Published online Feb 14, 2025. doi: 10.3748/wjg.v31.i6.96782
Revised: October 25, 2024
Accepted: December 11, 2024
Published online: February 14, 2025
Processing time: 240 Days and 1.7 Hours
Mixed lineage kinase domain-like protein (MLKL) serves as a critical mediator in necroptosis, a form of regulated cell death linked to various liver diseases. This study aims to specifically investigate the role of MLKL’s adenosine triphosphate (ATP)-binding pocket in facilitating necroptosis-independent pathways that may contribute to liver disease progression. By focusing on this mechanism, we seek to identify potential therapeutic targets that can modulate MLKL activity, offering new strategies for the prevention and treatment of liver-related pathologies.
To investigate the possibility of using the ATP-binding pocket-associated, necro
Cell death following necroptosis stimuli was evaluated using cell proliferation assays, flow cytometry, and electron microscopy in various cells. The human liver organoid system was used to evaluate whether the MLKL ATP pocket-binding inhibitor could attenuate inflammation. Additionally, alcoholic and non-alcoholic fatty liver diseases animal models were used to determine whether MLKL ATP pocket inhibitors could attenuate liver injury.
While an MLKL ATP pocket-binding inhibitor did not prevent necroptosis-induced cell death in RAW 264.7 cells, it did reduce the necroptosis-led expression of CXCL2, ICAM, and VCAM. Notably, MLKL ATP pocket inhibitor diminishes the expression of CXCL2, ICAM, and VCAM by inhibiting the IκB kinase and nuclear factor kappa-B pathways without inducing necroptosis-induced cell death in two-dimensional cell culture as well as the human-derived liver organoid system. Although MLKL ATP-binding inhibitor was ineffective in non-alcoholic fatty liver disease animal models, MLKL ATP-binding inhibitor attenuated hepatic inflammation in the alcoholic liver disease model.
MLKL ATP pocket-binding inhibitor exerted anti-inflammatory effects through the necroptosis-independent MLKL pathway in an animal model of alcoholic liver disease.
Core Tip: Targeting the adenosine triphosphate-binding pocket of mixed lineage kinase domain-like protein may provide a novel therapeutic strategy for reducing inflammation in alcoholic liver disease, independent of its role in necroptosis, highlighting its potential as a unique target in liver disease management.
- Citation: Xuan Yuan HN, Kim HS, Park GR, Ryu JE, Kim JE, Kang IY, Kim HY, Lee SM, Oh JH, Yoon EL, Jun DW. Adenosine triphosphate-binding pocket inhibitor for mixed lineage kinase domain-like protein attenuated alcoholic liver disease via necroptosis-independent pathway. World J Gastroenterol 2025; 31(6): 96782
- URL: https://www.wjgnet.com/1007-9327/full/v31/i6/96782.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i6.96782
Necroptosis is triggered by a complex formed by the binding of tumor necrosis factor (TNF) and the TNF receptor 1 Ligand. The activated receptor interacts with serine/threonine protein kinase 1 (RIPK1) to form complex IIb with receptor-interacting serine/threonine kinase 3 (RIPK3), which binds to the mixed lineage kinase domain-like protein (MLKL), an effector protein of the necroptosis pathway[1,2]. Phosphorylated MLKL (p-MLKL) then translocates to the nucleus and plasma membrane, leading to membrane puncture, rupture, and death[3,4]. Many studies have shown that MLKL is associated with various liver diseases, including non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and liver fibrosis[5-7]. According to a published report, the mRNA levels of RIPK3 and phosphorylated MLKL are increased in the liver tissue of patients with NASH. RIPK3 deletion significantly reduces liver fibrosis[8]. In addition, RIPK3 deficiency reduces steatosis, inflammation, and fibrosis in a NAFLD model induced by a methionine-choline-deficient diet[9-11].
MLKL is known to be a key molecule of necroptosis and the “RIPK3–MLKL” axis is recognized as a key pathway of necroptosis-induced cell death. MLKL is the most important protein for the necroptosis pathway and is also a novel target for various liver diseases[12,13]. However, an effective drug targeting MLKL has not yet been developed. MLKL-mediated necroptosis results from oligomerization of MLKL at the 86th cysteine (Cys86) of the N-terminus. Therefore, MLKL targets have so far focused on Cys86 in the N-terminus. However, the presence of Cys86 in the N-terminus of MLKL has several limitations. First, the interspecies variations in Cys86 of N-terminus is too high. Most animals lack a cysteine at the 86th position in the N-terminus. Second, cysteine-based covalent-binding compounds have difficulty avoiding non-specific binding. Therefore, other researchers focused on the adenosine triphosphate (ATP)-binding pocket instead of the N-terminal Cys86 residue in MLKL. But, the inhibition of the MLKL ATP pocket did not inhibit necroptosis in previous studies[14].
MLKL has been considered a key molecule in necroptosis-induced cell death triggered by TNF-α stimulation[15]. However, recent studies have revealed that the outcomes of TNF-α-induced necroptosis stimulation vary significantly depending on the cell type. According to the study by Oh et al[4], among the cells that constitute the liver, necroptosis stimuli induce cell death in hepatocytes and inflammatory cells, while stellate cells evade cell death and instead undergo activation. This mechanism of cell death evasion is thought to be associated with nuclear factor kappa-B (NF-κB) activation.
So, our hypothesis is whether ATP pocket inhibitor can regulate inflammation via necroptosis independent pathway[4]. In this study, we investigated the role of the ATP-binding pocket of MLKL as a possible novel target of MLKL. The purpose of this study was to investigate the necroptosis-independent role of MLKL and the role of the MLKL ATP-binding pocket in this cell death-independent pathway.
This study presents several significant advancements compared to previous research on MLKL.
In contrast to earlier studies that primarily focused on the role of MLKL in mediating necroptosis and resulting cell death, this research investigates the ATP-binding pocket of MLKL as a potential target for regulating inflammation without triggering necroptosis. This shift highlights the investigation of MLKL’s functions beyond cell death, suggesting that it may play a critical role in inflammatory processes independently of its traditional necroptotic functions.
Furthermore, we employed a variety of animal models, including both alcoholic and NAFLD models, to rigorously test and validate the anti-inflammatory effects of the MLKL ATP-binding inhibitor, compound-4 (CPD4). This approach allows for a thorough examination of the compound’s efficacy across different pathological contexts, providing stronger evidence for its potential therapeutic applications in liver diseases.
Cell death after necroptosis stimuli was evaluated using cell proliferation assay, flow cytometry, and electron microscopy in various cells. The human liver organoid system was used to evaluate whether MLKL ATP pocket binding inhibitor can attenuate inflammation although it does not inhibit necroptosis induced cell death caused by necroptosis stimulation. Additionally, alcoholic and fatty liver animal models were conducted to determine whether MLKL ATP pocket inhibitor could attenuate liver injury.
RAW 264.7 (TIB-71; American Type Culture Collection) mouse macrophages were cultured in Dulbecco’s Modified Eagle’s medium, a low-glucose medium containing 10% fetal bovine serum and 1% penicillin. When the cell density exceeded 70%, subculturing was performed. The original medium was removed, and the cells on top were scraped off using a cell scraper. After adding a new medium, centrifugation was performed at 125 r for 5 minutes. The supernatant was removed, and 3 mL of medium was added for mixing. The usual passage ratio was 1:3, a 150-cm dish was used for subculture, and the culture medium was replaced once every 2-3 days. Thereafter, RAW 264.7 were seeded onto a 96-well plate the number of cells per well was 3 × 103, and the cells were treated with 5-aza-2’-deoxycytidine (5AD), (189825, Sigma-Aldrich; 20, 10, 5, 2.5, or 1 μmol/L) for 48 hours for demethylation.
The cells were treated with the SMAC mimetics (Selleck Chemicals ,Houston, TX, United States; S7597, 2 μmol/L), Z-VAD-FMK (R and D Systems, United States; FMK001, 20 μmol/L), and TNF-α (10 ng/mL; R and D Systems; 210-TA). After 24 hours, the cells were harvested for protein extraction, RNA extraction, immunofluorescence, cell viability, incucyte, and flow cytometry analysis.
The human-derived organoids were cultured as follows: Human liver specimens were minced and incubated with a digestion solution at 37 °C. The suspension was separated by filtration and centrifugation. The pellets were washed and mixed with Matrigel (CORNING, NY, United States). Cell clusters were then seeded onto plates. Thereafter, the organoid culture medium was added to the cells. Additionally, it contained recombinant human epidermal growth factor (Peprotech, Cranbury, NJ, United States), recombinant human growth factor (Peprotech), and forskolin (Selleck Chemicals). The culture medium was changed twice a week. To evaluate the anti-inflammatory efficacy of the MLKL ATP-binding inhibitor in a steatohepatitis liver organoid model, the steatohepatitis-induced organoid was treated with CPD4 30 µm for 1 day after necroptotic stimuli. Polymerase chain reaction (PCR) and immunochemical staining were performed to determine the degree of inflammation and fibrosis in the liver organoid system.
After seeding in 96-well plates, RAW 264.7 mouse macrophages were divided into seven groups according to treatment: Control [dimethyl sulfoxide (DMSO) only], (SMAC mimetics + ZVAD + TNF-α)-treated, 20, 10, 5, 2.5, or 1 μM 5AD-treated groups. After demethylation with 5AD for 48 hours, necroptosis was induced for 24 hours. Subsequently, the viability of RAW 264.7 mouse macrophages was tested using a cell counting kit (Dojindo, Japan). After adding 10 μL solution, the cells were incubated for 2 hours. Cell viability were assessed using a spectrophotometer (Microplate Manager Software, Bio-Rad, Japan), and absorbance was measured at 450 nm.
RAW 264.7 and HT29 cells were seeded onto 96-well plates with 100 µL growth medium, at a density of 4 × 103 cells/ per well. The cells were then treated with SMAC mimetic (Selleck Chemicals; S7597, 2 μmol/L), zvad-fmk (R and D Systems; FMK001, 20 μmol/L), and TNF-α (10 ng/mL; R and D Systems, 210-TA). The 96-well plate was inserted into the Incucyte (MCO-230AIC; PHCbi, Japan) for real-time detection of cell activity. Four images were obtained from each well at 4 × magnification every 24 hours for 72 hours. Subsequently, each image was saved and analyzed.
Changes in the activities of RAW 264.7 and HT29 cells after induction of necroptosis were determined by BD Biosciences Pharmingen™ fluorescein Isothiocyanate (FITC) Annexin V apoptosis detection kit I (556547; Korea) double staining with a live/dead assay. In a 24-well plate, RAW 264.7 and HT29 cells were seeded (3 × 105 cells) in a supplementary medium and incubated for 24 hours. Subsequently, necroptotic stimuli were applied to the cells for 24 hours. Thereafter, propidium iodide (PI) and Annexin V double staining were performed, and PI/FITC live/read were evaluated using fluorescence-activated cell sorting Canto Anto II flow cytometry (BD Biosciences) at the Biospecimen-Multiomics Digital Bioanalysis Core Facility of Hanyang University.
RAW 264.7 and HT29 cells were seeded in a 24-well plate (1 × 104 cells/well), incubated for 24 hours, and treated with a concentration of SMAC mimetic (Selleck Chemicals; S7597, 2 μmol/L), zvad-fmk (R and D Systems; FMK001, 20 μmol/L), and TNF-α (10 ng/mL; R and D Systems; 210-TA) for 24 hours to induce necroptosis. After 24 hours, the cells were fixed at room temperature in 4% formaldehyde for 15 minutes. The cells were blocked with 3% bovine albumin (BSA) in Dulbecco’s phosphate-buffered saline for 1 hour. Then, the cells were incubated with the primary antibody P-MLKL (ab196436, 3% BSA, 1:1000) at 4 °C overnight. The following day, a secondary antibody (Alexa Fluor 488 goat anti-rabbit, IgG, A11008; Invitrogen, Carlsbad, CA, United States) was added. The immunolabeling procedure was followed by washing with PBS and incubation with 4′,6-diamidino-2-phenylindole (H-1500, Vector Laboratories, Burlingame, CA, United States). Finally, a confocal fluorescence microscope (TCS SP5, Leica) was used to capture representative images.
The Total mRNA of RAW 264.7 cells was isolated using TRIzol RNA isolation protocol (Invitrogen). A Nanodrop 2000 spectrophotometer (Thermo Scientific ND2000USCAN) was used to determine the RNA concentration. The purity of the RNA was determined using the A260/A280 values measured using a spectrophotometer. PrimeScript™ RT reagent kit (TaKaRa, Japan) was used to reverse-transcribe the RNA into cDNA. The prepared PCR solution was placed on a real-time PCR instrument for PCR amplification. The reaction conditions were: Pre-denaturation at 95 °C for 10 minutes, followed by 45 cycles at 60 °C for 10 seconds, 72 °C for 10 seconds, and 65 °C for 15 seconds. The quantification cycle value of each sample was automatically calculated using the LightCycler program. All tests were performed in triplicate. The measured values were normalized to those obtained for glyceraldehyde 3-phosphate dehydrogenase.
Proteins were extracted from the cells using radio immunoprecipitation assay cell lysis buffer containing ethylene diamine tetraacetic acid (1 ×) (R4100-010; Gendepot, United States). The centrifuge was pre-cooled to 4 °C (13000 RPM,
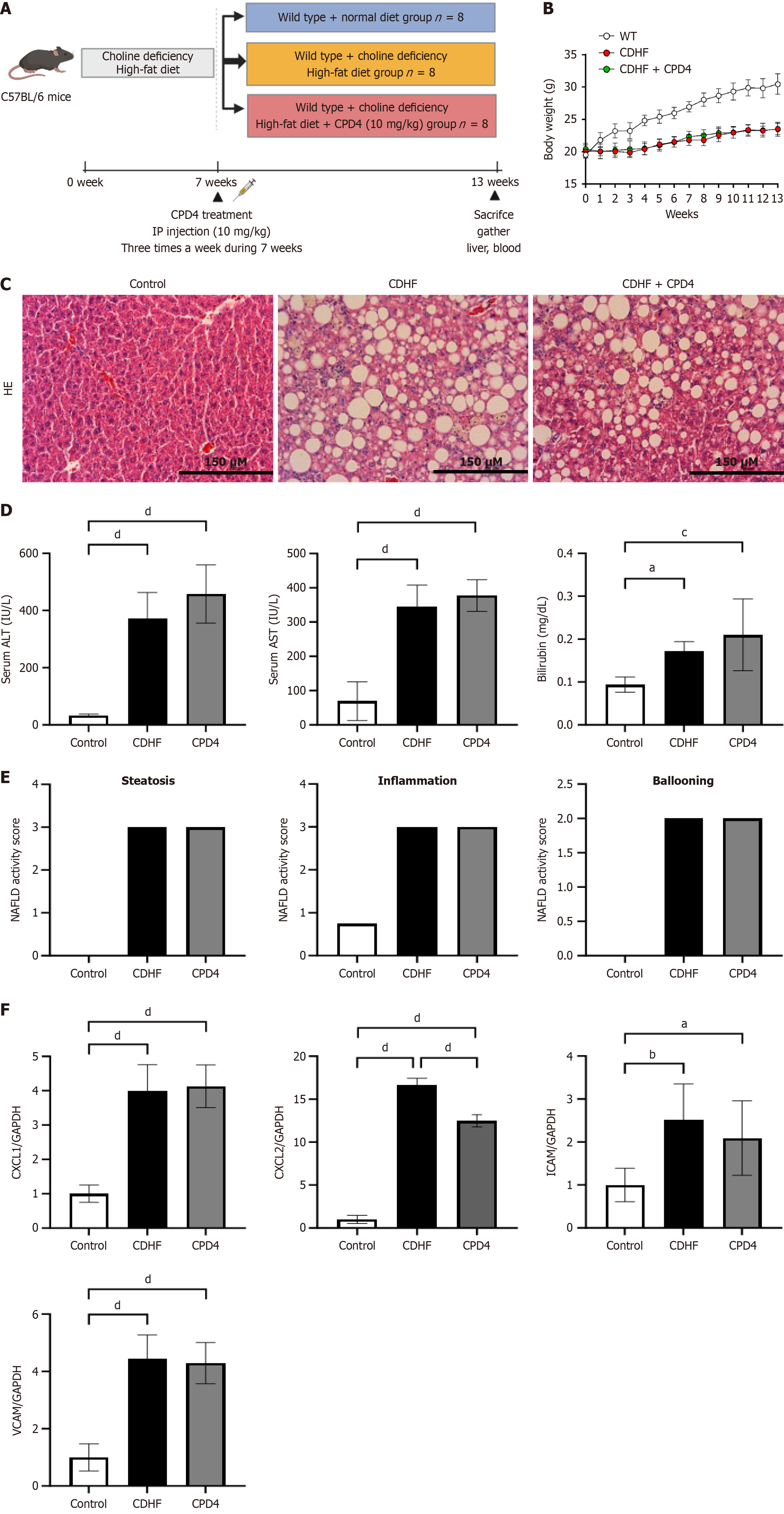
Six-week-old C57BL/6 mice (n = 8) were sourced from Central Lab Animal Inc. in Seoul, South Korea, and housed in the animal laboratory at Hanyang University School of Medicine. Prior to the start of the experiment, all mice underwent a one-week acclimatization period to minimize stress and ensure a stable environment. During this period, the mice were kept at a controlled temperature of 23 ± 2 °C with 55% ± 5% relative humidity. The facility maintained a specific pathogen-free environment, and a consistent light-dark cycle (12 hours light/12 hours dark) was implemented. All experimental procedures were approved by the Animal Committee of Hanyang University (approval numbers: HY-IACUC-2022-0217A and HY-IACUC-2023-0276A). To model NAFLD, the mice were fed a choline-deficient high-fat diet (CDHFD) for six weeks, which is known to induce hepatic steatosis and inflammation. Following the diet period, the MLKL ATP pocket-binding inhibitor (CPD4), which was dissolved in a solution of 10% DMSO and 90% corn oil, was administered via intraperitoneal injection at a dosage of 10 mg/kg. This treatment was given three times a week for a duration of seven weeks. Throughout the study, body weight was monitored weekly to assess overall health and response to treatment. At the conclusion of the experiment (week 13), all mice were humanely euthanized. Liver tissues and serum samples were collected for further analysis of inflammatory markers and histological examination, allowing for a comprehensive evaluation of the effects of the MLKL ATP pocket-binding inhibitor on liver pathology.
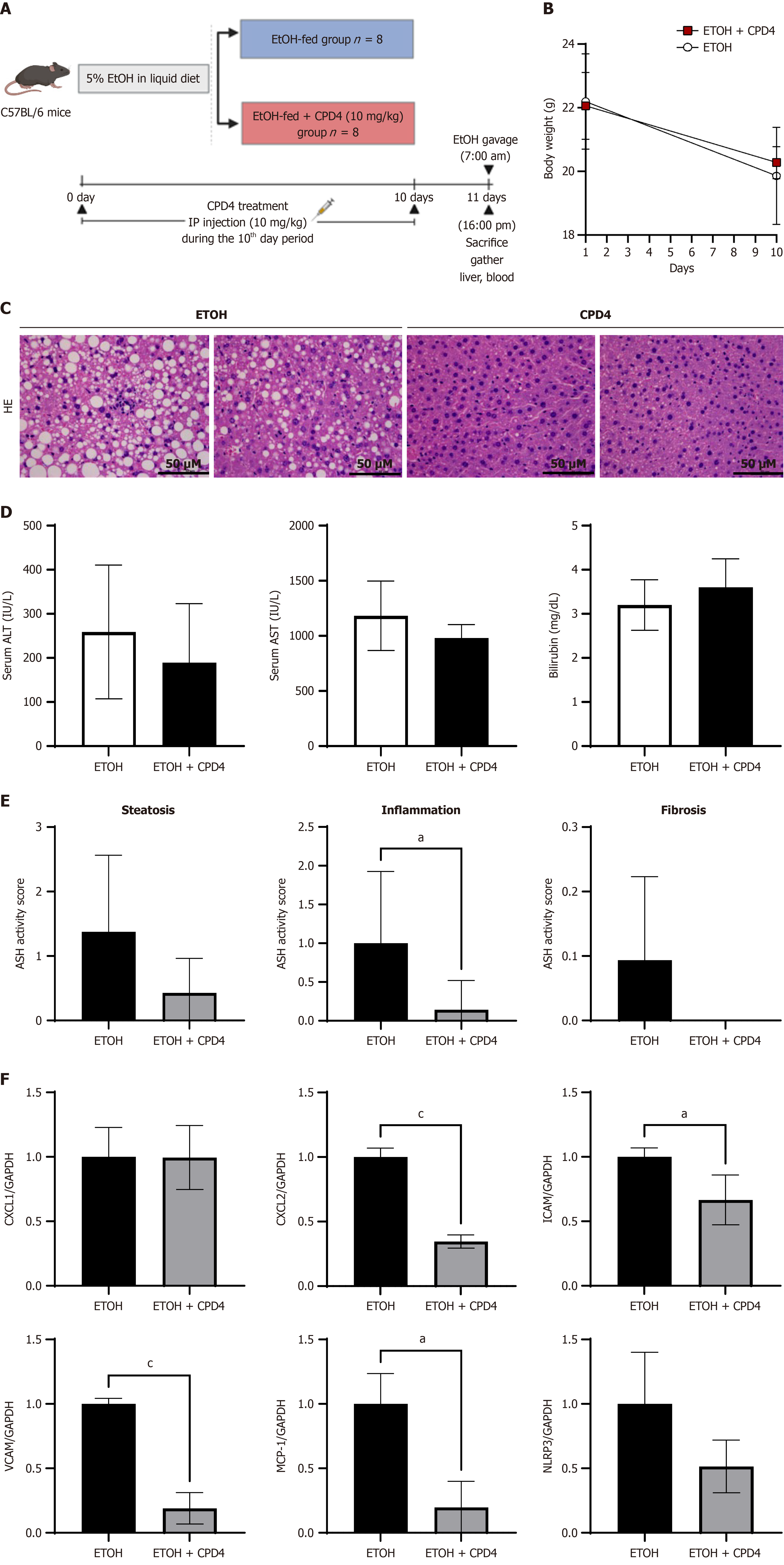
To establish an animal model of alcoholic liver disease, the mice were fed 5% ethanol. The mice received a 5% ethanol diet for 10 days, and the MLKL ATP pocket-binding inhibitor (10 mg/kg CPD4) was administered intraperitoneally every day for 10 days. On the 11th day at 7 AM, a binge dose of ethanol was administered orally (5 g/kg) for 9 hours before eutha
For hematoxylin-eosin (HE) staining, a 10% formalin solution was used to fix mouse liver tissue. Paraffin was used to embed the tissues, which were then cut into 4-μm thick slices. To evaluate fibrosis, the liver tissues were stained with picrosirius red solution (Abcam, Cambridge, MA, United States) for 25 minutes. A light microscope was used to capture photographs of the stained sections, and all histological data were evaluated by researchers.
Blood was collected from the heart by using an insulin syringe and stored in serum-separating tubes. The serum was collected via centrifugation at 3000 r for 20 minutes and then stored at -80 °C. Glucose, bilirubin, alanine transaminase (ALT), and aspartate transaminase (AST) levels were also measured.
Values are expressed as mean ± SD. Statistical analyses were performed using the GraphPad Prism 10 software (GraphPad Software, CA, United States). All experiments were repeated thrice. One-way analysis of variance and inde
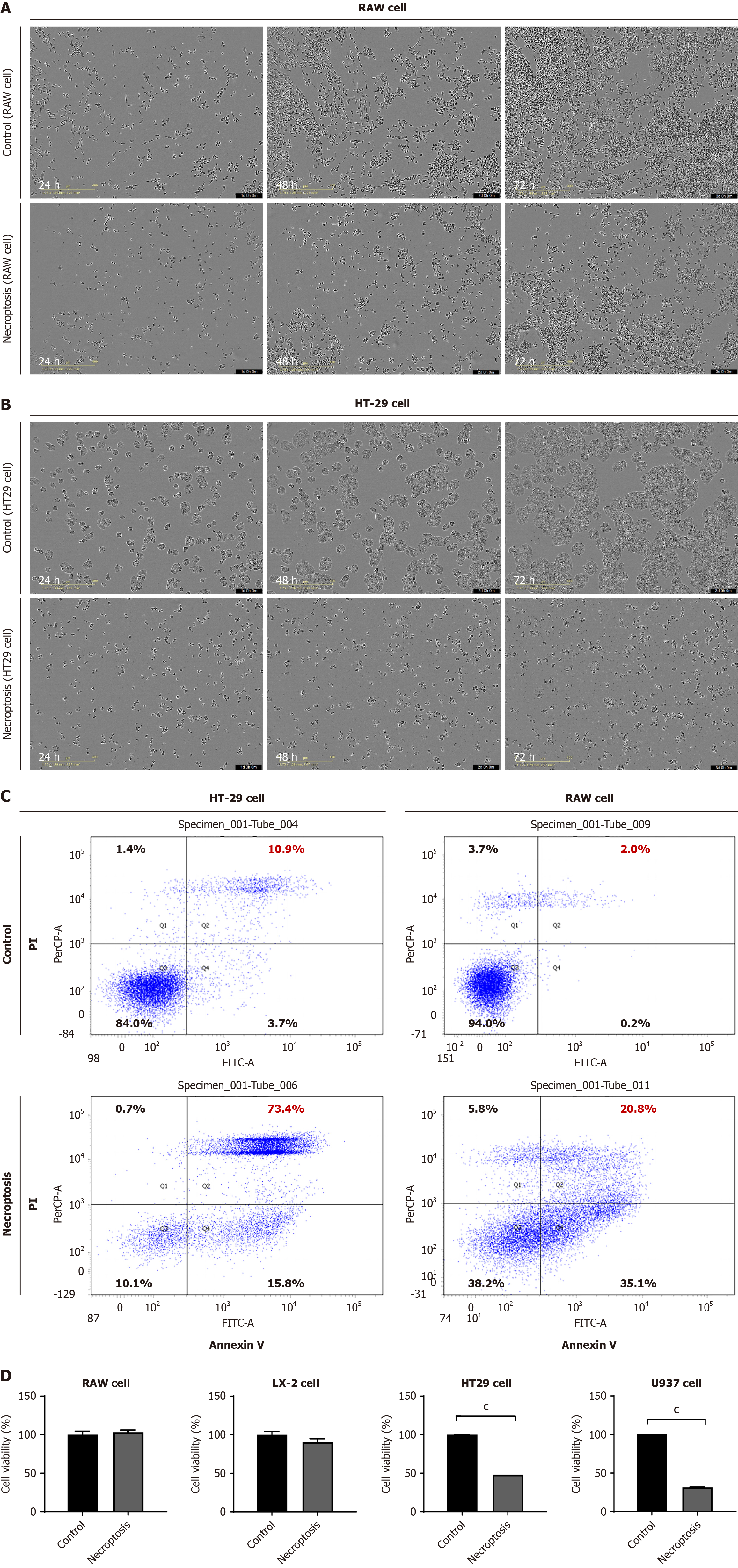
Although HT29 cells exhibited a noticeable decline in cell density and an increase in cell death after necroptotic stimuli, RAW 264.7 cells did not undergo cell death after necroptotic stimuli for over three days (Figure 1A and B). Flow cytometry analysis data indicated that the necroptotic cell death rate was notably lower in RAW 264.7 cells compared to HT29 cells following necroptotic stimuli (Figure 1C). In contrast, significant cell death was observed in U937 cells (human monocytes) upon necroptotic stimulation, whereas LX-2 cells did not exhibit cell death (Figure 1D). In summary, RAW 264.7 and LX-2 cells did not exhibit a pronounced increase in cell death following necroptosis, indicating a significant difference in the fate of cells after necroptotic stimuli based on cell type.
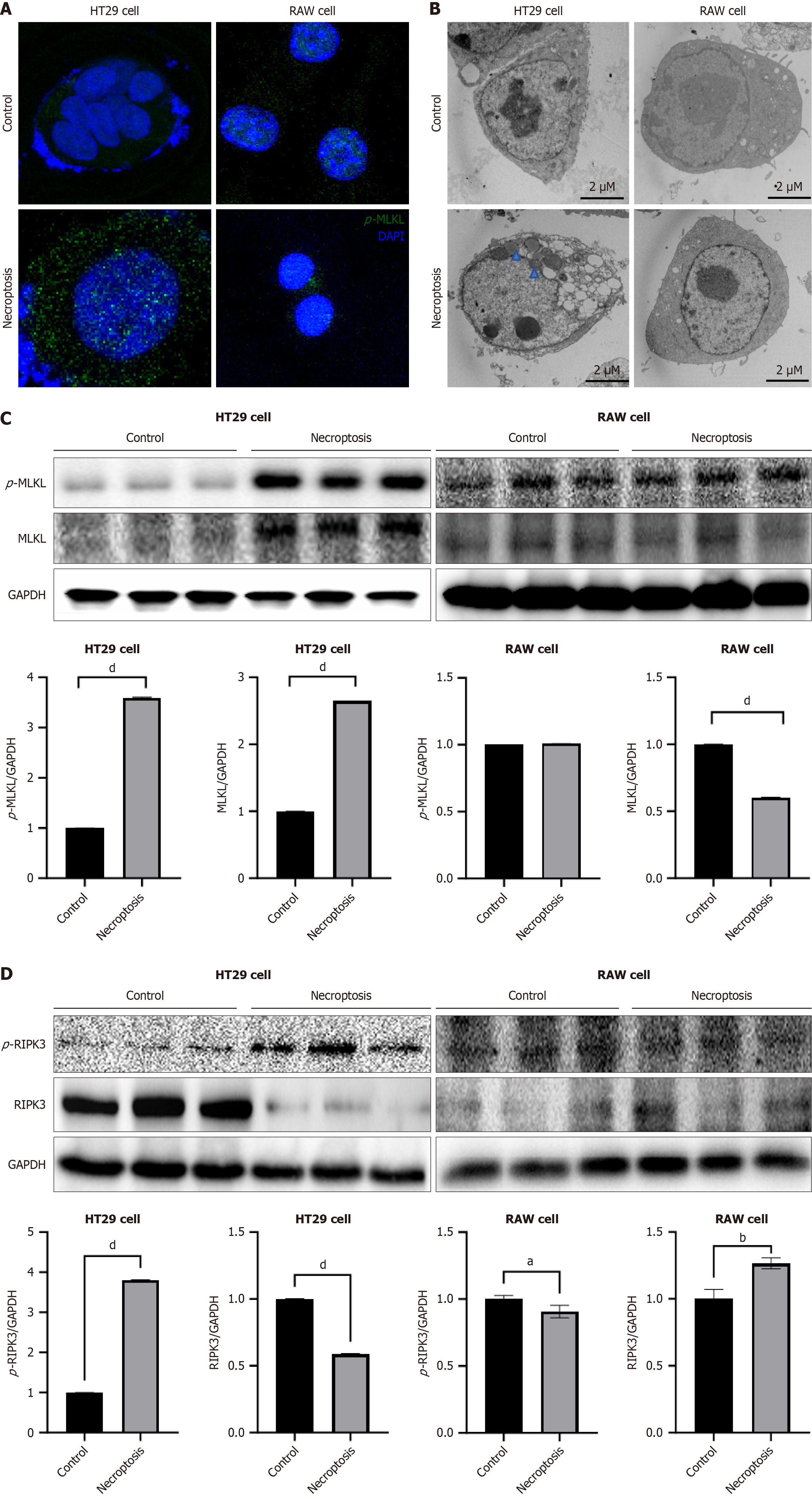
Next, we assessed the phosphorylation of MLKL and the intracellular localization of p-MLKL using immunofluorescence microscopy. The expression of p-MLKL in the cytoplasm of HT29 cells increased after necroptosis. In contrast, the expression of p-MLKL did not increase after the induction of necroptosis in RAW 264.7 cells (Figure 2A). We performed transmission electron microscopy 24 hours after inducing necroptosis in HT29 cells. Ruptured membranes, swollen mitochondria, and cytoplasmic vacuolization were observed in the HT29 cells. However, RAW 264.7 cells did not exhibit any morphological changes after necroptotic stimulation (Figure 2B). Western blot analysis demonstrated an elevated expression of p-MLKL and p-RIPK3 in HT29 cells following necroptotic stimulation. In contrast, the expression of p-MLKL and p-RIPK3 in RAW 264.7 cells did not increase after necroptotic stimulation (Figure 2C and D). Overall, necroptotic stimulation induced cell death in HT-29 cells in a p-MLKL-dependent manner but failed to induce phosphorylation of MLKL in RAW 264.7 cells.
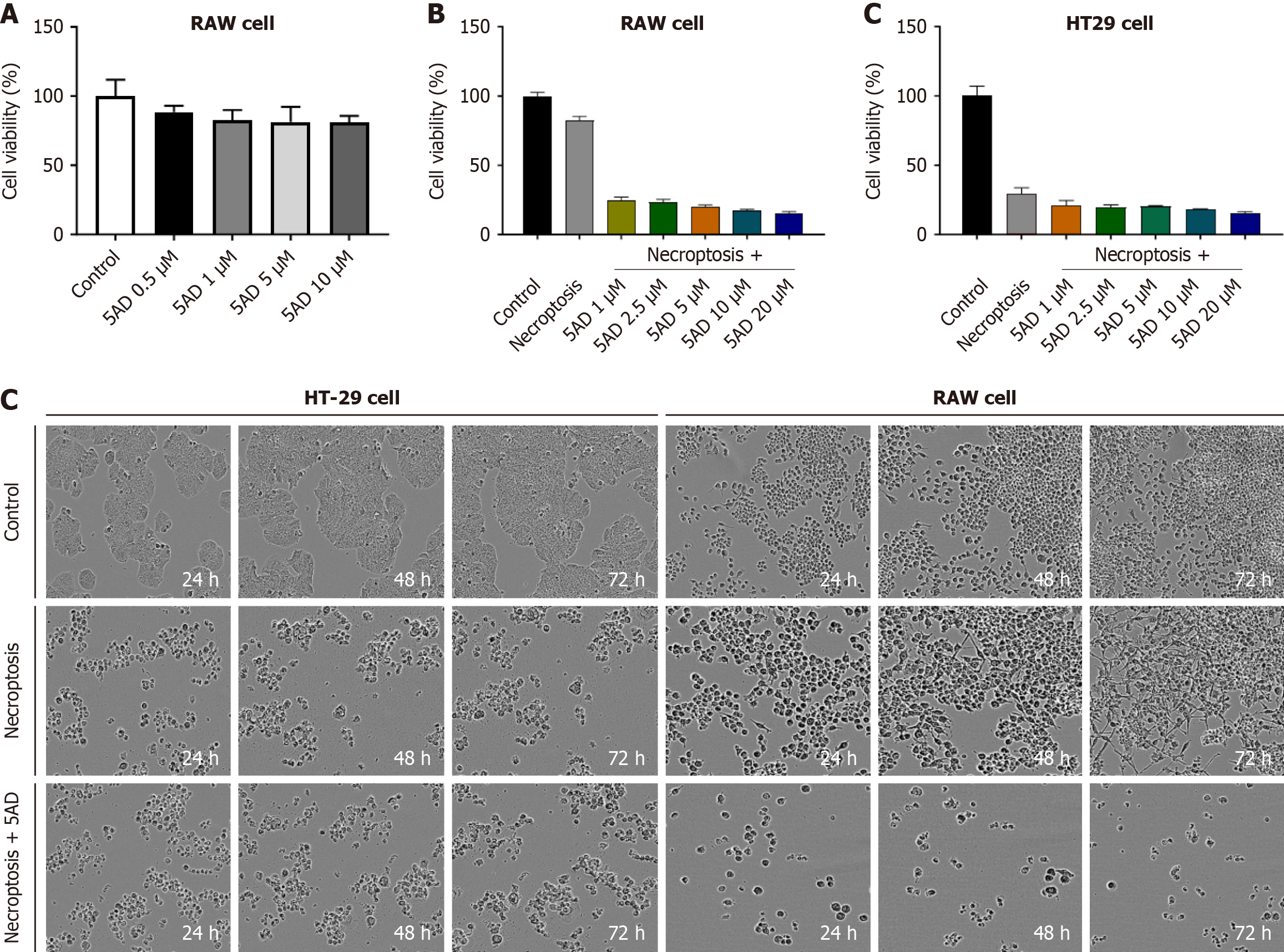
We initially conducted toxicity tests using the methylation inhibitor 5AD, also known as decitabine), an epigenetic drug that inhibits DNA methylation. This choice was based on a previous study indicating that high levels of methylation in liver cells do not lead to necroptosis. Our findings suggest that RAW 264.7 cells do not undergo cell death when stimulated for necroptosis, which we speculate is due to their high methylation status. The results demonstrated that, compared to the untreated group, 5AD did not show significant cytotoxicity at concentrations up to 10 μM (Figure 3A). Following this, RAW 264.7 and HT29 cells were treated with 5AD for 72 hours to induce demethylation, after which necroptosis was induced. Post-treatment, HT29 cells exhibited no changes in necroptosis-induced cell death, while RAW 264.7 cells showed a dose-dependent increase in cell death in response to necroptosis stimulation. This indicates that the induction of cell death in RAW 264.7 cells following necroptosis stimulation is attributed to the inhibition of DNA methylation (Figure 3B and C).
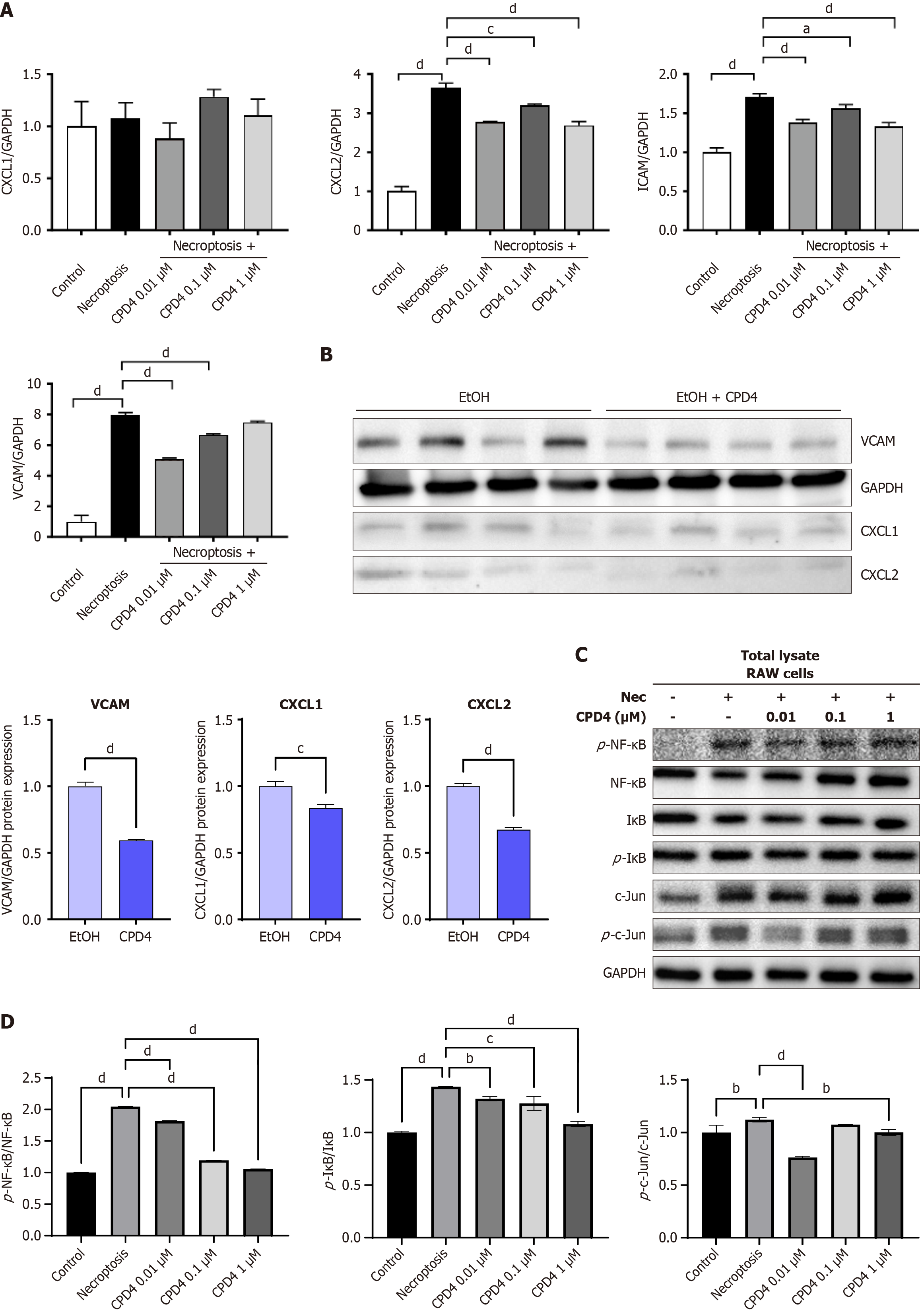
After inducing necroptosis in RAW 264.7 cells, MLKL ATP-binding inhibitor (Compound 4) was administered at concentrations of 0.01, 0.1, and 1 μmol/L. While the MLKL ATP-binding inhibitor (Compound 4) did not prevent cell death following necroptosis stimulation, MLKL ATP-binding inhibitor (Compound 4) significantly reduced the expression of key inflammatory markers. A similar downward trend in these markers was also observed in liver tissue from mice (Figure 4A and B). Additionally, treatment with MLKL ATP-binding inhibitor (Compound 4) led to decreased levels of NF-κB, c-Jun, and IκB following necroptosis stimulation in RAW 264.7 cells. These results suggest that MLKL ATP-binding inhibitors exert anti-inflammatory effects through modulation of the NF-κB signaling pathway (Figure 4C and D). In summary, while MLKL ATP-binding inhibitors may not directly inhibit necroptosis-induced cell death, they effectively regulate inflammation, highlighting their potential as therapeutic agents in inflammatory conditions.
As shown in Figure 5A, the mice were fed a CDHFD for 13 weeks to establish a NAFLD model (Figure 5A). There were no significant differences in weight were noted between the CDHF and CPD4 treatment groups (Figure 5B). HE staining of the livers of the CDHFD-fed mice revealed a significant increase in lipid content, ballooning, and liver inflammation. However, no histological or biochemical changes were observed in the animals with CPD4 treatment (Figure 5C-F).
As illustrated in Figure 6A, an alcoholic steatohepatitis (ASH) model was established by administering ethyl alcohol (EtOH) to mice. The mice received an EtOH diet for 10 days. Concurrently, CPD4 (10 mg/kg) was administered intraperitoneally daily for 10 days (Figure 6A and B). The HE staining of the livers of EtOH-fed mice revealed a significant increase in lipid content, balloons, and liver inflammation. However, CPD4-treated mice exhibited a reduction in lipid droplets, and reduced inflammation, indicating that CPD4 can ameliorate liver steatosis and inflammation (Figure 6C). According to the biochemical analysis of serum AST and ALT, CPD4-treated mice showed a decreasing trend, although this difference was not statistically significant (Figure 6D and E). Next, we extracted RNA from the liver tissue to test the anti-inflammatory effect of CPD4. The mRNA levels of inflammatory factors CXCL2, ICAM, VCAM, MCP-1, and NLRP3 showed a decreasing trend, indicating that CPD4 can improve liver inflammation in the ASH model (Figure 6F).
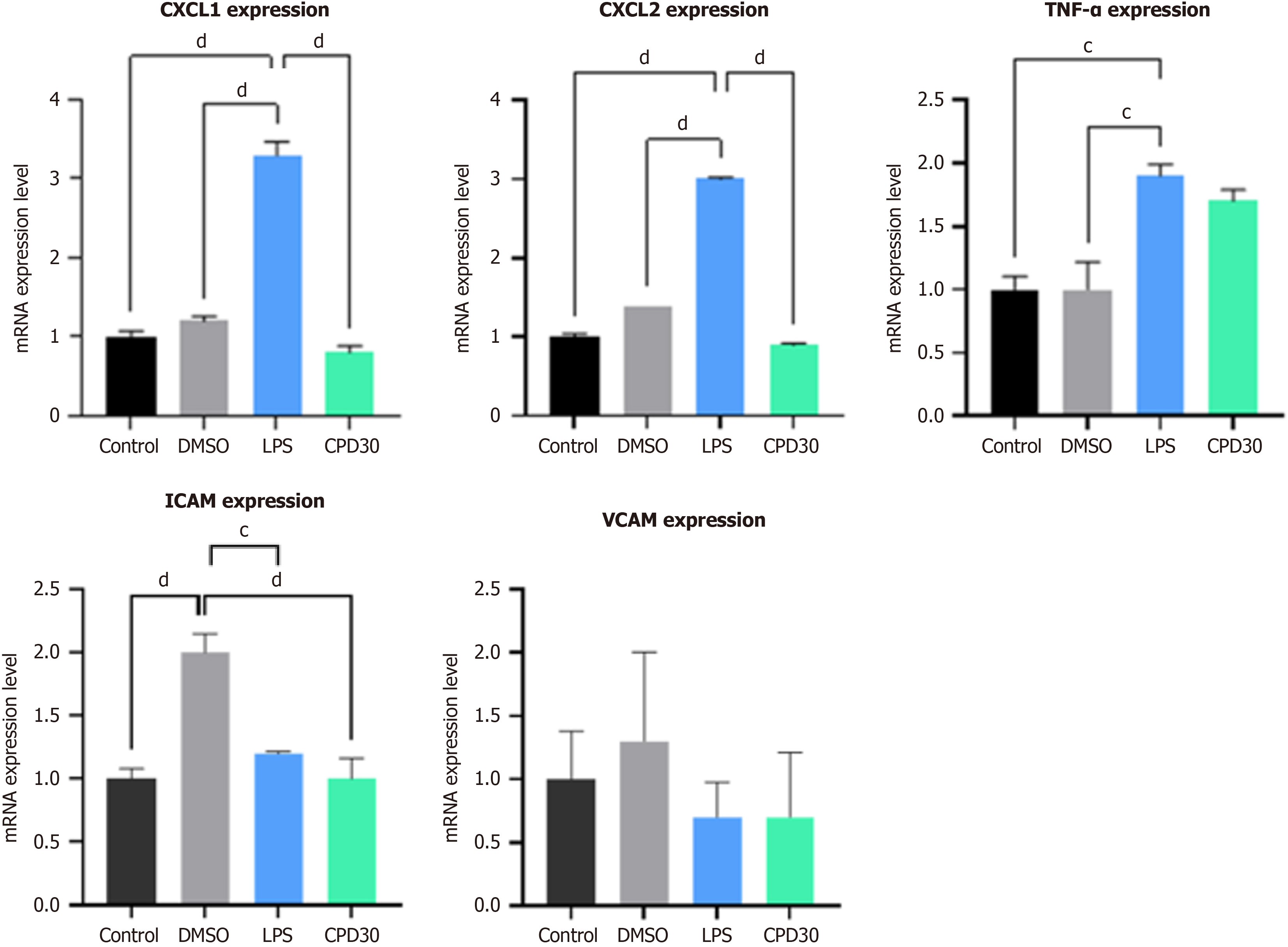
We tried to reconfirm the effect of the MLKL APT pocket inhibitor in the human derived organoid system. Liver organoids were treated with lipopolysaccharide (LPS) to establish an alcohol-induced liver disease model (Figure 7). After LPS treatment, the expression of TNF-α, CXCL1/CXCL2, ICAM, and VCAM increased in the organoid. In addition, the administration of an MLKL ATP pocket-binding inhibitor decreased the expression of TNF-α, CXCL1/CXCL2, ICAM, and VCAM in the liver organoid.
Our data indicate that RAW 264.7 cells do not undergo cell death after the induction of necroptosis. However, induction of necroptosis in RAW 264.7 cells after demethylation causes cell death. In addition, the MLKL ATP-binding inhibitor (CPD4) did not inhibit necroptosis but reduced the expression of inflammation-related genes (CXCL2, ICAM, and VCAM) in RAW 264.7 cells[16-18]. The MLKL ATP-binding inhibitor did not improve steatosis and inflammation in NAFLD and ASH models[19-21].
Most cells eventually show necrosis-like changes such as cell swelling and organelle dissolution under necroptotic stimulation. However, according to our recent research, some cells (RAW 264.7 and LX-2 cells) do not undergo cell death under the stimulation of necroptosis. Based on our data, RAW 264.7 cells were activated after necroptosis rather than cell death. Unlike MLKL, RIPK3 is not universally expressed but is predominantly found in the hematopoietic system, skin, and gastrointestinal tract[22]. RIPK3 protein and transcripts have not been detected in some primary tumors or cancer cell lines. This was attributed to excessive methylation of the RIPK3 promoter region, which is associated with transcriptional inhibition[23]. Therefore, we speculate that the failure of RAW 264.7 cells to undergo cell death is also due to DNA methylation, whereas in RAW 264.7 cells, the epigenetics of the interaction with the key necroptosis activator, RIPK3, are silenced, resulting in the inability of RAW 264.7 cells to undergo necroptosis. Previous literature has mentioned that DNA hypermethylation prevents RIPK3 transcription, which may explain the low expression or deletion of RIPK3 in certain cancer cells. Mechanistically, the methylation of RIPK3 inhibits the interaction between RIPK3 and RIPK1, thereby preventing the formation of the RIPK3–RIPK1 necrotic complex and inhibiting the phosphorylation of RIPK3, RIPK1, and MLKL, and subsequent necrotic cell death. Therefore, epigenetic silencing of RIPK3 in cells may prevent MLKL-mediated necroptosis[24-26]. In contrast, our study revealed that HT29 cells maintained high RIPK3 expression and exhibited characteristics of cellular necrosis after stimulation of necroptosis. Overall, high RIPK3 expression was not associated with DNA methylation. However, the results indicate that the low expression of RIPK3 is caused by the inhibition of RIPK3 by DNA methylation, which is why RAW 264.7 cells can evade cell death after being stimulated by necroptosis.
An important finding from our study data is that in addition to the necroptotic cell death pathway mediated by p-MLKL, necroptosis stimulation also leads to increased expression of ICAM and VCAM through the NF-κB pathway. This increase in the expression of ICAM and VACM depends on a pathway other than the phosphorylation- and oligomerization-induced cell death pathways of MLKL. In other words, our data clearly show that alternative pathways, other than the necroptotic cell death pathway of MLKL, exist in response to necroptotic stimuli. TNF-α stimulates and increases the expression of ICAM, activates signaling cascades, and regulates the redox-sensitive NF-κB activation and translocation. Previous studies have shown that NF-κB activation is necessary for the upregulation of adhesion molecules such as ICAM-1 and VCAM-1, which are responsible for monocyte adhesion and increase cell adhesion and vascular inflammation[27]. Therefore, the current data suggest that necroptosis-induced NF-κB activation is associated with the MLKL ATP pocket rather than the MLKL N-terminus, which is involved in the phosphorylation of MLKL.
Next, we investigated whether MLKL ATP-binding inhibitors exert protective effects against various liver diseases. Remarkably, the MLKL ATP-binding inhibitor was not effective in the NAFLD model but showed a protective effect in the alcoholic liver disease model. This difference is presumably due to the pathophysiology of steatotic liver disease. Monocytes and macrophages play important roles in the development of intrahepatic inflammation in the pathophysiology of NAFLD. However, in the alcohol-liver disease models, neutrophils play an important role in the occurrence and progression of the disease[28]. In alcoholic liver disease, neutrophils infiltrate the liver tissue through ICAM and VCAM and interact with other inflammatory cells through CXCL1/CXCL2. ICAM and VCAM are endothelial cell adhesion molecules of the immunoglobulin superfamily that play key roles in various acute and chronic inflammatory diseases. Our data showed that the MLKL ATP-binding inhibitor reduced ICAM, VCAM, and CXCL2 expression in animal models as well as in the liver organoid system. These chemokines, which are powerful neutrophil activators, play crucial roles in many immune reactions, including wound healing in alcoholic liver diseases, compared to the NAFLD model[29-31].
Necroptosis plays an important role in the pathogenesis of liver disease. Therefore, MLKL has received considerable attention as an attractive target for the treatment of liver diseases. To date, research targeting MLKL has primarily concentrated on the N-terminal cysteine, which is implicated in MLKL-associated cell death. Conversely, the ATP pocket, which does not influence cell death or MLKL phosphorylation, has received relatively little attention. This study identified a novel necroptosis-independent, ICAM/VCAM-activated pathway of MLKL, in addition to the previously known MLKL-induced necroptosis cell death pathway. Furthermore, it is also believed that these results may provide new insight and possibilities for MLKL ATP pocket as a novel target in the future.
In this study, we present a novel mechanism for CPD4 as an ATP-competitive binder of MLKL, providing critical insights into its role in necroptosis. Our findings reveal that, contrary to prior research, CPD4 does not alter the functional pathway of necroptotic cell death, thus challenging established beliefs about the interactions of MLKL. Specifically, we demonstrate through biochemical assays that CPD4 binding does not inhibit MLKL oligomerization or membrane translocation, key processes in necroptosis.
Moreover, our research highlights the potential of CPD4 as a tool for investigating MLKL’s regulatory pathways, suggesting that it may serve as a framework for developing targeted therapies for conditions associated with dysregulated necroptosis, such as inflammatory disorders. This work lays the groundwork for further studies aimed at elucidating the complex mechanisms governing MLKL activity and its implications in disease.
In conclusion, the MLKL ATP pocket-binding inhibitor demonstrated significant anti-inflammatory effects by modulating the necroptosis-independent MLKL pathway in an animal model of alcoholic liver disease. Specifically, the inhibitor reduced the expression of key inflammation-related markers, such as ICAM-1 and VCAM-1, by disrupting the activation of the NF-κB signaling pathway. This action led to decreased recruitment of inflammatory cells, particularly neutrophils, to the liver tissue. By targeting this necroptosis-independent mechanism, the MLKL ATP-binding inhibitor effectively mitigated hepatic inflammation and contributed to improved liver function in the context of alcoholic liver disease, highlighting its potential as a therapeutic strategy for.
The author would like to acknowledge Jun DW and Yoon EL for all support and assistance.
| 1. | Saeed WK, Jun DW, Jang K, Koh DH. Necroptosis signaling in liver diseases: An update. Pharmacol Res. 2019;148:104439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1923] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 3. | He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1930] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 4. | Oh JH, Saeed WK, Kim HY, Lee SM, Lee AH, Park GR, Yoon EL, Jun DW. Hepatic stellate cells activate and avoid death under necroptosis stimuli: Hepatic fibrosis during necroptosis. J Gastroenterol Hepatol. 2023;38:2206-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Saeed WK, Jun DW, Jang K, Oh JH, Chae YJ, Lee JS, Koh DH, Kang HT. Decrease in fat de novo synthesis and chemokine ligand expression in non-alcoholic fatty liver disease caused by inhibition of mixed lineage kinase domain-like pseudokinase. J Gastroenterol Hepatol. 2019;34:2206-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Sun HJ, Jiao B, Wang Y, Zhang YH, Chen G, Wang ZX, Zhao H, Xie Q, Song XH. Necroptosis contributes to non-alcoholic fatty liver disease pathoetiology with promising diagnostic and therapeutic functions. World J Gastroenterol. 2024;30:1968-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 7. | Wu X, Poulsen KL, Sanz-Garcia C, Huang E, McMullen MR, Roychowdhury S, Dasarathy S, Nagy LE. MLKL-dependent signaling regulates autophagic flux in a murine model of non-alcohol-associated fatty liver and steatohepatitis. J Hepatol. 2020;73:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (2)] |
| 8. | Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, Imai H, Yuet-Yin Kok C, Okochi H, Nakano H, Miyajima A, Tanaka M. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 405] [Article Influence: 57.9] [Reference Citation Analysis (3)] |
| 9. | Miyata T, Wu X, Fan X, Huang E, Sanz-Garcia C, Ross CKC, Roychowdhury S, Bellar A, McMullen MR, Dasarathy J, Allende DS, Caballeria J, Sancho-Bru P, McClain CJ, Mitchell M, McCullough AJ, Radaeva S, Barton B, Szabo G, Dasarathy S, Nagy LE. Differential role of MLKL in alcohol-associated and non-alcohol-associated fatty liver diseases in mice and humans. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Mohammed S, Nicklas EH, Thadathil N, Selvarani R, Royce GH, Kinter M, Richardson A, Deepa SS. Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radic Biol Med. 2021;164:315-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 11. | Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, Castro RE, Rodrigues CM. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci (Lond). 2015;129:721-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Ai Y, Meng Y, Yan B, Zhou Q, Wang X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol Cell. 2024;84:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 236] [Reference Citation Analysis (0)] |
| 13. | Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 743] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 14. | Ma B, Marcotte D, Paramasivam M, Michelsen K, Wang T, Bertolotti-Ciarlet A, Jones JH, Moree B, Butko M, Salafsky J, Sun X, McKee T, Silvian LF. ATP-Competitive MLKL Binders Have No Functional Impact on Necroptosis. PLoS One. 2016;11:e0165983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Lambrecht R, Delgado ME, Gloe V, Schuetz K, Plazzo AP, Franke B, San Phan T, Fleming J, Mayans O, Brunner T. Liver receptor homolog-1 (NR5A2) orchestrates hepatic inflammation and TNF-induced cell death. Cell Rep. 2023;42:113513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Liu H, Liu Y, Fan W, Fan B. Fusobacterium nucleatum triggers proinflammatory cell death via Z-DNA binding protein 1 in apical periodontitis. Cell Commun Signal. 2022;20:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 17. | Luo Q, Song Y, Kang J, Wu Y, Wu F, Li Y, Dong Q, Wang J, Song C, Guo H. mtROS-mediated Akt/AMPK/mTOR pathway was involved in Copper-induced autophagy and it attenuates Copper-induced apoptosis in RAW264.7 mouse monocytes. Redox Biol. 2021;41:101912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019;14:e0216711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 19. | Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 646] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 20. | Cheng ML, Nakib D, Perciani CT, MacParland SA. The immune niche of the liver. Clin Sci (Lond). 2021;135:2445-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Zheng Y, Wang S, Wu J, Wang Y. Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: new insights from pathogenic mechanisms to clinically targeted therapy. J Transl Med. 2023;21:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 22. | Preston SP, Stutz MD, Allison CC, Nachbur U, Gouil Q, Tran BM, Duvivier V, Arandjelovic P, Cooney JP, Mackiewicz L, Meng Y, Schaefer J, Bader SM, Peng H, Valaydon Z, Rajasekaran P, Jennison C, Lopaticki S, Farrell A, Ryan M, Howell J, Croagh C, Karunakaran D, Schuster-Klein C, Murphy JM, Fifis T, Christophi C, Vincan E, Blewitt ME, Thompson A, Boddey JA, Doerflinger M, Pellegrini M. Epigenetic Silencing of RIPK3 in Hepatocytes Prevents MLKL-mediated Necroptosis From Contributing to Liver Pathologies. Gastroenterology. 2022;163:1643-1657.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Tan Y, Sementino E, Cheung M, Peri S, Menges CW, Kukuyan AM, Zhang T, Khazak V, Fox LA, Ross EA, Ramanathan S, Jhanwar SC, Flores RM, Balachandran S, Testa JR. Somatic Epigenetic Silencing of RIPK3 Inactivates Necroptosis and Contributes to Chemoresistance in Malignant Mesothelioma. Clin Cancer Res. 2021;27:1200-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Martens S, Bridelance J, Roelandt R, Vandenabeele P, Takahashi N. MLKL in cancer: more than a necroptosis regulator. Cell Death Differ. 2021;28:1757-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 25. | Wang Q, Wang P, Zhang L, Tessema M, Bai L, Xu X, Li Q, Zheng X, Saxton B, Chen W, Willink R, Li Z, Zhang L, Belinsky SA, Wang X, Zhou B, Lin Y. Epigenetic Regulation of RIP3 Suppresses Necroptosis and Increases Resistance to Chemotherapy in NonSmall Cell Lung Cancer. Transl Oncol. 2020;13:372-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Zhang L, He Y, Jiang Y, Wu Q, Liu Y, Xie Q, Zou Y, Wu J, Zhang C, Zhou Z, Bian XW, Jin G. PRMT1 reverts the immune escape of necroptotic colon cancer through RIP3 methylation. Cell Death Dis. 2023;14:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Kurpios-Piec D, Grosicka-Maciąg E, Woźniak K, Kowalewski C, Kiernozek E, Szumiło M, Rahden-Staroń I. Thiram activates NF-kappaB and enhances ICAM-1 expression in human microvascular endothelial HMEC-1 cells. Pestic Biochem Physiol. 2015;118:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Park S, Song J, Baek IJ, Jang KY, Han CY, Jun DW, Kim PK, Raught B, Jin EJ. Loss of Acot12 contributes to NAFLD independent of lipolysis of adipose tissue. Exp Mol Med. 2021;53:1159-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Wu X, Fan X, McMullen MR, Miyata T, Kim A, Pathak V, Wu J, Day LZ, Hardesty JE, Welch N, Dasarathy J, Allende DS, McCullough AJ, Jacobs JM, Rotroff DM, Dasarathy S, Nagy LE. Macrophage-derived MLKL in alcohol-associated liver disease: Regulation of phagocytosis. Hepatology. 2023;77:902-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Tong J, Lan XT, Zhang Z, Liu Y, Sun DY, Wang XJ, Ou-Yang SX, Zhuang CL, Shen FM, Wang P, Li DJ. Ferroptosis inhibitor liproxstatin-1 alleviates metabolic dysfunction-associated fatty liver disease in mice: potential involvement of PANoptosis. Acta Pharmacol Sin. 2023;44:1014-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 31. | Majdi A, Aoudjehane L, Ratziu V, Islam T, Afonso MB, Conti F, Mestiri T, Lagouge M, Foufelle F, Ballenghien F, Ledent T, Moldes M, Cadoret A, Fouassier L, Delaunay JL, Aït-Slimane T, Courtois G, Fève B, Scatton O, Prip-Buus C, Rodrigues CMP, Housset C, Gautheron J. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J Hepatol. 2020;72:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (1)] |