INTRODUCTION
Hepatitis B virus (HBV) infection is still a major health concern around the world. Globally, more than 350 million people are infected by HBV, and some of them will evolve into liver cirrhosis and hepatocellular carcinoma (HCC)[1-3]. Although vaccination can elicit effective immunity in about 95% of inoculated people, the non-responders to vaccination who may contact HBV infection in their life, the threat of escape mutants, and the huge amount of currently infected people call for an effective treatment[4-6]. Interferon-α (IFN-α) is the first choice for the treatment of chronic HBV infection. However, the sustained response rate to IFN-α treatment is only about 30%[7]. IFN-α can also cause many adverse effects, such as fatigue, fever, neutropenia, autoimmune disease, psychological problems, and so on[8]. Nucleotide analogues such as lamivudine can inhibit replication of HBV via inhibiting the synthesis of minus-strand DNA of HBV, but cease of the treatment usually leads to relapse of the infection and drug-resistant virus variants have emerged[9-11]. Antisense nucleotides and ribozymes have been reported to suppress HBV replication in vitro[12-17]. However, up to now, antisense nucleotides and ribozymes can generally only moderately inhibit HBV replication intracellularly. In a word, the limited efficacy of current treatment for HBV infection justifies the search for new treatment strategy.
Capsid-targeted viral inactivation (CTVI), first proposed by Natsoulis and Boeke in 1991, is a conceptually powerful antiviral approach[18]. The principle of CTVI is to construct a fusion protein of viral capsid protein and some degradative enzyme. The capsid part of the fusion protein serves to target the degradative enzyme to virions and the degradative enzyme can specifically destruct the component of the virions. The degradative enzymes used presently are nucleases though other enzymes such as lipases and proteinases can also be used in CTVI in principle. CTVI has been thoroughly investigated in the experimental treatment for retrovirus, such as Moloney murine leukemia virus (MMLV) and HIV, showing a promising prospect as an antiviral treatment[19-26].
The replication of Hepadnavirus, including HBV, is unique in DNA virus in that it needs a RNA intermediate and a reverse transcription process[1]. This 3.5 kb RNA intermediate contains all genetic information of HBV and is so called pregenomic RNA (pgRNA). The pgRNA is first translated to both the capsid, or core, protein (c protein) and the DNA polymerase protein (P protein). Then the pgRNA is bound first by P protein and cellular chaperones to form a complex which is then encapsidated by C protein[27-29]. Inside the nucleocapsids, P protein catalyzes the synthesis of minus-strand DNA via reverse transcription and then the incomplete plus-strand DNA. The nucleocapsids containing minus- and plus-strand DNA can reenter nucleus or bud into endoplasmic reticulum and then be released via secretory pathway out of host cells[30]. C-terminal 144-164 amino acids of C protein, rich in arginine, is necessary for pregenome encapsidation since particles formed by C mutant deleting this domain contain no pgRNA[31].
The replication characteristic of HBV hints CTVI can also be applied to this malicious virus. Previously, we reported the construction of a fusion protein of HBV C protein and a ribonuclease, human eosinophil-derived neurotoxin (hEDN)[32-34]. In this paper, we found that this fusion protein, which was named as targeted ribonuclease in this paper, could effectively inhibit the replication of HBV, while had no cytotoxicity on host cells.
MATERIALS AND METHODS
Cell culture
2.2.15 cell line, human hepatoblastoma Hep G2 cell line stably transfected by HBV genome[35-37], was kindly provided by Dr. Cheng (Chinese PLA 302 Hospital) and cultured in Dulbecco's modified Eagle's medium (DMEM, purchased from Gibco Life Technologies, Grand Island, NY) supplemented with 100 mL/L fetal calf serum (Sijiqing Biotech Company, Hangzhou).
Plasmid construction
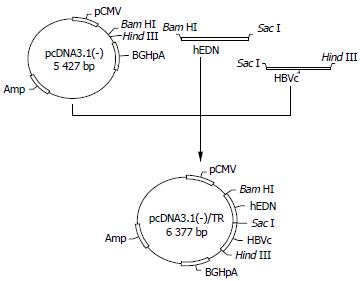
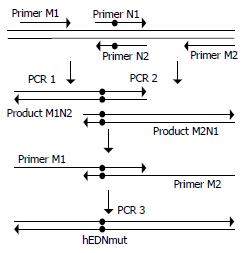
Targeted ribonuclease eukaryotic expression plasmid p/TN (Figure 1) and control plasmids p/hEDN and p/HBVc were constructed with the method previously reported[34]. Briefly, cDNA coding for hEDN or HBVc was amplified by RT-PCR from the total RNA isolated from HL60 cell line (kindly provided by Professor Boquan Jin, Department of Immunology, Fourth Military Medical University) or 2.2.15 cell line. Then, the PCR product was cloned into pUC18. After verification of the correctness of the open reading frames of the cloned hEDN and HBVc by sequencing, they were subcloned into pcDNA3.1 (-) (Gibco Life Technologies), to generate p/hEDN and p/HBVc, respectively, or they were ligated together by T4 ligase (Gibco Life Technologies) and the ligated DNA fragment was cloned into pcDNA3.1 (-) to form p/TN. To construct control plasmid p/TNmut in which the DNA fragment encoding hEDNmut (hEDN mutated for just one amino acid, Lys113→Arg, which eliminates the ribonuclease activity[38]) substituted for hEDN in p/TN, sequential PCR was used to obtain hEDNmut-encoding DNA fragment (Figure 2) . For the first round of two separate PCR reactions, primers M1, N2 and M2, N1 (all synthesized by Sangon Company, Shanghai) were used, respectively, and pUC18/hEDN was used as template. The sequences of the primers are as follows:
Figure 1 Construction of p/TN.
cDNA coding for hEDN or HBVc was amplified by RT-PCR, ligated together, and then cloned into pcDNA3.1 (-) to form p/TN.
Figure 2 Generation of hEDNmut gene by sequential PCR.
For PCR 1 and 2, pUC18/hEDN was used as template. Primers pair M1 and N2 were used in PCR 1 and N1 and M2 in PCR 2. For PCR 3, the mixture of PCR 1 and 2 product was used as template and M1 and M2 as primers. The black dots in the figure denote the nucleotides introducing the Lys113→Arg mutation which eliminates the ribonuclease activity.
N1: 5'-GCA GAA ACC AAA ATA CTT TCC T-3'
N2: 5'-TCT GCA TCG CCG TTG ATA ATT-3'
M1: 5'-GCG CGG ATC CAC CAT GAA ACC TCC ACA GTT TAC-3'
M2: 5'-GCG CGA GCT CGA TGA TTC TAT CCA GGT G-3'
The underlined nucleotides in N1 and N2 were used to introduce the mutation. The PCR products of the two reaction were mixed together. Two mL of the mixture was used as the template, M1 and M2 were used as primers for the next round of PCR. The PCR product of this round amplification was cloned into pUC18 and sequenced for confirmation of the mutation. Then the hEDNmut gene was subcloned into p/TN to substitute hEDN gene to generate recombinant plasmid p/TNmut. pcDNA3.1 (-)/GFP was generated by transferring green fluorescent protein (GFP) gene from pEGFP-N3 MCS (Clontech, Palo Alto, CA) into pcDNA3.1 (-).
Transfection
Twenty-four h before transfection, 2.2.15 cells were seeded into the culture plate at a density of 2 × 108/L. LipofectamineTM 2000 reagent (Gibco Life Technologies) was used for the transfection of 2.2.15 cells by p/TN, p/TNmut, p/hEDN, p/HBVc, pcDNA3.1 (-), or mock solution (DMEM plus LipofectamineTM 2000 reagent containing no plasmid) according to the manufacturer's protocol. The amount of LipofectamineTM 2000 reagent (approximately 3.1 μL/cm2 surface area in this study) and the concentration of DNA (approximately 1 μg/cm2 surface area in this study) were first optimized in a preliminary transfection of 2.2.15 cells by pcDNA3.1 (-)/GFP in which GFP gene was used as a reporter gene.
Confirmation of transgene expression by RT-PCR
Forty-eight h after the transfection, total RNA was isolated from transfected 2.2.15 cells using Trizol® reagent (Gibco Life Technologies) according to the manufacturer's protocol. One μL of total RNA was used as template. RT-PCR was performed using SuperScriptTM One-step RT-PCR kit (Gibco Life Technologies) as recommended by the manufacturer. Forward primer was T7 promoter primer which is upstream to the multicloning site of pcDNA3.1 (-). Reverse primer was complementary to pcDNA3.1/BGH reverse priming site which is downstream to multicloning site of pcDNA3.1 (-). The sequences of the two primers are as follows:
Forward primer: 5'-TAA TAC GAC TCA CTA TAG GGA GA-3'
Reverse primer: 5'-TAG AAG GCA CAG TCG-3'
The condition of RT-PCR was: 50 °C 30 min, 94 °C 2 min, then 40 cycles at 94 °C 30 s, 60 °C 30 s, and 72 °C 2 min. To ensure that the amplified product was from RNA, but not DNA, a control was set up for each RT-PCR reaction, in which the reverse transcriptase was omitted.
Quantification of HBsAg in supernatant of cell culture
Forty-eight h after the transfection, the supernatant of the cell culture was collected, and HBsAg in the supernatant was quantified using the Solid Phase Radio-immunoassay Kit for Quantification of HBsAg (Beimian Dongya Biotech Institute, Beijing) according to the manufacturer's protocol. Briefly, 200 μL of the supernatant was incubated with plastic bead coated by anti-HBs Ab at 45 °C for 1.5 h. After thorough washing, the bead with deionized water, 200 μL of 125I-labelled secondary antibody was added and incubated at room temperature overnight. The bead was thoroughly washed, and cpm was measured. The concentration of HBsAg of each sample was obtained by comparing the cpm of each sample with a standard curve.
MTT assay
Forty-eight h after the transfection, 20 μL of MTT solution (the concentration of MTT was 5 g/L) was added to each well and the culture plate was incubated at 37 °C for 4 h, then 150 μL of DMSO was added to each well and the plate was shaken for 10 min. The A490 value of each well was obtained by an ELISA reader.
Statistical analysis
Variance analysis and Student's t test were used for data analysis. Differences were considered significant when P < 0.05.
RESULTS
Transgene expression in 2.2.15 cells
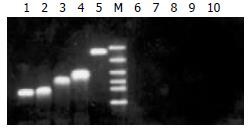
As shown in Figure 3, the results of the RT-PCR indicated that the transgenes were expressed in transfected 2.2.15 cells.
Figure 3 Confirmation of transgenes expression in transfected 2.
2.15 cell line. Forty-eight h after the transfection, total RNA was isolated from transfected 2.2.15 cells and RT-PCR was performed to confirm the expression of transgenes. The RT-PCR products were then electrophoresed in 1.2% agarose gel. Lanes 1-5 represent the RT-PCR results for total RNA isolated from 2.2.15 cells transfected by p/TN, p/TNmut, p/HBVc, p/hEDN, and pcDNA3.1 (-), respectively. Lanes 6-10 represent controls corresponding to lanes 1-5, respectively, in which reverse transcriptase was omitted in RT-PCR. M: DNA Marker (2000, 1000, 750, 500, 250, 100bp from top to bottom).
The effect of targeted ribonuclease on cell viability
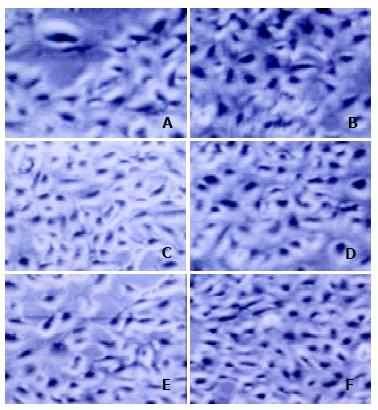
The morphological alterations of the transfected 2.2.15 cells were observed under microscope. There were no discernible morphological differences of 2.2.15 cells transfected with p/TN, as compared with the controls (Figure 4). To further analyze the effect of targeted ribonuclease on cell viability, MTT assay was performed. The A490 value of 2.2.15 cells transfected with p/TN, p/hEDN, p/HBVc, p/TNmut, pcDNA3.1 (-), and mock transfection was 0.425 ± 0.065, 0.465 ± 0.050, 0.410 ± 0.075, 0.438 ± 0.042 0.413 ± 0.063, and 0.430 ± 0.065, respectively (-x ± s, n = 4).
Figure 4 Morphology of 2.
2.15 cells 48 h after transfection. A-F represent 2.2.15 cells transfected by p/TN, p/hEDN, p/HBVc, p/TNmut, pcDNA3.1 (-), or mock transfection, respectively. There were no discernible morphological differences of 2.2.15 cells transfected with p/TN, as compared with the controls.
There were no significant differences between A490 value of 2.2.15 cells transfected with p/TN and those of controls (P > 0.05). Taken together, these results indicated that the targeted ribonuclease had no adverse effects on cell viability and proliferation.
Inhibition of HBV replication by targeted ribonuclease
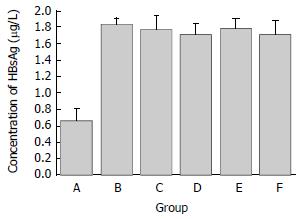
As shown in Figure 5, the concentration of HBsAg in the supernatant of 2.2.15 cells transfected with p/TN was significantly lower than controls (P < 0.05), while that of 2.2.15 cells transfected with p/TNmut, p/hEDN, p/HBVc, pcDNA3.1 (-), or mock transfection did not significantly different from each other (P > 0.05). Compared with that of mock transfected 2.2.15 cells, the concentration of HBsAg in the supernatant of 2.2.15 cells transfected with p/TN was decreased by 58%.
Figure 5 The HBsAg concentration in supernatants of transfected 2.
2.15 cells. Groups A-F represent 2.2.15 cells transfected by p/TN, p/hEDN, p/HBVc, p/TNmut, pcDNA3.1 (-), or mock transfection, respectively. The concentration of HBsAg in the supernatant of 2.2.15 cells transfected with p/TN was decreased by 58% as compared with that of mock transfected 2.2.15 cells.
DISCUSSION
Theoretically, CTVI has many advantages in the treatment of viral infection[39]. First, CTVI is highly specific. This high specificity is conferred by the encapsidating capacity and self-assembly property of the viral capsid protein which is used as a targeting molecule in CTVI. Second, CTVI is highly efficient. The nuclease, which is used as an effector molecule in CTVI, is a protein enzyme, and its catalytic efficiency is higher than ribozyme. Third, escape mutants may rarely if not impossibly arise in case of CTVI since viral capsid proteins generally play important roles in virus assembly and infection. Fourth, while ribozyme and antisense RNA can only be used clinically in the form of gene therapy, CTVI can be used in the form of either gene therapy or recombinant protein drug. The results of experimental research also indicate that CTVI is a promising antiviral strategy. Boeke et al[21,24] reported that the fusion protein of Gag of murine Moloney leukemia virus (MMLV) and staphylococcus nuclease could reduce the infection titer of MMLV by 20%-60%, while that of Gag and ribonuclease H1 of E. coli could inhibit the production of MMLV by 97%-99%. Recently, Schumann et al[26] tested the antiviral effect of CTVI in vivo using transgenic murine models. They found Gag-nuclease fusion protein could significantly inhibit MMLV replication, ameliorate the symptoms, and increase the life span up to 2.5 fold in transgenic mice. Furthermore, they found that the fusion protein was nontoxic for transgenic mice, confirming the previous in vitro cell culture results[19-25]. In this report, we demonstrated that targeted ribonuclease constructed by us (the fusion protein of HBVc and hEDN) could reduce the concentration of HBsAg in the supernatant of the transfected 2.2.15 cells by 58%. Although it is controversial that the decrease of HBsAg concentration is caused by some factors other than the inhibition of HBV replication by targeted ribonuclease, our results strongly disprove this argument. First, the fact that transfection of p/TN decreased HBsAg concentration in the supernatant while p/HBVc and p/TNmut, which are identical to p/TN except for only one amino acid mutation but lose ribonuclease activity, indicates that the reduction of HBsAg is dependent on the activity of the ribonuclease in the fusion protein targeted ribonuclease. Second, transfection of p/hEDN did not affect the HBsAg concentration, showing that the reduction of HBsAg relies on the existence of HBVc in the fusion protein. Our present results are most consistent with a model that the targeted ribonuclease encapsidates the pgRNA together with wild type capsid protein and then degrades it (also see below). This model has been corroborated by some recent research reports[19-26,40]. On the other hand, 58% reduction of HBsAg in the supernatant of 2.2.15 cells caused by the targeted ribonuclease might be an underestimate for the inhibition of HBV replication since besides present in mature virions as envelope protein, a large amount of HBsAg was synthesized and secreted as 22 nm spherical and filamentous particles by 2.2.15 cells using 2.1kb HBV mRNA as transcript which, unlike pgRNA, can not be encapsidated and therefore may not be degraded by the targeted ribonuclease[35,37]. Therefore, even if the targeted ribonuclease can strongly inhibit HBV replication, there might still be a large amount of HBsAg secreted extracellularly. The mechanism for the decrease of HBsAg concentration caused by the targeted ribonuclease might be as follows: the degradation of HBV pgRNA by targeted ribonuclease leads to the decrease of P and C protein of HBV since pgRNA also acts as mRNA for the translation of both proteins. The reduction of pgRNA, P and C protein will impair the assembly of viral capsid and then reduce the mature virions released from host cells. Further analysis including Northern and Southern blot is being performed in this laboratory to clarify the degree of inhibition on HBV replication by the targeted ribonuclease and to elucidate at what stage of HBV replication the targeted ribonuclease exerts its antiviral role.
In the process of this study, Beterams and Nassal reported their CTVI application for inhibition of HBV replication in vitro[40]. Although both their research and ours used the same strategy against HBV replication and both used HBVc as the target molecule, the effector molecules adopted were different. Bererams et al[40] used staphylococcal nuclease (SN) and we used hEDN. Compared with SN, hEDN may be more suitable for the treatment of HBV infection in human beings because as a human-origin molecule, hEDN will not induce specific immunity which may not only decrease the efficacy of CTVI but also induce immunopathological response. The difference of the effector molecules also means the targets of CTVI in the two studies were different. SN is generally believed to be active only extracellularly because it requires a high Ca2+ concentration present extracellularly but not intracellularly for its activity[41]. Therefore, SN-capsid protein incorporated into the virion is only active in degrading the viral nucleic acids upon release of the virion from the cell into an extracellular milieu[18-19,21,23,25-26]. According to this scheme, the targeted SN in Beterams et al[40]'s study can only degrade the relaxed circular (RC) DNA of mature virion released form the cell. Although they suggested in their reports that targeted SN might be mildly activated intracellularly by somewhat unknown mechanism and then cut pgRNA and minus-strand DNA of HBV, their results showed targeted SN had no significant effect on pgRNA. In contrast, the effector molecule in our study, hEDN, is a ribonuclease. Therefore, the target molecule degraded by the targeted ribonuclease constructed by us is most probably HBV pgRNA, the only RNA stage in the replication of HBV. As stated above, this notion is also supported by our experimental results, since the ribonuclease activity of hEDN is necessary for the anti-HBV effect of targeted ribonuclease. Similarly, onconase, an amphibian ribonuclease was reported to inhibit HIV replication intracellularly by degrading HIV RNA[42]. The degradation of HBV pgRNA will not only lead to less mature virions released from host cells, which means secreted viral particles had lower infectivity, but also inhibit the amplification of HBV closed circular DNA (cccDNA) which is downstream to pgRNA in HBV replication[43]. This inhibition on HBV cccDNA amplification is of pivotal significance for the treatment of chronic HBV infection since the amplification of cccDNA, the template for all HBV transcripts, plays a major role in the persistence of HBV in infected hepatocytes[1,30].
In summary, we constructed a novel targeted ribonuclease which can specifically inhibit HBV replication but has no cytotoxicity for host cells. Our results raise the possibility of using the targeted ribonuclease as a therapeutic agent for human HBV infection. For this purpose, we are generating recombinant adenovirus vector carrying the targeted ribonuclease to test its antiviral efficacy in HBV murine model.