Published online Apr 15, 2003. doi: 10.3748/wjg.v9.i4.645
Revised: December 29, 2002
Accepted: January 5, 2003
Published online: April 15, 2003
AIM: To investigate the alteration of the annexin I subcellular localization in esophageal squamous cell carcinoma (ESCC) and the correlation between the translocation and the tumorigenesis of ESCC.
METHODS: The protein localization of annexin I was detected in both human ESCC tissues and cell line via the indirect immunofluorescence strategy.
RESULTS: In the normal esophageal epithelia the annexin I was mainly located on the plasma membrane and formed a consecutive typical trammels net. Annexin I protein also expressed dispersively in cytoplasm and the nuclei without specific localization on the nuclear membrane. In esophageal cancer annexin I decreased very sharply with scattered disappearance on the cellular membrane, however it translocated and highly expressed on the nuclear membrane, which was never found in normal esophageal epithelia. In cultured esophageal cancer cell line annexin I protein was also focused on the nuclear membrane, which was consistent with the result from esophageal cancer tissues.
CONCLUSION: This observation suggests that the translocation of annexin I protein in ESCC may correlate with the tumorigenesis of the esophageal cancer.
- Citation: Liu Y, Wang HX, Lu N, Mao YS, Liu F, Wang Y, Zhang HR, Wang K, Wu M, Zhao XH. Translocation of annexin I from cellular membrane to the nuclear membrane in human esophageal squamous cell carcinoma. World J Gastroenterol 2003; 9(4): 645-649
- URL: https://www.wjgnet.com/1007-9327/full/v9/i4/645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i4.645
Annexin I is a member of annexins, an evolutionary conserved multigene family, which are calcium and phospholipid-binding proteins. Annexins consist of a conserved C-terminal domain that confers calcium-dependent phospholipid binding and a variable N-terminal domain that is responsible for the specific properties of each annexin I[1,2]. As a steroid-regulated protein annexin I has been found to participate in the cell differentiation and anti-inflammatory effects[3,4]. It is also a major substrate of EGF receptor, which related to endocytic trafficking and sorting of EGFR in multivesicular endosomes[5]. The annexin I modulates the signal transduction through MAPK/ERK pathway and, specifically, inhibits the activities of phospholipase A2[6,7]. Recent studies describe increased expression of annexin I in human hepatocellular carcinoma but it is absent in several types of carcinomas, such as human esophageal cancer and prostatic neoplasm[8,9]. We have found that annexin I is clearly lost in ESCC, a kind of major diseases and the 4th killer of malignant tumors in China, and it seems that the annexin I protein plays an important role in the carcinogenesis.
It is well known that the protein subcellular localization is a very important way to better understand protein functions. The alteration of protein subcellular localizations and the membrane trafficking will facilitate the specific cellular functions as well as signal transduction. To detect the subcellular localizations of annexin I in both normal epithelia and ESCC mucosa will give us some clues to address the functions of annexin I in malignant tumors.
Tissues specimens The esophageal specimens used for immunohistochemical (IHC) staining were obtained from patients who presented to the Cancer Hospital of Chinese Academy of Medical Sciences, Beijing, China and were diagnosed as esophageal squamous cell carcinoma without chemotherapy and radiotherapy by two senior pathologists. After surgical resections the specimens were fixed in 70% ethanol or 40 mg·L-1neutral formalin and embedded in paraffin.
Cell line Human ESCC cell line, EC0156 was generated in our laboratory from an ESCC tissue.
Antibodies Commercial available antibodies included annexin I monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), goat anti-mouse TRITC and goat anti-mouse FITC antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). All other reagents were of analytical grade.
Cell cultures The human esophageal cancer cell line EC0156 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and antibiotics (penicillin and streptomycin) at 37 °C in a humidified atmosphere with 5% CO2.
Indirect immunofluorescence staining[10] For immunostaining, EC0156 cells were grown on glass-coverslips at 80% confluence and fixed in 4% paraformaldehyde in 100 mmol·L-1 PBS (pH = 7.4) for 15 min at room temperature. After three washes with the buffer (25 mmol·L-1 HEPES, 1 mmol·L-1 CaCl2, 1 mmol·L-1 MgCl2 and 10 g·L-1 BSA), the paraffin embedded tissue sections were deparaffinized and hydrated through xylenes and graded alcohol series, and then rinsed for 5 min in water. The fixed cells and deparaffinized tissue sections were incubated in blocking solution (0.1% horse serum and 0.06% Triton-X 100 in PBS) for 1 hour to decrease the non-specific binding of the antibodies and to improve the penetration of the antibodies through membranes. The blocking solution was also used for diluting the primary and secondary antibodies. After 1 hour, the blocking solution was changed for the primary antibody solution (anti-annexin I monoclonal antibody was diluted to 1:200 and PBS was used as negative control) and the cells were incubated at 4 °C overnight. After three washes, cells and tissues were incubated with the fluorescence-labeled secondary antibodies (1:300 diluted goat anti-mouse TRITC or the goat anti-mouse FITC) for 30 min at room temperature. This was followed by a last washing step (3 × 5 min, in PBS), then the cells were rinsed with distilled water, air dried and mounted on glass slides using Cytoseal 60 mounting medium (Stephens Scientific). Cells were then analyzed and images were obtained with a fluorescence microscope (Olympus BX51, OLYMPUS OPTICAL CO., LTD., Japan).
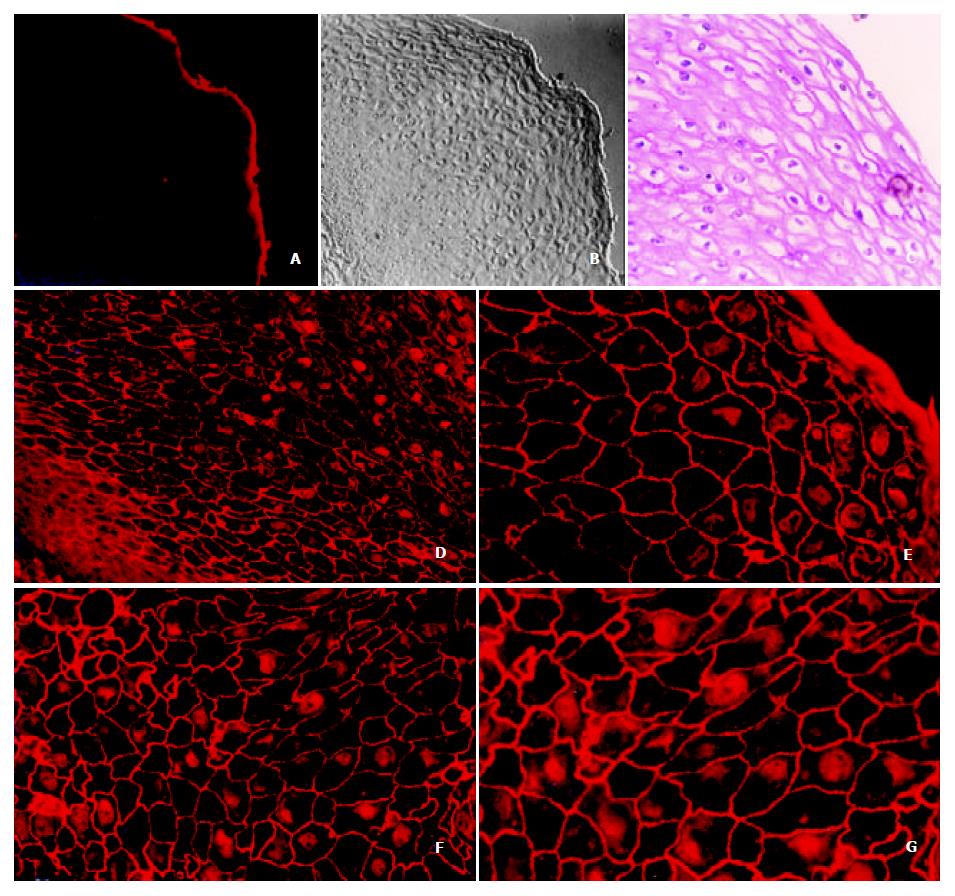
Annexin I protein was mostly located on the cell membrane in a granular pattern and some of them on the nucleus and cytoplasm as well through immunofluorescence staining in the normal esophagus epithelia (Figure 1. d, e, f and g). Consecutively and symmetrically expressed annexin protein on the cellular membrane makes typical trammel net. Annexin I also expressed in the cytoplasm and nuclei dispersively without specific localization on the nuclear membrane.
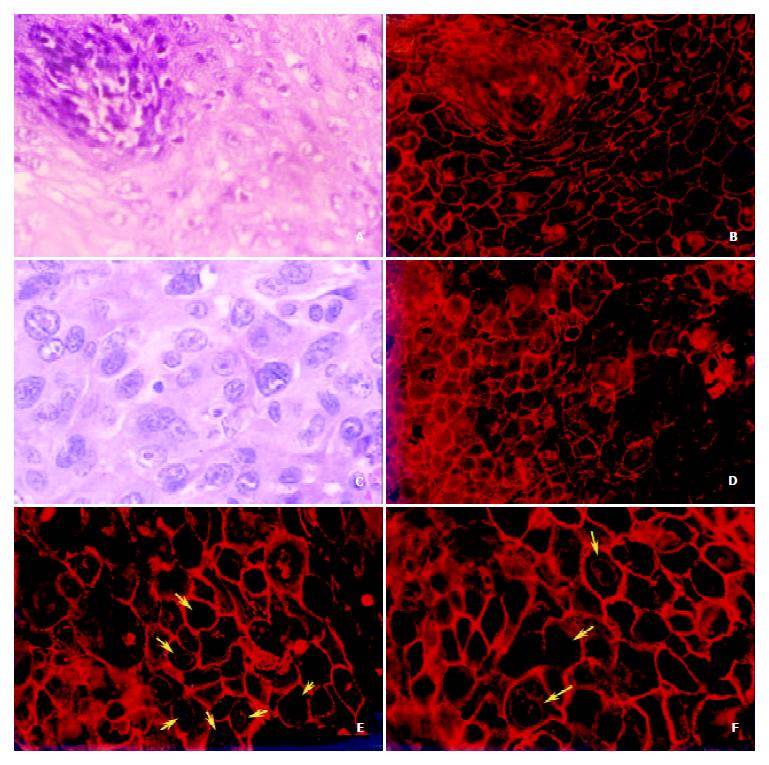
Figure 2 (c, d, e and f) showed clearly that the beautiful trammel net of annexin I protein on the esophageal epithelia had been broken and the holes of the net had been fused each other on the ESCC cellular membrane. The expression of annexin I on the cellular membrane decreased very sharply with unequal distribution or scattered disappearance. In the meantime, we also found the nuclear membrane localization of annexin I protein had appeared and increased very obviously (shown by the yellow arrows), which has never been found in normal esophageal epithelia. However, the expression of annexin I in nuclear plasma had been decreased distinctly.
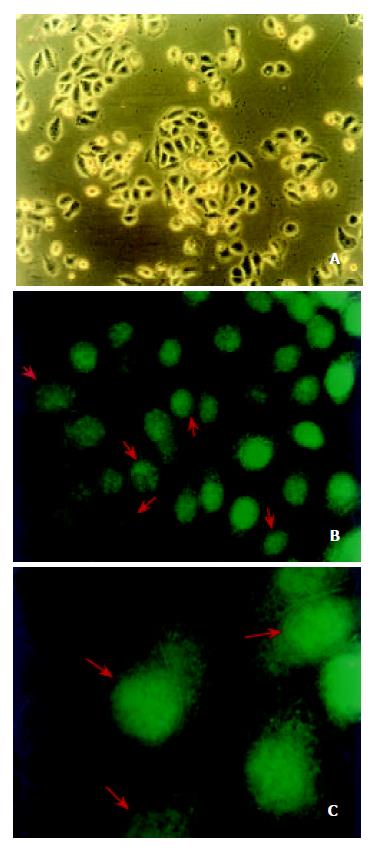
In order to further confirm the localization of annexin I protein in ESCC, a human esophageal cancer cell line, EC0156 was used to inspect the distributions of annexin I by indirect immunofluorescence staining. Annexin I protein was distinctly focused on the nuclear membrane (Figure 3, indicated by red arrows) of the EC0156 cells and it was consistent with what we saw in esophageal cancer tissues. On the cell line, annexin I proteins were also expressed on cytoplasm, cellular membrane and nuclear plasma as well.
Annexin I protein belongs to a family of calcium-dependent phospholipid-binding proteins whose functions are presumed to participate in various membrane related events including membrane fusion in exocytosis and endocytosis as well as membrane organization. Previously studies have reported that in human epidermis annexin I was stained in a granular pattern in the monolayer but in an envelope pattern in the stratified keratinocytes[11]. The subcellular localization of annexins in rat was mainly found in the cytoplasm and nucleus of the mesangial cells[12], and in cytoplasm of mast cells[13]. By the localization of annexin I, II, VI and XIII in epithelial cells of intestinal, hepatic and pancreatic tissues of the rabbit, Massey-Harroche et al[14] showed that the basolateral domain of polarized cells appears to be the main site where annexins are located, and annexins may therefore be involved in the important cellular events occurring at this level. It suggests that the different localizations of annexin I contribute to its versatile functions.
Recently it has been found that annexin I is drastically down-regulated in esophageal cancer and assumed that the it may play a key role in the tumorigenesis of ESCC[8,15,16]. To gain the insight into the localization of annexin I, its relationship with the processing of esophageal cancer and the possible functions of this protein, we investigated the subcellular localization of annexin I in ESCC cell line, normal and cancer esophageal epithelia by indirect immunofluorescence strategy. It was found that annexin I was mainly located on the membrane of the normal esophageal epithelia in granular pattern and formed a typical trammel net between the esophageal epithelia. Annexin I also expressed dispersively in the nuclei and cytoplasm without specific localization on the nuclear membrane. However, on the cellular membrane of ESCC the beautiful trammel net of annexin I had been broken and the holes on the net had been fused together. The expression of annexin I on the cellular membrane and nuclear plasma decreased very sharply, and the protein distributed unequally with scattered disappearance. Meanwhile, the nuclear membrane localization of annexin I had been appeared and increased obviously, which was never been found in normal esophageal epithelia. Additionally the distribution of annexin I on the human esophageal cancer cell line, EC0156 was also distinctly focused on the nuclear membrane. This finding suggests that the translocation of the subcellular localization of annexin I may be correlated with the tumorigenesis of ESCC. An IHC analysis of annexin I in ductal epithelial cells of various human breast tissues indicated that this annexin was not demonstrable in both the ductal luminal cells of normal breast and benign tumors, but was generally expressed in various types of breast cancer. Therefore it is most likely involved in an early stage of human breast cancer development. Annexin I expression might also correlate with breast cancer progress[17].
Annexin I appeared to be cleaved by neutrophil elastase at the N-terminal portion between Val-36 and Ser-37 to yield a 33 kDa protein. Cleavage of the N-terminal portion of annexin I was accompanied by a marked change in the annexin I isoelectric point (pI) value (from 6.0 to 8.5-9.0) and greatly diminished its functional activities. The findings demonstrate that annexin I degradation in epithelial lining fluid is closely related to lung inflammation[18].
The mechanisms of annexin I localization are a complexity. A study showed calcium induced translocation of annexin I into subcellular organelles and secretory vesicles in human neutrophils, which suggested annexin I might contribute to the secretory process in neutrophils[19]. Other studies also showed that dexamethasone and IL-6 could affect the localization of annexin I. In A549 human adenocarcinoma cell line dexamethasone could inhibit EGF-stimulated cytosolic PLA2 activation and arachidonic acid release. Annexin I participated in this regulated pathway. Dexamethasone induced annexin I phosphorylation and translocation mediated by glucocorticoids receptor, then brought about a competition between annexin I and Grb2 leading to a failure of recruitment of signaling factors to EGFR[6]. In U937 cells dexamethasone also caused translocation of annexin I from the intracellular compartment to the cell membrane[20]. Solito et al[21] reported that induction of annexin I protein and its translocation to the cell membrane were stimulated by interleukin 6 and a unique 30 bp region of the annexin I promoter, which was critical for the responsiveness of the reporter gene to IL-6 and dexamethasone. IL-6 stimulation was mediated by a C/EBP beta-like transcriptional factor. Annexin I might participate in host defence system as a new acute class II phase protein.
Another study showed that stresses, treatment of A549 and Hela cells with heat, hydrogen peroxide or arsenate, resulted in the translocation of annexin I from cytoplasm to nucleus and perinuclear region. There were different intracellular distributions of annexins in macrophage-like cells in phagocytosis, and reactions to hydrogen peroxide and sodium arsenate[22].
In summary, we have first detected the translocation of annexin I from cellular membrane to nuclear membrane in ESCC cells. It seems that the subcellular localizations of annexin I are closely related to its functions and the alterations are most likely involved in the tumorigenesis of ESCC, especially at the early stage. More investigations are doing to further clarify the mechanisms of annexin I subcellular-localization changes during tumorigenesis of human ESCC.
| 1. | Kourie JI, Wood HB. Biophysical and molecular properties of annexin-formed channels. Prog Biophys Mol Biol. 2000;73:91-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Rosengarth A, Wintergalen A, Galla HJ, Hinz HJ, Gerke V. Ca2+-independent interaction of annexin I with phospholipid monolayers. FEBS Lett. 1998;438:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. Human annexin 1 is highly expressed during the differentiation of the epithelial cell line A 549: involvement of nuclear factor interleukin 6 in phorbol ester induction of annexin 1. Cell Growth Differ. 1998;9:327-336. [PubMed] |
| 4. | Perretti M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen Pharmacol. 1998;31:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 164] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Alldridge LC, Harris HJ, Plevin R, Hannon R, Bryant CE. The annexin protein lipocortin 1 regulates the MAPK/ERK pathway. J Biol Chem. 1999;274:37620-37628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Croxtall JD, Choudhury Q, Newman S, Flower RJ. Lipocortin 1 and the control of cPLA2 activity in A549 cells. Glucocorticoids block EGF stimulation of cPLA2 phosphorylation. Biochem Pharmacol. 1996;52:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J. Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000;60:6293-6297. [PubMed] |
| 9. | Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M. Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology. 1996;24:72-81. [PubMed] |
| 10. | Zhao X, Várnai P, Tuymetova G, Balla A, Tóth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183-40189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Ma AS, Ozers LJ. Annexins I and II show differences in subcellular localization and differentiation-related changes in human epidermal keratinocytes. Arch Dermatol Res. 1996;288:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Vervoordeldonk MJ, Schalkwijk CG, Vishwanath BS, Aarsman AJ, van den Bosch H. Levels and localization of group II phos-pholipase A2 and annexin I in interleukin- and dexamethasone-treated rat mesangial cells: evidence against annexin mediation of the dexamethasone-induced inhibition of group II phospholi-pases A2. Biochim Biophys Acta. 1994;1224:541-550. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Oliani SM, Christian HC, Manston J, Flower RJ, Perretti M. An immunocytochemical and in situ hybridization analysis of annexin 1 expression in rat mast cells: modulation by inflammation and dexamethasone. Lab Invest. 2000;80:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Massey-Harroche D, Mayran N, Maroux S. Polarized localizations of annexins I, II, VI and XIII in epithelial cells of intestinal, hepatic and pancreatic tissues. J Cell Sci. 1998;111:3007-3015. [PubMed] |
| 15. | Zhou G, Li H, DeCamp D, Chen S, Shu H, Gong Y, Flaig M, Gillespie JW, Hu N, Taylor PR. 2D differential in-gel electrophoresis for the identification of esophageal scans cell cancer-specific protein markers. Mol Cell Proteomics. 2002;1:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 270] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Emmert-Buck MR, Gillespie JW, Paweletz CP, Ornstein DK, Basrur V, Appella E, Wang QH, Huang J, Hu N, Taylor P. An approach to proteomic analysis of human tumors. Mol Carcinog. 2000;27:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997;15:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Tsao FH, Meyer KC, Chen X, Rosenthal NS, Hu J. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 1998;18:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Sjölin C, Stendahl O, Dahlgren C. Calcium-induced translocation of annexins to subcellular organelles of human neutrophils. Biochem J. 1994;300:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Solito E, Nuti S, Parente L. Dexamethasone-induced translocation of lipocortin (annexin) 1 to the cell membrane of U-937 cells. Br J Pharmacol. 1994;112:347-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998;10:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Rhee HJ, Kim GY, Huh JW, Kim SW, Na DS. Annexin I is a stress protein induced by heat, oxidative stress and a sulfhydryl-reactive agent. Eur J Biochem. 2000;267:3220-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
Edited by Zhang JZ