INTRODUCTION
Antibiotic resistance in Helicobacter pylori (H. pylori) remains a global challenge, undermining eradication success and complicating empiric therapy. The present multicenter Taiwanese study, analyzing 1408 treatment-naive isolates from 2019 to 2024, offers a comprehensive view of evolving resistance patterns[1]. It documents a significant rise in tetracycline resistance, a decline in metronidazole resistance, and stable but moderate resistance to clarithromycin and levofloxacin. These findings carry substantial implications for clinical practice, public health policy, and international guideline alignment.
TETRACYCLINE RESISTANCE: A NEW CLINICAL THREAT
Historically, tetracycline resistance in H. pylori has been rare, with global prevalence estimates below 5%[2,3]. In Taiwan, prior surveillance reported near-zero resistance[4], making the observed rise from 0% in 2019 to 3.5% in 2024 statistically and clinically significant. This trend threatens the efficacy of bismuth-based quadruple therapy, long considered the cornerstone of empiric treatment in regions with high clarithromycin resistance[5].
Mechanistically, tetracycline inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit, specifically the 16S rRNA[6]. Resistance arises from point mutations in the 16S rRNA gene, with high-level resistance requiring multiple substitutions[7]. A well-characterized triple-base substitution (AGA926-928 TTC) has been linked to elevated minimum inhibitory concentrations and clinical failure[8]. Additionally, efflux pump upregulation, particularly via the hefA gene, contributes to reduced intracellular drug concentrations[9].
Environmental exposure to tetracycline via aquaculture, livestock, and food residues may have contributed to resistance emergence. Studies have detected residual tetracyclines in Taiwanese water sources and food products[10,11], suggesting non-clinical reservoirs that exert selective pressure on microbial populations.
METRONIDAZOLE RESISTANCE: A DECLINING TREND
In contrast to tetracycline, metronidazole resistance declined from 35.5% to 13.0% over the study period. This reversal is noteworthy given that metronidazole resistance typically ranges from 30% to 60% globally[12,13]. The decline may reflect changes in prescribing practices, improved stewardship, or shifts in microbial ecology[14]. Clinically, reduced metronidazole resistance may help preserve the efficacy of bismuth quadruple therapy, even as tetracycline resistance rises. Prior studies have shown that this regimen remains effective despite dual clarithromycin/metronidazole resistance[15,16], but the impact of tetracycline-metronidazole dual resistance remains uncertain.
CLARITHROMYCIN AND LEVOFLOXACIN: STABILITY AMID GLOBAL ESCALATION
Clarithromycin resistance in this cohort ranged from 13.7% to 24.3%, remaining relatively stable compared to Taiwan’s historical baseline of approximately 15%[3]. This contrasts with rising resistance rates reported in mainland China (approximately 50%)[17], Japan (approximately 30%)[18], and Europe (approximately 23%)[19]. The stability may reflect reduced use of clarithromycin-based triple therapy in Taiwan, a practice aligned with international guidelines such as Maastricht VI, which discourage its use when resistance exceeds 15%[5].
Levofloxacin resistance also remained stable, though moderately elevated at approximately 28.7%. This is clinically relevant, as levofloxacin-containing regimens are often used as second-line therapy[20]. Resistance to fluoroquinolones has been linked to community-level antibiotic consumption, with higher rates observed in regions with greater outpatient use[21]. The study’s regional analysis revealed significantly higher levofloxacin resistance in southern Taiwan (30.9%) compared to eastern (20.2%) and northern (26.5%) regions, likely reflecting local prescribing patterns rather than biological variation[22].
DUAL RESISTANCE: A GROWING THREAT
The rise in dual resistance particularly combinations involving tetracycline warrants close attention. Dual resistance to clarithromycin plus tetracycline and metronidazole plus tetracycline both increased significantly from 0% to 1.7%. Although absolute rates remain low, the upward trajectory is concerning. Dual resistance is associated with markedly reduced eradication rates; for example, clarithromycin/metronidazole resistance can lower success to approximately 50%[23].
Importantly, no first-line regimen currently includes clarithromycin plus tetracycline, and dual tetracycline-metronidazole resistance could compromise even bismuth quadruple therapy. While increasing metronidazole doses may overcome resistance[24], the presence of dual resistance implies that empiric therapy may increasingly require culture-based susceptibility testing[25].
REGIONAL UNIFORMITY AND STEWARDSHIP SUCCESS
Aside from levofloxacin, no significant regional differences in resistance rates were observed. This likely reflects Taiwan’s centralized healthcare system and consistent prescribing practices under the National Health Insurance framework[26]. Uniform adherence to national guidelines may help contain resistance spread and ensure equitable treatment outcomes.
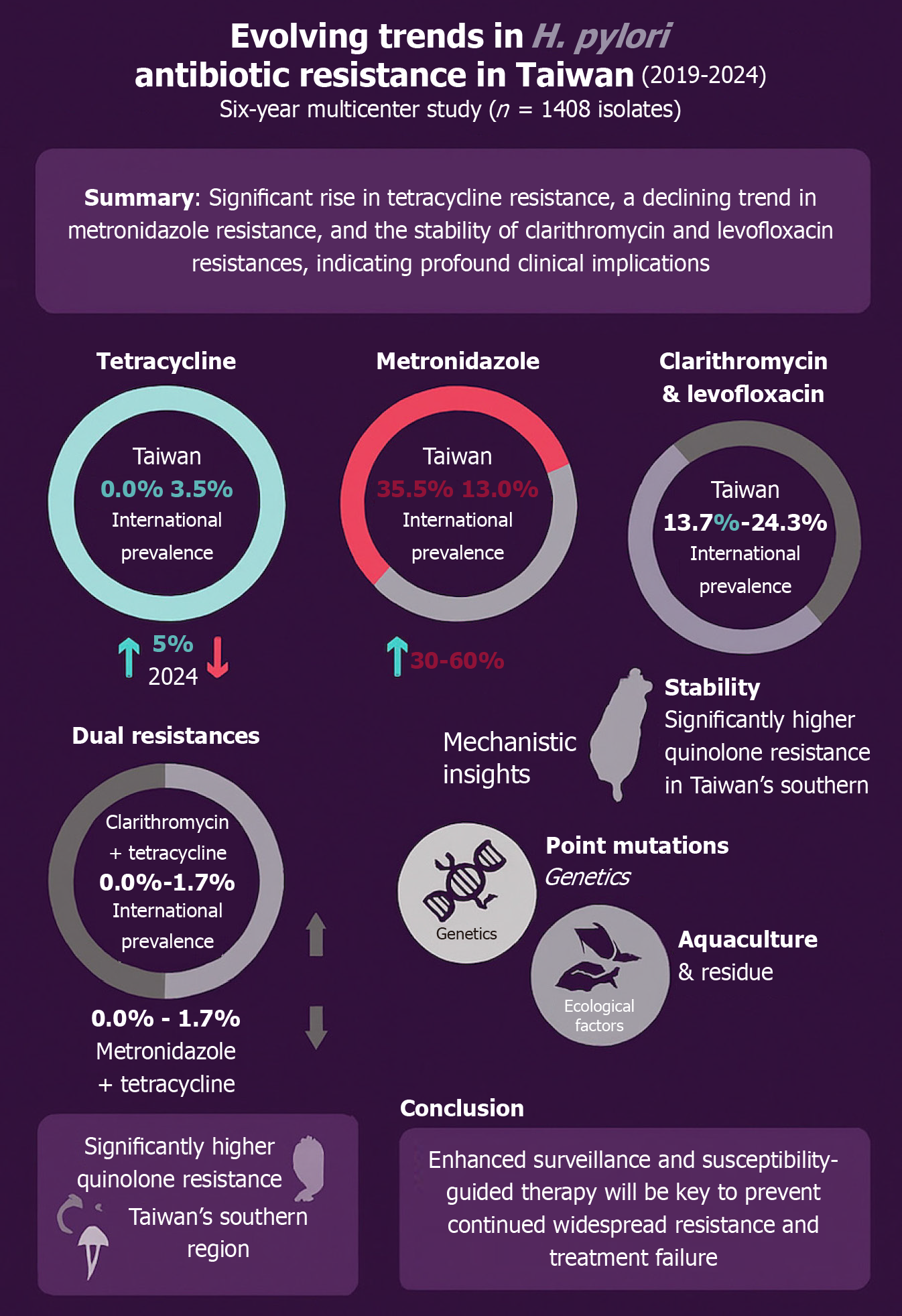
The regional variation in levofloxacin resistance underscores the importance of localized surveillance. Southern Taiwan’s higher resistance may be driven by outpatient fluoroquinolone use[21], while eastern Taiwan’s lower rates may reflect its rural character and reduced antibiotic exposure[27]. Figure 1 highlights the key findings from the published trial with a comparison to international trends and a summary of the suspected causative agents for the changes.
Figure 1 Evolving trends in Helicobacter pylori antibiotic resistance in Taiwan (2019-2024).
This infographic summarizes six-year surveillance data from 1408 treatment-naive patients across Taiwan. It highlights the rising prevalence of tetracycline resistance, the decline in metronidazole resistance, and the relative stability of clarithromycin and levofloxacin resistance. Dual resistance involving tetracycline is emerging, and regional variation in levofloxacin resistance is noted. Mechanistic insights include genetic mutations and environmental exposure contributing to resistance evolution. H. pylori: Helicobacter pylori.
LIMITATIONS AND FUTURE DIRECTIONS
The study’s retrospective design limits causal inference. Isolates were obtained from medical centers, potentially underrepresenting community clinics and introducing selection bias. The absence of molecular analyses precludes identification of specific resistance mutations or clonal spread[28]. Additionally, clinical outcomes were not assessed, leaving the impact of resistance trends on eradication success speculative[29]. Geographic coverage was uneven, with eastern regions underrepresented.
In clinical practice, non-biological factors such as inappropriate antibiotic usage and poor patient adherence have also contributed to the development of resistance in H. pylori eradication. Overprescription, self-medication, and incomplete treatment courses can foster subtherapeutic exposure, promoting selective pressure and resistance mutations[30]. A recent consensus report emphasized that patient noncompliance particularly with complex regimens significantly reduces eradication rates and may accelerate the emergence of resistance[5]. These behavioral and systemic contributors underscore the necessity of enhanced patient education, simplified treatment regimens, and stricter antibiotic stewardship to maintain eradication success.
Future research should incorporate whole-genome sequencing to elucidate resistance mechanisms, track strain evolution, and link resistance profiles to treatment outcomes[31]. Environmental surveillance of antibiotic residues could clarify non-clinical drivers of resistance[10,11].
POTASSIUM-COMPETITIVE ACID BLOCKERS: A PROMISING PHARMACOLOGIC PIVOT
In response to rising antibiotic resistance particularly to tetracycline and clarithromycin potassium-competitive acid blockers (PCABs) have emerged as a potent adjunct in H. pylori eradication therapy[32]. Unlike traditional proton pump inhibitors (PPIs), PCABs such as vonoprazan and tegoprazan provide rapid, sustained acid suppression, which enhances antibiotic stability and mucosal penetration critical factors for overcoming resistance-related treatment failure. A comprehensive meta-analysis of 28 randomized controlled trials involving 8818 patients found that PCAB-based regimens achieved significantly higher eradication rates than both PPI-based therapy and bismuth quadruple therapy [intention-to-treat: 87.0% vs 79.8%; risk ratio (RR) = 1.08, 95% confidence interval (CI): 1.04-1.12; P < 0.0001][33]. This benefit was especially pronounced in patients with clarithromycin-resistant strains, where PCAB-based therapy achieved a 73.7% eradication rate compared to 41.5% with PPI-based regimens (RR = 1.53, 95%CI: 1.07-2.20; P = 0.02). Another network meta-analysis of 21 randomized controlled trials confirmed that vonoprazan-based quadruple therapy ranked highest in both efficacy and safety, with high-dose dual therapy showing the lowest incidence of adverse events (surface under the cumulative ranking curve: 0.952)[34]. These findings underscore the pharmacologic advantages of PCABs, including their independence from CYP2C19 polymorphisms and superior acid control, which collectively improve antibiotic performance even in resistant infections. While most data originate from East Asian populations, the consistency across trials suggests PCABs may offer a globally relevant solution. As Taiwan faces increasing dual resistance, integrating PCABs into empiric and susceptibility-guided regimens could help preserve eradication success and reduce reliance on failing antibiotic combinations. However, broader implementation will require consideration of cost-effectiveness, insurance coverage, and regulatory approval status. In Taiwan, vonoprazan was approved in 2022 but remains more expensive than PPIs, potentially limiting access in community settings. Ensuring equitable availability especially in rural or under-resourced regions will be essential if PCABs are to fulfill their promise as a cornerstone of future eradication strategies.
CONCLUSION
The Taiwanese multicenter study reveals a shifting H. pylori resistance profile that demands both clinical and policy recalibration[1]. The rise in tetracycline resistance, though modest, threatens the durability of bismuth-based quadruple therapy long a cornerstone in high-resistance settings. Meanwhile, the decline in metronidazole resistance and stability of clarithromycin and levofloxacin contrast with global trends, reflecting relative stewardship success. Yet the emergence of dual resistance involving tetracycline foreshadows an era where empiric therapy may fail without susceptibility testing. Environmental antibiotic exposure and regional prescribing patterns underscore that resistance is shaped by both medical and non-medical ecosystems. National uniformity in prescribing has contained some threats, but regional levofloxacin variability highlights the need for localized strategies. PCABs offer cautious optimism: By enhancing antibiotic efficacy regardless of resistance drivers, they may restore eradication rates and prolong empiric options. Their integration in Taiwan could serve as a model for other regions confronting rising multidrug resistance. In short, tetracycline resistance is now clinically relevant, dual resistance threatens standard regimens, and empiricism is nearing its limits. Sustaining eradication will require a blended approach molecular-guided therapy, evolved stewardship, and adoption of PCAB-based regimens.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: United Arab Emirates
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Huang ZP, PhD, Associate Professor, China S-Editor: Fan M L-Editor: A P-Editor: Lei YY