INTRODUCTION
Hepatitis B virus is closely related to human hepatocellular carcinoma, but the mechanism of HBV in tumorigenesis of hepatocellular carcinoma is still unclear. HBV-associated hepatocellular carcinoma is a dominant type in China. It is reported that the X protein of HBV plays a key role in the tumorigenesis of HBV-associated hepatocellular carcinoma and it participates in many signal tranduction pathway of hepatocellular carcinoma cells[1,2].
NF-κB was extensively studied since it was first found in 1986. It plays an important role in physiologic and pathologic conditions as an inducible nuclear factor. More than 60 proinflammatory genes involved in controling. The differentiation, immuno-stimulation, apoptosis, chemoattraction, cell adhension, extracellular matrix degradation have been shown to be regulated by NF-κB[3]. However, concerning the relatetionship of the X protein of HBV to the activation of NF-κB and the role of NF-κB in the tumorigenesis of hepatocellular carcinoma is unclean. In this study we investigated the expression of NF-κB in hepatocellular carcinoma tissues and its relationship with the X protein of HBV.
MATERIALS AND METHODS
Material
Human hepatocellular carcinoma cell line HCC-9204 was established by the Department of Pathology, the Fourth Military Medical University. 52 cases of hepatocellular carcinoma tissues were collected from on Department. The main reagents such as rabbit NF-κB p65 antibody was purchased from Sant Cruz Company. DNA random primer labelled reagent kit and ALLN inhibitor were purchased from Bornglinman Company. The primer of HBV x gene was synthesized by Shengon Biology Company in Shanghai. G418 were purchased from Promega Company. Lipofectamin was purchased from GIBCORL Company. Other reagents were bought from Zhongshan Company, Beijing.
Method
Detection of NF-κB in hepatocellular carcinoma Immunohistochemistry SP method was used to detect the expression of NF-κB in 52 cases of human hepatocellular carcinoma tissues. The main steps were as follows: ① Tissues were treated with 3% H2O2 methanol at room temperature for 30 min and then incubated in 5% goat antiserum for 30 min at 37 °C. ② Rabbit anti p65 and HBX antibody were added to adjacent tissue sections respectively and incubated overnight at 4 °C. The dilution of p65 and HBX were 1:80 and 1:50. ③ Biotin-labelled goat anti-rabbit IgG was added to the sections and incubuated at 37 °C for 30 min. ④ SP complex was added and then DAB-H2O2 was used for the colour reaction. The tissues sections were washed with PBS (0.01 M, pH7.4) between each step.
Gene transfection and selection The eukaryotic expression vector pCDNA3.1-HBX of HBV x gene and the empty vector pCDNA3.1 were transfected into human hepatocellular carcinoma cell line HCC-9204 respectively by gene transfection mediated by lipofectamine. The main steps were as follows. ① HCC-9204 cells were seeded in culture plate. A liquid (100 μL 1640 serum free medium containing 2 μg plasmid) and B liquid (100 μL 1640 medium free of serum containg 10 μL lipofectamine) were mixed at room temperature for 30 minues to form DNA-lipofectamine complex. ② HCC-9204 cells were washed with 2 mL 1640 serum free medium twice. The DNA-lipofectamine complex were diluted with 0.8 mL 1640 medium and added to cells. The cells were cultured at 37 °C 5% CO2 condition for 5 h. ③ These the cells were cultured in 10% vitulary serum for 72 h. ④ Cells were cultured in the selective medium containing G418 700 g·L-1 for 1 week, and then cultured in the selective medium containing G418 500 g·L-1. Untransfected HCC-9204 cells were cultured in the same selective medium as control test.
Detection of HBV x mRNA in HCC-9204 cellIn situ hybridization was used to detect HBV x mRNA in the fourth passage of HCC-9204 cells which were transfected with the eukaryotic expression vector pCDNA3.1-HBX of HBV x gene. DNA probe was labelled by using DNA random primer method. Control experiment: HCC-9204 cells which were not transfected served as negative control. Empty controls were those without adding any probes. The main steps were as follows: ① The cells were fixed in 4% paraformaldehyde for 30 min and were digested in 25 g·L-1 protease for 5 min at 37 °C. ② The forehibridization liquids were added to the cells for 2 h at 42 °C, and then the hybridization liquids were added to the cells at 42 °C overnight (the constitutions of the hybridization liquid was 50% mathane amide, 1 x Denharts liquids, 10% sulfuric acid dextran, 0.5 g·L-1 herring DNA, probes labled with digoxin). ③ The cells were washed with 2 × SSC, 1 × SSC, and buffer 1 sequencesly. ④ Anti-digoxin alkaline phosphatase complex (1:500) diluted with 1% goat serum -0.3% Triton X 100 was added to the cells for 2 h at room temperature and then washed with buffer 1 and buffer 3 respectivily. ⑤ Chromogenic liquids (100 μL buffer III, 4.5 μL NBT, 3.5 μL BCIP) was added to the cells in the darkness and observed under microscope. Buffer IV was used to terminate the chromogenic reaction.
Detection of the X protein of HBV in HCC-9204 cell Immunofluorimetry was used to detect the X protein of HBV in the fourth passage of HCC-9204 cells which were transfected with pCDNA3.1-HBX or the empty vector pCDNA3.1. HCC-9204 cells untransfected served as the negative control. The main steps were as conventional. The dilution of HBx antibody was 1:50.
Detection of NF-κB in HCC-9204 cells Immunohistochemistry SP method was used to detect NF-κB in cells which were transfected or not transfected by HBV x gene. The dilution of rabbit p65 NF-κB is 1:80. The detailed steps were as the above.
RESULTS
Expression of NF-κB in hepatocellular carcinoma tissues
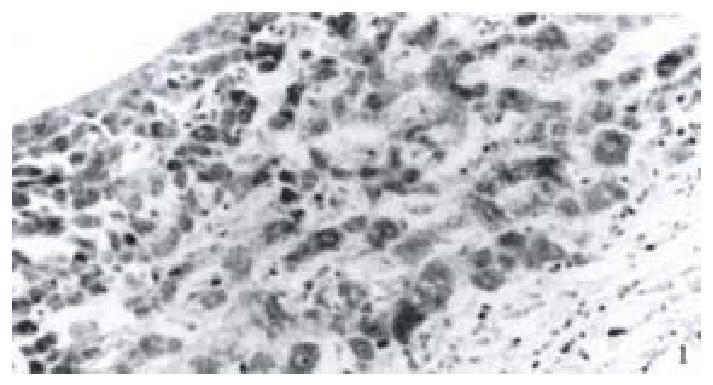
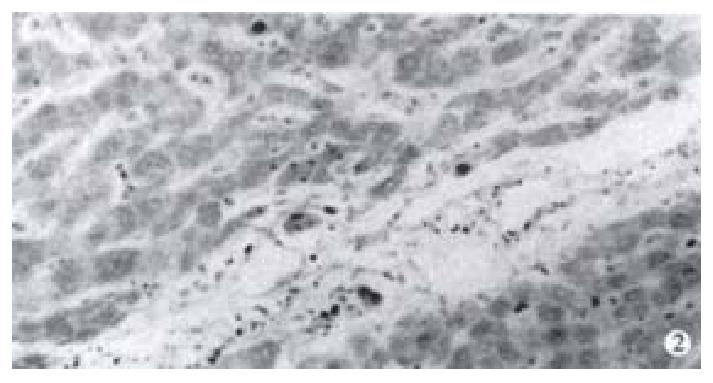
Immunohistochemistry SP method was used to detect the expression of NF-κB in 52 cases of hepatocellular carcinoma tissues. It was found that NF-κB was widely expressed in hepatocellular carcinoma tissues. The cells which were positive for NF-κB distributed diffusely. The cells used were hepatocellular carcinoma cells and the expression of NF-κB was closely related to the expression of the X protein of HBV. In 11 cases of hepatocellular carcinoma tissues which were positive for X protein of HBV, NF-κB was localized both in the cytoplasm and the nuclei of hepatocellular carcinoma cells, while in 41 cases of hepatocellular carcinoma tissues which were negative for X protein of HBV, NF-κB was localized only in the cytoplasm of hepatocellular carcinoma cells (Figure 1, Figure 2).
Figure 1 Expression of NF-κB in hepatocellular carcinoma which is positive of the X protein.
Figure 2 Expression of NF-κB in hepatocellular carcinoma which is negative of the X protein.
Expression of NF-κB in HCC-9204 cells which were transfected with the eukaryotic expression vector pCDNA3.1-HBX of HBV x gene
Gene transfection and selection Gene transfection which was mediated by lipofectamine was used to transfect the eukaryotic expression vector pCDNA3.1-HBX of HBV x gene and the blank vector pCDNA3.1 respectively into HCC-9204 cells. The cells were cultured in the selective medium containing G418. Most of the cells died after 2 weeks, only a few anchorage-dependent cells grew clonely. All of the control cells untransfected died 2 weeks after they were cultured in the same condition. It was suggested that gene transfection were successful primarily.
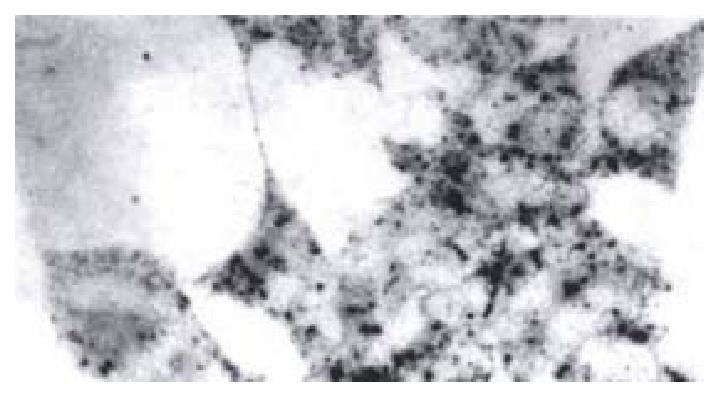
Detection of HBV x mRNA in HCC-9204 cellsIn situ hybridization was used to detect HBV x mRNA in cells which were transfected with the eukaryotic expression vector pCDNA3.1-HBX of HBV x gene. It was shown that positive siganls were loacted in the cytoplasm of HCC-9204 cells, while the nuclei were negative. There was no positive signal in both cytoplasm and nuclei of HCC-9204 cells untransfected served as the negative control (Figure 3).
Figure 3 HBV x mRNA in HCC-9204 cells which were transfected with pCDNA3.
1-HBX. (× 400)
Detection of the X protein of HBV gene in HCC-9204 cells Expression of the X protein of HBV gene in the fourth passage of HCC-9204 cells which were transfected with pCDNA3.1-HBX or the blank vector pCDNA3.1 were detected by using immunofluorimetry. It was observed under fluorescent micrscope that the yellow-green fluorescence was localized in both cytoplasm and nuclei of hepatocellular carcinoma cells which were transfected with pCDNA3.1-HBX, but none in the cell not transfected (Figure 4).
Figure 4 X protein in HCC-9204 cells which were transfected with pCDNA3.
1-HBX. (× 400)
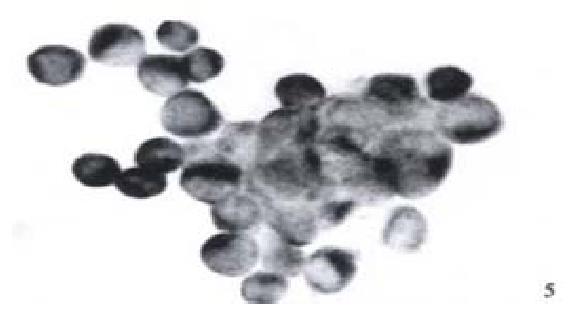
Expression of NF-κB in HCC-9204 cells which was transfected with eukaryotic expression vector of HBV x gene It was found NF-κB was localized in both cytoplasm and nuclei of hepatocellular carcinoma cells. NF-κB was localized only in cytoplasm of hepatocellular cells which were not transfected by the eukaryotic expression vector pCDNA3.1-HBX (Figure 5, Figure 6).
Figure 5 Expression of NF-κB in hepatocellular carcinoma cells which is positive for X protein of HBV.
(× 400)
Figure 6 Expression of NF-κB in hepatocellular carcinoma cells which is negative for X protein of HBV.
(× 400)
DISCUSSION
An estimated 300 million hepatitis B virus (HBV) carriers in the world are at an increased risk for the development of chronic active hepatitis (CAH), cirrhosis, and hepatocellular carcinoma[4]. HBV associated HCC is among the 10 most frequent cancers worldwide. At least 250000 cases of HCC are diagnosed annually, less than 3% survive 5 years. The relative risk of HBV carrier developing HCC approaches 200:1, which is one of the highest relative risks known for human cancers[5,6]. Among the four proteins translated from the hepatitis B virus geneome, the x gene produt (the X protein) has drawn much attention for its role as a transacting factor for exploitation of the host cell machinery. Up to now, several significant discoveries had been made regarding the functions of the X protien, and from these studies, the X protein was established as essential in viral replication, HCC, and activation of certain signal transduction pathyway[7-11]. Although there is a cotroversy about the role of the X protein in viral replication, the x-gene product was shown to be required for the replicatiion of woodchuck hepatitis virus in animal studies, which were considered to reflect the in vivo phenomenon more precisely. Generally, it is believed that the X protein contributes to the generation of hepatocellular carcinoma. The X protein was reported to induce transformation of NIH3T3 cells. Forthermores, the development of HCC was observed in HBx transgenenic liver. The possible mechanism is based on the ability of the X protein to activate cellular signal transduction pathways. The X protein stimulates the ras/raf/mitogen-activated protein kinase cascade, leading to the activation of AP-1-dependent transcriptional activation[1,2]. In addition, c-Jun, N-terminal kinase were shown to be activated by the X protein[4,12-15]. Probably, through the combined actions of the above mechanisms, the X protein contributes to tumorigenesis[16-20]. In fact, the X protein has been shown to deregulate the cell cycle check point cotrols through the activation of ras.
Originally defined as the nuclear factor bound to the B site of the immunoglobin κ light chain gene enhancer in B lymphocytes[3], NF-κB is now known to be a family of dimeric transcription factors. NF-κB is ubiquitously expressed in non-B cells inactive form sequestered in the cytoplasm with specific inhibitory proteins termed IêBs[21-25]. NF-κB/Rel have been implicated in the inflammatory response and synthesis of adhesion molecule. When cells are stimulated by TNF-α and interleukin-1 (IL-1), I-κB proteins associated with NF-κB in the cytoplasm become phosphorylated, ubiquitinated, and degraded. Degradation of I-κB proteins frees NF-κB proteins, which then translocate into the nucleus, where they activate transcription[26-28]. Recently, it was demonstrated a role for the NF-κB/Rel family in survival of B lymphocytes. Downregulation of NF-κB/Rel activity induces apoptosis of normal and transformed murine B cells, whereas ectopic expression of c-Rel leads to survival of B lymphoma cells. Interestingly, mice lacking the p65 subunit of NF-κB displayed embryonic lethality, accompanied by massive liver apoptosis on the 15th and 16th days of gestation[29]. These findings suggest that expression of NF-κB/Rel activity in murine hepatocytes acts directly to promote survival of these cells and suggest that apoptosis observed in the hepatocytes of mice lacking relA is a direct effect of p65 deficiency[30,31]. In addition, many cells are resistant to stimuli that can induce apoptosis. The activation of the transcription factor nuclear NF-κB by tumour necrosis factor (TNF), ionizing radiation was found to protect from cell killing. Inhibition of NF-κB nuclear translocation enhanced apoptotic killing by these reagents but not by apoptotic stimuli that do not activate NF-κB[32-34]. Furthermore, NF-κB is also related to cell proliferation, transformation[35]. The abnorma activation of NF-κB was ralated to the tumorigensis of maglinant cancer, for example, in Hodgkin's lymphtoma, breast carcinoma and other cancers[36-49].
The X protein of HBV has been shown to be closely related to human hepatocellular carcinoma, but the relationship of the X protein of HBV and NF-κB and its role in the tumorigenesis of hepatocellular carcinoma, has not been elucidation. We firstly detected the expression of NF-κB in hepatocellular carcinoma tissues using immunohistochemistry SP method. It was shown that NF-κB was expressed diffusely in hepatocellular carcinoma tissues and its localization in cells was related to the expression of the X protein of HBV. In hepatocellular carcinoma tissues which was positive for X protein of HBV, the NF-κB was expressed in both cytoplasm and nuclei of hepatocellular carcinoma cells, whereas in hepatocellular carcinoma tissues which were negative for X protein of HBV, the NF-κB was expressed only in the cytoplasm of hepatocellular carcinoma cells. Gene transfection further indicated that the X protein of HBV could activate NF-κB translocate it to the nucleus.
This finding strongly indicated that there was abnormal activation of NF-κB in human hepatocellular carcinoma tissues which was probably related to the X protein of HBV. The X protein can activate NF-κB and translocate it to the nuclei from the cytoplasm of hepatocellular carcinoma cells. Gene transfection further demonstrated that the X protein of HBV can activate NF-κB. Activated NF-κB probably regulate the target gene transcription in the nuclei of hepatocellular carcinoma cells. The role of NF-κB in tumorigenesis of hepatocellular carcinoma was probably related to the cell cycle. Recently, it was reported that cyclinD1 is a target gene of NF-κB and activated NF-κB can initate the transcription of cyclinD1 and regulation of G0/G1 to-S phase transition. This led to loss of the normal regulation of cell cyccle and cell proliferation and transformation.
In conclusion, NF-κB is abnormally activated in huamnn hepatocellular carcinoma, which is related to the role of the X protein of HBV, but the detailed mechanism of NF-κB in the tumorigensis of HBV-associated hepatocellular carcinoma worths further study. This study has important significance, because it sheds new light in revealing the role of the key nuclear factor NF-κB in the tumorigenesis of HBV-associated hepatocellular carcinoma from a new primt of view.