©Author(s) (or their employer(s)) 2026.
World J Stem Cells. Feb 26, 2026; 18(2): 113694
Published online Feb 26, 2026. doi: 10.4252/wjsc.v18.i2.113694
Published online Feb 26, 2026. doi: 10.4252/wjsc.v18.i2.113694
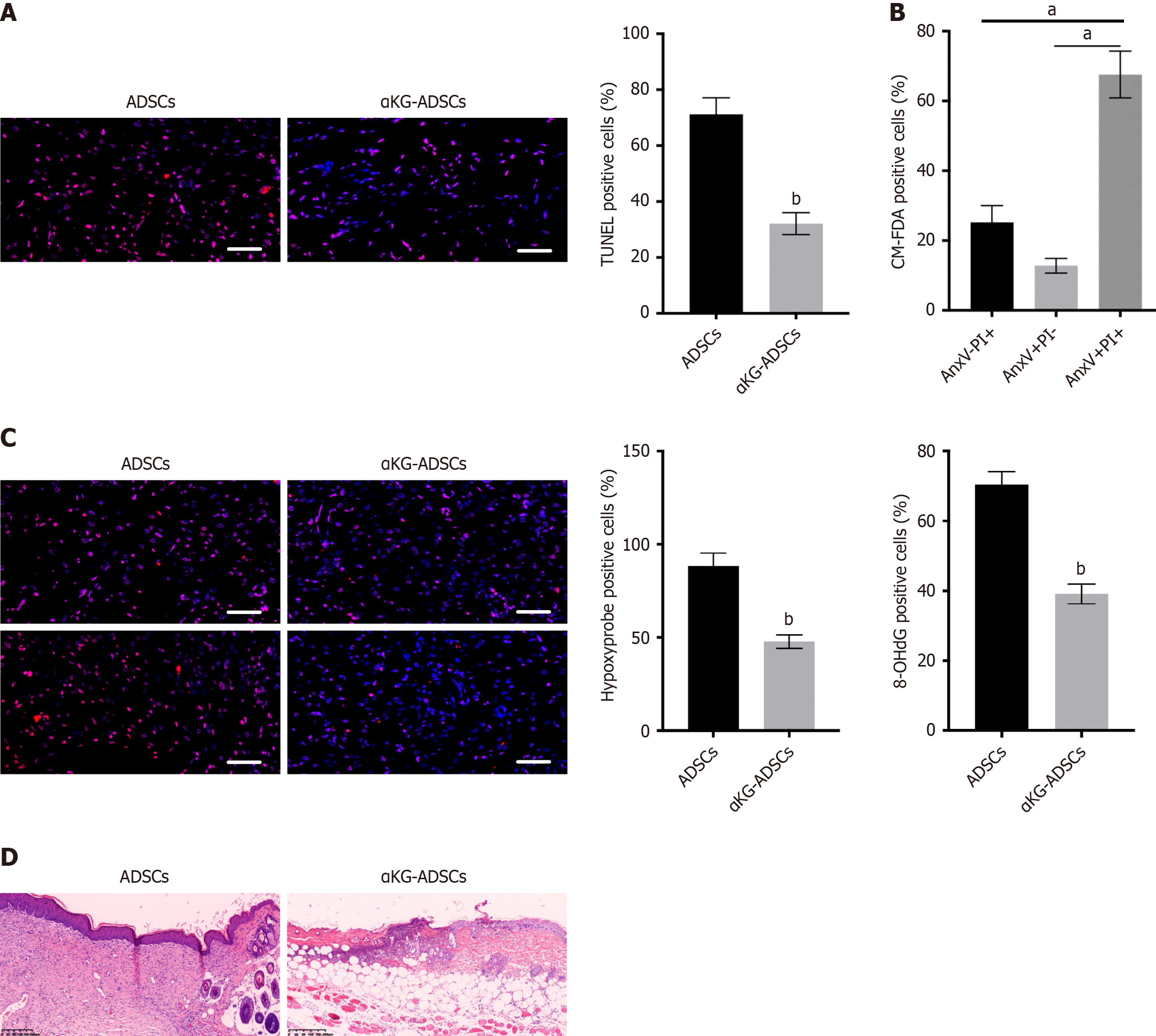
Figure 1 Alpha-ketoglutarate improves cell survival and burn wound healing.
A: Terminal deoxynucleotidyl transferase dUTP nick end labeling immunostaining of the mouse burn wounds treated with adipose-derived stem cells (ADSCs) and α-ketoglutarate-ADSCs (αKG-ADSCs), with quantification; B: Ex vivo annexin V-propidium iodide flow-cytometry analysis of CM-FDA-labeled mouse ADSCs and αKG-ADSCs; C: Representative images of hypoxyprobe (upper panel) and 8-hydroxy-2’-deoxyguanosine (lower panel) immunostaining of mouse ADSCs and αKG-ADSCs, with quantification; D: Hematoxylin-eosin staining of the mouse burn wounds treated with ADSCs and αKG-ADSCs. Data are presented with means ± SEM (aP < 0.05). ADSCs: Adipose-derived stem cells; αKG-ADSCs: Alpha-ketoglutarate adipose-derived stem cells.
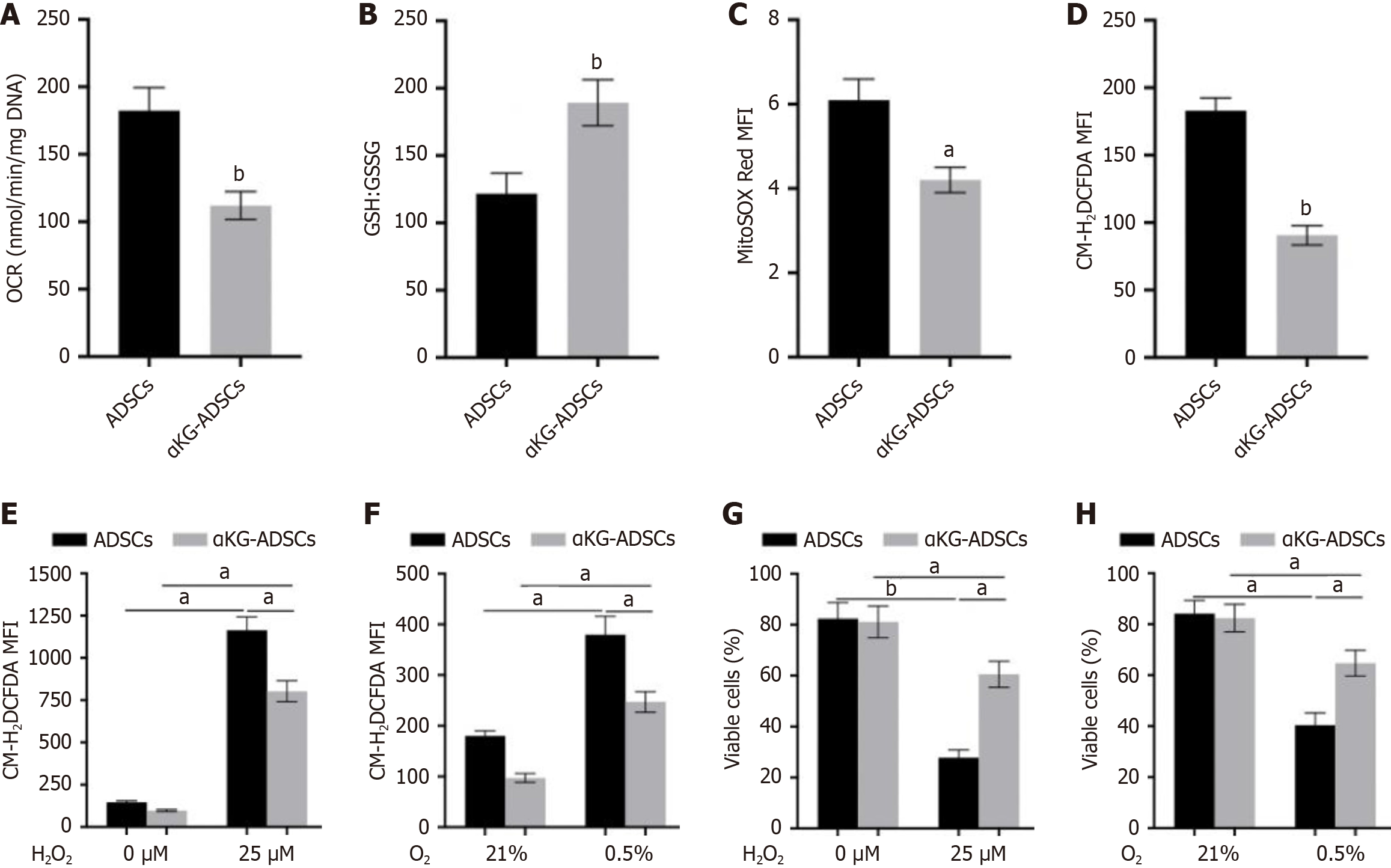
Figure 2 Alpha-ketoglutarate-adipose-derived stem cells are metabolically reprogrammed.
A: Basal oxygen consumption rate of mouse adipose-derived stem cells and α-ketoglutarate adipose-derived stem cells; B: Ratio of reduced glutathione to glutathione disulfide; C and D: Mean fluorescent intensity of mitochondrial (MitoSOX Red) (C) or intracellular reactive oxygen species levels (CM-H2DCFDA) (D) by flow-cytometry analysis; E and F: Intracellular reactive oxygen species levels after treatment with 25 μmol/L H2O2 (E) or culture in 0.5% O2 (F); G and H: Cell viability analysis after treatment with 25 μmol/L H2O2 (G) or culture in 0.5% O2 (H), by annexin V-propidium iodide flow cytometry. Data are presented with means ± SEM (aP < 0.05, bP < 0.01). OCR: Oxygen consumption rate; ADSCs: Adipose-derived stem cells; αKG-ADSCs: Alpha-ketoglutarate adipose-derived stem cells; GSH: Glutathione; GSSG: Glutathione disulfide; MFI: Mean fluorescent intensity.
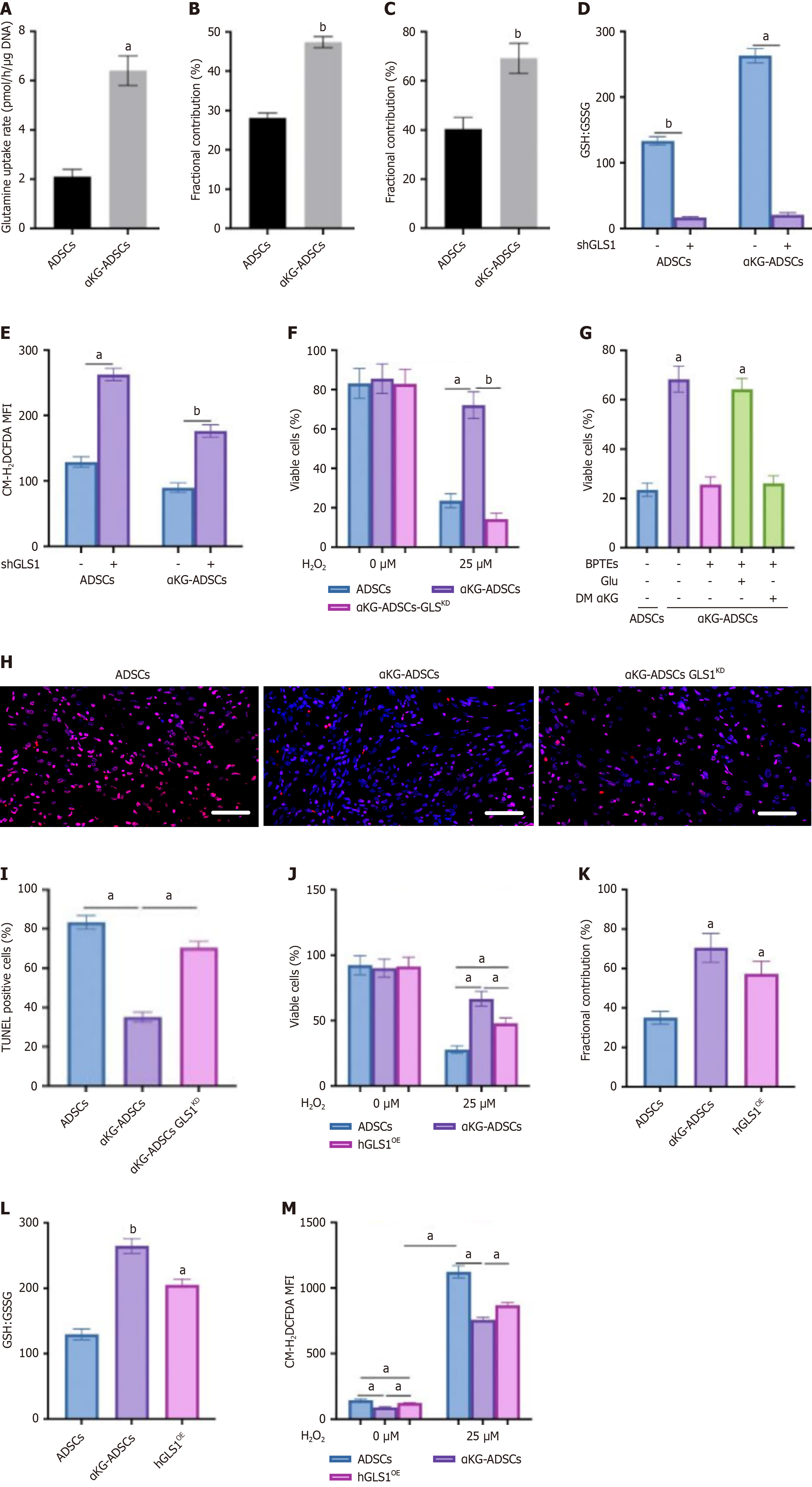
Figure 3 GLS1 regulates glutamine-mediated reactive oxygen species scavenging in adipose-derived stem cells treated with α-ketoglutarate and contributes to cell survival.
A: Glutamine uptake rate in mouse adipose-derived stem cells (ADSCs) and α-ketoglutarate-ADSCs (αKG-ADSCs); B and C: Fractional contribution of [U-13C]-glutamine to glutamate (B) and glutathione (GSH) (C) in mouse ADSCs and αKG-ADSCs; D and E: GSH to GSSG disulfide levels (D) and total intracellular reactive oxygen species levels (E) in mouse ADSCs and αKG-ADSCs after genetic silencing of GLS1 [scrambled shRNA(-) or shGLS1 (+)]; F: Cell viability of mouse ADSCs and αKG-ADSCs after treatment with 25 μmol/L H2O2. αKG-ADSCs-GLSKD are αKG-ADSCs transduced with shGLS1; G: Rescue of cell viability of BPTES-treated αKG-ADSCs cells during oxidative stress (25 μmol/L H2O2) with glutamate, but not with dimethyl α-ketoglutarate; H and I: Terminal deoxynucleotidyl transferase dUTP nick end labeling immunostaining (H) of the mouse burn wounds treated with ADSCs, αKG-ADSCs, and ADSCs-GLSKD, with quantification (I); J: Cell viability of ADSCs, αKG-ADSCs, and hGLS1-overexpressing (hGLS1OE) αKG-ADSCs after treatment with 25 μmol/L H2O2; K: Fractional contribution of [U-13C]-glutamine to GSH; L: Ratio of GSH to GSH disulfide; M: Total intracellular reactive oxygen species levels with or without H2O2 treatment. Data are presented with means ± SEM (aP < 0.05, bP < 0.01). ADSCs: Adipose-derived stem cells; αKG-ADSCs: Alpha-ketoglutarate adipose-derived stem cells; GSH: Glutathione; GSSG: Glutathione disulfide; MFI: Mean fluorescent intensity; DM-αKG: Dimethyl α-ketoglutarate; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
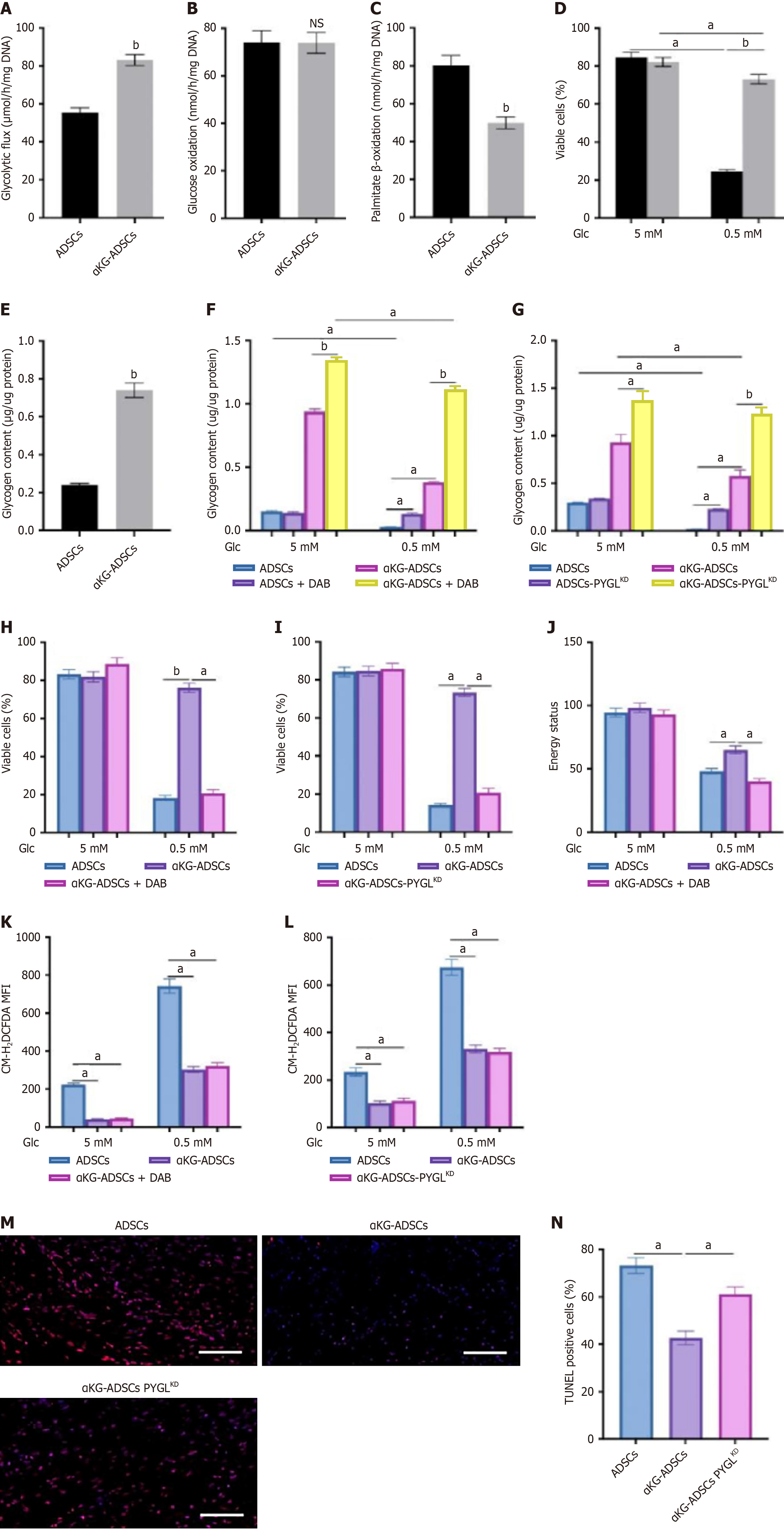
Figure 4 Glycogen storage contributes to survival of adipose-derived stem cells treated with α-ketoglutarate via energy supply.
A: Glycolytic flux of mouse adipose-derived stem cells (ADSCs) and α-ketoglutarate-ADSCs (αKG-ADSCs); B: Glucose oxidation rate; C: Palmitate β-oxidation; D: Cell viability during normal (5 mmol/L glucose) or glucose-deprived conditions (0.5 mmol/L glucose); E: Intracellular glycogen deposition; F and G: Cellular glycogen content after inhibition of PYGL with 1,4-dideoxy-1,4-imino-d-arabinitol (DAB) (F) or shRNA (PYGLKD) (G); H and I: Cell viability of ADSCs, αKG-ADSCs, and αKG-ADSCs after inhibition of PYGL with DAB (H) or shRNA (PYGLKD) (I); J: Energy status (ratio of ATP to AMP levels); K and L: Intracellular reactive oxygen species levels in ADSCs, αKG-ADSCs, and αKG-ADSCs after inhibition of PYGL with DAB (K) or shRNA (PYGLKD) (L); M and N: Terminal deoxynucleotidyl transferase dUTP nick end labeling immunostaining (M) at day 3 after cell implantation, with quantification of TUNEL+ cells (N). Data are presented with means ± SEM (aP < 0.05, bP < 0.01, NS: Not significant). ADSCs: Adipose-derived stem cells; αKG-ADSCs: Alpha-ketoglutarate adipose-derived stem cells; MFI: Mean fluorescent intensity; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
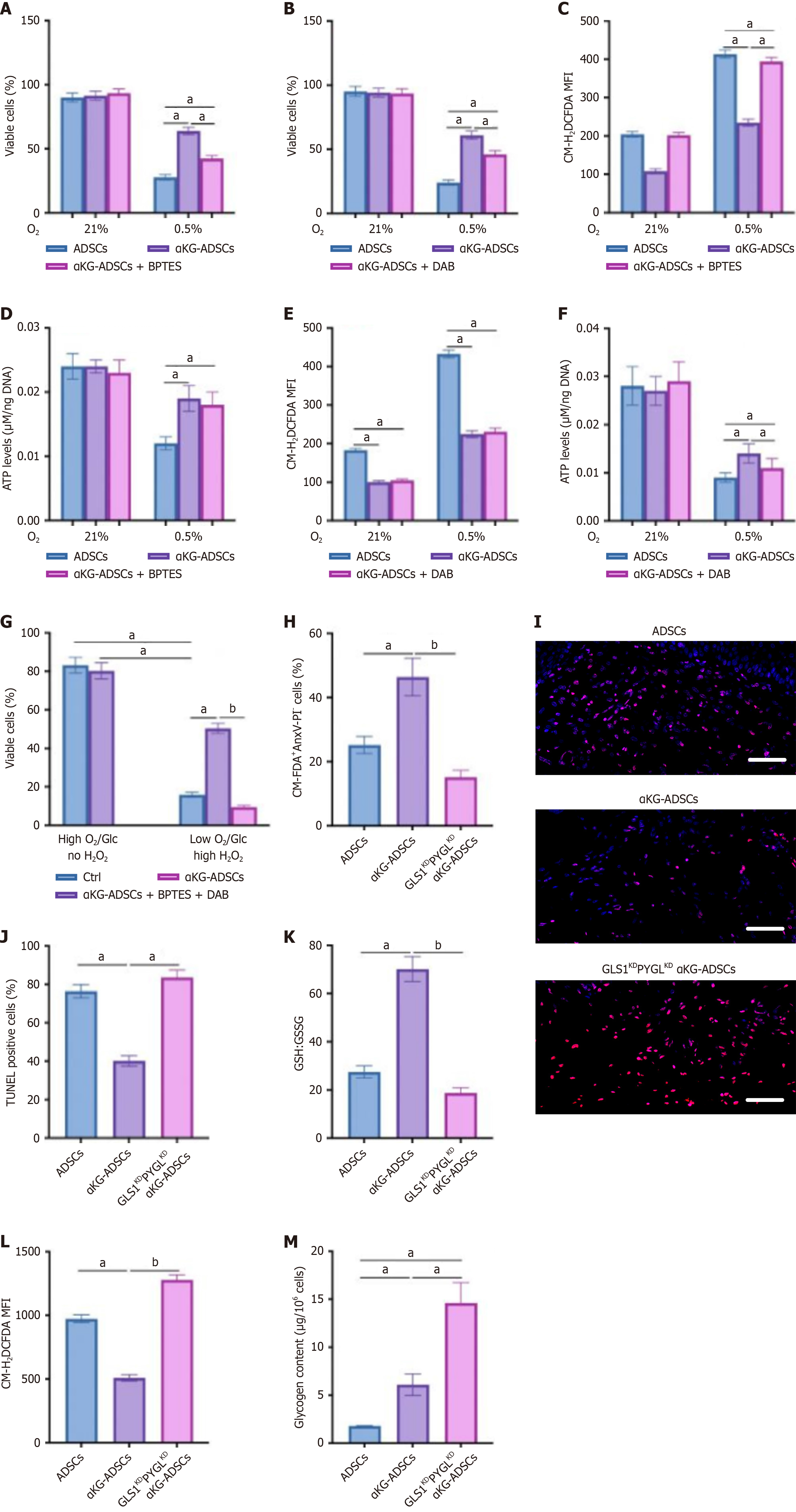
Figure 5 The combinational changes in glutamine and glycogen metabolism are necessary for cell viability in α-ketoglutarate-adipose-derived stem cells.
A and B: Analysis of cell viability of adipose-derived stem cells (ADSCs), α-ketoglutarate-ADSCs (αKG-ADSCs), and αKG-ADSCs treated with BPTES (A) or 1,4-dideoxy-1,4-imino-d-arabinitol (DAB) (B) during hypoxia (0.5% O2); C-F: Intracellular reactive oxygen species levels (C and E) and quantification of intracellular ATP levels (D and F) in ADSCs, αKG-ADSCs, and αKG-ADSCs treated with BPTES (C and D) or DAB (E and F) during hypoxia (0.5% O2); G: Cell viability of ADSCs, αKG-ADSCs, and αKG-ADSCs treated with BPTES and DAB during normal or combined stress conditions (1% O2), 1 mmol/L glucose, and 12 mmol/L H2O2 (high H2O2); H: Cell viability of αKG-ADSCs in 3 days after in vivo ectopic ADSCs implantation. Viable cells are CM-FDA+AnxV-PI-; I and J: Terminal deoxynucleotidyl transferase dUTP nick end labeling immunostaining (I) at day 3 after cell implantation, with quantification of the percentage of TUNEL+ cells (J); K: Ratio of glutathione to glutathione disulfide in implanted ADSCs, αKG-ADSCs, and GLS1KDPYGLKD αKG-ADSCs; L: In vivo intracellular reactive oxygen species levels in implanted ADSCs, αKG-ADSCs, and GLS1KDPYGLKD αKG-ADSCs; M: Glycogen content in implanted ADSCs, αKG-ADSCs, and GLS1KDPYGLKD αKG-ADSCs. Data are presented with means ± SEM (aP < 0.05, bP < 0.01). ADSCs: Adipose-derived stem cells; αKG-ADSCs: Alpha-ketoglutarate adipose-derived stem cells; GSH: Glutathione; GSSG: Glutathione disulfide; MFI: Mean fluorescent intensity; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
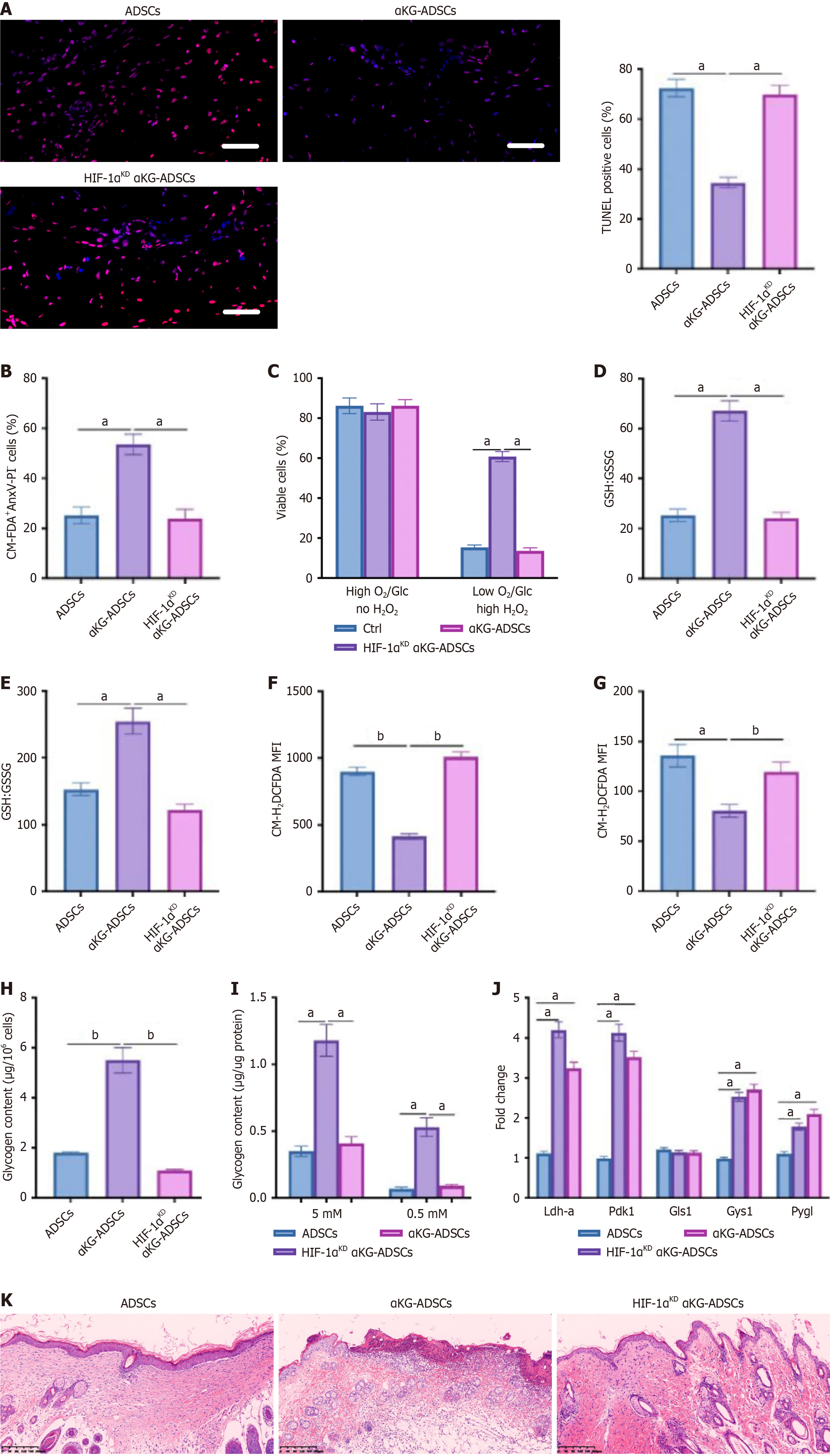
Figure 6 Hypoxia-inducible factor 1-alpha promotes cell survival, burn wound healing, and angiogenesis in α-ketoglutarate-adipose-derived stem cells.
A: Terminal deoxynucleotidyl transferase dUTP nick end labeling immunostaining at day 3 after cell implantation, with quantification of TUNEL+ cells; B: Ex vivo cell viability of CM-FDA-labeled cells 3 days after implantation. Viable cells are CM-FDA+AnxV-PI-; C: Cell viability analysis on cells cultured in combined stress conditions [1% O2, 1 mmol/L glucose, 12 mmol/L H2O2 (high H2O2)]. Viable cells are AnxV-PI-; D and E: Ratio of glutathione to glutathione disulfide in in vivo implanted (D) or in vitro cultured (E) adipose-derived stem cells (ADSCs), α-ketoglutarate-ADSCs (αKG-ADSCs), and HIF-1αKD αKG-ADSCs; F and G: In vivo intracellular reactive oxygen species levels in ADSCs, αKG-ADSCs, and HIF-1αKD αKG-ADSCs (F) or in vitro cultured cells (G); H: Glycogen content of implanted ADSCs, αKG-ADSCs, and HIF-1αKD αKG-ADSCs; I: Intracellular glycogen deposition determined after extraction from cells during normal culture conditions or glucose deprivation; J: Fold enrichment of HIF-1β binding to promoter regions of Ldh-a, Pdk1, Gls1, Gys1, and Pygl in ADSCs, αKG-ADSCs, and HIF-1αKD αKG-ADSCs, as determined by chromatin immunoprecipitation followed by quantitative polymerase chain reaction; K: Hematoxylin-eosin staining of the mouse burn wounds treated with ADSCs, αKG-ADSCs, and HIF-1αKD αKG-ADSCs. Data are presented with means ± SEM (aP < 0.05, bP < 0.01). ADSCs: Adipose-derived stem cells; αKG-ADSCs: Alpha-ketoglutarate adipose-derived stem cells; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling; GSH: Glutathione; GSSG: Glutathione disulfide; MFI: Mean fluorescent intensity; HIF-1α: Hypoxia-inducible factor 1-alpha.
- Citation: Dilimulati D, Dilimulati D, Cui L. Alpha-ketoglutarate enhances adipose-derived stem cells survival in wound healing by hypoxia-inducible factor 1-alpha-mediated redox homeostasis and glycogen-dependent bioenergetics. World J Stem Cells 2026; 18(2): 113694
- URL: https://www.wjgnet.com/1948-0210/full/v18/i2/113694.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v18.i2.113694