Copyright
©The Author(s) 2021.
World J Stem Cells. Oct 26, 2021; 13(10): 1417-1445
Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1417
Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1417
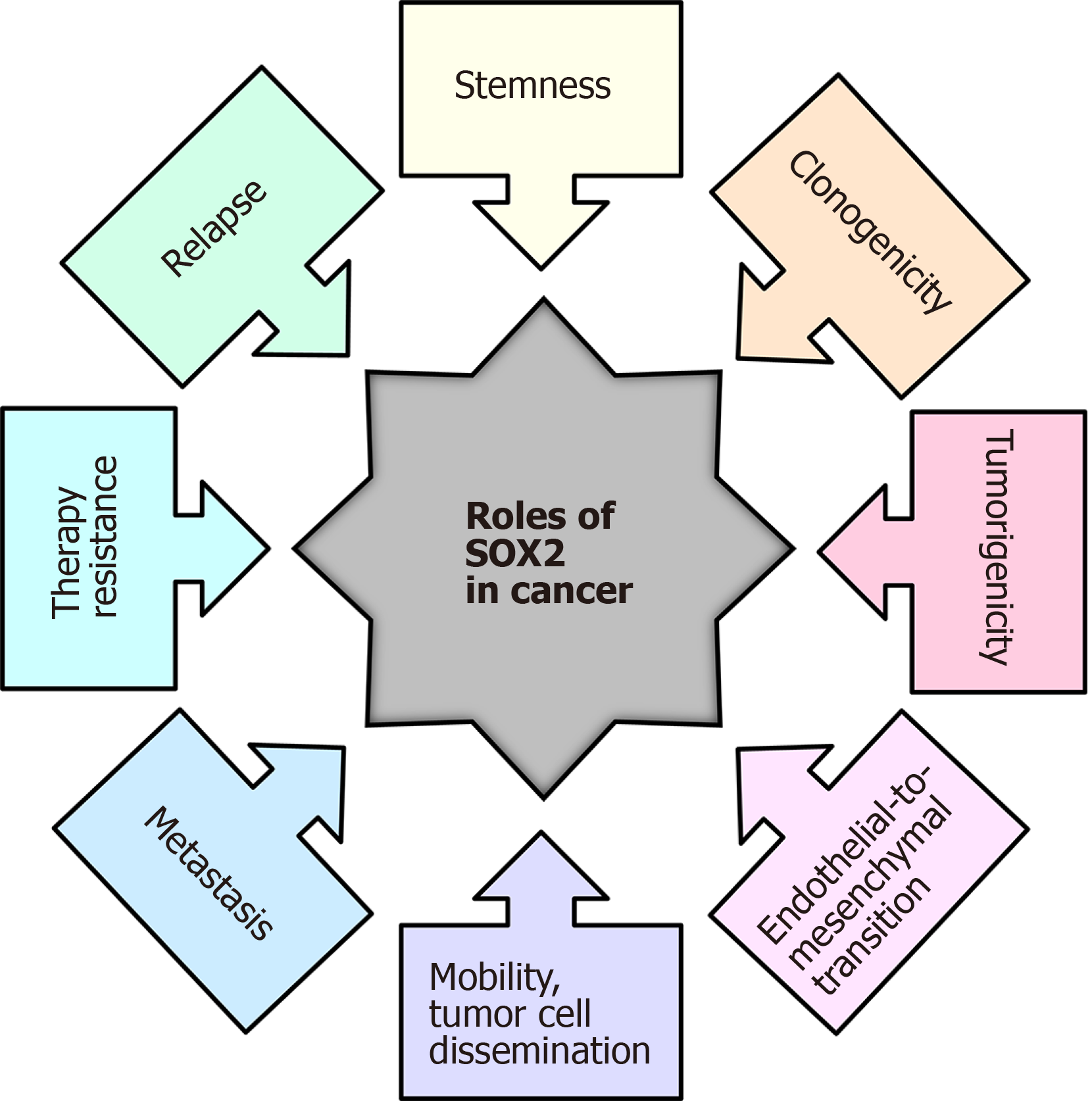
Figure 2 The roles of the SOX2 transcription factor in cancer.
Modified from[223].
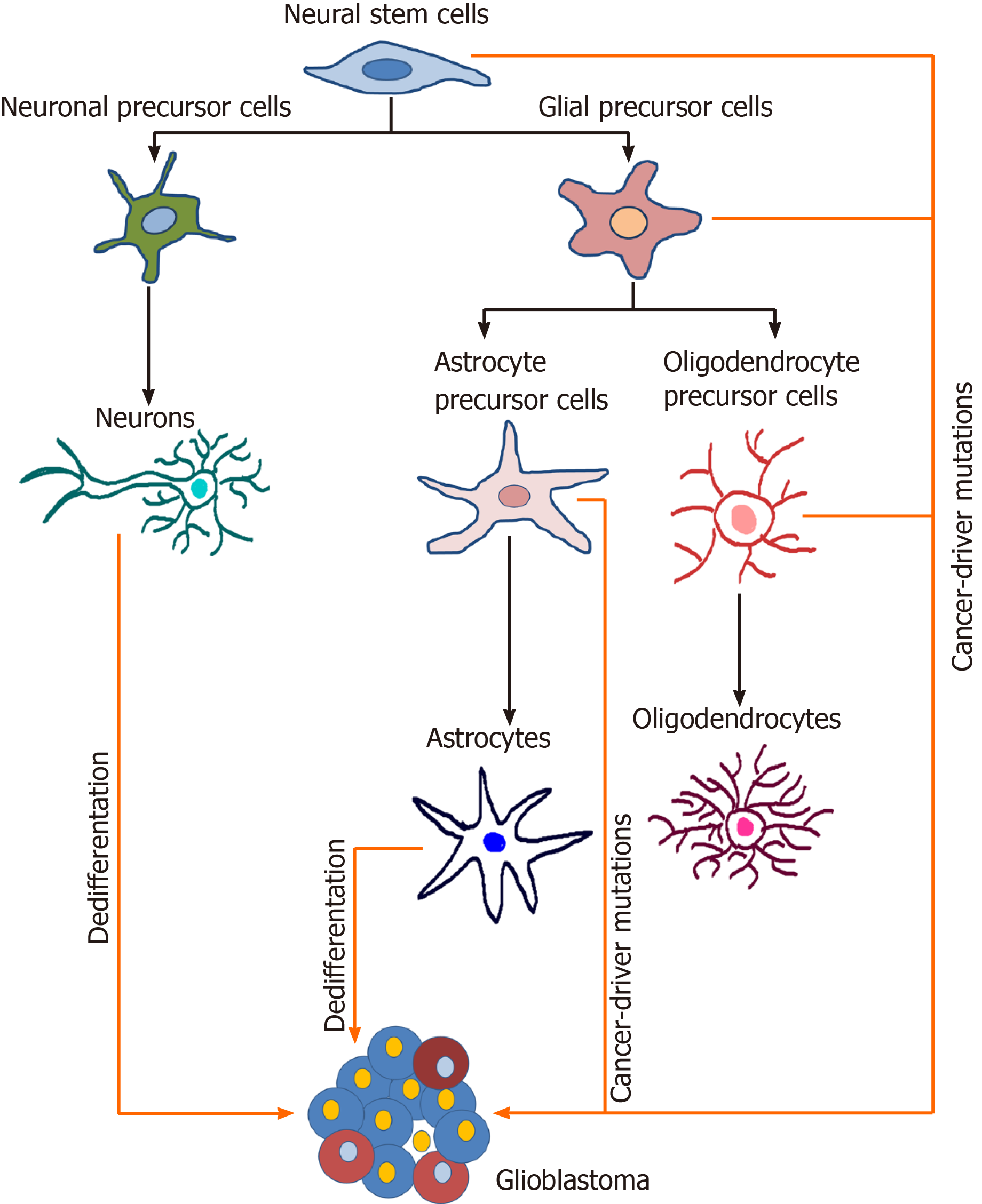
Figure 3 Potential cells of origin of glioblastoma.
During the neural differentiation process (black arrows), neural stem cells differentiate into fate-restricted precursors that are capable of differentiating into neurons, astrocytes or oligodendrocytes. Glioblastoma cells can arise due to the accumulation of cancer driver mutations in different cell types or due to the dedifferentiation of mature cells (orange arrows). Modified from[224]. References are included in the main text.
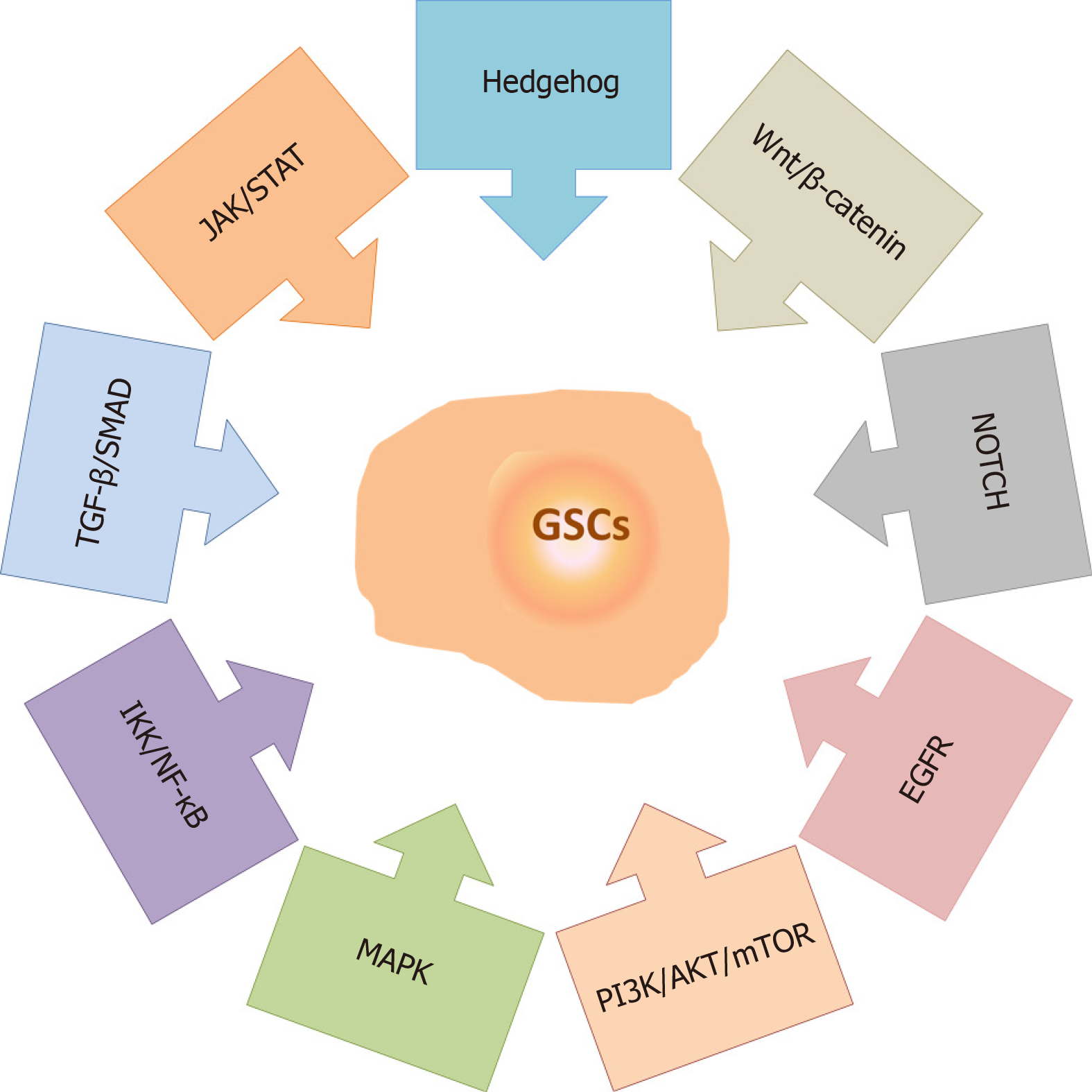
Figure 4 Signaling pathways involved in the maintenance of glioma stem cell properties.
The main signaling pathways involved in the maintenance of glioma stem cell properties include Hedgehog, Wnt/β-catenin, NOTCH, Epidermal growth factor receptor, Phosphatidylinositol-3-phosphate kinase/AKT/Mammalian target of rapamycin, Mitogen-activated protein kinase, Inhibitory kappa B kinase/Nuclear factor-kappa B, Transforming growth factor-beta/Small mothers against decapentaplegic and Janus kinase/Signal transducers and activators of transcription. References are included in the main text. MAPK: Mitogen-activated protein kinase; JAK/STAT: Janus kinase/Signal transducers and activators of transcription; EGFR: Epidermal growth factor receptor; PI3K/AKT/mTOR: Phosphatidylinositol-3-phosphate kinase/AKT/Mammalian target of rapamycin; IKK/NF-κB: Inhibitory kappa B kinase/Nuclear factor-kappa B; TGF-β/SMAD: Transforming growth factor-beta/Small mothers against decapentaplegic; GSCs: Glioma stem cells.
Figure 5 The roles of SOX transcription factors in glioma stem cells.
SOX transcription factors exert numerous functions in glioma stem cells (GSCs). They influence GSC self-renewal, stemness, tumorigenicity, sphere-forming capacity, proliferation rate, viability, migration and invasion capacities, and apoptosis. Some of the members of the SOX gene family are involved in the resistance of GSCs to therapy. Blue arrows represent stimulation, and orange bars represent inhibition. References are included in the main text. GSCs: Glioma stem cells.
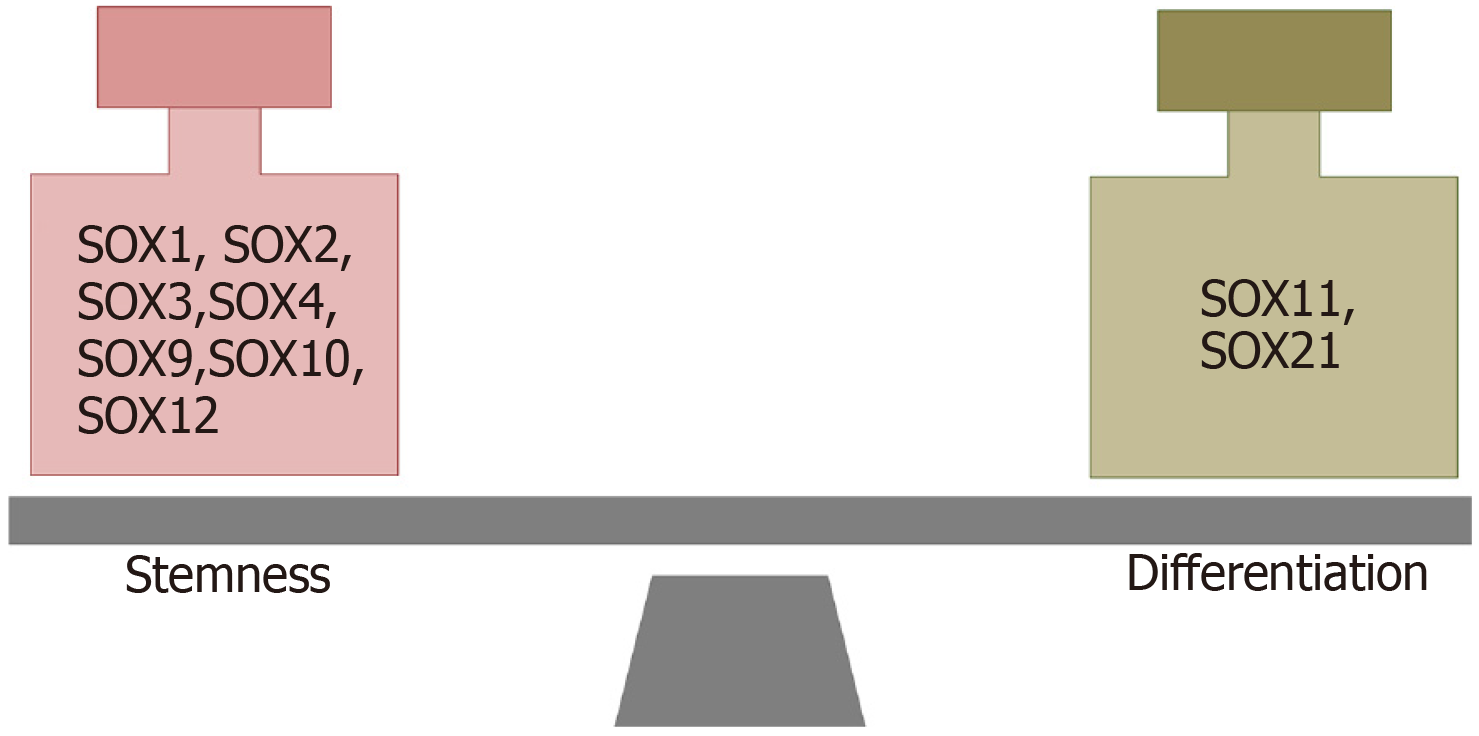
Figure 6 SOX transcription factors involved in the maintenance of stemness and differentiation of glioma stem cells.
References are included in the main text.
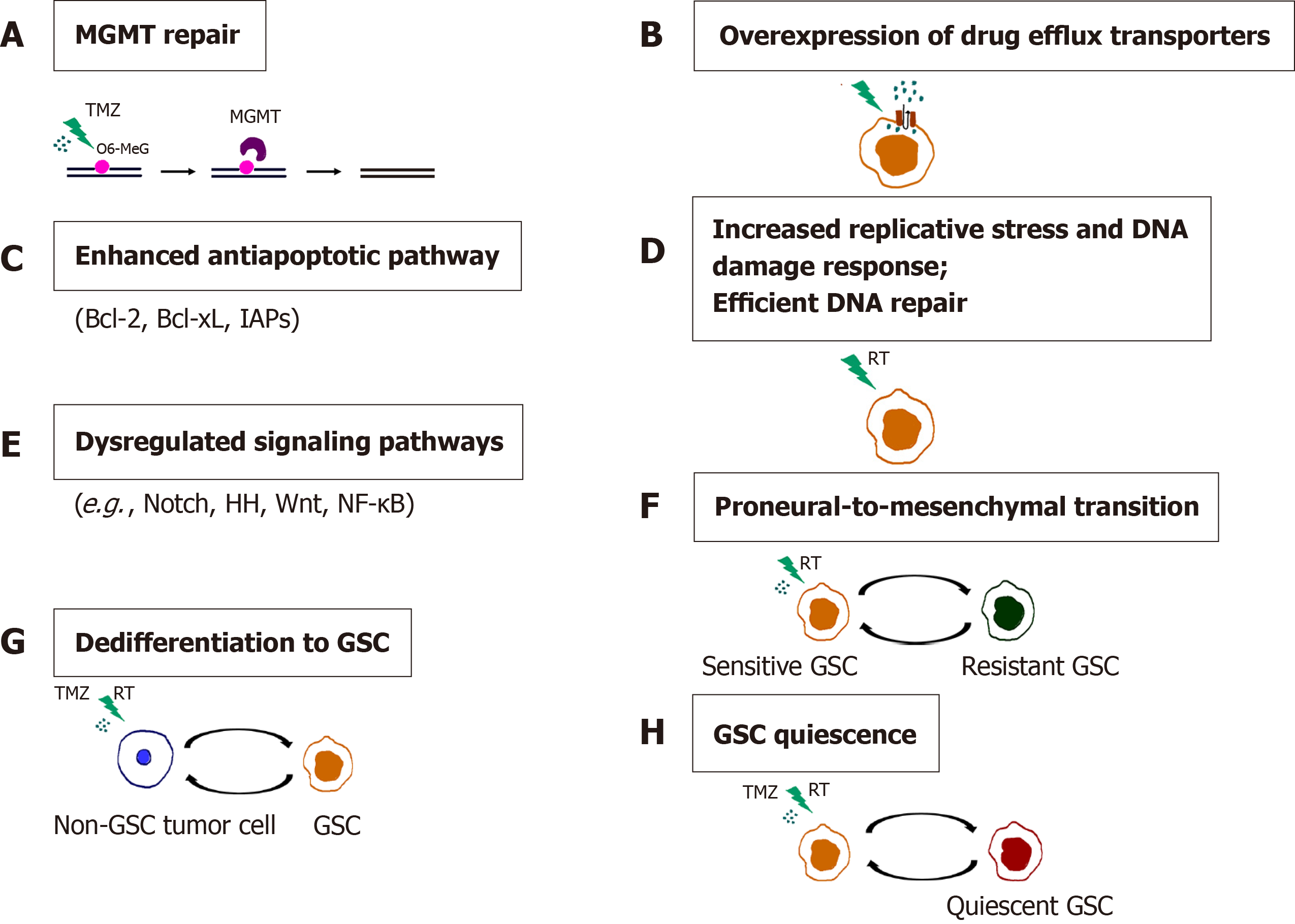
Figure 7 Mechanisms of chemo- and radioresistance of glioma stem cells.
A: O6-methylguanine-DNA methyltransferase (MGMT) repair; B: Overexpression of drug efflux transporters; C: Enhanced antiapoptotic pathway; D: Increased replicative stress and DNA damage response. Efficient DNA repair; E: Dysregulated signaling pathways; F: Proneural-to-mesenchymal transition; G: Dedifferentiation to GSC; H: Glioma stem cells (GSC) quiescence. Resistance of GSCs to temozolomide is achieved through increased expression of MGMT, drug efflux transporters and antiapoptotic proteins. Replicative stress, constitutively active DNA damage response and efficient DNA repair confer radioresistance to GSCs. Aberrantly activated signaling pathways, the ability of non-GSC tumor cells to dedifferentiate, proneural-to-mesenchymal transition and the capacity of GSCs to become quiescent contribute to the chemo- and radioresistance of glioblastoma. References are included in the main text. RT: radiation; O6-MeG: O-6-methylguanine; GSC: Glioma stem cell; TMZ: Temozolomide.
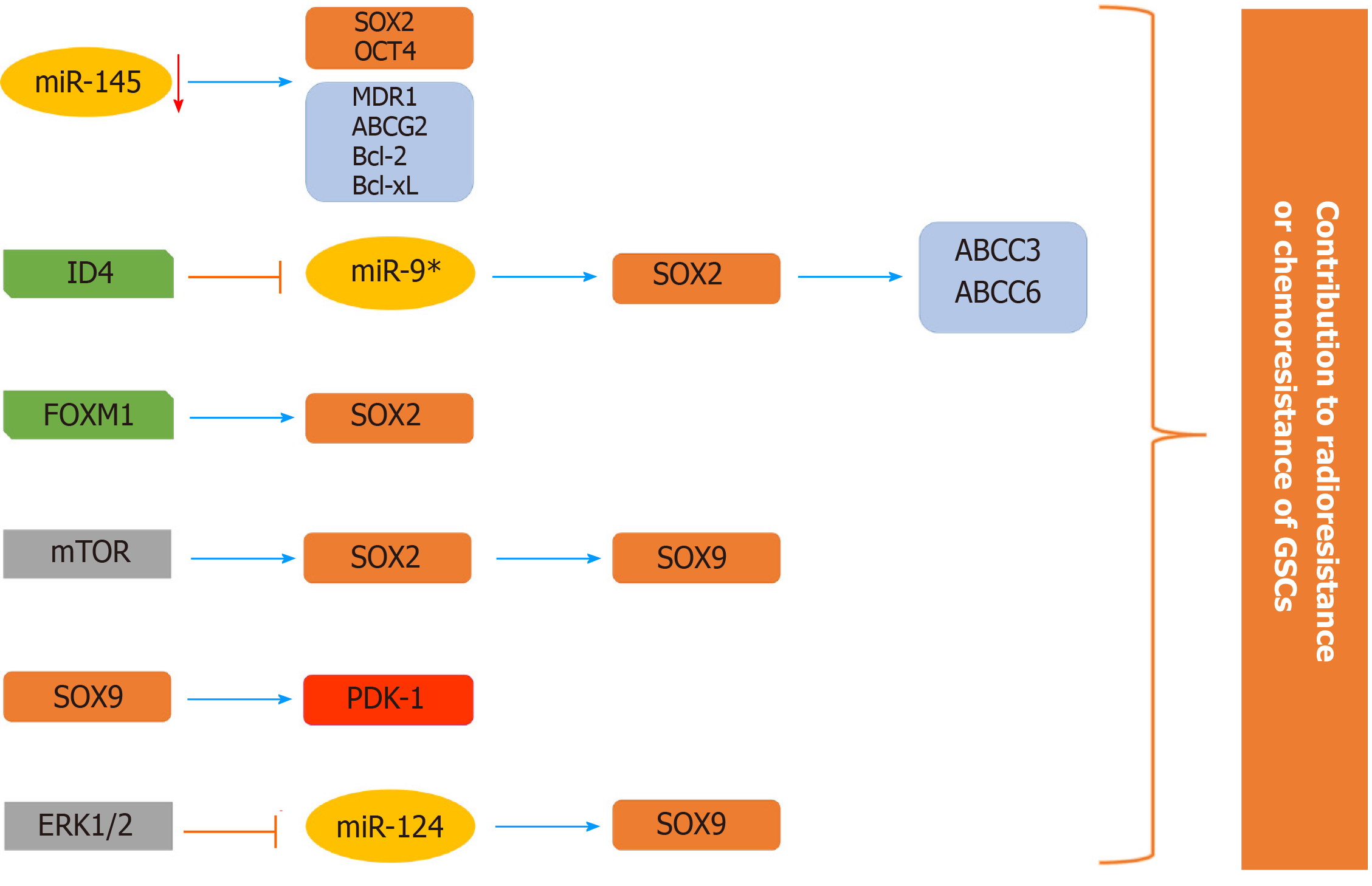
Figure 8 Schematic illustration of the involvement of SOX2 and SOX9 in the chemoresistance and radioresistance of glioma stem cells.
The expression of stemness-related transcription factor SOX2 is elevated in glioma stem cells (GSCs) through various mechanisms (decreased expression of miR-145 and miR-9* that directly target SOX2, direct transcriptional activation by Forkhead box M1 and through mammalian target of rapamycin signaling) contributing to chemo- and radioresistance of GSCs. SOX2 directly activates the expression of ABCC3 and ABCC6 transporters. mTOR signaling affects the SOX2/SOX9 axis, contributing to chemoresistance. The ERK1/2/miR-124/SOX9 axis and direct targeting of PDK1 (pyruvate dehydrogenase kinase 1) by SOX9 have a role in resistance to radiation and temozolomide, respectively. References are included in the main text. GSCs: Glioma stem cells; FOXM1: Forkhead box M1; mTOR: Mammalian target of rapamycin; PDK1: Pyruvate dehydrogenase kinase 1.
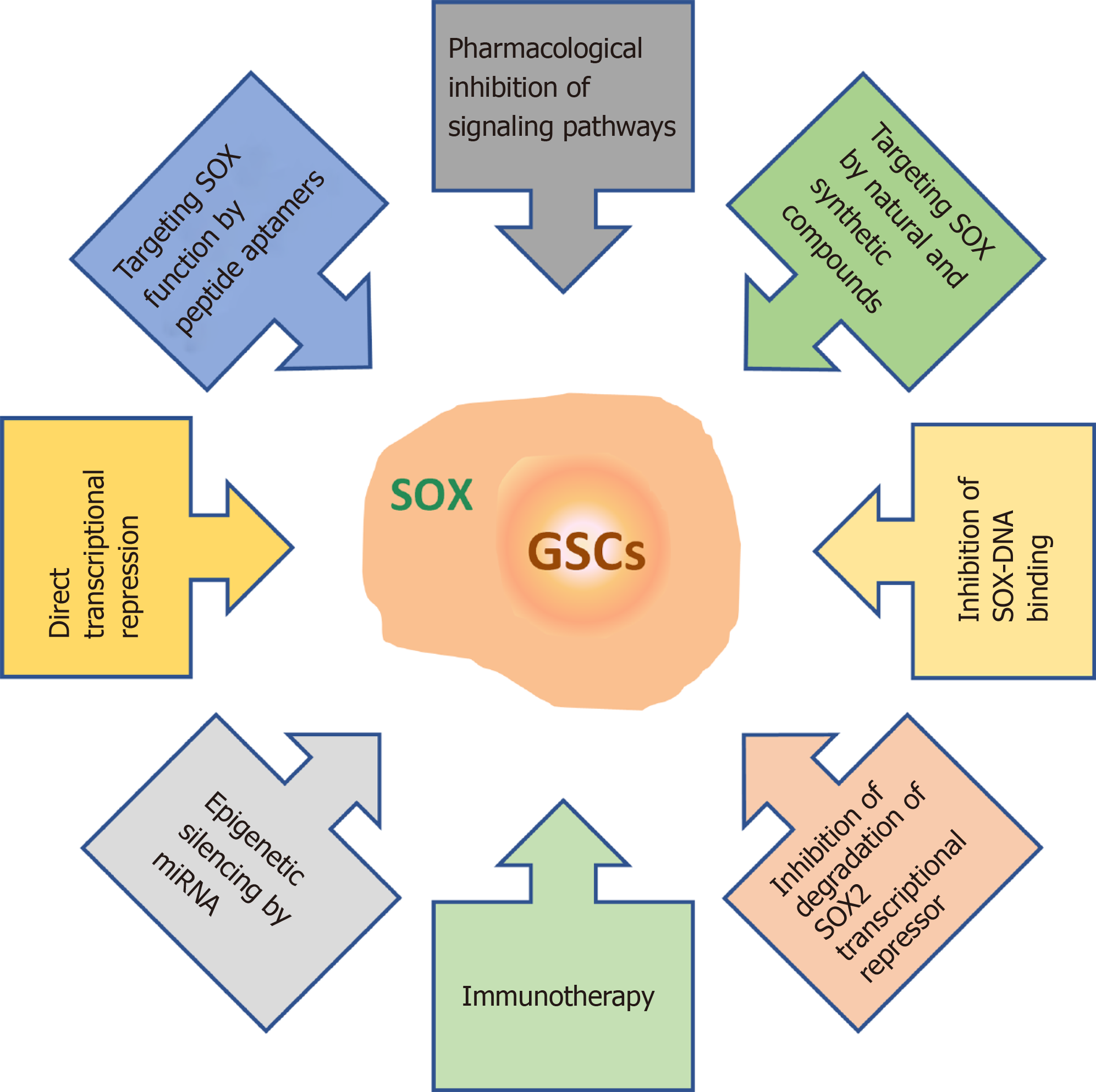
Figure 9 Overview of the therapeutic strategies for targeting SOX in glioma stem cells.
References are included in the main text. GSCs: Glioma stem cells.
- Citation: Stevanovic M, Kovacevic-Grujicic N, Mojsin M, Milivojevic M, Drakulic D. SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation. World J Stem Cells 2021; 13(10): 1417-1445
- URL: https://www.wjgnet.com/1948-0210/full/v13/i10/1417.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i10.1417