular pathophysiology underlying neurodegeneration in AD. Whilst Down syndrome and familial AD iPSC models faithfully reproduce features of AD such as accumulation of Aβ and tau, oxidative stress and mitochondrial dysfunction, sporadic AD is much more difficult to model in this way due to its complex etiology. Nevertheless, iPSC-based modelling of AD has provided invaluable insights into the underlying pathophysiology of the disease, and has a huge potential for use as a platform for drug discovery.
Core tip: Alzheimer’s disease (AD) is a huge burden on the healthcare system and on society. At present, there are no therapeutic approaches that address the underlying causes of this devastating disease, largely because we lack understanding of the underlying molecular mechanisms. Induced pluripotent stem cells (iPSCs) from AD or Down syndrome patients can be used to elucidate these molecular mechanisms, therefore presenting a novel approach to this problem. In this review, we focus on the ability of iPSC models to gain insight into the mitochondrial dysfunction that occurs during AD and therefore identify novel drug targets.
- Citation: Hawkins KE, Duchen M. Modelling mitochondrial dysfunction in Alzheimer’s disease using human induced pluripotent stem cells. World J Stem Cells 2019; 11(5): 236-253
- URL: https://www.wjgnet.com/1948-0210/full/v11/i5/236.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i5.236
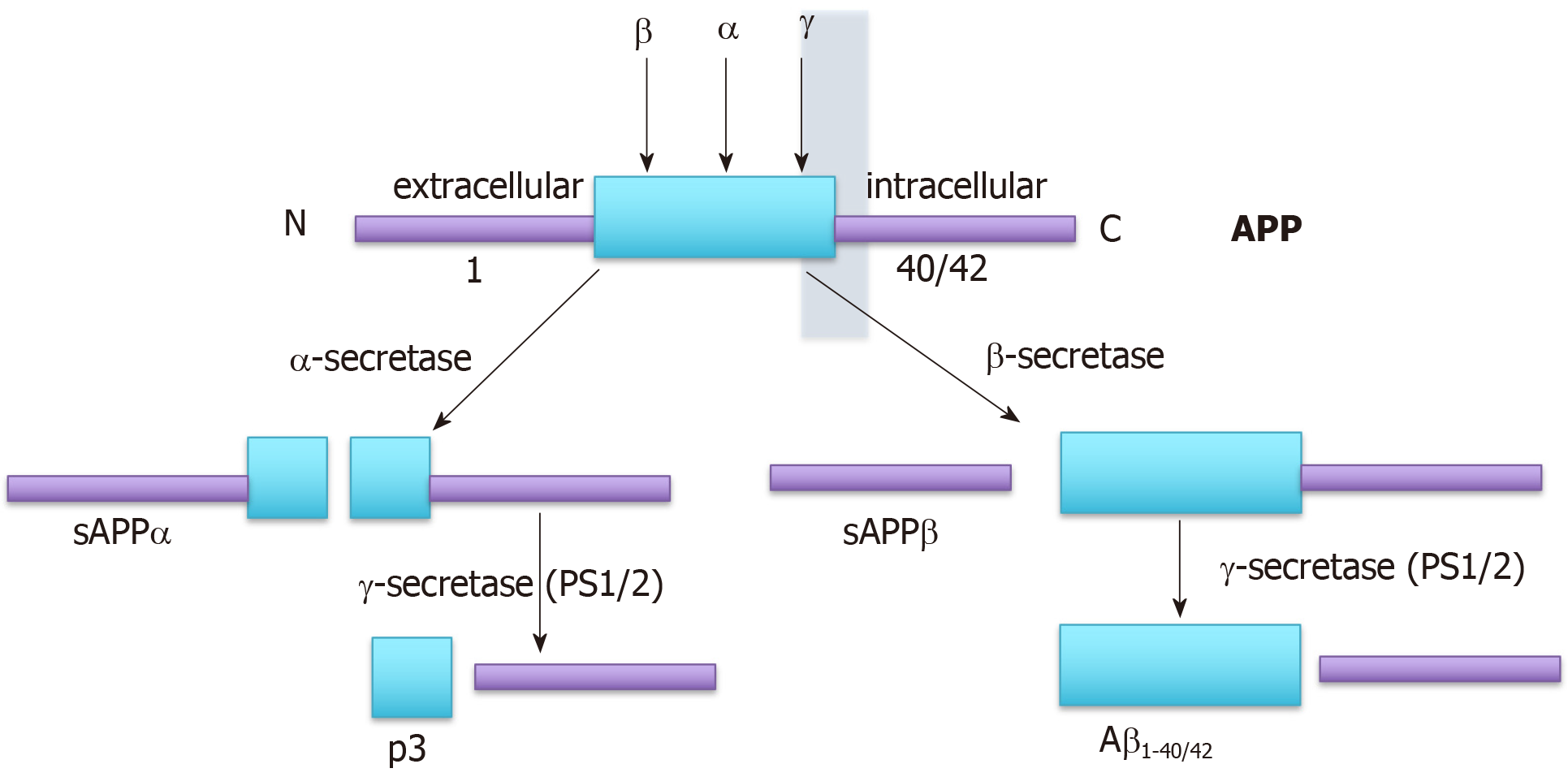
Alzheimer’s disease (AD) is characterized by the presence of tangles of hyper-phosphorylated tau and plaques of beta-amyloid (Aβ) in the central nervous system (CNS). However, it is not clear whether the tangles and plaques drive the pathophysiology of AD or whether they are symptomatic, caused by a common underlying process. The vast majority of people with AD present at 65 or older with “sporadic” AD (sAD). Around 1% of subjects present with atypical early onset familial AD (fAD), generally diagnosed between the ages of 30-60[1,2]. Despite this, most research has focused on fAD since its etiology is the most straightforward to model. fAD is most frequently caused by mutations in the genes encoding the three components of the amyloid precursor protein (APP) processing pathway (Figure 1), the γ-secretase-component, encoding the genes presenilin (PSEN)-1 and PSEN-2, or the APP gene itself, whereas a growing consensus suggests that sAD is more likely to be caused by impaired clearance of Aβ[3-7].
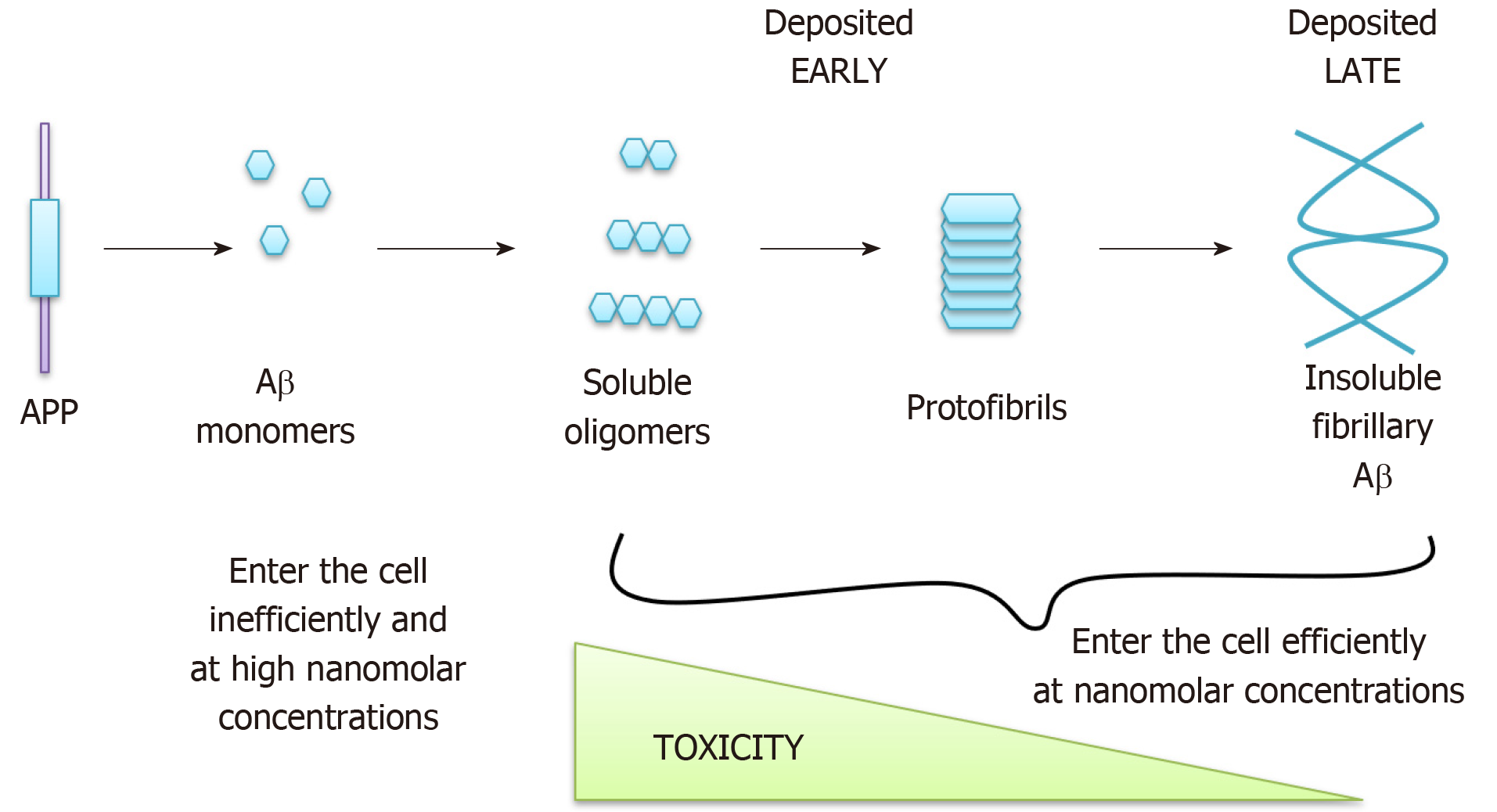
The genetic basis of fAD suggests that the accumulation of Aβ in plaques is one, if not the only, cause of the disease, as was suggested by the “amyloid hypothesis” of AD[8]. The amyloid hypothesis has evolved over the years and the most recent version distinguishes between soluble forms of Aβ, which are likely to accumulate in cells early in AD and be highly toxic, and insoluble fibrillary Aβ which is deposited later in the disease and is less toxic (reviewed in[9-12]) (Figure 2). Interestingly, tau tangles are generally no longer posited as a primary cause for AD, despite being a major cause of neuronal death, since mutations in the tau gene (MAPT) do not cause AD (reviewed in[13]), instead leading to frontotemporal dementia and parkinsonism. MAPT knockout mice are also relatively normal[14]. Instead Aβ accumulation is thought to cause accumulation of tau tangles[15], since treatment of AD neurons in vitro with Aβ-specific antibodies reverses the tau accumulation phenotype[16], although the mechanism for this association is currently unknown[17]. In support of the amyloid hypothesis, exposure of astrocytes and neurons to exogenous Aβ causes mitochondrial dys-function, impaired glucose uptake and ultimately cell death[18,19] whilst injecting Aβ42 into the CNS of healthy rats[20] and primates[21] causes impaired memory. In addition, APP duplications cause fAD[22] and the incidence of AD-like dementia is almost universal in ageing Down’s syndrome (DS) subjects, who have three copies of chromosome 21 and therefore of the APP gene[23]. Approximately two thirds of people with DS will develop a dementia by the age of 60[23], compared to an incidence closer to 1 in 10 in the general population at a similar age. Furthermore, Prasher et al[24] described a 78-year-old woman with DS but without AD, in which the distal segment of chromosome 21 was translocated so that the APP gene, amongst others, was not triplicated[24]. Despite extensive evidence for the role of Aβ in AD aetiology, various anti-amyloid drugs have failed in clinical trials[25,26], as have anti-tangle drugs, which have also all failed phase II clinical trials[27]. This, along with the observations that sAD patients do not harbor APP or PSEN mutations[28], that many ageing individuals also have plaques and tangles at post mortem without signs of dementia[29,30], and that triplication of all genes on chromosome 21 except APP in mice still leads to Aβ deposition and cognitive deficits in mice[31], suggests that the pathophysiology underlying AD progression likely to be more complex. Thus, the search for the underlying mechanisms driving the pathophysiology of sAD and identification of novel candidate drug targets is urgent.
Swerdlow and Khan[32] proposed the mitochondrial cascade hypothesis, suggesting that AD develops as a consequence of an individual’s baseline mitochondrial function coupled with a decline in mitochondrial function with age[33,34]. This might explain the role of ageing in the aetiology of sAD and is supported by various forms of experi-mental evidence. For example, evidence of oxidative stress can precede plaque formation in the brain[35], AD has a strong maternal genetic contribution[36,37] and cybrid cells, in which platelets from AD patients were fused with neuro-blastoma/teratocarcinoma cell lines lacking mtDNA, develop molecular features of AD including Aβ production[38]. Exposure of HEK293 cells to the mitochondrial respiratory chain inhibitor antimycin A was associated with increased reactive oxygen species (ROS) generation, Aβ deposition and toxicity and this was reduced by expression of the alternative oxidase, which prevents antimycin A-induced ROS production[39]. Furthermore, normal astrocytes exhibit intracellular accumulation of Aβ similar to that observed in DS astrocytes when mitochondrial metabolism is prevented by the treatment with the uncoupler carbonyl cyanide mchlorophenyl-hydrazone (CCCP)[40].
The role of Aβ in AD remains controversial since, despite its toxicity, it can also protect cells, perhaps by virtue of an antioxidant role (reviewed in[41,42]). This role, evidenced by the ability of aggregated Aβ42 peptide to abolish ROS formation in rat mitochondria exposed to FeSO4 and ascorbate, has been proposed to be mediated by metal chelation by the peptide[43]. In addition, soluble (s)APPα, generated by the non-amyloidogenic processing of APP (Figure 1), has been shown to be neuroprotective[44]. It has been suggested that accumulation of Aβ40 and Aβ42 in AD may be a protective response to the oxidative damage caused by mitochondrial dysfunction[45], consistent with the mitochondrial cascade theory. This idea is supported by the observation that the survival of DS neurons was increased by recombinant or astrocyte-produced Aβ[40]. It seems plausible that ageing (or premature ageing in DS[46,47]) causes both Aβ accumulation, as a result of neurodegeneration, and mitochondrial dysfunc-tion/oxidative stress and therefore that a vicious cycle develops whereby accumulation of Aβ into plaques causes oxidative stress which in turn increases the amyloidogenic processing of APP and Aβ deposition[45]. Interestingly, tau phosphorylation also increases in response to disruption of mitochondrial function through inhibition of the electron transport chain[48-50]. Both hypotheses are therefore likely to be correct at least to some extent.
Various mechanisms by which Aβ plaques may cause oxidative stress have been proposed. For example, it has long been suggested that Aβ generates oxygen radicals directly in solution[51], since Aβ coordinates with iron and copper, which can generate ROS[52,53]. Aβ also has the capacity to form Ca2+-permeant channels in lipid bilayers[54,55]. This property is dependent on the membrane cholesterol content of the bilayer[56,57], leading to the selective generation of Ca2+ signals in astrocytes, but not neurons after exposure to Aβ, reflecting differences in membrane cholesterol content between the two cell types[58]. This phenomenon may explain our previous observations, detailed below, in which we described mitochondrial dysfunction in astrocytes in response to Aβ, followed only later by the death of neurons. Interestingly, reactive astrocytes have been shown to actively induce neuronal death in the context of many neurode-generative diseases, including AD[59,60].
We have previously shown that exogenous Aβ-mediated Ca2+ influx into rat astrocytes activate the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase which generates superoxide. This results in DNA damage and large transient depolarizations of the mitochondrial membrane potential, driven by Ca2+ signals and opening of the mitochondrial permeability transition pore (mPTP)[18,19,61]. We showed that overactivation of Poly (ADP-ribose) polymerase (PARP)-1 in astrocytes and neurons in response to superoxide-driven DNA damage caused NAD+ depletion, failure of glycolysis in neurons and neuronal death. Neurons were rescued by inhibition of each step of this pathway - by NADPH oxidase inhibitors, PARP-1 inhibitors and by supply of metabolic substrates that bypass glycolysis, such as supplementation with methyl succinate or pyruvate[18] (Figure 3). That these mechanisms are not simply an artefact of the experimental design and operate in the intact AD nervous system is suggested by a number of observations. For example, intercellular Ca2+ waves passing between astrocytes and initiated at Aβ plaques were described in vivo in a double transgenic mouse model of AD expressing APP and mutant PSEN[62] and we found evidence for increased activation of the NADPH oxidase in the hippocampus of a triple transgenic AD mouse model[18]. Similarly, Love et al[63] reported evidence of increased PARP activity in post-mortem AD brains.
Impaired mitochondrial substrate supply may be exacerbated by decreased glucose uptake, a feature of the AD brain[64], likely due to Aβ exposure, which impairs glucose uptake in astrocytes[18]. This effect has been modelled in stem cell-derived neurons and astrocytes upon exposure to Aβ, which resulted in decreased levels of glucose uptake[65]. Interestingly, glucose levels have been shown in some studies to increase in the AD brain[66], which has been proposed to lead to decreased glucose uptake as an adaptive response. Whilst the mechanisms remain uncertain, Liu et al[67] demonstrated decreased expression of the glucose transporters GLUT-1 (the blood-brain barrier and astrocytic glucose transporter) and GLUT-3 in the AD brain and Prapong et al[68] have shown that Aβ inhibits neuronal glucose uptake by preventing the fusion of GLUT-3-containing vesicles with the plasma membrane.
Mitochondrial dysfunction in AD is well-established (reviewed in[69]) and res-piratory capacity is generally decreased across AD models[70,71]. Mitochondrial dynamics also appear to be dysregulated in AD. Expression of the proteins mitofusin-1 and -2 and optic atrophy-1, which are involved in mitochondrial fusion, and dynamin-like protein-1, which mediates fission, are all downregulated in pyramidal neurons of AD patients[72,73]. In addition, genes associated with autophagy and mitophagy are downregulated in fibroblasts derived from sAD patients[73]. Despite this, Birnbaum et al[74] demonstrated an upregulation of mitochondrial complex protein expression. Mechanistically, PTEN-induced putative kinase (PINK)1, which promotes removal of damaged mitochondria by mitophagy, is downregulated in AD and restoring its expression decreases Aβ production, oxidative stress and mito-chondrial dysfunction in APP-overexpressing mouse brains[75]. PINK1 mutations are associated with Parkinson’s disease (reviewed in[76,77]), highlighting the common mechanisms underlying the various neurodegenerative disorders.
Supraphysiological increases in intra-mitochondrial Ca2+ can trigger opening of the mPTP, causing mitochondrial depolarization and cell death, especially if the Ca2+ signal is coincident with oxidative stress[18,78-81]. It has been suggested that Aβ may directly contribute to the formation of the mPTP by binding cyclophilin D, the major regulator of mPTP opening, resident in the mitochondrial matrix[82]. Alternatively, it has been suggested that Aβ upregulates another putative regulator of mPTP opening, the voltage-dependent anion-selective channel-1[83]. The concept that Aβ can be internalized into the cell was recently supported by a study which visualized its uptake using confocal microscopy[84]. Interestingly, mitochondria in DS astrocytes were described as shorter, consistent with mitochondrial fragmentation and, possibly, mitochondrial swelling due to mPTP formation[85]. Furthermore, a double transgenic AD mouse model crossed with a cyclophilin D knockout mouse (in which mPTP opening is suppressed), performed significantly better in various cognitive tasks[82,86]. Aβ can also disrupt the mitochondrial respiratory chain directly through the inhibi-tion of complex IV[87-89], complex V (reviewed in[42]) and/or by binding to Aβ-binding alcohol dehydrogenase[88,90-94], all of which would contribute to mitochondrial dysfunction and potentially to increased ROS production[95]. Moreover, deregulation of complex I has been shown to be regulated by tau[96].
Much insight has been gained through animal models. However, the lack of effective disease modifying drugs for AD largely reflects the failure of these studies to translate to efficacy in humans[97]. Reasons for this failure remain unclear, but certainly, the anatomy and genetics of the brain in rodents differ significantly from that of the human[1]. sAD is especially difficult to model, as we know so little about the un-derlying mechanisms, and mouse models have been generated through genetic manipulation and are therefore representative of fAD than sAD[98], with the hope that these will give insights into mechanisms of sAD. Even in the case of fAD they do not accurately mimic AD progression, for example by exhibiting full tau tangle pathology[99]. Animal models do have the unique advantage of being able to model systemic physiological factors such as diet, obesity and hypertension, all of which play important roles in sAD (reviewed in[100]). Mouse models also cannot realistically lend themselves to drug screens. Postmortem brain tissue from AD patients has also been used as a research tool. However, this is difficult to obtain[101] and the ability to generate neural cultures from postmortem tissue is highly dependent on the quality of the tissue, which is often compromised during the later stages of the disease[102].
Adding Aβ exogenously to cell cultures has been widely employed as a strategy and may have generated interesting data on the mechanisms of Aβ toxicity, but is also fraught with interpretational difficulties. It is difficult to know whether the levels or forms of Aβ that are used experimentally are (patho) physiologically relevant. In addition, various groups have added pre-aggregated tau fibrils to induced pluri-potent stem cell (iPSC)-derived neurons to model AD, demonstrating that these fibrils efficiently enter the neurons[103], are propagated intracellularly[104] and that the tau aggregation phenotype that they induce can be rescued by treatment with autophagy inducers[105]. The advantages and disadvantages of the different model systems are summarized in Table 1.
| Model system | Advantages | Disadvantages |
| Animal models | Can be used to model physiological factors such as diet, obesity and hypertension | Findings may not be able to be directly extrapolated to humans |
| Postmortem tissue | Human-derived | Difficult to obtain; May be of poor quality due to the destructive effects of AD in its later stages |
| iPSC-based models | Human-derived; More easily obtained than post-mortem tissue | Cannot be used to model physiological or epigenetic factors; Large variation between sAD iPSC lines (may not exhibit phenotype); Neuronal derivatives may be akin to ‘younger’ neurons |
The use of patient-derived iPSCs may be able to address many of these challenges, since they are derived from human subjects and are easier to obtain than postmortem tissue. In addition, tau phosphorylation has been demonstrated both in AD patient iPSC-derived neurons[106,107] and cerebral organoids generated from these cells[108] while GSK3β, a major tau kinase, has been shown to be upregulated in AD iPSC-derived neurons[17,107].
iPSCs were first generated from mouse[109] and human[110] fibroblasts in 2006 and 2007 respectively. The pioneering work of Prof. Yamanaka’s group in Japan de-monstrated that pluripotency, the ability to give rise to the three germ layers, could be induced in these cells through the forced expression of four key “Yamanaka factors”, OCT4, SOX2, KLF4 and cMYC. The original Yamanaka factors are still in use today, with the optional addition of LIN28, p53 shRNA and NANOG to increase efficacy and the substitution of LMYC in the place of cMYC. The substitution of the latter factor is for safety reasons, since cMYC is a known oncogene[111]. In addition, pTAT-mcMYC[112] or fluorescence-activated cell sorting for differentiation markers[102] can be used to prevent uncontrolled proliferation.
Importantly, the epigenetic landscape is largely reset by the reprogramming pro-cess[113]. This will inevitably limit the use of iPSC-derived neurons to study the role of epigenetic factors in AD. Despite this phenomenon, some iPSC lines have been shown to exhibit an “epigenetic memory” of their cell type of origin[114]. This observation, along with the high degree of variability between iPSC clones (reviewed in[115]), may mean that they exhibit differing abilities to differentiate down a particular lineage, which should be taken into account when using iPSCs in this way. To address these issues, various groups have published protocols for the direct conversion of fibroblasts into induced neural precursor cells (iNPCs[116]) and induced neuronal cells (iNs[117]), in which case the epigenetic changes a cell has obtained over the lifespan of the individual are maintained. The choice of whether to use iPSC-derived neurons or iNPCs/iNs will depend on whether the researchers intend to study the genetic basis of the disease only or both genetic and epigenetic factors. However, the cell type of origin, usually fibroblasts, may still be an issue depending to what extent this cell type is affected by the disease and ageing in comparison to the neurons and astrocytes of the brain that are directly affected by the disease in the patient.
Since their original discovery a decade ago, iPSCs have proven to be an invaluable tool for studying disease progression “in a dish”. Diseases that have been modelled in this way include amyotrophic lateral sclerosis[118], familial dysautonomia[119], Rett syndrome[120], schizophrenia[121], spinal muscular atrophy[122,123], DS[124], Huntingdon’s disease[125], Duchenne muscular dystrophy, Parkinson’s disease, AD, type 1 dia-betes[126] and Gaucher disease[125]. These diseases all have a genetic basis, which is necessary to allow recapitulation of the disease phenotype in iPSCs and their derivatives.
sAD may be included in this category to some degree since it is linked to SNP variants in particular genes in 60%-80% of cases[102] (reviewed in[127]). Interestingly, in two recent studies only one of two sAD patient lines studied demonstrated an AD phenotype in the iPSC-derived neurons, including altered APP expression and Aβ secretion[17,53], demonstrating the high variability of results obtained using sAD patient-derived iPSCs. This variability is likely to reflect the different genetic backgrounds of the two different patients and highlights the importance of maxi-mising the number of cell lines used, particularly in the case of sAD where phenotypes are so variable. The maximum number of cell lines used in the studies described here is Young et al[128] who use seven lines. However, studies of other diseases have been identified that use almost 30 disease lines to ensure that the statistical power of their findings is sufficient[129]. These types of studies will be important, at least initially, to identify subtypes of patients with similar phenotypes and therefore to potentially allow particular therapies to be targeted to these subtypes. Variations in the neural differentiation protocols used are also likely to represent another potential source of variation between studies. This may lead to different neural cell phenotypes and therefore possibly to different mechanistic findings.
Encouragingly, however, Hossini et al[130] have demonstrated AD-like gene ex-pression profiles in sAD patient iPSC-derived neurons, including alterations in the response to oxidative stress. In addition, AD-associated phenotypes such as the presence of large RAB5+ early endosomes, which indicate impaired autophagy, increased susceptibility to cell death, abnormal calcium influx and altered axonal transport have all been observed in cells derived from patients with both fAD and sAD[17,53,131,132], reinforcing the validity of iPSC sAD models.
Many groups have modelled AD using fAD[15-17,53,98,101,108,131,133-140], sAD[17,53,98,128,129,136,141] or DS[15,124,142-147] iPSCs (Table 2). Indeed, fAD iPSC-derived neurons appear to faithfully reproduce the Aβ overproduction/tau hyperphosphorylation phenotype[15,16]. Interestingly, AD iPSCs differentiate into NPCs with indistinguishable growth rate and morphology to control cells and show a comparable efficacy of terminal differentiation into neurons[98], as do DS iPSCs[124].
| Study | Disease | Key findings | Advantages | Disadvantages |
| Yagi et al[133], 2011 | fAD | Relevant expression of APP and secretase subunits in iPSC-derived neurons | Obvious AD phenotype observed | fAD only represents ~ 5% patients |
| Shi et al[124], 2012a | DS | AD pathology (such as aberrant Aβ production and hyperphosphorylated Tau) developed over months in culture, as opposed to years in vivo | Show tau (advanced) phenotype | Findings may not be able to be extrapolated to AD |
| Israel et al[17], 2012 | fAD, sAD | fAD neurons and one out of two sAD neurons exhibit altered APP expression and Aβ secretion and swollen endosomes | Comparison of fAD and sAD, in essence using fAD lines as positive control | High levels of variation between cell lines |
| Koch et al[101], 2012 | fAD | Key steps in proteolytic APP processing are recapitulated in hES and iPSC-derived neurons | Obvious AD phenotype observed | High levels of variation between cell lines |
| Maclean et al[146], 2012 | DS | Disturbance of multilineage myeloid haematopoiesis in T21 at fetal liver stage | Reproducible phenotype because clear genetic link | Findings may not be able to be extrapolated to AD |
| Kondo et al[53], 2013 | fAD, sAD | Aβ oligomers accumulated in iPSC-derived neurons and astrocytes in fAD and one out of two sAD patients, also observed ROS | Comparison of fAD and sAD, in essence using fAD lines as positive control | High variation between sAD cell lines |
| Xu et al[66], 2013 | Exogenous Aβ | Cell cycle re-entry in iPSC-derived neurons treated with Aβ | Used pharmacological inhibitors to demonstrate rescue of phenotype | May not be physiologically relevant |
| Weick et al[142], 2013 | DS | Compensatory responses to oxidative stress in T21 neurons, also reduced synaptic activity | Reproducible phenotype because clear genetic link | Findings may not be able to be extrapolated to AD |
| Woodruff et al[139], 2013 | fAD | PSEN1 mutations impair γ-secretase activity but do not disrupt γ-secretase-independent functions | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Hibaoui et al[143], 2014 | DS | Abnormal neural differentiation, likely caused by DYRK1A on chromosome 21 | Used fetal fibroblasts to generate iPSCs (less acquired mutations) | Findings may not be able to be extrapolated to AD |
| Muratore et al[16], 2014 | fAD | iPSC-derived neurons have increased Aβ42 and Aβ38, along with increased levels of both tau and phosphorylated tau | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Mahairaki et al[134], 2014 | fAD | Increased Aβ42:Aβ40 ratio in fAD iPSC-derived neurons | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Sproul et al[135], 2014 | fAD | Identified 14 genes that are differentially regulates in PSEN1 mutant NPCs relative to controls | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Duan et al[131], 2014 | fAD | iPSC-derived neurons with ApoE3/4 mutations showed typical AD features | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Liu et al[67], 2014 | fAD | Treatment with NSAID reduced Aβ42:Aβ40 ratio | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Young et al[128], 2015 | sAD | Human neurons with SORL1 mutations associated with sAD show a reduced response to BDNF, at the level of both SORL1 expression and APP processing | Many cell lines used (n = 7) | Only one type of sAD mutation examined; unlikely to be able to be extrapolated to a large patient cohort |
| Hossini et al[130], 2015 | sAD | Genes associated with AD expressed in sAD iPSC-derived neurons (including oxidative stress response). Treatment with a γ-secretase inhibitor reduced levels of Tau. | Show AD-like gene expression patterns | Only one patient line used (n = 1) |
| Chang et al[147], 2015 | DS | Tau mislocalisation | Show advanced (tau) phenotype | Findings may not be able to be extrapolated to AD |
| Murray et al[144], 2015 | DS | Slower proliferation of NPCs, increased Aβ production, a decrease in mitochondrial membrane potential and increased no. and abnormal appearance of mitochondria, also increased no. of ds DNA breaks in T21 neurons | Reproducible phenotype because clear genetic link | Findings may not be able to be extrapolated to AD |
| Moore et al[15], 2015 | fAD, DS | APP mutations increase levels of tau and phosphorylated tau whereas PSEN mutations do not | Obvious AD phenotype observed | Tested drugs (β-secretase and ɣ-secretase inhibitors) that have failed clinical trials |
| Tubsuwan et al[177], 2016 | fAD | Description of model | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Raja et al[108], 2016 | fAD | Brain organoids from AD patients exhibit amyloid aggregation, pTau and endosome abnormalities, treatment with β and γ-secretase inhibitors reduced this pathology | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Li et al[140], 2016 | fAD | Characterisation of an iPSC line | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Lee et al[119], 2016 | sAD | Secretase inhibtors decreased Aβ generation but less potency in 3D | High number of sAD lines used (n = 5) | Tested generic drugs (BACE1 and ɣ-secretase inhibitors) that have failed clinical trials |
| Yang et al[136], 2017 | fAD | Premature neuronal differentiation with decreased proliferation and increased apoptosis in AD-NPCs, Wnt-Notch pathway involvement | Obvious AD phenotype observed | fAD only represents ~5% patients |
| Dashinimaev et al[145], 2017 | DS | Increased Aβ secretion and upregulation of APP gene, also increased BACE2, RCAN1, ETS2, TMED10 expression in T21 neural cells compared to controls | Reproducible phenotype because clear genetic link | Findings may not be able to be extrapolated to AD |
| Jones et al[98], 2017 | fAD, sAD | Astrocytes derived from iPSCs from both fAD and sAD patients exhibit a pronounced pathological phenotype | Comparison of fAD and sAD, in essence using fAD lines as positive control | Only one line each fAD and sAD used (n = 1) |
| Armijo et al[137], 2017 | fAD, sAD | fAD neurons have increased susceptibility to Aβ in comparison to sAD (and control) neurons | Comparison of fAD and sAD, in essence using fAD lines as positive control | Only one line each fAD and sAD used (n = 1) |
| Ochalek et al[107], 2018 | fAD, sAD | sAD iPSC-derived neurons reveal elevated tau hyperphosphorylation, increased amyloid levels and GSK3β activation | Show tau (advanced) phenotype | Differentiation protocol requires 10 weeks at least |
| Birnbaum et al[74], 2018 | sAD | sAD iPSC-derived neurons display oxidative stress and increased mitochondrial protein expression which doesn’t correlate with Aβ/tau | Occurs in ~95% of AD cases | Hard to explain why the oxidative stress and increased mitochondrial protein expression don’t correlate with Aβ/tau |
Neural differentiation of iPSCs also presents the unique opportunity to model disease progression from an early stage. For example, it has been shown that Aβ secretion increases throughout neural differentiation of both fAD patient and control iPSCs[16]. Moreover, DS iPSC-derived neurons are electrophysiologically active; DS and control cell lines show no significant differences in this respect[144] and neural cultures develop AD-like pathologies after relatively short periods in culture. Despite this, iPSC-derived neurons have been shown to be more similar to late fetal neurons than late adult neurons which may limit the expression of tau isoforms[148]. In addition, iPSC-derived astrocytes from both sAD and fAD patients exhibited defective locali-zation of astroglial markers in comparison to control cell lines[98] and fAD iPSC-derived astrocytes exhibited increased Aβ production, dysregulated calcium homeostasis and were more inflammatory, producing more ROS[98,149]. Birnbaum et al[74] also showed oxidative stress in iPSC-derived neurons from sAD patients, even in the absence of Aβ and tau pathology, providing support for the mitochondrial cascade hypothesis. Hibaoui et al[143] showed that DS iPSC-derived neurospheres contained a reduced number of NPCs, likely related to the observation that NPC proliferation was decreased and levels of apoptosis increased in the patient-derived cells. Upon neural maturation, they observed decreased expression of neuronal markers and increased expression of astroglial markers in DS cells in comparison to isogenic controls. These defects could be rescued by inhibition of dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 1A, suggesting that its triplication in DS is responsible for the phenotypes observed.
In addition to 2D disease modelling, various groups are attempting to model AD in 3D cultures, in order to recreate the interactions between neurons and glia in the brain[99,108,141]. Lancaster et al[150] were the first to generate cerebral organoids, paving the way for 3D studies by demonstrating that these “mini brains” recapitulate the development of the fetal brain and can be used to model diseases such as micro-cephaly. 3D culture may have benefits over 2D culture. For example, Choi et al[99] describe accelerated Aβ and tau pathologies in 3D compared to 2D cultures, arguing that Aβ aggregates get “trapped” in the 3D structure rather than being released into the culture medium as they would in 2D and therefore that 3D cultures more accurately model the disease. This assumes that aggregated extracellular species are the toxic entity as opposed to soluble oligomers or intracellular accumulation. However, current drawbacks of 3D modeling include their heterogeneity and lack of developmental maturity[150-152]. Jorfi et al[153] have recently addressed the heterogeneity issue by demonstrating the derivation of more uniform neurospheroids which may be of use in future studies.
One of the major potential applications of AD derived iPSCs is in drug discovery. This relies on the establishment of a reliable and robust readout that associates unequi-vocally with AD pathophysiology that is suitable for screening on high throughput platforms. Various groups have used AD iPSC-derived neurons to test γ-secretase inhibitors, with some efficacy[16,132,154]. Additional drugs that have been tested in this way include docosahexaenoic acid (DHA), which reduces ROS production by an unknown mechanism. Interestingly, treatment with this drug increased the survival time of AD iPSC-derived neurons[53]. Since Aβ-induced toxicity has been linked to aberrant cell cycle re-entry, CDK2 inhibitors[155] and avermectins[156] have also been shown to be effective blockers of Aβ-induced toxicity in AD iPSC-derived neuronal models, although the mechanism of action of avermectins is unknown other than they increase the relative production of shorter Aβ peptides and that this action is unrelated to γ-secretase activity[156]. In addition, a combinatorial approach may be useful. For example, Kondo et al[154] have used human iPSC-derived neurons to identify three drugs (bromocriptine, cromolyn and topiramate) from a screen of 1258 compounds that had the most potent Aβ-reducing effects in both fAD and sAD iPSC-derived neurons.
One particular benefit of iPSC technology is the ability to model the heterogeneity of sAD. Many AD-linked SNPs have been identified by genome-wide association studies[157], and so use of iPSCs may allow particular treatments to be targeted to groups of individuals based on the SNPs they harbor. This field of personalized medicine, known as pharmacogenomics, may mean that drugs that have failed in clinical trials of large cohorts may be effective when applied to specific patient groups (as discussed in[158]).
Human cell models, including those based on iPSCs, are the most appropriate for modelling the human genetic variation underlying sAD since they are derived directly from sAD patient cells. Despite this, disease phenotypes are not observed in all sAD iPSC lines[17,53]. Moreover, some cell lines exhibit extracellular Aβ ac-cumulation whereas other lines exhibit intracellular Aβ and only the latter were responsive to DHA treatment[53], suggesting an additional parameter that should be considered when designing personalized treatments. Part of the issue here understands which of these readouts most reliably reflects meaningful AD patho-physiology. The lack of a “disease phenotype” observed in some cell lines is likely due to the “rejuvenation” of markers of ageing that occurs during iPSC repro-gramming and includes not only epigenetic signatures but also telomere length, mitochondrial function and the levels of oxidative stress[159-161]. To address this challenge it has been suggested that “ageing” could be accelerated in cell cultures by exposure to toxins including hydrogen peroxide or compounds that trigger mitochondrial stress such as CCCP or rotenone[162,163]. Interestingly, it has been suggested that rotenone (an inhibitor of complex I of the respiratory chain) treatment may mimic Parkinson’s disease (PD)[164], again showing similar molecular mechanisms underlying neurodegeneration between AD and PD. Alternatively, the epigenetic signature could be maintained by generating iNs instead of iPSCs as described previously[117,165,166]. Importantly, Mertens et al[167] showed that iNs from donors aged 0-89 retained ageing-associated molecular signatures whereas iPSCs did not. Another potential approach to combat this problem is to overexpress Progerin which re-establishes age-related markers in iPSC-derived fibroblasts and neurons[159].
Despite the huge promise of personalized medicine, therapeutics with wider applicability will be more cost-efficient. Due to the widespread mitochondrial dysfunction observed not only across sAD and fAD but also across various different neurodegenerative disorders it is likely that mitochondrial disease targets may constitute a more global approach.
Recent advances in iPSC technology have highlighted the importance of metabolic dysfunction in the progression of AD. Our hope and expectation is that under-standing the molecular mechanisms underlying this metabolic dysfunction will reveal novel therapeutic targets for this devastating disease[168-177].
| 1. | Sullivan SE, Young-Pearse TL. Induced pluripotent stem cells as a discovery tool for Alzheimer׳s disease. Brain Res. 2017;1656:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 3. | Wavrant-DeVrièze F, Lambert JC, Stas L, Crook R, Cottel D, Pasquier F, Frigard B, Lambrechts M, Thiry E, Amouyel P, Tur JP, Chartier-Harlin MC, Hardy J, Van Leuven F. Association between coding variability in the LRP gene and the risk of late-onset Alzheimer's disease. Hum Genet. 1999;104:432-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Myers A, Holmans P, Marshall H, Kwon J, Meyer D, Ramic D, Shears S, Booth J, DeVrieze FW, Crook R, Hamshere M, Abraham R, Tunstall N, Rice F, Carty S, Lillystone S, Kehoe P, Rudrasingham V, Jones L, Lovestone S, Perez-Tur J, Williams J, Owen MJ, Hardy J, Goate AM. Susceptibility locus for Alzheimer's disease on chromosome 10. Science. 2000;290:2304-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Ertekin-Taner N, Graff-Radford N, Younkin LH, Eckman C, Baker M, Adamson J, Ronald J, Blangero J, Hutton M, Younkin SG. Linkage of plasma Abeta42 to a quantitative locus on chromosome 10 in late-onset Alzheimer's disease pedigrees. Science. 2000;290:2303-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE. Evidence for genetic linkage of Alzheimer's disease to chromosome 10q. Science. 2000;290:2302-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 346] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Olson JM, Goddard KA, Dudek DM. The amyloid precursor protein locus and very-late-onset Alzheimer disease. Am J Hum Genet. 2001;69:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 1747] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 9. | Sengupta U, Nilson AN, Kayed R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine. 2016;6:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 578] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9775] [Cited by in RCA: 10418] [Article Influence: 434.1] [Reference Citation Analysis (2)] |
| 11. | Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4021] [Cited by in RCA: 4545] [Article Influence: 454.5] [Reference Citation Analysis (16)] |
| 13. | Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15 Spec No 2:R188-R195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Šimić G, Babić Leko M, Wray S, Harrington CR, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, de Silva R, Di Giovanni G, Wischik CM, Hof PR. Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol. 2017;151:101-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 15. | Moore S, Evans LD, Andersson T, Portelius E, Smith J, Dias TB, Saurat N, McGlade A, Kirwan P, Blennow K, Hardy J, Zetterberg H, Livesey FJ. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Rep. 2015;11:689-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet. 2014;23:3523-3536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 942] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 18. | Abeti R, Abramov AY, Duchen MR. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain. 2011;134:1658-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 466] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 20. | Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A. 2011;108:5819-5824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 718] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 21. | Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer's disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol Neurobiol. 1994;8:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 858] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 23. | Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VL, Fisher EM, Strydom A. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 24. | Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, Butler AC. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol. 1998;43:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Smith AD. Why are drug trials in Alzheimer's disease failing? Lancet. 2010;376:1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 26. | Wan HI, Jacobsen JS, Rutkowski JL, Feuerstein GZ. Translational medicine lessons from flurizan's failure in Alzheimer's disease (AD) trial: Implication for future drug discovery and development for AD. Clin Transl Sci. 2009;2:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Navarrete LP, Pérez P, Morales I, Maccioni RB. Novel drugs affecting tau behavior in the treatment of Alzheimer's disease and tauopathies. Curr Alzheimer Res. 2011;8:678-685. [PubMed] |
| 28. | Swerdlow RH. Pathogenesis of Alzheimer's disease. Clin Interv Aging. 2007;2:347-359. [PubMed] |
| 29. | Swerdlow RH. Is aging part of Alzheimer's disease, or is Alzheimer's disease part of aging? Neurobiol Aging. 2007;28:1465-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C; Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 654] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 31. | Wiseman FK, Pulford LJ, Barkus C, Liao F, Portelius E, Webb R, Chávez-Gutiérrez L, Cleverley K, Noy S, Sheppard O, Collins T, Powell C, Sarell CJ, Rickman M, Choong X, Tosh JL, Siganporia C, Whittaker HT, Stewart F, Szaruga M; London Down syndrome consortium, Murphy MP, Blennow K, de Strooper B, Zetterberg H, Bannerman D, Holtzman DM, Tybulewicz VLJ, Fisher EMC; LonDownS Consortium. Trisomy of human chromosome 21 enhances amyloid-β deposition independently of an extra copy of APP. Brain. 2018;141:2457-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 548] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 33. | Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670-C686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 501] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 34. | Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1878] [Cited by in RCA: 2036] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 35. | Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim Biophys Acta. 2010;1802:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 36. | Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer's disease cases: evidence for maternal inheritance. Neurology. 1996;47:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Duara R, Lopez-Alberola RF, Barker WW, Loewenstein DA, Zatinsky M, Eisdorfer CE, Weinberg GB. A comparison of familial and sporadic Alzheimer's disease. Neurology. 1993;43:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85:3416-3428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | El-Khoury R, Kaulio E, Lassila KA, Crowther DC, Jacobs HT, Rustin P. Expression of the alternative oxidase mitigates beta-amyloid production and toxicity in model systems. Free Radic Biol Med. 2016;96:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, Martins RN. Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res Brain Res Rev. 2003;43:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Carrillo-Mora P, Luna R, Colín-Barenque L. Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid Med Cell Longev. 2014;2014:795375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Sinha M, Bhowmick P, Banerjee A, Chakrabarti S. Antioxidant role of amyloid β protein in cell-free and biological systems: implication for the pathogenesis of Alzheimer disease. Free Radic Biol Med. 2013;56:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 468] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 44. | Smith MA, Drew KL, Nunomura A, Takeda A, Hirai K, Zhu X, Atwood CS, Raina AK, Rottkamp CA, Sayre LM, Friedland RP, Perry G. Amyloid-beta, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochem Int. 2002;40:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 46. | Horvath S, Garagnani P, Bacalini MG, Pirazzini C, Salvioli S, Gentilini D, Di Blasio AM, Giuliani C, Tung S, Vinters HV, Franceschi C. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 47. | Roth GM, Sun B, Greensite FS, Lott IT, Dietrich RB. Premature aging in persons with Down syndrome: MR findings. AJNR Am J Neuroradiol. 1996;17:1283-1289. [PubMed] |
| 48. | Escobar-Khondiker M, Höllerhage M, Muriel MP, Champy P, Bach A, Depienne C, Respondek G, Yamada ES, Lannuzel A, Yagi T, Hirsch EC, Oertel WH, Jacob R, Michel PP, Ruberg M, Höglinger GU. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci. 2007;27:7827-7837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 49. | Höglinger GU, Lannuzel A, Khondiker ME, Michel PP, Duyckaerts C, Féger J, Champy P, Prigent A, Medja F, Lombes A, Oertel WH, Ruberg M, Hirsch EC. The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. J Neurochem. 2005;95:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Szabados T, Dul C, Majtényi K, Hargitai J, Pénzes Z, Urbanics R. A chronic Alzheimer's model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav Brain Res. 2004;154:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 559] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 52. | Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609-7616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 827] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 53. | Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, Okita K, Takahashi K, Asaka I, Aoi T, Watanabe A, Watanabe K, Kadoya C, Nakano R, Watanabe D, Maruyama K, Hori O, Hibino S, Choshi T, Nakahata T, Hioki H, Kaneko T, Naitoh M, Yoshikawa K, Yamawaki S, Suzuki S, Hata R, Ueno S, Seki T, Kobayashi K, Toda T, Murakami K, Irie K, Klein WL, Mori H, Asada T, Takahashi R, Iwata N, Yamanaka S, Inoue H. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 54. | Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc Natl Acad Sci U S A. 1993;90:10573-10577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Kagan BL, Azimov R, Azimova R. Amyloid peptide channels. J Membr Biol. 2004;202:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Arispe N, Doh M. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AbetaP (1-40) and (1-42) peptides. FASEB J. 2002;16:1526-1536. [PubMed] |
| 57. | Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer's beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem. 2000;275:14077-14083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Abramov AY, Ionov M, Pavlov E, Duchen MR. Membrane cholesterol content plays a key role in the neurotoxicity of β-amyloid: implications for Alzheimer's disease. Aging Cell. 2011;10:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1729] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 60. | Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3268] [Cited by in RCA: 5829] [Article Influence: 647.7] [Reference Citation Analysis (0)] |
| 61. | Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176-9184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 62. | Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 574] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 63. | Love S, Barber R, Wilcock GK. Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer's disease. Brain. 1999;122:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Jagust WJ, Seab JP, Huesman RH, Valk PE, Mathis CA, Reed BR, Coxson PG, Budinger TF. Diminished glucose transport in Alzheimer's disease: dynamic PET studies. J Cereb Blood Flow Metab. 1991;11:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Tarczyluk MA, Nagel DA, Rhein Parri H, Tse EH, Brown JE, Coleman MD, Hill EJ. Amyloid β 1-42 induces hypometabolism in human stem cell-derived neuron and astrocyte networks. J Cereb Blood Flow Metab. 2015;35:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KA, Hooper NM, Vardy ER, Wu D, Unwin RD, Faull RL, Dowsey AW, Cooper GJ. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer's disease: metabolic basis for dementia. Sci Rep. 2016;6:27524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582:359-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 68. | Prapong T, Buss J, Hsu WH, Heine P, West Greenlee H, Uemura E. Amyloid beta-peptide decreases neuronal glucose uptake despite causing increase in GLUT3 mRNA transcription and GLUT3 translocation to the plasma membrane. Exp Neurol. 2002;174:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Swerdlow RH. Mitochondria and Mitochondrial Cascades in Alzheimer's Disease. J Alzheimers Dis. 2018;62:1403-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 616] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 70. | Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D'Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S, Counts SE, Auwerx J. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017;552:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 71. | Sonntag KC, Ryu WI, Amirault KM, Healy RA, Siegel AJ, McPhie DL, Forester B, Cohen BM. Late-onset Alzheimer's disease is associated with inherent changes in bioenergetics profiles. Sci Rep. 2017;7:14038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 72. | Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090-9103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 975] [Cited by in RCA: 1004] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 73. | Martín-Maestro P, Gargini R, García E, Perry G, Avila J, García-Escudero V. Slower Dynamics and Aged Mitochondria in Sporadic Alzheimer's Disease. Oxid Med Cell Longev. 2017;2017:9302761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 74. | Birnbaum JH, Wanner D, Gietl AF, Saake A, Kündig TM, Hock C, Nitsch RM, Tackenberg C. Oxidative stress and altered mitochondrial protein expression in the absence of amyloid-β and tau pathology in iPSC-derived neurons from sporadic Alzheimer's disease patients. Stem Cell Res. 2018;27:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 75. | Du F, Yu Q, Yan S, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, Yan SS. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain. 2017;140:3233-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 76. | Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1769] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 77. | Kumar A, Tamjar J, Waddell AD, Woodroof HI, Raimi OG, Shaw AM, Peggie M, Muqit MM, van Aalten DM. Structure of PINK1 and mechanisms of Parkinson's disease-associated mutations. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Abeti R, Duchen MR. Activation of PARP by oxidative stress induced by β-amyloid: implications for Alzheimer's disease. Neurochem Res. 2012;37:2589-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Briston T, Roberts M, Lewis S, Powney B, M Staddon J, Szabadkai G, Duchen MR. Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci Rep. 2017;7:10492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Canevari L, Abramov AY, Duchen MR. Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res. 2004;29:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 81. | Briston T, Selwood DL, Szabadkai G, Duchen MR. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol Sci. 2019;40:50-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 82. | Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 784] [Cited by in RCA: 769] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 83. | Smilansky A, Dangoor L, Nakdimon I, Ben-Hail D, Mizrachi D, Shoshan-Barmatz V. The Voltage-dependent Anion Channel 1 Mediates Amyloid β Toxicity and Represents a Potential Target for Alzheimer Disease Therapy. J Biol Chem. 2015;290:30670-30683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 84. | Jin S, Kedia N, Illes-Toth E, Haralampiev I, Prisner S, Herrmann A, Wanker EE, Bieschke J. Amyloid-β(1-42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity. J Biol Chem. 2016;291:19590-19606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 85. | Helguera P, Seiglie J, Rodriguez J, Hanna M, Helguera G, Busciglio J. Adaptive downregulation of mitochondrial function in down syndrome. Cell Metab. 2013;17:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 86. | Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 87. | Aliev G, Smith MA, de la Torre JC, Perry G. Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer's disease. Mitochondrion. 2004;4:649-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Canevari L, Clark JB, Bates TE. beta-Amyloid fragment 25-35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett. 1999;457:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Readnower RD, Sauerbeck AD, Sullivan PG. Mitochondria, Amyloid β, and Alzheimer's Disease. Int J Alzheimers Dis. 2011;2011:104545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 585] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 91. | Gibson GE, Karuppagounder SS, Shi Q. Oxidant-induced changes in mitochondria and calcium dynamics in the pathophysiology of Alzheimer's disease. Ann N Y Acad Sci. 2008;1147:221-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 1044] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 93. | Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 94. | Yan SD, Stern DM. Mitochondrial dysfunction and Alzheimer's disease: role of amyloid-beta peptide alcohol dehydrogenase (ABAD). Int J Exp Pathol. 2005;86:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Izzo A, Nitti M, Mollo N, Paladino S, Procaccini C, Faicchia D, Calì G, Genesio R, Bonfiglio F, Cicatiello R, Polishchuk E, Polishchuk R, Pinton P, Matarese G, Conti A, Nitsch L. Metformin restores the mitochondrial network and reverses mitochondrial dysfunction in Down syndrome cells. Hum Mol Genet. 2017;26:1056-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E, Czech C, Götz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A. 2009;106:20057-20062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 549] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 97. | Duncan T, Valenzuela M. Alzheimer's disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 98. | Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L. Aberrant iPSC-derived human astrocytes in Alzheimer's disease. Cell Death Dis. 2017;8:e2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 99. | Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 912] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 100. | Businaro R, Ippoliti F, Ricci S, Canitano N, Fuso A. Alzheimer's disease promotion by obesity: induced mechanisms-molecular links and perspectives. Curr Gerontol Geriatr Res. 2012;2012:986823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Koch P, Tamboli IY, Mertens J, Wunderlich P, Ladewig J, Stüber K, Esselmann H, Wiltfang J, Brüstle O, Walter J. Presenilin-1 L166P mutant human pluripotent stem cell-derived neurons exhibit partial loss of γ-secretase activity in endogenous amyloid-β generation. Am J Pathol. 2012;180:2404-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 102. | Mungenast AE, Siegert S, Tsai LH. Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2016;73:13-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 103. | Evans LD, Wassmer T, Fraser G, Smith J, Perkinton M, Billinton A, Livesey FJ. Extracellular Monomeric and Aggregated Tau Efficiently Enter Human Neurons through Overlapping but Distinct Pathways. Cell Rep. 2018;22:3612-3624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 104. | Karikari TK, Nagel DA, Grainger A, Clarke-Bland C, Hill EJ, Moffat KG. Preparation of stable tau oligomers for cellular and biochemical studies. Anal Biochem. 2019;566:67-74. [PubMed] |
| 105. | Verheyen A, Diels A, Dijkmans J, Oyelami T, Meneghello G, Mertens L, Versweyveld S, Borgers M, Buist A, Peeters P, Cik M. Using Human iPSC-Derived Neurons to Model TAU Aggregation. PLoS One. 2015;10:e0146127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 106. | Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477-486, S1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 627] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 107. | Ochalek A, Mihalik B, Avci HX, Chandrasekaran A, Téglási A, Bock I, Giudice ML, Táncos Z, Molnár K, László L, Nielsen JE, Holst B, Freude K, Hyttel P, Kobolák J, Dinnyés A. Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers Res Ther. 2017;9:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 108. | Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, Tsai LH. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer's Disease Phenotypes. PLoS One. 2016;11:e0161969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 409] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 109. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18593] [Article Influence: 929.7] [Reference Citation Analysis (1)] |
| 110. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14570] [Article Influence: 809.4] [Reference Citation Analysis (0)] |
| 111. | Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 112. | Devineni A, Tohme S, Kody MT, Cowley RA, Harris BT. Stepping back to move forward: a current review of iPSCs in the fight against Alzheimer's disease. Am J Stem Cells. 2016;5:99-106. [PubMed] |
| 113. | Frobel J, Hemeda H, Lenz M, Abagnale G, Joussen S, Denecke B, Sarić T, Zenke M, Wagner W. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports. 2014;3:414-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 114. | Rouhani F, Kumasaka N, de Brito MC, Bradley A, Vallier L, Gaffney D. Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014;10:e1004432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 115. | Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (3)] |
| 116. | Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838-7843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 117. | Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2235] [Cited by in RCA: 2309] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 118. | Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1412] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 119. | Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 657] [Cited by in RCA: 655] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 120. | Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1012] [Article Influence: 63.3] [Reference Citation Analysis (10)] |
| 121. | Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1058] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 122. | Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1169] [Cited by in RCA: 1046] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 123. | Yoshida M, Kitaoka S, Egawa N, Yamane M, Ikeda R, Tsukita K, Amano N, Watanabe A, Morimoto M, Takahashi J, Hosoi H, Nakahata T, Inoue H, Saito MK. Modeling the early phenotype at the neuromuscular junction of spinal muscular atrophy using patient-derived iPSCs. Stem Cell Reports. 2015;4:561-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 124. | Shi Y, Kirwan P, Smith J, MacLean G, Orkin SH, Livesey FJ. A human stem cell model of early Alzheimer's disease pathology in Down syndrome. Sci Transl Med. 2012;4:124ra29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 125. | Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1775] [Cited by in RCA: 1618] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 126. | Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, Kim DS, Park CY, Hwang DY, Kim HS, Kang HC, Kim DW. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44:202-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 127. | Chouraki V, Seshadri S. Genetics of Alzheimer's disease. Adv Genet. 2014;87:245-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 128. | Young JE, Boulanger-Weill J, Williams DA, Woodruff G, Buen F, Revilla AC, Herrera C, Israel MA, Yuan SH, Edland SD, Goldstein LS. Elucidating molecular phenotypes caused by the SORL1 Alzheimer's disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell. 2015;16:373-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 129. | Cook CN, Hejna MJ, Magnuson DJ, Lee JM. Expression of calcipressin1, an inhibitor of the phosphatase calcineurin, is altered with aging and Alzheimer's disease. J Alzheimers Dis. 2005;8:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 130. | Hossini AM, Megges M, Prigione A, Lichtner B, Toliat MR, Wruck W, Schröter F, Nuernberg P, Kroll H, Makrantonaki E, Zouboulis CC, Adjaye J. Induced pluripotent stem cell-derived neuronal cells from a sporadic Alzheimer's disease donor as a model for investigating AD-associated gene regulatory networks. BMC Genomics. 2015;16:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 131. | Duan L, Bhattacharyya BJ, Belmadani A, Pan L, Miller RJ, Kessler JA. Stem cell derived basal forebrain cholinergic neurons from Alzheimer's disease patients are more susceptible to cell death. Mol Neurodegener. 2014;9:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 132. | Lacovich V, Espindola SL, Alloatti M, Pozo Devoto V, Cromberg LE, Čarná ME, Forte G, Gallo JM, Bruno L, Stokin GB, Avale ME, Falzone TL. Tau Isoforms Imbalance Impairs the Axonal Transport of the Amyloid Precursor Protein in Human Neurons. J Neurosci. 2017;37:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 133. | Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20:4530-4539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 134. | Mahairaki V, Ryu J, Peters A, Chang Q, Li T, Park TS, Burridge PW, Talbot CC, Asnaghi L, Martin LJ, Zambidis ET, Koliatsos VE. Induced pluripotent stem cells from familial Alzheimer's disease patients differentiate into mature neurons with amyloidogenic properties. Stem Cells Dev. 2014;23:2996-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 135. | Sproul AA, Jacob S, Pre D, Kim SH, Nestor MW, Navarro-Sobrino M, Santa-Maria I, Zimmer M, Aubry S, Steele JW, Kahler DJ, Dranovsky A, Arancio O, Crary JF, Gandy S, Noggle SA. Characterization and molecular profiling of PSEN1 familial Alzheimer's disease iPSC-derived neural progenitors. PLoS One. 2014;9:e84547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 136. | Yang J, Zhao H, Ma Y, Shi G, Song J, Tang Y, Li S, Li T, Liu N, Tang F, Gu J, Zhang L, Zhang Z, Zhang X, Jin Y, Le W. Early pathogenic event of Alzheimer's disease documented in iPSCs from patients with PSEN1 mutations. Oncotarget. 2017;8:7900-7913. [PubMed] |
| 137. | Armijo E, Gonzalez C, Shahnawaz M, Flores A, Davis B, Soto C. Increased susceptibility to Aβ toxicity in neuronal cultures derived from familial Alzheimer's disease (PSEN1-A246E) induced pluripotent stem cells. Neurosci Lett. 2017;639:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 138. | Liu Q, Waltz S, Woodruff G, Ouyang J, Israel MA, Herrera C, Sarsoza F, Tanzi RE, Koo EH, Ringman JM, Goldstein LS, Wagner SL, Yuan SH. Effect of potent γ-secretase modulator in human neurons derived from multiple presenilin 1-induced pluripotent stem cell mutant carriers. JAMA Neurol. 2014;71:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 139. | Woodruff G, Young JE, Martinez FJ, Buen F, Gore A, Kinaga J, Li Z, Yuan SH, Zhang K, Goldstein LS. The presenilin-1 ΔE9 mutation results in reduced γ-secretase activity, but not total loss of PS1 function, in isogenic human stem cells. Cell Rep. 2013;5:974-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 140. | Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Holst B, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer's disease patient carrying a M146I mutation in PSEN1. Stem Cell Res. 2016;16:334-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Lee HK, Velazquez Sanchez C, Chen M, Morin PJ, Wells JM, Hanlon EB, Xia W. Three Dimensional Human Neuro-Spheroid Model of Alzheimer's Disease Based on Differentiated Induced Pluripotent Stem Cells. PLoS One. 2016;11:e0163072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 142. | Weick JP, Held DL, Bonadurer GF, Doers ME, Liu Y, Maguire C, Clark A, Knackert JA, Molinarolo K, Musser M, Yao L, Yin Y, Lu J, Zhang X, Zhang SC, Bhattacharyya A. Deficits in human trisomy 21 iPSCs and neurons. Proc Natl Acad Sci U S A. 2013;110:9962-9967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 143. | Hibaoui Y, Grad I, Letourneau A, Sailani MR, Dahoun S, Santoni FA, Gimelli S, Guipponi M, Pelte MF, Béna F, Antonarakis SE, Feki A. Modelling and rescuing neurodevelopmental defect of Down syndrome using induced pluripotent stem cells from monozygotic twins discordant for trisomy 21. EMBO Mol Med. 2014;6:259-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 144. | Murray A, Letourneau A, Canzonetta C, Stathaki E, Gimelli S, Sloan-Bena F, Abrehart R, Goh P, Lim S, Baldo C, Dagna-Bricarelli F, Hannan S, Mortensen M, Ballard D, Syndercombe Court D, Fusaki N, Hasegawa M, Smart TG, Bishop C, Antonarakis SE, Groet J, Nizetic D. Brief report: isogenic induced pluripotent stem cell lines from an adult with mosaic down syndrome model accelerated neuronal ageing and neurodegeneration. Stem Cells. 2015;33:2077-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 145. | Dashinimaev EB, Artyuhov AS, Bolshakov AP, Vorotelyak EA, Vasiliev AV. Neurons Derived from Induced Pluripotent Stem Cells of Patients with Down Syndrome Reproduce Early Stages of Alzheimer's Disease Type Pathology in vitro. J Alzheimers Dis. 2017;56:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 146. | Maclean GA, Menne TF, Guo G, Sanchez DJ, Park IH, Daley GQ, Orkin SH. Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc Natl Acad Sci U S A. 2012;109:17567-17572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 147. | Chang CY, Chen SM, Lu HE, Lai SM, Lai PS, Shen PW, Chen PY, Shen CI, Harn HJ, Lin SZ, Hwang SM, Su HL. N-butylidenephthalide attenuates Alzheimer's disease-like cytopathy in Down syndrome induced pluripotent stem cell-derived neurons. Sci Rep. 2015;5:8744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 148. | Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I, Abdelrahim M, Matikainen-Ankney B, Chao SH, Mrksich M, Rakic P, Fang G, Zhang B, Yates JR, Gage FH. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 320] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 149. | Oksanen M, Petersen AJ, Naumenko N, Puttonen K, Lehtonen Š, Gubert Olivé M, Shakirzyanova A, Leskelä S, Sarajärvi T, Viitanen M, Rinne JO, Hiltunen M, Haapasalo A, Giniatullin R, Tavi P, Zhang SC, Kanninen KM, Hämäläinen RH, Koistinaho J. PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer's Disease. Stem Cell Reports. 2017;9:1885-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 150. | Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3083] [Cited by in RCA: 3749] [Article Influence: 288.4] [Reference Citation Analysis (0)] |
| 151. | Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1847] [Cited by in RCA: 1614] [Article Influence: 161.4] [Reference Citation Analysis (1)] |
| 152. | Bershteyn M, Kriegstein AR. Cerebral organoids in a dish: progress and prospects. Cell. 2013;155:19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 153. | Jorfi M, D'Avanzo C, Tanzi RE, Kim DY, Irimia D. Human Neurospheroid Arrays for In Vitro Studies of Alzheimer's Disease. Sci Rep. 2018;8:2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 154. | Kondo T, Imamura K, Funayama M, Tsukita K, Miyake M, Ohta A, Woltjen K, Nakagawa M, Asada T, Arai T, Kawakatsu S, Izumi Y, Kaji R, Iwata N, Inoue H. iPSC-Based Compound Screening and In Vitro Trials Identify a Synergistic Anti-amyloid β Combination for Alzheimer's Disease. Cell Rep. 2017;21:2304-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 155. | Xu X, Lei Y, Luo J, Wang J, Zhang S, Yang XJ, Sun M, Nuwaysir E, Fan G, Zhao J, Lei L, Zhong Z. Prevention of β-amyloid induced toxicity in human iPS cell-derived neurons by inhibition of Cyclin-dependent kinases and associated cell cycle events. Stem Cell Res. 2013;10:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 156. | Brownjohn PW, Smith J, Portelius E, Serneels L, Kvartsberg H, De Strooper B, Blennow K, Zetterberg H, Livesey FJ. Phenotypic Screening Identifies Modulators of Amyloid Precursor Protein Processing in Human Stem Cell Models of Alzheimer's Disease. Stem Cell Reports. 2017;8:870-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 157. | Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O'Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P; European Alzheimer's Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer's Disease; Alzheimer's Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3669] [Cited by in RCA: 3416] [Article Influence: 262.8] [Reference Citation Analysis (1)] |
| 158. | Robbins JP, Price J. Human induced pluripotent stem cells as a research tool in Alzheimer's disease. Psychol Med. 2017;47:2587-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 159. | Marion RM, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, Serrano M, Blasco MA. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 160. | Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, Ellisman MH, Perez GI, Cibelli JB. Mitochondrial rejuvenation after induced pluripotency. PLoS One. 2010;5:e14095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 161. | Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, Taldone T, Fusaki N, Tomishima MJ, Krainc D, Milner TA, Rossi DJ, Studer L. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 2013;13:691-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 596] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 162. | Byers B, Cord B, Nguyen HN, Schüle B, Fenno L, Lee PC, Deisseroth K, Langston JW, Pera RR, Palmer TD. SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS One. 2011;6:e26159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 163. | Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L, Xie Z, Osborn T, Hargus G, Deleidi M, Lawson T, Bogetofte H, Perez-Torres E, Clark L, Moskowitz C, Mazzulli J, Chen L, Volpicelli-Daley L, Romero N, Jiang H, Uitti RJ, Huang Z, Opala G, Scarffe LA, Dawson VL, Klein C, Feng J, Ross OA, Trojanowski JQ, Lee VM, Marder K, Surmeier DJ, Wszolek ZK, Przedborski S, Krainc D, Dawson TM, Isacson O. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci Transl Med. 2012;4:141ra90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 164. | Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34:279-290. [PubMed] |
| 165. | Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, Zhao H, Jin Y, Tang B, Yu Y, Zhao J, Pei G. Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell. 2015;17:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 383] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 166. | Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Südhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1225] [Article Influence: 94.2] [Reference Citation Analysis (15)] |
| 167. | Mertens J, Paquola ACM, Ku M, Hatch E, Böhnke L, Ladjevardi S, McGrath S, Campbell B, Lee H, Herdy JR, Gonçalves JT, Toda T, Kim Y, Winkler J, Yao J, Hetzer MW, Gage FH. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 582] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 168. | Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 169. | Ager RR, Davis JL, Agazaryan A, Benavente F, Poon WW, LaFerla FM, Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss. Hippocampus. 2015;25:813-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 170. | Yun HM, Kim HS, Park KR, Shin JM, Kang AR, il Lee K, Song S, Kim YB, Han SB, Chung HM, Hong JT. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Aβ1-42-infused mouse model of Alzheimer's disease. Cell Death Dis. 2013;4:e958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 171. | Park D, Yang G, Bae DK, Lee SH, Yang YH, Kyung J, Kim D, Choi EK, Choi KC, Kim SU, Kang SK, Ra JC, Kim YB. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J Neurosci Res. 2013;91:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 172. | Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, Wang P, Zhao C, Bi J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res Ther. 2013;4:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 173. | Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, Lim DS, Lee TH, Chopp M, Moon J. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer's disease model. Neurobiol Aging. 2013;34:2408-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 174. | Naaldijk Y, Jäger C, Fabian C, Leovsky C, Blüher A, Rudolph L, Hinze A, Stolzing A. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43:299-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 175. | Oh SH, Kim HN, Park HJ, Shin JY, Lee PH. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer's Disease Model. Cell Transplant. 2015;24:1097-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 176. | Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, Bae JS. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 177. | Tubsuwan A, Pires C, Rasmussen MA, Schmid B, Nielsen JE, Hjermind LE, Hall V, Nielsen TT, Waldemar G, Hyttel P, Clausen C, Kitiyanant N, Freude KK, Holst B. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer's disease patient carrying a L150P mutation in PSEN-1. Stem Cell Res. 2016;16:110-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 178. | Lee SJ, Nam E, Lee HJ, Savelieff MG, Lim MH. Towards an understanding of amyloid-β oligomers: characterization, toxicity mechanisms, and inhibitors. Chem Soc Rev. 2017;46:310-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 422] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang H, Saeki K S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ