©The Author(s) 2023.
World J Stem Cells. Nov 26, 2023; 15(11): 1017-1034
Published online Nov 26, 2023. doi: 10.4252/wjsc.v15.i11.1017
Published online Nov 26, 2023. doi: 10.4252/wjsc.v15.i11.1017
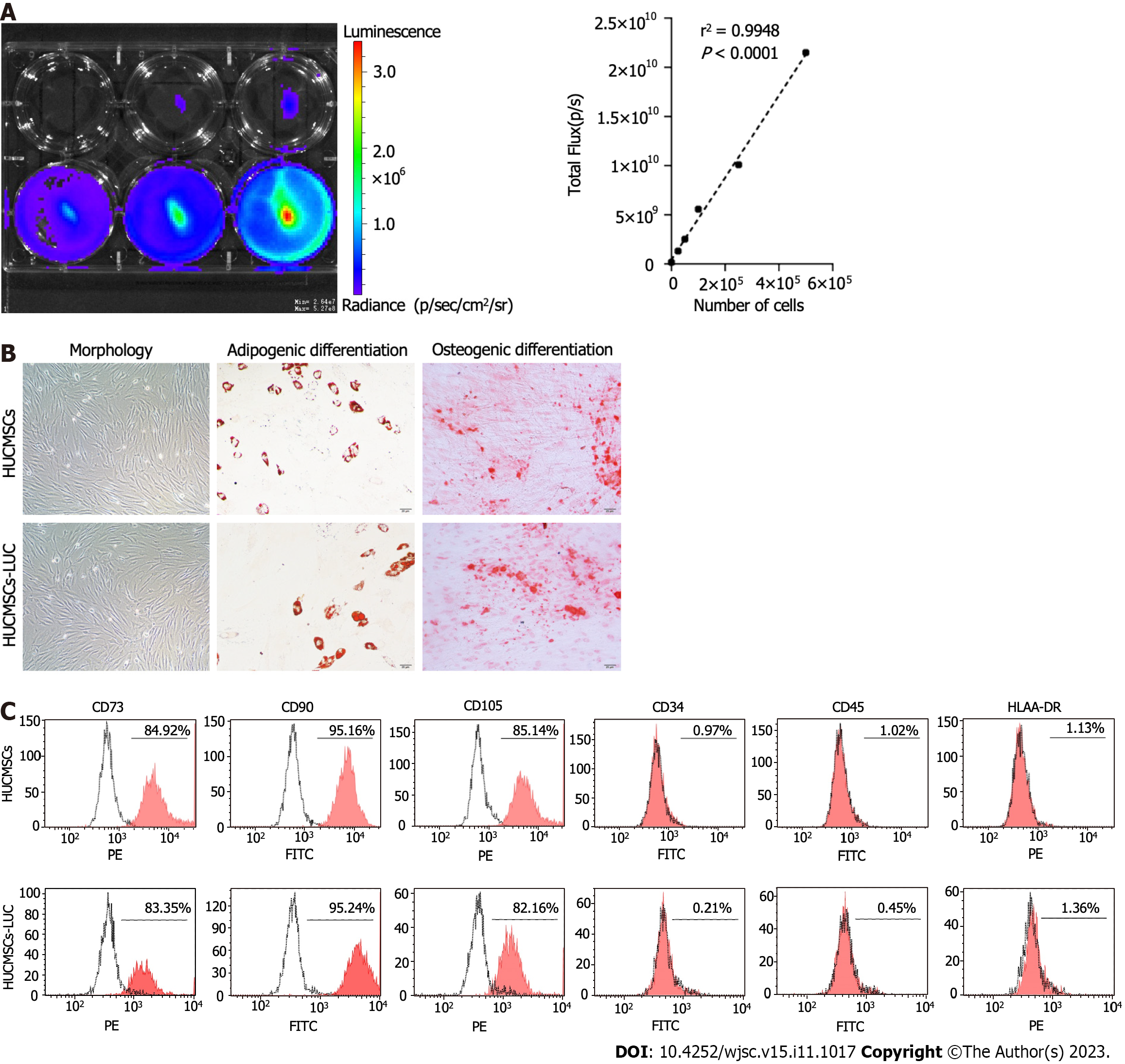
Figure 1 Characteristics of human umbilical cord mesenchymal stem cells expressing firefly luciferase gene.
A: Luciferase activity of human umbilical cord mesenchymal stem cells expressing firefly luciferase gene (HUCMSCs-LUC). Representative luciferase imaging is shown using in vivo imaging system; B: Morphologies of HUCMSCs-LUC. HUCMSCs-LUC differentiated into adipocytes (stained with oil red O for lipid droplets) and osteocytes (stained with alizaline red for mineral deposition); C: Surface marker expression of HUCMSCs-LUC in flow cytometry analysis. HUCMSCS: Human umbilical cord mesenchymal stem cells; HUCMSCs-LUC: Human umbilical cord mesenchymal stem cells expressing firefly luciferase gene; PE: Phycoerythrin; FITC: Fluorescein isothiocyanate.
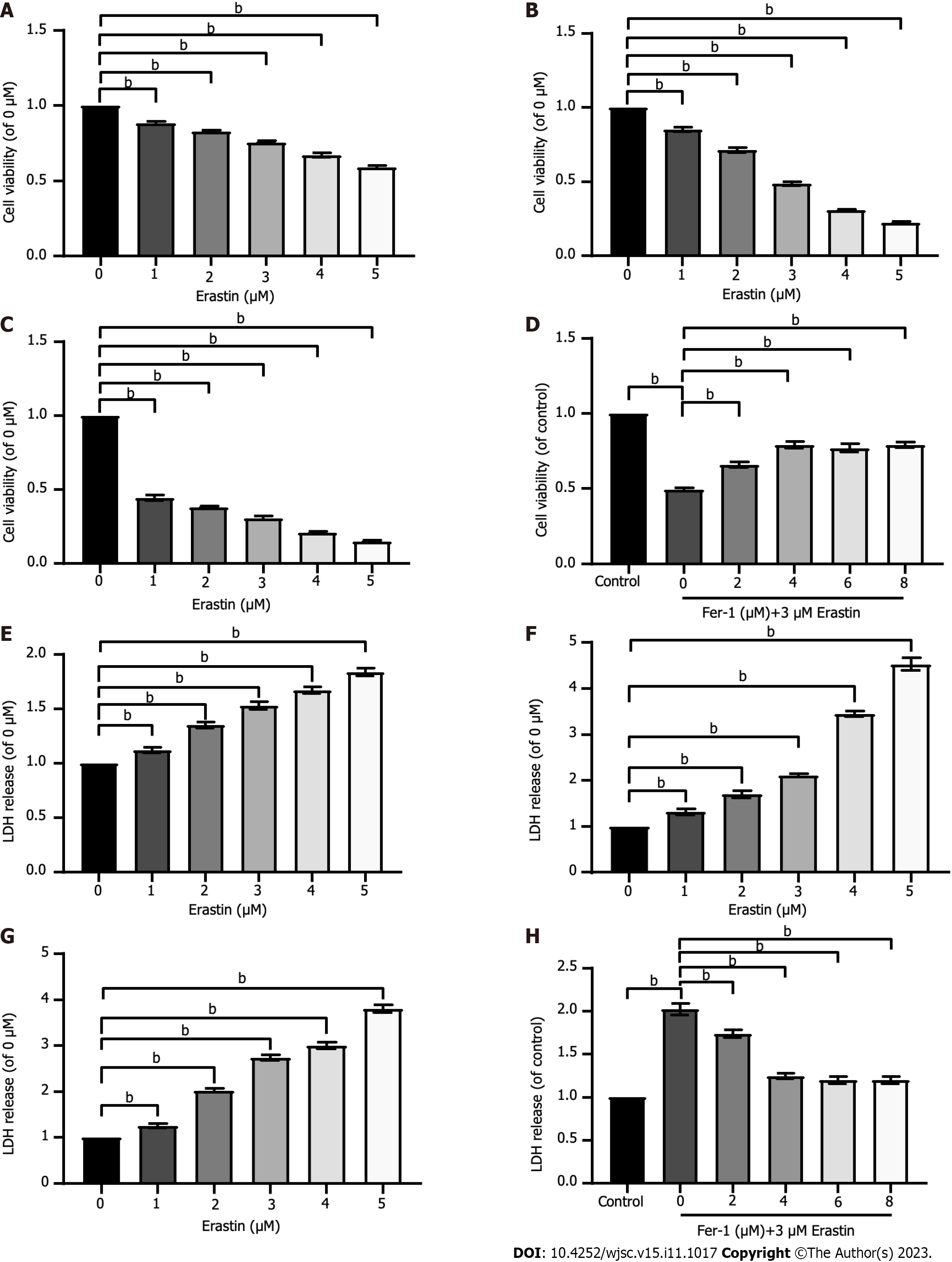
Figure 2 Ferroptosis exists in human umbilical cord mesenchymal stem cells.
A-C: Cell viability was assessed after exposure to different concentrations of erastin for different times (12 h, 24 h, and 48 h); D: Cell viability was assessed after exposure to 3 μM erastin and different concentrations of ferrostatin-1 (Fer-1). Cell viability was measured with Cell Counting Kit-8 kit; E-G: Lactic dehydrogenase (LDH) release was assessed after exposure to different concentrations of erastin for different times (12 h, 24 h, and 48 h); H: LDH release was assessed after exposure to 3 μM erastin and different concentrations of Fer-1. LDH release was measured with LDH cytotoxicity detection kit. The data were from at least three independent experiments. Data were quantified for cells subjected to erastin (0 μM), and values are represented as the mean ± SD. Fer-1: Ferrostatin-1; LDH: Lactic dehydrogenase. bP < 0.01.
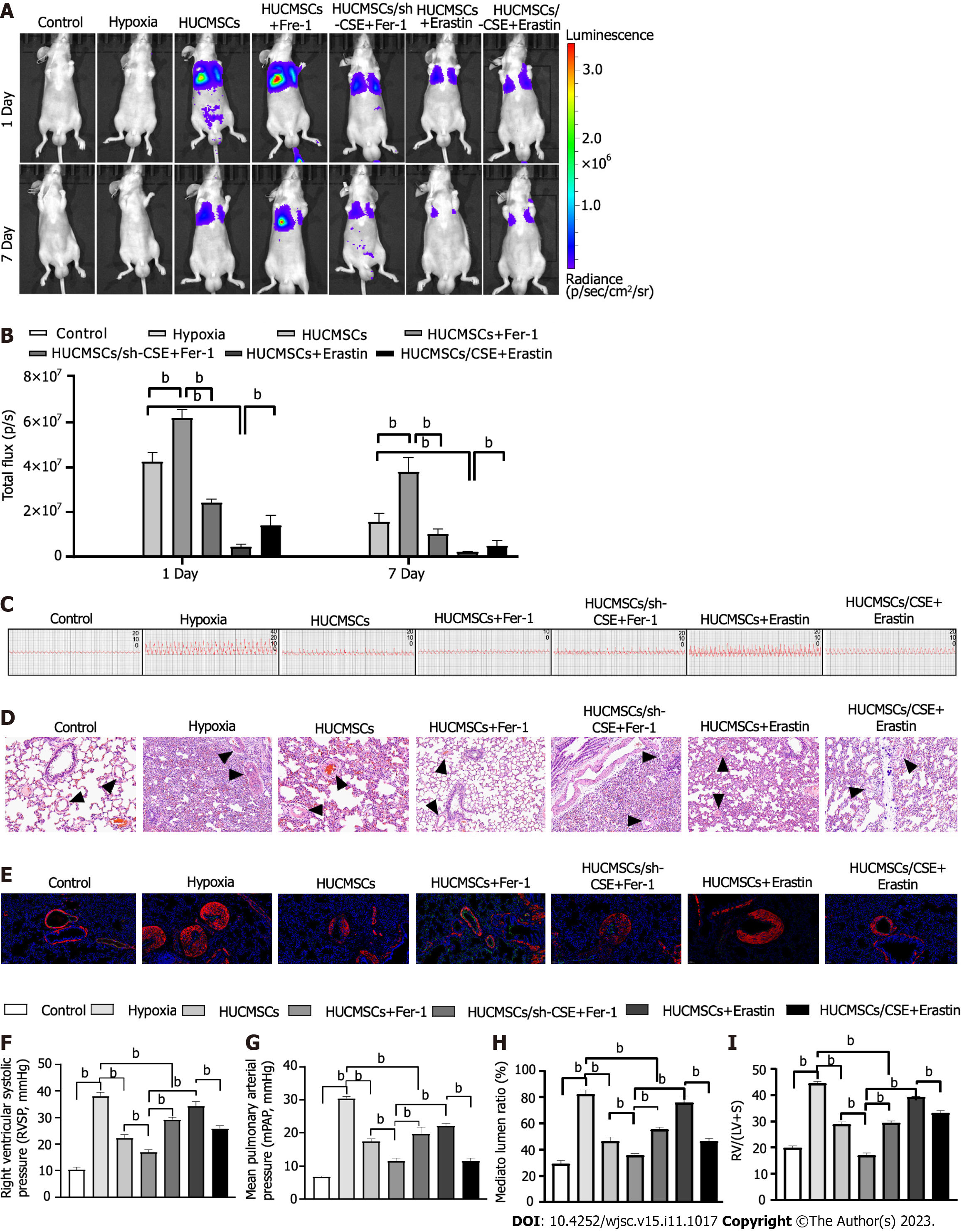
Figure 3 Change of pulmonary arterial remodeling.
A: In vivo bioluminescence imaging (BLI). Representative time course image showing in vivo BLI of mice grafted with living human umbilical cord mesenchymal stem cells expressing firefly luciferase gene in the lungs. Images were acquired at 24 h and 7 d post-implantation. Regions of interest are drawn on the mouse lung and on the mouse shoulder, considered as background signal; B: In vivo BLI. Quantitative analysis of in vivo BLI at days 1 and 7 post-implantation; C-H: Representing pulmonary arterial pressure (C, F, and G). Representative haematoxylin and eosin-stained lung sections and quantification of the ratio of the medial wall area to the total vessel cross sectional area of the distal pulmonary artery sections (D and H). Immunofluorescent staining (E). Green fluorescence represents VEcadherin, red fluorescence represents alpha-smooth muscle actin, and blue fluorescence indicates 4’,6-diamidino-2-phenylindole nuclear staining; I: Right ventricle/left ventricle plus septum. Data are expressed as the mean ± SEM (n = 6/group). HUCMSCS: Human umbilical cord mesenchymal stem cells; Fer-1: Ferrostatin-1; CSE: Cystathionine γ-lyase; RV/(LV + S): Right ventricle/(left ventricle plus septum). bP < 0.01.
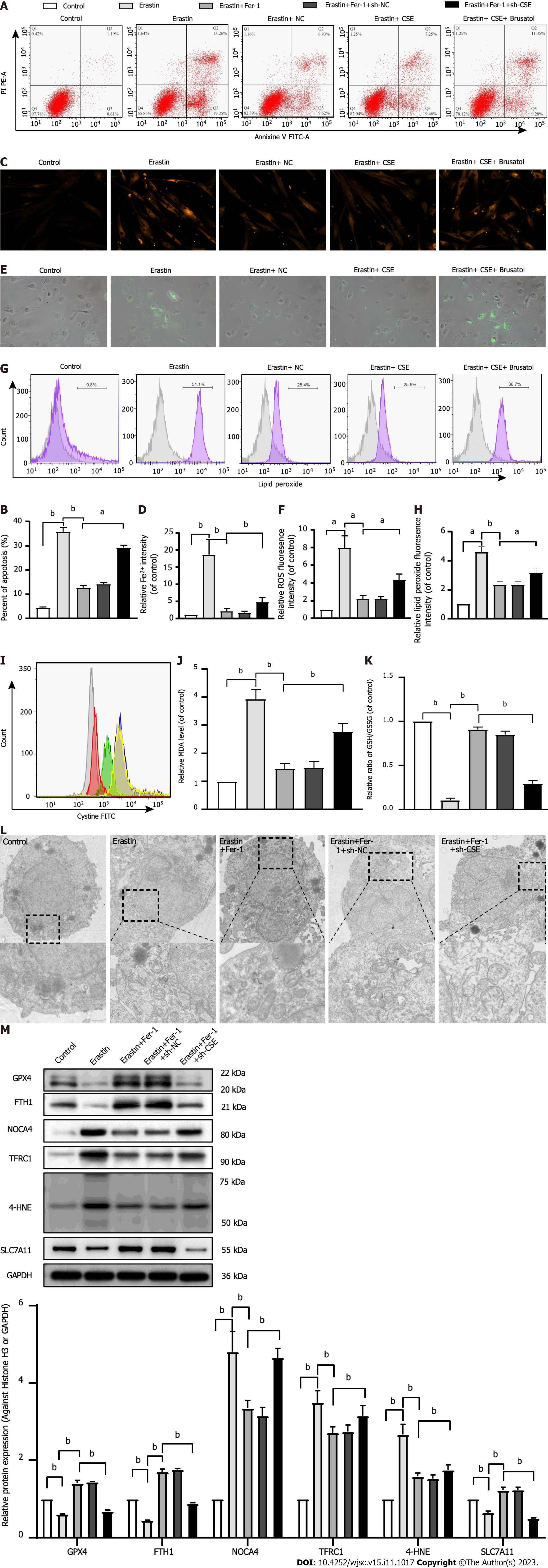
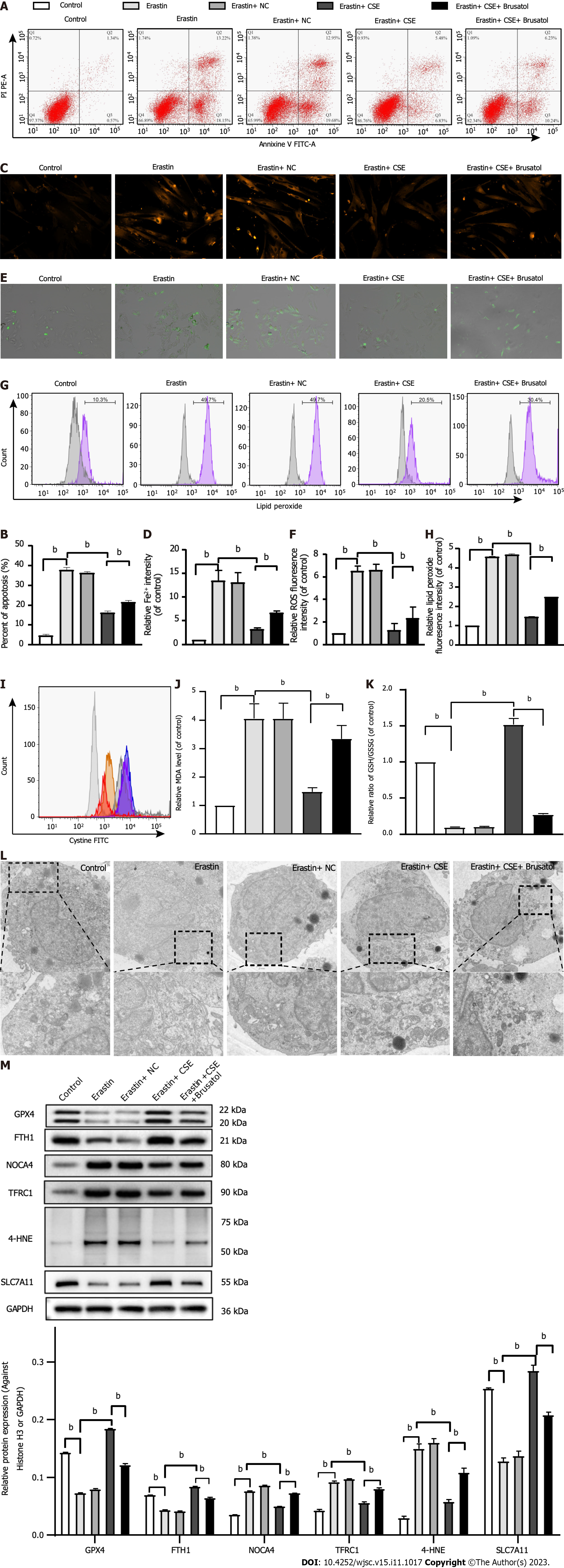
Figure 4 Effect of cystathionine γ-lyase overexpression on ferroptosis in human umbilical cord mesenchymal stem cells.
A and B: Cell apoptosis was analyzed by Annexin V-fluorescein isothiocyanate (FITC)/propidine iodide staining using flow cytometry analysis (n = 3), and quantified on the basis of apoptosis rate (n = 3); C and D: Iron level was detected by FeRhoNox-1 staining (C) and iron assay kit (D); E and F: Immunofluorescent staining of total reactive oxygen species; G and H: Level of lipid peroxidation detected by flow cytometry analysis after staining with C11-BODIPY; I: Intracellular cystine-FITC levels measured by flow cytometry. Results represent three independent experiments; J: Malonaldehyde level; K: Ratio of reduced glutathione/oxidized glutathione disulfide; L: Mitochondrial morphological changes detected by transmission electron microscopy; M: Western blot analysis of expression of the glutathione-dependent antioxidant enzyme glutathione peroxidase, ferritin heavy chain 1, nuclear receptor coactivator 4, Fe3+-bound transferrin receptor 1, 4-hydroxynonenal, and SLC7A11 protein. Glyceraldehyde-3-phosphate dehydrogenase was used for normalization for protein (n = 3). Fer-1: Ferrostatin-1; Annexin V-FITC/PI: Annexin V-fluorescein isothiocyanate/propidine iodide; ROS: Reactive oxygen species; sh-CSE: Short hairpin RNA targeting cystathionine γ-lyase; MDA: Malonaldehyde; GSH/GSSG: Reduced glutathione/oxidized glutathione disulfide; GPX4: Glutathione-dependent antioxidant enzyme glutathione peroxidase; FTH1: Ferritin heavy chain 1; NCOA4: Nuclear receptor coactivator 4; TFRC1: Fe3+-bound transferrin receptor 1; 4-HNE: 4-hydroxynonenal; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. aP < 0.05, bP < 0.01.
Figure 5 Effect of cystathionine γ-lyase inhibition on ferroptosis in human umbilical cord mesenchymal stem cells.
A and B: Cell apoptosis was analyzed by Annexin V-fluorescein isothiocyanate (FITC)/propidine iodide staining using FACS (n = 3), and quantified on the basis of apoptosis rate (n = 3); C and D: Iron level detected by FeRhoNox-1 staining (C) and iron assay kit (D); E and F: Immunofluorescent staining of total reactive oxygen species; G and H: Level of lipid peroxidation detected by flow cytometry after staining with C11-BODIPY; I: Intracellular cystine-FITC levels measured by flow cytometry. Results represent three independent experiments; J: Malonaldehyde level; K: Ratio of reduced glutathione/oxidized glutathione disulfide; L: Mitochondrial morphological changes detected by transmission electron microscopy; M: Western blot analysis of expression of glutathione-dependent antioxidant enzyme glutathione peroxidase, ferritin heavy chain 1, nuclear receptor coactivator 4, Fe3+-bound transferrin receptor 1, 4-hydroxynonenal, and SLC7A11 protein. Glyceraldehyde-3-phosphate dehydrogenase was used for normalization for protein (n = 3). Fer-1: Ferrostatin-1; Annexin V-FITC/PI: Annexin V-fluorescein isothiocyanate/propidine iodide; ROS: Reactive oxygen species; MDA: Malonaldehyde; CSE: Cystathionine γ-lyase; GSH/GSSG: Reduced glutathione/oxidized glutathione disulfide; GPX4: Glutathione-dependent antioxidant enzyme glutathione peroxidase; FTH1: Ferritin heavy chain 1; NCOA4: Nuclear receptor coactivator 4; TFRC1: Fe3+-bound transferrin receptor 1; 4-HNE: 4-hydroxynonenal; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. bP < 0.01.
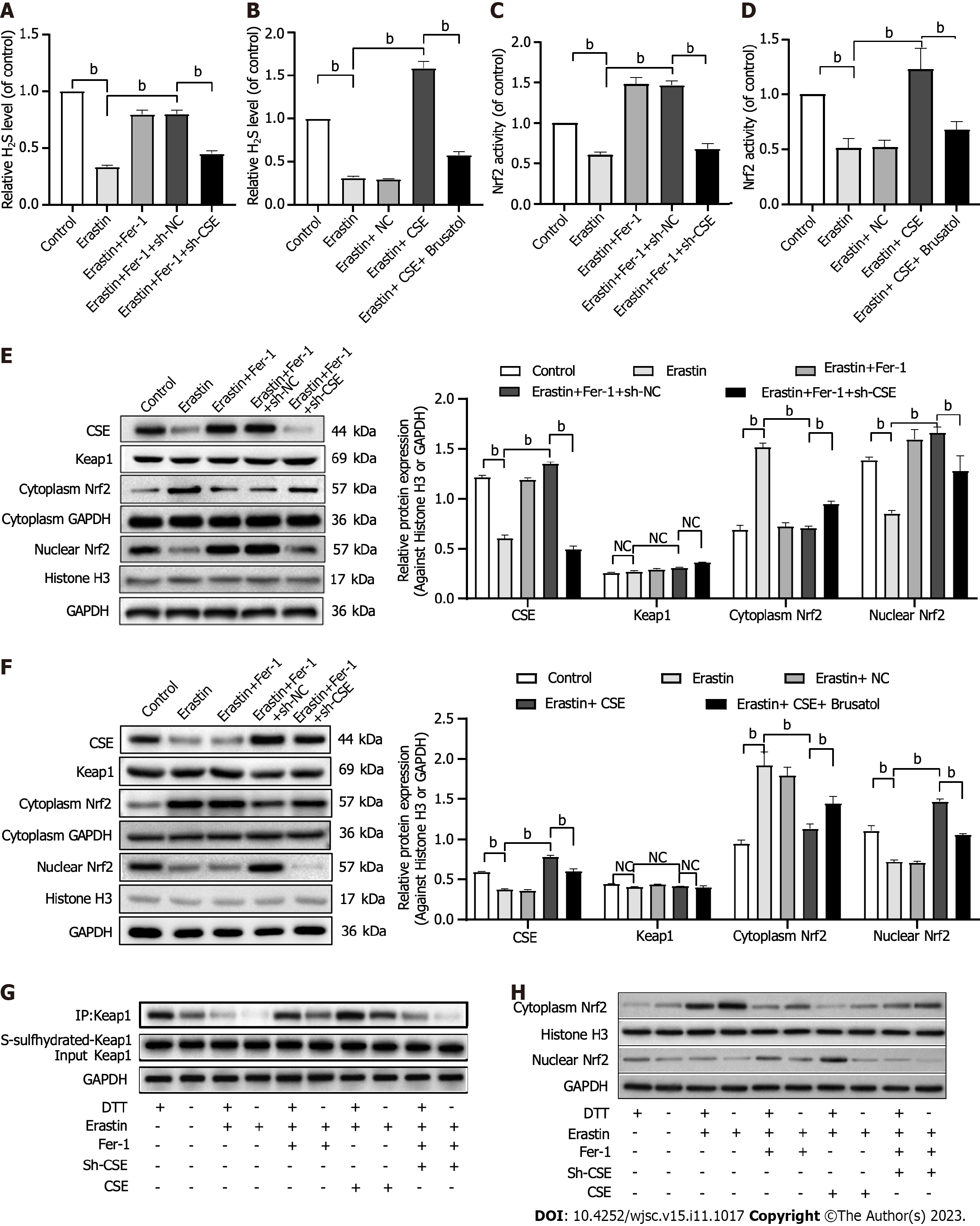
Figure 6 Effect of cystathionine γ-lyase/hydrogen sulfide pathway on nuclear factor erythroid 2-related factor 2 activation and ferroptosis in human umbilical cord mesenchymal stem cells.
A and B: Exogenous hydrogen sulfide (H2S) production; C and D: Nuclear factor erythroid 2-related factor 2 (Nrf2) activity; E and F: Western blot analysis and quantification of cystathionine γ-lyase (CSE) protein, Kelch-like ECH-associating protein 1 (Keap1), and cytoplasmic and nuclear Nrf2 protein; G: Change of Keap1 S-sulfhydration. 1,4-Dithio-DL-threitol (DTT, 1 mmol/L, 3 h) served as a negative control; H: Effect of DTT on cytoplasmic and nuclear Nrf2 expression. Histone H3 was used for normalization for nuclear proteins, and glyceraldehyde-3-phosphate dehydrogenase was used for normalization for whole and cytoplasmic protein (n = 3). CSE: Cystathionine γ-lyase; H2S: Hydrogen sulfide; Nrf2: Nuclear factor erythroid 2-related factor 2; HUCMSCs: Human umbilical cord mesenchymal stem cells; Fer-1: Ferrostatin-1; sh-CSE: Short hairpin RNA targeting cystathionine γ-lyase; Keap1: Kelch-like ECH-associating protein 1; DTT: 1,4-Dithio-DL-threitol; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. bP < 0.01.
- Citation: Hu B, Zhang XX, Zhang T, Yu WC. Dissecting molecular mechanisms underlying ferroptosis in human umbilical cord mesenchymal stem cells: Role of cystathionine γ-lyase/hydrogen sulfide pathway. World J Stem Cells 2023; 15(11): 1017-1034
- URL: https://www.wjgnet.com/1948-0210/full/v15/i11/1017.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i11.1017