Published online Feb 14, 2026. doi: 10.3748/wjg.v32.i6.113880
Revised: November 21, 2025
Accepted: December 29, 2025
Published online: February 14, 2026
Processing time: 150 Days and 4.7 Hours
Oral sulfate tablets (OSTs) are commonly used in South Korea, but the requirement to ingest 28 tablets and concerns regarding renal and gastrointestinal safety may reduce patient compliance. DWJ1609 is a novel OST formulation that contains sodium picosulfate and 25% less sulfate, requiring only 20 tablets. This modification is expected to improve tolerability while maintaining cleansing efficacy.
To evaluate the bowel cleansing efficacy and safety of DWJ1609 compared with conventional OSTs and to assess adverse events.
This prospective, randomized, single-blinded (investigator), multicenter, phase III clinical trial was conducted at seven university hospitals in South Korea. The primary endpoint was the non-inferiority of DWJ1609 based on the proportion of participants achieving a “successful” grade A or B on the Harefield Cleansing Scale, as assessed by independent central readers. Safety was monitored through adverse events and laboratory evaluations.
Overall, 215 participants were randomized, and 200 were included in the per-protocol analysis (DWJ1609: 99; OST: 101). Successful bowel cleansing was achieved in 96.97% of the DWJ1609 group, which was non-inferior to the OST group (100.00%), with a difference of 3.03%. DWJ1609 showed significantly higher tolerability, with lower inci
DWJ1609 demonstrated non-inferior bowel cleansing efficacy, improved tolerability, and a favorable safety profile compared with conventional. DWJ1609 has the potential to improve compliance and overall quality of colo
Core Tip: This multicenter randomized trial evaluated DWJ1609, a newly formulated oral sulfate tablet that incorporates sodium picosulfate with reduced sulfate content. DWJ1609 demonstrated non-inferior bowel cleansing efficacy compared with conventional oral sulfate tablets, while requiring fewer tablets and showing improved tolerability. These findings highlight DWJ1609 as a clinically meaningful alternative that may enhance patient acceptance and optimize colonoscopy preparation. This study provides evidence supporting its effectiveness and safety in routine practice, while noting the need for future research in older adults and patients with comorbidities.
- Citation: Park SB, Kang SB, Seo GS, Eun CS, Choi CH, Yang DH, Park JJ, Moon CM, Jung SH, Park H, Park MH, Yoo HK, Kim J, Heo JA, Park DI. Efficacy and safety of a novel sodium picosulfate oral tablet in a randomized controlled trial for bowel preparation. World J Gastroenterol 2026; 32(6): 113880
- URL: https://www.wjgnet.com/1007-9327/full/v32/i6/113880.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i6.113880
Common bowel preparations used to cleanse the colon before colonoscopy include polyethylene glycol (PEG) solutions, sodium picosulfate with magnesium citrate (SPMC), sodium sulfate-based preparations (oral sulfate solution/tablets), and combination laxatives[1,2]. Among these, oral sulfate tablets (OSTs) have recently gained widespread use in South Korea owing to their effectiveness and convenient administration. These tablets act as laxatives by increasing water absorption into the colon, inducing diarrhea, and effectively emptying the bowel to ensure clear visualization during the examination[1]. The recommended dosage for adults consists of 28 OSTs administered in two separate dosing sessions, with 14 tablets per session. However, concerns regarding renal safety and acute gastropathy have limited the widespread use of these preparations[3,4].
DWJ1609 is a novel, optimized OST formulation with reduced sulfate concentration in addition to sodium picosulfate incorporation. This modification reduces gastrointestinal discomfort and improves tolerability, decreasing salt content by approximately 75% of conventional OST preparations and thereby reducing the total number of tablets to 20.
This study aimed to demonstrate the non-inferiority of DWJ1609 relative to conventional OST by comparing the proportion of individuals who achieved a “successful” rating on the Harefield Cleansing Scale (HCS) for bowel cleansing, using a split-dosing regimen in adults undergoing colonoscopy[5]. Additionally, this study evaluated the efficacy, safety, and tolerability of DWJ1609 in comparison to OST.
This prospective, randomized, single-blinded (investigator), parallel, multicenter, active-control, phase III, non-inferiority trial was conducted at seven university hospitals across South Korea. Institutional Review Board (IRB) approval was obtained from each participating hospital (the approval numbers are provided in Supplementary Table 1). The study procedures adhered to the ethical standards outlined in the Declaration of Helsinki and regulations of the Ministry of Food and Drug Safety of South Korea. This trial is registered with the International Clinical Trials Registry Platform (No. ClinicalTrials.gov NCT 06062030). Participants voluntarily consented to take part in the clinical trial and signed the written informed consent form.
Eligible participants were men or women aged ≥ 19 years with a body mass index (BMI) between 19 kg/m2 and 30 kg/m2 scheduled to undergo colonoscopy for diagnosis, screening, or surveillance. Major exclusion criteria included individuals undergoing colonoscopy for therapeutic purposes, active gastrointestinal bleeding, gastrointestinal obstruction, perforation, inflammatory bowel diseases, significant cardiovascular diseases such as severe heart disease, acute respiratory failure, severe renal impairment, or a clinically significant history of gastrointestinal surgery. Complete exclusion criteria are provided in Supplementary Table 2.
Participants deemed eligible for this clinical trial were randomized in a 1:1 ratio by an independent statistician, who had no clinical involvement in the trial, to either the experimental group (DWJ1609; Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea; composition per tablet: Anhydrous sodium sulfate 1177.50 mg, potassium sulfate 211.13 mg, anhydrous magnesium sulfate 108.00 mg, sodium picosulfate 1.00 mg, simethicone 16.00 mg) or the control group (Orafang® tab; Pharmbio Korea Co., Seoul, South Korea; composition per tablet: Anhydrous sodium sulfate 1125.00 mg, potassium sulfate 201.07 mg, anhydrous magnesium sulfate 102.86 mg, simethicone 11.43 mg). The randomization sequence was generated using random block sizes of 4, 6, and 8 using the SAS® v9.4 statistical software (SAS Institute, Cary, NC, United States) and an interactive web response system. Each participant was provided with the investigational medicinal product according to their respective group.
Participants were administered the experimental substance through a split-dose regimen. In the DWJ1609 group, participants received an initial dose of 10 tablets with 425 mL of water in the evening (18:00-20:00), followed by two subsequent 425-mL water intake sessions over the next hour. Similarly, in the OST group, participants ingested 14 tablets with the same water volume and timing. A second administration occurred 10-12 hours later (approximately 04:00-08:00), following the same procedure as that of the initial dose. All participants completed their final dose at least 2 hours before the scheduled colonoscopy and subsequently underwent the procedure as part of the clinical trial. All colonoscopies were conducted in the morning. Sedation was not standardized in the protocol; each site administered sedatives according to its routine clinical practice, but sedation conditions were comparable across both groups. The investigator recorded the entire colonoscopy procedure on video in accordance with a specified protocol manual. A summary of chemical compositions of each preparation is shown in Supplementary Table 3.
The recorded colonoscopy procedures were subsequently uploaded to the Image Evaluation System. An Independent Review Committee, comprised of three gastroenterology specialists (Yang DH, Park JJ, and Moon CM), evaluated the videos to ensure objectivity and minimize bias. The primary endpoint was to compare and evaluate the bowel-cleansing efficacy of the investigational drug with that of the control drug based on the proportion of participants undergoing colonoscopy who achieved “successful” cleansing, defined as an HCS grade A or B rating[5]. This evaluation was done by independent central readers. The secondary endpoint was to evaluate the bubble score, cecal intubation rate, colonoscopy insertion and withdrawal times, polyp detection rate (PDR), adenoma detection rate (ADR), and compliance and safety of the investigational drug in individuals requiring bowel preparation before colonoscopy.
The bowel preparation scale was evaluated based on the HCS score. The overall HCS grade was categorized as: (1) A (all segments scored 3 or 4); (2) B (≥ 1 segment scored 2, but no segment scored < 2); (3) C (≥ 1 segment scored 1, but no segment scored < 1); and (4) D (≥ 1 segment scored 0). The bubble score was determined using a Numeric Rating Scale (NRS): (1) 0, no or minimal bubbles; (2) 1, bubbles covering at least half the luminal diameter; (3) 2, bubbles covering the circumference of the lumen; and (4) 3, bubbles filling the entire lumen)[6,7]. Safety was determined based on the oc
Tolerability was assessed based on the participants’ feedback on difficulty with the preparations, taste experience, and willingness to reuse the preparation. Difficulty with the preparations was assessed using a 5-point NRS, with scores of 1 (very easy), 2 (easy), 3 (tolerable), 4 (difficult), and 5 (very difficult). Taste experience was measured separately using a 4-point NRS, with scores of 1 (good), 2 (tolerable), 3 (poor), and 4 (bad). The willingness to reuse the preparation was evaluated using yes or no questions.
In this non-inferiority trial, the sample size was calculated to detect a difference in proportions between the treatment and control groups. Assuming a 90% successful cleansing rate, a one-sided 97.5% confidence interval (CI) and a statistical power of 80% were utilized for the analysis. Non-inferiority would be determined if the lower bound of the calculated CI exceeded the predefined non-inferiority margin of -15%, which was selected with reference to previous studies of bowel preparations[1,8,9]. Considering an anticipated dropout rate of 20%, the required sample size was adjusted. Based on these assumptions, a minimum of at least 214 patients was required to maintain sufficient power for detecting proportional differences between groups.
The safety set included participants who were administered at least one dose of the investigational medicinal product after randomization. The full analysis set (FAS) included participants who were eligible for efficacy evaluation (i.e., those who underwent colonoscopy). The FAS was analyzed based on the randomized treatment groups, regardless of the actual treatment received. The per-protocol set (PPS) 1 included participants from the FAS who underwent an efficacy evaluation by Independent Review Committee and had no major protocol violations that could impact the efficacy evaluation. The PPS2 population included participants from the PPS1 analysis set who completed the clinical trial without major protocol violations. Efficacy analyses were primarily conducted using PPS1 as the main analysis set, with additional analyses performed on the FAS and PPS2 populations. A safety evaluation was performed on the safety set population.
All statistical analyses were conducted using SAS® version 9.4, with a two-sided test at a significance level of 5%, unless otherwise stated in the protocol. When fewer than 20% of the participants had an expected frequency < 5, the Wald normal CI was used. However, when > 20% of the participants had an expected frequency < 5, the Chan-Zhang (Exact) CI was employed. For continuous variables, descriptive statistics (mean, standard deviation, median, minimum, and maximum) were calculated and presented, while categorical variables were summarized as frequencies and proportions.
For between-group comparisons, the analytical approach was determined based on the distributional characteristics of each variable. Continuous variables were compared using the two samples t-test when the assumption of normality was satisfied; otherwise, the Wilcoxon rank-sum test was applied. Categorical variables were evaluated using the χ2 test when its assumptions were met – specifically, when no more than 20% of expected cell counts were < 5, and all expected counts were ≥ 1. If these criteria were not met, Fisher’s exact test was used instead.
Between March and June 2024, a total of 221 patients were included in the study and randomly divided into two groups: (1) The DWJ1609 group; and (2) OST group. Participants who were randomized, received at least one dose of the investigational drug, and were eligible for efficacy evaluation were included in the analysis.
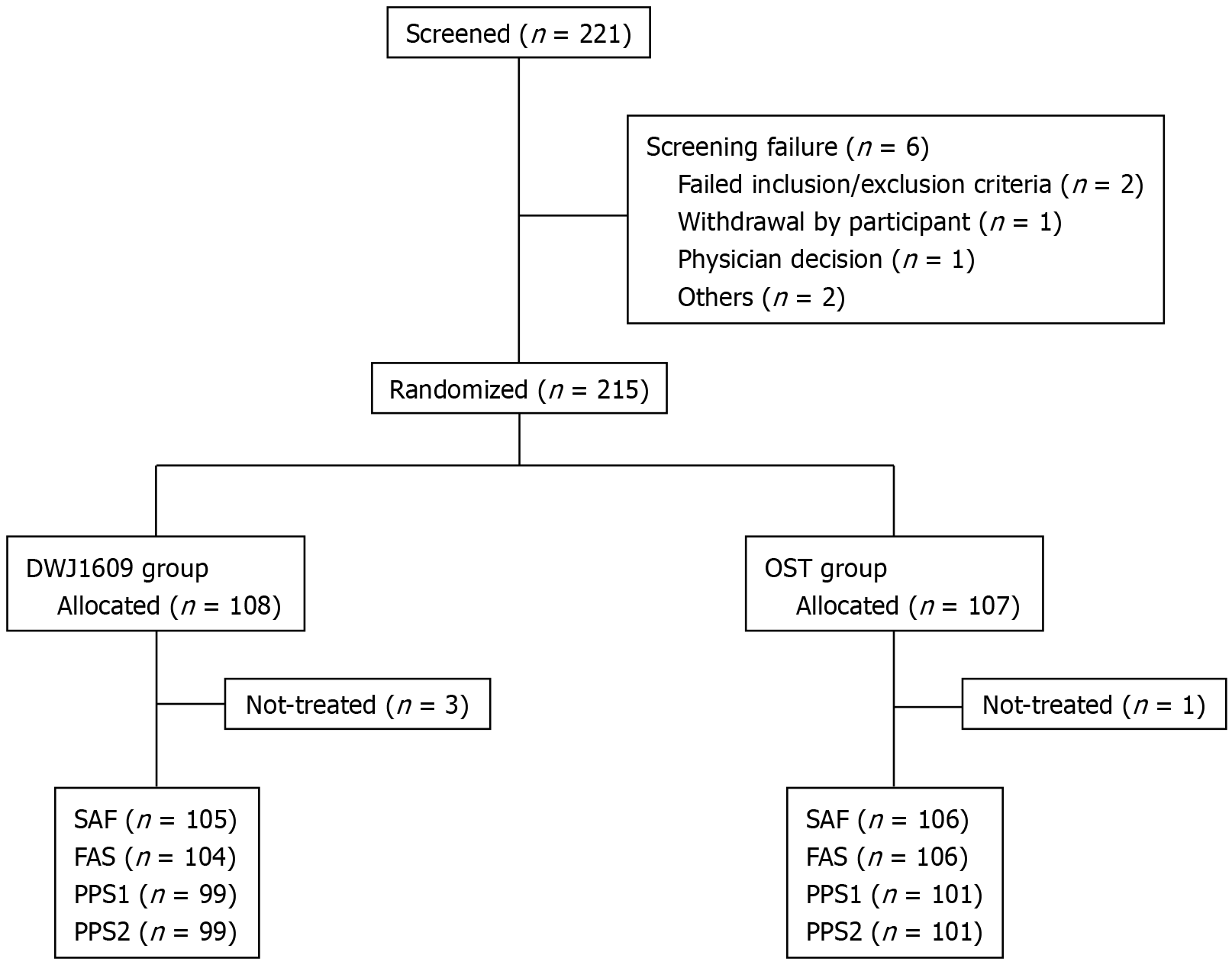
The study flowchart is presented in Figure 1. The mean age (mean ± SD) of the overall population was 43.3 ± 10.94 years, ranging from 21 to 75; 36.28% (78/215) of all participants were men. Demographics and clinical characteristics were balanced between the two groups in all randomized sets (Table 1).
| Total (n = 215) | DWJ1609 group (n = 108) | Oral sulfate tablet group (n = 107) | P value | |
| Age (years) | 43.3 ± 10.94 | 42.8 ± 11.83 | 43.8 ± 10.00 | 0.5631 |
| Age < 65 years | 208 (96.74) | 103 (95.37) | 105 (98.13) | 0.4452 |
| Age ≥ 65 years | 7 (3.26) | 5 (4.63) | 2 (1.87) | |
| Sex | ||||
| Men | 78 (36.28) | 36 (33.33) | 42 (39.25) | 0.3672 |
| Women | 137 (63.72) | 72 (66.67) | 65 (60.75) | |
| Body mass index (kg/m2) | 23.94 ± 3.1005 | 24.072 ± 3.1191 | 23.802 ± 3.0904 | 0.6611 |
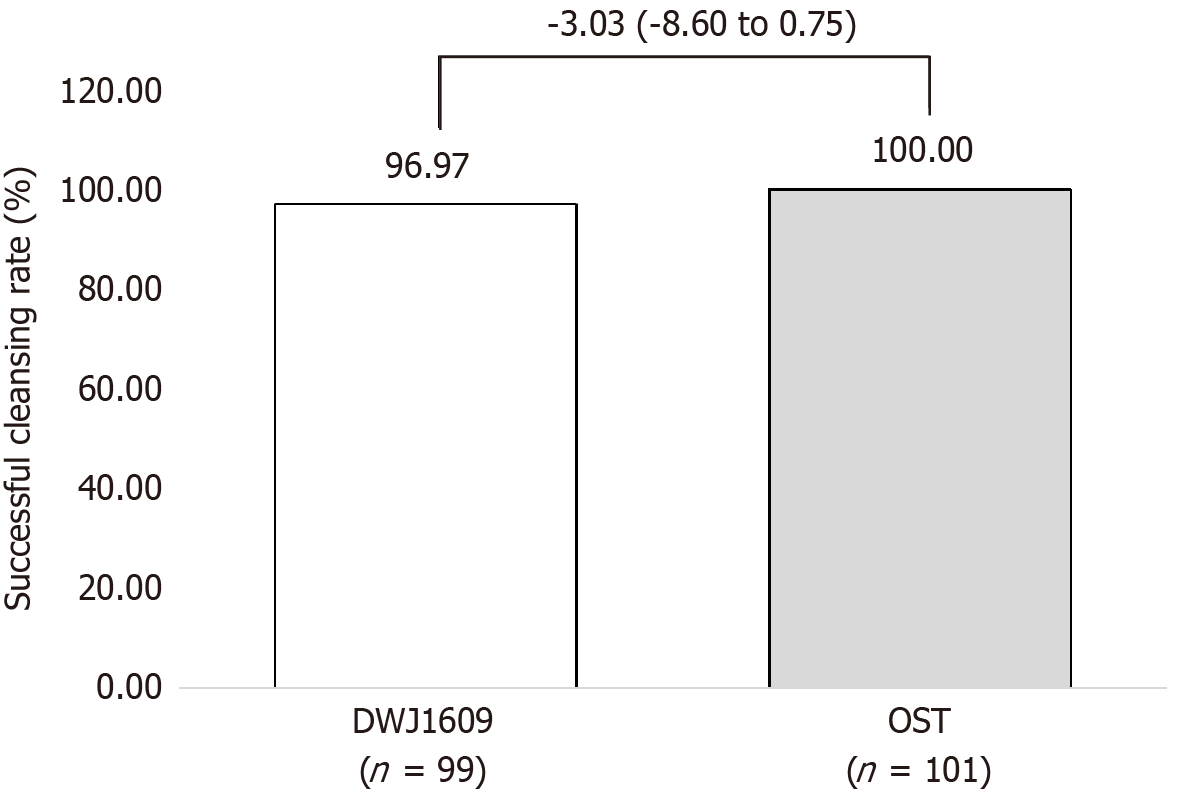
The DWJ1609 group was non-inferior to the OST group in terms of the primary efficacy endpoint. In PPS1, successful bowel cleansing (HCS grade A or B) was achieved in 96/99 (96.97%) of the DWJ1609 group and 101/101 (100.00%) of the OST group, with a difference of 3.03% [Chan-Zhang (Exact), 95%CI: -8.60 to 0.75], as determined by independent central readers (P = 0.119; Figure 2).
All three participants in the DWJ1609 group who were classified as experiencing bowel cleansing failures (HCS grade C or D) were male and demonstrated 100% compliance with the investigational drugs. Participant A was a 59-year-old man (height: 173.1 cm, weight: 83.1 kg, BMI: 27.7 kg/m2) with multiple comorbidities, including type 2 diabetes mellitus, dyslipidemia, rheumatoid arthritis, gastroesophageal reflux disease, cataract, interstitial lung disease, gout, and a sliding hiatal hernia. Participant B was a 45-year-old man (height: 169.1 cm, weight: 75.4 kg, BMI: 26.4 kg/m2) with no notable medical history. Participant C was a 70-year-old man (height: 168.0 cm, weight: 71.0 kg, BMI: 25.2 kg/m2) with a history of pancreas divisum and atrial fibrillation. In all cases, a partially semi-solid stool was observed in certain colonic segments, leading to an overall inadequate assessment.
The overall cleansing rates in the DWJ1609 group based on the HCS grading were as follows: (1) Grade A was 62.63%; (2) Grade B was 34.34%; (3) Grade C was 2.02%; and (4) Grade D was 1.01%. In the OST group, grade A was 72.28%, and grade B was 27.72%, with no cases of grade C or grade D. The difference in overall cleansing rates based on HCS grading between the two groups was not statistically significant (P = 0.164) in PPS1 (Table 2).
| DWJ1609 group (n = 99) | Oral sulfate tablet group (n = 101) | P value1 | |
| Overall cleansing based on Harefield Cleansing Scale | 0.164 | ||
| Grade A | 62 (62.63) | 73 (72.28) | 0.145 |
| Grade B | 34 (34.34) | 28 (27.72) | 0.316 |
| Grade C | 2 (2.02) | 0 (0) | 0.244 |
| Grade D | 1 (1.0) | 0 (0) | 0.495 |
| Successful bowel cleansing (grade A/B) reader | 96/99 (96.97) | 101/101 (100.00) | 0.119 |
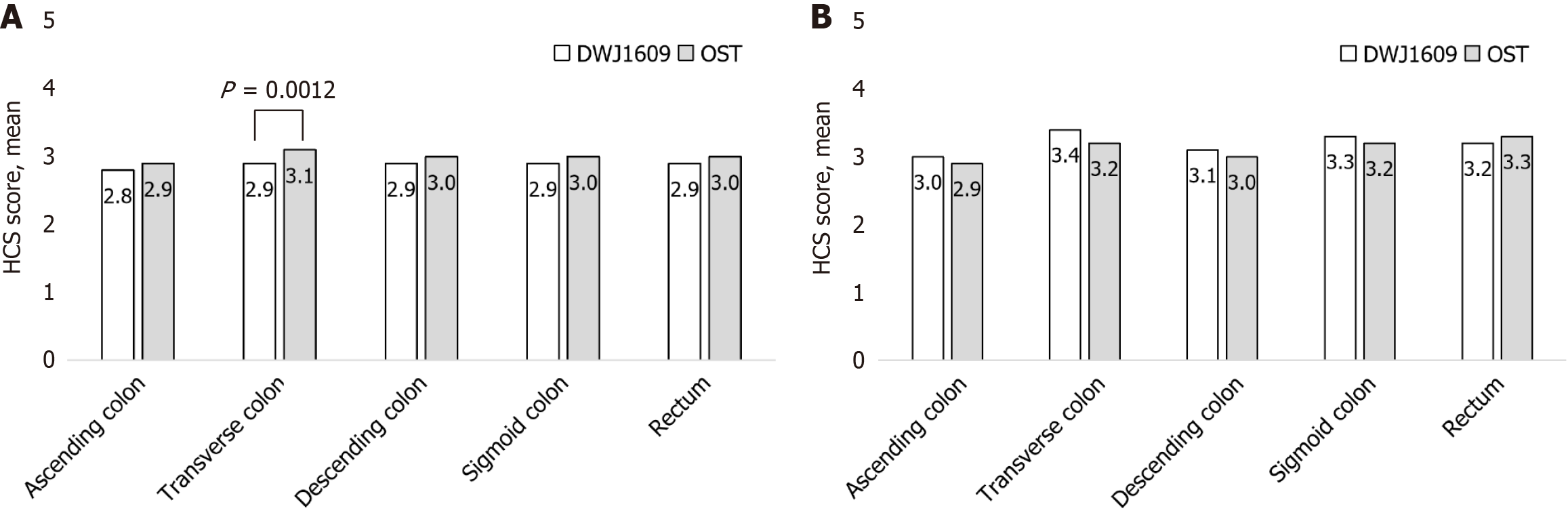
HCS scores for each of the five segments (ascending, transverse, descending, sigmoid colons, and rectum) were evaluated by independent central readers and investigators. The HCS score for the transverse colon was 2.9 ± 0.50 in the DWJ1609 group compared, with 3.1 ± 0.38 in the OST group as assessed by the independent central readers (P = 0.001). The HCS scores for ascending, descending, and sigmoid colons and rectum in the DWJ1609 group were not significantly different from those of the OST group (Figure 3). However, the HCS score for each segment did not significantly differ between the two groups (Supplementary Table 4). No residual air bubbles were observed (100%) in the colon and rectum, and no significant differences in intraluminal bubbles were observed between the two groups (Supplementary Table 5).
The endoscopic outcomes, including cecal intubation rate (100% vs 100%), insertion time (4.04 ± 2.42 minutes vs 4.25 ± 2.34 minutes, P = 0.407), withdrawal time (8.18 ± 3.39 minutes vs 8.21 ± 3.09 minutes, P = 0.889), PDR (40.4% vs 39.6%, P = 0.908), and ADR (14.1% vs 13.9%, P = 0.955), were not significantly different between the groups (Supplementary Table 6).
Participants in the DWJ1609 group experienced less difficulty with consumption compared to those in the OST group (1.9 ± 0.83 vs 2.2 ± 0.82, P = 0.040). Although the mean difficulty score for medication intake did not differ significantly, a score-based analysis revealed a higher proportion of participants in the DJW1609 group who gave a rating of 1 (very easy): 35 participants (35.35%) in the DWJ1609 group compared with 23 participants (22.77%) in the control group. Taste (1.4 ± 0.50 vs 1.5 ± 0.56, P = 0.302) and the rate of willingness to reuse the same agent during colonoscopy (94.95% vs 93.07%, P = 0.576) did not significantly differ between the groups (Supplementary Table 7).
The incidence of adverse drug reactions was 18.10% (19/105 participants, 23 events) in the DWJ1609 group and 33.02% (35/106 participants, 59 events) in the OST group, showing a significant difference between the groups (P = 0.013). Participants in the DWJ1609 group demonstrated a significantly lower incidence of nausea (7.62% vs 21.70%, P = 0.004) and headache (0.95% vs 8.49%, P = 0.019). Additionally, the incidence of dizziness was lower in the DWJ1609 group, though not statistically significant (1.90% vs 4.72%, P = 0.445). Conversely, reports of vomiting were higher in the DWJ1609 group, but this difference was also not statistically significant (5.71% vs 2.83%, P = 0.332). Chest discomfort, chills, and metabolism/nutrition disorders were not reported in the DWJ1609 group but occurred once each in the OST group (Table 3).
| DWJ1609 group (n = 105) | Oral sulfate tablet group (n = 106) | P value1 | |
| Adverse drug reaction | 19 (18.10) | 35 (33.02) | 0.013 |
| Gastrointestinal disorders | 16 (15.24) | 27 (25.47) | 0.065 |
| Nausea | 8 (7.62) | 23 (21.70) | 0.004 |
| Vomiting | 6 (5.71) | 3 (2.83) | 0.332 |
| Abdominal distension | 4 (3.81) | 6 (5.66) | 0.748 |
| Diarrhea | 1 (0.95) | 0 | 0.498 |
| Gastroesophageal reflux disease | 1 (0.95) | 0 | 0.498 |
| Abdominal pain | 0 | 1 (0.94) | 1.000 |
| Gastritis erosive | 0 | 1 (0.94) | 1.000 |
| Nervous system disorders | 3 (2.86) | 12 (11.32) | 0.017 |
| Dizziness | 2 (1.90) | 5 (4.72) | 0.445 |
| Headache | 1 (0.95) | 9 (8.49) | 0.019 |
| Hypoaesthesia | 0 | 1 (0.94) | 1.000 |
| General disorders and administration site conditions | 0 | 2 (1.89) | 0.498 |
| Chest discomfort | 0 | 1 (0.94) | 1.000 |
| Chills | 0 | 1 (0.94) | 1.000 |
| Metabolism and nutrition disorders | 0 | 1 (0.94) | 1.000 |
| Hyponatremia | 0 | 1 (0.94) | 1.000 |
Most colorectal cancers progress from precancerous lesions known as colorectal adenomas, the removal of which via colonoscopy is a well-established preventive measure[10,11]. Bowel cleansing is a prerequisite for colonoscopy, as it ensures clear visualization of colorectal lesions, thereby enhancing the effectiveness of the procedure. However, many individuals are reluctant to undergo the procedure owing to the discomfort associated with taking bowel preparation solutions. For several years, PEG has been the standard bowel cleansing agent, recognized for its effectiveness and safety[12,13]. However, despite the development of relatively lower-dose formulations recently, PEG remains subject to poor compliance owing to its unpleasant taste and flavor[14]. SPMC is an effective and well-tolerated bowel cleansing agent that combines a stimulant and osmotic laxative, requiring a lower liquid volume and offering better patient compliance than traditional preparations[15]. In response to reports indicating severe esophageal and gastric injuries caused by ingestion of partially dissolved or undissolved SPMC powder, the formulation has been modified to a liquid form[16,17]. Other reported side effects of SPMC administration include weight loss, dehydration, increased hemoglobin levels, hyponatremia, and syncope, along with concerns about hypermagnesemia[18-20]. oral sulfate solution, comprising sodium, potassium, and magnesium sulfates, has demonstrated an excellent bowel cleansing effect but reportedly causes mild gastrointestinal events such as abdominal pain and nausea[8,9,21,22]. OST, an oral sulfate agent in tablet form, was introduced in 2020 and contains simethicone to help removal of intraluminal air bubbles, thereby enhancing visualization during colonoscopy[1]. DWJ1609 is a novel formulation designed to reduce the number of tablets required compared to currently available OSTs by lowering the amount of sulfate and incorporating the stimulant laxative sodium picosulfate. In addition, DWJ1609 contains simethicone.
In this phase III randomized multicenter trial, the novel sulfate tablet preparation DWJ1609 displayed non-inferiority to an OST preparation in achieving successful bowel-cleansing rates in adults undergoing colonoscopy. Independent central readers confirmed successful bowel cleansing in 96.97% of the participants in the DWJ1609 group and 100.00% in the OST group, demonstrating non-inferiority with a difference of 3.03%.
The HCS score for the transverse colon was 2.9 ± 0.50 in the DWJ1609 group and 3.1 ± 0.38 in the OST group, as only one participant in the DWJ1609 group was classified as a failure (score of 1 or less). Although this difference reached statistical significance (P = 0.001), it was numerically small and unlikely to be clinically meaningful. These scores were close to 3, which corresponds to “clear liquid” and indicates a high level of bowel cleanliness. However, in the investigator-assessed scores for the entire bowel, the DWJ1609 and OST groups showed comparable results (3.4 ± 0.74 vs 3.2 ± 0.74), with no meaningful differences between the two groups. In several cases, small residual stool (< 10 mm) or stool retained within the diverticula led the independent central readers to rate the cleansing quality relatively lower than the investigators. This discrepancy may stem from interobserver variation rather than an actual difference in bowel cleanliness. Additionally, although DWJ1609 contains SPMC, both DWJ1609 and the conventional OST preparation share similar mechanisms of action, promoting bowel cleansing through osmotic effects and stimulation of colonic motility. Therefore, a physiological difference is unlikely.
Residual air bubbles in the colonic lumen were rarely observed in bowel preparation when DWJ1609 was administered, likely attributed to the inclusion of simethicone. Adding simethicone to bowel cleansing agents is known to reduce air bubbles[1,23,24].
The endoscopic outcomes, including cecal intubation, insertion, and withdrawal times, PDR, and ADR, did not significantly differ between the groups. The relatively lower ADR observed in this study compared with that in previous studies could be due to the recruitment of a relatively young and healthy population[25]. Crockett and Ladabaum[26] reported that the ADR for individuals aged ≥ 50 years was 35.8% and hypothesized that the ADR for those aged 45-49 years should range from 17% to 22%[26].
The ease of consumption is important for achieving successful bowel preparation. In this study, the DWJ1609 group was less likely to experience difficulty with consumption compared with the OST group (1.9 ± 0.83 vs 2.2 ± 0.82, P = 0.040), with a higher proportion of participants providing a rating of 1, indicative of great ease. However, the taste and rate of willingness to reuse the same agent for colonoscopy were similar between the groups.
Additionally, the incidence of adverse drug reactions in the DWJ1609 group was significantly lower than in the OST group, indicating better tolerability. Gastrointestinal and nervous system disorders were more frequent in the OST group, with nausea and headache being particularly notable. The most frequently reported adverse drug reaction in the DWJ1609 group was nausea at 7.62% (8/105 participants, 8 events), followed by vomiting at 5.71% (6/105 participants, 6 events) and abdominal distension at 3.81% (4/105 participants, 4 events). In the OST group, nausea was the most frequent adverse drug reaction at 21.70% (23/106 participants, 28 events), followed by headache at 8.49% (9/106 participants, 9 events), abdominal distension at 5.66% (6/106 participants, 7 events), and dizziness at 4.72% (5/106 participants, 6 events). Nausea and neurological AEs were significantly less frequent in the DWJ1609 group. The incidence of vomiting was numerically higher in the DWJ1609 group compared with the control group (5.71% vs 2.83%), although this difference did not reach statistical significance. The slight increase in vomiting frequency may be attributable to individual variability rather than chemical composition. However, because DWJ1609 contains SPMC as an additional component, a potential hypersensitivity reaction or stimulant-induced response cannot be entirely excluded. This observation warrants further investigation in future studies involving a larger population, particularly to clarify whether vomiting is related to the SPMC component or represents random variability across individuals. Nevertheless, when considered alongside the lower incidence of nausea in the DWJ1609 group, these findings suggest that the overall tolerability of DWJ1609 remains favorable.
No abnormal laboratory findings, including electrolyte-related AEs such as hypokalemia or hyponatremia, were observed after DWJ1609 consumption. In the control group, a single case of hyponatremia was reported. Therefore, DWJ1609 offers a more comfortable bowel cleansing experience for patients by reducing the overall incidence of adverse drug reactions and minimizing symptoms such as nausea and headache. DWJ1609 resulted in fewer gastrointestinal and neurologic adverse drug reactions attributed to its reduced salt composition, which is approximately 75% less than that in typical OSTs, while maintaining bowel cleansing efficacy. Designed to reduce gastrointestinal and neurologic discomfort and improve tolerability, it reduces both salt content and the total number of tablets to 20.
This study has several limitations. First, the study population consisted of relatively young and healthy individuals (mean age, approximately 43 years), which may not fully reflect real-world patients and is associated with a lower incidence of ADRs. Bowel preparation is typically more difficult in high-risk groups such as older adults and patients with diabetes, chronic constipation, or chronic kidney disease. Therefore, further studies are needed to evaluate the efficacy and safety of this regimen in these higher-risk populations, including elderly individuals and those with comorbidities. Second, while this study demonstrated non-inferiority, three cases of bowel cleansing failure were observed in the DWJ1609 group. Although no common factors were identified among the participants with failed bowel cleansing, the United States Multi-Society Task Force of Colorectal Cancer guidelines recommend achieving a bowel cleansing success rate of at least 85%[27]. This study confirmed a successful bowel cleansing rate in accordance with these guidelines. Third, acute gastropathy caused by ingesting a preparation agent, which was observed after the current OST consumption, was not evaluated. The reliability of the comparison is constrained by the limitations of a single study, highlighting the need for additional studies to further validate these results.
Despite these limitations, this study offers significant insights. DWJ1609 can maintain the effectiveness of bowel cleansing by reducing the dosage of conventional osmotic sulfate preparations and incorporating the stimulant laxative sodium picosulfate. Additionally, the included simethicone helps eliminate bubbles, improving visibility. The DWJ1609 group showed better tolerability and lower incidence rates of nausea, headache, dizziness, and AEs than those of current OSTs. Therefore, our results suggest that administering DWJ1609 can result in high-quality bowel preparation comparable with that achieved by OSTs in adults undergoing colonoscopy.
DWJ1609 demonstrated non-inferior bowel cleansing efficacy with improved tolerability and no additional safety concerns compared with conventional OSTs. These results support its suitability as a routine bowel preparation option, particularly given its reduced tablet burden and potential to enhance patient acceptance. Further studies in older adults and patients with comorbidities are warranted to confirm its broader clinical applicability.
The authors thank the study participants, their family members, and all participating investigators for their valuable contributions to this clinical trial.
| 1. | Yang HJ, Park DI, Park SK, Lee CK, Kim HJ, Oh SJ, Moon JR, Lee BJ, Koh JS, Kim HS, Park SY, Kim DH, Chun J, Kang EA, Kim J, Soh H, Eun CS, Kim YS, Jeen YT. Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: A randomized phase 3 trial. J Gastroenterol Hepatol. 2020;35:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Moulin B, Ponchon T. A comparative review of use of sulphate and phosphate salts for colonoscopy preparations and their potential for nephrotoxicity. Endosc Int Open. 2018;6:E1206-E1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Yoon JY, Park SB, Lee MH, Kwak MS, Cha JM. Acute Gastropathy Associated with Bowel Preparation According to Age: Oral Sulfate Tablets versus 1-L Polyethylene Glycol with Ascorbic Acid. Korean J Gastroenterol. 2024;84:177-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Halphen M, Heresbach D, Gruss HJ, Belsey J. Validation of the Harefield Cleansing Scale: a tool for the evaluation of bowel cleansing quality in both research and clinical practice. Gastrointest Endosc. 2013;78:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Zhang S, Zheng D, Wang J, Wu J, Lei P, Luo Q, Wang L, Zhang B, Wang H, Cui Y, Chen M. Simethicone improves bowel cleansing with low-volume polyethylene glycol: a multicenter randomized trial. Endoscopy. 2018;50:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Kim H, Ko BM, Goong HJ, Jung YH, Jeon SR, Kim HG, Lee MS. Optimal Timing of Simethicone Addition for Bowel Preparation Using Polyethylene Glycol Plus Ascorbic Acid. Dig Dis Sci. 2019;64:2607-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Yang HJ, Park SK, Kim JH, Im JP, Yeom DH, Seo GS, Park DI. Randomized trial comparing oral sulfate solution with 4-L polyethylene glycol administered in a split dose as preparation for colonoscopy. J Gastroenterol Hepatol. 2017;32:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Rex DK, Di Palma JA, Rodriguez R, McGowan J, Cleveland M. A randomized clinical study comparing reduced-volume oral sulfate solution with standard 4-liter sulfate-free electrolyte lavage solution as preparation for colonoscopy. Gastrointest Endosc. 2010;72:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, Lucas F, Brown JP, Kralj-Hans I, Greliak P, Pack K, Wood J, Thomson A, Veitch A, Duffy SW, Cross AJ. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18:823-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1663] [Article Influence: 138.6] [Reference Citation Analysis (1)] |
| 12. | Golub RW, Kerner BA, Wise WE Jr, Meesig DM, Hartmann RF, Khanduja KS, Aguilar PS. Colonoscopic bowel preparations--which one? A blinded, prospective, randomized trial. Dis Colon Rectum. 1995;38:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980;78:991-995. [PubMed] |
| 14. | Rivas JM, Perez A, Hernandez M, Schneider A, Castro FJ. Efficacy of morning-only 4 liter sulfa free polyethylene glycol vs 2 liter polyethylene glycol with ascorbic acid for afternoon colonoscopy. World J Gastroenterol. 2014;20:10620-10627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | van Lieshout I, Munsterman ID, Eskes AM, Maaskant JM, van der Hulst R. Systematic review and meta-analysis: Sodium picosulphate with magnesium citrate as bowel preparation for colonoscopy. United European Gastroenterol J. 2017;5:917-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Seo JY, Kang KJ, Kang HS, Kim SE, Park JW, Moon SH, Kim JH, Park CK. Corrosive esophagitis caused by ingestion of picosulfate. Clin Endosc. 2015;48:66-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Suh JP, Choi YS, Lee SH. Education and Imaging. Gastroenterology: acute mucosal injury of esophagus and stomach induced by sodium picosulfate/magnesium citrate for bowel preparation. J Gastroenterol Hepatol. 2014;29:1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. 2009;69:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 19. | Dillon CE, Laher MS. The rapid development of hyponatraemia and seizures in an elderly patient following sodium picosulfate/magnesium citrate (Picolax). Age Ageing. 2009;38:487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rahman A, Vanner SJ, Baranchuk A, Hookey LC. Serial monitoring of the physiological effects of the standard Pico-Salax® regimen for colon cleansing in healthy volunteers. Can J Gastroenterol. 2012;26:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ali IA, Roton D, Madhoun M. Oral sulfate solution versus low-volume polyethylene glycol for bowel preparation: Meta-analysis of randomized controlled trials. Dig Endosc. 2022;34:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Saito Y, Oka S, Tamai N, Kudo T, Kuniyoshi N, Shirakura T, Omae Y, Hamahata Y, Arai T, Tanaka S, Uedo N, Shimizu S, Fukuzawa M, Uraoka T, Ichinose S, Ogata H, Kobayashi K, Saito S, Tajiri H. Efficacy and safety of oral sulfate solution for bowel preparation in Japanese patients undergoing colonoscopy: Noninferiority-based, randomized, controlled study. Dig Endosc. 2021;33:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Tongprasert S, Sobhonslidsuk A, Rattanasiri S. Improving quality of colonoscopy by adding simethicone to sodium phosphate bowel preparation. World J Gastroenterol. 2009;15:3032-3037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 24. | Sudduth RH, DeAngelis S, Sherman KE, McNally PR. The effectiveness of simethicone in improving visibility during colonoscopy when given with a sodium phosphate solution: a double-bind randomized study. Gastrointest Endosc. 1995;42:413-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Liang PS, Williams JL, Dominitz JA, Corley DA, Zauber AG. Age-Stratified Prevalence and Predictors of Neoplasia Among U.S. Adults Undergoing Screening Colonoscopy in a National Endoscopy Registry. Gastroenterology. 2022;163:742-753.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Crockett SD, Ladabaum U. Potential Effects of Lowering Colorectal Cancer Screening Age to 45 Years on Colonoscopy Demand, Case Mix, and Adenoma Detection Rate. Gastroenterology. 2022;162:984-986.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Rex DK, Anderson JC, Butterly LF, Day LW, Dominitz JA, Kaltenbach T, Ladabaum U, Levin TR, Shaukat A, Achkar JP, Farraye FA, Kane SV, Shaheen NJ. Quality indicators for colonoscopy. Gastrointest Endosc. 2024;100:352-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/