Published online Jun 15, 2001. doi: 10.3748/wjg.v7.i3.345
Revised: November 24, 2000
Accepted: November 30, 2000
Published online: June 15, 2001
AIM: To evaluate antihepatoma effect of antisense phosphorothioate oligodeoxyribonucleotides (S-ODNs) targeted to alpha-fetoprotein (AFP) genes in vitro and in nude mice.
METHODS: AFP gene expression was examined by immunocytochemical method or enzyme-linked immunosorbent assay. Effect of S-ODNs on SMMC-7721 human hepatoma cell growth in vitro was determined using microculture tetrazolium assay. In vivo antitumor activities of S-ODNs were monitored by measuring tumor weight differences in treated and control mice bearing SMMC-7721 xenografts. Induction of cell apoptosis was evaluated by fluorescence-activated cell sorter (FACS) analysis.
RESULTS: Antisense S-ODN treatment led to reduced AFP gene expression. Specific antisense S-ODNs, but not control S-ODNs, inhibited the growth of heaptoma cells in vitro. In vivo, only antisense S-ODNs exhibited obvious antitumor activities. FACS analysis revealed that the growth inhibition by antisense S-ODNs was associated with their cell apoptosis induction.
CONCLUSION: Antisense S-ODNs targeted to AFP genes inhibit the growth of human hepatoma cells and solid hepatoma, which is related to their cell apoptosis induction.
- Citation: Wang XW, Yuan JH, Zhang RG, Guo LX, Xie Y, Xie H. Antihepatoma effect of alpha-fetoprotein antisense phosphorothioate oligodeoxyribonucleotides in vitro and in mice. World J Gastroenterol 2001; 7(3): 345-351
- URL: https://www.wjgnet.com/1007-9327/full/v7/i3/345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i3.345
Primary hepatocellular carcinoma (PHCC), one of the most common malignancies in the world, is an aggressive cancer. The mean survival time from establishment of the diagnosis is only about 4 months (2 months if the diagnosis is made late). It causes approximately 250000 deaths annually. Although much less common in western Europe, the Americas, and Australia than elsewhere, PHCC is responsible for approximately 10000 deaths per year in the United States. A number of strategies such as surgery, radiation, chemotherapy, and biological response modifiers have been applied for the treatment of PHCC, there is still no satisfactory method that can obviously improve the overall survival rate. Most of the treatment protocols are related to significant side effects and, at best, result in an overall median survival of 7-8 months[1]. Therefore, PHCC with a high fatality rate and short disease period has been one of the major health problems in the world.
Alpha-fetoprotein (AFP) is a major serum protein produced by the liver or yolk sac in fetal life in mammals and other vertebrates, and it is hardly detectable in normal adult life. However, AFP is often elevated to a significant level in association with development of PHCC, and has been defined as an oncofetal antigen[2]. Currently, AFP has become one of the most important markers in the diagnosis of PHCC[3]. The biological roles of AFP have been widely investigated. Two functions of AFP have withstood the test of time, i.e., the carrier-transport of various ligands and the regulation of certain aspects of the immune responses. AFP binds to a large variety of ligands such as fatty acids, estrogens and phytosteroids. The nature of AFP ligand(s) can orient cells toward multiplication or differentiation. The immunoregulatory activity of AFP is another important character. AFP has been found to inhibit T-cell-dependent mitogen responses and allogenic one-way mixed lymphocyte reaction[4,5]. AFP also suppresses the natural killer cell activity and induces suppressor T cells. AFP causes selective and rapid down-regulation of monocyte major histocompatibility complex class II molecules. The immunological activities of tumor necrosis factor, interleukin-1, transforming growth factor, and interferon are also decreased by the treatment with AFP[6,7]. The concept of AFP as an immuno regulatory agent provides an attractive explanation for the immunologic tolerance during pregnancy, since the fetus may be considered an allograft in the mother's body. Furthermore, it is also conceivable that AFP may some day be employed to treat certain autoimmune disorders such as rheumatoid arthritis[8]. The third function of AFP, only recently described, is the regulatory control of cell growth. AFP synergizes growth factors such as epidermal growth factor and insulin-like growth factor-I to cause a marked increase in the proliferation of porcine granulosa cells. Placental cells undergo increased proliferation in vitro in response to AFP. AFP has also been found to regulate the proliferation of human mammary tumor cells[9]. AFP can inhibit apoptosis in HL-60 human leukemia cells and AFP receptors may play a role in anti-cellular senescence.
However, the relationship between AFP and PHC is still far from clear. Our recent investigations indicate that human AFP can enhance the proliferation of mouse hepatoma H-22 and human hepatoma SMMC-7721, BEL-7404 or QGY-7703 cells in vitro[10-12]. Similar growth-stimulatory effect of low concentrations of AFP has also been obtained in human hepatoma Hep G2 cells. These results have an important implication that AFP may function as a hepatoma growth stimulator, thus suppression of AFP gene expression and its biological activities may become a new strategy for the treatment of AFP-associated tumors such as PHCC[13]. Antisense oligodeoxynucleotides can inhibit gene expression by forming RNA-DNA duplexes, thereby preventing mRNA translation and are now commonly used to investigate their role in the treatment of human malignancies, both ex vivo and in vivo[14]. In this study, we investigated the antiproliferative action of AFP antisense phosphorothioate oligodeoxyribonucleotides (S-ODNs) on an established SMMC-7721 human hepatoma cell line in vitro. We also evaluated the efficacy of AFP antisense S-ODNs in the treatment of human hepatoma xenografts growing in nude mice.
A previously characterized human hepatoma cell line SMMC-7721 derived from a male patient with liver cancer was used for the present studies. The cell line as monolayer cultures was maintained in RPMI-1640 medium supplemented with 10% heat-inactivated new-born calf serum, 100 units/mL of penicillin and 100 mg/L of streptomycin, at 37 °C in a 5% carbon dioxide atmosphere in a humidified incubator[15]. The culture medium was replaced with fresh medium every two to three days. The SMMC-7721 hepatoma cells were also employed for in vivo studies. The solid tumor was obtained by subcutaneous injection of 2.5 × 106 viable cells into the right flank of male Balb-c nude (nu/nu) mice. Subcutaneous implantation resulted in 100% tumor obtained by days 6-7 after tumor cell injection.
S-ODNs complementary to the translational initiation region of AFP mRNA (antisense: 5'-ACTTCATGGTTGCTA-3', 15 mer; sense: 5'-TAGCAACCATGAAGT-3', 15 mer) were used in the present study[16]. They were with phosphorothioate residues in the last two linkages at each end of the oligomers. Oligo-dT15 having the same modifications as the sense or antisense S-ODNs was used for controls. These oligomers were synthesized by β-cyanoethyl-phospho-ramidite chemistry using a model 381 A automated DNA synthesizer, as suggested by the manufacturer. Deprotection and purification were carried out according to the protocol on the user's manual (Applied Biosystems).
AFP gene expression in cells was determined by avidin-biotin-peroxidase complex immunocyto-chemical method. Briefly, adhesive cells were fixed in 4% paraformaldehyde. Rabbit anti-human antibody (Dako) was used as the first antibody, while normal rabbit serum served as negative control. The second antibody was biotinylated sheep anti-rabbit immunoglobulins. Final incubation was in rabbit avidin-biotin-peroxidase complex. All incubations were made at 37 °C. The positive intensities of AFP in cells were analyzed with a Leitz MPV-3 microspectrophotometer and expressed as absorbance at 460 nm.
AFP content in the serum of nude mice bearing SMMC-7721 xenografts was evaluated by two-site sandwich enzyme-linked immunosorbent assay. Briefly, the mice were killed by cervical dislocation the second day after the final injection of S-ODNs and the serum samples from the animals in each group were collected. AFP-containing serum was pipetted into the relevant wells of antiserum-precoated microtiter plate. The plate was incubated at 37 °C in a humid chamber for 1 h after which it was washed three times. Antibodies to AFP, labeled with horseradish peroxidase, were added to each well at a concentration of 1 mg antibody/L. After a 45-min incubation at 37 °C with the antibody conjugate and subsequent washing, 0.001% hydrogen peroxide and O-phenylenediamine 0.4 g/L were added. The color development was stopped 15 min later at 37 °C by the addition of sulfate 4 mol/L. The absorbance of each well was measured at 492 nm using an automatic microplate reader. Standards of defined concentrations were run in each assay allowing the construction of a calibration curve by plotting absorbance versus concentration. The AFP concentration in the serum was then calculated from this calibration curve and expressed into μg/L.
SMMC-7721 cells were seeded in the wells of 96-well culture plates. After 24 h, AFP antisense S-ODNs were added. Equimolar amounts of AFP sense and Oligo-dT 15 were used in control experiments. In this procedure, the culture medium was removed and the S-ODNs added directly to the cells for 30-60 s to facilitate uptake, whereafter the culture medium was added. Cell growth was determined using the microculture tetrazolium assay. Briefly, after culture, the cells were incubated with 800 mg/L 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Fluka, Switzerland), which is used to assay the activity of mitochondrial dehydrogenases. Four hours later, 10% sodium dodecyl sulphate-5% isobutanol-0.12% hydrochloric acid solution was added to solubilize the formazan product. The plate was then incubated at 37 °C for another 12 h. The absorbance at 570 nm was measured with a model EL × 800 enzyme-linked immunosorbent assay automated microplate reader (Bio-Tek).
Blab/c male nude (nu/nu) mice, 6-8 weeks in age and 22-24 g in body weight, were purchased from the Tumor Biology Laboratories, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. Mice were housed under pathogen-free conditions and given acidified, autoclaved water and a γ-irradiated commercial diet ad libitum. All manipulations were performed under sterile conditions in a laminar air flow hood. All procedures involving animals and their care were in accord with institutional ethical guidelines in compliance with national and international laws and policies. Each experimental group of mice included six animals. S-ODNs were dissolved in 50 μL sterile distilled water and administered 8 days after tumor cell implantation by injecting them directly into the tumor every 24 h for 8 consecutive days. Tumor weights were calculated from caliper measurements according to the previous method with the formula: tumor weight (mg) = length (mm) × width 2 (mm)/2. Antitumor activity of S-ODNs was assessed by using percent tumor weight inhibition, calculated by dividing the mean tumor weight of the treated group by the mean tumor weight of the control group, subtracting the resulting value from 1, and multiplying that value by 100. Toxicity was evaluated based on the apparent drug-related deaths and net body weight loss. Death in a treated mouse was presumed to be treatment-related if the mouse died within 7 days after the last treatment. Net body weight loss was calculated as a percentage of the mean net body weight of untreated mice.
Cell suspensions from in vitro cultures and tumor xenografts were used for fluoroescence-activated cell sorter (FACS) analysis. Cell sample preparation and propidium iodide staining for FACS analysis were performed according to the method reported previously. Briefly, the cells were fixed with 80% ethanol at 4 °C for 60 min. The ethanol-treated cells were washed twice with phosphate-buffered saline, pelleted by centrifugation and resuspended in a solution containing 50 mg/L propidium iodide and 100 mg/L RNase A. The percentage of apoptotic cells was determined by the fluorescence of individual cells measured by FACS flow cytometry (Becton Dickinson, San Jose, CA).
The experimental results summarized in Table 1 and the apoptosis data in Table 2 were analyzed by Student's t test for statistical significance. The statistical significance of AFP protein expression differences in Table 2 was evaluated using one-way analysis of variance. The results in Table 3 were analyzed by Mann-Whitney U-test for statistical significance. Differences were considered significant at P value < 0.05.
| Group (mg/day × day) | Mean tumor weight (mg) | Toxic deaths | AFP content (% inhibition) | |||||||
| 0* | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |||
| Control (0) | 96.7 ± 51.1 | 191.4 ± 48.3 | 336.9 ± 67.8 | 504.8 ± 80.2 | 636.3 ± 68.8 | 823.9 ± 187.6 | 1056.7 ± 182.0 | 1201.6 ± 208.0 | 0/6 | - |
| Antisense (0.25 ± 8) | 83.1 ± 33.7 | 145.2 ± 43.8 (24.2) | 215.8 ± 49.6 (35.9)a | 286.5 ± 49.2 (43.2)a | 356.7 ± 52.5 (43.9)a | 520.0 ± 56.1 (36.9)a | 653.1 ± 128.8 (38.2)a | 776.6 ± 61.3 (35.4)a | 0/6 | 68.9a |
| Antisense (0.50 ± 8) | 84.8 ± 34.6 | 130.3 ± 36.9 (31.9)a | 202.3 ± 47.9 (39.9)a | 254.2 ± 43.2 (49.6)a | 310.8 ± 56.9 (51.2)a | 449.9 ± 108.3 (45.4)a | 593.9 ± 72.3 (43.8)a | 737.3 ± 146.7 (38.6)a | 0/6 | 73.8a |
| Sense (0.25 ± 8) | 100.5 ± 33.5 | 201.4 ± 44.2 | 339.1 ± 71.1 | 510.4 ± 114.1 | 629.6 ± 109.4 | 829.5 ± 164.5 | 1034.9 ± 198.1 | 1191.9 ± 181.3 | 0/6 | 3.4 |
| Sense (0.50 ± 8) | 86.9 ± 25.6 | 187.7 ± 42.3 | 359.7 ± 59.6 | 509.5 ± 111.8 | 659.7 ± 101.7 | 837.7 ± 147.3 | 1011.0 ± 142.9 | 1232.3 ± 149.0 | 0/6 | 0 |
As shown in Table 1, when SMMC-7721 cells were exposed to AFP antisense S-ODNs for 48 h, the growth of the cells was significantly suppressed as compared with untreated cells. Moreover, the antisense oligomers showed concentration-dependent effect although not following a linear relationship. However, the inhibitory effect of the sense S-ODNs was much weaker than that of the antisense S-ODNs. No obvious inhibitory influence of Oligo-dT15 on SMMC-7721 cell growth was observed. The growth of unhepatoma HL-60 leukemia cells was not affected by the three above-mentioned oligomers (data not shown). It was suggested that the antiproliferative effect of the antisense S-ODNs may be due to a hybridization-based mechanism. In order to confirm it, a 10-fold excess of complementary sense S-ODNs was added to the culture system for 48 h. The obvious reversal of the SMMC-7721 cell growth inhibition of 10 μmol/L antisense S-ODNs was obtained by the hybridization competition. The addition of equal amounts of antisense and sense S-ODNs produced intermediate degrees of growth suppression (Table 4). The unmodified phosphodiester oligomers were not testable in vitro because of their poor stability in culture medium.
| Antisense (μmol/L) | Sense (μmol/L) | Hepatoma cell growth (absorbance at 570 nm) | Suppression (%) |
| 0 | 0 | 1.00 ± 0.08 | |
| 0 | 10 | 0.95 ± 0.07 | 5 |
| 10 | 0 | 0.46 ± 0.02 | 54 |
| 10 | 10 | 0.51 ± 0.05 | 49 |
| 10 | 100 | 0.95 ± 0.05 | 5 |
We examined AFP gene expression in SMMC-7721 cells by immunocytochemical method. The positive grains were found to diffuse throughout the cytoplasm of SMMC-7721 cells. Reaction products were visible in almost all the cells although in various amounts. In contrast, we could not detect positive grains when rabbit anti-human AFP primary antibody was replaced by normal rabbit serum. It is indicated that SMMC-7721 cells could express AFP protein. Similar results were obtained by using BEL-7404 human hepatoma cell line[17,18]. Treatment of SMMC-7721 cells with AFP antisense S-ODNs for 48 h markedly downregulated AFP protein expression. Downregulation of AFP protein expression by the antisense oligomers was specific, since the sense S-ODNs and Oligo-dT15 having the same modification did not downregulate AFP protein expression (Table 2). The results obtained from HuH-7 human hepatoma cell line were similar. These data argue strongly that the growth inhibition of AFP antisense oligomers is likely caused by the sequence-specific blocking of AFP protein expression in SMMC-7721 cells. To determine whether the decrease in AFP protein expression was due to a downregulation of AFP gene expression induced by antisense S-ODNs and not merely related to the apoptotic status of the cell population, AFP protein expression was analyzed 6 h after S-ODNs treatment. The results indicated that treatment with 25 μmol/L antisense S-ODNs resulted in an obvious inhibition of AFP protein expression, whereas no evidence of an increase in apoptosis was observed at this time point. The same concentrations of sense S-ODNs and oligo dT-15 had no effect on the AFP protein expression and hepatoma cell apoptosis (data not shown).
After exposure of exponentially growing SMMC-7721 cells to AFP antisense S-ODNs for 48 h, cells showed a hypodiploid DNA content indicative of apoptosis as determined by FACS analysis (Table 2). The presense of apoptosis was also confirmed by morphologic observation. It is concluded that SMMC-7721 cells are rapidly induced to undergo apoptosis by AFP antisense S-ODNs.
Nude mice bearing SMMC-7721 tumors were treated with 0.25 and 0.5 mg S-ODNs per day for 8 consecutive days. As shown in Table 3, a marked decrease in tumor weight was observed after antisense S-ODNs treatment. Moreover, treatment with 0.25 mg per day proved less effective than treatment with 0.5 mg per day. No obvious toxicity was observed in mice receiving either 0.25 or 0.5 mg per day of antisense S-ODNs for 8 days.
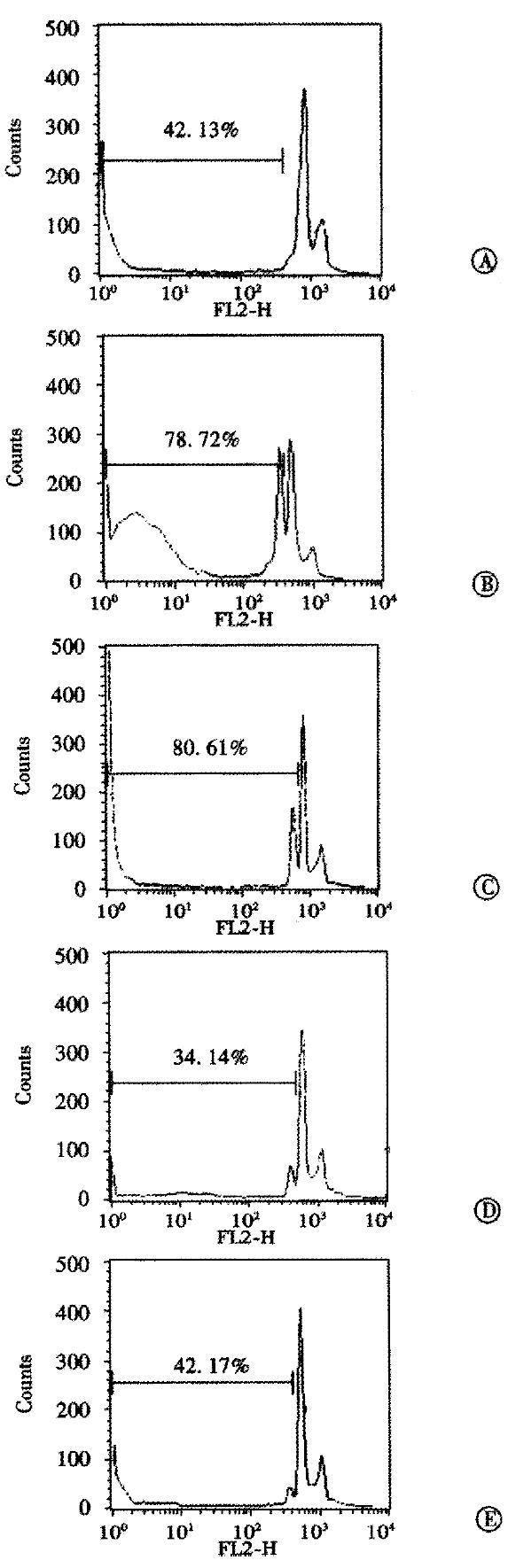
In order to verify whether the antitumor effect observed in vivo was due to a specific antisense effect, AFP protein content in the serum of untreated mice and mice treated with S-ODNs was measured by using avidin-biotin-peroxidase complex method. The results indicated that treatment with AFP antisense S-ODNs decreased the AFP protein in the serum (Table 3). FACS analysis of the DNA content histograms of hepatoma cells from tumors of untreated mice revealed an accumulation in the hypodiploid region representative of about 40% of the cell population, indicating apoptotic cell death. This cell death was expected, since the FACS analysis was performed when the tumors were at a late stage of growth (day 16 of growth). Similar levels of apoptosis were found in tumor cells from sense S-ODN-treated mice. Treatment with AFP antisense S-ODNs produced a marked increase of the percentage of apoptotic tumor cells to about 80% (Figure 1).
Since AFP was found in the mid 1950s, this oncofetal protein has demonstrated clinical utility as a PHCC marker. But its biological activities in mammals have still remained an enigma. To date, only two functional roles of AFP (i.e., ligand/carrier transport and modulation of immune responses) have been ascertained. In the last decade, the growth regulatory properties of AFP have aroused interests as a result of studies involving ontogenetic and oncogenic growth in both cell cultures and animal models. Particularly, the effect of AFP administration on the development of experimental tumors in mammals has been investigated in vivo with the use of carcinogens. The influence of murine AFP on the threshold dose, mean tumor size, regression time, and number of progress or for moloney sarcoma virus-induced tumors in mice was studied. AFP-treated mice developed larger tumors, required a longer period for regression and had a significantly higher mortality. Furthermore, AFP, but not murine albumin or transferrin, allowed the growth of tumors when normally subthreshold doses of virus were injected. The effect of AFP could be abrogated by pretreatment with an anti-AFP antibody. AFP, but neither albumin nor transferrin, also accelerated the appearance of plasmacytomas. Chicken-AFP-treated quails developed tumors with shorter latent periods than those of the tumors that developed in untreated quails after inoculation with Rous sarcoma virus[19]. The in vivo tumor growth stimulation by AFP can be explained by its immunosuppression. Cell-mediated immunity is an important and central mechanism of host resistance to malignant neoplasms. Recently, we found that antihepatoma effects of AFP antisense S-ODNs were more significant in normal mice than in nude mice. Moreover, thymus weight of the normal mice was obviously increased after AFP antisense S-ODNs treatment (data not shown). It is suggested that there exists a relationship between AFP and the susceptibility of hepatoma cells to immunity-mediated cytotoxicity. If the effective immune system does not exist, however, can AFP still enhance the growth of tumor cells It was found that AFP stimulated, in a dose-dependent manner, human mammary tumor cell growth induced by platelet-derived growth factor in vitro; ablation of endogenous AFP by anti-AFP monoclonal antibody affinity chromatograph significantly reduced the proliferative activity of human mammary tumor cells. Recent studies indicated that AFP did directly stimulate the proliferation of hepatoma cells in vitro, independent of its immunosuppression. In the authors'view, AFP should be considered as a direct or indirect factor associated with tumor growth.
The precise relationship between AFP and PHC is not known. AFP was highly expressed in populations of hepatic oval cells during the early stages of carcinogenesis. These cells did not show the histological patterns that were diagnostic of trabecular hepatocellular carcinoma[20,21]. AFP-expressing hepatic oval cells in hepatocellular foci and nodules proliferating after carcinogenic treatment seem to be precursor cells of the hepatic carcinoma. On the other hand, high levels of AFP in the fully developed PHCC, or in the serum of the host, are associated with more aggressive behavior, and increased anaplasia[22]. The present study also demonstrated that AFP antisense S-ODNs are effective inhibitors of hepatoma cell growth in vitro and in vivo. Based on all the data, it is strongly inferred that AFP may play a role in the generation and development of PHCC.
The present results indicated that AFP antisense S-ODNs exhibited significant antihepatoma activities in vitro and in vivo by the sequence-specific blocking of AFP gene expression. AFP sense oligomers and Oligo-dT15 as controls had no or at least much weaker effects. Likewise, AFP antisense S-ODNs could also inhibit BEL-7404 human hepatoma cell proliferation in vitro[23,24]. We thus propose the possibility of suppressing human hepatoma proliferation by specifically decreasing AFP gene expression with antisense oligomers. The recent use of antisense oligomers as a therapeutic tool represents a newly established strategy for treating diseases[25]. Therefore, the successful designing of antisense S-ODNs for blocking the AFP gene expression in human hepatoma cells may not only further confirm the growth stimulatory effect of AFP, but also become an important therapeutic approach for PHCC[26]. However, the antitumor effects of AFP antisense S-ODNs were obtained at relatively high concentrations that might be difficult to achieve clinically for a number of reasons, among which are high cost and difficulties in the large-scale production of S-ODNs. Hopefully, the development of new generation ODN analogues or a more efficient delivery of S-ODNs, perhaps using liposomes as vehicles, will lead to therapeutic applications in humans. Thus this antisense therapy awaits further investigations[27,28]. And, antihepatoma therapeutic strategies relying on the use of AFP antisense S-ODNs may be enhanced by using these compounds in combination with other antitumor agents[24].
It is well-known that activation of an endonuclease in apoptotic cells results in extraction of the low molecular weight DNA following cell permeabilization, which, in turn, leads to the decreased stainability with DNA-specific fluorochromes. Thus, measurements of DNA content by FACS make it possible to identify apoptotic cells. Using the FACS method, we have observed that AFP antisense S-ODNs induced apoptosis of SMMC-7721 hepatoma cells. Moreover, the presence of apoptotic cells has also been confirmed by morphologic observation. Therefore, although the mechanisms of antihepatoma action of AFP antisense S-ODNs are not fully understood, the induction of hepatoma cell apoptosis may be responsible for their growth-inhibitory effect.
The authors would like to thank Drs. Wei Dong Tang, Zhen De Zhang and Zu Ming Sun at Shanghai Institute of Materia Medica, Chinese Academy of Sciences for technical assistance.
| 1. | Colleoni M, Gaion F, Liessi G, Mastropasqua G, Nelli P, Manente P. Medical treatment of hepatocellular carcinoma: any progress. Tumori. 1994;80:315-326. [PubMed] |
| 2. | Otsuru A, Nagataki S, Koji T, Tamaoki T. Analysis of alpha-fetoprotein gene expression in hepatocellular carcinoma and liver cirrhosis by in situ hybridization. Cancer. 1988;62:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 288] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Wang XW, Xu B. Effect of alpha-fetoprotein on splenocyte proliferation of mice bearing ascites hepatoma-22 in vitro. Shanghai Mianyixue Zazhi. 1995;15:327-329. |
| 5. | Wang XW, Xu B. Effect of alpha-fetoprotein on immune functions of mice bearing ascites hepatoma-22 in vitro. Shanghai Mianyixue Zazhi. 1997;17:224-226. |
| 6. | Yamashita T, Nakane A, Watanabe T, Miyoshi I, Kasai N. Evidence that alpha-fetoprotein suppresses the immunological function in transgenic mice. Biochem Biophys Res Commun. 1994;201:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Wang W, Alpert E. Downregulation of phorbol 12-myristate 13-acetate-induced tumor necrosis factor-alpha and interleukin-1 beta production and gene expression in human monocytic cells by human alpha-fetoprotein. Hepatology. 1995;22:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Ogata A, Yamashita T, Koyama Y, Sakai M, Nishi S. Suppression of experimental antigen-induced arthritis in transgenic mice producing human alpha-fetoprotein. Biochem Biophys Res Commun. 1995;213:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Jacobson HI, Bennett JA, Mizejewski GJ. Inhibition of estrogen-dependent breast cancer growth by a reaction product of alpha-fetoprotein and estradiol. Cancer Res. 1990;50:415-420. [PubMed] |
| 10. | Wang XW, Xu B. Stimulation of tumor-cell growth by alpha-fetoprotein. Int J Cancer. 1998;75:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Wang XW, Xie H. [Effect of alpha-fetoprotein on the growth of human hepatoma cells in vitro]. Shiyan Shengwu Xuebao. 1999;32:15-22. [PubMed] |
| 13. | Wang XW, Xie H. Significance of α-fetoprotein in the development of novel therapeutic agents. Drugs Fut. 1999;24:55-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents--is the bullet really magical. Science. 1993;261:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1011] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 15. | Yuan JH, Zhang RP, Zhang RG, Guo LX, Wang XW, Luo D, Xie Y, Xie H. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol. 2000;6:210-215. [PubMed] |
| 16. | Morinaga T, Sakai M, Wegmann TG, Tamaoki T. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci USA. 1983;80:4604-4608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Wang XW, Xu B. In situ hybridization and immunocytochemistry for the investigation of alpha-fetoprotein gene expression in human BEL-7404 hepatoma cells. Zhongguo Zuzhihuaxue Yu Xibaohuaxue Zazhi. 1997;6:259-263. |
| 18. | Wang XW, Xu B. Expression of alpha fetoprotein messenger RNA in BEL-7404 human hepatoma cells and effect of L-4 oxalysine on the expression. World J Gastroenterol. 1998;4:294-297. [PubMed] |
| 19. | Yamada A, Hayami M. Suppression of natural killer cell activity by chicken alpha-fetoprotein in Japanese quails. J Natl Cancer Inst. 1983;70:735-738. [PubMed] |
| 20. | Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. In situ hybridization studies on expression of albumin and alpha-fetoprotein during the early stage of neoplastic transformation in rat liver. Cancer Res. 1987;47:5469-5475. [PubMed] |
| 21. | Koen H, Pugh TD, Nychka D, Goldfarb S. Presence of alpha-fetoprotein-positive cells in hepatocellular foci and microcarcinomas induced by single injections of diethylnitrosamine in infant mice. Cancer Res. 1983;43:702-708. [PubMed] |
| 22. | Matsumoto Y, Suzuki T, Asada I, Ozawa K, Tobe T, Honjo I. Clinical classification of hepatoma in Japan according to serial changes in serum alpha-fetoprotein levels. Cancer. 1982;49:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Wang XW, Xie H. Growth inhibition of human liver cancer cells by alpha-fetoprotein antisense strategy. In Vitro Cell Dev Biol Anim. 1999;35:118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Wang X, Zhang R, Xie H. Combined effect of alpha-fetoprotein antisense oligodeoxy-nucleotides and 5-fluorouracil on human hepatoma cell growth. Chin Med J (Engl). 1999;112:743-746. [PubMed] |
| 25. | Hélène C, Toulmé JJ. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990;1049:99-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 557] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Wang XW, Xu B. Several new targets of antitumor agents. Zhongguo Yaoli Xuebao. 1997;18:289-292. [PubMed] |
| 27. | Wang X, Xie H. [Study of alpha-fetoprotein]. Zhonghua Yixue Zazhi. 1998;78:723-724. [PubMed] |
| 28. | Wang XW, Xie H. Alpha-fetoprotein and biotherapy of liver cancer. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 1998;5:235. |
Dr. Xing-Wang Wang, earned PhD from Shanghai Institute of Materia Medical, Chinese Academy of Sciences in 1997. Now a professor at Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
Edited by Ma JY