Published online Feb 21, 2026. doi: 10.3748/wjg.v32.i7.111478
Revised: August 15, 2025
Accepted: December 29, 2025
Published online: February 21, 2026
Processing time: 220 Days and 21.9 Hours
Gallbladder cancer (GBC) is a rare but highly aggressive malignancy often diag
To examine temporal trends and clinicopathological features in post-chole
In this retrospective study, 15785 patients who underwent cholecystectomy over a 20-year period at a single center were reviewed, among whom 285 were diag
GBC cases increased over time (P = 0.002). Compared to benign or precancerous conditions, GBC patients were older (P = 0.000) and had higher hypertension and cholangitis rates (both P = 0.000). Total cholesterol, triglycerides, and albumin were lowest in GBC (P = 0.000, P = 0.010, P = 0.000), with higher levels linked to better survival (P = 0.003, P = 0.026, P = 0.016), and tumor budding, size, and poorly cohesive or undifferentiated components correlated with advanced stage and poorer prognosis (P = 0.015, P = 0.002, P = 0.002) on univariate analysis. Age, metabolic parameters, and inflammatory conditions influenced both GBC risk and prognosis.
The clinicopathologic characteristics of GBC highlight the value of recognizing high-risk profiles and underscore the prognostic significance of detailed histopathological evaluation.
Core Tip: This study examined temporal trends, clinicopathological features, and survival outcomes in post-cholecystectomy gallbladder cancer to identify risk factors for early detection and management. In this single-institutional study, our findings may be limited but still provide insights. Age, hypertension, cholangitis, and serological markers including total cholesterol, triglyceride, albumin were risk factors for cancer and related to survival outcomes. Pathological features, such as tumor budding, tumor size, and poorly cohesive or undifferentiated components were correlated with worse prognosis. These findings emphasize the value of early identification of high-risk profiles and the prognostic markers of gallbladder cancer.
- Citation: An J, Ahn SH, Kim KH, Heo JY, Yeo MK. Twenty-year temporal trends, risk profiles, and prognostic value of clinicopathological features in gallbladder cancer following cholecystectomy. World J Gastroenterol 2026; 32(7): 111478
- URL: https://www.wjgnet.com/1007-9327/full/v32/i7/111478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i7.111478
Gallbladder cancer (GBC) is the most common malignancy of the biliary tract and the fifth most prevalent cancer of the gastrointestinal system[1]. The disease accounts for approximately 1.3% of all cancer cases and 1.7% of cancer-related deaths worldwide[2]. Due to its non-specific clinical presentation and lack of early symptoms, GBC is frequently diagnosed at an advanced stage and associated with poor prognosis. Many patients present with unresectable tumors at the time of diagnosis and have limited therapeutic options, particularly in terms of effective chemotherapy.
Globally, the incidence and mortality rates of GBC increased by 76% and 65%, respectively, between 1990 and 2017[3]. Notably, Asian countries, such as China, Japan, and South Korea, have reported relatively high incidence rates (≥ 2.3 cases per 100000 population)[4]. In particular, South Korea recorded the highest age-standardized mortality rate for GBC among both sexes, at 4.1 per 100000[5]. The highest incidence rates have been reported in Chile (27 cases per 100000) and northern India (21.5 cases per 100000), indicating a growing burden in both developing and developed regions. Conversely, the incidence of GBC has shown a declining trend in the United States[5].
The pathogenesis and etiology of GBC remain poorly understood and insufficiently explored, primarily due to the rarity of the disease. Moreover, GBC is frequently grouped with other biliary tract cancers, which complicates the identification of its distinct biological and clinical features[6]. GBC development is considered multifactorial, arising from a complex interplay of genetic, environmental, dietary, and pathological influences. Established clinical risk factors include sex, age, ethnic background, and chronic gallbladder inflammation[7]. In particular, chronic inflammation and degenerative changes associated with gallstones are known to contribute to carcinogenic processes within the gallbladder mucosa[8].
Similar to other epithelial malignancies, GBC is preceded by a well-defined series of precancerous changes. Pathologically, its progression typically follows metaplasia-dysplasia-carcinoma in situ-invasive carcinoma sequence over a period of 5 years to 15 years[9]. Once invasive cancer develops, the progression of GBC is often rapid and asymptomatic, contributing to its poor prognosis. Early detection of GBC remains challenging due to its asymptomatic nature in the early stages and the current lack of reliable diagnostic tools[10]. Despite its clinical significance, knowledge of GBC and its precursor lesions remains limited and their clinicopathological features are insufficiently described in the existing literature. The identification of key clinicopathological risk factors is therefore essential for improving detection and prevention strategies and identifying high-risk patients.
The aim of this study was to investigate long-term temporal trends in the incidence, clinicopathological features, and survival outcomes of patients who underwent cholecystectomy and were diagnosed with GBC postoperatively. To evaluate the impact of clinicopathological factors, we conducted a 20-year, single-center retrospective analysis of GBC cases with available survival data. Data from four distinct periods (2004-2008, 2009-2013, 2014-2018, and 2019-2023) were compared, with the aim of assessing whether temporal changes have influenced the clinicopathological characteristics of the disease and survival outcomes. In addition, we compared the clinicopathological profiles of patients diagnosed with benign gallbladder conditions, precursor lesions, and GBC to identify the key factors associated with malignant transformation and prognosis.
This retrospective study included 15785 patients who underwent cholecystectomy at Chungnam National University Hospital in Daejeon, South Korea, between January 2004 and December 2023. Clinicopathological characteristics and survival data were obtained from medical records and pathology archives. The follow-up period ranged from 1 year to 21 years with survival assessed in December 2024. Collected data included sex, age, body mass index (BMI), hypertension (HTN), diabetes mellitus (DM), and history of gallstones and cholangitis. Serologic and laboratory parameters were additionally collected, including aspartate aminotransferase (AST, IU/L), alanine transaminase (ALT, U/L), alkaline phosphatase (ALP, U/L), gamma-glutamyl transferase (γ-GT, IU/L), total cholesterol (mg/dL), triglycerides (mg/dL), blood urea nitrogen (BUN, mg/dL), creatinine (mg/dL), total protein (g/dL), and amylase (U/L).
Pathological features were independently reviewed by two pathologists (Kim KH and Yeo MK), including the assessment of accompanying premalignant neoplasms, histologic differentiation, non-neoplastic changes, anaplastic differentiation (such as poorly cohesive carcinoma, signet ring cell carcinoma, squamous carcinoma, sarcomatous com
Statistical analyses were performed using SPSS version 30.0 and Microsoft Excel 2021. A significance level of P < 0.05 was used. Categorical variables were analyzed using the χ2 test and continuous variables compared using one-way analysis of variance (ANOVA). Kaplan-Meier survival analysis was employed to identify the pathological factors influencing survival. Additionally, logistic regression analysis was performed to compare the risk of clinical factors across the benign, precancerous, and GBC groups.
Among cholecystectomy patients, 285 patients (1.81%) were diagnosed with GBC, 248 (1.57%) with precancerous lesions, and 15252 (96.62%) with benign conditions. For the benign group, a representative sample of 601 patients was randomly selected using the Excel ‘RAND()’ function, because there were an excessive number of benign patients compared to GBC and precancerous patients. Benign conditions included acute and chronic cholecystitis, cholelithiasis, cholesterolosis, and other non-neoplastic gallbladder diseases. Precancerous lesions comprised adenoma, biliary intraepithelial neoplasia (BilIN), intracholecystic papillary neoplasm (ICPN), and (adeno)carcinoma in situ. GBC cases were classified according to the World Health Organization classification system for gallbladder tumors, with adenocarcinoma identified as the prevalent histologic subtype.
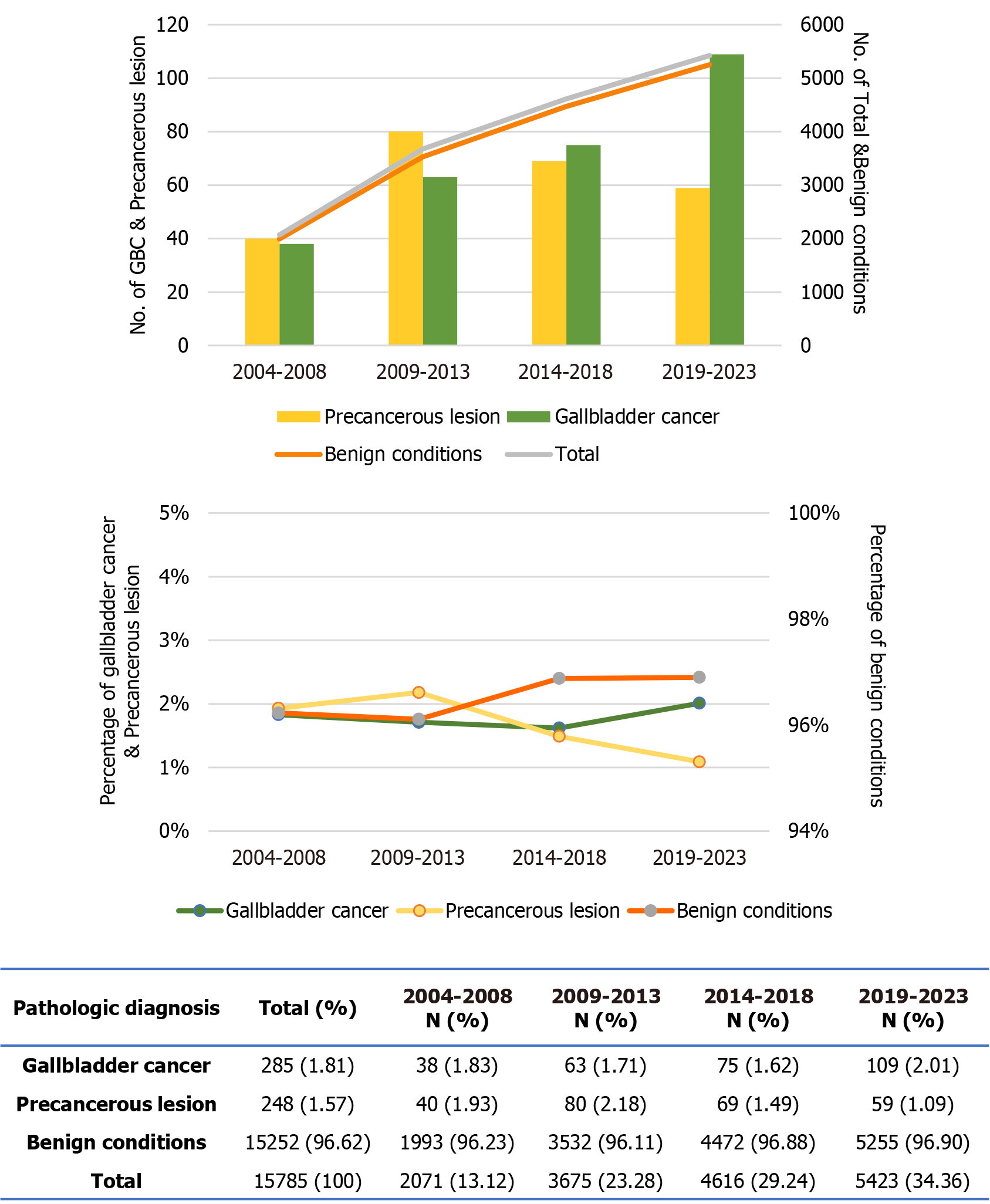
Over the past two decades, the number of cholecystectomies has steadily increased (Figure 1 and Supplementary Table 1). The number of patients who underwent surgery in 2019-2023 was more than double that in 2004-2008. While the absolute number of benign cases increased in parallel with the overall surgical volume, their proportion remained relatively stable throughout the study duration. In contrast, the incidence of precancerous lesions peaked in 2009-2013, nearly doubling compared to 2004-2008, but declined in subsequent years, resulting in an overall decrease in proportion over time (P = 0.002). The number of GBC cases recorded during 2019-2023 was 2.8 times higher than that in 2004-2008. Although the proportion of GBC cases declined across the first three time-periods, a notable increase was observed in the most recent interval.
The clinical characteristics of patients with benign conditions, premalignant lesions, and GBC are summarized in Table 1. Patients with GBC were significantly older than those in the other groups (mean age: 69.1 years vs 56.0 years and 57.3 years, P < 0.001) and had a higher male-to-female ratio (P = 0.337). The prevalence of HTN and cholangitis was highest in the GBC group (50.18% and 24.91%, respectively; both P < 0.001), whereas gallstones were predominantly observed in the benign group (72.05%, P < 0.001). No significant differences in the prevalence of DM were observed among the groups (P = 0.224). Among the serologic markers, ALT, ALP, and total protein levels were significantly higher in the benign group (P = 0.007, P = 0.035, and P = 0.002, respectively). In contrast, total cholesterol, triglyceride, albumin, and amylase levels were lowest in the GBC group (P < 0.001, P = 0.010, P < 0.001, and P = 0.026, respectively). BUN levels were markedly elevated in GBC patients compared to the other groups (P = 0.008). No significant differences were observed in BMI, AST, or creatinine levels across the groups.
| Clinical factors | Group | P value | ||
| Benign (n = 601) | Precancer (n = 248) | GBC (n = 285) | ||
| Sex ratio (male/female) | 0.89 | 0.85 | 1.08 | 0.337 |
| Age (years) | 56.03 | 57.26 | 69.10 | 0.000 |
| BMI (kg/m2) | 24.86 | 24.76 | 24.72 | 0.960 |
| AST (IU/L) | 36.24 | 33.16 | 33.18 | 0.544 |
| ALT (U/L) | 45.85 | 34.76 | 33.72 | 0.007 |
| ALP (U/L) | 96.60 | 84.68 | 96.52 | 0.035 |
| γ-GT (IU/L) | 119.10 | 77.12 | 111.29 | 0.050 |
| Total cholesterol (mg/dL) | 176.39 | 177.38 | 164.79 | 0.000 |
| Triglyceride (mg/dL) | 147.67 | 147.98 | 124.08 | 0.010 |
| BUN (mg/dL) | 13.67 | 13.97 | 15.01 | 0.008 |
| Creatinine (mg/dL) | 0.82 | 0.82 | 0.85 | 0.673 |
| Total protein (g/dL) | 6.98 | 6.95 | 6.82 | 0.002 |
| Albumin (g/dL) | 4.05 | 4.16 | 3.90 | 0.000 |
| Amylase (U/L) | 61.93 | 77.99 | 53.35 | 0.026 |
| HTN | 207 (34.44) | 82 (33.06) | 143 (50.18) | 0.000 |
| DM | 116 (19.30) | 41 (16.53) | 64 (22.46) | 0.224 |
| Gallstone | 433 (72.05) | 74 (29.84) | 83 (29.12) | 0.000 |
| Cholangitis | 105 (17.47) | 31 (12.50) | 71 (24.91) | 0.000 |
A total of 285 patients were diagnosed with GBC following cholecystectomy between 2004 and 2023. The clinical characteristics of GBC patients are summarized in Supplementary Table 2. In 48.4% of cases, surgery was performed for a suspected gallbladder mass, while the remaining cases (51.6%) underwent surgical resection for polypoid lesions (13.7%) or abdominal pain related to cholecystitis (30.9%) and were diagnosed as GBCs by pathologists during postoperative examination of gallbladder specimens. The patient ages ranged from 31 years to 91 years, with a mean age of 69 years, and the male-to-female ratio was 1.08. Adenocarcinoma was the predominant histological type, accounting for 96.5% of GBC cases. The average tumor size was 2.58 cm, with stage II representing the most frequently observed tumor-node-metastasis stage (present in 36.5% of patients). Among the GBC cases, 29.12% were associated with gallstones and 24.91% had a history of cholangitis.
The clinical characteristics of GBC patients were evaluated across four consecutive 5-year intervals (Table 2). While no statistically significant differences were observed in serologic markers between periods, several temporal trends emerged. The male-to-female ratio varied from 0.90 to 1.14, with a peak occurring in 2009-2013 (P = 0.820). Both age and BMI exhibited a gradual increase over time (P = 0.397 and P = 0.511, respectively). While liver function markers, such as AST, ALT, ALP, and γ-GT, showed minor fluctuations, no significant changes were evident. Additionally, levels of total cholesterol, triglycerides, and amylase displayed a declining trend over the study period (P = 0.675, P = 0.310, and P = 0.556, respectively).
| Clinical factors | Years | P value | |||
| 2004-2008 (n = 38) | 2009-2013 (n = 63) | 2014-2018 (n = 75) | 2019-2023 (n = 109) | ||
| Sex ratio (male/female) | 0.90 | 1.25 | 0.97 | 1.14 | 0.820 |
| Age (years) | 67.05 | 69.11 | 68.68 | 70.10 | 0.397 |
| BMI (kg/m2) | 24.47 | 23.61 | 24.36 | 25.75 | 0.511 |
| AST (IU/L) | 34.13 | 31.38 | 34.92 | 32.70 | 0.909 |
| ALT (U/L) | 31.47 | 28.90 | 37.44 | 34.72 | 0.710 |
| ALP (U/L) | 91.26 | 102.53 | 98.48 | 94.90 | 0.786 |
| γ-GT (IU/L) | 83.07 | 94.05 | 135.03 | 112.30 | 0.719 |
| Total cholesterol (mg/dL) | 170.03 | 163.90 | 167.95 | 161.29 | 0.675 |
| Triglyceride (mg/dL) | 131.97 | 134.25 | 130.65 | 110.94 | 0.310 |
| BUN (mg/dL) | 13.47 | 15.65 | 14.39 | 15.61 | 0.246 |
| Creatinine (mg/dL) | 0.94 | 0.80 | 0.79 | 0.90 | 0.480 |
| Total protein (g/dL) | 6.80 | 6.88 | 6.76 | 6.66 | 0.794 |
| Albumin (g/dL) | 3.96 | 3.98 | 3.79 | 3.92 | 0.132 |
| Amylase (U/L) | 67.21 | 54.51 | 53.85 | 47.51 | 0.556 |
| HTN | 22 (57.90) | 26 (41.30) | 34 (45.30) | 61 (56.00) | 0.167 |
| DM | 5 (13.20) | 11 (17.50) | 18 (24.00) | 30 (27.50) | 0.212 |
| Gallstone | 13 (34.20) | 21 (25.30) | 19 (25.30) | 30 (27.50) | 0.643 |
| Cholangitis | 4 (10.50) | 16 (25.40) | 19 (25.30) | 32 (29.40) | 0.147 |
| Stage I & II | 26 (68.4) | 34 (54.0) | 51 (68.0) | 76 (69.7) | 0.177 |
| Stage III & IV | 12 (31.6) | 29 (46.0) | 24 (32.0) | 33 (30.3) | |
| 5-year survival rate (%) | 52.6 | 51.6 | 52.0 | Not applicable | 0.876 |
Renal function indicators, including BUN and creatinine, and nutritional markers, such as total protein and albumin, also remained relatively stable across the study periods. The proportion of patients with DM increased from 13.2% to 27.5% (P = 0.212) and the prevalence of cholangitis rose from 10.5% to 29.4% (P = 0.147). Although these increases did not reach statistical significance, they represent a notable shift over time. In contrast, the prevalence of HTN and gallstones remained relatively unchanged (P = 0.167 and P = 0.643, respectively). No significant differences were observed over time in tumor-node-metastasis stage distribution (P = 0.177). The 5-year survival rates were 52.6% (2004-2008, 31.6% stage III/IV), 51.6% (2009-2013, 46% stage III/IV), and 52.0% (2014-2018, 32.0% stage III/IV) that over the 15-year period, the 5-year survival rate did not change significantly (P = 0.876).
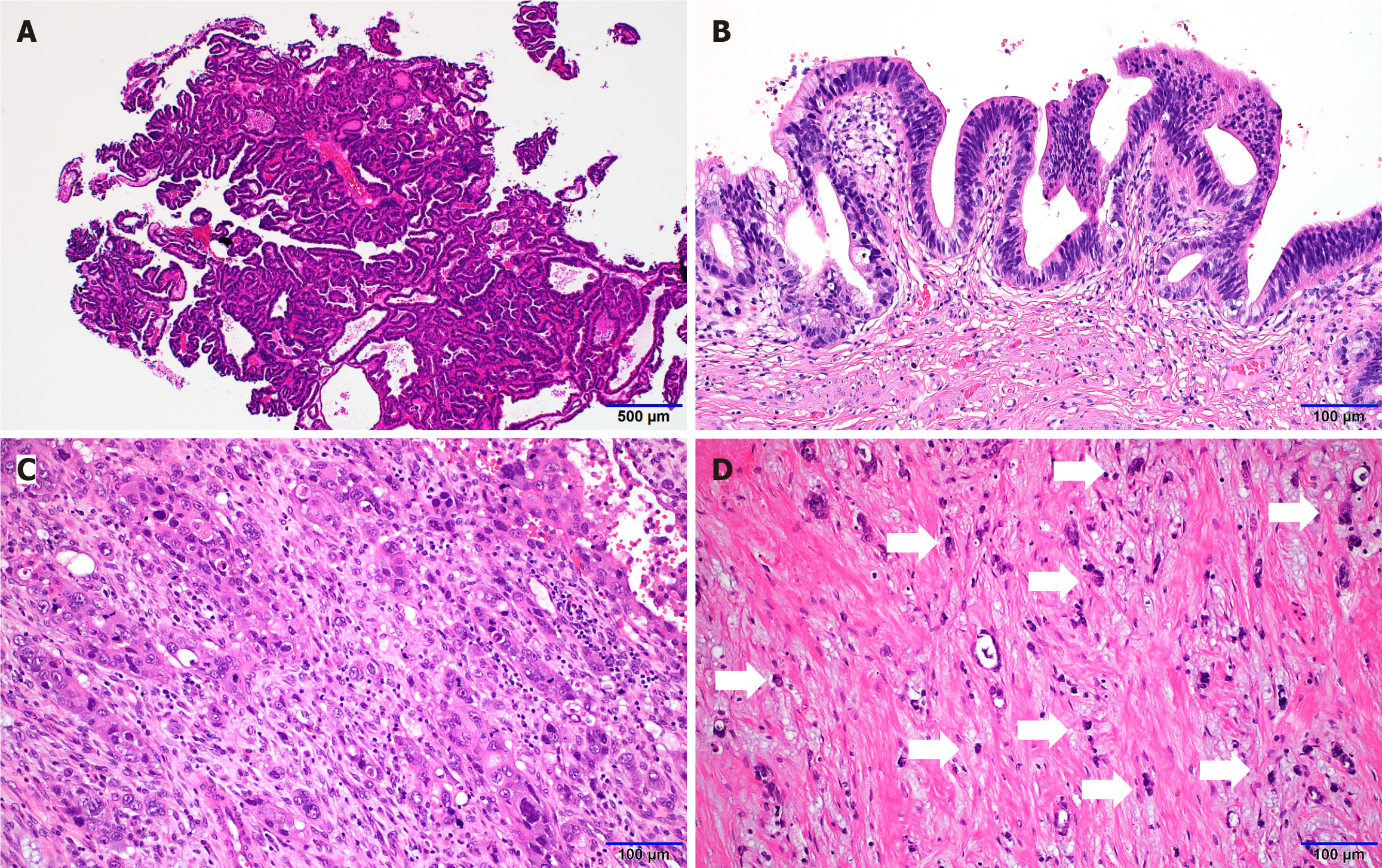
Pathological features were reviewed and quantitatively assessed, including the presence of accompanying premalignant neoplasms such as ICPN (Figure 2A) and BilIN (Figure 2B), histologic differentiation, non-neoplastic changes, undifferentiated components (Figure 2C), and poorly cohesive carcinoma components (Figure 2D). Additional parameters evaluated included depth of invasion, tumor size, and tumor staging.
The distribution of clinicopathologic features of GBC was evaluated at 5-year intervals (Table 3). Notably, the pro
| Pathological features | Years | P value | |||
| 2004-2008 (n = 38) | 2009-2013 (n = 63) | 2014-2018 (n = 75) | 2019-2023 (n = 109) | ||
| Preinvasive neoplasm | 0.472 | ||||
| ICPN | 11 (28.95) | 20 (32.26) | 26 (35.14) | 27 (24.77) | |
| BilIN or adenoma | 27 (71.05) | 42 (67.74) | 48 (64.86) | 82 (75.23) | |
| Histologic cell differentiation | 0.038 | ||||
| Pancreaticobiliary | 31 (81.58) | 57 (91.94) | 55 (74.32) | 82 (75.23) | |
| Intestinal | 7 (18.42) | 5 (8.06) | 19 (25.68) | 27 (24.77) | |
| Non-neoplastic change (hyperplasia or metaplasia) | 26 (68.42) | 51 (82.26) | 66 (89.19) | 89 (81.65) | 0.062 |
| Liver involvement | 4 (10.53) | 9 (14.29) | 5 (6.76) | 15 (13.76) | 0.445 |
| Poorly cohesive component (including signet ring cells) | 9 (23.68) | 17 (27.42) | 27 (36.49) | 27 (24.77) | 0.316 |
| Undifferentiated component (including squamous or sarcomatous) | 10 (26.32) | 7 (11.29) | 18 (24.32) | 20 (18.35) | 0.173 |
| Depth of invasion (cm) | 0.47 | 0.61 | 0.49 | 0.55 | 0.673 |
| Tumor size (cm) | 1.11 | 1.41 | 1.14 | 1.16 | 0.532 |
| Tumor budding (buds/0.785 mm2) | 4.92 | 3.66 | 5.41 | 3.33 | 0.128 |
Clinical features of GBC patients were compared between early-stage (stage I-II) and advanced-stage (stage III-IV) tumors (Supplementary Table 3). Among the variables analyzed, ALP, γ-GT, total cholesterol, and triglyceride levels were significantly different between the two groups (P = 0.027, P = 0.023, P = 0.040, and P = 0.024, respectively), with higher ALP and γ-GT levels and lower triglyceride levels observed in the advanced-stage disease group. HTN was linked to advanced stage significantly (P = 0.022).
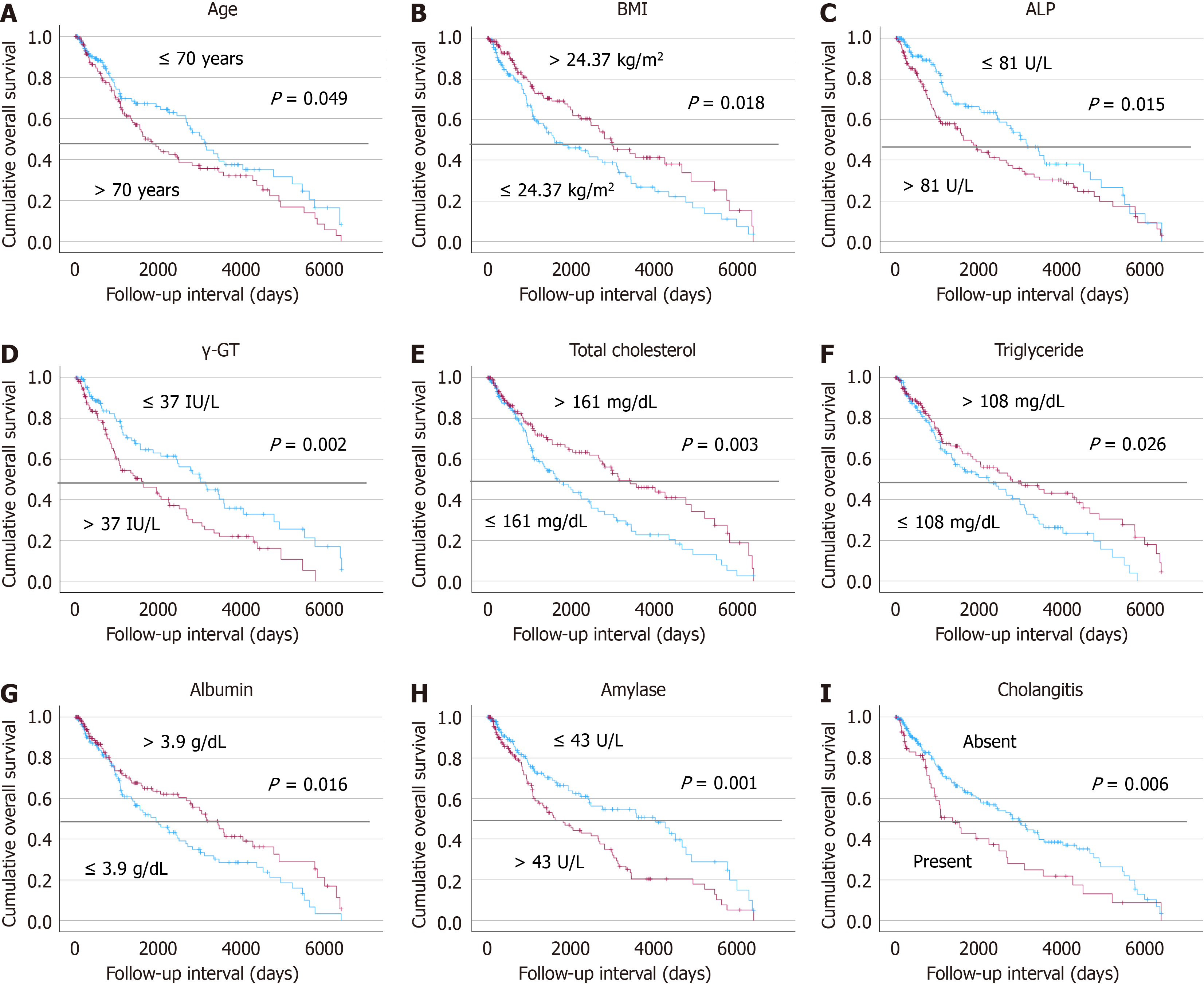
Survival analyses were conducted to evaluate the prognostic impact of a range of clinical factors in patients with GBC (Figure 3). The results indicated that older age, elevated ALP, γ-GT, and amylase levels were associated with poorer survival outcomes (P = 0.049, P = 0.015, P = 0.002, and P = 0.001, respectively). Additionally, the presence of cholangitis was linked to significantly poorer prognosis compared to patients without this condition (P = 0.006). Conversely, higher BMI, total cholesterol, triglyceride, and albumin levels were associated with better survival (P = 0.018, P = 0.003, P = 0.026, and P = 0.016, respectively). Cox regression of overall survival with metabolic factors including total cholesterol, triglyceride, and albumin were evaluated with age and stage, but did not attain prognostic significance (P = 0.104, P = 0.290, and P = 0.068, respectively) (Supplementary Table 4).
Pathologic features of GBC were compared between early-stage (stage I-II) and advanced-stage (stage III-IV) tumors (Table 4). Patients with advanced-stage disease were significantly more likely to present with preinvasive lesions of BilIN or adenoma than ICPN (P < 0.001). While pancreaticobiliary differentiation was identified as the predominant subtype in both groups, the distribution between pancreaticobiliary and intestinal types did not significantly differ (P = 0.722). Advanced-stage tumors were markedly associated with more aggressive features, including liver involvement (P < 0.001), poorly cohesive components such as signet ring cells (P < 0.001), and undifferentiated components, including squamous or sarcomatous elements (P = 0.025).
| Pathological features | Stage | P value | |
| I & II (n = 187) | III & IV (n = 98) | ||
| Preinvasive neoplasm | 0.000 | ||
| ICPN | 70 (37.4) | 15 (15.3) | |
| BilIN or adenoma | 117 (62.6) | 83 (84.7) | |
| Histologic cell differentiation | 0.722 | ||
| Pancreaticobiliary | 147 (78.6) | 79 (80.6) | |
| Intestinal | 40 (21.4) | 19 (19.4) | |
| Non-neoplastic change (hyperplasia or metaplasia) | 152 (81.3) | 80 (81.6) | 0.943 |
| Liver involvement | 11 (5.9) | 22 (22.4) | 0.000 |
| Poorly cohesive component (including signet ring cells) | 38 (20.3) | 42 (43.8) | 0.000 |
| Undifferentiated component (including squamous or sarcomatous) | 29 (15.5) | 26 (26.5) | 0.025 |
| Depth of invasion (cm) | 0.38 | 0.88 | 0.000 |
| Tumor size (cm) | 0.83 | 1.93 | 0.000 |
| Tumor budding (buds/0.785 mm2) | 2.97 | 6.47 | 0.000 |
Quantitative measures of tumor aggressiveness, such as depth of invasion (0.38 cm vs 0.88 cm), tumor size (0.83 cm vs 1.93 cm), and tumor budding (2.97 buds/0.785 mm² vs 6.47 buds/0.785 mm²), were significantly higher in advanced-stage cases (P < 0.001 for all). In contrast, the presence of non-neoplastic changes such as hyperplasia or metaplasia did not differ significantly between groups (P = 0.943).
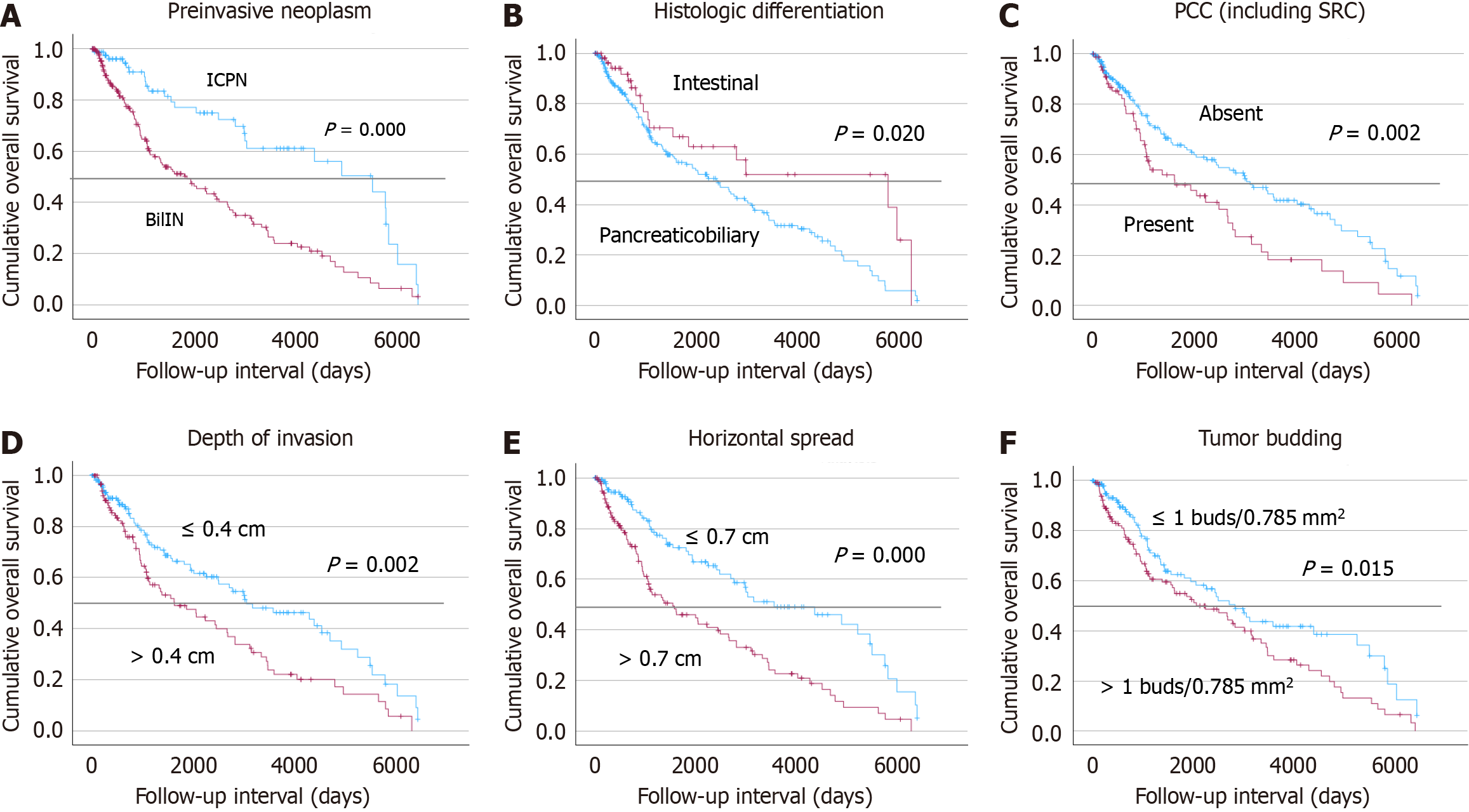
Kaplan-Meier survival analysis was conducted to evaluate overall survival based on various pathological features (Figure 4). Significantly improved survival outcomes were observed in patients with preinvasive lesions such as ICPN (P < 0.001), intestinal-type histologic differentiation (P = 0.002), absence of poorly cohesive components (P = 0.002), shallow depth of invasion (≤ 0.4 cm; P = 0.002), limited tumor size (≤ 0.7 cm; P < 0.001), and lower tumor budding scores (P = 0.015). Cox regression of overall survival with metabolic factors including tumor budding, poorly cohesive and undifferentiated components were evaluated with age and stage, but did not attain prognostic significance (P = 0.317, P = 0.916, and P = 0.490, respectively) (Supplementary Table 5).
Early detection of GBC poses a significant challenge due to its typically asymptomatic presentation. The majority of cases are diagnosed incidentally following cholecystectomy performed for symptomatic conditions, such as gallstones[17]. For instance, in this study, half of GBC cases were identified incidentally by pathologists based on gallbladder specimens after cholecystectomy. Identification of clinical factors associated with GBC may enhance screening processes and risk stratification. A comparative analysis of benign, precancerous, and GBC groups revealed notable variations in clinical factors, including age (> 70 years), HTN, and cholangitis. Patients with GBC were significantly older and showed a higher prevalence of HTN and cholangitis. HTN is not traditionally recognized as a direct risk factor for GBC, its role within the context of metabolic syndrome and chronic systemic inflammation may contribute to carcinogenesis[18]. In contrast to previous studies reporting a high prevalence of gallstones among GBC patients, our findings indicated a lower incidence of gallstones in the GBC cohort[19]. This observation may be attributable to the fact that most patients undergoing cholecystectomy for benign conditions presented with symptoms, such as gallstone-related pain, whereas a subset of GBC cases underwent surgery primarily based on preoperative imaging findings, including gallbladder wall thickening or mass-like lesions. Biochemical analyses revealed higher levels of total cholesterol, triglycerides, and liver enzymes (AST, ALT, ALP) in the benign than the GBC group. Previous studies on bile duct cancers (including GBC) have reported significantly lower serum albumin levels in cancer patients compared with those with gallstones, along with elevated ALP levels, findings that are in concordance with our results[20]. Dyslipidemia, characterized by high triglyceride and high-density lipoprotein cholesterol levels, has been reported as an independent risk factor for GBC; however, this finding was contrary to our results[21,22]. The low triglyceride and cholesterol levels observed in patients with GBC may also reflect decreased appetite or reduced dietary intake associated with cancer development. Despite the increasing prevalence of hypercholesterolemia and dyslipidemia in South Korea[23], total cholesterol and triglyceride levels exhibited a declining trend among GBC patients over the study period.
In our study, the number of patients undergoing total cholecystectomy increased from 2004 to 2023, which could be attributed to improved access to healthcare services[24]. Although the proportion of GBC diagnoses among chole
The number of precancerous lesions showed no significant temporal increase, peaking in 2009-2013, followed by a gradual decline in proportion over subsequent periods. The persistently high proportion of advanced-stage diagnoses may be attributed to the challenges associated with detecting GBC through radiologic evaluation. However, the observed decrease in precancerous lesions may reflect the increased awareness and proactive management of benign gallbladder conditions.
Within the GBC cohort, the average male-to-female incidence rate ratio was 1.08, indicating no significant differences in GBC incidence between genders. On a global scale, GBC occurs more frequently in females, with reported incidence rates being three to six times higher than in males[4]. However, studies conducted in South Korea have demonstrated lower sex ratios, with rates reported at 0.96 and 1.33, respectively[26,28]. Consistent with the stable proportion of GBC cases over time, no significant temporal changes were observed in the clinical characteristics of GBC patients. Nevertheless, several clinical factors [including age (> 70 years), ALP, γ-GT, amylase, BMI, total cholesterol, triglycerides, albumin levels, and the presence of cholangitis] were found to be associated with prognosis. These factors may serve as potentially useful prognostic indicators and aid in predicting patient survival. Cholesterol, triglyceride, and albumin levels demonstrated an inverse association with adverse prognosis, suggesting that higher levels of these metabolic factors may be associated with improved survival outcomes.
In our study, the distribution of the several histopathological features of GBC patients varied over time. The proportion of GBCs with intestinal differentiation increased during the 2019-2023 compared to 2004-2008. Although the changes were not statistically significant, the percentage of ICPN gradually increased in the first three periods before experiencing a decline in the final period, which diverges from the trends observed for BilIN and adenoma. Additionally, while liver involvement did not show statistical significance, its proportion more than doubled in 2019-2023 compared to 2014-2018. Other prognostic factors that showed no significant temporal variation may reflect limited statistical power during the early time periods due to smaller sample sizes. Although the temporal differences were not statistically significant, the overall patient profile remained stable across two decades, suggesting consistent underlying epidemiology. A modest decline in triglyceride and cholesterol levels may reflect cancer-related systemic effects, while slight increases in HTN, DM, and cholangitis likely mirror broader population health trends. The small increase in early-stage detection, though not significant, may still hold clinical relevance.
Advanced-stage tumors showed significantly higher rates of liver involvement, poorly cohesive and undifferentiated components, greater depth of invasion, tumor size, and tumor budding. In contrast, histologic subtype and non-neoplastic epithelial changes did not differ by stage. These features strongly support their role as markers of aggressive tumor biology. The presence of preinvasive lesions, intestinal histologic differentiation, poor differentiation histology including poorly cohesive components, liver invasion, increased depth of invasion, bigger tumor size, and tumor budding were all associated with poorer overall survival in patients with GBC. GBCs harboring ICPN or papillary neoplasia as preinvasive lesions were linked to poorer prognosis and exhibited distinct expression patterns of tumor suppressor proteins, suggesting a biologically different profile compared to tumors lacking such lesions[29]. Similarly, in periampullary adenocarcinoma, the pancreaticobiliary subtype has been associated with poorer outcomes compared to the intestinal subtype[30]. Our study highlights the prognostic value of several pathological factors in GBC. Notably, since these features can only be assessed following surgical resection, they are not applicable as predictive markers for preoperative decision-making, but could serve as useful prognostic markers in a postoperative setting.
In our study, metabolic factors including albumin, and lipid profiles and histologic factors were evaluated multivariate analysis with age and stages. They showed significant associations with GBC in the univariate analysis, however, many of these relationships were lost significance in the multivariate models after adjustment for tumor stage. This discrepancy suggests that tumor stage may act as a major determinant influencing these variables. We propose advanced-stage GBC is often accompanied by systemic changes including metabolic alterations and cachexia. Therefore, factors that appear to correlate with GBC in unadjusted analyses may, in fact, be driven primarily by stage-related physiological derangements rather than independent etiologic roles.
Our findings may be limited in generalizability but still provide meaningful insights. Age, HTN, cholangitis, and metabolic factors were identified as risk factors, supporting the importance of early identification of GBC. The clinicopathologic characteristics of GBC further highlight the value of recognizing high-risk profiles and underscore the prognostic significance of detailed histopathological evaluation. Treatment strategies for biliary tract cancers have evolved, with targeted agents such as HER2 and IDH1 inhibitors demonstrating promising efficacy in advanced-stage disease[31]. Because biliary tract cancers exhibit diverse genetic alterations and biological behaviors depending on subtype and anatomical location, dedicated studies focusing specifically on GBC remain essential[32]. Overall, this research contributes to a deeper understanding of the evolving landscape of GBC and provides a foundation for improving risk stratification and patient outcomes in clinical practice.
| 1. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 517] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20722] [Article Influence: 1883.8] [Reference Citation Analysis (23)] |
| 3. | Ouyang G, Liu Q, Wu Y, Liu Z, Lu W, Li S, Pan G, Chen X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer. 2021;127:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Schmidt MA, Marcano-Bonilla L, Roberts LR. Gallbladder cancer: epidemiology and genetic risk associations. Chin Clin Oncol. 2019;8:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol. 2019;5:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (1)] |
| 6. | Butte JM, Matsuo K, Gönen M, D'Angelica MI, Waugh E, Allen PJ, Fong Y, DeMatteo RP, Blumgart L, Endo I, De La Fuente H, Jarnagin WR. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg. 2011;212:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 780] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 8. | Espinoza JA, Bizama C, García P, Ferreccio C, Javle M, Miquel JF, Koshiol J, Roa JC. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016;1865:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Rakić M, Patrlj L, Kopljar M, Kliček R, Kolovrat M, Loncar B, Busic Z. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 105] [Reference Citation Analysis (0)] |
| 10. | Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 11. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2758] [Article Influence: 459.7] [Reference Citation Analysis (3)] |
| 12. | Roa JC, Basturk O, Adsay V. Dysplasia and carcinoma of the gallbladder: pathological evaluation, sampling, differential diagnosis and clinical implications. Histopathology. 2021;79:2-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Kai K, Kohya N, Kitahara K, Masuda M, Miyoshi A, Ide T, Tokunaga O, Miyazaki K, Noshiro H. Tumor budding and dedifferentiation in gallbladder carcinoma: potential for the prognostic factors in T2 lesions. Virchows Arch. 2011;459:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | American Joint Committee on Cancer. AJCC Cancer Staging Manual. New York: Springer, 2002. [DOI] [Full Text] |
| 15. | Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 16. | Kim HN, Lee SY, Kim BH, Kim CY, Kim A, Kim H. Prognostic value of tumor budding in gallbladder cancer: application of the International Tumor Budding Consensus Conference scoring system. Virchows Arch. 2021;478:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Rathanaswamy S, Misra S, Kumar V, Chintamani, Pogal J, Agarwal A, Gupta S. Incidentally detected gallbladder cancer- the controversies and algorithmic approach to management. Indian J Surg. 2012;74:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Sun L, Wang X, Chen Z. The association between hypertension and the risk of gallstone disease: a cross-sectional study. BMC Gastroenterol. 2022;22:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94:343-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 20. | Yadav SK, Mittal A, Sapkota K, Gupta SP, Sathian B. Ability of biochemical parameters to distinguish between bile duct cancer and gall bladder stones--a case control study in a tertiary care hospital of Pokhara Valley. Asian Pac J Cancer Prev. 2013;14:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Andreotti G, Chen J, Gao YT, Rashid A, Chang SC, Shen MC, Wang BS, Han TQ, Zhang BH, Danforth KN, Althuis MD, Hsing AW. Serum lipid levels and the risk of biliary tract cancers and biliary stones: A population-based study in China. Int J Cancer. 2008;122:2322-2329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Deng Z, Xuan Y, Li X, Crawford WJ, Yuan Z, Chen Z, Brooks A, Song Y, Wang H, Liang X, Chen T. Effect of metabolic syndrome components on the risk of malignancy in patients with gallbladder lesions. J Cancer. 2021;12:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Jin ES, Shim JS, Kim SE, Bae JH, Kang S, Won JC, Shin MJ, Jin HY, Moon J, Lee H, Kim HC, Jeong IK; Committee of Public Relation of the Korean Society of Lipid and Atherosclerosis. Dyslipidemia Fact Sheet in South Korea, 2022. J Lipid Atheroscler. 2023;12:237-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 24. | Barré A, Gusto G, Cadeau C, Carbonnel F, Boutron-Ruault MC. Diet and Risk of Cholecystectomy: A Prospective Study Based on the French E3N Cohort. Am J Gastroenterol. 2017;112:1448-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Su J, Liang Y, He X. Global, regional, and national burden and trends analysis of gallbladder and biliary tract cancer from 1990 to 2019 and predictions to 2030: a systematic analysis for the Global Burden of Disease Study 2019. Front Med (Lausanne). 2024;11:1384314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 26. | Kang MJ, Yun EH, Jung KW, Park SJ. Incidence, mortality and survival of gallbladder, extrahepatic bile duct, and pancreatic cancer using Korea central cancer registry database: 1999-2019. Ann Hepatobiliary Pancreat Surg. 2022;26:220-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 27. | Xiao YX, Chen J, Li ZY, Zou YX, Zhou XH, Zhang W, Li HL, Bischof EY, Xiang YB. Global trends in gallbladder cancer survival: A 30-year analysis of cancer registry data. Heliyon. 2025;11:e42853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Wi Y, Woo H, Won YJ, Jang JY, Shin A. Trends in Gallbladder Cancer Incidence and Survival in Korea. Cancer Res Treat. 2018;50:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Mochidome N, Koga Y, Ohishi Y, Miyazaki T, Matsuda R, Yamada Y, Aishima S, Nakamura M, Oda Y. Prognostic implications of the coexisting precursor lesion types in invasive gallbladder cancer. Hum Pathol. 2021;114:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Kehmann L, Jördens M, Loosen SH, Luedde T, Roderburg C, Leyh C. Evolving therapeutic landscape of advanced biliary tract cancer: from chemotherapy to molecular targets. ESMO Open. 2024;9:103706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 32. | LaPelusa M, Heumann T, Goff L, Agarwal R. Targeted therapies in advanced biliary tract cancers-a narrative review. Chin Clin Oncol. 2023;12:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/