Published online Feb 7, 2025. doi: 10.3748/wjg.v31.i5.98732
Revised: October 8, 2024
Accepted: December 4, 2024
Published online: February 7, 2025
Processing time: 179 Days and 5.4 Hours
Inflammatory bowel disease (IBD) is a common chronic intestinal inflammatory disease. High oxidative stress is a treatment target for IBD. Cerium oxide (CeO2) nanomaterials as nanozymes with antioxidant activity are potential drugs for the treatment of colitis.
To synthesize hollow cerium (H-CeO2) nanoparticles by one-step method and to validate the therapeutic efficacy of H-CeO2 in IBD.
H-CeO2 was synthesized by one-step method and examined its characterization and nanoenzymatic activity. Subsequently, we constructed dextran sulfate so
H-CeO2 nanoparticles prepared by the one-step method were uniform, monodi
The one-step synthesis of H-CeO2 nanomaterials had good antioxidant activity, biosafety, and inhibited deve
Core Tip: Inflammatory bowel disease (IBD) is a common chronic intestinal inflammatory disease. High oxidative stress is a treatment target for IBD. Cerium oxide nanomaterials as nanozymes with antioxidant activity are potential drugs for the treatment of colitis. To synthesize hollow cerium (H-CeO2) nanoparticles by one-step method and to study the physicochemical properties, biosafety, and therapeutic efficacy of H-CeO2 in IBD. In this study, we found that a one-step synthesis of H-CeO2 nanomaterials has good antioxidant activity, biosafety, and can inhibit the development of dextran sulphate sodium-induced IBD in mice by scavenging reactive oxygen species.
- Citation: Mi L, Zhang K, Ma JX, Yao JF, Tong YL, Bao ZJ. Hollow cerium nanoparticles synthesized by one-step method for multienzyme activity to reduce colitis in mice. World J Gastroenterol 2025; 31(5): 98732
- URL: https://www.wjgnet.com/1007-9327/full/v31/i5/98732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i5.98732
Inflammatory bowel disease (IBD) is a common disease characterized by chronic inflammation of the intestine. IBD includes two subtypes: Crohn’s disease (CD) and ulcerative colitis (UC). IBD can present with bloody or non-bloody diarrhea, abdominal pain, weight loss, or extraintestinal symptoms such as a rash or arthritis[1]. Due to the damage of intestinal mucosal barrier and the increase of inflammatory cells, the content of fecal calprotectin is increased in IBD patients. Currently, there are at least three main classes of drugs for the treatment of IBD: Anti-inflammatory drugs, immunosuppressants/immunomodulators, and biologics/biosimilars (e.g., anti-tumor necrosis factor-α antibodies, anti-integrins, etc.). However, these drugs have the defects of non-specific distribution and short retention time. Patients taking these drugs for a long time may suffer from side effects, and the symptoms of a considerable number of patients are not relieved. Safe and effective drugs for IBD treatment remain to be discovered and developed[2].
Although the exact etiology of IBD remains uncertain, hyperoxidative stress caused by excess production of reactive oxygen species (ROS) is a major key to the progression of colitis and is considered a therapeutic target for the treatment of colitis. Oxidative stress generated by inflammatory activity is induced by the accumulation of ROS beyond the regulation of mechanisms of antioxidant protection. Oxidative stress causes damage to proteins, lipids, cell membranes, organelles, etc., making cell function and cell signaling difficult. In addition, the cell damage caused by the accumulation of ROS induces the production of proinflammatory mediator molecules, which further amplifies inflammation and aggravates the symptoms of IBD. Unfortunately, traditional reductive drugs are not sufficient to clear a large amount of ROS indu
With the development of nanomaterials, nanomaterials such as Prussian blue, selenium, and cerium oxide (CeO2) have been explored and synthesized for the treatment of IBD. These nanomaterials have reductive properties, good biocompatibility, few side effects, and easy surface modification for enhanced targeting capabilities[6-8]. In addition, microenvironmental changes such as decreased intestinal tight junctions and increased permeability due to intestinal inflammation lead to reduced effectiveness of traditional therapeutic drugs. Nanomaterials with appropriate sizes are passively en
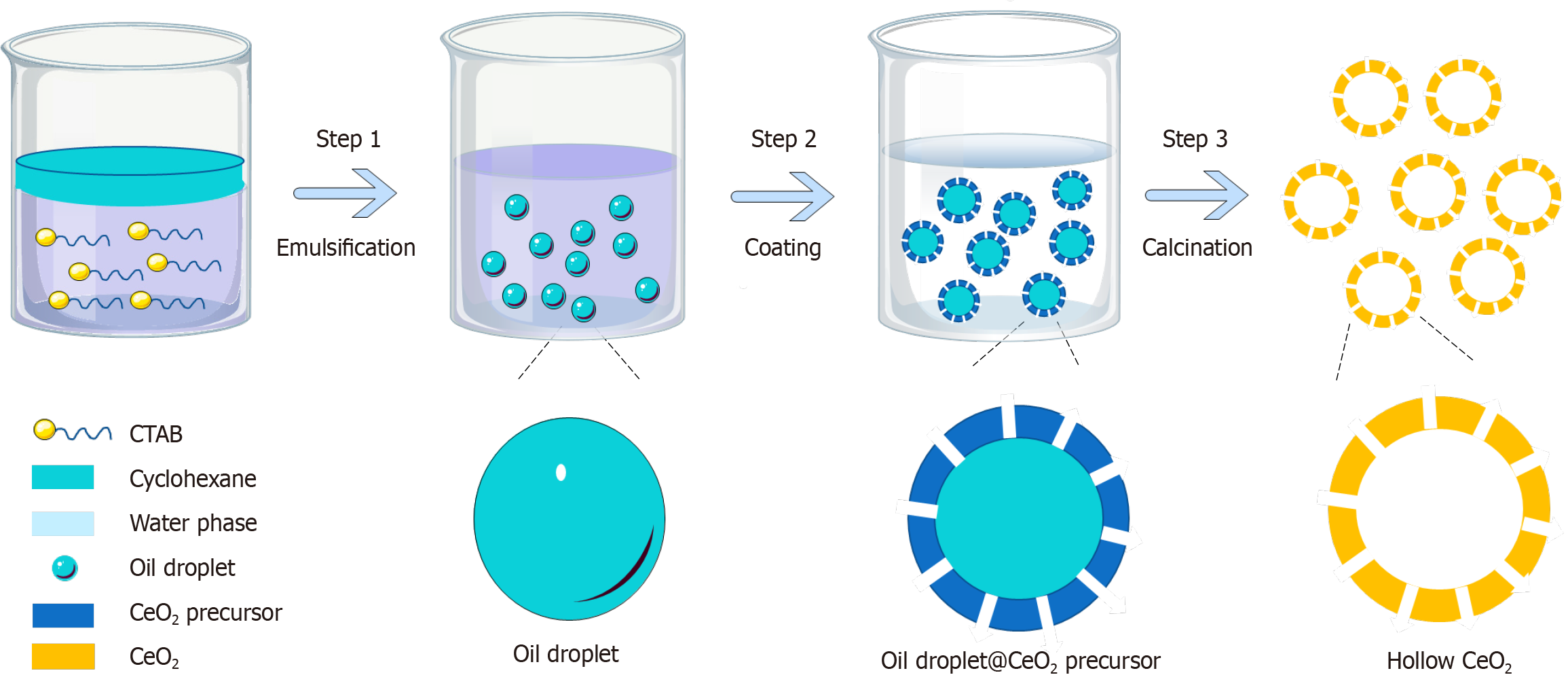
However, previously well-designed materials have limited applications due to uncertain biocompatibility and complex fabrication processes. Various synthetic methods such as electrochemical deposition, hydrothermal/solvothermal treatment and thermal decomposition of complex sources in solution phase have been developed for the preparation of shape-controllable CeO2 nanomaterials. The traditional synthesis method of CeO2 nanomaterials involves more experimental steps. For example, the steps of the hydrothermal method include: (1) Dissolving Ce(NO3)3∙6H2O and NaOH in deionized water; (2) The two solutions are combined and stirred continuously to form a milky white mixture; (3) The mixture is treated under hydrothermal conditions for 6-72 hours; and (4) Centrifugation to separate the white precipitate, wash with deionized water, and dry to obtain a yellow powder[15]. To enhance feasibility, in this study, we synthesized homogeneous, monodisperse hollow CeO2 nanomaterials (H-CeO2) by a simple and economical one-step method (Figure 1), and validated their multienzyme activity to effectively treat murine colitis by scavenging ROS.
The H-CeO2 were synthesized by a one-step method as follows: Dissolve cerium nitrate (1.5 mg/mL), urea (0.6 mg/mL), citric acid (0.2 mg/mL) and cetyltrimethyl ammonium bromide (2 mg/mL) in a mixture of 5 mL water and 5 mL absolute ethanol to form a transparent solution. Add 1 mL cyclohexane into the solution and stir rapidly to form a white microe
Scanning electron microscopy pictures were obtained using a Hitachi S-4800 high-resolution scanning electron micro
POD is an ROS scavenging enzyme. Using 3,3’,5,5’-tetramethylbenzidine (TMB) as the detection substrate, the POD-like activity of H-CeO2 was detected in an HAc-NaAc buffer solution of 0.2 M H2O2 at room temperature. We measured the absorbance of the sample at 650 nm with a microplate reader. The reaction system was 30% H2O2, 10 mg/mL TMB, 10 μg/mL H-CeO2 solution and buffer solution.
We added 0.6 mL of H2O2 solution (0.6 M) to the pH = 7.4 phosphate buffered saline, then 0.2 mL of H-CeO2 solution (100 μg/mL) and mixed well. The oxygen content of H2O2 catalyzed by H-CeO2 was measured on a multiparameter analyzer with a specific oxygen electrode.
Various concentrations of H-CeO2 (0, 5, 10, 20, and 40 μg/mL) were added to solutions containing 0.1 mg/mL TiO2 and 50 mmol/L 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO). The mixture was placed in a quartz capillary and exposed to 300 nm UV for 10 minutes. Electron spin resonance and Bruker EMX Plus were used for recording.
The xanthine/xanthine oxidase system was used as the substrate to generate superoxide anion, and BMPO was used as the superoxide anion detector. Electron spin resonance spectra were obtained after adding different concentrations of H-CeO2 to the xanthine/xanthine oxidase system.
To examine the effect of H-CeO2 on ROS elimination at the cellular level, we used H2O2 to induce excessive ROS pro
BALB/c mice (8-10 weeks, 22-25 g, male, n = 21) were purchased from Shanghai Animal Experiment Center and raised in a specific pathogen-free facility. Mice were randomly divided into four groups: Control group, dextran sulfate sodium (DSS)-induced group, DSS + H-CeO2 group, DSS + sulfasalazine (SASP) positive control group. The mice were fed with 3% (weight%) DSS solution instead of water for 1 week to construct a DSS-induced colitis mouse model. The colitis group and the treatment group were injected with normal saline and H-CeO2 (20 mg/kg) intravenously on days 3-10. The positive control group was given SASP (100 mg/kg) by gavage. During the treatment period, the body weight of each mouse was determined. On day 11, mice were anesthetized with isoflurane, and disease activity index (DAI) was mea
Colon tissue samples were paraffin-embedded and sectioned. Paraffin tissue sections were rehydrated and stained with aqueous hematoxylin solution for several minutes. After the samples were dehydrated with alcohol, they were incubated in alcohol-eosin staining solution for 2-3 minutes. Finally, the tissue sections were dehydrated with alcohol, mounted and observed under a microscope.
Fresh colon tissue of control, DSS and treatment groups was taken for frozen sectioning. Tissue sections were stained with DCFH-DA and developed using a chromogenic reagent for 15 minutes at 37 °C in the dark. Finally, tissue sections were incubated with stop solution and observed under a microscope.
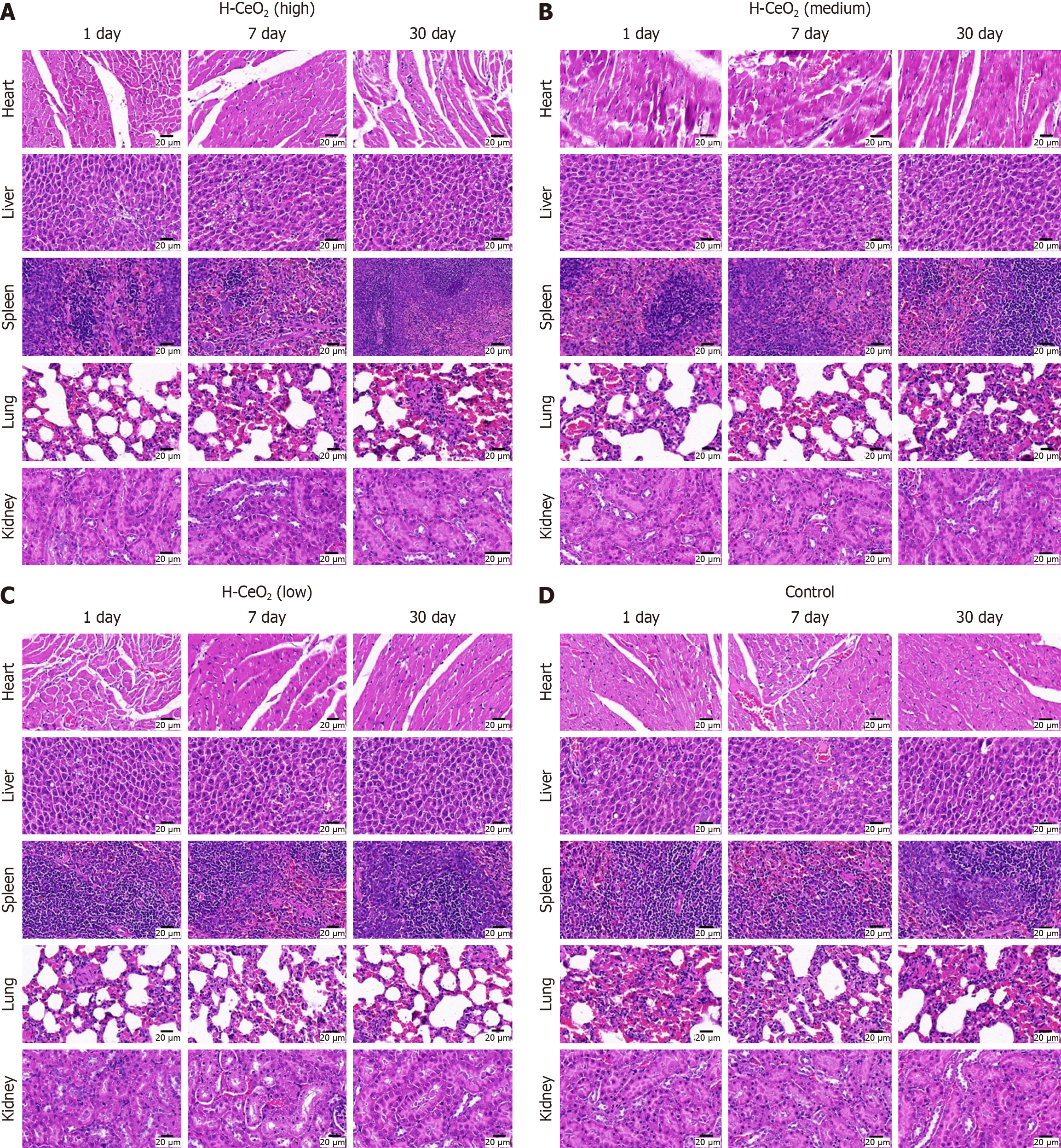
Healthy BALB/c mice were injected with 100 μL normal saline and different concentrations of H-CeO2 (0.01 mg/mL, 1 mg/mL, and 2 mg/mL) via the tail vein. The main organs (heart, liver, spleen, lung and kidney) of nude mice were collected on 7 and 30 days after injection, and the damage to specific organs by nanomaterials was observed by hema
The experimental data were expressed as mean ± SD. The results were analyzed by one-way analysis of variance (ANOVA) with the statistical software SPSS version 16.0. The t-test was used for comparison between the two groups, and one-way ANOVA was used for the comparison of the means of multiple samples. P < 0.05 represented a statistically significant difference.
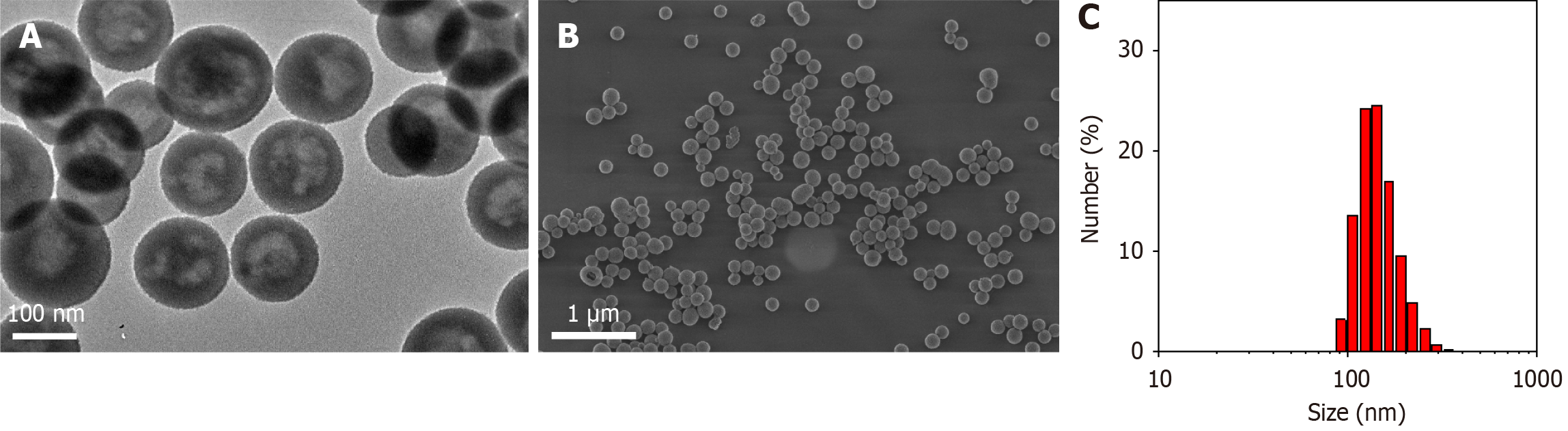
The morphology of the nanoparticles was observed by transmission and scanning electron microscopy (Figure 2A and B). It can be seen that the prepared H-CeO2 nanoparticles had a uniform, monodisperse and hollow morphology. The cavity of H-CeO2 was well maintained without collapse. Dynamic light scattering showed that the average particle size of H-CeO2 was about 140 nm (Figure 2C).
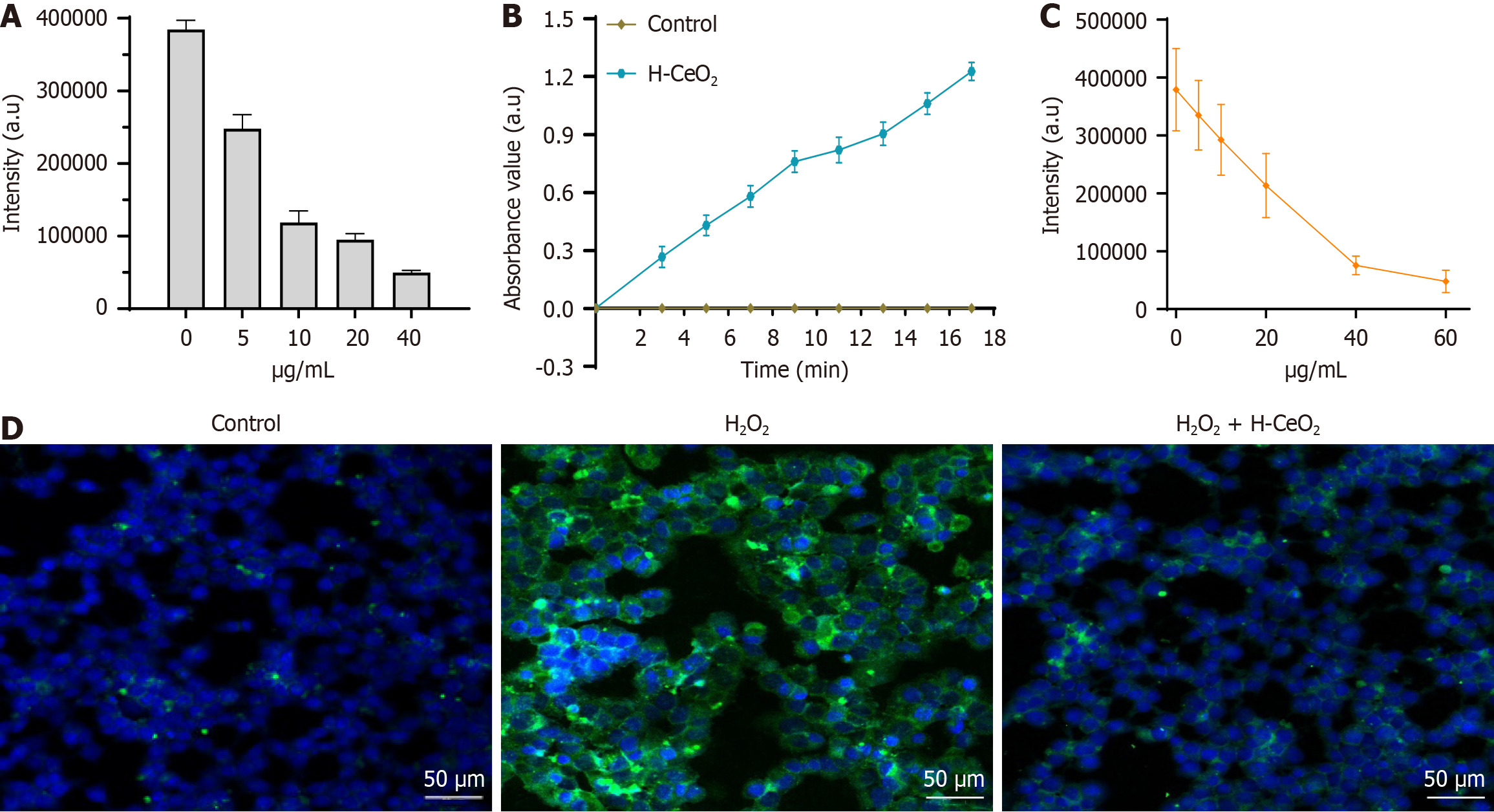
We investigated the scavenging effect of H-CeO2 on ROS in the TiO2/UV system generated by hydroxyl radicals in vitro. On the TiO2/UV system without H-CeO2 (control, 0 μm/mL), the signal intensity of BMPO/∙OH was the strongest. When the concentration of H-CeO2 increased from 0 to 10 μm/mL, the signal intensity of BMPO/∙OH had a sharp drop to 30.8% of the control group. When the concentration of H-CeO2 increased from 10 μg/mL to 40 μg/mL, the signal intensity of BMPO/∙OH still showed a decreasing trend, and the signal intensity at 40 μg/mL was only 12.9% of the control group. The results show that H-CeO2 had good ability to scavenge ∙OH (Figure 3A).
The POD-like activity of H-CeO2 was investigated with TMB; a natural POD substrate. After treatment with H-CeO2 nanoparticles, the absorbance of TMB at 650 nm rapidly and significantly increased from 0 to 0.9 within 13 minutes, indicating that H-CeO2 possessed POD-like activity (Figure 3B). In addition, the signal intensity of BMPO/∙OOH was significantly decreased in a H-CeO2 concentration-dependent manner, suggesting that H-CeO2 has SOD-like enzymatic activity for scavenging ∙OOH (Figure 3C).
Finally, we examined the scavenging ability of H-CeO2 on ROS in HCT116 cells. Cells were incubated with 100 μM
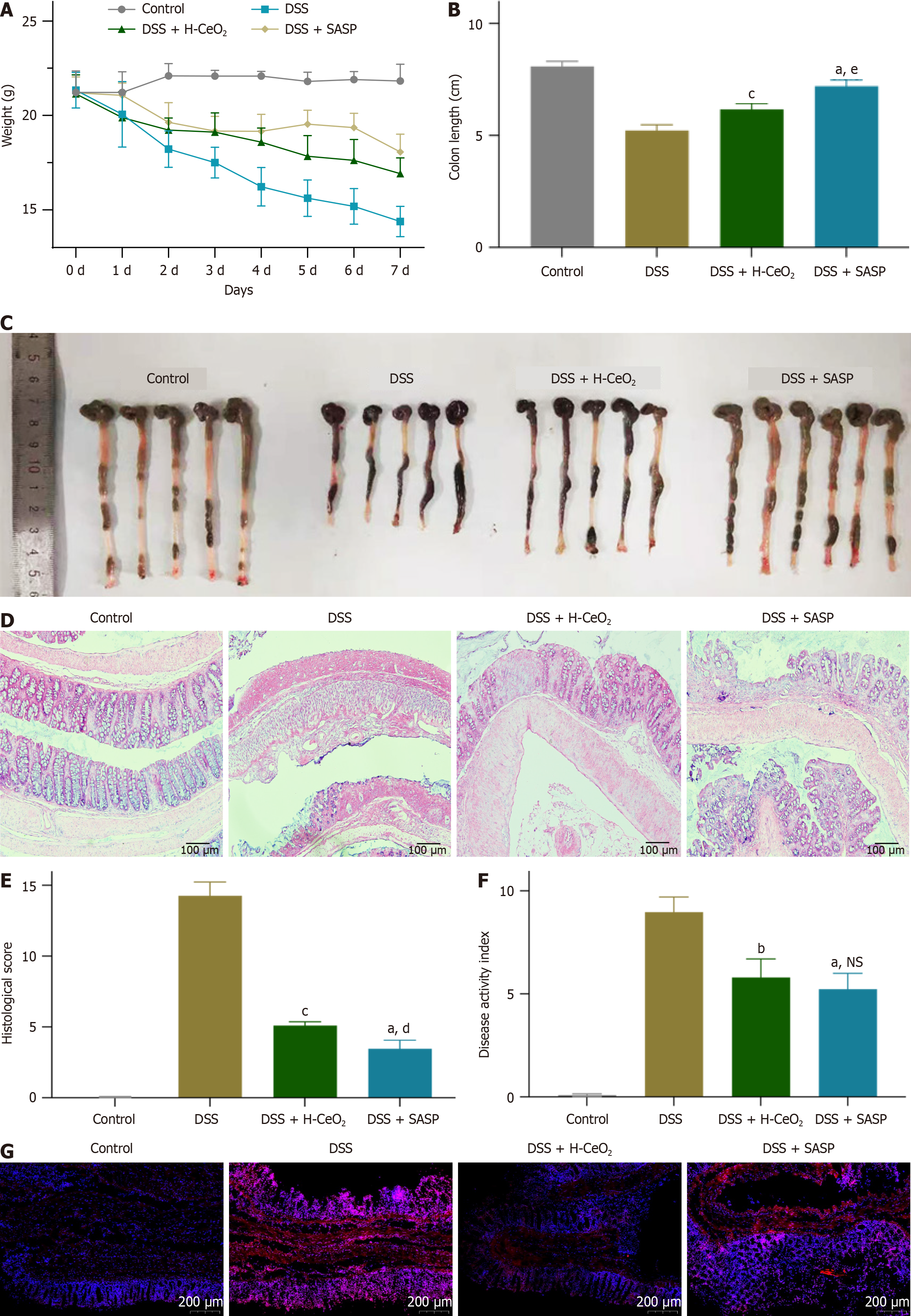
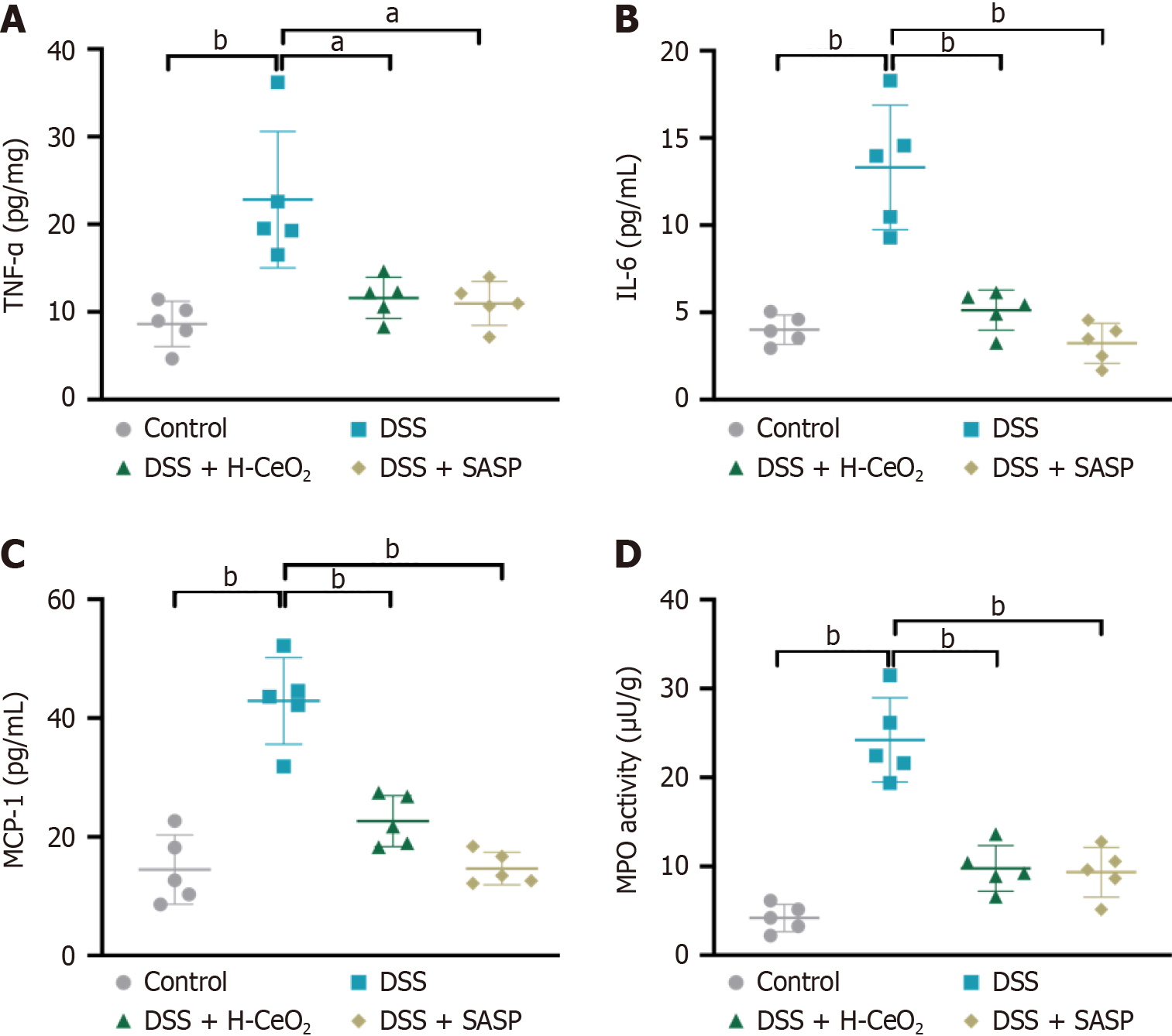
Based on the good POD-like and SOD-like activities of H-CeO2 nanoparticles, it is speculated that H-CeO2 scavenges ROS in inflammation-related diseases. DSS-induced mice exhibited weight loss, indicating the establishment of colitis. We found that H-CeO2 nanoparticle treatment inhibited the DSS-induced substantial decrease in body weight and colon length (Figure 4A-C). At the same time, the colon was collected for histological analysis, and the changes in colon structure after different treatments were observed. Histological analysis showed that the mucosal area of the colitis mice was severely damaged, a large number of inflammatory cells were infiltrated, and the colonic epithelium was severely damaged. However, after H-CeO2 treatment, only mild damage and inflammatory infiltration were observed in the mucosa or muscle. As a positive control, SASP, like H-CeO2, reduced DSS-induced colon tissue damage and inflammatory infiltration (Figure 4D and E). The DAI value of DSS-induced mice was about 8, while H-CeO2 treatment alleviated the DAI value to about 5, which means that H-CeO2 is effective in treating colitis (Figure 4F). Finally, we found that ROS was significantly increased in the tissues of the DSS-induced group compared with the control group, while ROS levels were significantly decreased after H-CeO2 treatment. This indicates that H-CeO2 has a good therapeutic effect on colitis by effectively scavenging ROS (Figure 4G). Compared with the control group, the levels of inflammatory factors tumor necrosis factor-α, interleukin 6, monocyte chemoattractant protein-1, and myeloperoxidase were significantly increased in the DSS group (Figure 5). These results suggested that H-CeO2 alleviated inflammation by eliminating ROS, although the effect was as good as that of the positive control.
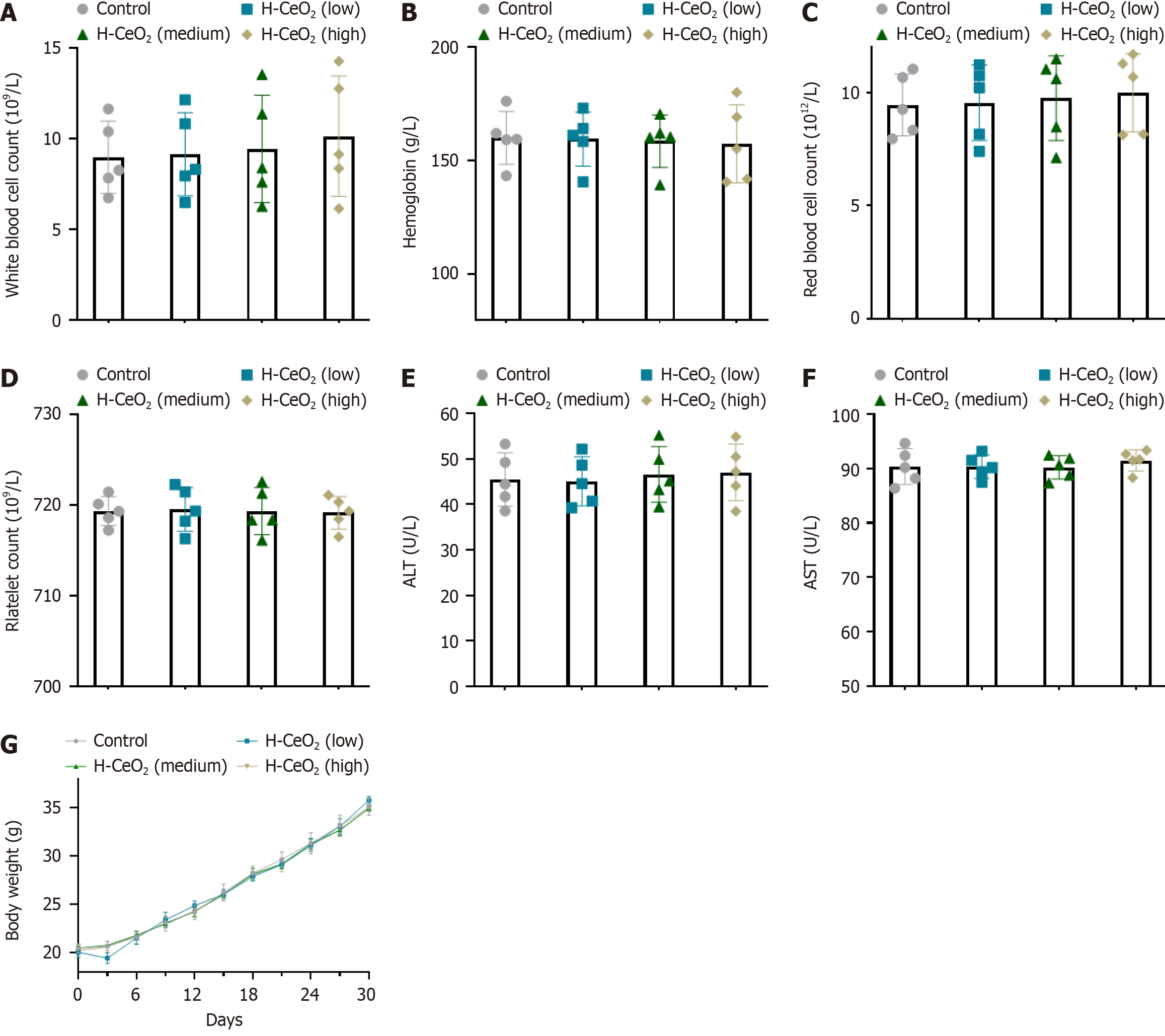
We randomly divided 20 BALB/c mice (6-8 weeks, female) into four groups of five, and injected 100 μL saline, or 0.01 mg/mL, 1 mg/mL or 2 mg/mL H-CeO2 intravenously. Thirty days after the first drug injection, serum was collected to measure blood biochemical and blood routine indexes. The total number of platelets, hemoglobin, white blood cells and red blood cells were not affected by H-CeO2, and there were no significant changes in liver cell injury markers, alanine aminotransferase and aspartate aminotransferase after H-CeO2 administration. In addition, no significant change in body weight was found (Figure 6). Finally, we took the main organs (heart, liver, spleen, lung and kidney) of one mouse on 1, 7, and 30 days, and the histopathological changes of the main organs were observed by hematoxylin and eosin staining. The results showed no obvious damage to the main organs of the H-CeO2 administration groups (Figure 7). These results confirmed the negligible toxicity of H-CeO2 after administration and demonstrated that H-CeO2 has good biocompatibility and biosafety.
CeO2 nanoparticles are potential nanomedicines for the treatment of colitis[16]. In this study, we reported that the one-step synthesis technology of H-CeO2; clarification that the H-CeO2 particles synthesized by the one-step method had the characteristics of uniform particle size, monodispersity, and hollowness; H-CeO2 synthesized by the one-step method had antioxidant activity in vitro, and effectively scavenged ROS in colon tissue of DSS-induced mice in vivo, inhibit colonic inflammatory injury, reduce DAI index, and showed good biocompatibility and security.
CeO2 nanomaterials are widely used in biology, catalysis, gas sensors, and other fields. Through different methods and parameter adjustment, CeO2 nanomaterials with different structures and shapes such as rods, flowers and tubes can be obtained[17-20]. The morphology of CeO2 nanomaterials is closely related to their catalytic activity and physicochemical properties. It has been reported that adjusting the temperature parameters of the hydrothermal method can control the formation of spherical or cubic morphologies of nanoparticles, and the morphology of nanoparticles affects their antibacterial biological activities[21]. Other researchers have synthesized CeO2 nanomaterials with different morphologies and sizes such as nanospheres, nanorods, and flower-like microspheres by hydrothermal and solvothermal methods[22]. We constructed uniform, monodisperse, and H-CeO2 nanomaterials by a one-step method. The simplification of the synthesis method of medical nanomaterials is beneficial to the control of large-scale production costs and reduces the economic burden on patients. The one-step synthesis scheme in this study can provide technical support for the application of H-CeO2.
Several studies have shown that CeO2 has good antioxidant and anti-inflammatory effects, and can relieve the inflammation and clinical symptoms of IBD in mice. Yang et al[16] reported that H-CeO2 synthesized by hydrothermal method as an ROS scavenger reduced the level of proinflammatory cytokines and slowed DSS-induced colitis. Zhang et al[7] found that CeO2 modified by polydopamine and polyethylene glycol could scavenge ROS and regulate macrophage polarization, with antioxidant and anti-inflammatory properties, thus treating colitis. Zeng et al[23] developed a drug-free CeO2 nanoparticles that can inhibit the coactivation of nuclear factor-kappaB and Janus kinase 2/signal transducer and activator of transcription 3 pathways, thereby continuously scavenging ROS, downregulating the levels of a variety of proinflammatory cytokines, inhibiting the proinflammatory profile of macrophages and T helper 1/T helper 17 response, and improving the proinflammatory microenvironment. These studies suggest that changes in the material process may alter its physicochemical properties and catalytic activity, which in turn may alter its therapeutic effect on diseases. Our study demonstrates that the H-CeO2 constructed by the one-step method still has good nanoenzyme activity and can inhibit DSS-induced colonic inflammation in IBD mice. We also proved that H-CeO2 has good biocompatibility and safety.
Although the physicochemical properties and antioxidative and anti-inflammatory effects of H-CeO2in vitro and in vivo were studied in this study, the oral acceptability of the drug needs to be assessed to realize the clinical transformation of H-CeO2[10]. There is currently no evidence that cerium is naturally present in the human body, and no studies have reported how the human body clears CeO2 nanoparticles, leaving doubts about the safety of CeO2 for use in medicine[24]. Therefore, we will continue to explore the oral acceptability and in vivo metabolic pathways of CeO2 nanoparticles in future studies.
H-CeO2 nanoparticles prepared by the one-step method are homogeneous, monodisperse, hollow, and have good antioxidant activity. In an IBD mouse model, we demonstrated that H-CeO2 nanoparticles have good biosafety and inhibits the development of colonic inflammation by effectively scavenging ROS.
| 1. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 708] [Article Influence: 101.1] [Reference Citation Analysis (1)] |
| 2. | Liu P, Li Y, Wang R, Ren F, Wang X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines. 2021;10:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 3. | Teruel AH, Gonzalez-Alvarez I, Bermejo M, Merino V, Marcos MD, Sancenon F, Gonzalez-Alvarez M, Martinez-Mañez R. New Insights of Oral Colonic Drug Delivery Systems for Inflammatory Bowel Disease Therapy. Int J Mol Sci. 2020;21:6502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 4. | Zhang T, Jiang J, Liu J, Xu L, Duan S, Sun L, Zhao W, Qian F. MK2 Is Required for Neutrophil-Derived ROS Production and Inflammatory Bowel Disease. Front Med (Lausanne). 2020;7:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 5. | Fan W, Zhang S, Wu Y, Lu T, Liu J, Cao X, Liu S, Yan L, Shi X, Liu G, Huang C, Song S. Genistein-Derived ROS-Responsive Nanoparticles Relieve Colitis by Regulating Mucosal Homeostasis. ACS Appl Mater Interfaces. 2021;13:40249-40266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 6. | Zhao J, Cai X, Gao W, Zhang L, Zou D, Zheng Y, Li Z, Chen H. Prussian Blue Nanozyme with Multienzyme Activity Reduces Colitis in Mice. ACS Appl Mater Interfaces. 2018;10:26108-26117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 7. | Zhang B, Li Q, Xu Q, Li B, Dong H, Mou Y. Polydopamine Modified Ceria Nanorods Alleviate Inflammation in Colitis by Scavenging ROS and Regulating Macrophage M2 Polarization. Int J Nanomedicine. 2023;18:4601-4616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 8. | Zeeshan M, Ali H, Khan S, Khan SA, Weigmann B. Advances in orally-delivered pH-sensitive nanocarrier systems; an optimistic approach for the treatment of inflammatory bowel disease. Int J Pharm. 2019;558:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 9. | Zhang S, Langer R, Traverso G. Nanoparticulate Drug Delivery Systems Targeting Inflammation for Treatment of Inflammatory Bowel Disease. Nano Today. 2017;16:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 10. | Li DF, Yang MF, Xu HM, Zhu MZ, Zhang Y, Tian CM, Nie YQ, Wang JY, Liang YJ, Yao J, Wang LS. Nanoparticles for oral delivery: targeted therapy for inflammatory bowel disease. J Mater Chem B. 2022;10:5853-5872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 11. | Li M, Liu J, Shi L, Zhou C, Zou M, Fu D, Yuan Y, Yao C, Zhang L, Qin S, Liu M, Cheng Q, Wang Z, Wang L. Gold nanoparticles-embedded ceria with enhanced antioxidant activities for treating inflammatory bowel disease. Bioact Mater. 2023;25:95-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (1)] |
| 12. | Jiang P, Zhang L, Liu X, Ye C, Zhu P, Tan T, Wang D, Wang Y. Tuning oxidant and antioxidant activities of ceria by anchoring copper single-site for antibacterial application. Nat Commun. 2024;15:1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (1)] |
| 13. | Asgharzadeh F, Hashemzadeh A, Rahmani F, Yaghoubi A, Nazari SE, Avan A, Mehr SMH, Soleimanpour S, Khazaei M. Cerium oxide nanoparticles acts as a novel therapeutic agent for ulcerative colitis through anti-oxidative mechanism. Life Sci. 2021;278:119500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 14. | Apte A, Bardill JR, Canchis J, Skopp SM, Fauser T, Lyttle B, Vaughn AE, Seal S, Jackson DM, Liechty KW, Zgheib C. Targeting Inflammation and Oxidative Stress to Improve Outcomes in a TNBS Murine Crohn's Colitis Model. Nanomaterials (Basel). 2024;14:894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Sakthivel T, Das S, Kumar A, Reid DL, Gupta A, Sayle DC, Seal S. Morphological Phase Diagram of Biocatalytically Active Ceria Nanostructures as a Function of Processing Variables and Their Properties. Chempluschem. 2013;78:1446-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Yang J, Zhou J, Zhao Y, Zhu L, Luo G, Ge B. Hollow CeO(2) with ROS-Scavenging Activity to Alleviate Colitis in Mice. Int J Nanomedicine. 2021;16:6889-6904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Qian X, Qu Q, Li L, Ran X, Zuo L, Huang R, Wang Q. Ultrasensitive Electrochemical Detection of Clostridium perfringens DNA Based Morphology-Dependent DNA Adsorption Properties of CeO₂ Nanorods in Dairy Products. Sensors (Basel). 2018;18:29890646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Zhang X, Wei Y, Song Z, Liu W, Gao C, Luo J. Silicotungstic acid modified CeO2 catalyst with high stability for the catalytic combustion of chlorobenzene. Chemosphere. 2021;263:128129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 19. | Matsumori A, Kishimoto C, Kawai C, Sawada S. Right ventricular aneurysms complicating encephalomyocarditis virus myocarditis in mice. Jpn Circ J. 1983;47:1322-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Miran HA, Jaf ZN, Altarawneh M, Jiang ZT. An Insight into Geometries and Catalytic Applications of CeO(2) from a DFT Outlook. Molecules. 2021;26:6485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Nithya P, Sundrarajan M. Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO(2) nanoparticles from Justicia adhatoda for pharmaceutical applications: Antibacterial and anti-cancer activities. J Photochem Photobiol B. 2020;202:111706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Dong F, Meng Y, Han W, Zhao H, Tang Z. Morphology effects on surface chemical properties and lattice defects of Cu/CeO(2) catalysts applied for low-temperature CO oxidation. Sci Rep. 2019;9:12056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Zeng F, Shi Y, Wu C, Liang J, Zhong Q, Briley K, Xu B, Huang Y, Long M, Wang C, Chen J, Tang Y, Li X, Jiang M, Wang L, Xu Q, Yang L, Chen P, Duan S, Xie J, Li C, Wu Y. A drug-free nanozyme for mitigating oxidative stress and inflammatory bowel disease. J Nanobiotechnology. 2022;20:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Singh KR, Nayak V, Sarkar T, Singh RP. Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv. 2020;10:27194-27214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/