Published online Jan 28, 2026. doi: 10.3748/wjg.v32.i4.114906
Revised: November 23, 2025
Accepted: December 18, 2025
Published online: January 28, 2026
Processing time: 113 Days and 5.3 Hours
Portal vein thrombosis (PVT) is a rare condition and is often associated with cir
A 67-year-old male without a prior history of cirrhosis or malignancy presented with acute abdominal pain and peritonitis. Contrast-enhanced computed to
Early low-dose catheter-directed thrombolysis provided a safe and effective treatment for a patient with noncirrhotic, nonmalignant PVT associated with me
Core Tip: Systemic anticoagulation is currently the first-line treatment for noncirrhotic portal vein thrombosis, and endo
- Citation: Chiu YC, Chang WC, Chiu YC. Early catheter-directed portal vein thrombolysis in myeloproliferative disorder-related diffuse mesenteric venous ischemia: A case report. World J Gastroenterol 2026; 32(4): 114906
- URL: https://www.wjgnet.com/1007-9327/full/v32/i4/114906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i4.114906
The portal vein collects venous return from all digestive organs and supplies 75% of the blood flow to the liver[1]. Portal vein thrombosis (PVT) is a rare condition with an incidence of 0.7-3.79 per 100000. Patients with noncirrhotic, non
Anticoagulation is the first-line therapy recommended for recent thrombi (i.e., those having presented within 6 months)[3]. Anticoagulation achieves recanalization in 30% of patients. Unfractionated heparin or low-molecular-weight heparin is initially prescribed, followed by vitamin K antagonist or direct oral anticoagulants, which have been shown to increase recanalization rates[4,5]. Alternative interventional therapies, including thrombus aspiration, catheter-direct thrombolysis (CDT), or transjugular intrahepatic portosystemic shunt, have been reported in selected patients with noncirrhotic PVT. Currently, the evidence for the success of these therapies remains limited, and comparative outcomes are unclear.
We presented a case in this report in which CDT was initiated within 1 day of PVT diagnosis. The patient made a complete recovery within 2 weeks, emphasizing the potential benefit of timely interventional therapy. We also performed a literature search of the terms “recent noncirrhotic portal vein thrombosis”, “direct thrombolysis”, and “endovascular treatment” to summarize the safety and efficacy of direct thrombolysis.
A 67-year-old male from Taiwan, who was an active smoker, presented to the emergency department with persistent right upper quadrant abdominal pain that had persisted for 3 days.
The patient denied experiencing nausea or vomiting. He reported normal bowel movements with smooth defecation noted the day before admission.
The patient had a history of coronary artery disease. He had undergone coronary artery stenting in 2016 but was lost to follow-up for 5 years. At the time of admission, he was not taking any medication. He had no history of hepatic disease or malignancy.
The patient’s family history was unremarkable.
The patient weighed 86 kg. Upon presentation his vital signs were as follows: Temperature: 36.6 °C; blood pressure: 156/116 mmHg; heart rate: 102 beats/minute; and respiratory rate: 18 breaths/minute. Abdominal examination revealed diffuse tenderness that was greatest in the right upper quadrant. This tenderness improved after administering analgesia. No rebound tenderness or muscle guarding was detected. Bowel sounds were decreased, and the liver and spleen were not palpable.
Serology revealed hyperbilirubinemia [total bilirubin concentration: 2.3 mg/dL (normal range: 0.3-1.0 mg/dL)] and leukocytosis [white blood cell: 14560/μL (normal range: 4500-11000 μL); neutrophil count: 78.5% (normal range: 40.0%-74.0%)]. Fibrinogen levels were elevated to 501.6 mg/dL (normal range: 200.0-400.0 mg/dL), and D-dimer levels were markedly increased [> 100000 ng/mL (normal range: < 0.5 ng/mL)]. Creatine kinase and lactate levels were within normal limits. The prothrombin time and partial thromboplastin time were normal. The patient’s procalcitonin level was mildly elevated [0.63 ng/mL (normal range: < 0.05 ng/mL)].
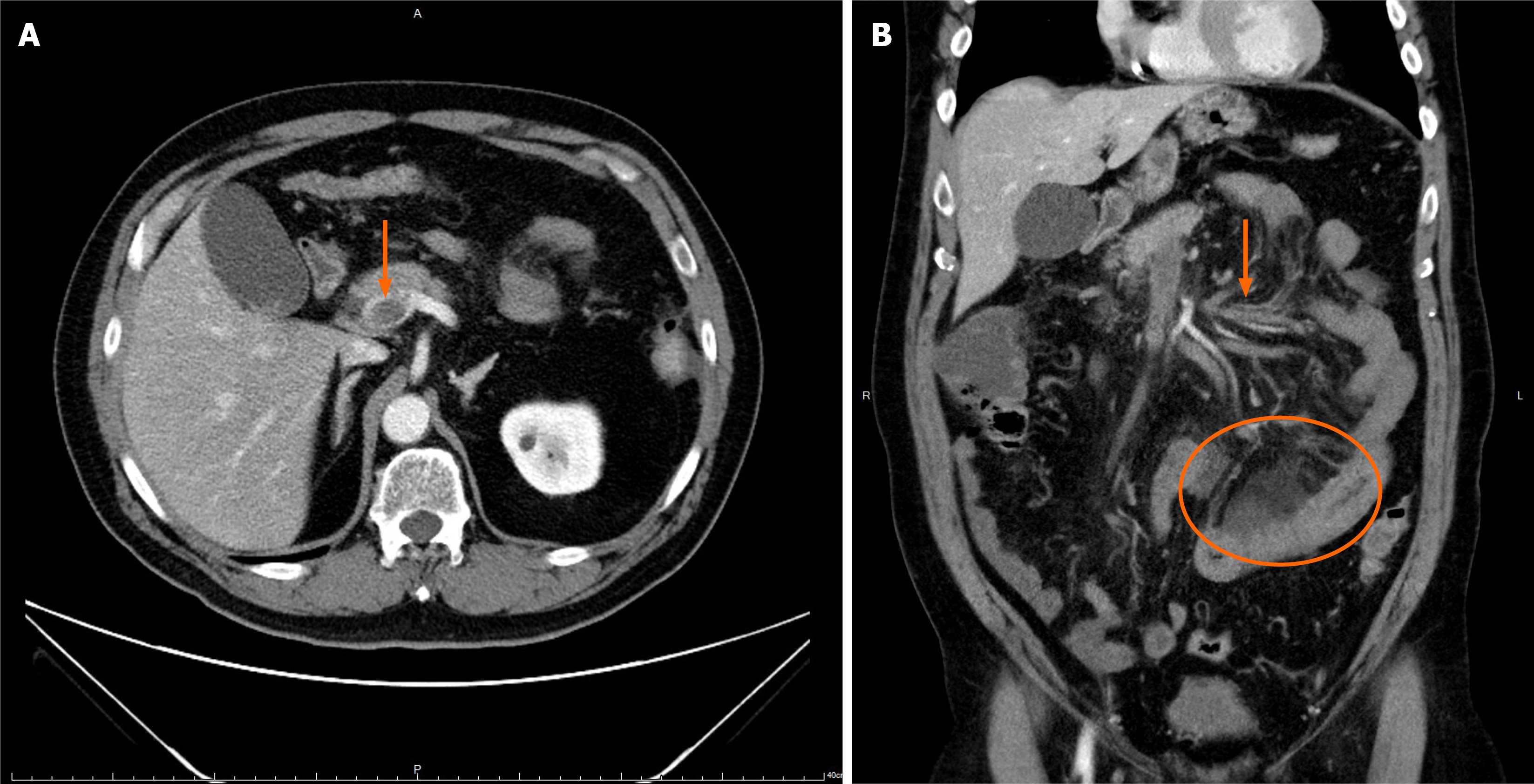
Contrast-enhanced abdominal computed tomography (CT) demonstrated a completely occlusive PVT extending into the superior mesenteric vein without evidence of cavernous transformation. Segmental small bowel wall thickening with fatty stranding, suggestive of an ischemic process, was observed (Figure 1).
A multidisciplinary discussion was conducted involving the general surgery, radiology, and hematology teams. The radiology team recommended and performed CDT. They also provided serial venography during hospitalization to evaluate the therapeutic response and guide the duration of the infusion. The hematology team was consulted for diagnosis and management of the underlying myeloproliferative disorder. The coordinated input from these specialty teams optimized the patient’s treatment strategy while monitoring potential complications.
The patient was diagnosed with PVT and imminent ischemic bowel secondary to JAK2 V617F mutation-related polycythemia vera after a comprehensive hypercoagulability survey.
Systemic anticoagulation with unfractionated heparin was initiated immediately, targeting an activated partial thromboplastin time of 50-70 seconds. Due to persistent symptoms, interventional radiology was consulted the day after admission. The general surgeon recommended CDT and discussion with the radiologist led to the latter performing placement of a 4.0 Fr Fountain catheter in the superior mesenteric vein using a transhepatic approach. A continuous urokinase infusion was initiated at a rate of 12000 U/hour. Fibrinogen levels were closely monitored to assess the blee
| Parameter | Time to treatment initiation (days) | ||||||||||
| D - 0 | D + 1 | D + 2 | D + 3 | D + 4 | D + 5 | D + 6 | D + 7 | D + 8 | D + 13 | D + 15 | |
| CDT with urokinase (U/hour) | 12000 | 12000 | 12000 | 14000 | 16000 | 16000 | 16000 | 12000 | 10000 | Off | |
| Systemic anticoagulant | |||||||||||
| Heparin pump (U/hour) | 440 | 440 | 320 | 320 | 200 | Off | |||||
| Oral apixaban | 2.5 mg twice/day | ||||||||||
| Fibrinogen (mg/dL) | 684 | 501 | 624 | 624 | 551 | 342 | 460 | 668 | |||
| APTT (second) | 32.8 | 44.9 | 38.2 | 35.9 | 39.6 | 41.5 | 41.3 | 36.0 | 45.5 | 39.8 | |
| Lactate (mmol/L) | 2.2 | 2.5 | 1.6 | 1.0 | 0.7 | 0.9 | |||||
| CK (U/L) | 57 | 47 | 220 | 195 | 76 | 48 | |||||
| WBC (103/μL) | 12480 | 14180 | 12030 | 10830 | 10720 | 8980 | 11110 | 9140 | 8570 | ||
| Hemoglobin (g/dL) | 18.8 | 17.0 | 13.7 | 13.5 | 13.4 | 12.4 | 13.7 | 11.1 | 11.4 | ||
| CRP (mg/dL) | 11.10 | 10.58 | 1.77 | 3.85 | 4.30 | ||||||
| Imaging | Abdominal CT | Venography | Venography | ||||||||
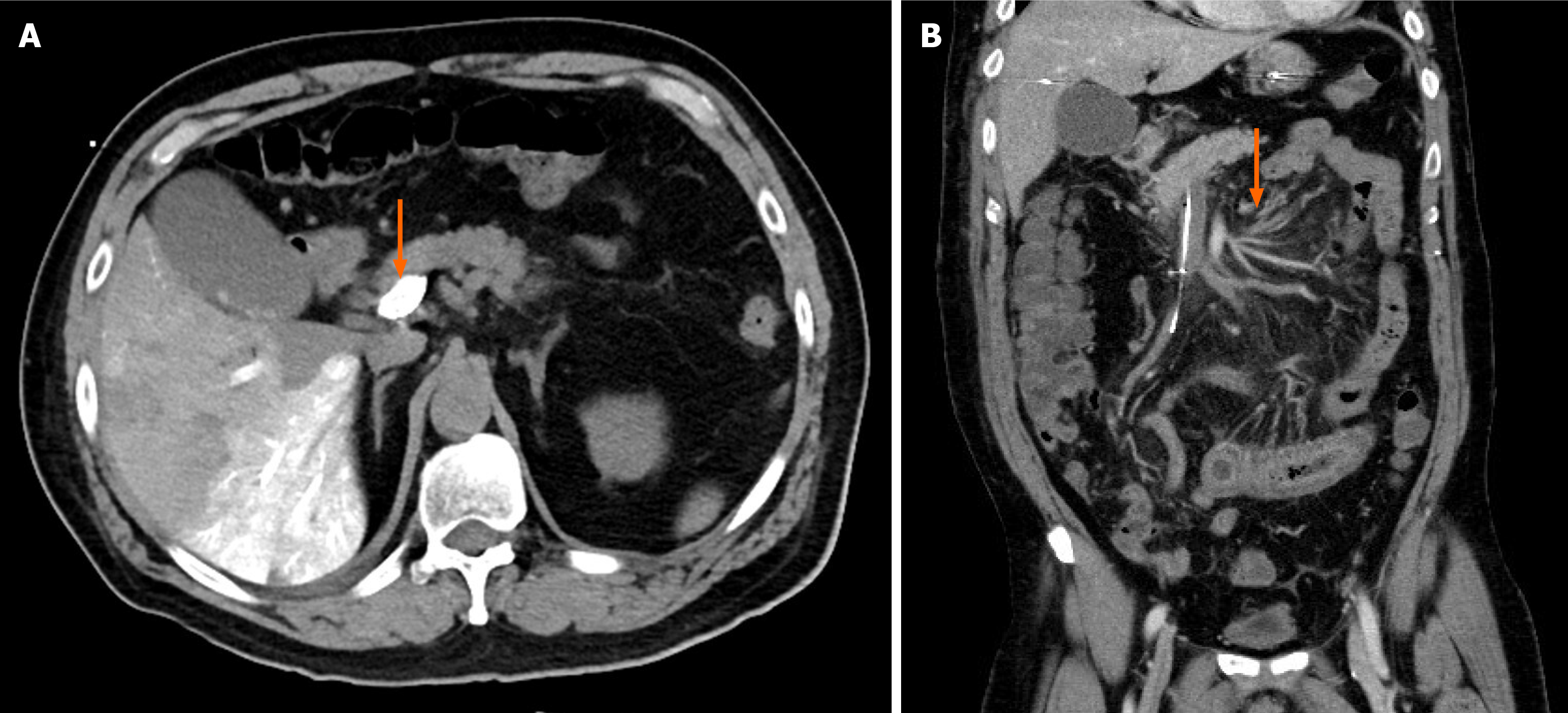
The patient experienced a self-limited episode of melena, resolving after urokinase tapering. His abdominal pain also subsided. The fibrinogen concentration decreased steadily throughout the course of treatment but remained above 100 mg/dL. A follow-up portal vein venography demonstrated portal vein patency with residual filling defects in the superior mesenteric vein branches, indicating partial thrombus resolution (Figure 2). After completing 14 days of CDT, the patient tolerated a soft diet and resumed normal bowel function. He was discharged on a therapeutic regimen of clopidogrel and apixaban.
Hematological evaluation confirmed a JAK2 V617F mutation, establishing polycythemia vera as the underlying pro
Noncirrhotic, nonmalignant PVT tends to present as a more acute course compared with cirrhotic PVT. Acquired hematological disorders account for up to 32% of extrahepatic PVT cases in Western and Asian populations[6]. Among these disorders myeloproliferative neoplasms with JAK2 V617F mutations are the most significant risk factors. Screening for this mutation is recommended for all adult patients who present with splanchnic vein thrombosis[1].
Although systemic anticoagulation is the standard first-line therapy for acute noncirrhotic PVT, patients with signs of impending bowel ischemia require advanced interventions such as CDT or thrombectomy[2]. Surgical resection of necrotic bowel is necessary after failure to achieve timely reperfusion. Complete recanalization is achieved in 30% of patients with acute PVT after treatment with systemic anticoagulation[2]. Factors associated with treatment failure include the onset of thrombosis, the presence of ascites, splenic vein involvement, and underlying prothrombotic disorders[4]. Endovascular therapy is typically a rescue option after anticoagulation fails, but the risk of bleeding complications remains the primary concern[2].
Recent studies have reported improved safety of endovascular therapy due to refined techniques and the adoption of lower-dose thrombolysis regimens. Our literature search identified six relevant studies: Five retrospective series and one prospective study (Table 2)[7-12]. Although endovascular therapy achieved higher complete recanalization rates (48%-70%) than anticoagulation alone (particularly when performed within 10 days of diagnosis), this benefit was offset by a significant risk of major bleeding. The thrombolytic regimens varied across the studies [urokinase: 20000-100000 IU/hour; recombinant tissue plasminogen activator (rtPA): 0.5-1.0 mg/hour; duration 2-14 days]. Complications have been reported in 19.1%-37.5% of patients, especially with higher-dose rtPA regimens. In response to this risk, lower-dose thrombolytic protocols have been developed and appear safer. One study used a sequential protocol of urokinase (20000 IU/hour, first day) followed by rtPA (0.5 mg/hour) and reported a 52% recanalization rate. Similarly, three additional studies[7,8,10] reported that low-dose urokinase regimens were associated with fewer complications[13] while maintaining acceptable efficacy (Table 2).
| Ref. | Case | MPD (%) | Total occlusive PVT | CDT timing (day) | CDT | Complete recanalization rate, AC/CDT (%) | Failure rate, AC/CDT (%) | Bleeding complications[13] | |||
| Transhepatic approach | TIPS | Grade 1/2, AC/CDT (%) | Grade 3/4, AC/CDT (%) | Death, AC/CDT (%) | |||||||
| Rössle et al[7], 2020 | 65 | 23 | 52 | 8.9 | 35 | 30.0/54.0 | 15.0/14.3 | 0/22.5 | 0/2.5 | 0/0 | |
| Benmassaoud et al[8], 2019 | 22 | 22.7 | 17 | 4 | 11 | 13.7/70.0 | 63.6/9.1 | Total 318 | 0/18.1 | 0/0 | |
| Wu et al[9], 2025 | 25 | 12 | 18 | 6 | 25 | -/48 | -/16 | -/16 | 0/0 | 0/0 | |
| Klinger et al[10], 2017 | 29 | 20.7 | 16 | 7 | 17 | -/52.9 | -/11.8 | -/5.9 | -/0 | -/0 | |
| Mansour et al[11], 2022 | 21 | 14.3 | 14 | 13 | 9 | 12 | -/35.0 | 9.5 | -/23.8 | -/19.0 | -/1.0 |
| Gerwing et al[12], 2019 | 8 | 0 | 5 | 0 | 6 | 2 | -/62.5 | -/12.5 | -/12.5 | -/37.5 | -/1.0 |
In our case, several factors contributed to the successful outcome. First, CDT was initiated immediately upon recognition of PVT and clinical signs suggesting early bowel ischemia rather than waiting until systemic anticoagulation failed. Most published studies reported CDT as a rescue intervention after deterioration or nonresponse to anticoagulation, a scenario in which bowel ischemia can already be advanced and higher-dose thrombolytic regimens are required. Early intervention before irreversible mesenteric injury occurred allowed the use of a relatively low-dose thrombolytic protocol while still achieving effective reperfusion in our patient. This approach likely reduced the bleeding risk com
Two studies identified in our literature search specifically compared percutaneous and transjugular approaches for CDT in acute noncirrhotic PVT[11,12]. The transjugular intrahepatic portosystemic shunt approach demonstrated higher rates of complete recanalization and fewer severe adverse events. However, differences in major bleeding did not reach statistical significance. There may be a safety and efficacy advantage of the transjugular approach, but current evidence remains limited and heterogeneous. Further comparative studies are needed to clarify the optimal access strategies and patient selection criteria for CDT.
Although our patient had a favorable outcome, this case highlighted the significant challenges that remain in managing acute noncirrhotic PVT. The choice of a low-dose urokinase regimen and a transhepatic approach was guided by institutional experience and the limited current evidence from small, retrospective studies. Further large-scale, multicenter prospective trials are urgently needed to establish optimal treatment strategies, including ideal thrombolysis dosages and intervention timing.
Early transhepatic CDT with low-dose urokinase infusion can be an effective and safe intervention for completely occlusive PVT with impending bowel ischemia, potentially avoiding extensive bowel resection. However, further prospective studies are urgently needed to determine the optimal dosage and timing for this intervention.
| 1. | Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG Clinical Guideline: Disorders of the Hepatic and Mesenteric Circulation. Am J Gastroenterol. 2020;115:18-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Willington AJ, Tripathi D. Current concepts in the management of non-cirrhotic non-malignant portal vein thrombosis. World J Hepatol. 2024;16:751-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1871] [Article Influence: 467.8] [Reference Citation Analysis (3)] |
| 4. | Elkrief L, Payancé A, Plessier A, d'Alteroche L, Ronot M, Paradis V, Valla D, Rautou PE. Management of splanchnic vein thrombosis. JHEP Rep. 2023;5:100667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Monaco G, Bucherini L, Stefanini B, Piscaglia F, Foschi FG, Ielasi L. Direct oral anticoagulants for the treatment of splanchnic vein thrombosis: A state of art. World J Gastroenterol. 2023;29:4962-4974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Boccatonda A, Gentilini S, Zanata E, Simion C, Serra C, Simioni P, Piscaglia F, Campello E, Ageno W. Portal Vein Thrombosis: State-of-the-Art Review. J Clin Med. 2024;13:1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 7. | Rössle M, Bettinger D, Trebicka J, Klinger C, Praktiknjo M, Sturm L, Caca K, Mücke VT, Radecke K, Engelmann C, Zipprich A, Heinzow H, Meyer C, Tappe U, Appenrodt B, Schmidt A, Lange C, Strassburg C, Zeuzem S, Grandt D, Schmidt H, Moessner J, Berg T, Lammert F, Thimme R, Schultheiß M. A prospective, multicentre study in acute non-cirrhotic, non-malignant portal vein thrombosis: comparison of medical and interventional treatment. Aliment Pharmacol Ther. 2020;52:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Benmassaoud A, AlRubaiy L, Yu D, Chowdary P, Sekhar M, Parikh P, Finkel J, See TC, O'Beirne J, Leithead JA, Patch D. A stepwise thrombolysis regimen in the management of acute portal vein thrombosis in patients with evidence of intestinal ischaemia. Aliment Pharmacol Ther. 2019;50:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Wu B, Yang W, Xie Y, Zhou H, Shi H, Liu S, Zhou W. Transjugular intrahepatic portosystemic shunt combined with dual-access thrombolysis for acute severe non-cirrhotic portal-mesenteric vein thrombosis. Dig Liver Dis. 2025;57:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Klinger C, Riecken B, Schmidt A, De Gottardi A, Meier B, Bosch J, Caca K. Transjugular local thrombolysis with/without TIPS in patients with acute non-cirrhotic, non-malignant portal vein thrombosis. Dig Liver Dis. 2017;49:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Mansour N, Öcal O, Gerwing M, Köhler M, Deniz S, Heinzow H, Steib C, Angele MK, Seidensticker M, Ricke J, Wildgruber M. Interventional recanalization therapy in patients with non-cirrhotic, non-malignant portal vein thrombosis: comparison between transjugular versus transhepatic access. Abdom Radiol (NY). 2022;47:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Gerwing M, Wilms C, Heinzow H, Sporns PB, Heindel W, Schmidt H, Wildgruber M, Köhler M. Escalating interventional recanalization therapy in non-cirrhotic, non-malignant acute portal vein thrombosis. Eur J Gastroenterol Hepatol. 2019;31:1584-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26200] [Article Influence: 1190.9] [Reference Citation Analysis (2)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/