©The Author(s) 2024.
World J Stem Cells. Jun 26, 2024; 16(6): 641-655
Published online Jun 26, 2024. doi: 10.4252/wjsc.v16.i6.641
Published online Jun 26, 2024. doi: 10.4252/wjsc.v16.i6.641
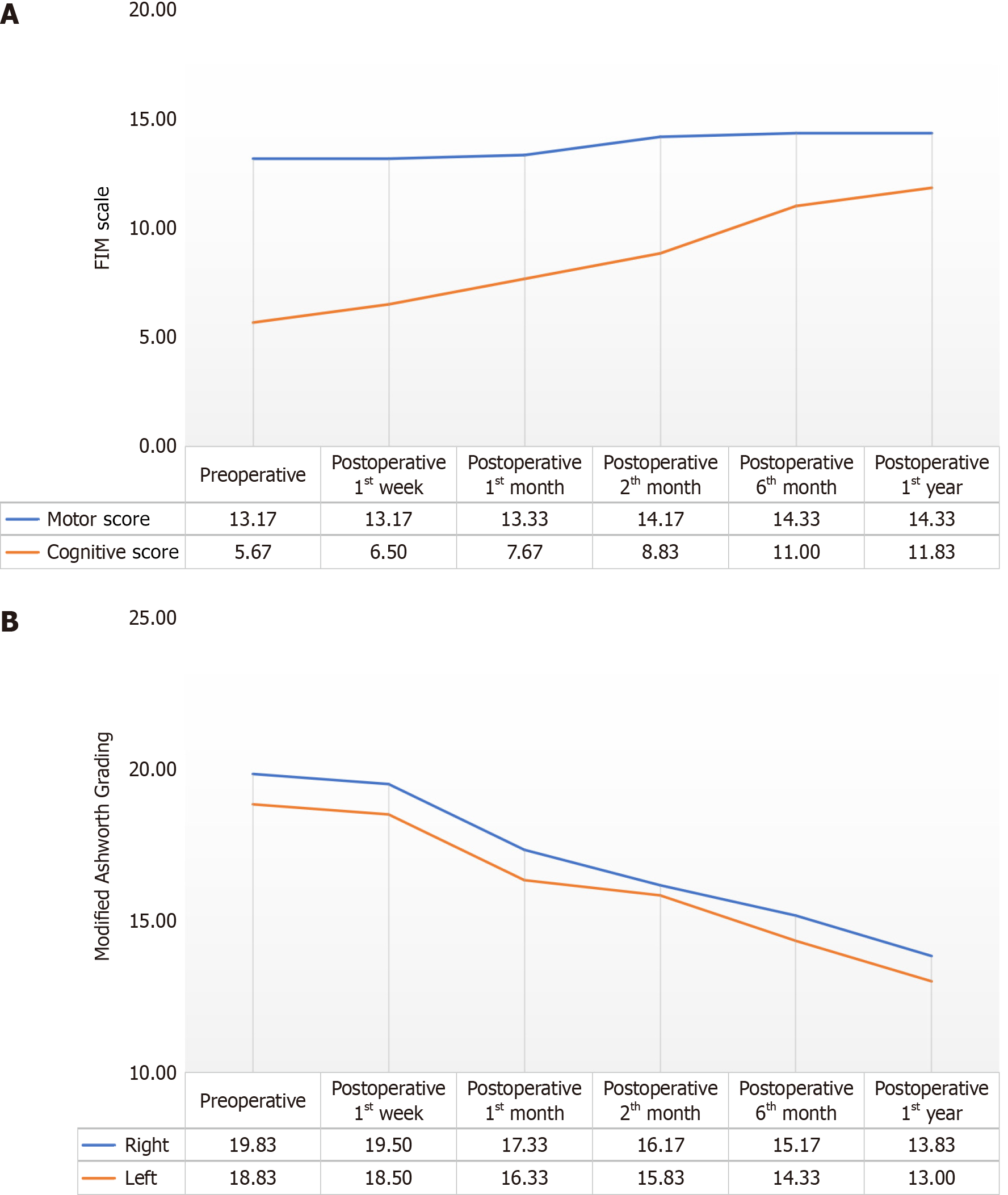
Figure 1 The changes observed in the patients’ average scores of Functional Independence Measure Motor and Cognitive Score values and Modified Ashworth Score right and left values before the procedure, 1st wk after the procedure, and at the 1st month, 2nd month, 6th month and 1st year.
A: The changes observed in the patients’ average scores of Functional Independence Measure Motor and Cognitive Score values before the procedure, 1st wk after the procedure, and at the 1st month, 2nd month, 6th month and 1st year; B: The changes observed in the patients’ average scores of Modified Ashworth Score right and left values before the procedure, 1st wk after the procedure, and at the 1st month, 2nd month, 6th month and 1st year. FIM: Functional Independence Measure.
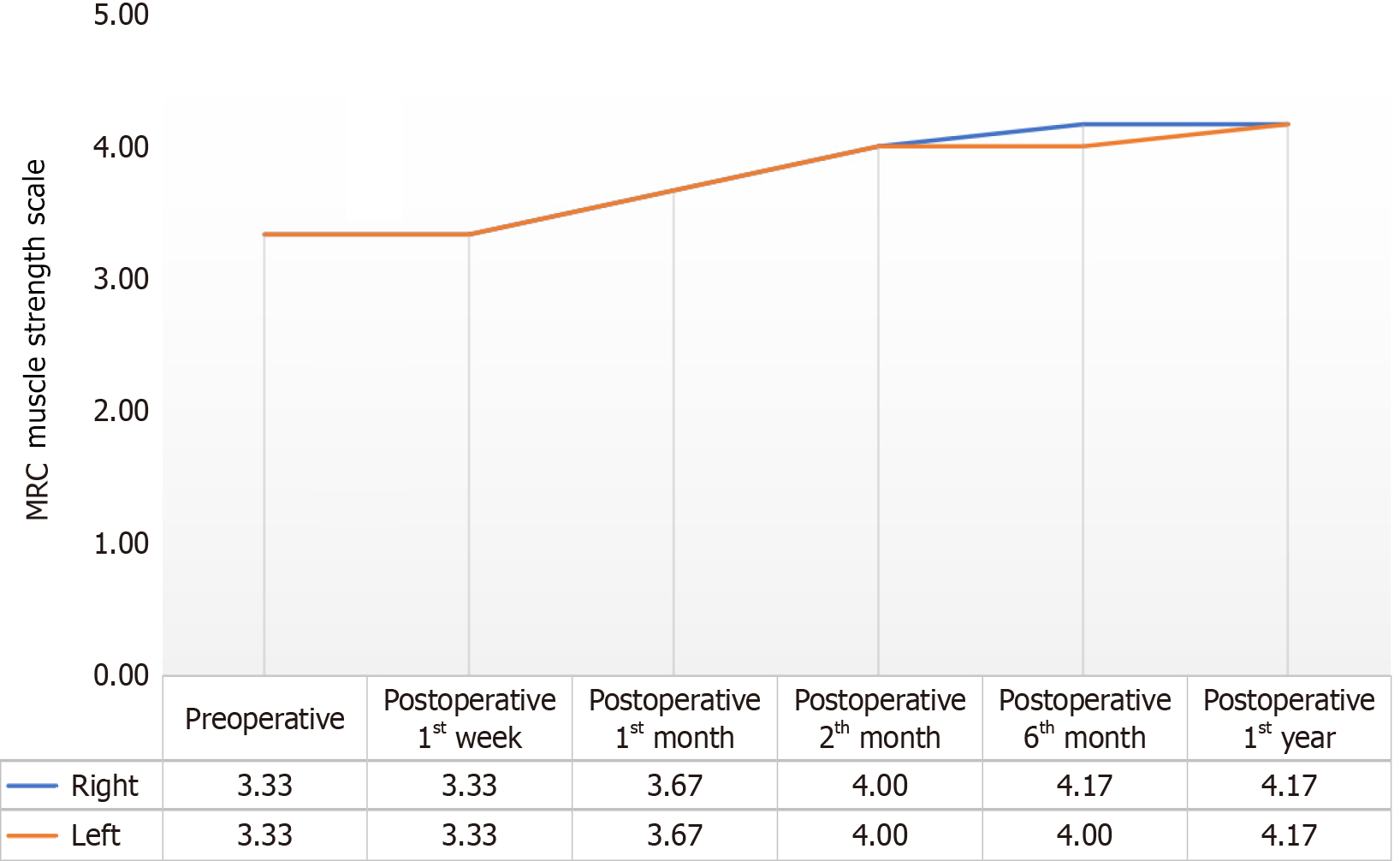
Figure 2 The changes observed in the average scores of the patients before the procedure, at the 1st wk, 1st month, 2nd month, 6th month and 1st year after the procedure, regarding the Medical Research Council muscle strength scale right and left values.
MRC: Medical Research Council.
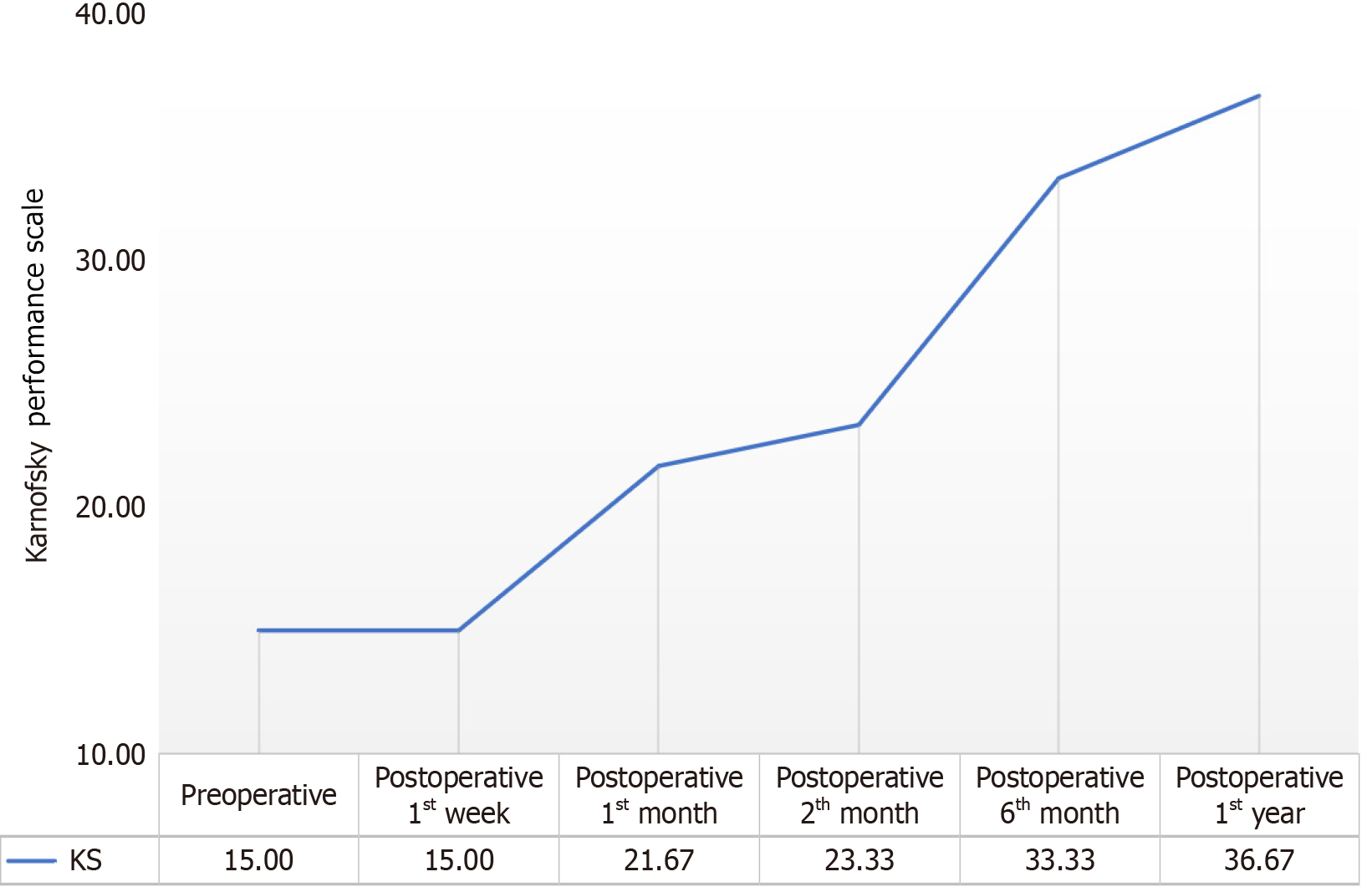
Figure 3 Changes observed in the pretest and posttest averages of the patients’ Karnofsky performance scale values.
- Citation: Kabatas S, Civelek E, Boyalı O, Sezen GB, Ozdemir O, Bahar-Ozdemir Y, Kaplan N, Savrunlu EC, Karaöz E. Safety and efficiency of Wharton’s Jelly-derived mesenchymal stem cell administration in patients with traumatic brain injury: First results of a phase I study. World J Stem Cells 2024; 16(6): 641-655
- URL: https://www.wjgnet.com/1948-0210/full/v16/i6/641.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i6.641