©The Author(s) 2025.
World J Stem Cells. Aug 26, 2025; 17(8): 107124
Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107124
Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107124
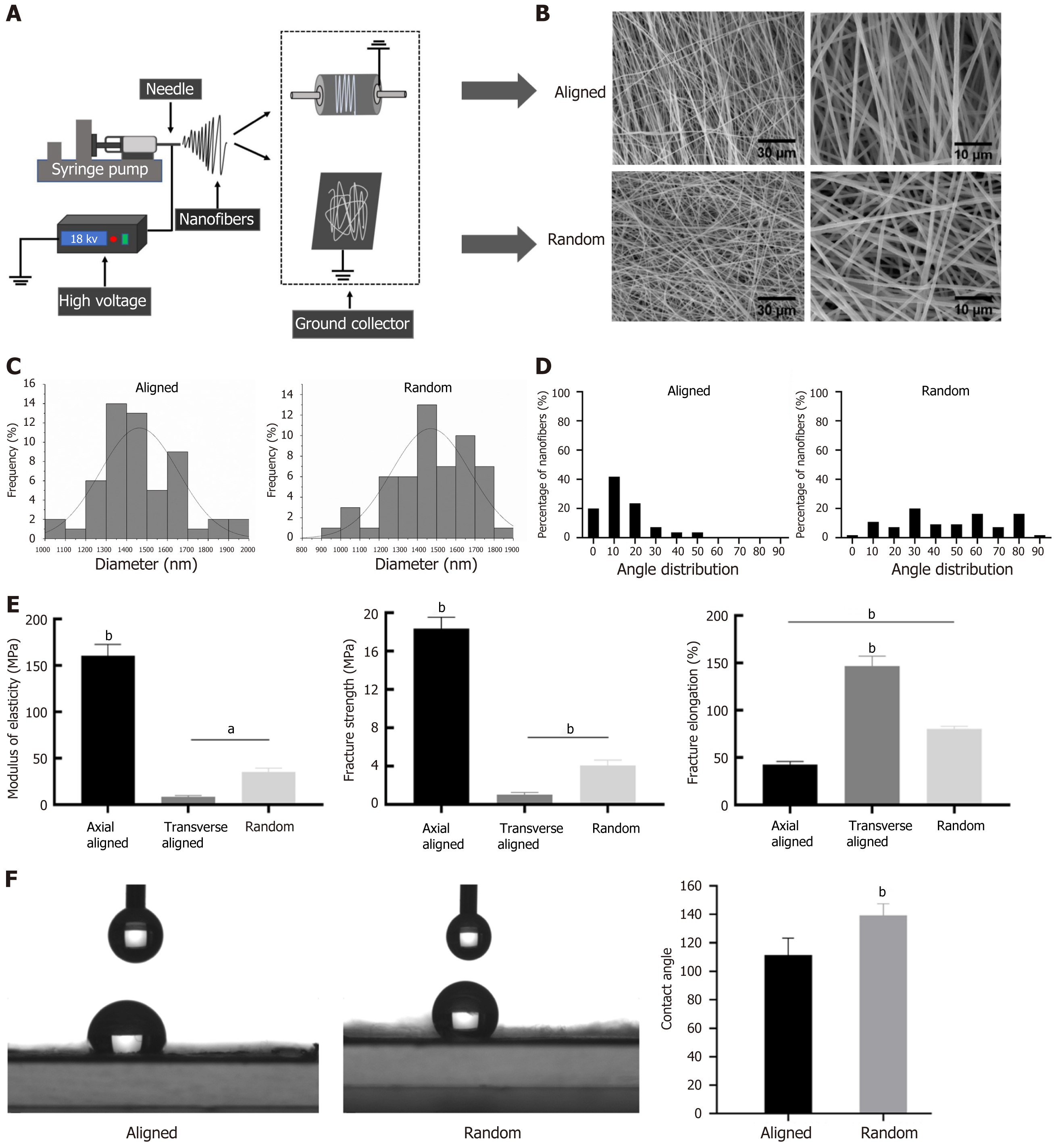
Figure 1 Preparation and performance testing of random and aligned nanofiber scaffolds.
A: Schematic of the electrospinning setup used for fabricating random and aligned nanofiber scaffolds, utilizing a rotating collector for aligned fibers and a stationary collector for random fibers; B: Scanning electron microscopy images showing the morphology of aligned (top) and random (bottom) nanofiber scaffolds, with aligned fibers displaying a parallel structure and random fibers exhibiting a disordered configuration. Scale bars: 30 μm and 10 μm; C: Diameter distribution of fibers in both types of scaffolds, showing consistent diameters; D: Angle distribution of fibers, with aligned fibers having a narrow angular dispersion, confirming uniform orientation, while random fibers display a broader angle range; E: Mechanical properties measured as modulus of elasticity, fracture strength, and fracture elongation, with aligned scaffolds showing higher values compared to transverse aligned and random scaffolds; F: Contact angle measurements showing differences in surface wettability, with random fibers having a higher contact angle, indicative of greater hydrophobicity. aP < 0.05, bP < 0.01.
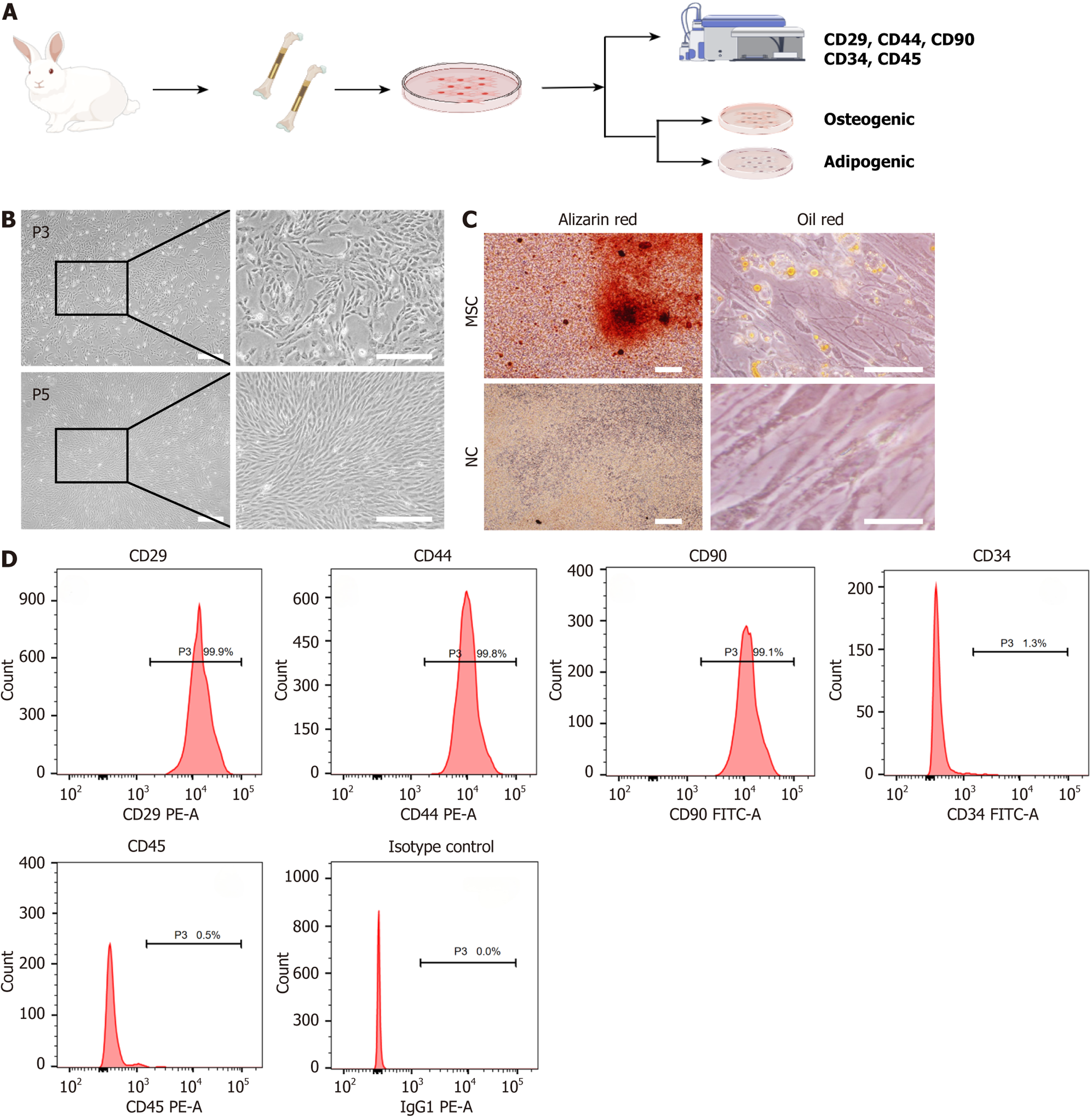
Figure 2 Isolation and characterization of rabbit mesenchymal stem cells.
A: Schematic representation of the mesenchymal stem cell (MSC) isolation and culture process from rabbit bone tissue, followed by phenotypic characterization through surface markers and differentiation assays; B: Phase-contrast images of MSCs at passage 3 and passage 5 showing spindle-shaped morphology. Scale bar: 100 μm; C: Differentiation assays demonstrating osteogenic and adipogenic potential, with positive Alizarin Red staining for osteogenesis and Oil Red O staining for adipogenesis in the MSC group; the negative control shows no staining. Scale bar: 100 μm; D: Flow cytometry analysis of surface markers, showing high expression of CD29, CD44, and CD90, and low expression of CD34 and CD45, confirming the MSC phenotype. P3: Passage 3; P5: Passage 5; MSC: Mesenchymal stem cell; NC: Negative control.
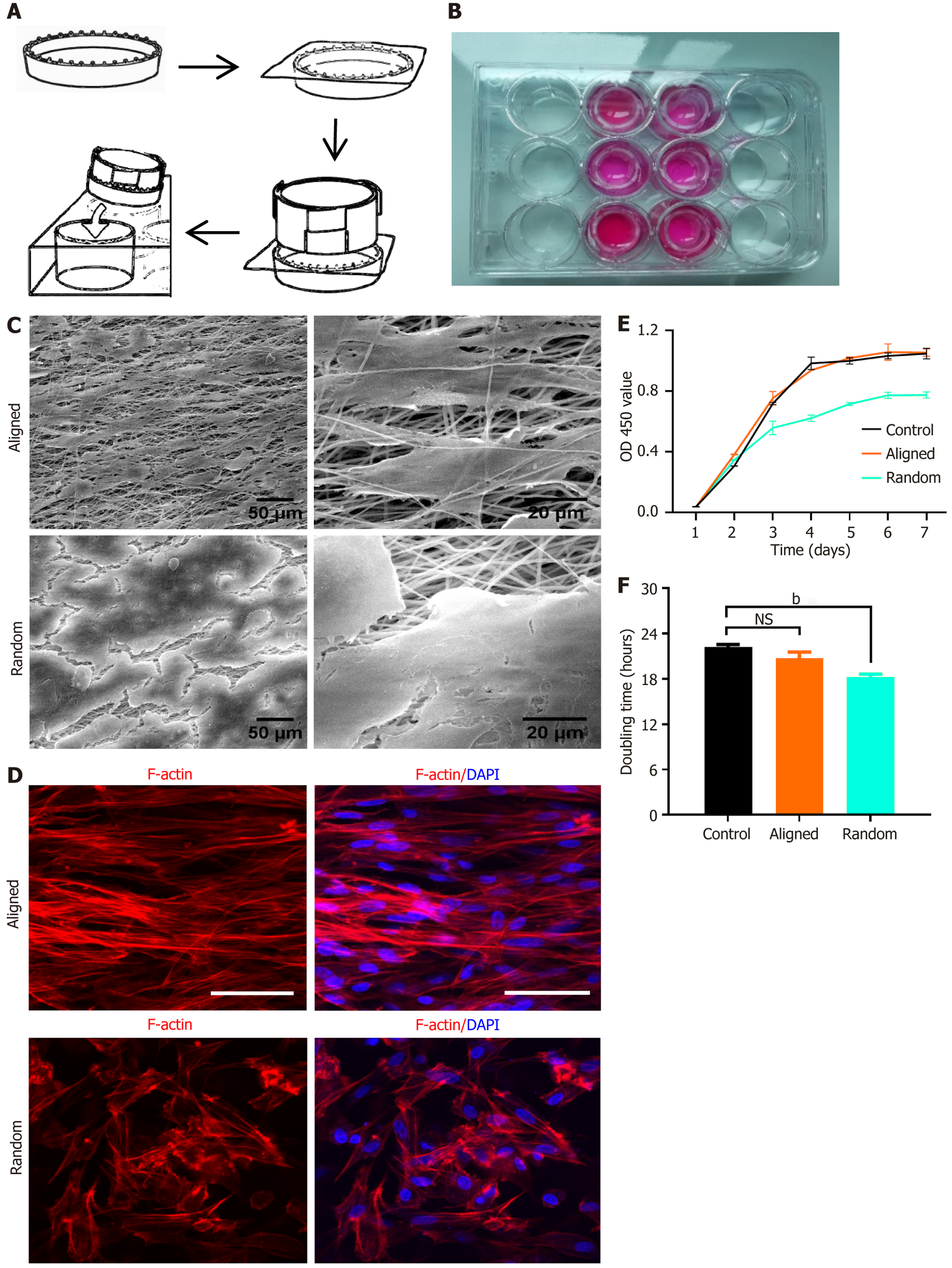
Figure 3 Bone marrow-derived mesenchymal stem cell seeding, morphology, and viability on aligned and random nanofiber scaffolds.
A: Schematic illustration of bone marrow-derived mesenchymal stem cell (BMSC) seeding on aligned and random nanofiber scaffolds placed in culture wells; B: Image of culture wells containing scaffolds with uniform BMSC seeding; C: Scanning electron microscopy images of aligned (top) and random (bottom) nanofiber scaffolds, highlighting organized orientation in aligned fibers and disordered structure in random fibers. Scale bars: 50 μm and 20 μm; D: Immunofluorescent staining for F-actin (red) and nuclei (4’,6-diamidino-2-phenylindole, blue) illustrating BMSC morphology and cytoskeletal arrangement on aligned vs random scaffolds. BMSCs on aligned scaffolds show an elongated, aligned cytoskeletal organization, while those on random scaffolds exhibit a more dispersed morphology; E: Cell viability assay using CCK-8 over 7 days, showing OD values at 450 nm for BMSCs on aligned, random, and control surfaces; F: Doubling time analysis of BMSCs on each substrate, with aligned scaffolds supporting faster proliferation than the random and control conditions. bP < 0.01, NS: Not significant. DAPI: 4’,6-diamidino-2-phenylindole.
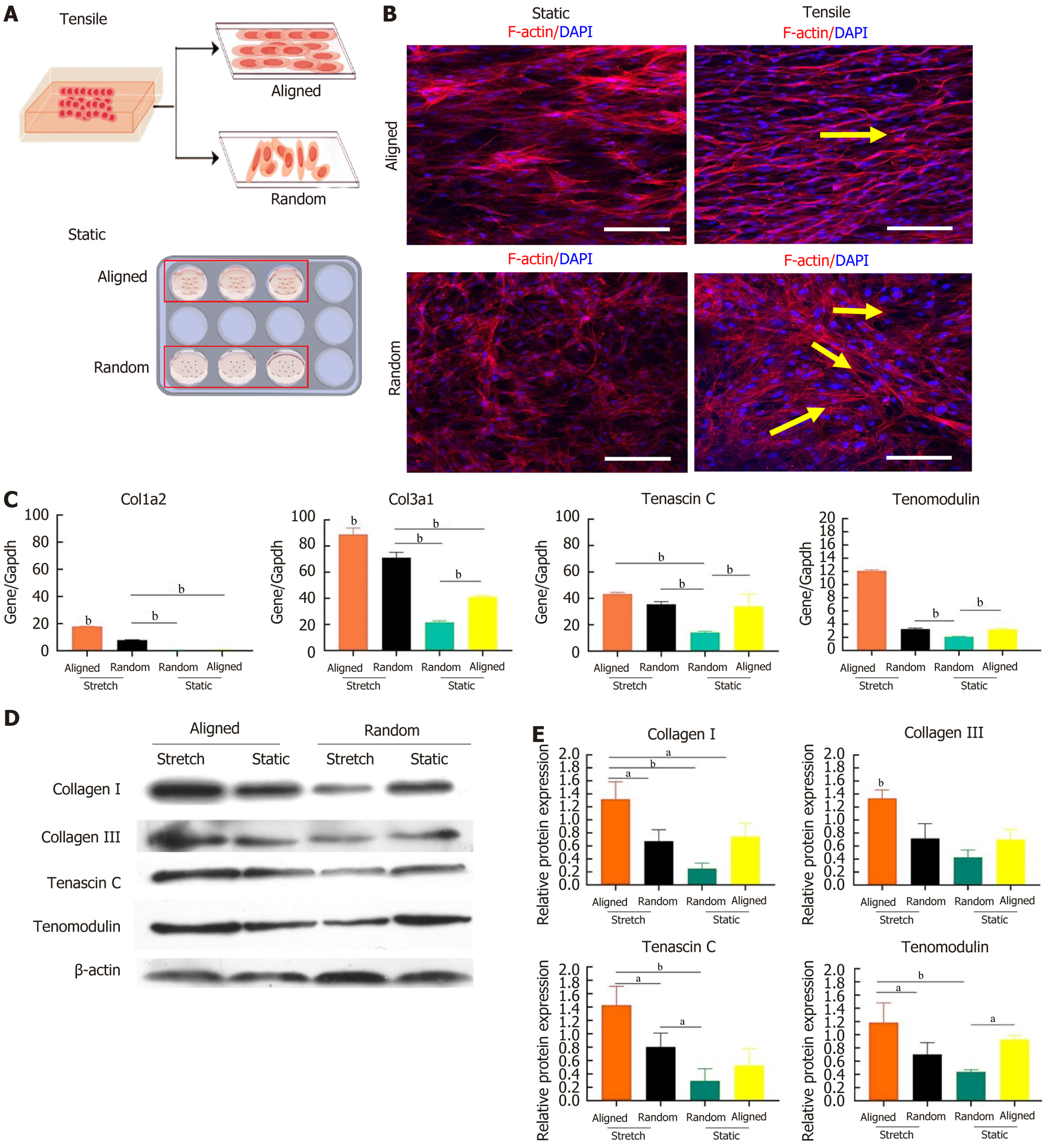
Figure 4 Effects of static and tensile conditions on bone marrow-derived mesenchymal stem cell alignment and tenogenic differentiation on aligned and random nanofiber scaffolds.
A: Schematic showing the experimental setup for culturing bone marrow-derived mesenchymal stem cells (BMSCs) on aligned and random nanofiber scaffolds under static and tensile strain conditions; B: Immunofluorescent staining of F-actin (red) and nuclei (4’,6-diamidino-2-phenylindole, blue) illustrating cytoskeletal alignment of BMSCs in response to static and tensile conditions. Under tensile strain, BMSCs on both scaffold types align along the direction of strain (yellow arrows). Scale bars: 50 μm; C: Gene expression levels of tenogenic markers collagen type I alpha 2, collagen type III alpha 1, tenascin C, and tenomodulin in BMSCs on aligned and random scaffolds under static and tensile conditions, showing upregulation of these markers with tensile strain; D: Western blot analysis of tenogenic proteins (collagen I, collagen III, tenascin C, and tenomodulin) in BMSCs cultured on aligned and random scaffolds under static and tensile conditions, with β-actin as the loading control; E: Quantified protein expression levels from western blot, demonstrating scaffold orientation and mechanical strain effects on tenogenic protein expression. aP < 0.05, bP < 0.01. DAPI: 4’,6-diamidino-2-phenylindole; Col1a2: Collagen type I alpha 2; Col3a1: Collagen type III alpha 1.
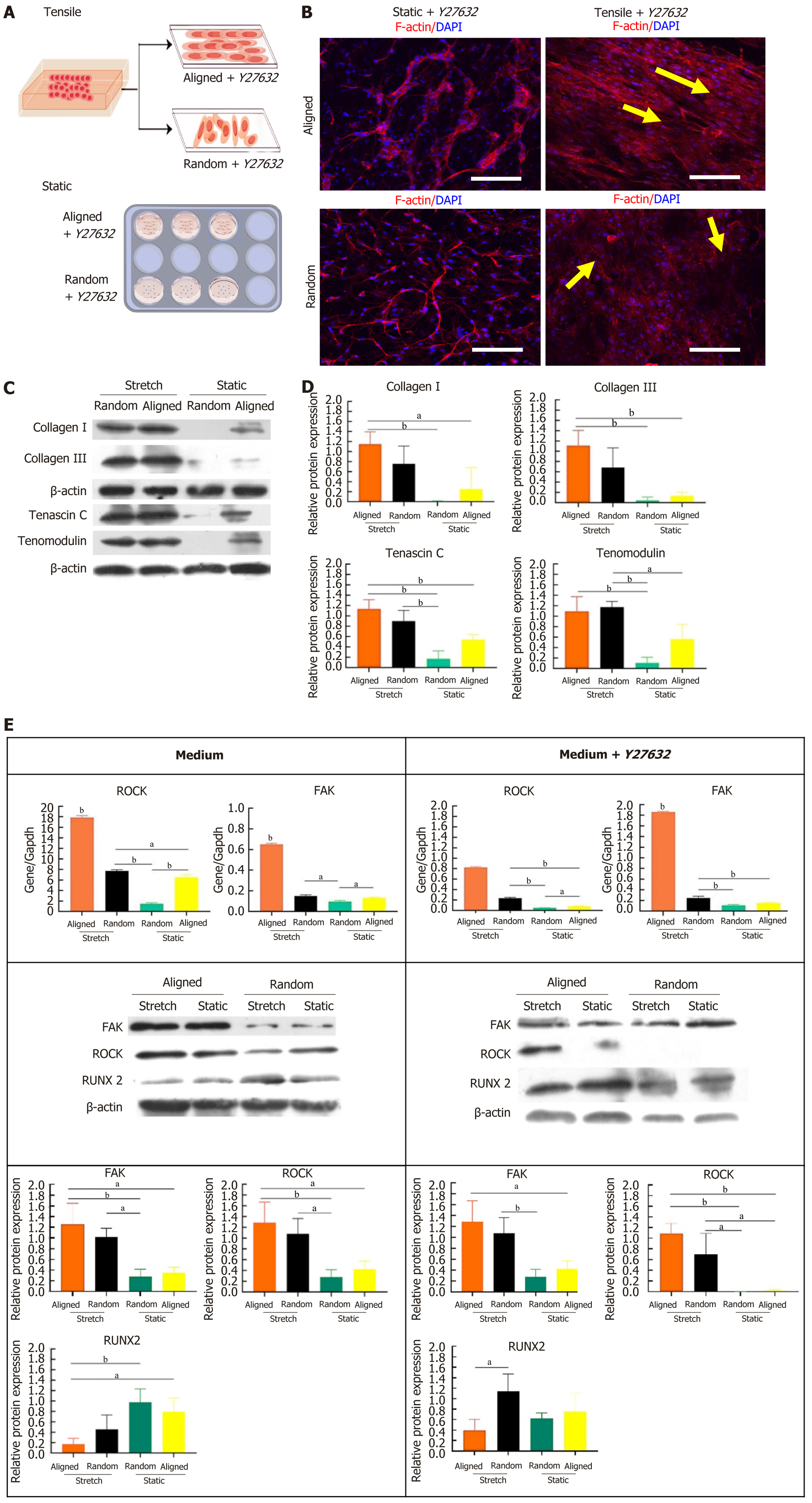
Figure 5 Effects of Ras homolog gene family (Rho)-associated coiled coil-containing kinase inhibition with Y27632 on bone marrow-derived mesenchymal stem cell tenogenic differentiation and cytoskeletal organization under static and tensile conditions.
A: Schematic showing the experimental setup for culturing bone marrow-derived mesenchymal stem cell (BMSCs) on aligned and random nanofiber scaffolds under static and tensile conditions with Y27632 treatment; B: Immunofluorescent staining of F-actin (red) and nuclei (4’,6-diamidino-2-phenylindole, blue) illustrating the cytoskeletal alignment of BMSCs in response to static and tensile conditions with Y27632. Under tensile strain, BMSCs on both scaffold types align along the strain direction (yellow arrows). Scale bars: 50 μm; C: Western blot analysis of tenogenic proteins (collagen I, collagen III, tenascin C, and tenomodulin) in BMSCs on aligned and random scaffolds under static and tensile conditions with Y27632, with β-actin as the loading control; D: Quantification of protein expression levels, showing scaffold orientation and mechanical strain effects on tenogenic protein expression in the presence of Y27632; E: Expression analysis of Ras homolog gene family (Rho)-associated coiled coil-containing kinase, focal adhesion kinase, and runt-related transcription factor 2 at both gene and protein levels, demonstrating the effects of Y27632 on tenogenic signaling pathways in BMSCs under static and tensile conditions on aligned and random scaffolds. aP < 0.05, bP < 0.01. DAPI: 4’,6-diamidino-2-phenylindole; ROCK: Ras homolog gene family (Rho)-associated coiled coil-containing kinase; FAK: Focal adhesion kinase; RUNX2: Runt-related transcription factor 2.
- Citation: Yang CW, Zhang YQ, Chang H, Gao R, Chen D, Yao H. Aligned nanofiber scaffolds combined with cyclic stretch facilitate mesenchymal stem cell differentiation for ligament engineering. World J Stem Cells 2025; 17(8): 107124
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/107124.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.107124