Published online Feb 28, 2026. doi: 10.3748/wjg.v32.i8.115291
Revised: December 23, 2025
Accepted: February 3, 2026
Published online: February 28, 2026
Processing time: 121 Days and 5.2 Hours
Biologics are the preferred treatment for patients with moderate to severe Crohn’s disease (CD). Four biologics included in China’s National Reimbursement Drug List are available for CD treatment. Due to loss of response, patients need swit
To assess the cost-effectiveness of sequential treatment strategies with National Reimbursement Drug List-included biologics for moderate to severe CD in China.
From a healthcare system perspective, a Markov model was constructed to evaluate the cost-effectiveness of four biologics [infliximab, adalimumab (ADA), ustekinumab (UST), and vedolizumab] applied in different treatment sequences for moderate to severe CD patients. Using one times the GDP per capita ($13444.68, 2024) in China as the willingness-to-pay threshold, the absolute net monetary benefit (NMB) and incremental cost-effectiveness ratio (ICER) were calculated. Both costs and utilities were discounted at an annual rate of 5%. Sensitivity analyses were conducted on key parameters.
With $13444.68 as willingness-to-pay, among the 17 treatment sequences evaluated for biologic-naïve patients, sequence 3 (ADA-UST) yielded the highest absolute NMB of $35850.93. Compared with sequence 1 (vedolizumab-UST), sequence 3 had the most favorable ICER of $2285.38/quality-adjusted life year. For biologic-exposed patients, sequence 3 still demonstrated the optimal NMB and ICER results.
Adding biologic treatment lines provides greater health benefits for patients with moderate to severe CD. Among the various sequential strategies, the treatment sequence combining ADA and UST is more likely to be the optimal cost-effective option in China.
Core Tip: Using a Markov model, this study evaluated the cost-effectiveness of 17 sequential biologic strategies for moderate-to-severe Crohn’s disease in China. Under a willingness-to-pay threshold of one time the 2024 GDP per capita, the findings suggest that while additional lines of biologic therapy improve health outcomes, they concurrently increase costs. The sequence initiating with adalimumab and followed by ustekinumab emerged as the most cost-effective option, yielding the highest net monetary benefit and a favorable incremental cost-effectiveness ratio for both biologic-naïve and biologic-exposed patients.
- Citation: Wu Y, Tao LB, Liu C, Wang FX, Yan Y, Sun S. Cost-effectiveness of different strategies with biologics for the treatment of moderate to severe Crohn’s disease in China. World J Gastroenterol 2026; 32(8): 115291
- URL: https://www.wjgnet.com/1007-9327/full/v32/i8/115291.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i8.115291
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that can affect the entire digestive tract from the mouth to the anus, with predominant involvement of the ileum, cecum, and colon[1]. CD presents with complex clinical manifestations, including abdominal pain, diarrhea, intra-abdominal abscesses, recurrent fistula formation, and perianal lesions, all of which significantly impair patients’ quality of life[2]. Over the past three decades, the global prevalence of IBD has shown a continuous upward trend, potentially imposing substantial social and economic burdens on govern
The development of biologics has revolutionized the treatment of moderate to severe CD, shifting the paradigm from the use of corticosteroids, 5-aminosalicylic acid, and non-targeted immunosuppressants aimed at symptom control, to the early application of biologics to achieve clinical and endoscopic remission[8]. As of 2025, four biologics have been included in China’s National Reimbursement Drug List (NRDL) for the treatment of moderate to severe CD: (1) Infliximab (IFX); (2) Adalimumab (ADA); (3) Ustekinumab (UST); and (4) Vedolizumab (VDZ). Although biologics can control clinical symptoms and promote mucosal healing, their high cost may increase the economic burden on patients. Additionally, due to the risk of loss of response, patients may need switching therapies during the course of treatment. While previous studies have evaluated the cost-effectiveness of individual biologics in the Chinese CD population[9-12], there is a lack of cost-effectiveness analyses on sequential treatment strategies involving multiple biologics.
Therefore, from a healthcare system perspective, this study aims to evaluate the cost-effectiveness of different sequential treatment strategies involving the four NRDL-covered biologics for patients with moderate to severe CD in China. The findings are expected to inform clinical decision-making, optimize therapeutic outcomes, and ultimately improve long-term prognosis and quality of life for patients.
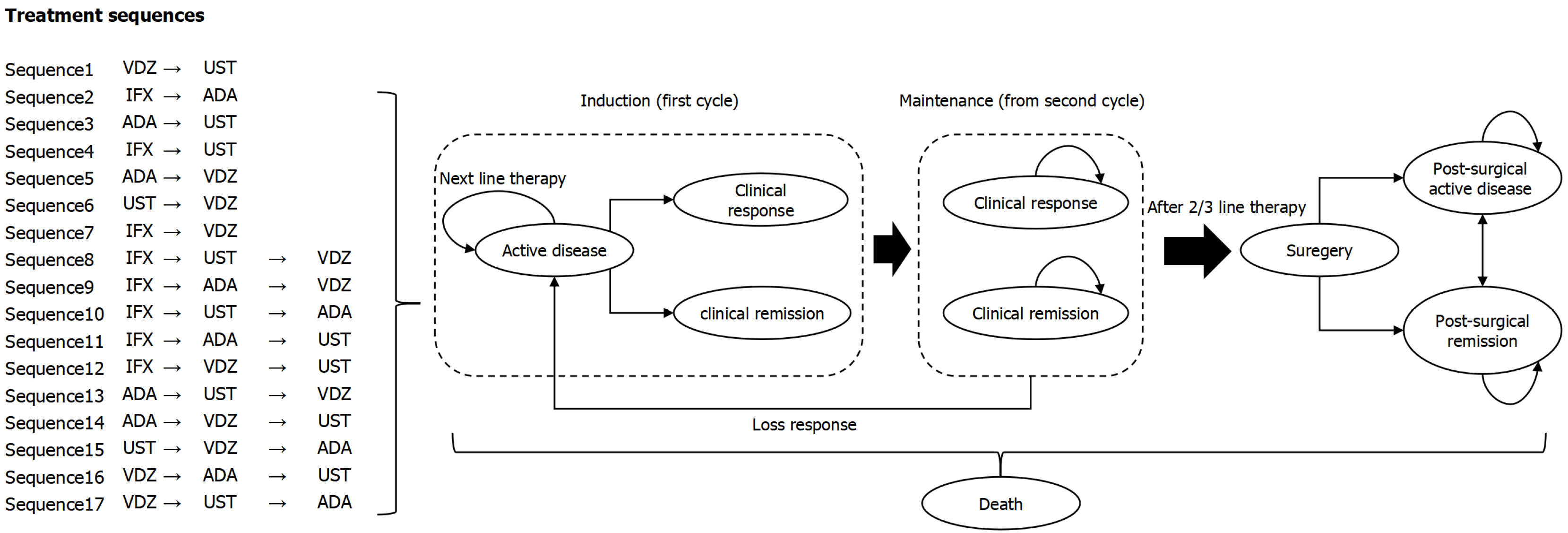
The four biologics selected in the study (IFX, ADA, UST, VDZ) have been both included in NRDL and clinical practice guidelines[2]. However, existing guidelines did not explicitly recommend specific treatment sequences or detailed switch strategies. Therefore, in order to systematically and comprehensively evaluate the possible sequencing of different biologics in the real world and their economics, 17 biologic treatment sequences were defined. Crohn’s Disease Activity Index (CDAI) score was used to evaluate the efficacy and disease status, which was a commonly used efficacy endpoint in clinical trials. Specifically, clinical remission was defined as a post-treatment CDAI score < 150, and clinical response was defined as a reduction in CDAI score ≥ 100 points from baseline to the end of treatment. Based on the disease progression and clinical practice of CD, as well as reference to internationally published pharmacoeconomic evaluations[13], a Markov model (Figure 1) was constructed to simulate the disease state transitions in patients with moderate to severe CD. Patients entered the model in the “active disease” state and initiate treatment with a first-line biologic. Treatment typically consisted of two phases: (1) Induction phase; and (2) Maintenance phase. The induction phase employed a higher dose or increased frequency of administration for one cycle, followed by the maintenance phase, during which treatment continued at a reduced dosing regimen until loss of response. During induction, patients who achieved a clinical response or clinical remission entered corresponding states in the maintenance phase, where they continued the same biologic therapy. Patients remained in the “clinical response” or “clinical remission” state until loss of response, at which point they returned to the “active disease” state and switched to another biologic.
Patients who did not achieve clinical response or clinical remission during the induction phase remained in the “active disease” state and switched to another biologic, repeating the “induction + maintenance” treatment process. Those who remained in the “active disease” state after two or three treatment switches underwent surgical intervention, which involved resection of the affected bowel segment. Most patients experienced symptom relief after surgery and transitioned to the “post-surgical remission” state, while some experienced ongoing disease activity and entered the “post-surgical active disease” state. Since CD could affect the entire digestive tract, recurrence was possible even after intestinal resection; therefore, patients in “post-surgical remission” could also transition to the “post-surgical active disease” state. “Death” was an absorbing state, and transition to this state was possible from any health state.
In accordance with the dosing patterns of biologics for CD, the model cycle length was set at 8 weeks, and the time horizon was set to 10 years or a lifetime.
Patients were categorized into two groups based on prior exposure to biologic therapy: (1) Biological-naïve; and (2) Biological-exposed. The efficacy of each biologic in treating biological-naïve and biological-exposed patients was calculated based on the odds ratios (ORs) compared to placebo (PBO). The ORs for clinical response rates and clinical remission rates were derived from two network meta-analyses[14,15]. For biologics with alternative administration regimens resulting in different OR values, the OR corresponding to the dosage and frequency consistent with the cost calculations in the Markov model was selected for analysis. The probabilities for the biologics were calculated based on the formula: Pbio= (Ppbo × ORbiovspbo)/(1 - Ppbo+ Ppbo × ORbiovspbo), where Pbio and Ppbo were the probability of clinical response or clinical remission for biologics and PBO, separately. The probability for PBO came from a meta-analysis of clinical trials in moderate to severe CD[16]. For missing efficacy data of biologic-exposed populations related to IFX and ADA, to avoid overestimating the efficacy of these two biologics, a conservative assumption was adopted[13], using the lowest reported OR among other biologics as a substitute (Table 1 and Supplementary Table 1).
| Biological-naïve | Biological-exposed | Serious adverse event (%) | |||
| Response rate (%) | Remission rate (%) | Response rate (%) | Remission rate (%) | ||
| Induction phase | |||||
| IFX | 86.12 | 43.27 | 30.68 | 12.28 | 6.61 |
| ADA | 41.26 | 33.63 | 35.38 | 28.29 | 4.46 |
| UST | 44.04 | 30.67 | 38.18 | 17.96 | 5.28 |
| VDZ | 32.67 | 25.09 | 30.68 | 12.28 | 6.87 |
| PBO | 22.00 | 11.00 | 20.00 | 10.00 | 7.00 |
| Maintenance phase | |||||
| IFX | 62.17 | 47.34 | 34.06 | 17.45 | 16.14 |
| ADA | 87.80 | 84.51 | 34.06 | 17.45 | 11.27 |
| UST | 89.84 | 84.90 | 69.90 | 49.76 | 13.16 |
| VDZ | 61.13 | 46.81 | 34.06 | 17.45 | 16.72 |
| PBO | 39.00 | 32.00 | 26.00 | 16.00 | 17.00 |
Safety parameters focused specifically on the incidence of serious adverse events (SAEs). The SAE rates were calculated based on the ORs of SAEs for each biologic compared to PBO[14], and the incidence rates of SAEs for PBO during both the induction and maintenance phases (Table 1 and Supplementary Table 2)[16].
The efficacy and safety parameters for both the induction and maintenance phases were converted into transition probabilities per model cycle and incorporated into the model, following the approach by Lohan et al[17] (Supplementary Table 3). Other transition probability parameters, including transitions probabilities between post-surgical health states and mortality, were presented in Table 2[2,5,11,13,18-20].
| Parameters | Value | Ref. |
| Cohort characteristics | ||
| Age | 30 | Xu et al[5], 2023 |
| Female (%) | 46.65 | Xu et al[5], 2023 |
| Weight (kg) | 60 | |
| Transition probability | ||
| Post-surgical remission | 52.68% | Bouhnik et al[13], 2023 |
| Post-surgical active disease | 47.32% | Bouhnik et al[13], 2023 |
| Recurrence after surgery | 3.50% | Bouhnik et al[13], 2023 |
| Death rate | Age-gender-specific death rate | Cai[20], 2012 |
| Costs | ||
| Infliximab | $204.40/100 mg IV | Induction: 5 mg/kg, 0/2/6 weeks; maintenance: 5 mg/kg, every 8 weeks |
| Adalimumab | $144.71/40 mg SC | Induction: 160 mg 0 week, 80 mg 2 weeks; maintenance: 40 mg, every 2 weeks |
| Ustekinumab | $302.11/130 mg IV $958.59/90 mL SC | Induction: 390 mg; maintenance: 90 mg, every 12 weeks |
| Vedolizumab | $699.27/300 mg IV | Induction: 300 mg, 0/2/6 weeks; maintenance: 300 mg, every 8 weeks |
| SAE | $1507.46/cycle | Zhou et al[11], 2021 |
| Surgery | $9849.27/cycle | Average colectomy cost of China Healthcare Security Diagnosis Related Group 2.0 |
| Conventional therapy (induction) | $125.42/cycle | Inflammatory Bowel Disease Group[2], 2024 |
| Conventional therapy (maintenance) | $103.79/cycle | Inflammatory Bowel Disease Group[2], 2024 |
| Utility | ||
| Active disease | 0.40 | Bouhnik et al[13], 2023; Lindsay et al[18], 2008 |
| Clinical remission | 0.83 | Bouhnik et al[13], 2023; Lindsay et al[18], 2008 |
| Clinical response | 0.55 | Bouhnik et al[13], 2023; Lindsay et al[18], 2008 |
| Surgery | 0.40 | Bouhnik et al[13], 2023 |
| Post-surgical remission | 0.67 | Bouhnik et al[13], 2023; Lindsay et al[18], 2008 |
| Post-surgical active disease | 0.40 | Bouhnik et al[13], 2023 |
| Disutility of SAE | 0.07 | Worbes-Cerezo et al[19], 2019 |
The cost parameters from the healthcare system perspective included expenses for biologics, management of adverse events, surgery, and conventional therapy for recurrence patients after surgery. All biologic costs were calculated using the latest publicly available winning bid prices. For the four biologics included in the NRDL, these bid prices correspond to the official medical insurance payment prices. For IFX and ADA, which have several biosimilars available, the average market price was used to calculate the drug cost. For UST and VDZ, which are original products, the cost was calculated based on brand name drug price. All the biologics costs were calculated based on the recommended adult dosage specified in the prescribing information. The model assumed that patients received the standard recommended maintenance dose and changed the treatment directly after loss of response, without dose adjustment. For weight-based biologics, the dosage and cost were calculated assuming a standard patient body weight of 60 kg.
The cost of intestinal resection was the average cost of China Healthcare Security Diagnosis Related Group 2.0 for this procedure, representing a bundled payment for the entire surgical procedure. Patients who experienced postoperative recurrence received conventional therapy recommended by the Chinese guidelines for the diagnosis and treatment of CD[2], with adverse events monitored each cycle.
All costs were adjusted to 2024 values using the Chinese healthcare price index and converted to United States dollars based on the 2024 average exchange rate ($1 = CNY 7.12) (Table 2)[2,5,11,13,18-20].
As health state preferences in the Chinese population have not been reported, the utility values for each state were derived from published literatures[13,18], which estimated utility values for relevant health states in adult CD patients in the United Kingdom. The disutility associated with SAEs was obtained from Worbes-Cerezo et al[19] (Table 2).
The base-case analysis was conducted with the initial biologic-naïve patient cohort. Using one times the GDP per capita ($13444.68, 2024) in China as the willingness-to-pay (WTP) threshold, the absolute net monetary benefit (NMB) was calculated for each treatment sequence, with the formula: NMB = cost × WTP - quality-adjusted life year (QALY)[21]. With sequence 1 as the reference, the incremental cost-effectiveness ratio (ICER) of other sequences was calculated, representing the additional cost required to gain one additional QALY. Both costs and utilities in the model were discounted at an annual rate of 5%, and a half-cycle correction was applied.
One-way sensitivity analysis and probabilistic sensitivity analysis were employed to evaluate the impact of variations in single or multiple key parameters on the economic evaluation results. For the one-way sensitivity analysis, the OR parameters were assigned ranges based on the 95%CI of the OR values obtained from the literature, and other parameters were varied by assuming plausible ranges of the base-case values (± 20%). The probabilistic sensitivity analysis was conducted, using Monte Carlo simulations of 5000 iterations by randomly sampling key parameters from the preset probability distributions. Specifically, cost parameters followed a gamma distribution, OR values were assigned a lognormal distribution, transition probabilities were modeled using a normal distribution, and utility values were assumed to follow a beta distribution. A cost-effectiveness acceptability curve (CEAC) was plotted to illustrate the probability of each treatment sequence being cost-effective compared to sequence 1 across a range of WTP thresholds (Supplementary Table 4).
In addition to the biologic-naïve patients included in the base-case analysis, a scenario analysis was further conducted on biologic-exposed patients. Given that prior biologic exposure has a significant impact on treatment efficacy, and considering that approximately 37%[22] of CD patients in China have already received biologic therapy, this patient population still requires switching to alternative treatments after loss of response. Therefore, a scenario analysis was performed with biologic-exposed patients as the initial cohort. Since IFX was the earliest biologic introduced into China, and accounts for over 73.7%[22] of biologic exposures, treatment sequences containing IFX were excluded in this scenario analysis. This approach assumed that the initial patient population consisted entirely of those who had experienced treatment failure with IFX.
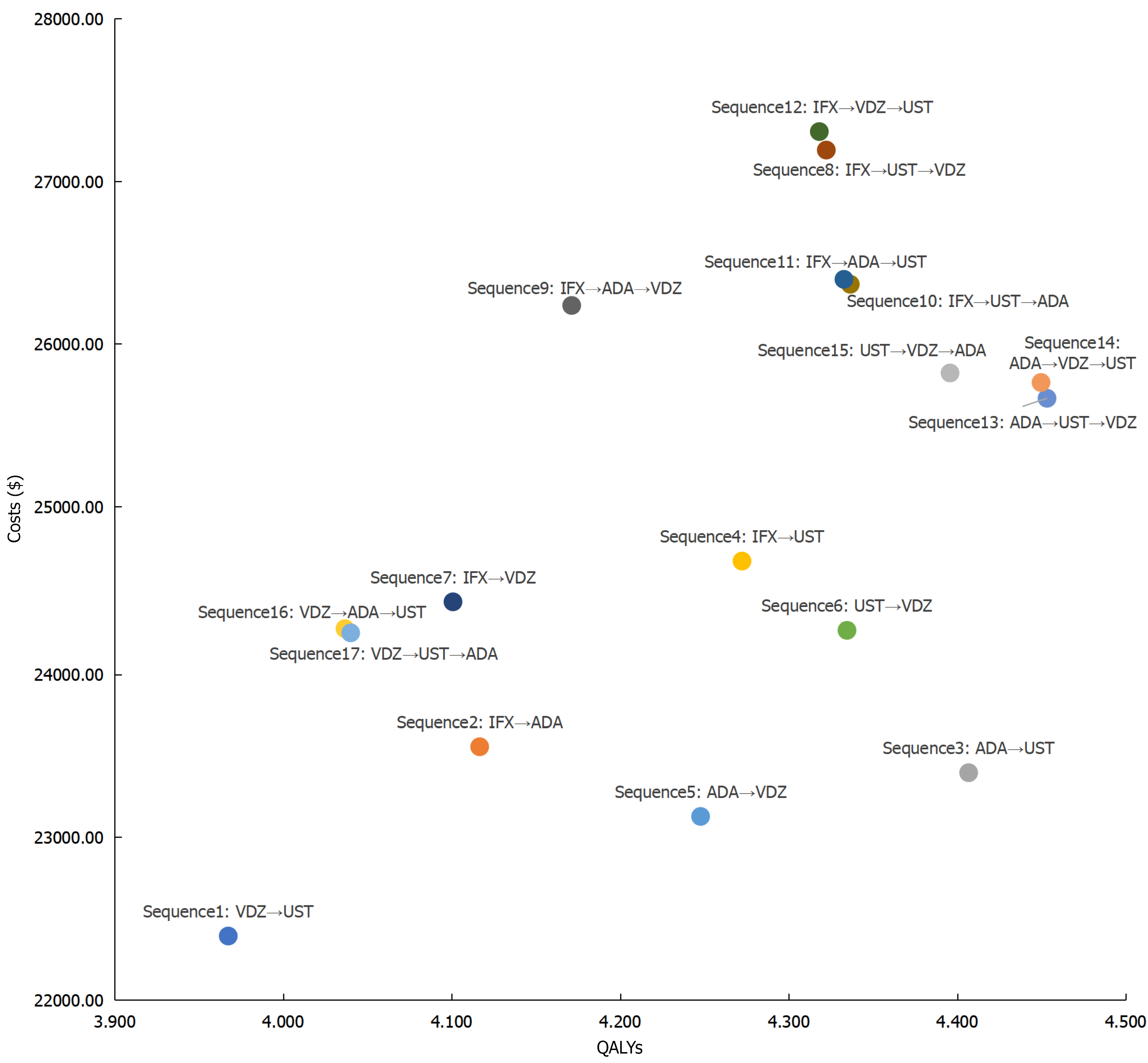
From a healthcare system perspective and over a ten-year time horizon, the base-case analysis results for Chinese patients with moderate to severe CD were presented in Table 3 and Figure 2. The NMB analysis showed that for biologic-naïve patients, sequence 3 (ADA-UST) yielded the highest absolute NMB at $35850.93, followed by sequence 13 (ADA-UST-VDZ, $34207.27) and sequence 14 (ADA-VDZ-UST, $34062.39). In terms of QALYs, compared to sequence 1 (VDZ-UST), the incremental QALYs of other treatment sequences ranged from a minimum of 0.069 (sequence 16: VDZ-ADA-UST) to a maximum of 0.486 (sequence 13: ADA-UST-VDZ).
| Sequence | Costs ($) | QALY | Net monetary benefit ($) | Incremental costs ($) | Incremental QALYs | Incremental cost-effectiveness ratio ($/QALY)1 |
| Sequence 1: VDZ-UST | 22392.37 | 3.967 | 30948.45 | - | - | - |
| Sequence 2: IFX-ADA | 23555.90 | 4.117 | 31790.65 | 1163.52 | 0.149 | 7799.27 |
| Sequence 3: ADA-UST | 23396.38 | 4.407 | 35850.93 | 1004.01 | 0.439 | 2285.38 |
| Sequence 4: IFX-UST | 24697.41 | 4.272 | 32741.92 | 2305.04 | 0.305 | 7561.41 |
| Sequence 5: ADA-VDZ | 23127.51 | 4.248 | 33981.33 | 735.13 | 0.280 | 2623.04 |
| Sequence 6: UST-VDZ | 24271.51 | 4.335 | 34005.35 | 1879.14 | 0.367 | 5118.36 |
| Sequence 7: IFX-VDZ | 24446.82 | 4.101 | 30686.86 | 2054.45 | 0.133 | 15406.35 |
| Sequence 8: IFX-UST-VDZ | 27191.67 | 4.322 | 30920.52 | 4799.30 | 0.355 | 13523.37 |
| Sequence 9: IFX-ADA-VDZ | 26236.50 | 4.171 | 29844.52 | 3844.12 | 0.204 | 18861.04 |
| Sequence 10: IFX-UST-ADA | 26366.71 | 4.337 | 31936.78 | 3974.34 | 0.369 | 10767.13 |
| Sequence 11: IFX-ADA-UST | 26397.00 | 4.333 | 31856.02 | 4004.63 | 0.365 | 10960.66 |
| Sequence 12: IFX-VDZ-UST | 27305.37 | 4.318 | 30751.11 | 4913.00 | 0.351 | 14007.32 |
| Sequence 13: ADA-UST-VDZ | 25665.78 | 4.453 | 34207.27 | 3273.41 | 0.486 | 6737.35 |
| Sequence 14: ADA-VDZ-UST | 25762.83 | 4.450 | 34062.39 | 3370.46 | 0.482 | 6988.27 |
| Sequence 15: UST-VDZ-ADA | 25821.32 | 4.396 | 33277.94 | 3428.95 | 0.428 | 8005.84 |
| Sequence 16: VDZ-ADA-UST | 24281.88 | 4.037 | 29989.99 | 1889.51 | 0.069 | 27285.02 |
| Sequence 17: VDZ-UST-ADA | 24256.07 | 4.040 | 30061.27 | 1863.70 | 0.073 | 25659.35 |
As shown in Table 3, among the ICERs for all treatment sequences compared to sequence 1, sequence 3 (ADA-UST) had the lowest ICER at $2285.38 per QALY, indicating that it required the lowest additional cost to gain one additional QALY. This was followed by sequence 5 (ADA-VDZ) and sequence 6 (UST-VDZ), with ICERs of $2623.04/QALY and $5118.36/QALY, respectively. The ICERs for sequences 2-17 were all below three times the GDP per capita of China.
When the time horizon was extended to a lifetime, sequence 3 remained the strategy with the highest absolute NMB and the most favorable ICER results (Supplementary Table 5).
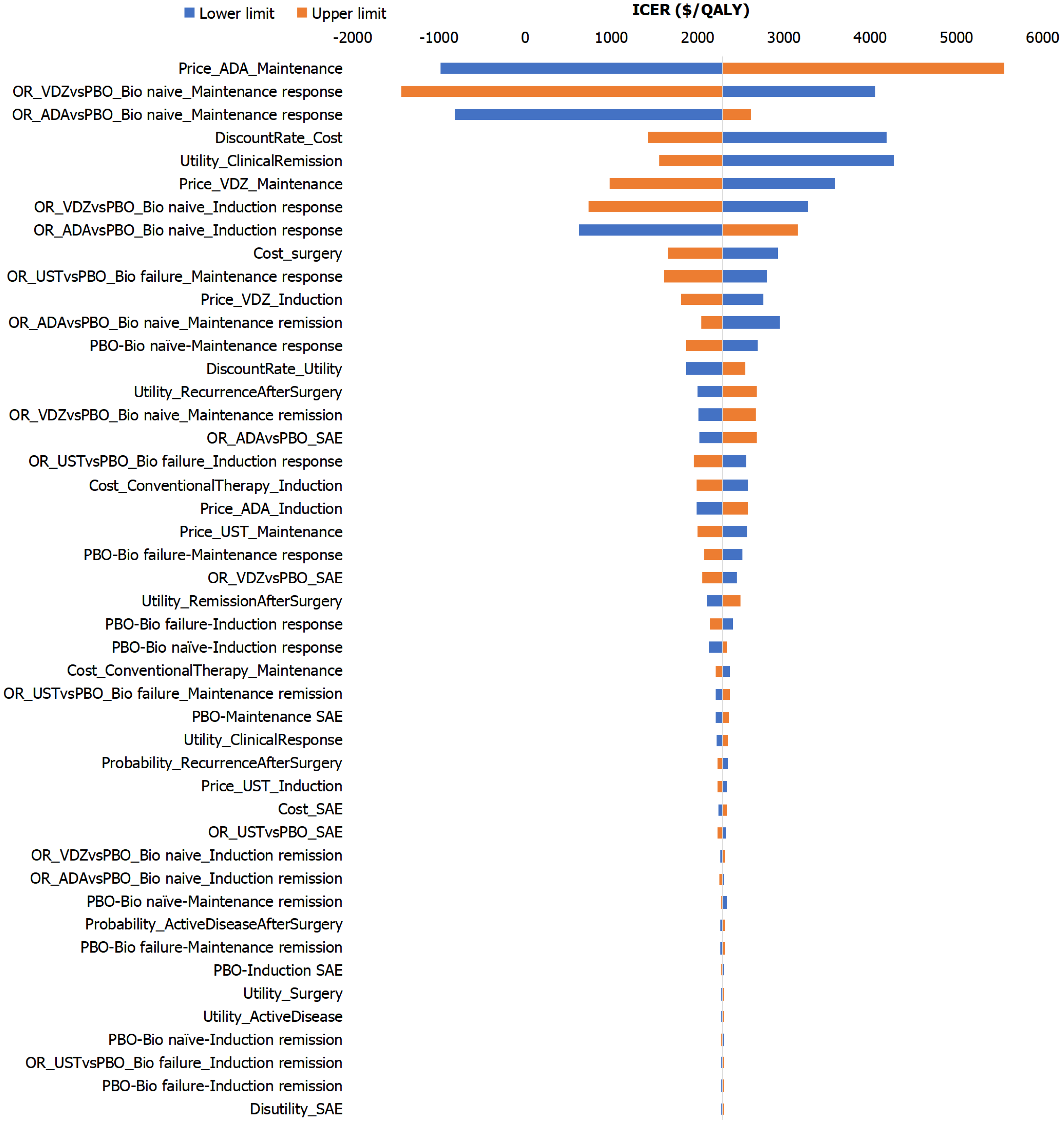
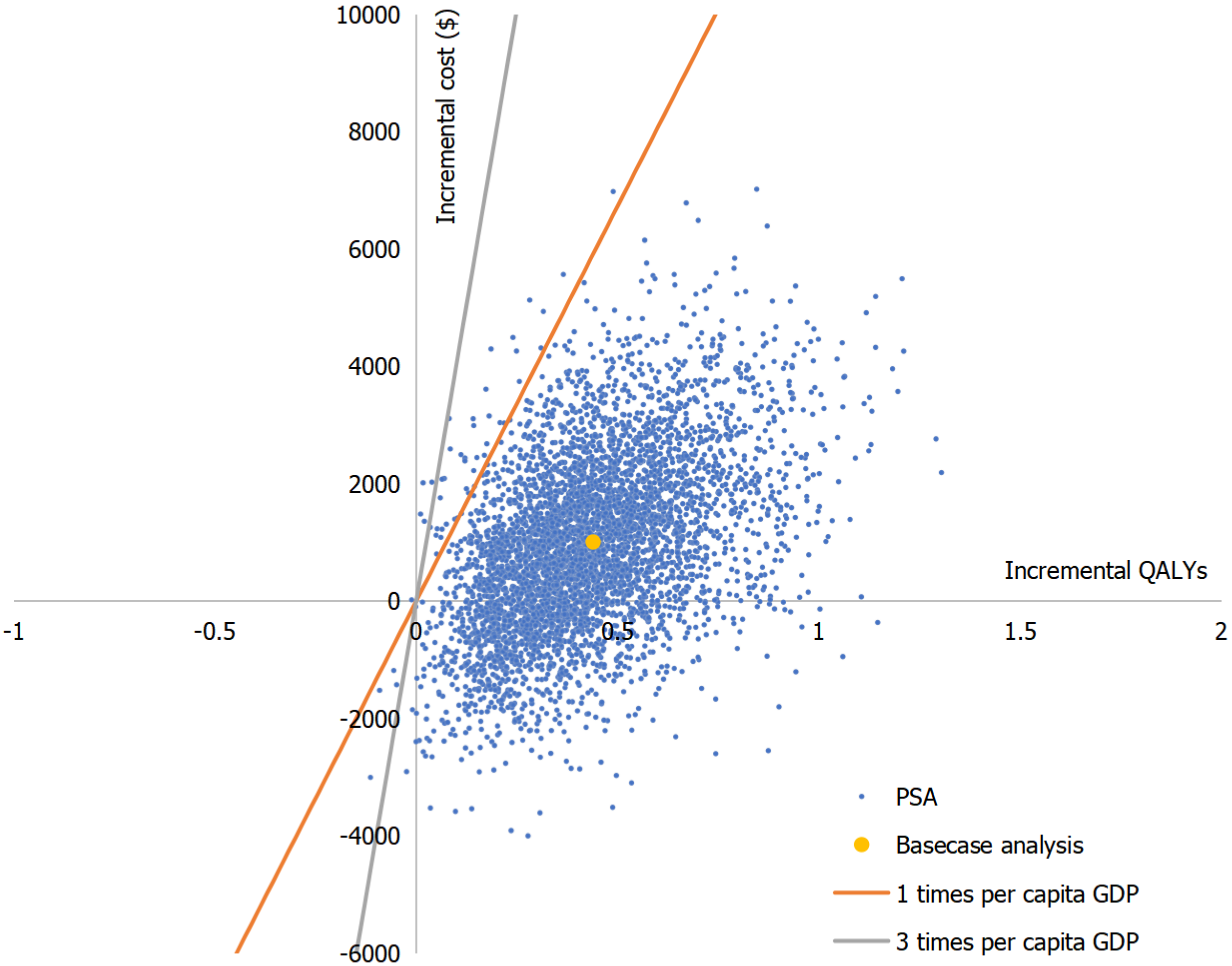
Taking the one-way sensitivity analysis comparing sequence 1 (VDZ-UST) and sequence 3 (ADA-UST) as an example (Figure 3), the tornado diagram indicated that the maintenance phase drug costs of biologics, the OR for clinical response rate of biologics vs PBO during the maintenance phase, and the utility value of the clinical remission state were the key factors influencing the model results. From the results of probabilistic sensitivity analysis between sequence 3 (ADA-UST) and sequence 1 (VDZ-UST), the scatter plot results showed that most of the points were in the first quadrant and within one times the GDP per capita (Figure 4).
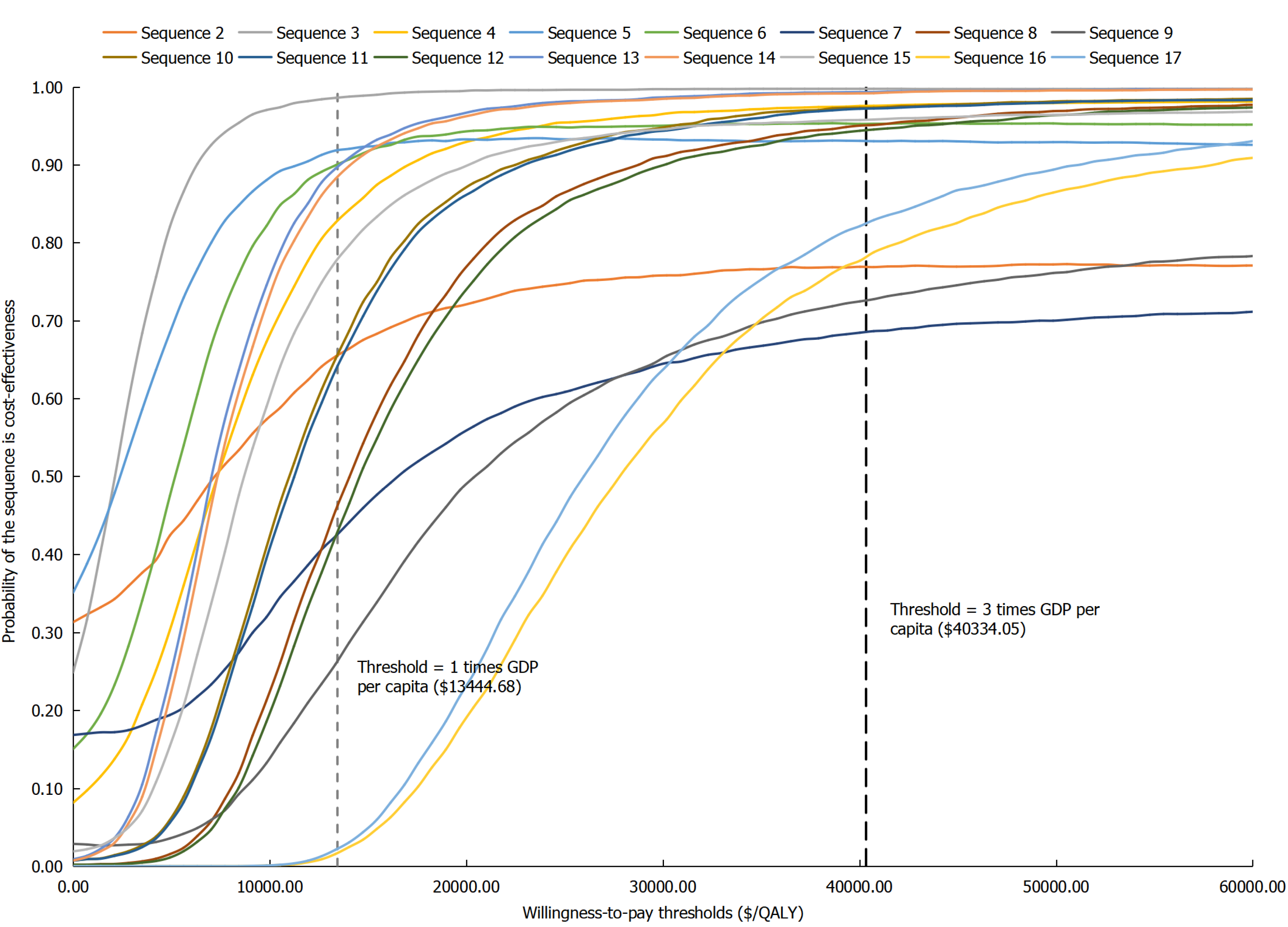
According to the CEAC derived from the probabilistic sensitivity analysis (Figure 5), for biologic-naïve CD patients, at a WTP threshold equivalent to one time the GDP per capita, the probabilities of sequences 3, 5, and 6 being cost-effective compared to sequence 1 were 98.60%, 91.84%, and 90.04%, respectively. According to the CEAC derived from the probabilistic sensitivity analysis (Figure 5), for biologic-naïve CD patients, at a WTP threshold equivalent to one time the GDP per capita, the probabilities of sequences 3, 5, and 6 being cost-effective compared to sequence 1 were 98.60%, 91.84%, and 90.04%, respectively.
The scenario analysis results (Table 4) showed that after excluding sequences containing IFX, for biologic-exposed patients over a 10-year time horizon, sequence 3 (ADA-UST) yielded the highest absolute NMB at $31150.98, followed by sequence 1 (VDZ-UST, $30363.95) and sequence 6 (UST-VDZ, $29883.92). In terms of QALYs, compared to sequence 1 (VDZ-UST), the incremental QALYs of other treatment sequences ranged from a minimum of -0.184 (sequence 5: ADA-VDZ) to a maximum of 0.079 (sequence 15: UST-VDZ-ADA). Regarding the ICER, sequence 3 (ADA-UST) was the dominant strategy. When the time horizon was extended to a lifetime, sequence 3 (ADA-UST) remained the treatment strategy with the highest absolute NMB and the most favorable ICER results (Supplementary Table 6).
| Sequence | Costs ($) | QALY | Net monetary benefit ($) | Incremental costs ($) | Incremental QALYs | Incremental cost-effectiveness ratio ($/QALY)1 |
| Sequence 1: VDZ-UST | 21375.37 | 3.848 | 30363.95 | - | - | - |
| Sequence 3: ADA-UST | 20813.64 | 3.865 | 31150.98 | -561.73 | 0.017 | -33520.57 |
| Sequence 5: ADA-VDZ | 20304.88 | 3.664 | 28962.46 | -1070.49 | -0.184 | 5822.22 |
| Sequence 6: UST-VDZ | 21899.71 | 3.852 | 29883.92 | 524.34 | 0.003 | 159090.08 |
| Sequence 13: ADA-UST-VDZ | 23620.33 | 3.924 | 29137.76 | 2244.97 | 0.076 | 29626.51 |
| Sequence 14: ADA-VDZ-UST | 23714.52 | 3.921 | 28995.66 | 2339.16 | 0.072 | 32392.94 |
| Sequence 15: UST-VDZ-ADA | 23814.60 | 3.927 | 28983.03 | 2439.23 | 0.079 | 30987.73 |
| Sequence 16: VDZ-ADA-UST | 23315.89 | 3.920 | 29393.93 | 1940.52 | 0.072 | 26882.61 |
| Sequence 17: VDZ-UST-ADA | 23291.21 | 3.924 | 29462.02 | 1915.84 | 0.075 | 25404.50 |
Patients with moderate to severe CD often experience a variety of symptoms, including diarrhea, abdominal pain, extraintestinal manifestations, and anorectal complications, which significantly impair their quality of life. Therefore, selecting optimal treatment strategies to achieve symptomatic relief and minimize the risks of surgery, hospitalization, and disease-related complications is essential. While previous studies have compared the efficacy of various biologics through indirect methods such as network meta-analysis, there remains a lack of evidence regarding the comparative health outcomes and cost-effectiveness of different sequential biologic therapy regimens.
This study evaluated the cost-effectiveness of 17 treatment sequences involving four biologics included in the NRDL. The results demonstrated that for both biologic-naïve and biologic-exposed patients, sequence 3 (ADA-UST) yielded the highest absolute NMB. According to the ICER results, multiple treatment sequences (sequences 2-6, 10-11, and 13-15 in biologic-naïve patients; sequence 3, 5 in biologic-exposed patients) exhibited ICER values below one times the GDP per capita of China, indicating that these sequences were more cost-effective relative to sequence 1 (VDZ-UST).
Sensitivity analysis identified the cost and efficacy of biologics as key factors influencing the ICER. When comparing the NMB across different treatment sequences, those incorporating both ADA and UST demonstrated higher absolute NMB values, particularly sequences in which ADA was used as first-line therapy and UST was administered as either first-line or second-line treatment. These results highlight the efficacy and cost advantages of ADA and UST. Consistent with previously reported comparative efficacy outcomes in published studies[14,15], ADA showed superior outcomes in biologic-naïve populations, while UST demonstrated favorable results in both biologic-naïve and biologic-exposed patients, particularly during the maintenance phase. In contrast, VDZ exhibited relatively weaker clinical efficacy, leading to poorer cost-effectiveness performance in treatment sequences containing VDZ. In terms of treatment costs, the maintenance phase expenses of all four biologics were comparable, with ADA being the lowest.
Regarding health outcomes across treatment sequences, four of the five regimens with the highest total QALYs involved the sequential use of three biologics (sequence 10, 13-15), suggesting that utilizing a greater number of treatment lines may improve health outcomes. This finding was consistent with other cost-effectiveness analyses in IBD[13,23], which indicated that the superior efficacy of biologics over conventional therapy made additional treatment lines beneficial for patient health outcomes. However, the significantly higher cost of biologics compared to conventional therapy also increased the total costs of therapy. As a result, sequences containing two biologics (sequences 3, 5, and 6) demonstrated more favorable ICER results.
The scenario analysis results indicated that for biologic-exposed patients, increasing the number of biologic treatment lines also contributed to improved health outcomes. However, due to the significantly higher treatment costs, sequences containing two biologics yielded greater NMB and more favorable ICERs, particularly sequence 3 (ADA-UST) and sequence 1 (VDZ-UST). Sequence 3 (ADA-UST) demonstrated the highest NMB in both biologic-naïve and biologic-exposed populations, suggesting that the efficacy advantages of these two biologics in different patient groups, especially the superior clinical response and remission rates of UST during the maintenance phase, positively influenced the economic outcomes.
In recent years, the diagnosis and treatment of CD in China have improved significantly with the introduction of new therapies and updates to treatment paradigms. However, against the growing patient population, the healthcare system still faced multiple challenges: (1) Relatively insufficient resources and training in IBD subspecialties[24]; (2) Weak patient self-management capabilities and low treatment adherence[25]; and (3) Delays in diagnosis and treatment among some patients due to socioeconomic factors[26]. Together, these factors contributed to increased disease relapse rates, hospitalization rates, and surgical risks. Although the NRDL has consistently included several CD biologics since 2020, substantially enhancing drug accessibility and affordability, the overall utilization rate of biologics in China remained relatively low, with notable regional disparities[27]. Low biologics utilization could be influenced by a variety of factors. Compared with small molecule drugs, biologics have higher requirements for storage, transportation, etc., which may increase the costs for hospitals, and reduce patient compliance. In addition, there were several barriers to switching biologics in clinical practice, including clinical inertia or physician preference, the complex dosage adjustment regimens of biologics, administrative barriers to new drug admission, and patient concerns about the unknown side effect spectrum of new drugs, especially biologics with a short time to market, such as VDZ. Therefore, establishing a comprehensive support system, including standardized training for physicians, systematic education for patients and their families, multi-channel social support, is essential[24].
This study has several limitations. Due to the absence of head-to-head trials comparing different treatment regimens, a recently published network meta-analysis of clinical trials in patients with moderate to severe CD was used to estimate efficacy. Partial efficacy data were lacking for IFX and ADA in biologic-exposed populations; therefore, assumptions were made regarding their efficacy as second-line therapies. Given the significant differences inefficacy between biologic-naïve and biologic-exposed patients, the missing subsequent efficacy values were not assumed to be equivalent to those of first-line treatment but were instead set equal to the efficacy of the least effective drug in the corresponding population to avoid overestimating the effectiveness of the treatment. Although the four biologics could be combined into a greater number of treatment sequences, only the most clinically plausible regimens were selected for analysis based on real-world practice. Even though, several sequences still appeared theoretically possible but may not reflect routine clinical decision making. However, considering the complexity and variability of clinical decision-making, providing therapeutic sequences can still help provide are reference for optimizing the clinical pathway from an economic perspective. The study assumed that all treatment-switching behaviors were related to clinical efficacy. However, in the real world, the complexity of patient decision-making – particularly the interplay of social, economic, and personal factors affecting treatment adherence – may introduce additional uncertainty to the outcomes. Future research could further integrate these multidimensional decision-making factors to study the cost-effectiveness of different treatment sequences.
Overall, the findings indicate that for Chinese patients with moderate to severe CD, increasing the number of biologic treatment lines could lead to greater health improvements. In the comparison of cost-effectiveness across different treatment sequences, the combination of ADA and UST yielded the highest NMB and the most favorable ICER results, and is likely to be the most cost-effective option in China. However, our findings indicate that further studies are needed to evaluate the long-term efficacy and safety of different biologics in Chinese CD patients, as well as the economic and epidemiological impacts of CD, using more robust data on costs, quality of life, and treatment adherence.
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (113)] |
| 2. | Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association; Inflammatory Bowel Disease Quality Control Center of China. 2023 Chinese national clinical practice guideline on diagnosis and management of Crohn's disease. Chin Med J (Engl). 2024;137:1647-1650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1615] [Article Influence: 269.2] [Reference Citation Analysis (0)] |
| 4. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4449] [Article Influence: 494.3] [Reference Citation Analysis (111)] |
| 5. | Xu L, He B, Sun Y, Li J, Shen P, Hu L, Liu G, Wang J, Duan L, Zhan S, Wang S. Incidence of Inflammatory Bowel Disease in Urban China: A Nationwide Population-based Study. Clin Gastroenterol Hepatol. 2023;21:3379-3386.e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Nguyen NH, Singh S, Sandborn WJ. Positioning Therapies in the Management of Crohn's Disease. Clin Gastroenterol Hepatol. 2020;18:1268-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Zhou WP, Mu N, Jian WY, Wang HH. [Economic burden and factors associated with Crohn's disease]. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 179] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 9. | Shi JH, Luo L, Chen XL, Pan YP, Zhang Z, Fang H, Chen Y, Chen WD, Cao Q. Real-world cost-effectiveness associated with infliximab maintenance therapy for moderate to severe Crohn's disease in China. World J Gastroenterol. 2020;26:6455-6474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Wang X, Li Q, Sun S, Liang X, Li H, Huang J, Zhao T, Hu J, Liu J, Hu Z, Duan Y, He J. Network meta-analysis and cost-effectiveness analysis of infliximab, cyclosporine and tacrolimus for ulcerative colitis. Medicine (Baltimore). 2022;101:e31850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Zhou T, Sheng Y, Guan H. Cost-Effectiveness of Vedolizumab in the Treatment of Moderate-to-Severe Crohn's Disease in China. Adv Ther. 2021;38:4233-4245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Yao J, Jiang X, You JHS. Proactive therapeutic drug monitoring of adalimumab for pediatric Crohn's disease patients: A cost-effectiveness analysis. J Gastroenterol Hepatol. 2021;36:2397-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Bouhnik Y, Atreya R, Casey D, Górecki M, Baik D, Yoon SW, Kwon TS, Jang M. Cost-effectiveness Analysis of Subcutaneous Infliximab for Inflammatory Bowel Diseases in Sequential Biologic Treatment. Inflamm Bowel Dis. 2023;29:898-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Singh S, Murad MH, Fumery M, Sedano R, Jairath V, Panaccione R, Sandborn WJ, Ma C. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn's disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:1002-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 15. | Disher T, Naessens D, Sanon M, Bonner A, Ellis J, Bartlett M, Hooper B, Yang Z, Allegretti JR, Dignass A. One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis. Adv Ther. 2025;42:2708-2727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Solitano V, Hogan M, Singh S, Danese S, Peyrin-Biroulet L, Zou G, Yuan Y, Sands BE, Feagan BG, Dulai PS, Narula N, Ma C, Jairath V. Placebo Rates in Crohn's Disease Randomized Clinical Trials: An Individual Patient Data Meta-Analysis. Gastroenterology. 2025;168:344-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Lohan C, Diamantopoulos A, LeReun C, Wright E, Bohm N, Sawyer LM. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol. 2019;6:e000302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Lindsay J, Punekar YS, Morris J, Chung-Faye G. Health-economic analysis: cost-effectiveness of scheduled maintenance treatment with infliximab for Crohn's disease--modelling outcomes in active luminal and fistulizing disease in adults. Aliment Pharmacol Ther. 2008;28:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Worbes-Cerezo M, Nafees B, Lloyd A, Gallop K, Ladha I, Kerr C. Disutility Study for Adult Patients with Moderate to Severe Crohn's Disease. J Health Econ Outcomes Res. 2019;6:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Cai F. Chapter Five Growing Pains: What Employment Dilemma Does China Face at Its Lewis Turning Point? The China Population and Labor Yearbook. Leiden: Brill, 2012; 3: 113-134. [DOI] [Full Text] |
| 21. | Messori A, Trippoli S. The results of a pharmacoeconomic study: incremental cost-effectiveness ratio versus net monetary benefit. Heart. 2017;103:1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Wan J, Shen J, Wu X, Zhong J, Chen Y, Zhu L, Miao Y, Hu N, Chen J, Liang J, Wu K. Geographical heterogeneity in the disease characteristics and management of patients with inflammatory bowel disease, the preliminary results of a Chinese database for IBD (CHASE-IBD). Therap Adv Gastroenterol. 2023;16:17562848231210367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Wu B, Wang Z, Zhang Q. Cost-Effectiveness of Different Strategies for the Treatment of Moderate-to-Severe Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:2291-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Peng L, Hu S, Yu Q, Chen Y. Challenging the Surge of Inflammatory Bowel Disease: The Role of the China Crohn's and Colitis Foundation in the Healthcare Landscape of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2022;28:S9-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Hu W, Hu S, Zhu Y, Chen H, Chen Y. Assessing Oral Medication Adherence and Identifying Predictors of Low Adherence in Chinese Inflammatory Bowel Disease Patients. Patient Prefer Adherence. 2020;14:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Yu Q, Zhu C, Feng S, Xu L, Hu S, Chen H, Chen H, Yao S, Wang X, Chen Y. Economic Burden and Health Care Access for Patients With Inflammatory Bowel Diseases in China: Web-Based Survey Study. J Med Internet Res. 2021;23:e20629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Li Y, Chen B, Gao X, Hu N, Huang M, Ran Z, Liu Z, Zhong J, Zou D, Wu X, Ren J, Sheng J, Zheng P, Wang H, Chen M, Chen J, Xi P, Lu J, Handel M, Liu Y, Fan H, Qian J. Current diagnosis and management of Crohn's disease in China: results from a multicenter prospective disease registry. BMC Gastroenterol. 2019;19:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/