©The Author(s) 2022.
World J Stem Cells. Feb 26, 2022; 14(2): 183-199
Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.183
Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.183
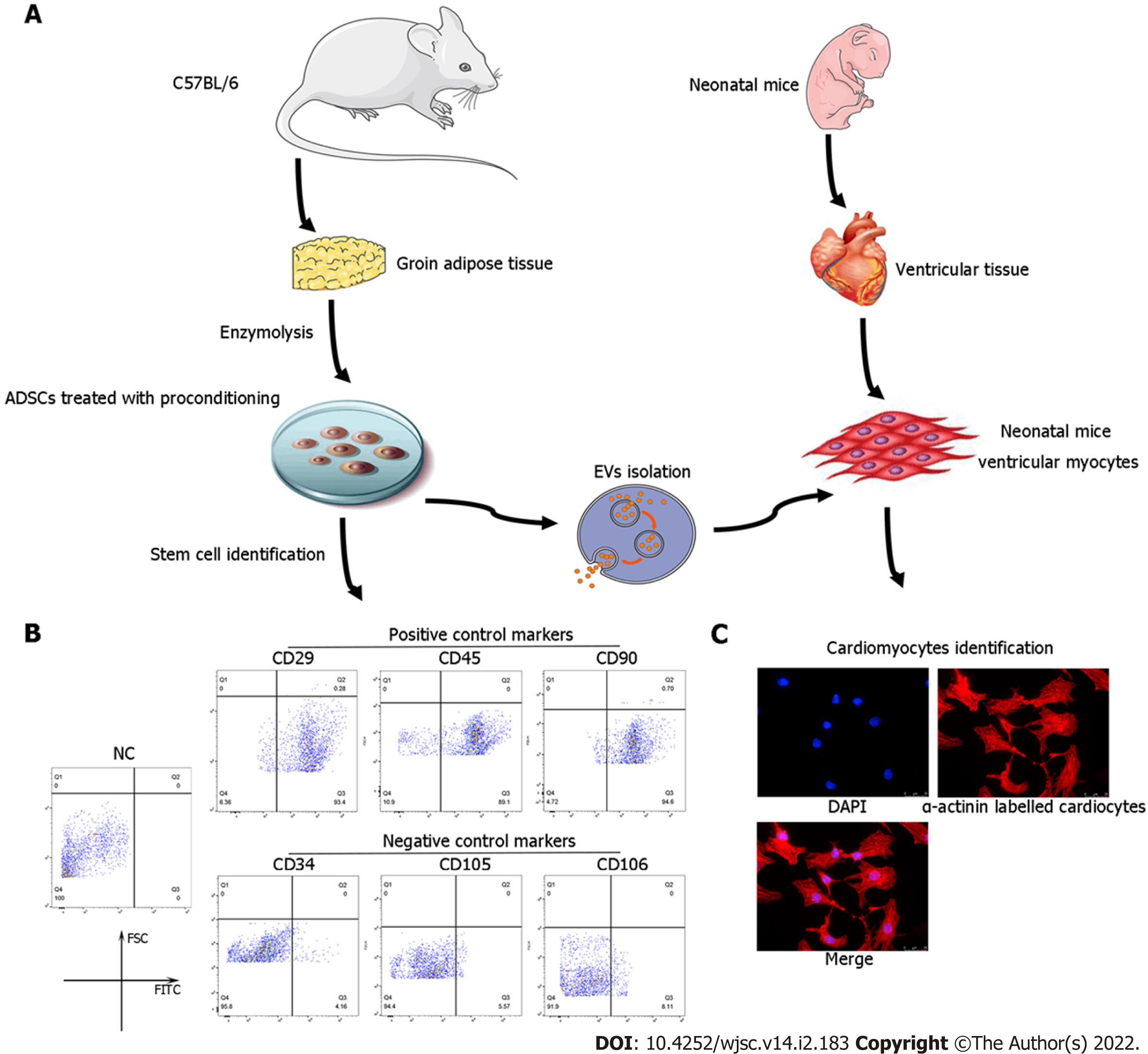
Figure 1 Identification of adipose-derived mesenchymal stem cells and neonatal mouse cardiomyocytes.
A: Flow chart of extracellular vesicles and neonatal mouse cardiomyocyte (CM) isolation; B: Characterization of adipose-derived mesenchymal stem cells (ADSCs) was performed by flow cytometry analyses of cluster of differentiation 34 (CD34), CD105, and CD106 (negative controls) and CD29, CD45, and CD90 (positive cell surface markers); C: Identification of neonatal mouse CMs, which were specifically stained with α-actinin. CM: Cardiomyocyte; ADSC: Adipose-derived mesenchymal stem cells; CD: Cluster of differentiation.
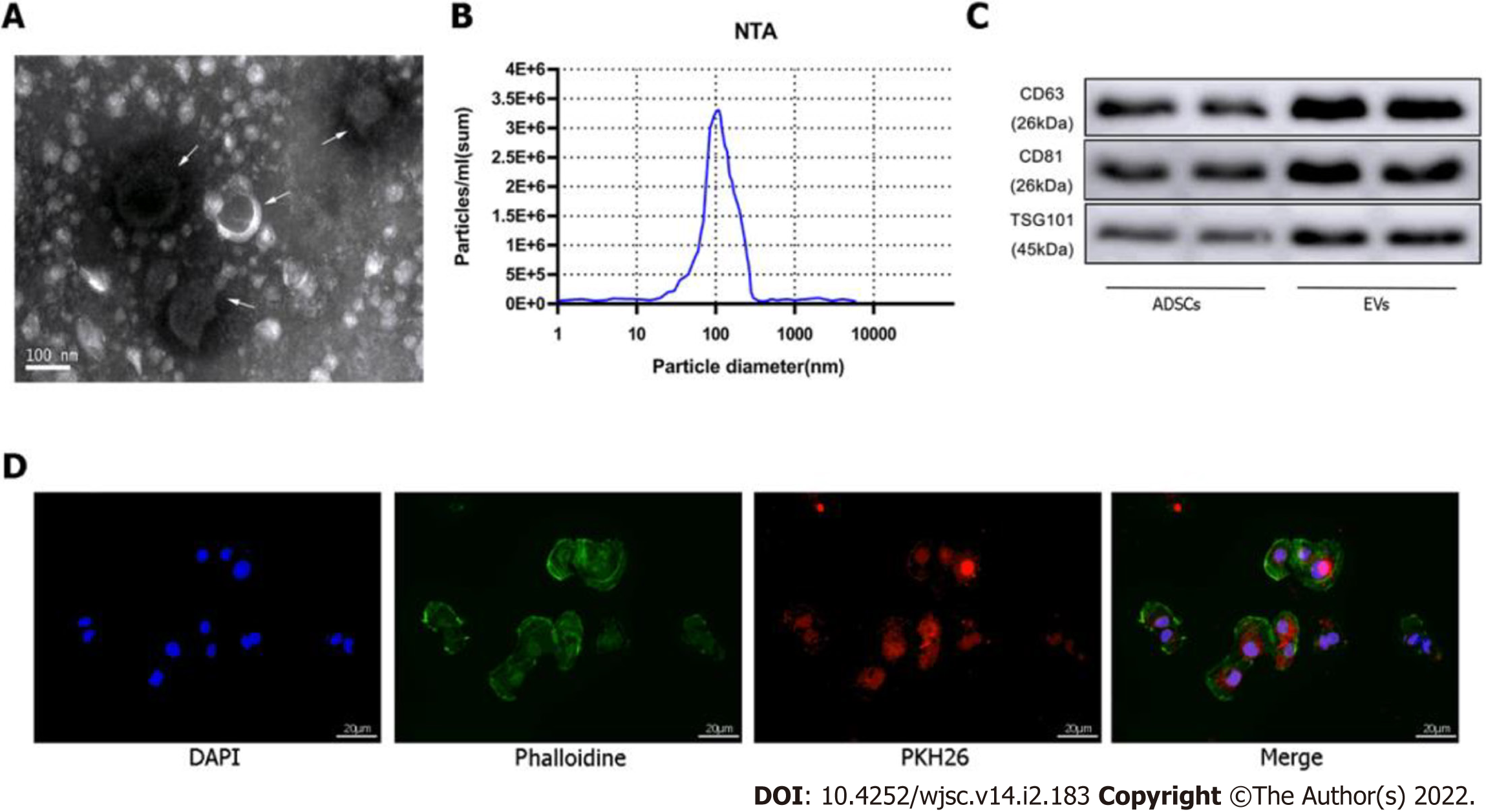
Figure 2 Identification of extracellular vesicles.
A: Transmission electron microscopy characterization of extracellular vesicles (EVs); B: Size distribution by intensity was detected using the NanoSight Instrument; C: EV biomarkers cluster of differentiation 63 (CD63), CD81, and tumor susceptibility gene 101 were identified by western blotting of adipose-derived mesenchymal stem cell (ADSC)-derived EVs and ADSCs; D: EV tracer assay was used to identify exosomes phagocytosed by cardiomyocytes. ADSC: Adipose-derived mesenchymal stem cell; EV: Extracellular vesicles.
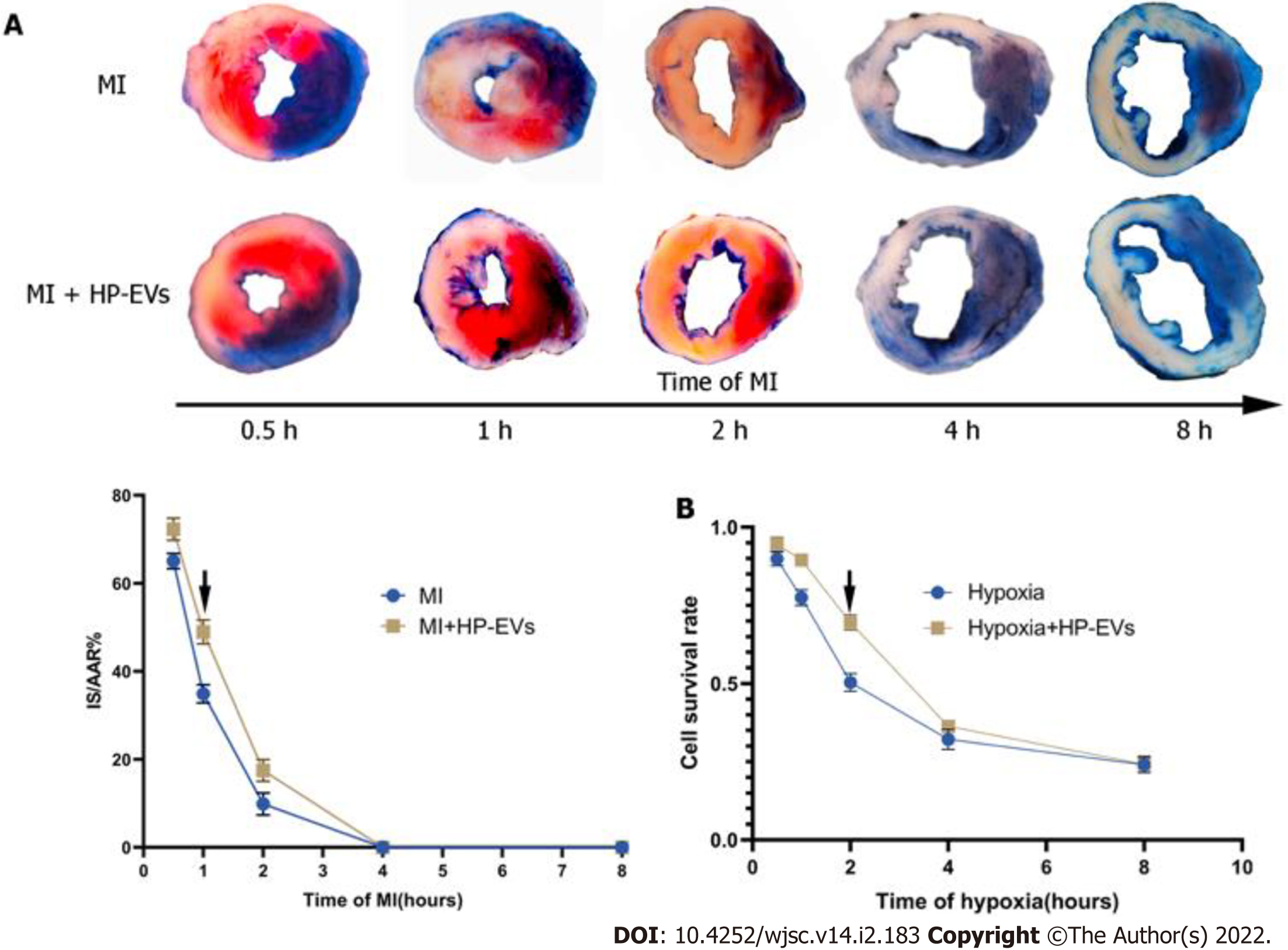
Figure 3 Evaluation of the temporal relationship of the cardioprotective effects of hypoxia-preconditioned extracellular vesicles.
A: Temporal relationship of the cardioprotective effects of hypoxia-preconditioned extracellular vesicles (HP-EVs) in vivo. Myocardial infarction (MI) models were established with left coronary artery ligation for 0.5, 1, 2, 4, and 8 h followed by 12 h of reperfusion. Evans Blue/2,3,5-triphenyltetrazolium chloride staining was used to evaluate the area of viable myocardium in the HP-EV and MI groups (n = 5); B: Temporal relationship of the HP-EV cardioprotective effects in vitro. Hypoxia injury models were established for 0.5, 1, 2, 4, and 8 h of hypoxia followed by 12 h of reoxygenation. Cell Counting Kit-8 assay was used to evaluate the cell survival rate in the HP-EV and hypoxia groups (x axis was time of hypoxia, y axis was the value of absorbance of experimental/control group, which reflected the survival rate of cells in the experimental condition; n = 5). The arrows indicate the most significant difference, and the corresponding time points were adopted for subsequent research. MI: Myocardial infarction; HP-EV: Hypoxia-preconditioned extracellular vesicles.
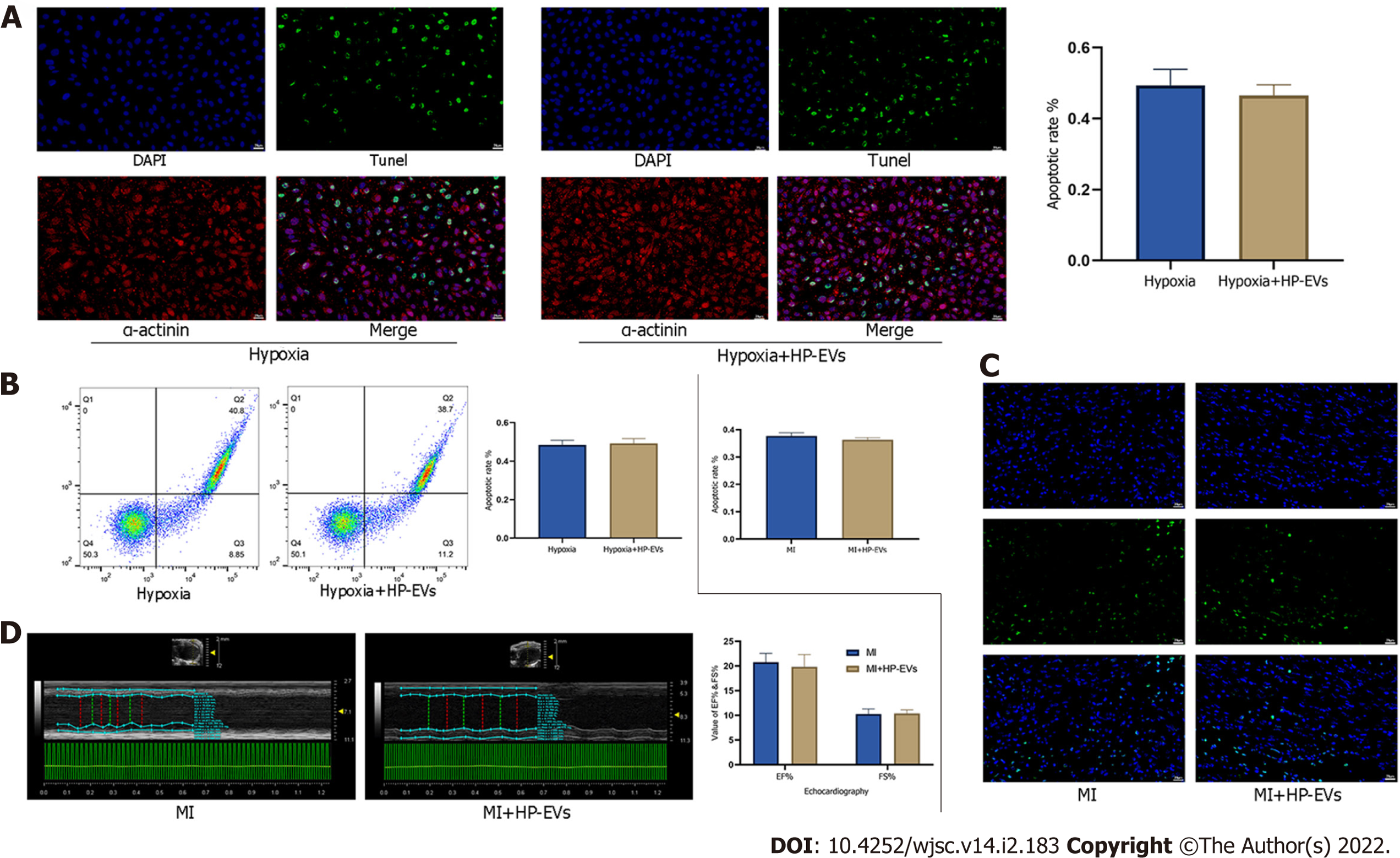
Figure 4 Cardioprotective effects of hypoxia-preconditioned extracellular vesicles after long periods of hypoxia and ischemia.
A: Degree of in situ apoptosis of cardiomyocytes (CMs) in hypoxia and hypoxia-preconditioned extracellular vesicle (HP-EV) groups after a long period (8 h) of hypoxia followed by 12 h of reoxygenation, as determined by the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay (n = 5); B: Degree of CM apoptosis in the hypoxia and HP-EVs groups after a long period (8 h) of hypoxia followed by 12 h of reoxygenation, as determined by flow cytometry (n = 5); C: Degree of in situ apoptosis of CMs in the myocardial infarction (MI) and HP-EVs groups after a long period of MI [left coronary arteries (LCAs) were ligated for 8 h followed by 12 h of reperfusion], as determined by the TUNEL assay (n = 5); D: Echocardiography was used to examine the heart function of the MI and HP-EVs groups on Day 3 after a long period of MI (LCAs were ligated for 8 h followed by 12 h of reperfusion); ejection fraction and fractional shortening were detected (n = 5). MI: Myocardial infarction; HP-EV: Hypoxia-preconditioned extracellular vesicles.
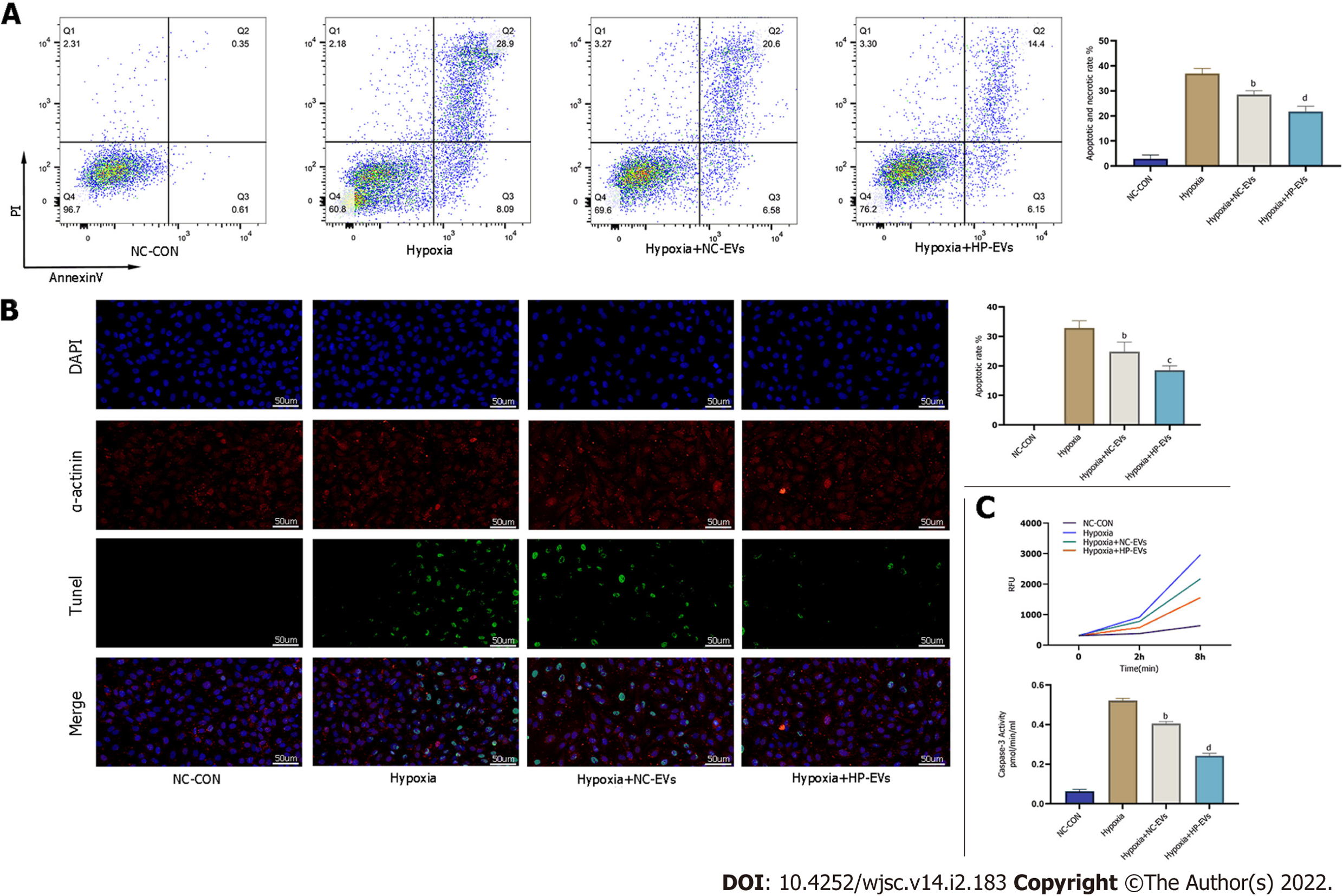
Figure 5 Hypoxia-preconditioned extracellular vesicles alleviated hypoxia/reoxygenation-induced apoptosis in vitro.
A: Degree of cardiomyocyte (CM) apoptosis in control, hypoxia, normoxic extracellular vesicle (NC-EV), and hypoxia-preconditioned EV (HP-EV) groups after 2 h of hypoxia followed by 12 h of reoxygenation, as determined by the Annexin V/PI assay (n = 5); B: Degree of CM apoptosis in control, hypoxia, NC-EV, and HP-EV groups after 2 h of hypoxia followed by 12 h of reoxygenation, as determined by the terminal deoxynucleotidyl transferase dUTP nick end-labeling assay (n = 5); C: Caspase-3 activity of CMs in the control, hypoxia, NC-EV, and HP-EV groups after 2 h of hypoxia followed by 12 h of reoxygenation (n = 5). All data are expressed as the mean ± SD. aP < 0.05, bP < 0.01 compared with Hypoxia group; cP < 0.05, dP < 0.01 compared with Hypoxia + NC-EVs group. HP-EV: Hypoxia-preconditioned extracellular vesicles; NC-EV: Normoxic extracellular vesicle.
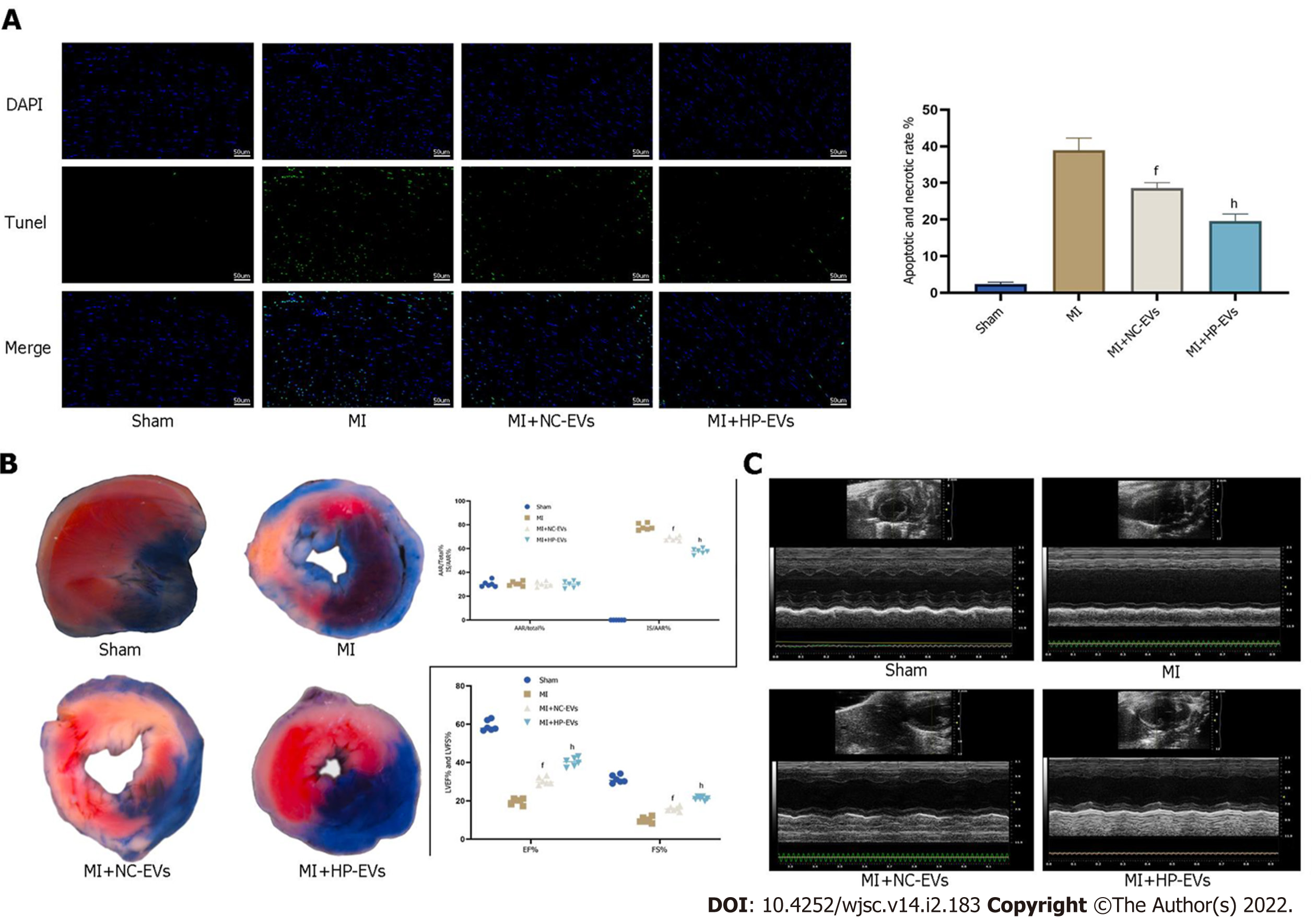
Figure 6 Hypoxia-preconditioned extracellular vesicles alleviated hypoxia/reoxygenation-induced apoptosis in vivo.
A: Degree of cardiomyocyte (CM) apoptosis in the control, sham, myocardial infarction (MI), normoxic extracellular vesicle (NC-EV), and hypoxia-preconditioned EV (HP-EV) groups after 2 h of left coronary artery (LCA) ligation followed by 12 h of reperfusion, as determined by the terminal deoxynucleotidyl transferase dUTP nick end-labeling assay (n = 5); B: HP-EV cardioprotective effects were assessed in vivo, and MI models were established with LCA ligation for 1 h followed by 12 h of reperfusion. Evans Blue/2,3,5-triphenyltetrazolium chloride staining was used to evaluate the area of viable myocardium in the sham, MI, NC-EV, and HP-EV groups (n = 5); C: Heart function of the sham, MI, NC-EV, and HP-EV groups was evaluated by echocardiography; ejection fraction and fractional shortening were detected (n = 6). All data are expressed as the mean ± SD. eP < 0.05, fP < 0.01 compared with MI group; gP < 0.05, hP < 0.01 compared with MI + NC-EVs group. MI: Myocardial infarction; HP-EV: Hypoxia-preconditioned extracellular vesicles; NC-EV: Normoxic extracellular vesicle.
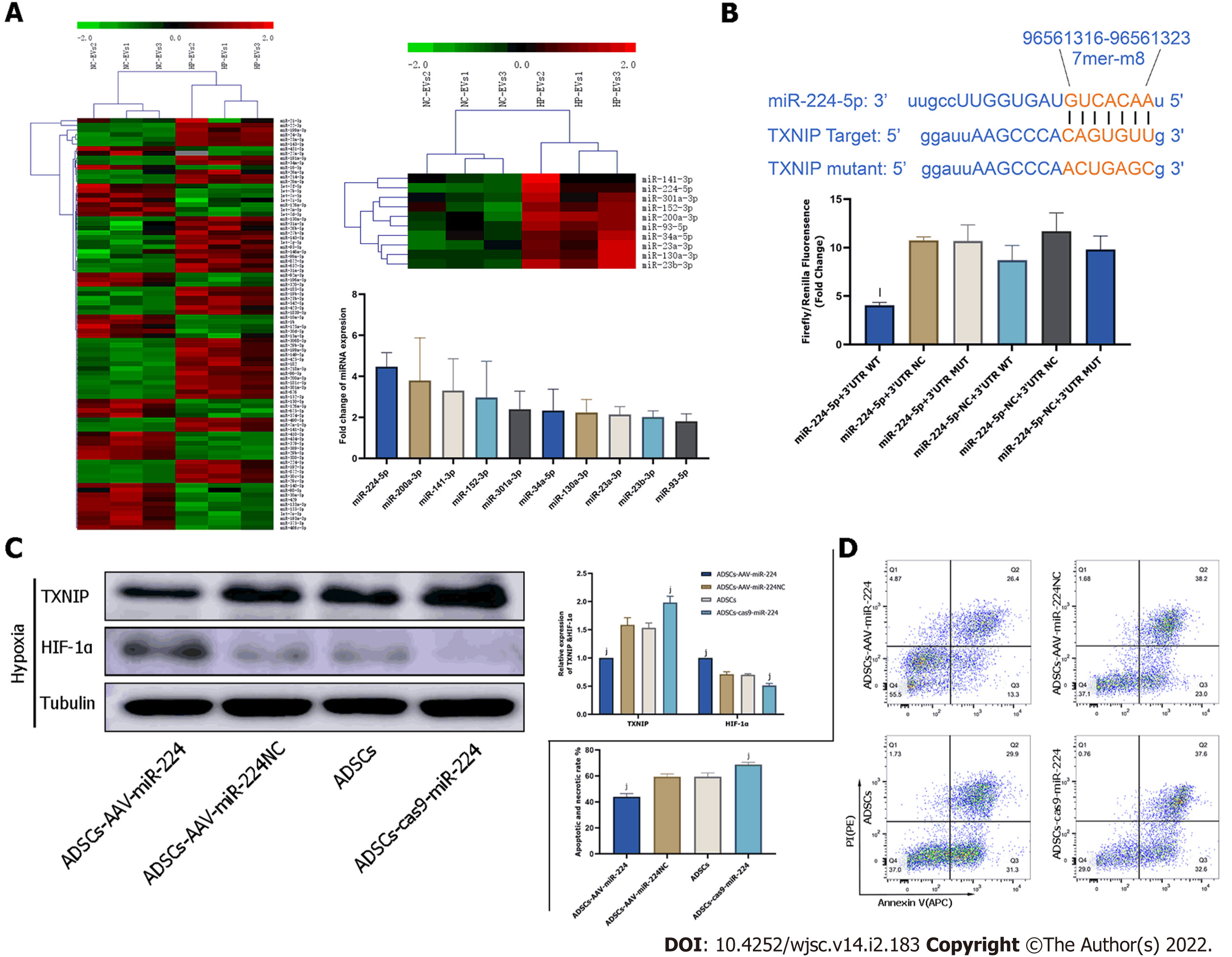
Figure 7 Extracellular vesicle-associated miR-224-5p inhibits hypoxia-induced apoptosis in cardiomyocytes by downregulating thioredoxin-interacting protein.
A: MicroRNA (miRNA) sequencing analysis in adipose-derived stem cell-extracellular vesicles (ADSC-EVs). A total of 88 miRNAs were upregulated in hypoxia preconditioned EVs (HP-EVs) compared to normoxic EVs (NC-EVs), of which 10 (shown in the heatmap) were predicted to associate with thioredoxin-interacting protein (TXNIP). Validation of differential EV-associated miRNA expression through quantitative PCR. U6 small nuclear RNA served as the internal reference; B: Dual luciferase reporter assay. HEK 293 T cells were co-transfected with miR-224-5p mimics and PGL3 Luciferase reporter plasmids containing wild-type (WT) or mutated TXNIP 3′-untranslated region (3’-UTR). mutated-TXNIP 3′-UTR served as the control. Dual luciferase reporter assay (All data are expressed as the mean ± SD , kP < 0.05, lP < 0.01, miR-224-5p + TXNIP WT 3′-UTR vs miR-224-negative + 3’-UTR-mutant groups; n = 3); C: Neonatal mouse CMs subjected to hypoxia were pre-treated with EVs derived from ADSCs (ADSCs group), ADSCs overexpressing miR-224 (ADSCs-AAV-miR-224 group), ADSCs overexpressing miR-224-NC (ADSCs-AAV-miR-224NC group) and ADSCs with miR-224 knocked-out (ADSCs-cas9-miR-224 group). Western blotting was used to detect the expression of hypoxia-inducible factor-1 alpha and TXNIP (n = 5); D: Neonatal mouse CMs subjected to hypoxia were pre-treated with EVs derived from ADSCs (ADSCs group), ADSCs overexpressing miR-224 (ADSCs-AAV-miR-224 group), ADSCs overexpressing miR-224-NC (ADSCs-AAV-miR-224NC group) and ADSCs with miR-224 knocked-out (ADSCs-cas9-miR-224 group). Annexin V/PI assay was used to assess the degree of apoptosis in four groups (n = 5). All data are expressed as the mean ± SD, iP < 0.05, jP < 0.01, compared with CMs subjected to hypoxia were pre-treated with EVs derived from ADSCs group(ADSCs group). HP-EV: Hypoxia-preconditioned extracellular vesicles; NC-EV: Normoxic extracellular vesicle; ADSC: Adipose-derived mesenchymal stem cell; WT: Wild-type.
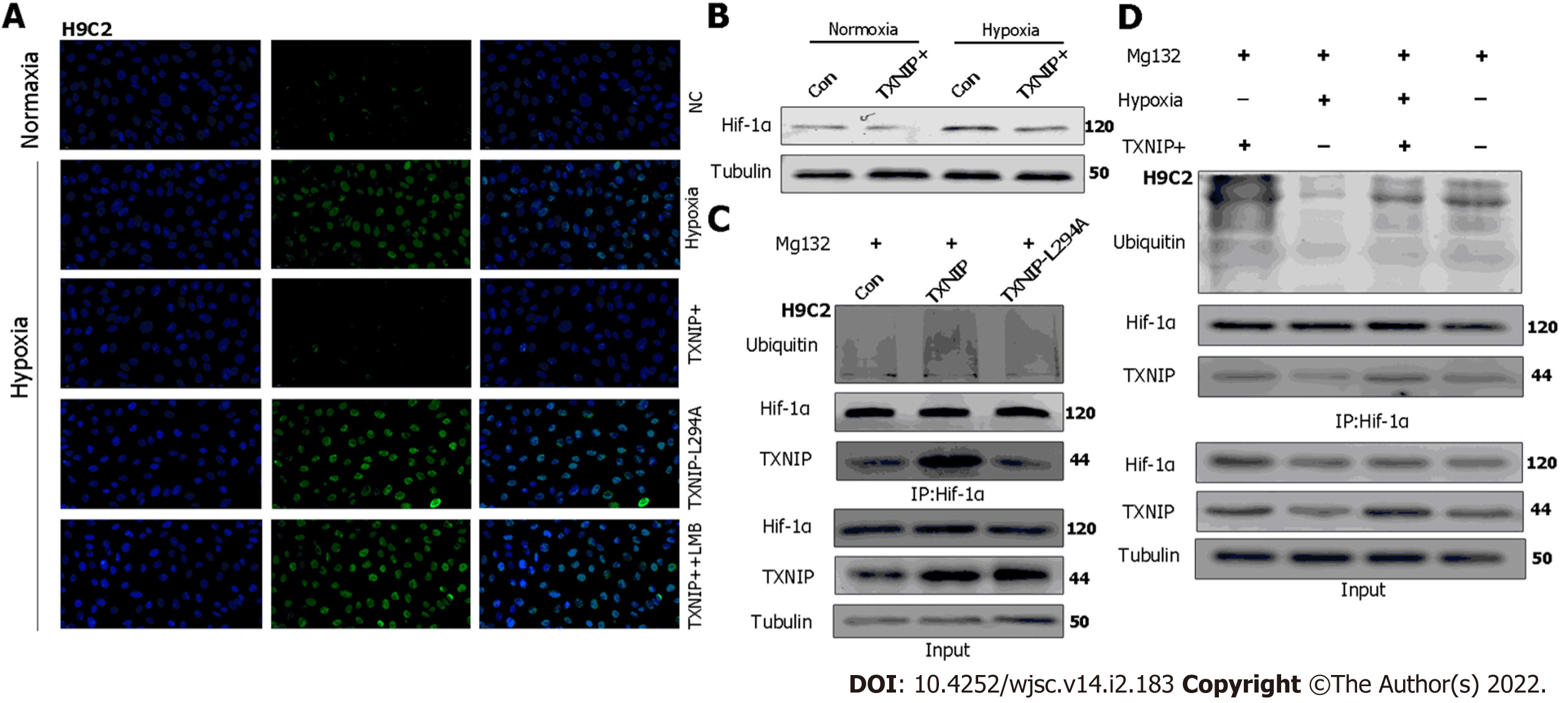
Figure 8 Thioredoxin-interacting protein promotes the ubiquitination of hypoxia-inducible factor-1 alpha through the chromosomal region maintenance-1 nuclear export pathway.
A: H9C2 cardiomyocytes (CMs) overexpressing thioredoxin-interacting protein (TXNIP) or TXNIP-L294A mutant as indicated and leptomycin B were used to inhibit the chromosomal region maintenance 1 nuclear export pathway. The expression and cellular localization of hypoxia-inducible factor-1 alpha (HIF-1α) was analyzed by immunofluorescence staining; B: H9C2CMs were treated with hypoxia or normoxia and TXNIP was overexpressed as indicated. The expression of HIF-1α was analyzed by western blotting; C: H9C2 CMs were pretreated with MG132 for 2 h and TXNIP or TXNIP-L294A-mutant were overexpressed as indicated. The interaction of TXNIP and HIF-1α, and HIF-1α ubiquitination were assessed by immunoprecipitation with an anti-HIF-1α antibody; D: H9C2 CMs were pretreated with MG132 for 2 h. TXNIP was overexpressed and hypoxia treatment was applied as indicated. The interaction of TXNIP and HIF-1α, and HIF-1α ubiquitination were assessed by immunoprecipitation with an anti-HIF-1α antibody.
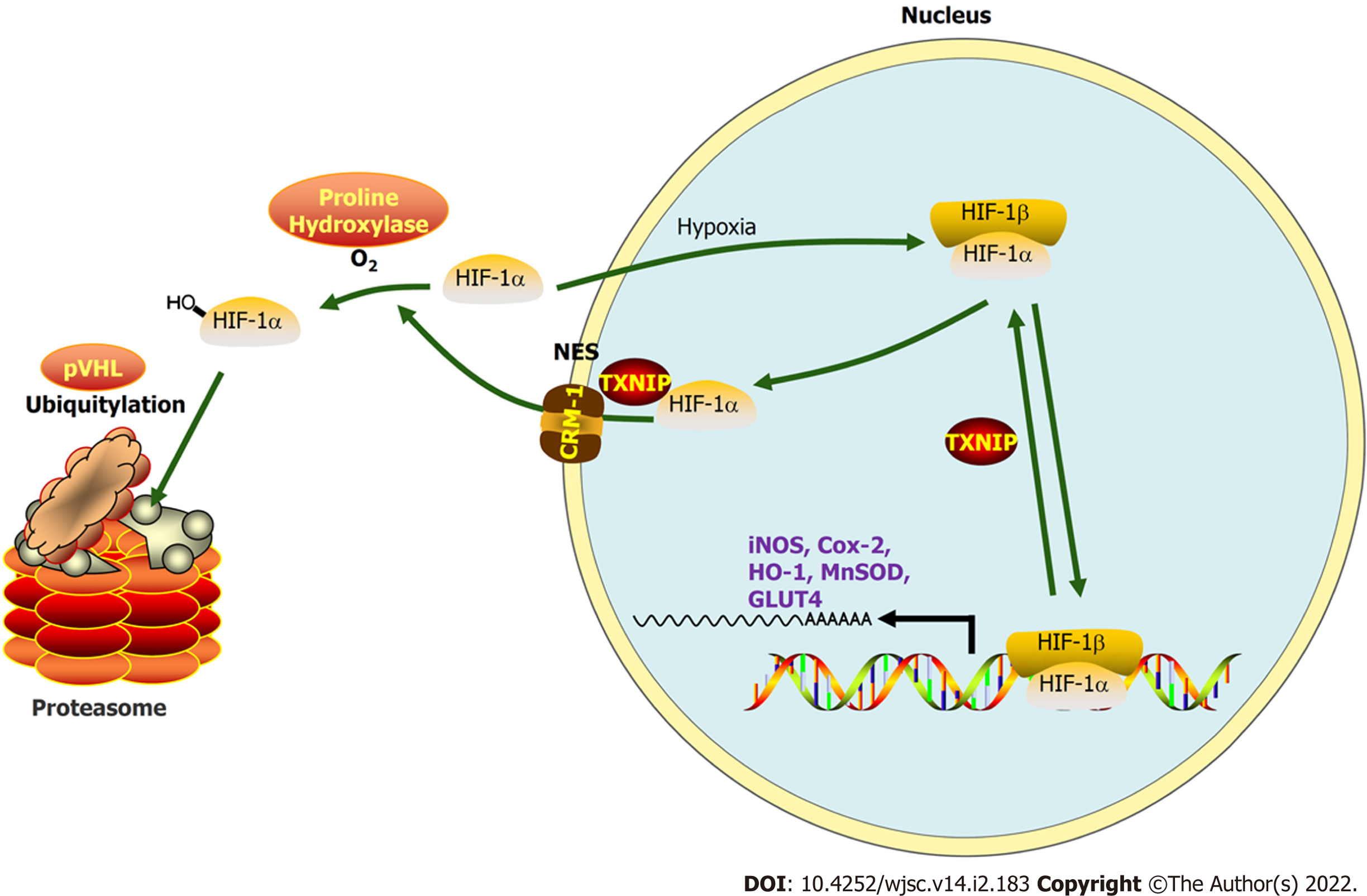
Figure 9 During myocardial infarction or hypoxia, hypoxia-inducible factor-1 alpha export is chromosomal region maintenance-1-dependent in the presence of thioredoxin-interacting protein, based on the association of thioredoxin-interacting protein with chromosomal region maintenance-1.
Thioredoxin-interacting protein (TXNIP)-induced hypoxia-inducible factor-1 alpha (HIF-1α) nuclear export may be hypoxia-independent, which triggers ubiquitination and degradation by the proteasome in cardiomyocytes. As a transcription factor, the function of HIF-1 in promoting targeted gene transcription is abolished by hypoxia/myocardial infarction-triggered TXNIP activation. Our research aimed to clarify this process by hypoxia-preconditioned extracellular vesicles and the underlying mechanism.
- Citation: Mao CY, Zhang TT, Li DJ, Zhou E, Fan YQ, He Q, Wang CQ, Zhang JF. Extracellular vesicles from hypoxia-preconditioned mesenchymal stem cells alleviates myocardial injury by targeting thioredoxin-interacting protein-mediated hypoxia-inducible factor-1α pathway. World J Stem Cells 2022; 14(2): 183-199
- URL: https://www.wjgnet.com/1948-0210/full/v14/i2/183.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i2.183