Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.1946
Revised: April 5, 2003
Accepted: April 14, 2003
Published online: September 15, 2003
AIM: To clarify the location and distribution of Kupffer cells in hepatocellular carcinoma (HCC), and to investigate their role in hepatocarcinogenesis.
METHODS: Kupffer cells were immunohistochemically stained by streptavadin-peroxidase conjugated method (S-P). The numbers of Kupffer cells in cancerous, para-cancerous and adjacent normal liver tissues of 48 HCCs were comparatively examined.
RESULTS: The mean number of Kupffer cells in cancerous, para-cancerous and adjacent normal liver tissues was 12.7 ± 6.8, 18.1 ± 8.2 and 18.9 ± 7.9 respectively. The number of Kuppfer cells in cancerous tissues was significantly lower than that in para-cancerous tissues (t = 2.423, P < 0.05) and adjacent normal liver tissues (t = 2.521, P < 0.05). As tumor size increased, the number of Kupffer cells in cancerous tissues significantly decreased (F = 4.61, P < 0.05). Moreover, there was also a significant difference in the number of Kupffer cells among well-differentiated, moderately-differentiated and poorly-differentiated cases(F = 4.49, P < 0.05).
CONCLUSION: This study suggests that decrease of Kupffer cells in HCCs may play an important role in the carcinogenesis of HCC, the number of Kupffer cells in HCC is closely related to the size and differentiation grade of the tumor.
- Citation: Liu K, He X, Lei XZ, Zhao LS, Tang H, Liu L, Lei BJ. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol 2003; 9(9): 1946-1949
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/1946.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.1946
Kupffer cells are important in maintaining homeostasis and in host anti-tumor defense mechanism[1-5]. It is thought that Kupffer cells are resident macrophages in the liver and are one kind of the sinusoidal endothelial cells[6]. Previously, Kupffer cells were not considered to exist in hepatocellular carcinoma (HCC) tissues. Recently, some studies revealed that Kupffer cells were also present in early-stage and well-differentiated HCC. Because small and well-differentiated HCCs could maintain an environment similar to that of normal sinusoids, Kupffer cells may also exist in these HCCs[7,8].
Unfortunately, whether Kupffer cells could exist in poorly-differentiated or large HCCs remaines unidentified. Nevertheless, the difference of Kupffer cells among variable tissue types is not clear. The relationship between the decrease of Kupffer cells and carcinogenesis of HCC also needs to be clarified.
In the present study, we pathomorphologically examined the localization and distribution of Kupffer cells in 48 cases of HCCs samples embracing cancerous tissues, corresponding para-cancerous tissues and adjacent normal liver tissues.
Tissues of forty-eight primary HCCs including cancerous tissues, corresponding para-cancerous tissues and adjacent normal liver tissues were obtained with the informed consent of patients who underwent hepatectomy at the West China Hospital of Sichuan University. The surgically resected tissues were fixed in 10% formalin, embedded in paraffin, cut into 5 μm sections and stained with hematoxylin-eosin. Histopathological diagnosis and classification were made by the same pathologist.
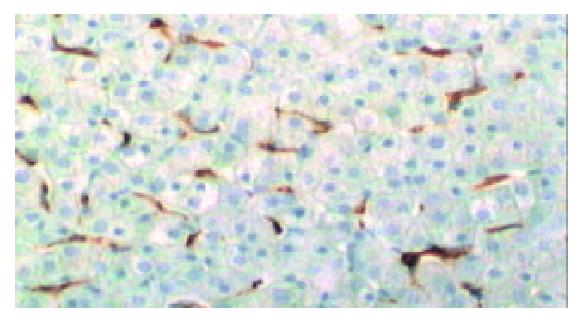
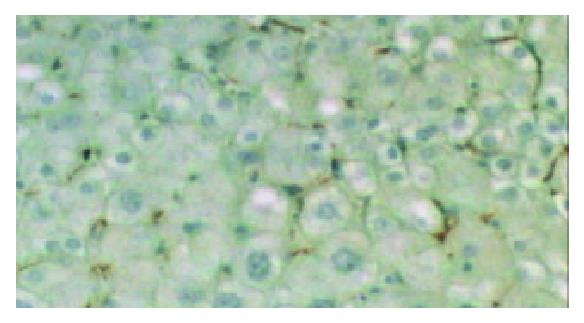
The SP method was used, and the first antibody was mouse-anti-human monoclonal CD68 antibody (DAKO, dilution 1: 50). The operation procedure was according to the instructions of SP kit which was purchased from DAKO, Glostrup, Denmark. DAB was used for coloration. The dark brown granules in cytoplasm were taken as CD68 positive reaction. Negative mouse serum and PBS were respectively used to replace 1st antibody as negative control and blank control.
Among the cells which were anti-CD68 antibody positive, those in the blood space of cancerous tissues or the sinusoids of noncancerous tissues with a stellate or spindle shape were evaluated as Kupffer cells.
The number of Kupffer cells was counted in five randomly selected visual fields under a microscope (× 200) for each specimen. Then the average Kupffer cell number of each specimen was determined.
The data of Kupffer cells were expressed as mean ± standard deviation (Mean ± SD), and the differences in the values of different groups were analyzed by t test and F test. The criterion of significance was set at P < 0.05.
Kupffer cells were present in the cancerous tissues of 45 out of 48 cases of HCCs (93.8%). The only 3 specimens in which no Kupffer cells were found were all poorly-differentiated HCCs. Kupffer cells were found in the para-cancerous tissues and adjacent normal liver tissues of all 48 HCCs.
The mean number of Kupffer cells in cancerous, para-cancerous and adjacent normal liver tissues was 12.7 ± 6.8, 18.1 ± 8.2 and 18.9 ± 7.9 respectively. The number of Kuppfer cells in cancerous tissues was significantly lower than that in para-cancerous tissues and adjacent normal liver tissues (P < 0.05) (Table 1). The number of Kupffer cells in cancerous tissues of well, moderately and poorly differentiated HCCs was 18.4 ± 4.2, 11.2 ± 6.2 and 5.2 ± 4.9, respectively (Table 2).The number of Kupffer cells in cancerous tissues significantly decreased as the histological grades decreased (P < 0.05) (Figure 1).
Among the 48 HCCs, 12 cases had a tumor size < 3 cm, 17 between 3-5 cm and 19 > 5 cm. Mean Kupffer cell number in the cancerous tissues of the three groups was 17.4 ± 4.8, 12.5 ± 6.3 and 7.9 ± 5.8 respectively (Table 2).The three poorly-differentiated cases of HCCs in which there was no Kupffer cell in cancerous tissues all had a diameter > 5 cm. Mean Kupffer cell number in cancerous tissues of HCCs which had a diameter < 3 cm was significantly higher than that of the cases of HCCs which had a diameter between 3-5 cm or > 5 cm (P < 0.05). In addition, Kupffer cell number in cancerous tissues of tumors which had a diameter between 3-5 cm differed significantly from that of the cases of HCCs which had a diameter > 5 cm (Figure 2 and Figure 3). As the tumor size increased, the Kupffer cell number decreased significantly (P < 0.05).
Kupffer cells are important in the host defense mechanism including normal metabolism, phagocytosis, cytokine generation and anti-tumor effects. They are also involved in the pathogenesis of liver diseases such as viral hepatitis, alcohoic liver injury, chemically mediated liver injury, liver fibrosis, ischemia and reperfusion injury in liver transplantation, and hepatocyte regeneration[9-23]. Functional capability of Kupffer cells is considered to decrease when the liver is impaired. Usually, light-microscope is used to identify Kupffer cells by confirming the presence of lipofuscin and hemosiderin pigments in the cytoplasm. Moreover, Kupffer cells can be identified by monitoring their unique ultra-structures. For example, they have numerous vesicles, well-developed lamillipodia in cytoplasm and lysosomes in various sizes. In this study, we used anti-CD68, an anti-human macrophage antibody, to identify macrophages. CD68 was expressed not only in the residential macrophages such as Kupffer cells, but also in the migrating macrophages. In view of this fact, Kupffer cells cannot be identified merely by being anti-CD68 positive. Morphological observations are also required to distinguish between these two cell types. For example, migrating macrophages are usually oval and contain abundant cytoplasm, while Kupffer cells usually have spindle or stellate-shaped cytoplasm and partly adhere to the sinusoidal endothelial cells[24-29].
The origin of Kupffer cells in normal liver tissues remains unidentified. One hypothesis postulates that Kupffer cells are originated from the macrophages which have been present in the premordial liver at the embryonal stage. The other hypothesis proposes that monocytes which are originated from bone marrow arrive, settle in the sinusoids and then differentiate into Kupffer cells. There are two possible mechanisms of Kupffer cells existing in cancerous tissues of HCC. (1) Under the environmental condition similar to normal sinusoids, migrating macrophages in the blood space change into Kupffer cell-like cells. (2) Kupffer cells in normal liver tissue are maintained in the cancerous tissues. Our results showed that all the three poorly differentiated HCCs contained no Kupffer cell in cancerous tissues. Moreover, Kupffer cells in cancerous tissues of poorly differentiated HCCs were significantly less than those of well or moderately differentiated HCCs. In view of the fact that the morphology of poorly differentiated cancerous tissues is quite different from that of normal liver tissue, the former hypothesis may be more reasonable. We also found Kupffer cell number in para-cancerous and adjacent normal liver tissues had no statistical difference, probably due to the fact that para-cancerous tissues present in the blood space closer to the normal sinusoids. On the other hand, Kupffer cells were found to be activated in the pathogenesis of liver injuries such as early-stage fibrosis and fatty liver hepatitis. Under these conditions the sinusoid structures usually were not destroyed seriously and Kupffer cell number did not decrease significantly[30-32].
The mechanism responsible for the tumoricidal activities of Kupffer cells is not completely known. Kupffer cells may execute their anti-tumor effect via increasing the production of some cytotoxic molecules such as NO, TNF-α and IFN-γ, which may inhibit the growth of tumor by damaging cellular DNA and inducing apoptosis. When implanted into normal and cirrhotic rat livers, rat HCC cells grew much more progressively in cirrhotic livers than in normal livers. Meanwhile, Kupffer cells were decreased profoundly in cirrhotic livers, resulting in markedly impaired phagocytic activity. Furthermore, profound decrease production of Kupffer cell-related cytokines was found to in cirrhotic livers[1,5,18]. Previous studies also found that Kupffer cells might play an important role in controlling occurrence and progression of liver metastasis. The possible pathway of Kupffer cells against liver metastasis might be that tumor cells were apoptotic via the Fas-Fas ligand system induced by TNF-α released from Kupffer cells[33-35]. When the liver is chemically injured, Kupffer cells may release biologically active mediators that promote the pathogenic process. Though there is evidence that indicates Kupffer cells play a stimulatory role in liver regeneration, presently Kupffer cells are thought to have the potential to exert both stimulatory and inhibitory influences on hepatocyte proliferation[14,36-38]. Some research suggested that in viral hepatitis, Kupffer cells were activated and expressed high levels of CD80, CD40 and class-II MHC molecules, thus acquiring the phenotype of antigen presenting cells (APCs)[39].
Enhanced magnetic resonance imaging (MRI) has been used to detect hepatic tumors[40]. The method utilizes selective taken-up mechanism of superparamagnetic iron oxide (SPIO) or chondroitin sulfate iron colloid into the reticuloendothelial cells such as Kupffer cells of the liver. Our findings indicated that the number Kupffer cells in cancerous tissues decreased significantly as the tumor size increased and histological grade decreased. Therefore, the enhanced MRI which utilizes the function of Kupffer cells can be useful in estimation of histological degree of HCC. Imai et al[41] studied histologically proven tumors including 31 HCCs and 6 dysplastic nodules by SPIO-enhanced MRI, and proposed that SPIO-enhanced MRI reflect Kupffer cell number in HCCs and dysplastic nodules and be useful in assessing the histological grades of HCCs, especially poorly-differentiated and moderately-differentiated cases. Recently, Kitamura et al[42] reported 18 HCCs detected by color Doppler sonography had either a marked reduction in the number or absence of Kupffer cells. In conclusion, the present study has shown that Kupffer cells are important in preventing development of HCCs, and tumor metastases. They are also involved in the pathogenesis of chemically mediated liver injury and viral hepatitis. Further study on the biological characteristics and function of Kupffer cells will contribute to the early diagnosis of hepatic tumors and new treatment strategies.
| 1. | Chen GG, Lau WY, Lai PB, Chun YS, Chak EC, Leung BC, Lam IK, Lee JF, Chui AK. Activation of Kupffer cells inhibits tumor growth in a murine model system. Int J Cancer. 2002;99:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Johnson SJ, Burr AW, Toole K, Dack CL, Mathew J, Burt AD. Macrophage and hepatic stellate cell responses during experimental hepatocarcinogenesis. J Gastroenterol Hepatol. 1998;13:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 276] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Zhu HZ, Ruan YB, Wu ZB, Zhang CM. Kupffer cell and apoptosis in experimental HCC. World J Gastroenterol. 2000;6:405-407. [PubMed] |
| 6. | Toth CA, Thomas P. Liver endocytosis and Kupffer cells. Hepatology. 1992;16:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 69] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Torimura T, Ueno T, Inuzuka S, Kin M, Ohira H, Kimura Y, Majima Y, Sata M, Abe H, Tanikawa K. The extracellular matrix in hepatocellular carcinoma shows different localization patterns depending on the differentiation and the histological pattern of tumors: immunohistochemical analysis. J Hepatol. 1994;21:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Nakashima O, Sugihara S, Kage M, Kojiro M. Pathomorphologic characteristics of small hepatocellular carcinoma: a special reference to small hepatocellular carcinoma with indistinct margins. Hepatology. 1995;22:101-105. [PubMed] |
| 9. | Tsujimoto T, Kuriyama S, Yamazaki M, Nakatani Y, Okuda H, Yoshiji H, Fukui H. Augmented hepatocellular carcinoma progression and depressed Kupffer cell activity in rat cirrhotic livers. Int J Oncol. 2001;18:41-47. [PubMed] |
| 10. | Takeishi T, Hirano K, Kobayashi T, Hasegawa G, Hatakeyama K, Naito M. The role of Kupffer cells in liver regeneration. Arch Histol Cytol. 1999;62:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Sakaida I, Hironaka K, Terai S, Okita K. Gadolinium chloride reverses dimethylnitrosamine (DMN)-induced rat liver fibrosis with increased matrix metalloproteinases (MMPs) of Kupffer cells. Life Sci. 2003;72:943-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71:226-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, Groothuis GM, Meijer DK, Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Bautista AP. Impact of alcohol on the ability of Kupffer cells to produce chemokines and its role in alcoholic liver disease. J Gastroenterol Hepatol. 2000;15:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15 Suppl:D20-D25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Vollmar B, Siegmund S, Richter S, Menger MD. Microvascular consequences of Kupffer cell modulation in rat liver fibrogenesis. J Pathol. 1999;189:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Yamaguchi R, Yano H, Nakashima Y, Ogasawara S, Higaki K, Akiba J, Hicklin DJ, Kojiro M. Expression and localization of vascular endothelial growth factor receptors in human hepatocellular carcinoma and non-HCC tissues. Oncol Rep. 2000;7:725-729. [PubMed] |
| 18. | Nakopoulou L, Stefanaki K, Vourlakou C, Manolaki N, Gakiopoulou H, Michalopoulos G. Bcl-2 protein expression in acute and chronic hepatitis, cirrhosis and hepatocellular carcinoma. Pathol Res Pract. 1999;195:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Wang JY, Zhang QS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol. 2001;7:115-119. [PubMed] |
| 20. | Hsu CM, Wang JS, Liu CH, Chen LW. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock. 2002;17:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] |
| 22. | Schauer RJ, Bilzer M, Kalmuk S, Gerbes AL, Leiderer R, Schildberg FW, Messmer K. Microcirculatory failure after rat liver transplantation is related to Kupffer cell-derived oxidant stress but not involved in early graft dysfunction. Transplantation. 2001;72:1692-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363-369. [PubMed] |
| 24. | Sharifi S, Hayek J, Khettry U, Nasser I. Immunocytochemical staining of Kupffer and endothelial cells in fine needle aspiration cytology of hepatocellular carcinoma. Acta Cytol. 2000;44:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Brown KE, Brunt EM, Heinecke JW. Immunohistochemical detection of myeloperoxidase and its oxidation products in Kupffer cells of human liver. Am J Pathol. 2001;159:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Longchampt E, Patriarche C, Fabre M. Accuracy of cytology vs. microbiopsy for the diagnosis of well-differentiated hepatocellular carcinoma and macroregenerative nodule. Definition of standardized criteria from a study of 100 cases. Acta Cytol. 2000;44:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Lührs H, Illert B, Timmermann W, Volk H, Scheppach W, Menzel T. Ultrastructural alterations of primary human liver sinusoidal cells in patients treated for peritonitis. J Invest Surg. 2002;15:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Klockars M, Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem. 1975;23:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 196] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Mathew J, Hines JE, Toole K, Johnson SJ, James OF, Burt AD. Quantitative analysis of macrophages and perisinusoidal cells in primary biliary cirrhosis. Histopathology. 1994;25:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Yamamoto T, Hirohashi K, Kaneda K, Ikebe T, Mikami S, Uenishi T, Kanazawa A, Takemura S, Shuto T, Tanaka H. Relationship of the microvascular type to the tumor size, arterialization and dedifferentiation of human hepatocellular carcinoma. Jpn J Cancer Res. 2001;92:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Baldus SE, Zirbes TK, Weidner IC, Flucke U, Dittmar E, Thiele J, Dienes HP. Comparative quantitative analysis of macrophage populations defined by CD68 and carbohydrate antigens in normal and pathologically altered human liver tissue. Anal Cell Pathol. 1998;16:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Huang X, Li DG, Wang ZR, Wei HS, Cheng JL, Zhan YT, Zhou X, Xu QF, Li X, Lu HM. Expression changes of activin A in the development of hepatic fibrosis. World J Gastroenterol. 2001;7:37-41. [PubMed] |
| 33. | Song E, Chen J, Ouyang N, Wang M, Exton MS, Heemann U. Kupffer cells of cirrhotic rat livers sensitize colon cancer cells to Fas-mediated apoptosis. Br J Cancer. 2001;84:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Miyagawa S, Miwa S, Soeda J, Kobayashi A, Kawasaki S. Morphometric analysis of liver macrophages in patients with colorectal liver metastasis. Clin Exp Metastasis. 2002;19:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Lau WY, Chen GG, Lai PB, Chun YS, Leung BC, Chak EC, Lee JF, Chui AK. Induction of Fas and Fas ligand expression on malignant glioma cells by Kupffer cells, a potential pathway of antiliver metastases. J Surg Res. 2001;101:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Nanji AA. Role of Kupffer cells in alcoholic hepatitis. Alcohol. 2002;27:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Hoebe KH, Witkamp RF, Fink-Gremmels J, Van Miert AS, Monshouwer M. Direct cell-to-cell contact between Kupffer cells and hepatocytes augments endotoxin-induced hepatic injury. Am J Physiol Gastrointest Liver Physiol. 2001;280:G720-G728. [PubMed] |
| 38. | Schümann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, Tiegs G. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157:1671-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | 41 Burgio VL, Ballardini G, Artini M, Caratozzolo M, Bianchi FB, Levrero M. Expression of co-stimulatory molecules by Kupffer cells in chronic hepatitis of hepatitis C virus etiology. Hepatology. 1998;27:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Lim JH, Choi D, Cho SK, Kim SH, Lee WJ, Lim HK, Park CK, Paik SW, Kim YI. Conspicuity of hepatocellular nodular lesions in cirrhotic livers at ferumoxides-enhanced MR imaging: importance of Kupffer cell number. Radiology. 2001;220:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Imai Y, Murakami T, Yoshida S, Nishikawa M, Ohsawa M, Tokunaga K, Murata M, Shibata K, Zushi S, Kurokawa M. Superparamagnetic iron oxide-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading. Hepatology. 2000;32:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Kitamura H, Kawasaki S, Nakajima K, Ota H. Correlation between microbubble contrast-enhanced color doppler sonography and immunostaining for Kupffer cells in assessing the histopathologic grade of hepatocellular carcinoma: preliminary results. J Clin Ultrasound. 2002;30:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Edited by Zhu LH and Wang XL