©The Author(s) 2024.
World J Stem Cells. May 26, 2024; 16(5): 591-603
Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.591
Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.591
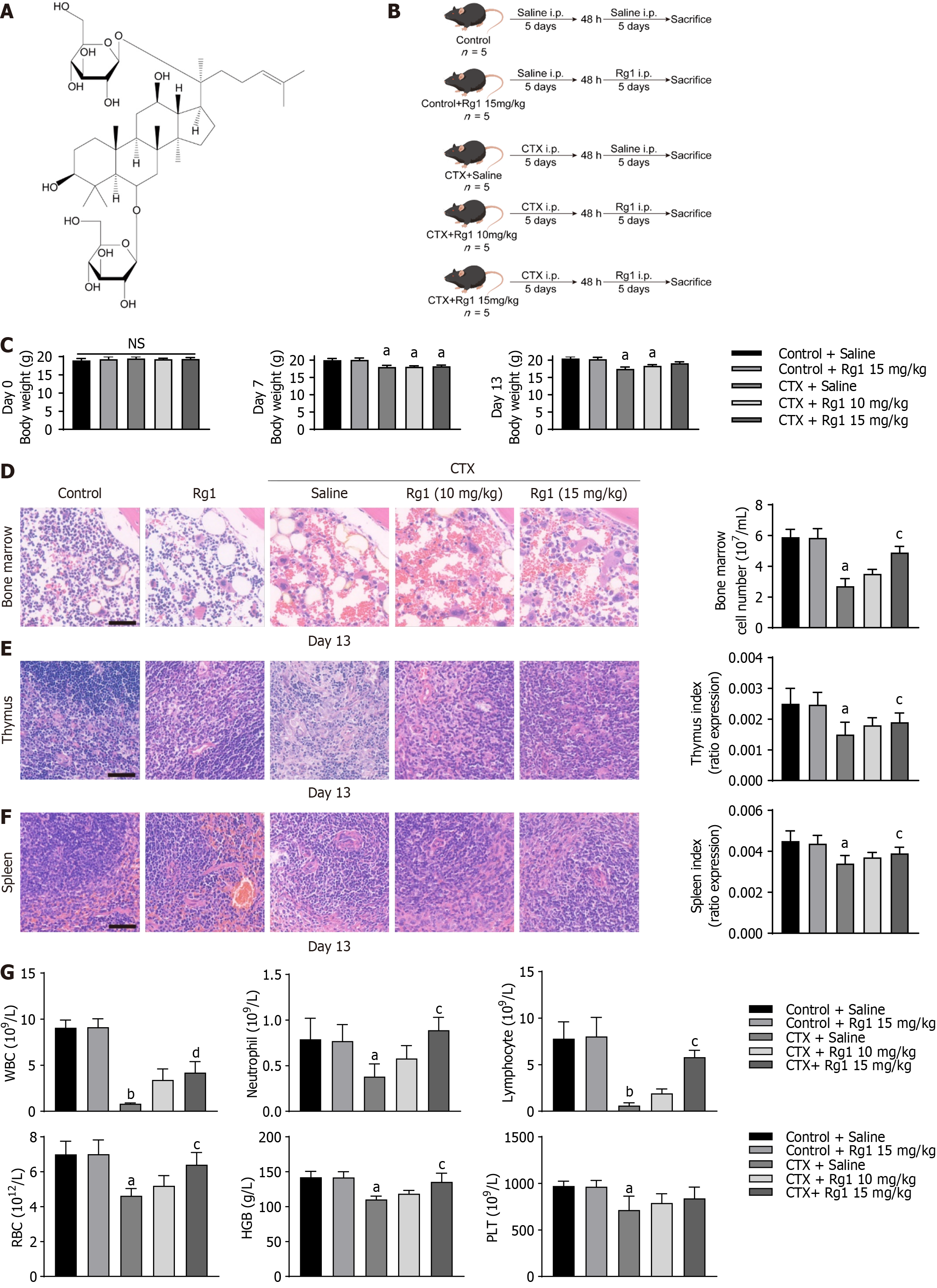
Figure 1 Ginsenoside Rg1 prevents myelosuppression.
A: Chemical structure of ginsenoside Rg1; B: Flowchart of experiments; C: Body weight plot of mice; D: Hematoxylin-eosin (HE) staining of marrow sample; E: HE staining of thymus; F: HE staining of spleen; G: Rg1 increased cell numbers in the peripheral blood of mice with myelosuppression. For all the statistical graphs: n = 5 mice per group. aP < 0.05, bP < 0.01, compared to the control group; cP < 0.05, dP < 0.01, compared to the cyclophosphamide (CTX) group. HGB: Hemoglobin; PLT: Platelets; RBC: Red blood cells; WBC: White blood cells.
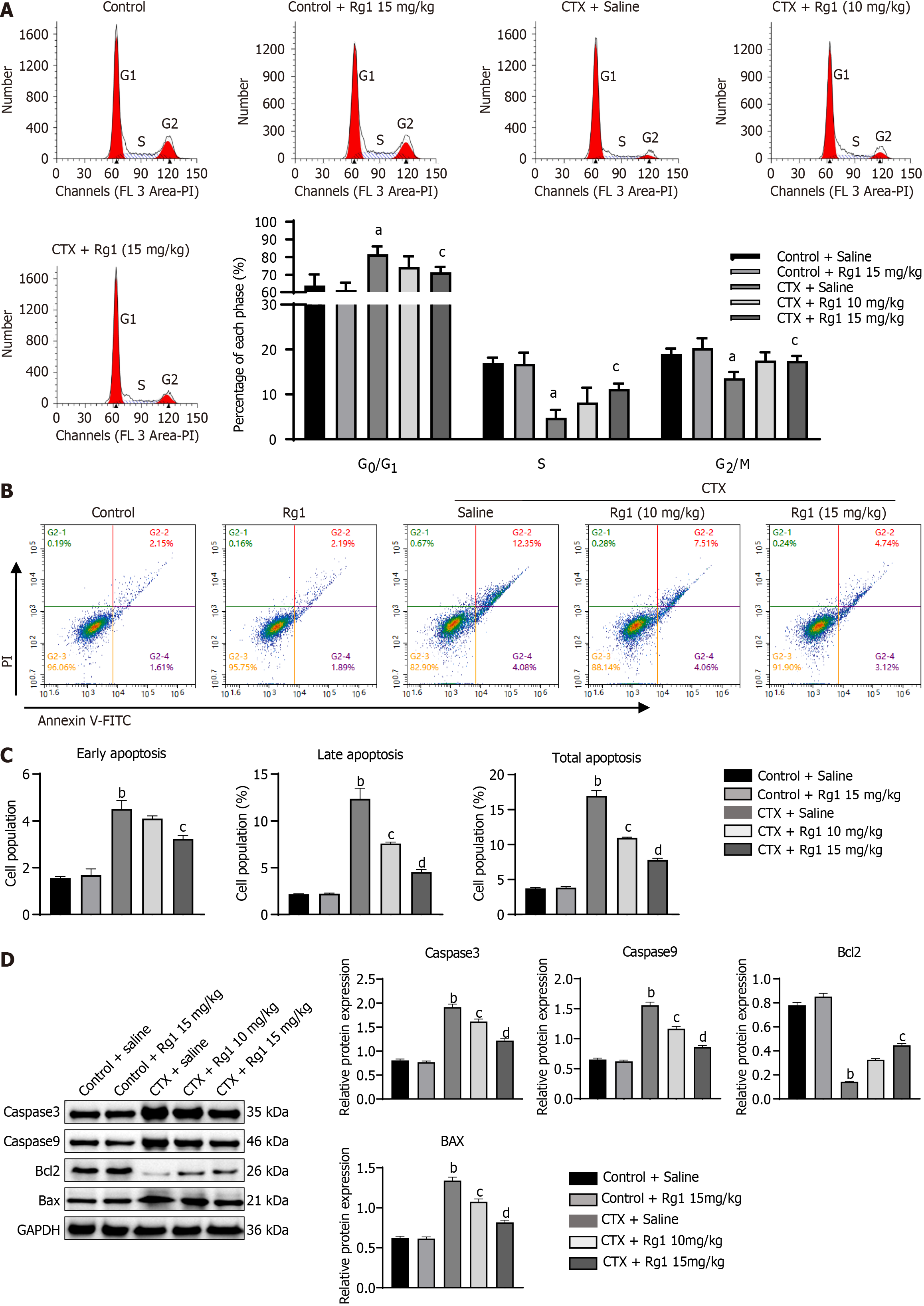
Figure 2 Impact of ginsenoside Rg1 on the cell cycle and apoptosis of hematopoietic stem cells in mice with aplastic anemia.
A: Representative flow cytometry results; B: Representative flow cytometry results; C: Statistical figure of flow cytometry; D: Using western blot detects the expression of Caspase3, Caspase9, Bcl2 and Bax. The endogenous control used was GAPDH. aP < 0.05, bP < 0.01, compared to the control group; cP < 0.05, dP < 0.01, compared to the cyclophosphamide (CTX) group.
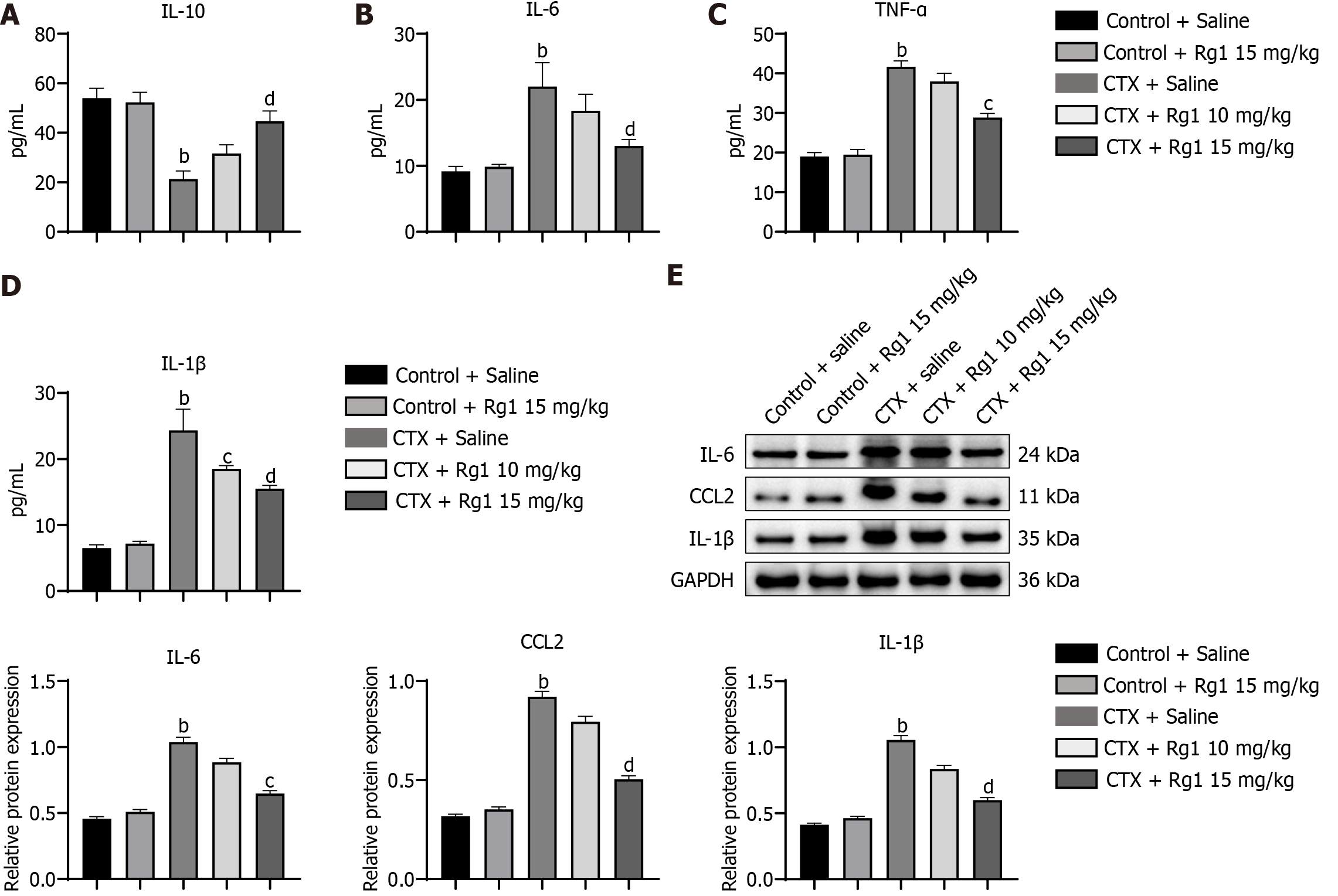
Figure 3 Impact of ginsenoside Rg1 on inflammatory cytokines in mice with aplastic anemia in the bone marrow was examined.
A: Interleukin (IL)-10; B: IL-6; C: Tumor necrosis factor (TNF)-α; D: IL-1β; E: Western blot assay of IL-6, CCL2 and IL-1β in different group, the endogenous control used was GAPDH. bP < 0.01, compared to the control group; cP < 0.05, dP < 0.01, compared to the cyclophosphamide (CTX) group.
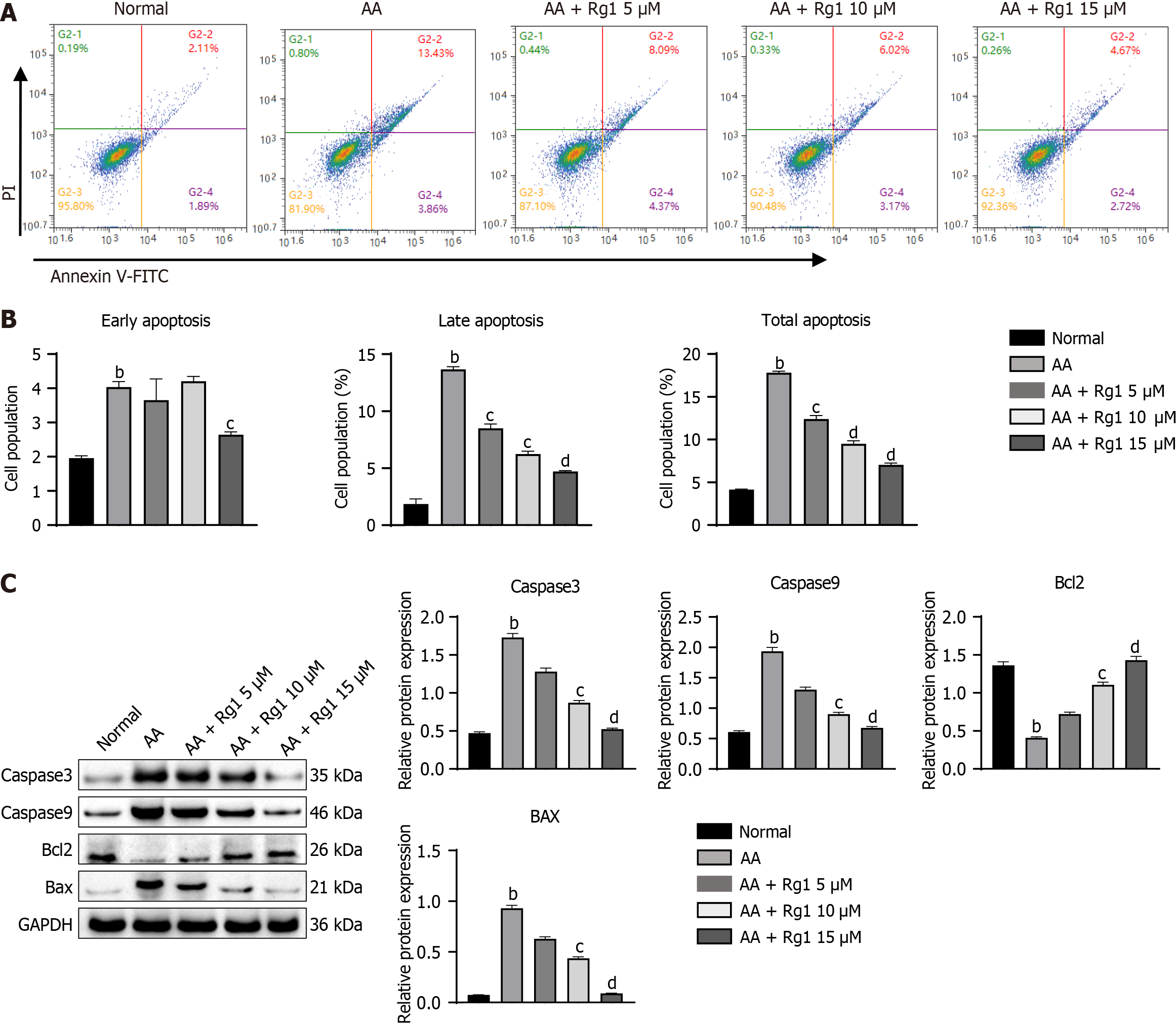
Figure 4 Ginsenoside Rg1 can inhibit apoptosis of hematopoietic stem cells from aplastic anemia mice in vitro.
A: This figure shows representative results from flow cytometry analysis; B: Statistical figure of flow cytometry; C: The expression of Caspase3, Caspase9, Bcl2, and Bax was detected using western blot. GAPDH was used as the endogenous control. bP < 0.01, compared to the normal group; cP < 0.05, dP < 0.01, compared to the aplastic anemia (AA) group.
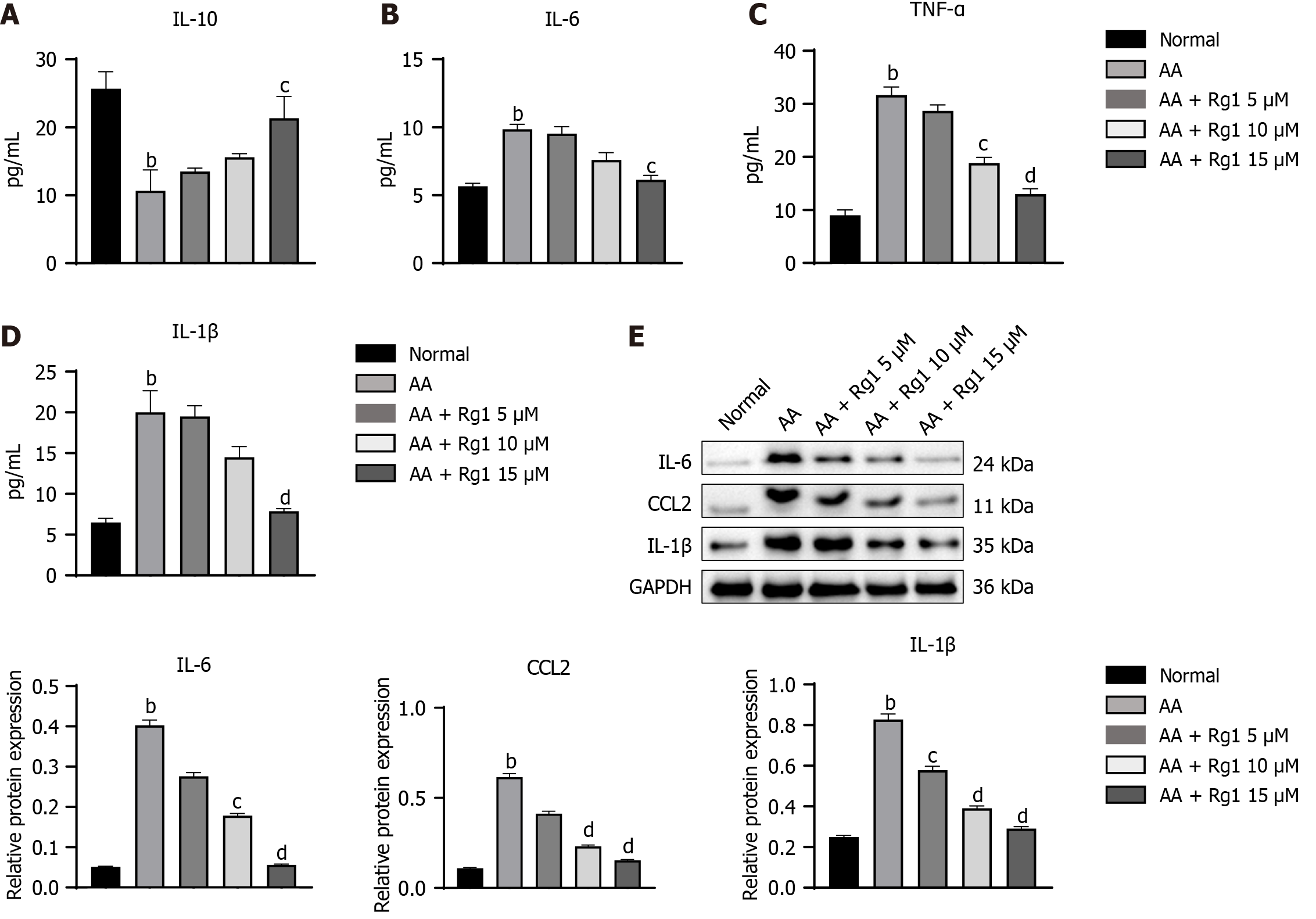
Figure 5 Ginsenoside Rg1 have the ability to inhibit inflammation of hematopoietic stem cells from aplastic anemia mice in vitro.
A: The content of interleukin (IL)-10; B: The content of IL-6; C: The content of tumor necrosis factor (TNF)-α; D: The content of IL-1β; E: Western blot assay of IL-6, CCL2, and IL-1β in different group, the endogenous control used was GAPDH. bP < 0.01, compared to the normal group; cP < 0.05, dP < 0.01, compared to the aplastic anemia (AA) group.
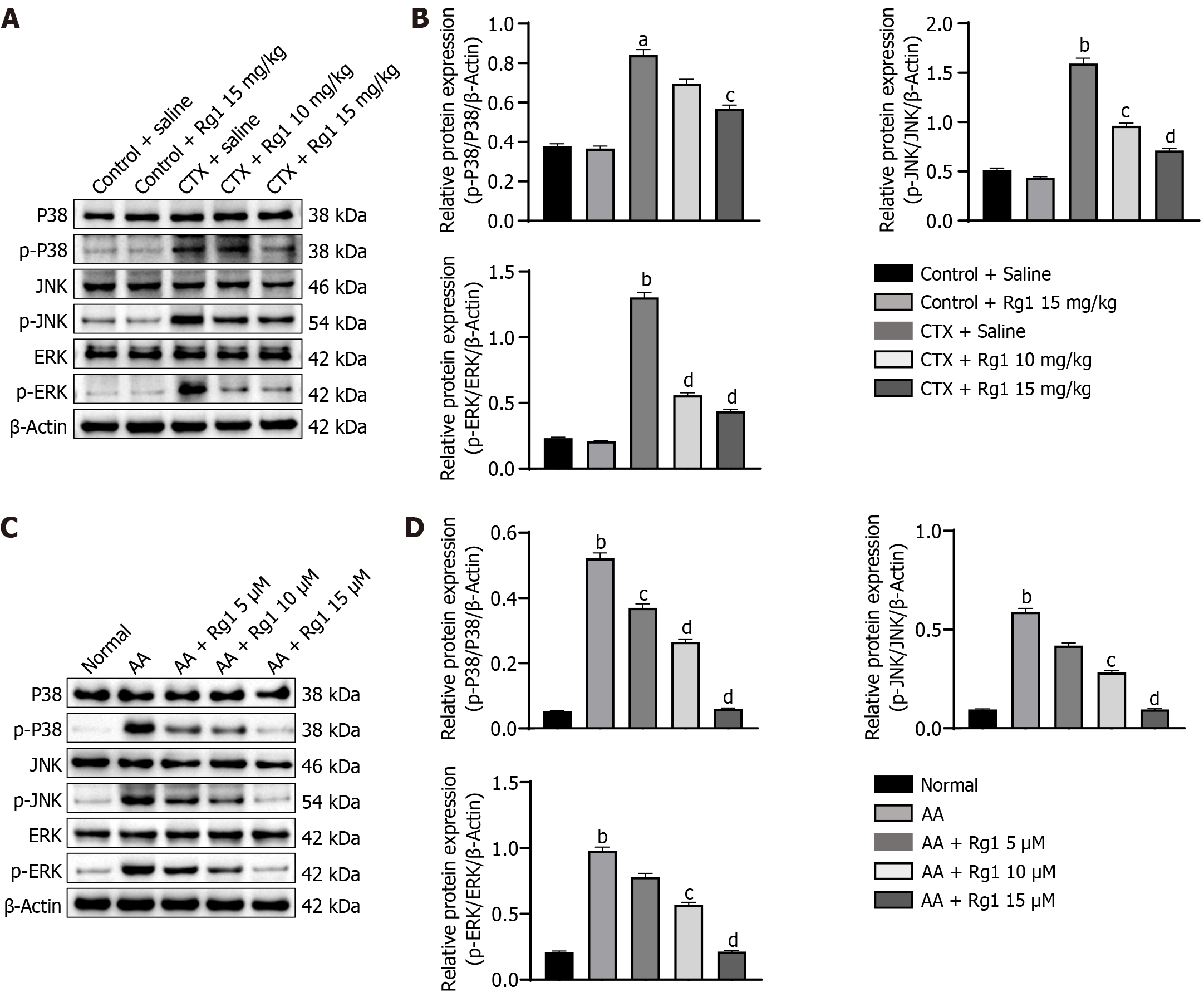
Figure 6 Ginsenoside Rg1 alleviated myelosuppression in mice by inhibiting MAPK signaling pathway in vivo and in vitro.
A: Western blot was used to detect the expression of p-p38, p38, p-JNK, JNK, p-ERK, and ERK in different groups in vivo, the endogenous control used was β-actin; B: Quantification of western blot images, aP < 0.05, bP < 0.01, compared to the control group; cP < 0.05, dP < 0.01, compared to the cyclophosphamide (CTX) group; C: Western blot was used to detect the expression of p-p38, p38, p-JNK, JNK, p-ERK, and ERK in different groups in vitro, the endogenous control used was β-Actin; D: Quantification of western blot images, bP < 0.01, compared to the normal group; cP < 0.05, dP < 0.01, compared to the aplastic anemia (AA) group.
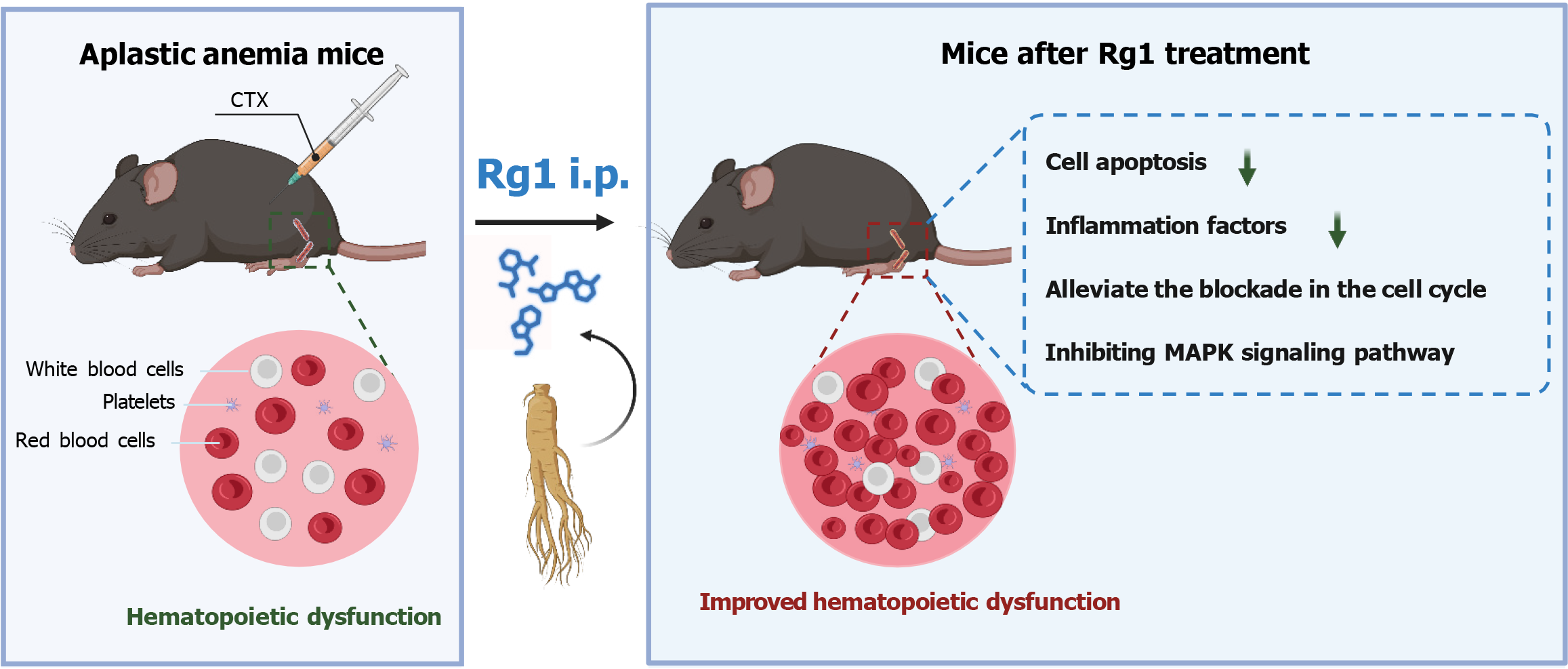
Figure 7 Effect of ginsenoside Rg1 on hematopoietic stem cells in the treatment of aplastic anemia in mice through the MAPK pathway.
The bone marrow, thymus, and spleen suffered severe damage from cyclophosphamide (CTX), leading to a decrease in hematopoietic cell count. Ginsenoside Rg1 effectively countered the hematopoietic issues in mice, showing superior results compared to the aplastic anemia control group. It reduced cell apoptosis, inflammation factors, and cell cycle disruption caused by CTX. By inhibiting the MAPK signaling pathway, ginsenoside Rg1 greatly improved myelosuppression in mice.
- Citation: Wang JB, Du MW, Zheng Y. Effect of ginsenoside Rg1 on hematopoietic stem cells in treating aplastic anemia in mice via MAPK pathway. World J Stem Cells 2024; 16(5): 591-603
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/591.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.591