Published online Feb 14, 2025. doi: 10.3748/wjg.v31.i6.101163
Revised: November 12, 2024
Accepted: December 19, 2024
Published online: February 14, 2025
Processing time: 126 Days and 18.5 Hours
Percutaneous cholecystostomy (PC) can be used as a bridging therapy for moderately severe acute biliary pancreatitis (MSABP). Currently, there are only a limited number of reports of MSABP using PCs.
To assess the short-term outcomes of early PC in MSABP and factors associated with recurrence and death in MSABP.
Patients who received conservative treatment or PC for acute biliary pancreatitis (ABP) in Liaoning Provincial People’s Hospital from January 2017 to July 2022 were collected. A total of 54 patients with MSABP who received early-stage PC and 29 patients who received conservative treatment. The short-term efficacy of PC was evaluated. Depending on whether there is a recurrence, compare the characteristics of the pre-PC and explore the factors of recurrence. Pre-PC features were compared and predictors were discussed, depending on the outcome.
After 3 days of PC treatment, patients experienced a reduction in inflammatory markers compared to the conservative group. After PC, patients were divided into non-recurrence (n = 37) and recurrence (n = 10) groups, and the results showed that age was an independent correlation affecting ABP recurrence [odds ratio (OR) = 0.937, 95% confidence interval (CI): 0.878-0.999; P = 0.047 < 0.05]. Patient outcomes were divided into non-lethal (n = 47) and lethal (n = 7) groups, and Charlson Comorbidity Index (CCI) was a risk factor for mortality (OR = 2.397, 95%CI: 1.139-5.047; P = 0.021 < 0.05). CCI was highly accurate in predicting death in MSABP (area under the curve = 0.86 > 0.7). When the Youden index maximum was 0.565, the cut-off value was 5.5, the sensitivity was 71.4%, and the specificity was 85.1%.
PC is an important method in the early years (< 72 hours) of MSABP. Age is a protective factor against recurrence of ABP. High pre-PC CCI is significantly associated with mortality.
Core Tip: We conducted a comparison of percutaneous cholecystostomy before treatment with 3 days of treatment, and between the two modalities after 3 days of treatment, and explored the factors associated with the occurrence of recurrence or death after percutaneous cholecystostomy in patients with moderate to severe acute biliary pancreatitis to deepen our understanding of moderate to severe acute biliary pancreatitis.
- Citation: Yan X, Xie F, Zhao XD, Li L, Meng JX. Short-term efficacy of early percutaneous cholecystostomy for pancreatitis and factors associated with recurrence and mortality. World J Gastroenterol 2025; 31(6): 101163
- URL: https://www.wjgnet.com/1007-9327/full/v31/i6/101163.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i6.101163
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases in the world, with rapid onset and high morbidity and mortality[1]. Biliary cholangitis, alcoholism, hypertriglyceridemia, transendoscopic retrograde cholangiopancreatography, endoscopic retrograde cholangiopancreatography (ERCP) and certain medications are common etiologic factors. After the incidence data are stratified according to etiology, the epidemiologic trends indicate that the incidence of biliary pancreatitis appears to be increasing linearly[2], with gallstone disease being the most prevalent cause of AP[3].
The pathogenesis of acute biliary pancreatitis (ABP) is still inconclusive and may be multifactorial, with multiple theories associated with it. Currently, the common channel theory is favored, i.e., blockage of the common outlet of the bile duct and the pancreatic duct, elevated pressure, mixing of bile acids or bacteria and inflammatory mediators in the bile duct with pancreatic fluid in the pancreatic duct[4], leading to an abnormal release of inflammatory factors in the pancreatic duct and excessive and abnormal activation of the pancreatic alveoli to produce a cascade of inflammatory responses, which in turn leads to the development of inflammation in the pancreas and the peripancreatic area. After patients were evaluated via the Revised Atlanta classification (RAC), which is often used to predict the severity of AP in clinical practice, although 80% of patients with ABP presented with mild disease, some patients with moderately severe ABP (MSABP)[5] were identified, and those patients who developed moderately severe disease presented more severe clinical symptoms, with a mortality rate of approximately 20%[6] and more aggressive disease.
The initial treatment of ABP consists of rehydration and pain control, followed by cholecystectomy, the gold standard for the treatment of most gallbladder-related diseases. However, owing to the timing of cholecystectomy, selection of the population for which it is indicated, and voluntary will, the treatment plan for patients with ABP is more individualized[7]. Aggressive fluid resuscitation and early emergency ERCP do not have a strong evidence base for a direct and significant benefit in patients with ABP[8,9]. This group of patients with MSABP cannot tolerate invasive procedures due to severe inflammation but needs to minimize the severity to survive the dangerous period. At this point, percutaneous cholecystostomy (PC), owing to its relative simplicity of operation, becomes particularly important for patients with MSABP as a rapid approach to drain accumulated bile, decompress the enlarged gallbladder, and reduce the pressure on the biliary system.
To date, insights into the application of PC for the treatment of MSABP are incomplete, and reports on the impact of outcomes after PC are scarce. PC is often used as the treatment of choice after failure to apply ERCP or as a valid option for patients who cannot tolerate endoscopic treatment[10,11]. Although urgent ERCP should be performed (within 24 hours) in patients with gallstone pancreatitis and cholangitis, it is not indicated in patients without cholangitis, and one study demonstrated that it also failed to significantly reduce major complications or mortality in patients with predicted severe ABP but without cholangitis[12]. As a result, there is a growing preference for less risky strategies.
Currently, there is no standardized measure of “early” treatment for pancreatitis, and some studies have shown that initial treatment within the first 72 hours after the diagnosis of AP is crucial, which may affect the clinical outcome of the disease[13,14]. Thus, our study defined “early” as the first 72 hours of admission to the hospital and “early” as the first 72 hours of admission to the hospital. As PC treatment within 72 hours of hospital admission[6]. Our current retrospective study emphasized the role of PC in the early treatment of MSABP by analyzing the clinical data of patients with MSABP who were treated conservatively or who underwent early PC therapy and by performing a comparison between pre-PC treatment and after 3 days of treatment and a comparison between the two modalities after 3 days of treatment.
In addition, progression after an episode of ABP has varied widely in previous studies[15]. The impact of patients’ pre-PC clinical characteristics and the presence of comorbidities on ABP recurrence and death is unclear, and there is a lack of research on the correlates of the occurrence of recurrence or death and patient characteristics that increase the risk of recurrence or death in patients with MSABP. Therefore, we investigated the clinical data of these patients on the basis of PC treatment through telephone calls and follow-up visits to explore the factors associated with the occurrence of relapse or death after PC in MSABP patients to increase the understanding of MSABP.
This was a retrospective study, and the medical records of all patients included in this study were obtained from the electronic medical records system of Liaoning Provincial People’s Hospital. In accordance with the Declaration of Helsinki (revised in 2013), this study was approved by the ethics committee of a hospital [approval number: (2023)K037]. All patients who underwent PC signed an informed consent for surgery. Patients with ABP who were treated conservatively and underwent PC at an early stage during their hospitalization at Liaoning Provincial People’s Hospital from January 2017 to July 2022 were selected for the study.
The inclusion criteria were as follows: (1) Patients diagnosed with MSABP; (2) Patients who were treated conservatively during hospitalization or received PC at an early stage; (3) Patients who were older than 18 years[16]; (4) Patients with normal or enlarged gallbladders; (5) Patients who were older than 6 days at the time of hospitalization; and (6) Patients with an allowable follow-up time of 20 months.
The exclusion criteria were as follows: (1) Patients with structural changes in the biliary tract; (2) Patients with combined pancreatic occupancy; (3) Patients who underwent ERCP during the same period; (4) Patients who underwent other abdominal drainage procedures or emergency laparotomies during the same period after PC; (5) Patients lost to follow-up; (6) Patients whose first diagnosed disease was not biliary pancreatitis; and (7) Patients whose medical records were incomplete.
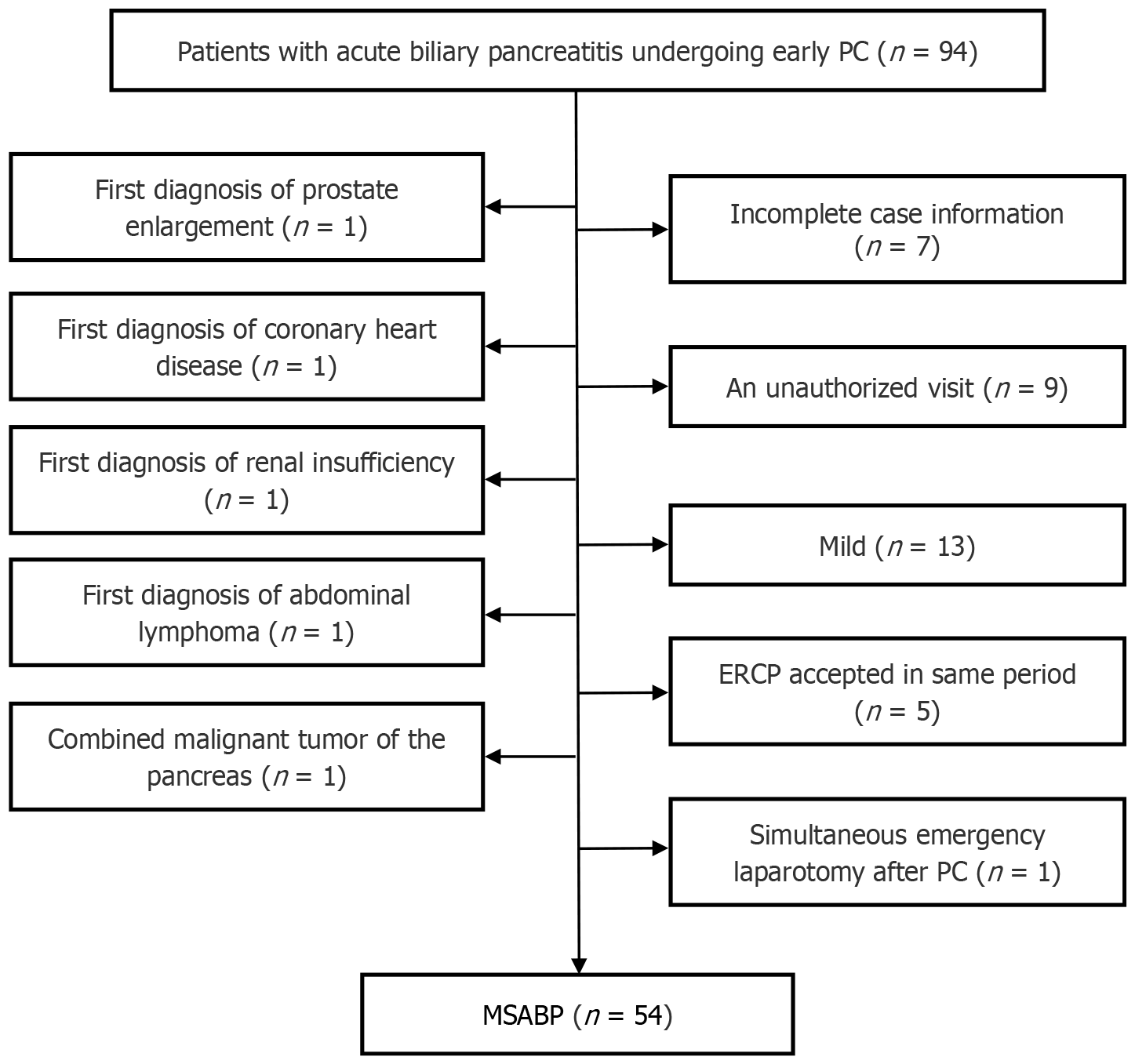
We retrieved data from a total of 123 patients who were treated conservatively or received early PC for MSABP, including 94 patients with early PC and 29 patients who were treated conservatively at an early stage. The 94 patients who underwent PC included patients with a first diagnosis of prostatic hyperplasia (n = 1), coronary artery disease (n = 1), abdominal lymphoma (n = 1), renal insufficiency (n = 1), combined pancreatic malignancy (n = 1), patients who underwent transendoscopic retrograde cholangiopancreatography during the same period (n = 5), and patients with simultaneous emergency laparotomy after PC (n = 1). Patients with incomplete medical records (n = 7) and patients who were lost to follow-up (n = 9) were excluded, and of the remaining 67 patients, those determined to be mildly ill according to the RAC (n = 13) were also excluded. A total of 54 patients with MSABP who underwent early PC and 29 patients who were treated conservatively were included. Figure 1 shows the PC patient screening process.
The diagnosis of all MSABP patients should meet the ABP diagnostic criteria[17]. Specifically, clinical symptoms manifested as intense and persistent epigastric pain, abdominal pain was more often located in the left upper abdomen, serum amylase (AMS) and/or lipase activity was at least three times higher than the upper limit of the normal value, and an abdominal imaging examination revealed changes in the AP. The diagnosis of ABP is confirmed when two of the above three criteria are met and when there is a combination of biliary diseases, such as gallbladder stones, bile duct stones, and cholestasis, and when other etiologies are ruled out. The severity of MSABP is based on the RAC[18], which includes moderately severe AP (SAP), in which patients have transient (≤ 48 hours) organ dysfunction and/or localized complications, and SAP, in which patients have persistent (> 48 hours) organ dysfunction.
Conservative treatment includes water fasting, nutritional support, and gastrointestinal decompression. The disturbances in water and electrolyte balance, analgesia, anti-inflammatory effects, inhibition of pancreatic enzyme activity, and symptomatic treatment should be implemented when combined with other systemic diseases.
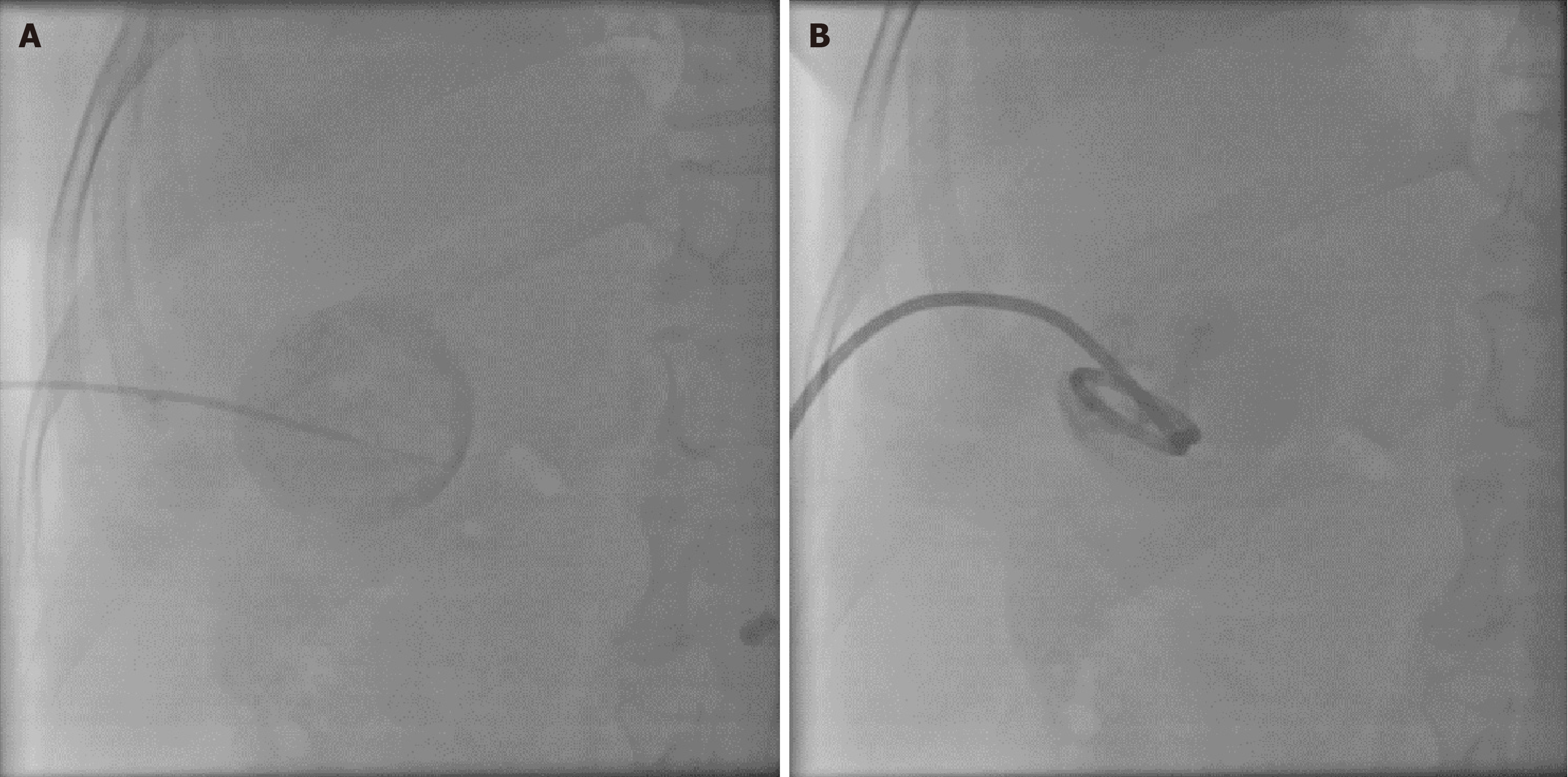
On the basis of conservative treatment within 72 hours of admission, patients with MSABP who failed to improve their clinical symptoms or subjective wishes were evaluated by the treating physician for PC. The procedure was performed via ultrasound guidance under aseptic technique and local anesthesia. The procedure was performed via percutaneous puncture of the gallbladder via a 0.035-inch guidewire (Terumo, loach guidewire), aspiration of bile and sending it to the laboratory for culture, placement of an 8-Fr pigtail catheter (Boston Scientific) in the gallbladder, and verification of the location of the gallbladder drainage catheter via X-ray after injection of a water-soluble contrast medium. Figure 2 shows the PC procedure. The PC was created by an interventionalist with 16 years of experience. Indications for removal of the fistula are as follows: Improvement of clinical symptoms, return to normal or improvement of laboratory values, and ultrasound or computed tomography (CT) imaging showing no signs of recurrence of drainage catheter entrapment for 2-3 days. Drainage tube removal usually occurs after 4-6 weeks.
The following demographic data were recorded: Sex, age, and body mass index. Length of hospitalization, previous history of disease, temperature, and use of antimicrobials. The relevant laboratory parameters included white blood cells (WBCs), the percentage of neutrophils (NEU%), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), serum total bilirubin (TBIL), direct bilirubin, indirect bilirubin (IBIL), and serum AMS, with the above data from the highest pretreatment value and the highest value 3 days after treatment. To clarify whether the patients were febrile, a temperature ≥ 37.3 °C was used as the cutoff. Imaging manifestations: On the basis of the presence of pancreatic edema, peripancreatic exudation, blurring of the peripancreatic fat space and/or peripancreatic tissue necrosis on the basis of pretreatment CT scanning, the CT severity index (CTSI) was calculated[19], and the gallbladder width was measured, which was taken from the widest part of the largest cross-section of the gallbladder in the abdominal ultrasound or CT images. Patients’ pre-PC comorbidities were classified according to the Charlson Comorbidity Index (CCI), and the RAC classification, which is often used in clinical practice to predict the severity of AP, was adopted to evaluate pre-PC patients as moderately severe or severe. The use of antimicrobial drugs, the presence or absence of comorbidities with other systemic diseases and past history were assigned values of 0 for “no” and 1 for “yes”.
SPSS27 and Graphpad Pism9.5 was used for data processing and drawing. The measurement data were tested for normality, those that conformed to a normal distribution were expressed as the mean ± SD, and independent samples t tests were used for between-group comparisons. Data that were not normally distributed were expressed as medians and interquartile ranges, and group comparisons were made via the Wilcoxon rank sum test or the Mann-Whitney U test. Count data are expressed as frequencies and percentages, and comparisons between groups were made via the one-way
A total of 83 patients with MSABP were included, including 54 in the PC group and 29 in the conservative group. The patients who underwent PC were operated on successfully, and there was no drain dislodgement or smooth drainage during hospitalization. The PC group included 30 males (55.6%) and 24 females (44.4%), and the average length of hospitalization for 54 patients was 17 days. The conservative group included 19 males (65.5%) and 10 females (34.5%), with an average length of hospitalization of 16 days in 29 patients. Between-group comparisons of the general characteristics, body temperature, infection-related indices, liver function-related indices, Charlson comorbidities, and use of antimicrobial drugs between the two groups of patients prior to treatment did not reveal statistically significant differences (P > 0.05). Bacterial culture of the bile drained from patients in the PC group revealed 14 cases (25.93%) of bacterial bile, including 7 cases of Escherichia coli, 3 cases of Enterococcus faecalis, 2 cases of Klebsiella pneumoniae, 1 case of Staphylococcus hemolyticus, and 1 case of Enterobacter cloacae. The morphology of the drained bile was categorized as black viscous in 46 cases and other traits in 8 cases, whereas the other traits included purulent in 5 cases, brown viscous in 2 cases, and green in 1 case. The application of antimicrobial drugs prior to treatment was not known in 20 patients. Forty-one patients were using at least one of the following antimicrobial drugs prior to undergoing PC treatment: Cephalosporins, nitroimidazoles, growth inhibitors, or proton pump inhibitors. Notably, 34 patients used growth inhibitors before PC. In our institution, the selection of antimicrobial agents for patients with positive bile cultures after PC treatment is adjusted in time to make it more favorable. Tables 1 and 2 show the pretreatment baseline data for both groups of patients.
| Variable | PC group (n = 54) | Conservative group (n = 29) | t/z value | P value |
| Age (years) | 62.7 ± 20.63 | 55.55 ± 15.92 | 1.624 | 0.108 |
| BMI | 25.31 ± 3.34 | 24.62 ± 2.47 | 0.983 | 0.329 |
| Hospitalization (days) | 17.28 ± 9.72 | 15.9 ± 4.36 | 0.726 | 0.47 |
| Temperature (°C) | 37.5 (36.8-38.18) | 37.8 (37.3-38.5) | -0.513 | 0.130 |
| WBC (× 109/L) | 13.6 (10.87-16.62) | 12.6 (8.95-16.73) | -0.817 | 0.414 |
| NEU (%) | 88.2 (82.8-90.9) | 85.1 (78.6-88.95) | -1.662 | 0.097 |
| ALT (U/L) | 49 (24.68-100.45) | 35.9 (28.5-48.9) | -1.027 | 0.305 |
| GGT (U/L) | 106.5 (51.5-238) | 71 (52.5-126.5) | -1.141 | 0.254 |
| TBIL (μmol/L) | 29.35 (17.57-54) | 22.2 (17.4-29.5) | -1.944 | 0.052 |
| DBIL (μmol/L) | 2.75 (0-12.95) | 0 (0-4.65) | -1.562 | 0.118 |
| IBIL (μmol/L) | 13.9 (8.58-27) | 11.7 (8.7-13.4) | -1.829 | 0.067 |
| AMS (U/L) | 394.5 (151.25-1060) | 211 (101.5-650) | -1.705 | 0.088 |
| CTSI | 3 (3-5) | 3 (3-3) | -0.282 | 0.778 |
| Variable | PC group (n = 54) | Conservative group (n = 29) | χ2 value | P value |
| Sex | 0.774 | 0.379 | ||
| Male | 30 (55.6) | 19 (65.5) | ||
| Female | 24 (44.4) | 10 (34.5) | ||
| Charlson comorbidities | ||||
| Congestive heart failure | 2 (3.7) | 0 | - | 0.540 |
| Diabetes mellitus (no comorbidities) | 14 (25.9) | 8 (27.6) | 0.027 | 0.870 |
| Solid tumors (no metastases) | 3 (5.6) | 0 | - | 0.548 |
| Cerebrovascular disease | 11 (20.4) | 2 (6.9) | 1.673 | 0.196 |
| Use of antimicrobials | ||||
| Cephalosporins | 19 (35.2) | 9 (31) | 0.145 | 0.703 |
| Nitroimidazoles | 30 (55.6) | 13 (44.8) | 0.870 | 0.351 |
| Growth inhibitors | 34 (63) | 17 (58.6) | 0.150 | 0.698 |
| Proton pump inhibitors | 25 (46.3) | 12 (41.4) | 0.185 | 0.667 |
| Combinations | 40 (74.1) | 22 (75.9) | 0.032 | 0.858 |
Compared with those before PC treatment, after 3 days of PC treatment, the temperature, WBC, NEU, GGT, TBIL, indirect bilirubin, and AMS of MSABP patients were significantly lower than those before treatment, and the difference was statistically significant (P < 0.05). The statistical results are shown in Table 3.
| Variable | Pre-treatment | Post-treatment | Z value | P value |
| Temperature (°C) | 37.5 (36.8-38.18) | 37 (36.95-37.63) | -2.447 | 0.014a |
| WBC (× 109/L) | 13.6 (10.87-16.62) | 10.65 (7.12-13.86) | -3.035 | 0.002a |
| NEU (%) | 88.2 (82.8-90.9) | 80.15 (73.2-85.93) | -5.132 | < 0.001a |
| ALT (U/L) | 49 (24.68-100.45) | 34.7 (23-72.05) | -3.306 | < 0.001a |
| GGT (U/L) | 106.5 (51.5-238) | 98.5 (48.75-170) | -2.603 | 0.009a |
| TBIL (μmol/L) | 29.35 (17.57-54) | 21.6 (13-34.68) | -4.064 | < 0.001a |
| DBIL (μmol/L) | 2.75 (0-12.95) | 2.75 (0-7.33) | -1.873 | 0.061 |
| IBIL (μmol/L) | 13.9 (8.58-27) | 10.1 (7.63-16.65) | -3.692 | < 0.001a |
| AMS (U/L) | 394.5 (151.25-1060) | 63 (41.75-89) | -6.169 | < 0.001a |
There was no statistically significant difference between the pretreatment temperature, infection-related indices or liver function levels of patients in the PC group and those in the conservative group (P > 0.05), and the detailed statistical results are shown in Table 1. The levels of temperature, WBC, ALT, GGT, TBIL, and direct bilirubin in patients in the PC group were significantly lower than those in the conservative group at 3 days after treatment, and the difference between the two groups was statistically significant (P < 0.05); the detailed statistical results are shown in Table 4.
| Variable | PC group (n = 54) | Conservative group (n = 29) | Z value | P value |
| Temperature (°C) | 37 (36.95-37.63) | 37.7 (37.35-38.05) | -3.135 | 0.002a |
| WBC (× 109/L) | 10.65 (7.12-13.86) | 13.42 (9.41-16.17) | -1.987 | 0.047a |
| NEU (%) | 80.15 (73.2-85.93) | 79.5 (68.95-85.45) | -0.812 | 0.417 |
| ALT (U/L) | 34.7 (23-72.05) | 52 (39.55-70.15) | -2.292 | 0.022a |
| GGT (U/L) | 98.5 (48.75-170) | 132 (102-190.5) | -2.230 | 0.026a |
| TBIL (μmol/L) | 21.6 (13-34.68) | 27.9 (21.6-32.65) | -1.968 | 0.049a |
| DBIL (μmol/L) | 2.75 (0-7.33) | 12.9 (0-16.85) | -2.271 | 0.023a |
| IBIL (μmol/L) | 10.1 (7.63-16.65) | 12.9 (8.6-17.25) | -1.012 | 0.311 |
| AMS (U/L) | 63 (41.75-89) | 64 (43-90) | -0.076 | 0.939 |
We followed the patients in the PC group for 2-20 months, with a median follow-up time of 6 months. Patients were categorized into a nonrelapse group (n = 37) and a relapse group (n = 10) according to whether they relapsed after PC treatment. Recurrence was based on follow-up by telephone and follow-up visits to clarify that patients were indeed rehospitalized for abdominal pain caused by ABP, and patients who underwent elective ERCP stone extraction or cholecystectomy in-hospital after treatment or during the follow-up period were included in the nonrecurrence group. The mean duration of recurrence in patients was 8 months.
The total number of patients who recurred was 10. There were 6 males (60%). Two patients recurred 2 months after PC, 2 patients recurred 3 months after PC, and 2 patients recurred during tube placement and were discharged from the hospital after replacement of the drainage tube and clinical remission with antibiotics only. One patient recurred 5 months after PC, one recurred 7 months after PC, one recurred 9 months after PC, one recurred 12 months after PC, and one recurred 19 months after PC. Nineteen months after PC surgery, 1 patient recurred 20 months after PC surgery; all 10 patients who recurred were discharged from the hospital after receiving only conservative treatment.
One-way analysis revealed that the age of patients in the recurrence group was lower than that of patients in the nonrecurrence group (P < 0.001), and patients in the recurrence group had a significantly greater CTSI (P = 0.028) and a lower CCI (P = 0.023) prior to PC than did those in the nonrecurrence group. Factors with P < 0.1 according to univariate analysis (age, CTSI, CCI) were included in the logistic regression equation, and multivariate analysis revealed that age was statistically significant for the recurrence of biliary pancreatitis (OR = 0.937, 95%CI: 0.878-0.999; P = 0.047 < 0.05). According to the multivariate analysis, the CTSI and CCI were not associated with the recurrence of biliary pancreatitis. Table 5 specifically shows the statistical results.
| Variables | Nonrecurrent group (n = 37) | Recurrent group (n = 10) | Single factor analysis | Multi-factor analysis |
| P value | P value [OR (95%CI)] | |||
| Age (years) | 66.14 ± 17.1 | 42.5 ± 21 | < 0.001a | 0.047 [0.937 (0.878-0.999)] |
| Sex | 0.897 | |||
| Male | 19 (51.4) | 6 (60) | ||
| Female | 18 (48.6) | 4 (40) | ||
| BMI | 25.03 ± 2.99 | 26.6 ± 3.5 | 0.164 | |
| RAC classification | 0.897 | |||
| Moderately severe | 19 (51.4) | 6 (60) | ||
| Severe | 18 (48.6) | 4 (40) | ||
| Bile duct stone type | 0.379 | |||
| Positive stones | 27 (73) | 4 (40) | ||
| Negative stones | 17 (45.9) | 6 (60) | ||
| Day of hospitalization (days) | 15.7 ± 10.31 | 19.9 ± 6.82 | 0.232 | |
| Fever (temperature ≥ 37.3 °C) | 20 (54.1) | 4 (40) | 0.117 | |
| WBC (× 109/L) | 13.09 (10.85-16.56) | 15.54 (10.01-17.08) | 0.697 | |
| NEU (%) | 87.9 (82.8-91.15) | 86.35 (79.25-89.23) | 0.176 | |
| ALT (U/L) | 53.3 (23.85-129.45) | 29 (21-105.83) | 0.311 | |
| GGT (U/L) | 108 (51-280) | 97 (46.25-174.25) | 0.659 | |
| TBIL (μmol/L) | 25.5 (15.85-68.8) | 22.9 (17.45-40.85) | 0.467 | |
| AMS (U/L) | 398 (156.5-983) | 420 (129.5-1327.25) | 0.938 | |
| Gallbladder width (cm) | 3.6 (3.1-4) | 3.45 (2.98-3.93) | 0.474 | |
| CTSI | 3 (1.5-3.5) | 4 (3-5) | 0.043a | 0.209 [1.596 (0.770-3.311)] |
| CCI | 4 (3-5) | 2.5 (2-4) | 0.023a | 0.925 [1.036 (0.491-2.188)] |
| Congestive heart failure | 2 (5.4) | 0 | 1 | |
| Diabetes mellitus (no comorbidities) | 8 (21.6) | 5 (50) | 0.167 | |
| Solid tumors (no metastases) | 2 (4.3) | 0 | 1 | |
| Cerebrovascular disease | 7 (18.9) | 0 | 0.322 | |
| History of pancreatitis | 7 (18.9) | 4 (40) | 0.329 | |
| Hypertension | 18 (48.6) | 4 (40) | 0.897 |
In addition, we recorded the mean values of the patients’ drainage volume on the day of PC and 3 days after PC, and the mean value of the drainage volume on the day of PC was 232.14 ± 133.5 mL in the patients in the nonrecurrent group and 184 ± 105.85 mL in the patients in the relapse group. The difference between the two groups was not statistically significant (P = 0.299). The mean value of 3-day post-PC drainage was 221.8 ± 122.27 mL in the nonrecurrent group and 153.67 ± 84.45 mL in the recurrent group, with no statistically significant difference between the two groups (P = 0.105).
To fully understand the factors associated with the occurrence of death after early PC treatment in MSABP patients during hospitalization, we included demographic data, pretreatment laboratory indices, severity grading and comorbidities. Patients who died in the hospital after treatment were included in the mortality group, and the remaining patients were included in the nonfatal group. The time to death in MSABP patients ranged from 8-35 days, with a median time to death of 20 days. A total of 7 patients died, including 5 males (71.4%). We investigated the causes of death in these patients and identified the following causes of death: Infectious shock due to MSABP in 4 patients, renal failure in 1 patient, gastrointestinal bleeding in 1 patient, and respiratory failure in 1 patient.
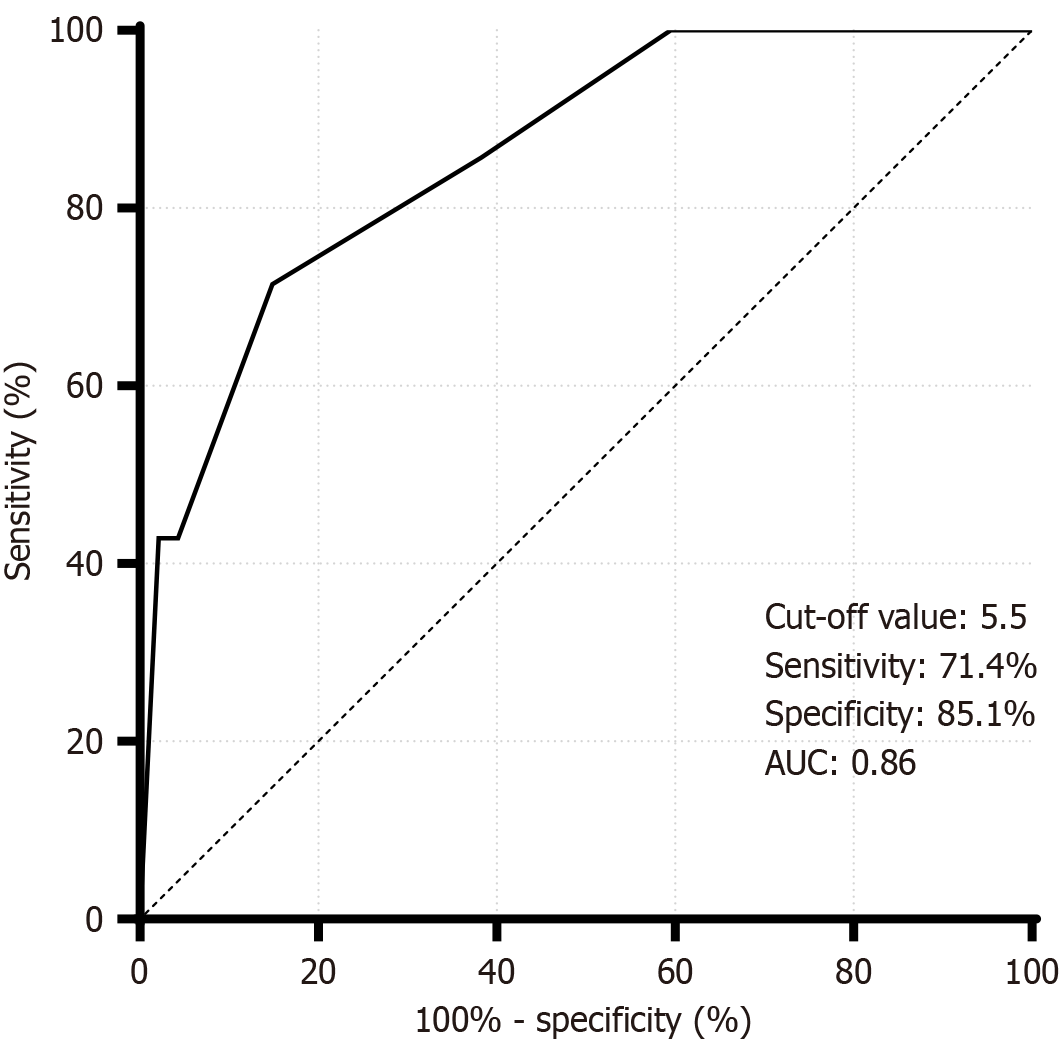
Compared with the nonfatal group, the nonfatal group had a greater pre-PC CCI (P < 0.001) and a smaller proportion of patients with cerebrovascular disease (P = 0.037), and the difference was statistically significant. Variables with P values < 0.1 (CCI, cerebrovascular disease) were included in logistic regression analysis, and multifactorial analysis revealed that a high pre-PC CCI was significantly positively associated with mortality (OR = 2.397, 95%CI: 1.139-5.047; P = 0.021 < 0.05). Multivariate analysis revealed that cerebrovascular disease comorbidities were not associated with mortality. The statistical results of the pre-PC clinical characteristics of the patients in the nonfatal group (n = 40) and the fatal group (n = 7) are shown in Table 6. The pre-PC CCI had a high accuracy for predicting death in MSABP patients (area under the curve = 0.86 > 0.7), and when the YI had a maximum value of 0.565, the cutoff value of the CCI was 5.5, with a sensitivity of 71.4% and a specificity of 85.1%. Figure 3 shows the receiver operating characteristic plot of the CCI for the prediction of death.
| Variables | Nonfatal group (n = 47) | Fatal group (n = 7) | Single factor analysis | Multi-factor analysis |
| P value | P value [OR (95%CI)] | |||
| Age (years) | 61.11 ± 20.27 | 73.43 ± 21.31 | 0.142 | |
| Sex | 0.443 | |||
| Male | 25 (53.2) | 5 (71.4) | ||
| Female | 22 (46.8) | 2 (28.6) | ||
| BMI | 25.37 ± 3.13 | 25.13 ± 4.68 | 0.861 | |
| RAC classification | 0.102 | |||
| Moderately severe | 25 (53.2) | 1 (14.3) | ||
| Severe | 22 (46.8) | 6 (85.7) | ||
| Days of hospitalization (days) | 16.6 ± 9.76 | 21.86 ± 8.69 | 0.184 | |
| Fever (≥ 37.3 °C) | 24 (51.1) | 6 (85.7) | 0.117 | |
| WBC (× 109/L) | 13.25 (10.88-16.52) | 16.28 (9.77-18.58) | 0.495 | |
| NEU (%) | 87.2 (82.8-90.7) | 90.3 (88.3-91.6) | 0.116 | |
| ALT (U/L) | 36 (23.7-115) | 59 (48-81) | 0.212 | |
| GGT (U/L) | 108 (52-265) | 105 (47-162) | 0.757 | |
| TBIL (μmol/L) | 25.5 (16.7-54.3) | 34.1 (29.5-36.2) | 0.374 | |
| DBIL (μmol/L) | 3 (0-14) | 2 (0-8.5) | 0.799 | |
| IBIL (μmol/L) | 14.3 (8.6-30.3) | 13.1 (4.2-23.5) | 0.361 | |
| AMS (U/L) | 398 (152-1051) | 173 (148-1087) | 0.643 | |
| Gallbladder width (cm) | 3.6 (3-4.1) | 3.4 (2.7-4.9) | 0.918 | |
| CTSI | 3 (1-5) | 3 (1-5) | 0.821 | |
| CCI | 4 (2-5) | 6 (5-8) | 0.001a | 0.021 [2.397 (1.139-5.047)] |
| Congestive heart failure | 2 (4.3) | 0 | 1 | |
| Diabetes mellitus (no comorbidities) | 13 (27.7) | 1 (14.3) | 0.771 | |
| Solid tumors (no metastases) | 2 (4.3) | 1 (14.3) | 0.346 | |
| Cerebrovascular disease | 7 (14.9) | 4 (57.1) | 0.037a | 0.659 [1.631 (0.186-14.336)] |
| History of pancreatitis | 11 (23.4) | 1 (14.3) | 0.957 | |
| Hypertension | 22 (46.8) | 3 (42.9) | 1 |
Management of PC for patients with MSABP most patients with gallstone pancreatitis will not benefit from ERCP. Urgent ERCP is only applicable to cholangitis or progressive cholestasis, which is defined as an elevation of bilirubin (bilirubin > 3-5 mg/dL) in the context of moderate or SAP. The guidelines of the American Gastroenterological Association point out that in most patients with gallstone pancreatitis, common bile duct stones will pass into the duodenum, and routine ERCP is not appropriate, including early ERCP[20].
The choice of the timing of surgical intervention has always been a hot issue in the treatment of SAP. Controlling the appropriate timing of surgical intervention is more important than selecting the appropriate intervention method. At present, the timing of surgical intervention has changed from the previously rather aggressive “early debridement” to “delayed debridement”, and the “3D” (delay, drain, debride) principle has been generally recognized. Dubina et al[7] believe that for patients with moderately SAP and SAP, surgery is usually performed after pancreatic recovery, and cholecystectomy can be carried out within 1 to 3 months after the onset of the disease. For patients with biliary SAP, cholecystectomy should be postponed until the active inflammation subsides, fluid accumulation regresses or stabilizes before implementation[7]. Patients with MSABP have a severe systemic inflammatory response and are often accom
Accurate identification of the severity of ABP is important for the choice of patient treatment, and the treatment method for PC may alter its clinical course. MSABP patients have transient or persistent organ failure and poor overall condition. Appropriate treatment strategies are important. Patients who cannot tolerate surgery should be promptly treated with biliary drainage and aggressive conservative treatment[11]. We retrospectively investigated the clinical characteristics of patients before and after early PC and after 3 days of treatment with both earlier methods. Patients with MSABP are considered at extremely high risk for anesthesia and surgery due to a combination of higher levels of infection and complex medical comorbidities when symptoms cannot be improved by medication early in the course of the disease. PC avoids the risks accompanying general anesthesia and invasive surgery and is less traumatic, with a shorter operative time, and allows for rapid drainage of sludged bile, thereby decompressing the gallbladder, performing a biliopancreatic shunt, and reducing the mixing of bile with pancreatic fluid. These advantages make PC the preferred choice for this group of patients. This finding is consistent with the report of Howard et al[22], who reported that PC is a safe option for biliary decompression in elderly patients and can be an early alternative to cholecystectomy for relief in high-risk pa
In this study, we showed that bile acids or other inflammatory substances in the gallbladder were directly drained from the body by a PC and that the patient’s temperature decreased. Some laboratory indices also change significantly. First, in terms of inflammation-related indices, such as immune cells involved in the entire process of AP, the levels of leukocytes and NEUs decrease significantly[23], indicating that the inflammatory state is controlled and alleviated and that PC effectively alleviates the inflammatory response of the pancreas. In addition, the changes in liver function-related indices were also notable, with TBIL, indirect bilirubin, transketolase, and glutamine transaminase decreasing signi
In conclusion, our study emphasizes the effectiveness of early PC for MSABP, which can benefit patients through gallbladder decompression, biliopancreatic shunting, and drainage of stagnant bile, which in turn can reduce infection and improve liver function. Therefore, for MSABP patients, PC is a reliable method based on conservative treatment, which is safe and effective, relatively less traumatic for patients, and can reduce the severity of the disease as soon as possible and more significantly than conservative treatment.
MSABP patients can drain the bile-pancreatic fluid mixture from the body, reduce the degree of inflammation, and delay the progression of the disease after timely intervention with PC at an early stage in the course of the disease. The recurrence of ABP may be because the etiology of the disease is not completely eradicated, and the stones repeatedly stimulate the biliary mucosa, resulting in local edema. It has been suggested that the expression of cell surface bile acid receptors by pancreatic alveolar cells also induces ABP[4]. For ABP, the cause of the disease can be removed by cholecystectomy or ERCP after the patient has passed the acute phase of the disease. However, in some patients, the stone diameter is small and difficult to detect by imaging. It has been suggested that post cholecystectomy patients are more prone to AP recurrence, which may be caused by tiny gallbladder stones falling into the common bile duct intraoperatively[26]. ERCP can be used to remove the cause of ABP combined with choledochal stones in a straightforward manner, but only in patients with coexisting cholangitis and persistent bile duct obstruction[18].
The reduction in biliary pressure after PC depends on the degree of patency of the cystic duct, and it has been reported that the presence of biliary sludge seems to increase the likelihood of pancreatitis recurrence[27]. Biliary sludge is a suspension of cholesterol-hydrate crystals or calcium bilirubin particles found primarily in the gallbladder. Management of the sludge itself is unnecessary unless further complications arise. In such patients, PC may be the best therapeutic option to facilitate recovery and provide definitive drainage in the event of potentially critical illness[28].
Although the majority of patients recover from a first episode of ABP, a subset of patients remain at risk for recurrent episodes. There is evidence that AP episodes and their recurrence constitute a disease continuum. However, the mechanisms and risk factors for disease progression are still poorly understood[29]. Since AP induced by biliary tract disease accounts for the majority of the causes of pancreatitis in our country, an in-depth understanding of which patients are at risk for recurrence is crucial, as preventive measures and more intensive follow-up can be offered to these patients.
In the multifactorial analysis of this study, there were no statistically significant differences in the patients’ past history, comorbidities with other diseases, or severity of the disease compared with the nonrecurrent group. During AP, activated pancreatic enzyme autodigestion increases the level of cell necrosis and death. Necrotic cells release their components, and a study by Nakahira et al[30] indicated that mitochondrial dysfunction is associated with the progression of pancreatic cell necrosis and that the CTSI was greater in patients with relapsed ABP than in nonrelapsed patients, but the difference was not statistically significant in a multifactorial analysis. One study reported that no significant difference was observed between the recurrence rates of AP and CTSI[31], and the same result was obtained in our study. Therefore, although CTSI is associated with the recurrence of ABP, large studies are needed to confirm our results. The CCI was lower in patients with relapsed ABP than in those with nonrelapsed ABP, but the difference was not statistically significant according to the multifactorial analysis. The inclusion of age among the scoring factors for the CCI may be because the age of the nonrelapsed group was significantly greater than that of the relapsed group.
Interestingly, there was a predominance of male patients in the relapse group, but there was no significant difference. However, the age of the 10 patients in the recurrent group was lower than that of the nonrecurrent patients, which is consistent with the results reported in earlier studies[15,31,32], in which a greater proportion of recurrent pancreatitis was found in males and younger patients. The recurrence rate of AP in the patient population is crucial for advanced research. Therefore, nonelderly patients admitted for MSABP should be given special attention and close follow-up.
PC, as an interventional treatment modality, may be accompanied by certain surgical complications, and studies have shown that the technical success rate of PC is 98%-100%, with few procedure-related complications (mortality and major complications 0%-6.5%, minor complications 0%-20%), resulting in complications caused by damage to peripheral structures, which mainly include biliary tract injury leading to hemorrhage, bile leakage, and pneumoperitoneum[22,33,34]. These complications may increase the risk of death in patients. In this study, PC was performed by experienced interventional radiologists with proper radiological support, and the risk of injury to adjacent structures was minimized. The PC operations were all successful, and there were no intraoperative deaths from the PC. None of the patients experienced complications caused by PC before extubation, so we concluded that the deaths of patients after PC were not related to PC as an operation.
According to the multifactorial analysis, the proportion of patients with more severe pancreatitis (RAC as severe) was greater in the nonsurviving group than in the surviving group, but the difference was not statistically significant because of the small sample size. The severity of a patient’s disease may have a direct impact on survival and, in some cases, may cause severe systemic inflammatory response syndrome or even the development of multiple organ dysfunction. In the present study, seven patients with MSABP died, all of whom had a combination of other systemic diseases or persistent ground organ failure (lasting > 48 hours) to varying degrees[18].
Our findings predict that a high CCI before patient admission is independently associated with patient death after PC, which may indicate that multiple comorbidities in MSABP patients contribute to poor outcomes. The CCI has been widely used as a predictor of mortality for a variety of diseases[35] but is currently less frequently used in predicting death in MSABP patients. The innovative aspect of our study is the use of the CCI. MSABP patients with higher CCIs are generally in poorer condition, and appropriate treatment strategies are important. In the early stages of MSABP, patients are at high risk of dying from cardiovascular or pulmonary failure[11]. Patients with a high preoperative CCI who are transferred to the intensive care unit for further treatment in the presence of deterioration of systemic organ function due to MSABP are associated with increased mortality, and PC may be used as a temporary medical treatment measure for such patients in their terminal phase. Our study revealed that a CCI ≥ 5.5 was independently associated with increased mortality in patients who received PC early in MSABP.
Notably, in this study, the number of cases of combined cerebrovascular disease was smaller in deceased patients than in nonfatal patients; however, the percentage of deceased patients with combined cerebrovascular disease was much greater than that of nonfatal patients. Patients with comorbid cerebrovascular disease may be susceptible to infections and immune-related complications because of impaired brain function due to previous cerebrovascular events or decreased immune function due to chronic stress. In addition, in our study, patients who died were older but not significantly different, and patients with comorbid cerebrovascular disease may themselves be older because of their age, which makes them more susceptible to complications from other serious diseases. However, owing to the restricted sample size of the study, no statistically significant difference was detected in the association between the deceased patients and their comorbid cerebrovascular diseases.
First, our study had a small sample size, a short follow-up period, and retrospective collection of follow-up data, which may have led to information bias, but here, we utilized prospective information obtained from electronic databases. Second, the effect of the use of anti-inflammatory drugs in patients with PC was not analyzed, which may have contributed to bias, but it is worth noting that in our institution, the selection of antimicrobial drugs for patients with positive bile cultures after treatment with PC is promptly adjusted and downgraded to make it preferable. Patients with early MSABP who underwent PC had a high short-term mortality rate, with 7 of 54 patients dying during hospitalization, which further reduced the number of recurrences in surviving patients. Therefore, the above conclusions are based on limited findings, and further large-sample, multicenter, high-quality clinical studies are needed in the future to validate the clinical effects and further explore the mechanisms underlying these changes.
Early PC (within 72 hours of admission) is safe and effective for MSABP patients, can rapidly reduce infection and improve liver function through biliopancreatic shunts, and can be used as an important method for early treatment of MSABP. Age is a protective factor against the recurrence of ABP, i.e., a lower age increases the risk of recurrence after PC. Therefore, nonelderly patients admitted for MSABP should be given special attention and close follow-up. PC is not associated with mortality in MSABP patients. According to the multifactorial analysis, lower survival in MSABP patients was significantly associated with a high CCI before PC.
| 1. | Matta B, Gougol A, Gao X, Reddy N, Talukdar R, Kochhar R, Goenka MK, Gulla A, Gonzalez JA, Singh VK, Ferreira M, Stevens T, Barbu ST, Nawaz H, Gutierrez SC, Zarnescu NO, Capurso G, Easler J, Triantafyllou K, Pelaez-Luna M, Thakkar S, Ocampo C, de-Madaria E, Cote GA, Wu BU, Paragomi P, Pothoulakis I, Tang G, Papachristou GI. Worldwide Variations in Demographics, Management, and Outcomes of Acute Pancreatitis. Clin Gastroenterol Hepatol. 2020;18:1567-1575.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 480] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Shah AP, Mourad MM, Bramhall SR. Acute pancreatitis: current perspectives on diagnosis and management. J Inflamm Res. 2018;11:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 4. | Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Alburakan AA, Alshunaifi AI, AlRabah RN, Alshammari SA, Aloraini AM, Nouh TA, AlShahwan NA. Early versus delayed cholecystectomy in biliary pancreatitis: Experience from a Local Acute Care Surgery Unit in Saudi Arabia. Medicine (Baltimore). 2023;102:e36491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Song Y, Lee SH. Recent Treatment Strategies for Acute Pancreatitis. J Clin Med. 2024;13:978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 7. | Dubina ED, de Virgilio C, Simms ER, Kim DY, Moazzez A. Association of Early vs Delayed Cholecystectomy for Mild Gallstone Pancreatitis With Perioperative Outcomes. JAMA Surg. 2018;153:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Gad MM, Simons-Linares CR. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? A meta-analysis of randomized control trials and cohort studies. World J Gastroenterol. 2020;26:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (6)] |
| 9. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 598] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 10. | Yu W, Li W, Wang Z, Ye X, Li N, Li J. Early percutaneous transhepatic gallbladder drainage compared with endoscopic retrograde cholangiopancreatography and papillotomy treatment for severe gallstone associated acute pancreatitis. Postgrad Med J. 2007;83:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Hayat U, Bakker C, Dirweesh A, Khan MY, Adler DG, Okut H, Leul N, Bilal M, Siddiqui AA. EUS-guided versus percutaneous transhepatic cholangiography biliary drainage for obstructed distal malignant biliary strictures in patients who have failed endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:4-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Hallensleben ND, Stassen PMC, Schepers NJ, Besselink MG, Anten MGF, Bakker OJ, Bollen TL, da Costa DW, van Dijk SM, van Dullemen HM, Dijkgraaf MGW, van Eijck B, van Eijck CHJ, Erkelens W, Erler NS, Fockens P, van Geenen EM, van Grinsven J, Hazen WL, Hollemans RA, van Hooft JE, Jansen JM, Kubben FJGM, Kuiken SD, Poen AC, Quispel R, de Ridder RJ, Römkens TEH, Schoon EJ, Schwartz MP, Seerden TCJ, Smeets XJNM, Spanier BWM, Tan ACITL, Thijs WJ, Timmer R, Umans DS, Venneman NG, Verdonk RC, Vleggaar FP, van de Vrie W, van Wanrooij RLJ, Witteman BJ, van Santvoort HC, Bouwense SAW, Bruno MJ; Dutch Pancreatitis Study Group. Patient selection for urgent endoscopic retrograde cholangio-pancreatography by endoscopic ultrasound in predicted severe acute biliary pancreatitis (APEC-2): a multicentre prospective study. Gut. 2023;72:1534-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Wen Y, Xu L, Zhang D, Sun W, Che Z, Zhao B, Chen Y, Yang Z, Chen E, Ni T, Mao E. Effect of early antibiotic treatment strategy on prognosis of acute pancreatitis. BMC Gastroenterol. 2023;23:431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Zhang XL, Sun JH, Wu Y, Li CC, Lv D, Yu W, Cui PL. Therapeutic outcomes of early and delayed ERCP and PTCD in patients with obstructive severe acute biliary pancreatitis. J Clin Transl Res. 2023;9:160-167. [DOI] [Full Text] |
| 15. | Cho JH, Jeong YH, Kim KH, Kim TN. Risk factors of recurrent pancreatitis after first acute pancreatitis attack: a retrospective cohort study. Scand J Gastroenterol. 2020;55:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Abu-El-Haija M, Hornung L, Lin TK, Nathan JD, Thompson T, Vitale DS, Nasr A, Husain SZ, Denson L. Drug induced pancreatitis is the leading known cause of first attack acute pancreatitis in children. Pancreatology. 2020;20:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Argaiz ER, de Moraes AG. Acute pancreatitis. Lancet. 2021;397:279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 613] [Article Influence: 122.6] [Reference Citation Analysis (1)] |
| 19. | Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 344] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 205] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 21. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 22. | Howard JM, Hanly AM, Keogan M, Ryan M, Reynolds JV. Percutaneous cholecystostomy--a safe option in the management of acute biliary sepsis in the elderly. Int J Surg. 2009;7:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kang H, Yang Y, Zhu L, Zhao X, Li J, Tang W, Wan M. Role of neutrophil extracellular traps in inflammatory evolution in severe acute pancreatitis. Chin Med J (Engl). 2022;135:2773-2784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | van Geenen EJ, van der Peet DL, Bhagirath P, Mulder CJ, Bruno MJ. Etiology and diagnosis of acute biliary pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Molla NW, Zaini RH, Alfaiz FA, Alkhayatt AM, AlJohani MA, Alomar MO, Aljohani AA, BinMayouf MS, Alyamani AA, Alsergani AH. Risk Factors Associated With the Development of Acute Peripancreatic Fluid Collections on Follow-Up Imaging After Acute Pancreatitis: What Physicians Need to Know. Cureus. 2023;15:e50471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | da Costa DW, Bouwense SA, Schepers NJ, Besselink MG, van Santvoort HC, van Brunschot S, Bakker OJ, Bollen TL, Dejong CH, van Goor H, Boermeester MA, Bruno MJ, van Eijck CH, Timmer R, Weusten BL, Consten EC, Brink MA, Spanier BWM, Bilgen EJS, Nieuwenhuijs VB, Hofker HS, Rosman C, Voorburg AM, Bosscha K, van Duijvendijk P, Gerritsen JJ, Heisterkamp J, de Hingh IH, Witteman BJ, Kruyt PM, Scheepers JJ, Molenaar IQ, Schaapherder AF, Manusama ER, van der Waaij LA, van Unen J, Dijkgraaf MG, van Ramshorst B, Gooszen HG, Boerma D; Dutch Pancreatitis Study Group. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet. 2015;386:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 27. | Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 313] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Ko CW, Lee SP. Gastrointestinal disorders of the critically ill. Biliary sludge and cholecystitis. Best Pract Res Clin Gastroenterol. 2003;17:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Machicado JD, Yadav D. Epidemiology of Recurrent Acute and Chronic Pancreatitis: Similarities and Differences. Dig Dis Sci. 2017;62:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Nakahira K, Hisata S, Choi AM. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid Redox Signal. 2015;23:1329-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Zhang J, Du JJ, Tang W, Zhang XY, Jiang R, Yang GQ, Zhang XM. CT characteristics of recurrent acute pancreatitis and acute pancreatitis in different stages-a retrospective cross-sectional study. Quant Imaging Med Surg. 2023;13:4222-4233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Boumezrag M, Harounzadeh S, Ijaz H, Johny A, Richards L, Ma Y, Le Saux MA, Kulie P, Davis C, Meltzer AC. Assessing the CT findings and clinical course of ED patients with first-time versus recurrent acute pancreatitis. Am J Emerg Med. 2019;37:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Pan YL, Wu PS, Chen JH, Chen LY, Fang WL, Chau GY, Lee KC, Hou MC. Early cholecystectomy following percutaneous transhepatic gallbladder drainage is effective for moderate to severe acute cholecystitis in the octogenarians. Arch Gerontol Geriatr. 2023;106:104881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Park JM, Kang CD, Lee M, Park SC, Lee SJ, Jeon YH, Cho SW. Percutaneous cholecystostomy for biliary decompression in patients with cholangitis and pancreatitis. J Int Med Res. 2018;46:4120-4128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Mnatzaganian G, Ryan P, Norman PE, Hiller JE. Accuracy of hospital morbidity data and the performance of comorbidity scores as predictors of mortality. J Clin Epidemiol. 2012;65:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/