Published online Dec 14, 2025. doi: 10.3748/wjg.v31.i46.114415
Revised: October 14, 2025
Accepted: October 29, 2025
Published online: December 14, 2025
Processing time: 83 Days and 6.5 Hours
Liver diseases caused by diverse inflammation or cancer have serious damaged people’s physical and mental health and become a heavy social burden. Stem cells possess unique properties of self-renewal and multi-directional differentiation, which are widely used for disease remodeling and regenerative medicine. Of them, human induced pluripotent stem cell-based liver organoids with self-organization of sinusoidal vessels have been reported for diverse liver cancer remodeling and drug sensitivity test, which provide unique platforms to dissect functional consequences of diverse levels of heteroplasmy of target gene mutation in liver cancers. Meanwhile, mesenchymal stem/stromal cells have been adopted for clinical treatment research on various liver diseases, including acute liver injury, decompensated liver cirrhosis, hepatic fibrosis and acute-on-chronic liver failure. In this review article, we mainly focus on the state-of-the-art literatures upon stem cell-based disease modeling and cell therapy for multifarious liver diseases from the view of basic research and clinical progress, which will provide references for the development of stem cell-based rege
Core Tip: Liver diseases of multitudinous types have caused great burden to public health and social economics. In this review article, we outline the fundamental and clinical progress of stem cell-based strategies for liver disease diagnosis and intervention, and in particular, the human induced pluripotent stem cells-based organoids for disease modeling and mesenchymal stem/stromal cells-based regimens for therapeutic purposes. Overall, our review will provide new references for facilitating the development of stem cell-based precision medicine for liver diseases.
- Citation: Wang YX, Ren YN, Zhang SS, Sun S, Xu MY, Wei T, Zhang LS. Application of stem cells in the precise diagnosis and treatment of liver diseases. World J Gastroenterol 2025; 31(46): 114415
- URL: https://www.wjgnet.com/1007-9327/full/v31/i46/114415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i46.114415
The liver is composed of primarily hepatocytes, hepatic parenchymal cells (e.g., hepatocytes) and non-parenchymal cells [e.g., hepatic stellate cells (HSCs), Kupffer cells, liver sinusoidal endothelial cells (LSECs)]. Proverbially, the liver plays a critical role in metabolism (e.g., protein synthesis, nutrient metabolism), detoxification and biochemical processes[1,2]. The dysregulation of homeostasis and abnormity in cellular interactions commonly results in metabolic zonation destruction and liver diseases[3], including liver injury[4], liver fibrosis[5], liver cirrhosis[6,7], metabolic associated fatty liver disease[8], metabolic dysfunction-associated steatotic liver disease (MASLD)[9,10], alcoholic steatohepatitis[11], acute-on-chronic liver failure (ACLF)[12], and even hepatocellular carcinoma (HCC)[13,14]. To date, diverse pathogenic factors have been identified as modulators of liver disease trajectory, including virus infection (e.g., hepatitis B virus[15], hepatitis C virus[16], hepatitis D virus[17]), gut microbiota[18], physical and chemical factors (ethanol[19], radiation[20]), oxidative stress (e.g., oxygen gradient within liver acinus[3]). Meanwhile, diverse liver disorders are also related with chronic inflammation and atherogenic dyslipidemia[21]. The liver disease-associated endothelial dysfunction and aging-associated cellular senescence[22] are orchestrated by a variety of proinflammatory signal transduction networks in the microenvironment, e.g., Wnt signaling[23], interleukin (IL) 6 trans-signaling[24], phosphoinositide 3-kinases (PI3K)-protein kinase B (AKT) signaling[25], nuclear factor-κB signaling[26,27], transforming growth factor-β (TGF-β) signaling[28]. Meanwhile, multifarious therapeutics and the relative interventions have been developed for liver disease administration, including surgical treatment (e.g., liver transplantation), pharmacotherapy, chemotherapy[20], and even chimeric antigen receptor-transduced T cell- and natural killer cells-based cell therapy (e.g., chimeric antigen receptor-modified T[29,30], chimeric antigen receptor natural killer[31-33]).
Stem cells are cell populations with self-renewal and multi-lineage differentiation properties, which play a prominent role in embryonic development, tissue repair and regeneration, disease modeling and drug screening[34-36]. Overall, stem cells can be classified in many ways. According to the developmental stages, stem cells can be divided into embryonic stem cells (ESCs)[37,38], adult stem cells[39] (e.g., dental pulp stem cells[40,41], bone marrow (BM)-derived mesenchymal stem/stromal cells (MSCs)[42], adipose tissue-derived stem cells[43,44]), and perinatal stem cells (e.g., umbilical cord-derived stem cells[45], placenta tissue-derived stem cells[46], amniotic stem cells[47]). According to the differentiation potential, stem cells include various subtypes such as totipotent stem cells, pluripotent stem cells [e.g., ESCs[35,48], induced pluripotent stem cells (iPSCs)[49]], multipotent stem cells (hematopoietic stem cells[50], MSCs[51]), and unipotent stem cells (e.g., neural stem cells[52]). Additionally, stem cells can be catalogued into natural stem cells (e.g., ESCs[53]) and artificial stem cells (e.g., haploid stem cells[54-56], iPSCs[57]), or classified into autologous stem cells[58] (e.g., autologous hematopoietic stem cells[59,60], autologous adipose tissue-derived stem cells[61], autologous iPSCs[62]) and allogeneic stem cells[63] (e.g., allogeneic germline stem cells[64], allogeneic iPSCs[65], allogeneic adipose-derived mesenchymal stem cells[66]) according to the origins.
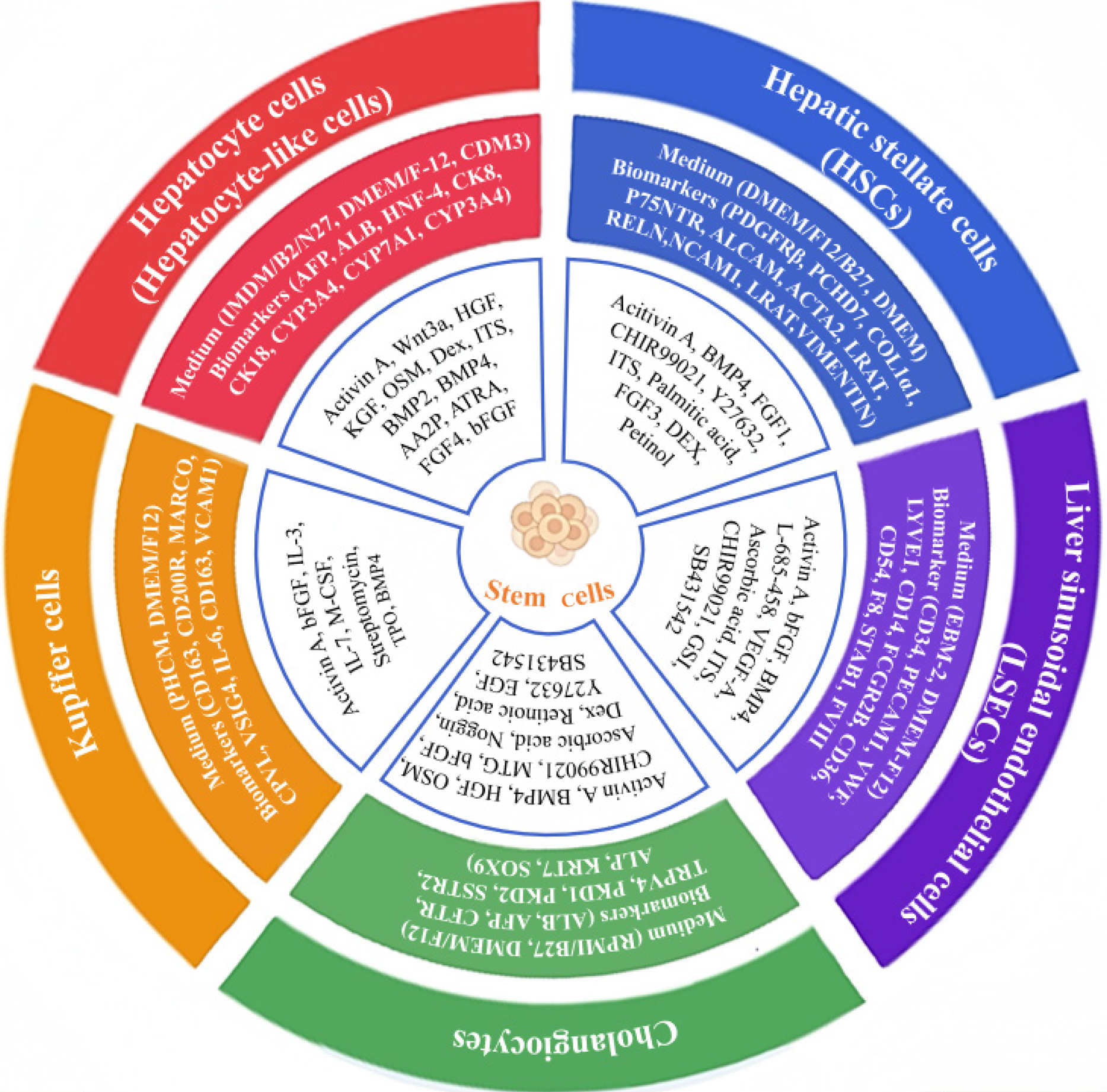
Longitudinal studies have indicated the generation of diverse functional liver cell lineages from human pluripotent stem cells (hPSCs) for precision medicine[67], including hepatocytes[68], HSCs, LSECs, and cholangiocytes[1]. Mean
Proverbially, hepatocytes are highly polarized epithelial cells and heterogeneous parenchymal cells within liver lobule that execute the main synthetic and metabolic functions of the liver[3,67]. Hepatocytes, together with cholangiocytes, are originally differentiated from ventral endoderm-derived hepatoblasts, which are recognized as the main cell type of liver as well as liver progenitor cells for liver development[2,70]. As to liver differentiation, Ang et al[71] detailed described the roadmap of hepatocyte induction from hPSCs via consecutive branching lineage choices orchestrated by spatiotemporally manipulation of extracellular signals (e.g., bone morphogenetic protein, TGF-β, basic fibroblast growth factor, Wnt)[67,71-73].
Currently, hepatocytes and fumarylacetoacetate hydrolase+ hepatocyte-like cells with biofunction have been generated from hPSCs [including human ESCs (hESCs) and human induced pluripotent stem cells (hiPSCs)] by utilizing cell programming and reprogramming technologies[4] (Table 1). For instance, Cai et al[74] reported the three-step induction of maturated hepatic cells with liver cell functions from hESCs within 18 days, while Song et al[75] took advantage of a four-step strategy for efficient differentiation of hiPSCs towards hepatocyte-like cells, including endoderm induction, hepatic specification, hepatoblast amplification, and hepatic maturation. Instead, Chen et al[76] reported a three-step protocol (endodermal induction, hepatic commitment and maturation) for the preparation of functional hepatocyte-like cells from hiPSCs (e.g., urea secretion, low-density lipoprotein uptake, glycogen store). For modeling hepatic tumorigenesis with distinct mutational patterns, single or combined oncogenic alterations were introduced into hepatocytes derived from hPSCs, thereby PI3KCA E542K mutant and c-MYC were demonstrated with liver neoplasia-promoting effects by facilitating cancer metabolic reprogramming and inhibiting hepatocyte functions[4]. Additionally, relative strategies have also applied for efficient hPSC-hepatocyte induction and preservation of the functionality, including the “5C” (short for a combination of five chemicals) culture method[77], the ProliHH (short for proliferation human hepa
| Liver lineages | Stem cell types | Culture medium | Supplements | Days of induction | Biomarkers | Ref. |
| Hepatocyte-like cells | hiPSCs | RPMI/B27, KO-DMEM with KSR, IMDM | Activin A, Wnt3a, HGF, OSM, Dex, ITS premix | 12 days | AFP, albumin, CK18, HNF4, CYP3A4, CYP7A1 | Chen et al[76] |
| Hepatocyte-like cells | hiPSCs | Hepatocyte culture medium, IMDM, N2/B27 | Acitivin A, FGF4, BMP2, HGF, KGF, OSM, Dex | 21 days | AFP, albumin, CK8, CK18, CK19, PEPCK, HNF4α, HNF6, CEBPα, GATA4, HEX | Song et al[75] |
| Hepatocyte cells | GATA6- hiPSCs | StemFit04, IMDM/Ham’s F-12K, N2/B27 | L-ascorbic acid, Dex, 2-phosphate, basic fibroblast growth factor, 1-thioglycerol, HGF, nicotinamide, OSM | 18 days | Albumin, AAT, AFP, HNF4A, CYP3A4, CYP3A5 | Luo et al[126] |
| Hepatic cells | hESCs | DMEM/F12, KSR, 1640 medium | Activin A, aFGF, basic fibroblast growth factor, FGF4, HGF, BMP2, BMP4, Noggin, OSM, Dex, Su5402 | 18 days | PEPCK, CYP7A1, CYP3A4, CYP2B6 | Cai et al[74] |
| Hepatocytes | hPSCs | DEI medium A/B, CDM3 medium, CDM5 medium | A8301, FGF2, Acitivin A, BMP4, ATRA, AAP, TTNPB, OSM, insulin, forskolin, SB505124, 8-bromo-cAMP, DAPT, CHIR99021, AA2P, RO4929097, Dex | 18 days | Fumarylacetoacetate hydrolase, albumin, HGD | Ang et al[71] |
| Hepatic stellate cells | hiPSCs | Liver differentiation medium | BMP4, FGF1, FGF3, retinol, palmitic acid | 12 days | PDGFRβ, PCHD7, COL1α1 | Vallverdú et al[2] |
| Hepatic stellate cells | hPSCs | DMEM low glucose | BMP4, FGF1, FGF3, retinol, palmitic acid, ITS, Dex | 12 days | DES, P75NTR, ALCAM, ACTA2, COL1α1, LRAT, RELN, PCDH7, PDGFRβ | Coll et al[91] |
| Hepatic stellate cells | hESCs | DMEM/F-12, B27, FBS | Activin A, CHIR99021, BMP4, FGF1, FGF3, Y-27632, palmitic acid, retinol | 12 days | PDGFRβ, NCAM1, ALCAM, LRAT, ACTA2, vimentin | Wilhelmsen et al[92] |
| LSECs | hPSCs | EBM-2 medium, IMDM | BMP4, basic fibroblast growth factor, VEGF-A, CHIR99021, cAMP, GSI, SB431542, ITS-X, ascorbic acid, transferrin | 22 days | CD34, PECAM1, VWF, LYVE1, CD14, FCGR2B, CD36, CD54, STAB1, FVIII, STAB2, CLEC1B | Gage et al[97] |
| LSECs | hPSCs | IMDM, DMEM-F12, StemPro34, FCS | BMP4, Activin A, ascorbic acid, basic fibroblast growth factor, L-685-458, ITS-X, VEGF-A, CHIR99021, transferrin | 21 days | FCGR2B, LYVE1, STAB2, F8, CD14, MRC1, CD36, RAMP3, CD32B, FVIII | Gage et al[96] |
| LSECs | Mouse ESCs | IMEM, EGM2-MV, FBS | Adrenomedullin, SB431542, β-ME, VEGF-A, ITS | 20 days | F8, Fcgr2b, Mrc1 | Arai et al[100] |
| Cholangiocytes | hPSCs | RPMI 1640 medium, low glucose DMEM, B27, DMEM/F12, a-MEM | MTG, Acitivin A, CHIR99021, ascorbic acid, basic fibroblast growth factor, BMP4, HGF, EGF, OSM, Dex, RA, Noggin, Y-27632, forskolin | 49 days | Albumin, AFP, CFTR, TRPV4, PKD1, PKD2 | Ogawa et al[101] |
| Cholangiocytes | hiPSCs | RPMI, B27, William’s E medium | Acitivin A, FGF2, FGF10, BMP4, Ly294002, SB431542, RA, EGF | 26 days | SSTR2, ALP, KRT7, SOX9 | Sampaziotis et al[102] |
| Kupffer cells | hiPSCs | X-VIVO 15 media, PHCM, advanced DMEM | M-CSF, IL-3, streptomycin | 4-5 weeks | CD163, CD200R, CD86, IL-6, CSF1, HLA-DRB1, IL-1β | Tasnim et al[106] |
| Kupffer cells | hESCs; cord blood | DMEM/F12, KSR, IMDM, RPMI 1640 | BMP4, basic fibroblast growth factor, Activin A, IL-3, IL-7, M-CSF, TPO, SCF | 9-17 days (cord blood); > 90 days (hESCs) | MARCO, CD5 L, TIMD4, VCAM1, CPVL, VSIG4, FOLR2, CD163 | Kent et al[99] |
Adult tissue-derived stem cells are also advantaged alternative sources for generating hepatocytes. For example, Park[83] reported the hepatic differentiation of human adipose-derived stem cells (hASCs) under the effective bio-stimulators (hypoxia, photobiomodulation therapy), and demonstrated the hepatic gene expression profiling (e.g., albumin, alpha-fetoprotein, cytokeratin 8/18) and growth factor secretion of generated hepatocytes. Taken together, both hPSCs and adult stem cells have served as alternative seeding cells for preparation of human hepatocyte source.
HSCs, including the quiescent HSCs and the activated counterparts, are nonparenchymal cells and specialized liver pericytes that mainly involve in extracellular matrix (ECM) homeostasis, vitamin A storage and the process of liver fibrosis after injury via communications with Kupffer cells, endothelial cells, and hepatocytes[2,84]. For example, HSCs act as the main contributors to the deposition of ECM and fibrosis progression during chronic liver injury in a fibrosis-independent manner, which thus renders HSCs as a primary candidate target for antifibrotic therapies[85,86]. Differ from hepatocytes, both HSCs and LSECs that originated from mesoderm for hepatocyte nuclear factor-4 alpha+ liver bud progenitor formation, which are essential for the maintenance of liver structure, homeostasis, and adequate response to inflammation and liver fibrosis[87]. Nowadays, HSCs have also been reported with fibropathogenic functions in the development of diverse liver disorders, including cirrhosis, liver fibrosis, portal hypertension, steatotic liver diseases (e.g., MASLD, and alcohol-associated liver disease), and HCC via modulating R-spondin-leucine-rich repeat containing G protein-coupled receptor 4/5-β-catenin signal cascades[86,88-90].
In recent years, investigators have benchmarked multiple culture techniques for functional HSC induction and long-term maintenance of mature HSC characteristics from hPSCs (Table 1). For instance, Vallverdú et al[2] reported a stepwise protocol with cytokine cocktail stimulation at different timepoints for the direct differentiation of hiPSCs into HSCs within 12 days. Coll et al[91] induced HSCs from both H9 hESCs and hiPSCs, which could further form liver spheroids with the in vitro HepaRG hepatocytes and thus competent for liver fibrosis and drug toxicity. Similarly, Wilhelmsen et al[92] detailed described the induction of hESC-HSCs benchmarked to human primary counterparts by integrating TGF-β-induced activation and vitamin A and palmitic acid starvation within 12 days, which highlighted the pivotal effect of energy metabolism in HSC activation. Hence, hPSC differentiation towards HSCs supplies an extensive framework for illuminating the physiological and pathological effects of HSCs during liver homeostasis and diseases[93].
LSECs are mesoderm derivatives and function essentially for preserving liver homeostasis, together with collagenization, immunologic responses, pathological adaptation, and pharmacological responses[94,95]. In chronic liver diseases such as MASLD and metabolic dysfunction-associated steatohepatitis (MASH), LSECs revealed cellular dysfunction and capillarization due to the hyperactivation of diverse pro-fibrotic signaling pathways (e.g., TGF-β). These data collectively propose the possibility of LSECs as alternative therapeutic targets in hepatic disorders[94,96].
Of late years, pioneering investigators in the field turned to hPSCs for LSEC generation and the concomitant research on pathogenesis (Figure 1, Table 1). For instance, Gage et al[97] developed a novel induction system for the generation of functional hPSC-LSECs by modulating hypoxia, activating cyclic adenosine monophosphate and TGF-β signaling. The hPSC-LSECs revealed scavenger functions and gene expression profiles of primary human LSECs. By integrating the organoid technology and Wnt 2 stimulation, liver sinusoidal endothelial progenitors and perfused vessels were induced from hiPSCs with functional sinusoid-like features[98]. Interestingly, Ken et al[99] verified that hPSC-LSECs facilitated the development of functional hPSC-derived kupffer cells by practicing the macrophage engraftment test. Additionally, Arai et al[100] reported the enhanced induction of functional LSECs (e.g., endocytosis of acetylated low-density lipoprotein, LSECs-specific biomarkers, and fenestrae-like structure) from mouse ESC-derived lymphatic vessel endothelial hyaluronan receptor 1 + stabilin-2 + endothelial cells by adrenomedullin stimulation and TGF-β signaling abolishment. Therefore, hPSCs supply as advantageous ex vivo models for dissecting LSEC generation and the concomitant pathoge
Cholangiocytes, also known as bile duct epithelial cells, are cell populations for the formation of the biliary system, which thus play an essential in the production of bile and the lipid digestion[101]. As described by Ogawa et al[101] and Sampaziotis et al[102], cholangiocytes in response to diverse sensory signaling (e.g., IL-6, Notch, epidermal growth factor, TGF-β, retinoic acid) or ion channel alterations in bile flow via primary cilia, and thus modulate liver homeostasis as well as cholangiocyte dysfunction[101,102].
The derivation of cholangiocytes from hPSCs extensively facilitates the study of the physiological cholangio-genesis and pathogenesis of cholangiopathies (e.g., Alagille syndrome, biliary atresia, cystic fibrosis, primary sclerosing cho
Kupffer cells, also known as resident liver macrophages, are structurally located at the luminal side of sinusoids and play a pivotal role in drug-induced liver injury and diverse liver diseases (e.g., drug-induced liver injury, cholestasis)[67,106]. In mice, Kupffer cells are also recognized as the first liver-resident macrophages derived from yolk sac hematopoietic progenitors prior to hematopoietic stem cell emergence in embryonic life[99,107-109].
For the recapitulation of yolk sac-like hematopoiesis, hPSC-derived kupffer cells were transplanted into NSG mice, and the homogeneous MARCO (macrophage receptor with collagenous structure)- expressing genetic signature and functional maturation (e.g., phagocytosis, erythrophagocytosis) were observed in the engrafted hPSC-derived kupffer cells according to single-cell RNA sequencing (scRNA-seq) analysis[99]. Instead, Tasnim et al[106] recapitulated Kupffer cell ontogeny in vitro from MYB-independent hiPSC-derived macrophage- precursors, which phagocytosed and secreted cytokines (e.g., IL-6, TNF-α) upon inflammation-associated drug stimulation (e.g., acetaminophen, chlorpromazine, and trovafloxacin) comparable to donor-mismatched counterpart. Of note, despite the signatures of Kupffer cells have been extensively explored and well established, yet the underlying molecular mechanisms modulating the derivation and maturation from hPSCs and the progenitor cells are largely obscure[110] (Figure 1, Table 1).
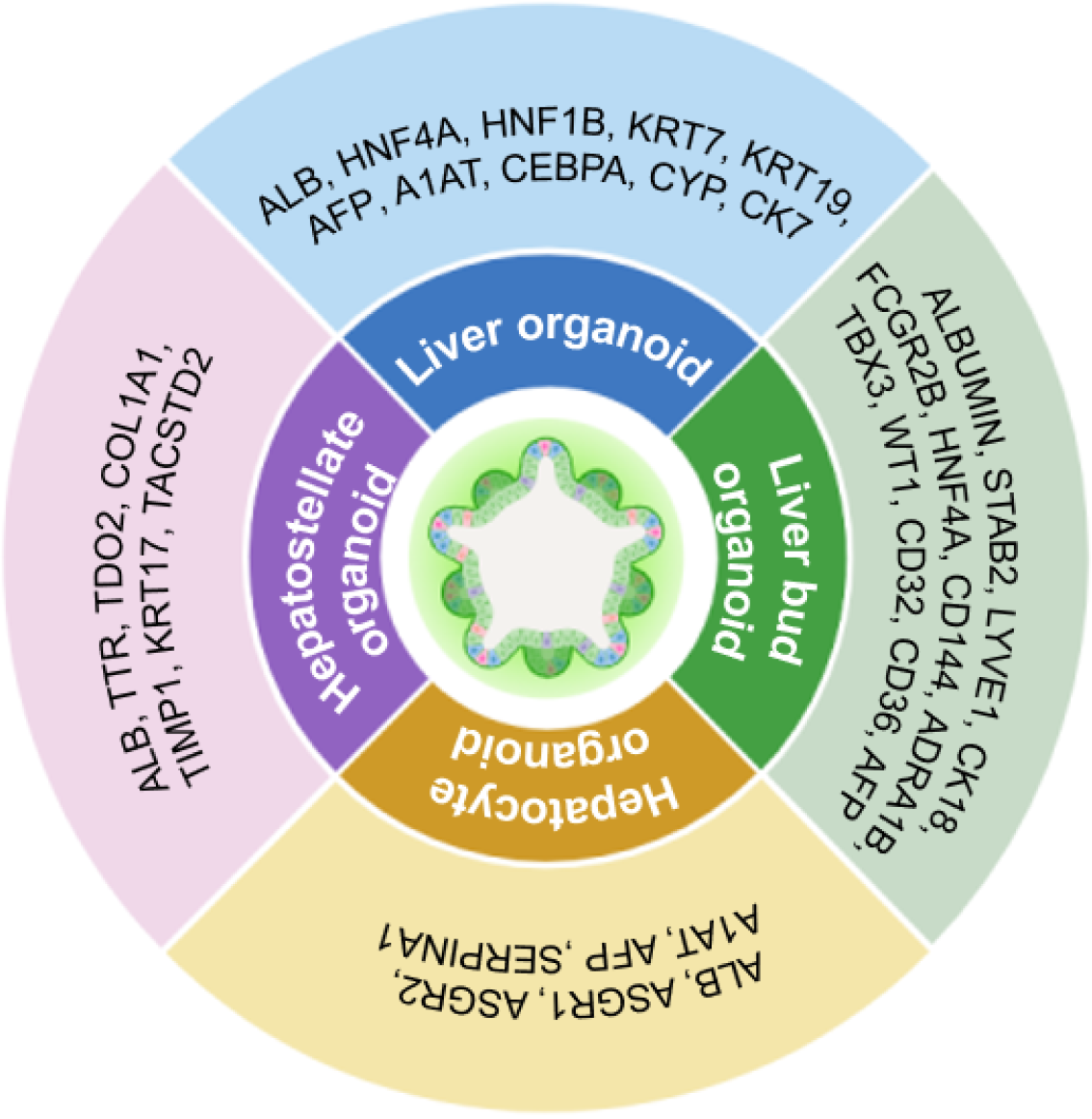
Organoids are three-dimensional (3D) “in a dish” models with remarkable self-organizing structures for exploring the physiological and pathological processes, which commonly generate from stem cells (e.g., adult stem cells, hPSCs) and recapitulate the key structural and functional signatures of the native organs[69]. For decades, liver organoids have been extensively reported for recapturing spatial liver architecture, cellular heterogeneity, and stimulations of liver microenvironment[69,111] (Figure 2, Table 2).
| Organoid types | Stem cells | Biomarkers | Characteristics | Ref. |
| Vascularized mLOs | hPSCs | HNF1B, HNF4A, albumin, CEBPA, CYP | The mLOs was superior to 2D Hep for HPC maturation; mLOs comprise hepatic- and non-parenchymal cells; mLOs with enhanced structural complexity and functionality | Chi et al[112] |
| Liver organoids | hPSCs | AFP, albumin, HNF4α, A1AT, KRT19, KRT7, albumin | The hPSC-LOs with various liver cell types highly simulated DENV infection and screened for antivirals | Li et al[69] |
| Liver organoids | hiPSCs | Albumin, HNF4α, KRT7, KRT19, CK7 | The in situ formation of hiPSC-LOs were achieved in microporous array chips; the hiPSC-LOs were competent for evaluating the efficacy of semaglutide for NAFLD | You et al[221] |
| Liver bud organoids (hLBOs) | hPSCs | STAB2, LYVE1, CD32, CD36, FCGR2B | The vascularized hLBOs contain hepatic and endothelial subpopulations that show key organ-specific functional features, and can restore coagulation factor deficiencies | Saiki et al[98] |
| Liver bud organoids (hLBOs) | hiPSCs | Albumin, CK18, HNF4α, AFP | The hiPSC-derived hLBOs were competent for whole body in vivo cell tracking and the resultant cell therapy development | Ashmore-Harris et al[119] |
| Liver bud organoids (hLBOs) | hPSCs | TBX3, ADRA1B, HNF4α, WT1, ADRA1B, LHX2, CD144, CD31 | The strategy fulfilled the mass production and batch validation of self-organizing hLBOs with in vitro functions and in vivo therapeutic potential from hPSCs | Takebe et al[118] |
| Hepatocyte organoids | hiPSCs | Albumin, ASGR1, ASGR2, A1AT, AFP, SERPINA1 | HiPSCs were transduced with adeno-associated virus vectors and the hepatocyte organoids enhanced the translation of gene therapies | Berreur et al[125] |
| Hepatostellate organoids | hiPSCs | Albumin, TTR, TDO2, COL1A1, TIMP1, KRT17, TACSTD2 | The hepatostellate organoids were derived from hiPSCs with dyskerin 1 mutation, and would benefit the studies upon telomere dysfunction–induced liver disease | Choi et al[128] |
Recently, Li et al[69] integrated the hPSC-derived liver organoid platform and scRNA-seq technology for recapitulating dengue virus infection and antiviral screening. The functional and expandable hPSC-derived liver organoids are consisted of multitudinous liver cell types (e.g., hepatocyte-like cells, intrahepatic CLCs, hepatocyte-like cells, and hepatic stellate-like cells), and thus competent for mimicking liver functional disability and validating drug response during infection. Multi-lineage liver organoids (mLOs) composed of hepatobiliary cell lineages are promising for simulating the functional maturity and structural complexity of the organ physiologically and pathologically[112]. By modulating the cyclic adenosine monophosphate/Wnt/Hippo signaling pathways, Chi et al[112] generated vascularized mLOs con
In recent years, talented investigators have reported the massive and reproducible induction of human liver bud organoids from hESCs and hiPSCs under the two-dimensional (2D) and 3D culture conditions[118] (Figure 2, Table 2). Very recently, Saiki et al[98] reported the direct differentiation of hPSCs into CD32b+ putative liver sinusoidal progenitors by utilizing a multilayered air-liquid interface culture for liver bud organoid formation. In detail, a stepwise differentiation system was established for the induction of vascularized 3D liver bud organoids and the derivatives from hPSCs, including LSEC progenitors, hepatic and endothelial subpopulations. Another study by Ashmore-Harris et al[119] turned to hiPSCs with a radionuclide reporter for the construction of in vivo trackable liver bud organoids in liver-injured mice, and demonstrated the differentiated function towards hepatocyte-like cells. Interestingly, with the aid of 2D culture and the organ-on-a-chip platform, Yoshimoto and the colleagues observed the promoting effect of cyclic mechanical stret
The ex vivo maintenance and expansion of primary hepatocytes with functional characteristics are long-standing challenges for investigators in the field[121]. Over the years, a variety of 2D and 3D methodologies have been developed for the construction and long-term expansion of primary hepatocyte organoids from stem cells and liver counterparts (e.g., hepatocytes, bile-duct epithelial cells)[122,123]. Longitudinal studies have demonstrated the feasibility of hPSCs for ex vivo hepatocyte organoid induction[124] (Figure 2, Table 2). For instance, Berreur et al[125] transduced eight serotypes of recombinant adeno-associated virus vectors into hiPSCs, and tested the efficiency and hepatotoxicity in iPSC-derived hepatocyte organoids. Differ from the conventional strategies with diverse limitations, Luo et al[126] recently turned to hiPSCs with a doxycycline-inducible GATA6 expression for the synchronous differentiation into homogeneous hepa
Compared to the abovementioned types of hPSC-derived liver organoids, there are minimal pieces of literatures upon hepatostellate organoid construction (Figure 2, Table 2). For example, Choi et al[128] reported the generation of hepatostellate organoids from patient hiPSCs with a causal dyskeratosis congenital mutation in dyskerin 1, which supplied novel platforms for dissecting telomere dysfunction-induced liver diseases. In details, the hiPSCs were incipiently programmed into hepatocytes or HSCs, and followed by the formation of genotype-admixed hepatostellate organoids[128]. Overall, the hepatostellate organoids provide new avenues for illuminating genotype-phenotype relationships and liver pathologies in telomeropathies.
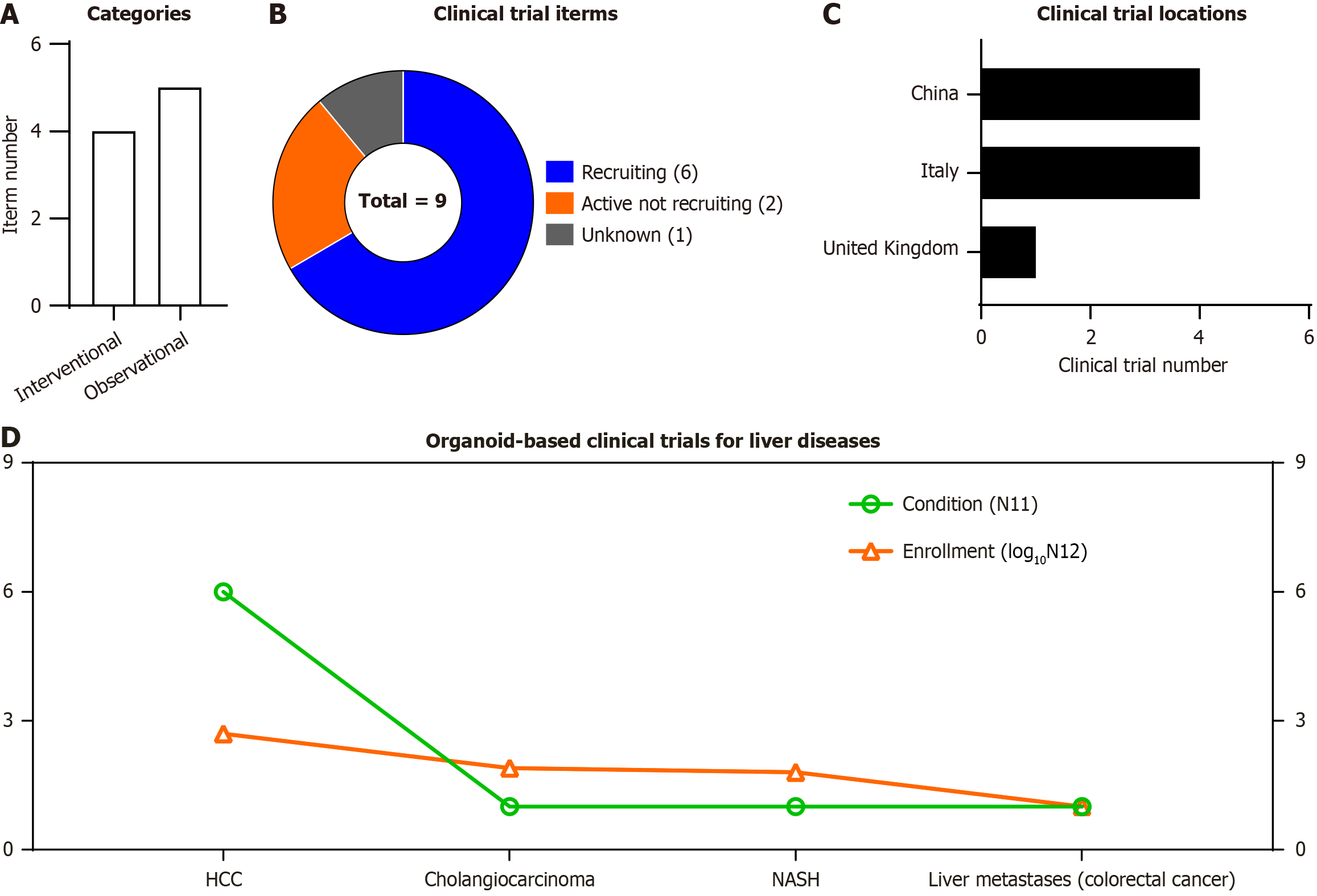
Due to the robust attributes of stem cell-derived organoids, clinicians are devoted to organoid-based precision medicine for liver disease prediction (e.g., liver resection, drug screen, the response to drugs, hepatic disease development). According to ClinicalTrials.gov website of National Institutes of Health (https://clinicaltrials.gov/), a total number of 9 trials (4 interventional trials and 5 observational ones) with 662 participants have been registered (up to September 10, 2025) for liver diseases (Figure 3A and B, Table 3). The registered clinical trials are distributed in China (4 trials), Italy (4 trials) and United Kingdom (1 trial) (Figure 3C). Of them, except one for non-alcoholic steatohepatitis, all the other trials were registered upon liver cancers, including HCC (n = 6), cholangiocarcinoma (n = 1) and liver metastases (n = 1) during colorectal cancer (n = 1). Furthermore, the enrolled participants (shown as log10N12) in the corresponding types of trials [shown as condition (N11)] were shown according to the line (Figure 3D, Table 3). Considering the recruiting and active (not recruiting) status of the abovementioned studies, there’s still far from adequate for large-scale application for patients with intractable liver diseases.
| NCT No. | Study title | Study status | Condition | Enrollment | Study type | Location |
| NCT06929845 | “Organoid Models of Hepatocellular Carcinoma” | Recruiting | HCC | 150 | Interventional | Italy |
| NCT02436564 | “In Vitro Models of Liver and Pancreatic Cancer” | Unknown | Cholangiocarcinoma | 75 | Observational | United Kingdom |
| NCT05913141 | “PDO/PDO-TIL/PDOTS for Drug Screen” | Recruiting | HCC | 30 | Observational | China |
| NCT06077591 | “Prospective Clinical Validation of Next Generation Sequencing and Patient-Derived Tumor Organoids (PDO) Guided Therapy in Patients with Advanced/ Inoperable Solid Tumors” | Recruiting | HCC | 40 | Interventional | China |
| NCT06355700 | “Hepatocellular Carcinoma Liver Organoids” | Recruiting | HCC | 10 | Interventional | Italy |
| NCT06699524 | “Construction of a Recurrence Risk Prediction Model for Liver Resection Based on Drug Sensitivity of Patient-derived Hepatocellular Carcinoma Organoid” | Active not recruiting | HCC | 122 | Observational | China |
| NCT06856252 | “Human Liver Organoids as a Model to Study the Development of NASH” | Recruiting | NASH | 60 | Interventional | Italy |
| NCT06787625 | “Development of Organoids From Primary Colorectal Tumors and Synchronous Liver Metastases” | Recruiting | Colorectal cancer | 10 | Observational | Italy |
| NCT05932836 | “An Organoid-on-chips Technique Based on Biopsy Samples and Its Efficacy in Predicting the Response to HAI in HCC” | Active not recruiting | HCC | 165 | Observational | China |
Acute liver injury (ALI) serves as a main inducement of liver failure and the concomitant microcirculatory dysfunction, which is commonly caused by drug-induced hepatotoxicity and proinflammatory factors[5,129]. For example, numerous literatures have indicated the drug overdose resultant ALI and the molecular mechanism by acetaminophen[130], together with the hepatoprotective effects of regimens (e.g., CpG-oligodeoxynucleotides[131], crizotinib[132], pristimerin[133]). Among them, formoterol acetaminophen reveals alleviative effect against acetaminophen-induced ALI via suppressing the lipid peroxidation-mediated ferroptosis, while liraglutide ameliorates radiation-induced hepatotoxicity in ALI via modulating the liver kinase B1/AMP-activated protein kinase/mammalian target of rapamycin axis[130,134].
State-of-the-art literatures have highlighted the feasibility of stem cells (e.g., MSCs) and the derivatives (e.g., microve
Meanwhile, current literatures have also indicated the ameliorative effect of MSC-extracellular vesicles (EVs) upon ALI[135,139,140]. For example, exosomes enriched from human exfoliated deciduous teeth-derived stem cells revealed notable regenerative effects in the context of ALI model, including mitigated damage, enhanced hepatocyte proliferation, macrophage polarization, and reduced inflammation[141]. With the aid of MSC-exosomes, Cai et al[140] verified the attenuated effect and potential mechanisms of MALAT1/miR-26a-5p axis-mediated ALI. Notably, the inherent interindividual and batch-to-batch variability in biological functions (e.g., differentiation capacity, immunomodulatory property) and genetic phenotypes (e.g., gene expression profiles) of MSCs and the derivatives including EVs for ALI administration should not be neglected in clinical translation.
ALF is recognized as a life-threatening disorder and critical clinical syndrome characterized by substantial hepatocyte loss, massive tissue necrosis, severe functional deterioration, hepatic encephalopathy, excessive oxidative stress, coa
Considering the bidirectional immunomodulatory capacity of MSCs, it is imperative to develop innovative cell therapy for triggering the progression of ALF[142,145]. For instance, MSCs manifested superior therapeutic efficacy upon ALF via modulating hepcidin-ferroportin axis and suppressing PI3K/AKT/nuclear factor erythroid-2-related factor 2 pathway or Notch1-yes-associated protein 1 circuit, which put forward the significance of liver management in ALF via iron homeostasis and ferroptosis[142,146]. Instead, Tao et al[147] demonstrated the alleviative effect of human umbilical cord-derived MSCs (hUC-MSCs) upon ALF and liver inflammation through synchronously inhibiting c-Jun N-terminal kinase/nuclear factor-кB activation-induced hepatocyte apoptosis and proinflammatory macrophage M1 polarization[147]. Interestingly, Zhang et al[143] integrated MSC spheroids and immunoregulatory decellularized EVs for the amelioration of ALF, which revealed enhanced anti-oxidative and pro-angiogenic effects, together with superior hepatic cell differentiation. Of note, MSC-derived EVs (MSC-EVs) alone or engineered with biomacromolecules like chimeric antigen receptor also reveal considerable efficacy in ALF[148-150]. In all, MSC-based regimens propose a novel multifaceted strategy dispense with liver transplantation for conquering refractory liver failure like ALF.
MASLD, previously known as NAFLD, is characterized by hepatocyte ballooning and liver damage in analogy with alcoholic fatty liver disease[113,151]. MASLD has been recognized as a metabolic and systemic disorder encompasses the metabolic dysfunction-related fatty liver and MASH subtypes based on hepatic steatosis[152,153]. The latest updates emphasize the superior attributes of MSCs and MSC-EVs in culture supernatant for MASLD management[154-157]. Very recently, Tunstead et al[158] combined MSCs with exogenous mitochondrial pretreatment or palmitate for enhancing the therapeutic efficacy or immunomodulation capacity of macrophages upon NAFLD, respectively. These findings suggest that stem cells like MSCs serve as novel avenues for chronic liver disease treatment including MASLD.
As to EVs of different origins including stem cells, investigators have also detailed described the applications for MASLD administration[159]. For instance, MSC-EVs were adequate to suppress inhibitor of κB alpha/nuclear factor-κB/angiopoietin-2 pathway hyperactivation-mediated LSEC angiogenesis in MASH mice, and the promoting effect could be attenuated by USP9X knockdown[160]. By conducting high-fat diet-induced MASLD model, researchers observed the inhibitory effect of MSC-EVs upon mitochondrial fission and lipid deposition in hepatocytes, which indicated the potential mechanism in hepatocyte steatosis and MASLD progression[161]. Meanwhile, MSC-EVs play an important role in counteracting oxidative stress in both in vivo and in vitro models, and represent a novel therapeutic avenue for halting or reversing liver disease progression including MASLD and MASH[154]. Instead, Kasahara et al[162] reported the controlled release of MSC conditioned medium encapsulated in hydrogel for MASLD rat treatment, which increased immunoregulatory cytokines and hepatocyte size and functionality (e.g., hepatic ATP, β-hydroxybutyrate). Even though the remarkable progress in preclinical investigations, the potential variations in clinical practice owing to the differences in species and infusion doses should be further dissected during MSCs-based regimens for MASLD administration.
As a complex progressive condition and critical health issue, liver fibrosis is characterized by excessive accumulation of ECM and scar tissue buildup, and commonly results in functional impairment and liver cirrhosis[163,164]. Generally, liver fibrosis initially encapsulates the injury and thus acts as a protective and reversible response, yet the prolonged damage usually leads to liver cirrhosis and even the life-threatening HCC[165]. There are two major approaches for liver fibrosis reversion in clinical practice, including the fibrosis process abolishment and the underlying insult elimination[166]. Despite diverse causes and underlying mechanisms have been identified for pathogenesis, yet the limitations in liver fibrosis treatment options largely hinder the reversibility and recovery of liver fibrosis[166].
Nowadays, stem cells like MSCs of different origins have showed robust prospects in the administration of liver fibrosis attribute to the intercellular communications and the suppression of key fibrotic pathways. For instance, BM-MSCs infusion is adequate to ameliorate liver fibrosis in tetrachloromethane-induced liver fibrosis model via anti-inflammation and immunoregulation[167,168]. Instead, Gunardi et al[169] compared the intrahepatic and intrasplenic infusion of MSCs for liver fibrosis treatment in a bile duct ligation rabbit model, and confirmed the facilitating effect upon hepatocyte proliferation whereas minimal differences were observed in liver function and liver fibrosis severity between the two routes. Of note, hepatocyte-like cells derived from hUC-MSCs revealed minimal effect upon the acceleration of the venularization and capillarization of hepatic sinusoids in a chronic liver fibrosis model[170]. Simultaneously, EVs derived from MSCs (MSC-exosomes) and liver-resident cells integrated with biomaterials stand out for their pharmacological and therapeutic potential against liver fibrosis. For example, Sani et al[171] put forward the synergistic effect of MSC-exosomes and anti-miR17-5p for the enhanced efficacy upon preclinical liver fibrosis model, and thus demonstrated the feasibility of MSC-exosome as promising therapeutic options against liver fibrosis. Despite the numerous progress in stem cell-based tactics for liver fibrosis intervention, the concomitant practical challenges should not be neglected such as efficacy heterogeneity, variations in cell sources, and post-transplantation survival rates. For example, we and Zhang et al[172] reported the variations of MSCs at diverse passages in long-term effectiveness and homing ability, which collec
Liver cirrhosis is the final phase of the reversible liver fibrosis, characterized by extensive liver scarring and the resultant hepatic dysfunction and fatal complications[174,175]. Patients with the inflammatory and fibrosing biliary atresia commonly lead to a rapid deterioration towards severe liver cirrhosis[176]. To overcome the issue in the chronic liver disease, Nguyen et al[177] conducted allogeneic UC-MSC administration, and confirmed the safety and improved liver function (e.g., improved alkaline phosphatase, better biochemical profiles) in patients with liver cirrhosis.
Currently, stem cell-based regimens have been recognized as encouraging innovative therapy for end-stage liver diseases including liver cirrhosis[178,179]. Of them, MSCs are splendid cell sources for liver cirrhosis management ascribe to the unique properties, including cytokine paracrine (e.g., vascular endothelial growth factor, hepatocyte growth factor) and multi-lineage lineage differentiation capacity towards hepatocyte-like cells[174,180]. The trophic signaling pathways of MSCs are vastly involved in angiogenesis, hepatocyte proliferation, liver recovery and regeneration such as PI3K/AKT pathway and TGF-β/Smad pathway[174,181]. In a randomized controlled clinical trial upon hepatitis B virus-related decompensated liver cirrhosis, Shi et al[178] observed the improved long-term survival rate and liver function in decompensated liver cirrhosis patients after infusion of UC-MSCs. In another phase Ia/Ib trials, Shi et al[178] recently verified the outcomes and dose-effect relationship of liver cirrhosis patients with three doses of MSC infusion, and observed the stronger immunomodulatory effects induced by higher MSC doses[182]. Therefore, stem cells like MSCs provide novel paradigms for irreversible liver cirrhosis and the severe complications. Meanwhile, it’s noteworthy that the safety and effectiveness of stem cell infusion for patients with liver cirrhosis should be further verified due to the limitations of small-scale enrollments.
ACLF, a severe form of cirrhosis, is a syndrome characterized by liver function decline, acute liver deterioration, multiorgan failure and high short-term mortality, which has become a major challenge in hepatology and global health burden[183-185]. Of the therapeutic regimens, liver transplantation serves as the most effective option for ACLF patients, yet multifaceted undeniable limitations should be further resolved such as high costs, surgical complications, liver donor shortage and immunosuppressive therapy[186].
Encouragingly, more and more investigators are devoted to developing the stem cell-based iatreusis for liver diseases including ACLF in preclinical disease models and clinical practice[187,188]. Of them, ACLF patients with MSC infusion have been reported with safety (e.g., adverse events, serious adverse events) and liver function improvement (elevated albumin level, decreased MELD score)[189,190]. For instance, Heo et al[186] reported the enhanced differentiation of hBM-MSCs into functional hepatocyte-like cells, while Liu et al[190] performed systematic meta-analysis and concluded the safety and efficacy of both hUC-MSCs- and BM-MSCs- based novel therapeutics upon ACLF and the concomitant clinical symptoms (e.g., encephalopathy, gastrointestinal hemorrhage events). As to the cell-free MSC-exosomes, the beneficial pleiotropic properties (e.g., low immunogenicity, good safety profile, anti-fibrotic cytokines) and hepatoprotetive effects (e.g., suppress HSC activation, improve metabolism, anti-apoptosis, anti-inflammatory, anti-ferroptosis) upon ACLF have also been repeatedly highlighted[185]. Additionally, the let-7a-5p/MAP4K3 (mitogen-activated protein kinase kinase kinase kinase 3) axis in MSC-exosomes were reported in mediating autophagy repairmen in ACLF, which would benefit the illumination of the intrinsic mechanisms and targeted cell therapy in future[191].
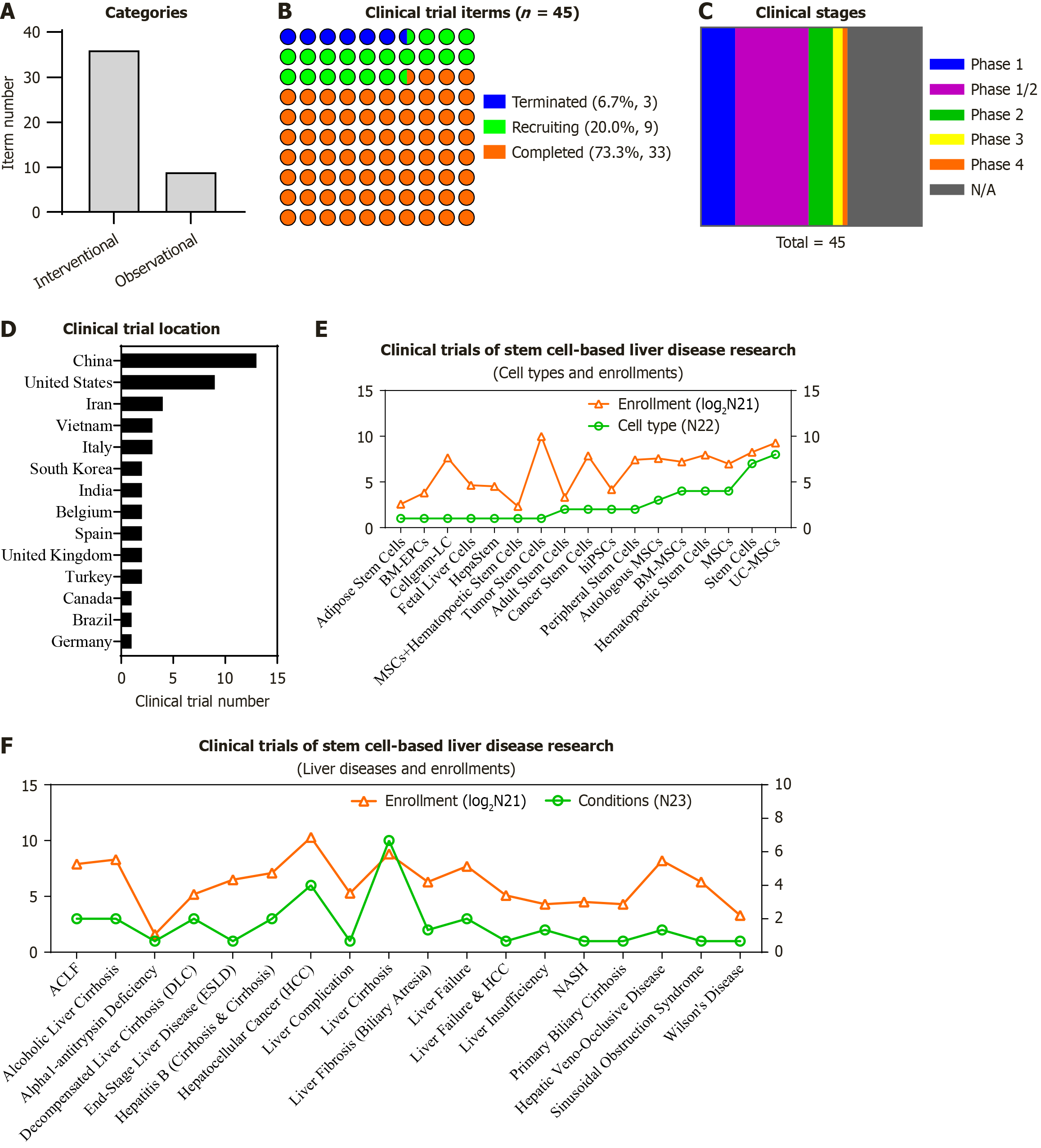
To present the systematic and detailed information of stem cell-based therapeutics, we turned to the registered clinical trials according to the ClinicalTrial.gov database (up to September, 2025). In total, 169 registered stem cell-related trials were gained according to the keywords (condition/disease: Liver diseases; intervention/treatment: Stem cells), and finally 45 trials with the “completed” (n = 33), “recruiting” (n = 9) or “terminated” (n = 3) status were further enriched for liver disease diagnosis and treatment including 36 interventional trials and 9 observational ones (Figure 4A and B, Table 4). Of them, most of the trials were under the early stage for verifying the safety and efficacy of stem cell transplantation upon liver diseases, including 7 in phase 1, 15 in phase 1/2, 5 in phase 2, 2 in phase 3, 1 in phase 4, and 15 with the unknown phase (N/A) (Figure 4C, Table 4).
| NCT No. | Study title | Cell types | Study status | Condition | Phase | Enrollment | Study type | Location |
| NCT00953693 | “Patient Specific Induced Pluripotency Stem Cells (PSiPS)” | hiPSCs | Completed | Liver insufficiency | N/A | 15 | Observational | Iran |
| NCT01591200 | “Dose Finding Study to Assess Safety and Efficacy of Stem Cells in Liver Cirrhosis” | MSCs | Completed | Alcoholic liver cirrhosis | Phase 2 | 40 | Interventional | India |
| NCT01429038 | “MSCs After Renal or Liver Transplantation” | MSCs | Completed | Liver failure | Phase 1/2 | 40 | Interventional | Belgium |
| NCT00655707 | “A Phase I/II Safety and Tolerability Dose Escalation Study of Autologous Stem Cells to Patients With Liver Insufficiency” | Adult stem cells | Completed | Liver insufficiency | Phase 1/2 | 5 | Interventional | United Kingdom |
| NCT02089919 | “Cancer Stem Cells Vaccine Therapy in Treating Hepatocellular Cancer Patients” | Cancer stem cells | Completed | HCC | Phase 1/2 | 40 | Interventional | China |
| NCT00147043 | “Adult Stem Cell Therapy in Liver Insufficiency” | Adult stem cells | Completed | Liver cirrhosis | N/A | 5 | Interventional | United Kingdom |
| NCT04243681 | “Combination of Autologous MSC and HSC Infusion in Patients With Decompensated Cirrhosis” | MSCs + hematopoetic stem cells | Completed | Decompensated liver cirrhosis | Phase 4 | 5 | Interventional | India |
| NCT01333228 | “Evaluate Safety and Efficacy of Autologous Bone Marrow-derived Endothelial Progenitor Cells in Advanced Liver Cirrhosis” | BM-EPCs | Completed | Liver cirrhosis | Phase 1/2 | 14 | Interventional | Spain |
| NCT05331872 | “Umbilical Cord-derived MSC Infusion in the Management of Adult Liver Cirrhosis” | UC-MSCs | Completed | Liver cirrhosis | Phase 1 | 20 | Interventional | Vietnam |
| NCT00420134 | “Improvement of Liver Function in Liver Cirrhosis Patients After Autologous MSC Injection: A Phase I-II Clinical Trial” | Autologous MSCs | Completed | Liver cirrhosis | Phase 1/2 | 30 | Interventional | Iran |
| NCT01454336 | “Transplantation of Autologous MSC in Decompensated Cirrhotic Patients With Pioglitazone” | Autologous MSCs | Completed | Decompensated liver cirrhosis | Phase 1 | 3 | Interventional | Iran |
| NCT02557724 | “Mobilization of MSCs During Liver Transplantation” | MSCs | Completed | Liver failure, HCC | N/A | 35 | Observational | United States |
| NCT01875081 | “REVIVE (Randomized Exploratory Clinical Trial to Evaluate the Safety and Effectiveness of Stem Cell Product in Alcoholic Liver Cirrhosis Patient)” | Stem cells | Completed | Alcoholic liver cirrhosis | Phase 2 | 72 | Interventional | South Korea |
| NCT01013194 | “Human Fetal Liver Cell Transplantation in Chronic Liver Failure” | Fetal liver cells | Completed | Liver cirrhosis | Phase 1/2 | 25 | Interventional | Italy |
| NCT02297867 | “Clinical Trial Study About Human Adipose-Derived Stem Cells in the Liver Cirrhosis” | ASCs | Completed | Liver cirrhosis | Phase 1 | 6 | Interventional | China |
| NCT01378182 | “Efficacy of In vitro Expanded Bone Marrow Derived Allogeneic MSC Transplantation Via Portal Vein or Hepatic Artery or Peripheral Vein in Patients With Wilson Cirrhosis” | BM-MSCs | Completed | Wilson’s disease | NA | 10 | Interventional | Turkey |
| NCT01120925 | “Autologous Bone Marrow Derived Stem Cells in Decompensated Cirrhotic Patients” | BM-MSCs | Completed | Decompensated liver cirrhosis | Phase 1/2 | 30 | Interventional | Iran |
| NCT01220492 | “Umbilical Cord MSCs for Patients With Liver Cirrhosis” | UC-MSCs | Completed | Liver cirrhosis | Phase 1/2 | 266 | Interventional | China |
| NCT01342250 | “Human Umbilical Cord MSCs Transplantation for Patients With Decompensated Liver Cirrhosis” | UC-MSCs | Completed | Liver cirrhosis | Phase 1/2 | 20 | Interventional | China |
| NCT03468699 | “Autologous Bone Marrow Mononuclear Stem Cell for Children Suffering From Liver Cirrhosis Due to Biliary Atresia” | BM-MSCs | Completed | Liver fibrosis (biliary atresia) | Phase 2 | 17 | Interventional | Vietnam |
| NCT04522869 | “Umbilical Cord Derived MSC (UC -MSC) Transplantation for Children Suffering From Biliary Atresia” | UC-MSCs | Completed | Primary biliary cirrhosis | Phase 1/2 | 20 | Interventional | Vietnam |
| NCT01625351 | “A Study of CD45RA+ Depleted Haploidentical Stem Cell Transplantation in Children With Relapsed or Refractory Solid Tumors and Lymphomas” | Hematopoetic stem cells | Completed | Hepatic tumor | Phase 1 | 23 | Interventional | United States |
| NCT00003966 | “Defibrotide in Treating Patients With Liver Damage Following Peripheral Stem Cell Transplantation” | Peripheral stem cells | Completed | Veno-occlusive disease | Phase 2 | 151 | Interventional | United States |
| NCT03132337 | “Sinusoidal Obstruction Syndrome for Stem Cell Transplant Patients Biomarker Study” | Stem cells | Completed | Sinusoidal obstruction syndrome | N/A | 80 | Observational | United States |
| NCT03963921 | “Safety and Tolerability of HepaStem in Patients With Cirrhotic and Pre-cirrhotic NASH Patients” | HepaStem | Completed | NASH | Phase 1/2 | 23 | Interventional | Belgium, Spain |
| NCT00956891 | “Therapeutic Effects of Liver Failure Patients Caused by Chronic Hepatitis B After Autologous MSCs Transplantation” | Autologous MSCs | Completed | Liver failure | N/A | 158 | Observational | China |
| NCT02727673 | “Relationship Between Circulating Tumor Stem Cells and the Clinical Pathology” | Tumor stem cells | Completed | HCC | N/A | 1000 | Observational | China |
| NCT00358501 | “Defibrotide for the Treatment of Severe Hepatic Veno-Occlusive Disease in Hematopoetic Stem Cell Transplant Patients” | Hematopoetic stem cells | Completed | Severe hepatic veno-occlusive disease | Phase 3 | 134 | Interventional | United States, Canada |
| NCT00007813 | “Peripheral Stem Cell Transplantation Plus Chemotherapy in Treating Patients With Malignant Solid Tumors” | Peripheral stem cells | Completed | Liver cancer | Phase 1 | 21 | Interventional | United States |
| NCT04423237 | “Risk Factors and Measures to Prevent Liver and Pancreas Complications in Pediatric Patients After HSCT” | Hematopoetic stem cells | Completed | Liver complication | N/A | 39 | Observational | Italy |
| NCT03511794 | “Effectiveness of the Hepatitis B Vaccine Post-Hematopoietic Stem Cell Transplant” | Hematopoetic stem cells | Completed | Hepatitis B | N/A | 52 | Observational | United States |
| NCT01481649 | “Risk of Hepatitis B Reactivation After Bone Marrow Transplantation With Prior Hepatitis B Virus (HBV) Exposure” | Stem cells | Completed | Exposure to hepatitis B virus | N/A | 69 | Observational | China |
| NCT00002515 | “Combination Chemotherapy Followed by Bone Marrow Transplantation in Treating Patients With Rare Cancer” | Stem cells | Completed | HCC | Phase 2 | N/A | Interventional | United States |
| NCT06892236 | “Preparation of IPSC for Cell Gene Editing for the Treatment of AATD” | hiPSCs | Recruiting | Alpha1-antitrypsin deficiency | NA | 3 | Interventional | Italy |
| NCT06242405 | “Effect of Different Frequencies of Umbilical Cord-MSCs Through Peripheral Vein in Patients with ESLD” | UC-MSCs | Recruiting | End-stage liver disease | NA | 92 | Interventional | China |
| NCT06564740 | “Stem Cell Applications in Biliary Atresia Patients” | Stem cells | Recruiting | Liver fibrosis (biliary atresia) | NA | 64 | Interventional | Turkey |
| NCT06740149 | “Efficacy and Safety of BMSCs (CG-BM1) for ACLF Patients” | BM-MSCs | Recruiting | ACLF | Phase 1/2 | 90 | Interventional | China |
| NCT06904755 | “Safety and Effectiveness of MSCs in Blood Purification for the Treatment of Liver Failure” | MSCs | Recruiting | Liver failure | Phase 1 | 10 | Interventional | China |
| NCT05106972 | “Umbilical Cord MSC Transplantation for Decompensated Hepatitis B Cirrhosis” | UC-MSCs | Recruiting | Liver cirrhosis | NA | 30 | Interventional | China |
| NCT05985863 | “Human Umbilical Cord MSC Transplantation for The Treatment of Acute-on-Chronic Liver Failure” | UC-MSCs | Recruiting | ACLF | Phase 1/2 | 150 | Interventional | China |
| NCT04689152 | “Clinical Trial to Evaluate the Efficacy and Safety of Cellgram-LC Administration in Patients With Alcoholic Cirrhosis” | Cellgram-LC | Recruiting | Alcoholic cirrhosis | Phase 3 | 200 | Interventional | South Korea |
| NCT03826433 | “hUC-MSCs (19#iSCLife®-LC) in the Treatment of Decompensated Hepatitis b Cirrhosis hepatitis b Cirrhosis” | UC-MSCs | Recruiting | Hepatitis B | Phase 1 | 20 | Interventional | China |
| NCT00382278 | “Safety Study of Autologous Stem Cell in Liver Cirrhosis” | Autologous stem cells | Terminated | Liver cirrhosis | Phase 1/2 | 15 | Interventional | Brazil |
| NCT03860155 | “Allogeneic ABCB5-positive Stem Cells for Treatment of Acute-on-Chronic Liver Failure” | Allogeneic stem cells | Terminated | ACLF | Phase 1/2 | 5 | Interventional | Germany |
| NCT00923052 | “The Natural History of Solid Organ Cancer Stem Cells (SOCSC)” | Cancer stem cells | Terminated | Hepatic cancer | N/A | 190 | Observational | United States |
As to the locations of the registered trials, China (n = 13), United States (n = 9) and Iran (n = 4) are the top three countries in the number of stem cell-based clinical studies for liver diseases (Figure 4D, Table 4). As to the subtypes of cell sources, both the autologous and allogeneic stem cells and the derivatives are applied, including adult stem cells, hBM-MSCs, hUC-MSCs, hASCs, patient-specific hiPSCs, circulating tumor stem cells, hematopoietic stem cells, solid organ cancer stem cells, peripheral stem cells, BM-derived endothelial progenitor cells, ABCB5-positive stem cells, bone barrow mononuclear stem cells, fetal liver cells, hematopoietic stem cells, cancer stem cells, and even the commercial stem cell products (e.g., HepaStem, Cellgram™, and 19#iSCLife®-LC) (Figure 4E, Table 4). As shown in Figure 4F (also see Table 4), a total number of 3337 participants are distributed in a variety of liver disorders and liver metastasis of the relative tumors, including ACLF and liver failure, decompensated or alcoholic liver cirrhosis, HCC, non-alcoholic steatohepatitis, primary biliary cirrhosis, severe hepatic veno-occlusive disease, sinusoidal obstruction syndrome, end-stage liver disease, and Wilson’s Disease.
Furthermore, of the abovementioned registered trials with completed status, only 7 interventional ones were with results for the indicated liver disorders. For instance, in a phase I/II study (NCT00655707), investigators reported the safety and tolerability dose escalation in 5 patients with liver insufficiency by infusion of autologous CD34+ hematopoie
Stem cell, and particularly patient-specific hiPSCs with unlimited expansion and multipotent differentiation capacity, are splendid alternatives and renewable sources for pathogenic mechanism exploration and liver cell preparation[86]. At the meantime, hPSCs- and MSCs-guided cell therapy and regenerative medicine hold remarkable prospects to revolutionize paradigms of traditional treatments for liver disorders including end-stage liver diseases[174]. For the purpose, in this review article, we outline the conception and the latest renewal of stem cells and the derivatives in liver disease diagnosis and modeling, together with novel cell therapy for improving the outcomes of intractable liver disorders.
As to stem cell-based cell therapy, the preparation of cell sources with outstanding attributes (e.g., purity, functional mature, long-term survival and expansion) is of prime importance for liver diseases. As to MSCs, longitudinal studies have highlighted the heterogeneity in cellular viability and efficacy upon liver diseases largely attributes to the wide range of sources and the absence of unique biomarker[192,193]. For example, Zhao et al[45] and Zhang et al[172] reported the multifaceted variations of hUC-MSCs both at the cellular and genomic levels after continuous in vitro culture. Interestingly, we and other investigators reported the identification and high-efficient preparation of a vascular cellular adhesion molecule-1+ subset of MSCs (also known as CD106+ MSCs) with preferable immunomodulatory signatures and improved angiogenesis over the negative counterparts[194-196], which were further confirmed by preclinical investigation upon diverse disease intervention (e.g., acute lung injury[197], cerebral Infarction[198], experimental autoimmune encephalomyelitis[199]). These data indicated the importance of stem cell heterogeneity as well as the limitations of current technological limitations for disease intervention. Primary hepatocytes in 2D cultures promptly dedifferentiate and lose their function[200]. Meanwhile, the current in vitro three-step or four-step protocols for hepatic differentiation might not precisely mirror body liver development attribute to the variations with the in vivo liver development[71]. Compared to the abovementioned 2D or 3D monolayer cultures, stem cell-based 3D liver organoids (e.g., mLOs, liver assembloids) facilitate the long-term ex vivo expansion of primary human hepatocytes and serve as unlimited sources for large-scale preparation of mature hepatic parenchymal and non-parenchymal cells[112,201]. Nevertheless, there’s still an urgency for engineering multicellular complexity in liver organoids to more faithfully recapture native structures of individual organs, and in particular, the mimicking of liver organ-specific vasculature (e.g., liver-specific capillary plexus, blood vessels)[98].
The stem cell-based liver organoids with self-organizing properties have dramatically prompted the progression of liver development, liver disease pathogenesis, pharmacological screening, functional liver lineage generation, and innovative cell therapy and targeted therapy[157,177,202-204]. Simultaneously, it’s of prime importance for the induction of mature hepatic organoids with structural complexity and functional maturity (e.g., vascularization, immune cells, and sinusoidal endothelial cells) by 3D bioprinting or modulating signaling pathways with non-gene-editing strategies (e.g., small molecules, cytokine cocktails) for fulfilling precise liver disease diagnosis and regenerative medicine[205,206]. For instance, the limitations in the current technologies for the vast network of capillaries and vessels and the resultant insufficient vascularization of organoids (e.g., endothelial specialization, angiocrine factors) largely hinder the faithful simulation of the in vivo organ development, homeostasis and immune response[207-209]. Meanwhile, the vascularized organoids with organ-specific vascular phenotypes would also provide platforms for dissecting the physiological and angiocrine interactions dispense from substrate provision and perfusion in vivo[210,211]. As to immune cells, the involvement of Kupffer cells in hepatic organoids would help enhance the understanding of liver immunity, the contribution to liver diseases, and hepatotoxicity of drugs and relative xenobiotics[212]. Additionally, the integration of liver organoid technology and multidisciplinary new technologies (e.g., scRNA-seq, organ-on-a-chip technology, nano-biomaterials, lineage tracing technology) will further facilitate the dissection of liver diseases and optimization of treatment schedules[69,213,214]. For example, investigators recently gained high-throughput generation of pre-vascularized hepatobiliary organoids from hiPSCs on a chip via nonparenchymal cell grafting, which offers novel technical route for high-fidelity liver organoids formation[214].
Small EVs (sEVs) of diverse kinds are prestigious vehicles for intercellular communication and nutrient delivery, which play a critical role in physiological liver metabolism and pathological hepatopathy[80,215,216]. To date, a variety of sEVs have been applied to modulating hepatic signal transduction such as MSC-exosomes, MSC-sEVs[173], and bioengineered sEVs[217]. Of them, it was observed the differential alteration between intracellular and extracellular inhibition of cathepsin D upon liver lipidome in MASLD mice[157]. Meanwhile, MSC-EVs exercise therapeutic effects upon liver diseases by regulating the mitochondrial functions of immune cells (e.g., macrophages, lymphocytes), which provides new evidence and novel insights for stem cell-related immunotherapeutic remedies by targeting immune microenvironment (e.g., cell polarization, reactive oxygen, programmed apoptosis, and tumor microenvironment)[202,218-221]. Collectively, stem cells allow for interrogating core insights governing liver-specific organogenesis and pathogenesis, and pave the way for conquering coagulation liver disorders and the concomitant precision medicine.
The co-authors thank the members in The Fourth People’s Hospital of Jinan Affiliated to Shandong Second Medical University, Qingdao Medical College of Qingdao University, Shandong Provincial Hospital Affiliated to Shandong First Medical University, School of Basic Medicine of Gannan Medical University, Shandong Provincial Key Medical and Health Laboratory of Blood Ecology and Biointelligence, Jinan Key Laboratory of Medical Cell Bioengineering, and Shandong Health Youth Science and Technology Innovation Team for their suggestions and technical supports.
| 1. | Koui Y, Tanaka M, Kido T. Human PSC-derived liver cells and their applications for disease models and drug discovery. Pharmacol Ther. 2025;274:108907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Vallverdú J, Martínez García de la Torre RA, Mannaerts I, Verhulst S, Smout A, Coll M, Ariño S, Rubio-Tomás T, Aguilar-Bravo B, Martínez-Sánchez C, Blaya D, Verfaillie CM, van Grunsven LA, Sancho-Bru P. Directed differentiation of human induced pluripotent stem cells to hepatic stellate cells. Nat Protoc. 2021;16:2542-2563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Kietzmann T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017;11:622-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 4. | Zhang L, Wang X, Yang X, Chi Y, Chu Y, Zhang Y, Gong Y, Wang F, Zhao Q, Zhao D. Genome Engineering of Primary and Pluripotent Stem Cell-Derived Hepatocytes for Modeling Liver Tumor Formation. Biology (Basel). 2024;13:684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, He Y, Deng R, Jiang Z, Zhang L, Zeng Y, Zou L. Multifaceted Characterization of Human Embryonic Stem Cell-Derived Mesenchymal Stem/Stromal Cells Revealed Amelioration of Acute Liver Injury in NOD-SCID Mice. Cell Transplant. 2024;33:9636897231218383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chen N, Liu S, Qin D, Guan D, Chen Y, Hou C, Zheng S, Wang L, Chen X, Chen W, Zhang L. Fate tracking reveals differences between Reelin(+) hepatic stellate cells (HSCs) and Desmin(+) HSCs in activation, migration and proliferation. Cell Prolif. 2023;56:e13500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Bozic D, Mamic B, Peric I, Bozic I, Zaja I, Ivanovic T, Gugic Ratkovic A, Grgurevic I. Assessment of Sarcopenia in Patients with Liver Cirrhosis-A Literature Review. Nutrients. 2025;17:2589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Yang Q, Zhang L, Li Q, Gu M, Qu Q, Yang X, Yi Q, Gu K, Kuang L, Hao M, Xu J, Yang H. Characterization of microbiome and metabolite analyses in patients with metabolic associated fatty liver disease and type II diabetes mellitus. BMC Microbiol. 2022;22:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Iruzubieta P, de Vega T, Crespo J. Overlooked determinants and unequal outcomes: rethinking metabolic dysfunction-associated steatotic liver disease beyond the biomedical model. Lancet Gastroenterol Hepatol. 2025;S2468-1253(25)00226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Wu K, Ji J, Pan J, Zhu M, Zhang J, Sun T, Lv D, Wei M, Wang M, Yao H. MSP-RON signaling in liver pathobiology and as an emerging therapeutic target: a review of the current evidence. Cell Commun Signal. 2025;23:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Omar DY, Willoughby MM, Mostafa N, Otakhor K, Bhatt S, Abbas Zaidi MA, Schott MB. Lipid Droplet Dynamics in Alcoholic Steatohepatitis. Am J Pathol. 2025;S0002-9440(25)00299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Yu H, Feng Y, Du W, Zhao M, Jia H, Wei Z, Yan S, Han Z, Zhang L, Li Z, Han Z. Off-the-shelf GMP-grade UC-MSCs as therapeutic drugs for the amelioration of CCl4-induced acute-on-chronic liver failure in NOD-SCID mice. Int Immunopharmacol. 2022;113:109408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Dai S, Su X, Li Z, Wang H, Liu L, Xie Y, Chai Y, Chen Y, Zhao Z, Luo B, Kong J, He Y, Cao H, Xin M, Shao G, Shi Y, Xiong F, Tang W, Song J. Phosphorus-32 microspheres: A dual-modality transarterial radioembolization approach for hepatocellular carcinoma therapy and Anti-PD1 immunotherapy potentiation. Mater Today Bio. 2025;34:102210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Lu H, Li Y, Chen J, Song D, Luo X, Lu X, Huang X, Tan W, Xie C, Wang C. In-situ-formed TCM-inspired dual-function nanocomposite hydrogel for intraoperative hemostasis and postoperative recurrence prevention in hepatocellular carcinoma. Mater Today Bio. 2025;34:102192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Sibiya T, Xaba L, Mthethwa L, Chuturgoon AA, Msomi N. The Evolution of Cell Culture Systems to Study Hepatitis B Virus Pathogenesis and Antiviral Susceptibility. Viruses. 2025;17:1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Toma D, Anghel L, Patraș D, Ciubară A. Hepatitis C Virus: Epidemiological Challenges and Global Strategies for Elimination. Viruses. 2025;17:1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Bardak AE, Ozturk NB, Gurakar M, Sequeira L, Yildiz E, Ozmert EH, Idilman R, Gurakar A. Updates on Recent Advancements in Hepatitis D Virus Treatment. Viruses. 2025;17:1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Cao J, Meltzer R, Cohn E, Benjamin A, Keskey R, Alverdy J. Friend or foe: the gut microbiota as a modulator of disease trajectory in trauma, surgery, and critical illness. Gut Microbes. 2025;17:2552346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Dourado TMH, Tirapelli CR. The Role of Perivascular Adipose Tissue, White Adipose Tissue, and Brown Adipose Tissue in the Pathophysiological Effects of Ethanol. Am J Pathol. 2025;S0002-9440(25)00301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Jin K, Zeng S, Li B, Zhang G, Wu J, Hu X, Chao M. Bicarbonate-integrated transarterial chemoembolization (TACE) in real-world hepatocellular carcinoma. Signal Transduct Target Ther. 2025;10:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Ratti C, Malaguti M, Emanuele D, Bellasi A, Sanna G. Understanding MASLD - from molecular pathogenesis to cardiovascular risk: A concise review for the clinical cardiologist. Atherosclerosis. 2025;409:120495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Du K, Umbaugh DS, Ren N, Diehl AM. Cellular senescence in liver diseases: From molecular drivers to therapeutic targeting. J Hepatol. 2025;S0168-8278(25)02454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, Huch M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11:1724-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 587] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 24. | Fazel Modares N, Polz R, Haghighi F, Lamertz L, Behnke K, Zhuang Y, Kordes C, Häussinger D, Sorg UR, Pfeffer K, Floss DM, Moll JM, Piekorz RP, Ahmadian MR, Lang PA, Scheller J. IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy. Hepatology. 2019;70:2075-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Nechemia-Arbely Y, Shriki A, Denz U, Drucker C, Scheller J, Raub J, Pappo O, Rose-John S, Galun E, Axelrod JH. Early hepatocyte DNA synthetic response posthepatectomy is modulated by IL-6 trans-signaling and PI3K/AKT activation. J Hepatol. 2011;54:922-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2227] [Cited by in RCA: 2462] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 27. | Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1412] [Cited by in RCA: 1426] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 28. | Balaphas A, Meyer J, Perozzo R, Zeisser-Labouebe M, Berndt S, Turzi A, Fontana P, Scapozza L, Gonelle-Gispert C, Bühler LH. Platelet Transforming Growth Factor-β1 Induces Liver Sinusoidal Endothelial Cells to Secrete Interleukin-6. Cells. 2020;9:1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Ren R, Yan L, Zhou Y, Sun R, Song H, Yan H, Li Y. From Bench to Bedside: Emerging Paradigms in CAR-T Cell Therapy for Solid Malignancies. Adv Sci (Weinh). 2025;e05822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Jaeckel E, Friedman SL, Hudecek M, Protzer U. Chimeric antigen receptor (CAR) T-cell therapy: Engineering immune cells to treat liver diseases. J Hepatol. 2025;83:1156-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Cheng Y, Gong Y, Li X, Zeng F, Liu B, Chen W, Zhang F, Chen H, Zhu W, Li H, Zhou L, Wu T, Zhou W. A spreadable self-gelling hemostatic powder sensitizes CAR-NK cell therapy to prevent hepatocellular carcinoma recurrence postresection. J Nanobiotechnology. 2025;23:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Thangaraj JL, Coffey M, Lopez E, Kaufman DS. Disruption of TGF-β signaling pathway is required to mediate effective killing of hepatocellular carcinoma by human iPSC-derived NK cells. Cell Stem Cell. 2024;31:1327-1343.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 33. | Peng L, Sferruzza G, Yang L, Zhou L, Chen S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol Immunol. 2024;21:1089-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 180] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 34. | Zhang L, Wei Y, Chi Y, Liu D, Yang S, Han Z, Li Z. Two-step generation of mesenchymal stem/stromal cells from human pluripotent stem cells with reinforced efficacy upon osteoarthritis rabbits by HA hydrogel. Cell Biosci. 2021;11:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Wang H, Liu C, Wu Q, Su P, Wu D, Guo J, Zhou W, Xu Y, Shi L, Zhou J. MSX2 Initiates and Accelerates Mesenchymal Stem/Stromal Cell Specification of hPSCs by Regulating TWIST1 and PRAME. Stem Cell Reports. 2018;11:497-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Ma D, Wei P, Cheng Q, Hao J, Li Z, Chen Z, Shi W, Yuan Z, Lo C, Luo Y, Qiao L, Gao J, Zhu J, Li Z. Immune checkpoint inhibitors use in liver transplantation for hepatocellular carcinoma: a global cohort study. BMC Med. 2025;23:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Jiang X, Yang J, Liu F, Tao J, Xu J, Zhang M. Embryonic stem cell-derived mesenchymal stem cells alleviate skeletal muscle injury induced by acute compartment syndrome. Stem Cell Res Ther. 2022;13:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 38. | Wei Y, Hou H, Zhang L, Zhao N, Li C, Huo J, Liu Y, Zhang W, Li Z, Liu D, Han Z, Zhang L, Song B, Chi Y, Han Z. JNKi- and DAC-programmed mesenchymal stem/stromal cells from hESCs facilitate hematopoiesis and alleviate hind limb ischemia. Stem Cell Res Ther. 2019;10:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Yu A, Ma C, Hu M. Rejuvenated Autologous Adult Stem Cells: Emerging Front Runners in the Fight Against Aging and Associated Diseases. Cells. 2025;14:1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Yao J, Chen N, Wang X, Zhang L, Huo J, Chi Y, Li Z, Han Z. Human Supernumerary Teeth-Derived Apical Papillary Stem Cells Possess Preferable Characteristics and Efficacy on Hepatic Fibrosis in Mice. Stem Cells Int. 2020;2020:6489396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Fernandes JMS, Pagani E, Wenceslau CV, Ynoue LH, Ferrara L, Kerkis I. Phase II trial of intravenous human dental pulp stem cell therapy for Huntington's disease: a randomized, double-blind, placebo-controlled study. Stem Cell Res Ther. 2025;16:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Huo J, Zhang L, Ren X, Li C, Li X, Dong P, Zheng X, Huang J, Shao Y, Ge M, Zhang J, Wang M, Nie N, Jin P, Zheng Y. Multifaceted characterization of the signatures and efficacy of mesenchymal stem/stromal cells in acquired aplastic anemia. Stem Cell Res Ther. 2020;11:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 43. | Wang L, Zhang L, Liang X, Zou J, Liu N, Liu T, Wang G, Ding X, Liu Y, Zhang B, Liang R, Wang S. Adipose Tissue-Derived Stem Cells from Type 2 Diabetics Reveal Conservative Alterations in Multidimensional Characteristics. Int J Stem Cells. 2020;13:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Tang J, Han Y, Ren P, Xu L, Lei Z, Li J, Cui Y, Zhang J, Li J, Li X. Mitochondria transplanted adipose-derived stem cells/decellularized adipose tissue hydrogel for adipose tissue regeneration. Mater Today Bio. 2025;34:102193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Zhao Q, Zhang L, Wei Y, Yu H, Zou L, Huo J, Yang H, Song B, Wei T, Wu D, Zhang W, Zhang L, Liu D, Li Z, Chi Y, Han Z, Han Z. Systematic comparison of hUC-MSCs at various passages reveals the variations of signatures and therapeutic effect on acute graft-versus-host disease. Stem Cell Res Ther. 2019;10:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 46. | Hou H, Zhang L, Duan L, Liu Y, Han Z, Li Z, Cao X. Spatio-Temporal Metabolokinetics and Efficacy of Human Placenta-Derived Mesenchymal Stem/Stromal Cells on Mice with Refractory Crohn's-like Enterocutaneous Fistula. Stem Cell Rev Rep. 2020;16:1292-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Sun Y, Wang TE, Hu Q, Zhang W, Zeng Y, Lai X, Zhang L, Shi M. Systematic comparation of the biological and transcriptomic landscapes of human amniotic mesenchymal stem cells under serum-containing and serum-free conditions. Stem Cell Res Ther. 2022;13:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | De Los Angeles A, Ferrari F, Xi R, Fujiwara Y, Benvenisty N, Deng H, Hochedlinger K, Jaenisch R, Lee S, Leitch HG, Lensch MW, Lujan E, Pei D, Rossant J, Wernig M, Park PJ, Daley GQ. Hallmarks of pluripotency. Nature. 2015;525:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 49. | Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell. 2018;23:181-192.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 50. | Chen Z, Duan M, Wang X, Liu B. SemiLT: A Multianchor Transfer Learning Method for Cross-Modality Cell Label Annotation from scRNA-seq to scATAC-seq. Adv Sci (Weinh). 2025;e07846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Guesdon R, Santoro S, Cras A, Pagin E, Serteyn D, Ceusters J, Guillemot F, Hagège A, Menasché P. Repair of infarcted myocardium by skeletal muscle-derived mesenchymal stromal cells delivered by a bioprinted collagen patch. Stem Cell Res Ther. 2025;16:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 52. | Fernandez-Muñoz B, Garcia-Delgado AB, Arribas-Arribas B, Sanchez-Pernaute R. Human Neural Stem Cells for Cell-Based Medicinal Products. Cells. 2021;10:2377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 53. | Hu R, Zhao J, Lai KC, Wang S, Zheng J, Stoddard C, Lai L. CHD7 regulates definitive endodermal and mesodermal development from human embryonic stem cells. Stem Cell Res Ther. 2025;16:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Li Q, Yu X. Telomere-to-telomere sequence of mouse haploid stem cells. Sci China Life Sci. 2025;68:3102-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Liu Z, Bartolomei MS. Overcoming gene dosage barriers in mammalian development: An imprinting balancing act. Cell Stem Cell. 2025;32:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Cashman R, Haim-Abadi G, Lezmi E, Philip H, Nissenbaum J, Viner-Breuer R, Kozulin C, Golan-Lev T, Gadban A, Spinner-Potesky S, Yanuka O, Kopper O, Benvenisty N. Genome-Wide Screening in Haploid Stem Cells Reveals Synthetic Lethality Targeting MLH1 and TP53 Deficient Tumours. Cell Prolif. 2025;e13788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Meng G, Gu J, Liew SY, Cao J, Wang Z, Ma C, Fu Z, Zhou H, Wang J, Wang S, Jing S, Wu Y, Lei Z, Zhi S, He Y, Li C, Deng H. Reconstruction of endocrine subtype-complete human pluripotent stem cell-derived islets with capacity for hypoglycemia protection in vivo. Cell Stem Cell. 2025;32:1438-1456.e7. [PubMed] [DOI] [Full Text] |
| 58. | Uzun D, Paul JM, Jensen A, Tamaresis J, Domingo W, Arai S, Ventura FA, Rocha J, Bennett L, Jones A, Bharadwaj S, Johnston L, Lowsky R, Rezvani A, Shiraz P, Shizuru J, Weng WK, Hosoya H, Sidana S, Miklos D, Muffly L, Mikkilineni L. The Impact of Anti-CD38 Monoclonal Antibody Therapy on Stem-Cell Mobilization Yields in Patients With Newly Diagnosed Multiple Myeloma (NDMM) Referred From Community and Academic Oncology Practices: Single Center, Real World Data 2021-2024. Transplant Cell Ther. 2025;S2666-6367(25)01380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 59. | Fokkema C, Appelman MK, Veldhoen N, Spelt A, van der Holt B, Grootes M, Wester R, de Pater E, Ypma PF, Broers AEC, Sonneveld P, Broijl A. Mobilization of hematopoietic stem cells using G-CSF with or without cyclophosphamide before autologous stem cell transplantation in patients with newly diagnosed multiple myeloma receiving induction therapy with or without daratumumab. Bone Marrow Transplant. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Meng X, Melsen JE, van der Holst R, de Mooij B, Vloemans S, van Eggermond M, Berghuis D, Lankester AC, Kivit S, Pike-Overzet K, Langerak AW, Staal FJ, Ott de Bruin LM, Canté-Barrett K. RAG1 lentiviral gene therapy restores T cell development of RAG1-SCID patient cells in artificial thymic organoids. Blood Adv. 2025;bloodadvances.2025016970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Riza SM, Porosnicu AL, Cepi PA, Parasca SV, Sinescu RD. Integrating Regenerative Medicine in Chronic Wound Management: A Single-Center Experience. Biomedicines. 2025;13:1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 62. | Kanie K, Ito T, Iguchi G, Matsumoto R, Muguruma K, Urai S, Kitayama S, Bando H, Yamamoto M, Fukuoka H, Ogawa W, Kaneko S, Takahashi Y. Modeling of T cell-mediated autoimmune pituitary disease using human induced pluripotent stem cell-originated organoid. Nat Commun. 2025;16:7900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Sun HL, Fu Q, Zhao XY, Yan CH, Zhang YY, Mo XD, Chen YH, Xu LP, Wang Y, Zhang XH, Huang XJ, Pei XY. Long-Term Efficacy of Epstein-Barr Virus-Specific T Cells for Epstein-Barr Virus-Associated Post-Transplantation Lymphoproliferative Disorder after Allogeneic Stem Cell Transplantation: A Real-World Study. Transplant Cell Ther. 2025;S2666-6367(25)01383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Morimoto H, Ogonuki N, Matoba S, Tokita S, Kanatsu-Shinohara M, Miyazaki T, Ogura A, Shinohara T. Allogeneic germline stem cell transplantation restores fertility in nonablated recipient mice†. Biol Reprod. 2025;ioaf183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Morizane A, Yamasaki E, Shindo T, Anazawa T, Sawamoto N, Shima A, Yamakado H, Nakanishi E, Sawamura M, Taruno Y, Doi D, Kikuchi T, Kawasaki Y, Saito MK, Kikuchi T, Arakawa Y, Miyamoto S, Nakamoto Y, Takahashi R, Takahashi J. Control of immune response in an iPSC-based allogeneic cell therapy clinical trial for Parkinson's disease. Cell Stem Cell. 2025;32:1346-1355.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Song Y, Liu Y, Yu Y, Wang Y, Mu Y, Wang S, Han W, Zhang H, Zhang W. Case Report: Allogeneic adipose-derived mesenchymal stem cells for severe feline chronic kidney disease. Front Vet Sci. 2025;12:1632324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 430] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 68. | Pozo Garcia V, Çobanoğlu TS, Hammer HS, Carlota R, Holm K, Verfaillie C, Poetz O, Jennings P, Moco S. Nutrient environment improves drug metabolic activity in human iPSC-derived hepatocytes and HepG2. Arch Toxicol. 2025;99:4493-4511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Li MQ, Xu YP, Li K, Zhou C, Fan XX, Wang H, Shi PD, Li RT, Wang ZX, Cao TS, Chen Q, Cui YJ, Deng YQ, Wu XY, Zhao H, Qin CF. Recapitulating dengue virus infection with human pluripotent stem cell-derived liver organoids for antiviral screening. Nat Commun. 2025;16:8069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 71. | Ang LT, Tan AKY, Autio MI, Goh SH, Choo SH, Lee KL, Tan J, Pan B, Lee JJH, Lum JJ, Lim CYY, Yeo IKX, Wong CJY, Liu M, Oh JLL, Chia CPL, Loh CH, Chen A, Chen Q, Weissman IL, Loh KM, Lim B. A Roadmap for Human Liver Differentiation from Pluripotent Stem Cells. Cell Rep. 2018;22:2190-2205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 72. | Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 528] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 73. | Palaria A, Angelo JR, Guertin TM, Mager J, Tremblay KD. Patterning of the hepato-pancreatobiliary boundary by BMP reveals heterogeneity within the murine liver bud. Hepatology. 2018;68:274-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 460] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 75. | Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, Yin X, Wu C, Che J, Lu S, Ding M, Deng H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 76. | Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 77. | Teufel A, Itzel T, Erhart W, Brosch M, Wang XY, Kim YO, von Schönfels W, Herrmann A, Brückner S, Stickel F, Dufour JF, Chavakis T, Hellerbrand C, Spang R, Maass T, Becker T, Schreiber S, Schafmayer C, Schuppan D, Hampe J. Comparison of Gene Expression Patterns Between Mouse Models of Nonalcoholic Fatty Liver Disease and Liver Tissues From Patients. Gastroenterology. 2016;151:513-525.e0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 78. | Ong J, Zhao JJ, Swift C, Markaki AE. Biopolymers for Liver Tissue Engineering: A Systematic Review. Gels. 2025;11:525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 79. | Kazemzadeh Dastjerd M, Merens V, Smout A, De Wolf R, Chesné C, Verfaillie C, Verhulst S, van Grunsven LA. 9-cis-Retinoic Acid Improves Disease Modelling in iPSC-Derived Liver Organoids. Cells. 2025;14:983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Ye L, Li S, Bi G, Li B, Cai Z, Jin M, Zhang Y, Yang W, Li Y, Li S, Hu W, Gao Y, Pan M, Zhou S, Zhang C, Bi H, Peng Q. Exosomes From Intestinal Epithelial Cells Promote Hepatic Differentiation of Liver Progenitor Cells in Gut-Liver-on-a-Chip Models. Adv Sci (Weinh). 2025;12:e17478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 81. | Mugaanyi J, Huang J, Fang J, Musinguzi A, Lu C, Chen Z. Developments and Applications of Liver-on-a-Chip Technology-Current Status and Future Prospects. Biomedicines. 2025;13:1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 555] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 83. | Park IS. In Vitro Hepatocyte Differentiation of Human Adipose-Derived Stem Cells Under Hypoxia and Photobiomodulation Irradiation. Cell Reprogram. 2025;27:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Chen CC, Hsu LW, Chen KD, Chiu KW, Kung CP, Li SR, Goto S, Chen CL, Huang KT. Extracellular calreticulin regulates fibrogenic and immunogenic properties of hepatic stellate cells. Int Immunopharmacol. 2025;148:114129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | de la Torre RAMG, Sancho-Bru P. Differentiation of Hepatic Stellate Cells from Pluripotent Stem Cells. Methods Mol Biol. 2023;2669:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 86. | Schwabe RF, Brenner DA. Hepatic stellate cells: balancing homeostasis, hepatoprotection and fibrogenesis in health and disease. Nat Rev Gastroenterol Hepatol. 2025;22:481-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 87. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1430] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 88. | Martini T, Naef F, Tchorz JS. Spatiotemporal Metabolic Liver Zonation and Consequences on Pathophysiology. Annu Rev Pathol. 2023;18:439-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 89. | Annunziato S, Sun T, Tchorz JS. The RSPO-LGR4/5-ZNRF3/RNF43 module in liver homeostasis, regeneration, and disease. Hepatology. 2022;76:888-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | Sugimoto A, Saito Y, Wang G, Sun Q, Yin C, Lee KH, Geng Y, Rajbhandari P, Hernandez C, Steffani M, Qie J, Savage T, Goyal DM, Ray KC, Neelakantan TV, Yin D, Melms J, Lehrich BM, Yasaka TM, Liu S, Oertel M, Lan T, Guillot A, Peiseler M, Filliol A, Kanzaki H, Fujiwara N, Ravi S, Izar B, Brosch M, Hampe J, Remotti H, Argemi J, Sun Z, Kendall TJ, Hoshida Y, Tacke F, Fallowfield JA, Blockley-Powell SK, Haeusler RA, Steinman JB, Pajvani UB, Monga SP, Bataller R, Masoodi M, Arpaia N, Lee YA, Stockwell BR, Augustin HG, Schwabe RF. Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature. 2025;640:752-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 91. | Coll M, Perea L, Boon R, Leite SB, Vallverdú J, Mannaerts I, Smout A, El Taghdouini A, Blaya D, Rodrigo-Torres D, Graupera I, Aguilar-Bravo B, Chesne C, Najimi M, Sokal E, Lozano JJ, van Grunsven LA, Verfaillie CM, Sancho-Bru P. Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell. 2018;23:101-113.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 92. | Wilhelmsen I, Combriat T, Dalmao-Fernandez A, Stokowiec J, Wang C, Olsen PA, Wik JA, Boichuk Y, Aizenshtadt A, Krauss S. The effects of TGF-β-induced activation and starvation of vitamin A and palmitic acid on human stem cell-derived hepatic stellate cells. Stem Cell Res Ther. 2024;15:223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 93. | Hsieh CC, Hung CH, Lu L, Qian S. Hepatic immune tolerance induced by hepatic stellate cells. World J Gastroenterol. 2015;21:11887-11892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Abdulmajeed RJ, Sergi CM. Liver Sinusoidal Endothelial Cells and Their Regulation of Immunology, Collagenization, and Bioreactivity in Fatty Liver: A Narrative Review. Int J Mol Sci. 2025;26:8006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 95. | Liu J, Wang Q, Chen H, Yin W, Diao J, Ma W, Su Y, Le Y, Jin B, Dong J, Wang Y. Construction of region-specific liver organoid and fabrication of hierarchical functional liver lobule for liver disease modeling and drug evaluation. Sci Bull (Beijing). 2025;S2095-9273(25)00741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Gage BK, Merlin S, Olgasi C, Follenzi A, Keller GM. Therapeutic correction of hemophilia A by transplantation of hPSC-derived liver sinusoidal endothelial cell progenitors. Cell Rep. 2022;39:110621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Gage BK, Liu JC, Innes BT, MacParland SA, McGilvray ID, Bader GD, Keller GM. Generation of Functional Liver Sinusoidal Endothelial Cells from Human Pluripotent Stem-Cell-Derived Venous Angioblasts. Cell Stem Cell. 2020;27:254-269.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 98. | Saiki N, Nio Y, Yoneyama Y, Kawamura S, Iwasawa K, Kawakami E, Araki K, Fukumura J, Sakairi T, Kono T, Ohmura R, Koido M, Funata M, Thompson WL, Cruz-Encarnacion P, Chen YW, Takebe T. Self-organization of sinusoidal vessels in pluripotent stem cell-derived human liver bud organoids. Nat Biomed Eng. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 99. | Kent GM, Atkins MH, Lung B, Nikitina A, Fernandes IM, Kwan JJ, Andrews TS, MacParland SA, Keller GM, Gage BK. Human liver sinusoidal endothelial cells support the development of functional human pluripotent stem cell-derived Kupffer cells. Cell Rep. 2024;43:114629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 100. | Arai T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Iinuma N, Iesato Y, Koyama T, Yoshizawa T, Uetake R, Yamauchi A, Yang L, Kawate H, Ogawa S, Kobayashi A, Miyagawa S, Shindo T. Induction of LYVE-1/stabilin-2-positive liver sinusoidal endothelial-like cells from embryoid bodies by modulation of adrenomedullin-RAMP2 signaling. Peptides. 2011;32:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Ogawa M, Jiang JX, Xia S, Yang D, Ding A, Laselva O, Hernandez M, Cui C, Higuchi Y, Suemizu H, Dorrell C, Grompe M, Bear CE, Ogawa S. Generation of functional ciliated cholangiocytes from human pluripotent stem cells. Nat Commun. 2021;12:6504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WT, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NRF, Vallier L. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 103. | Fabris L, Fiorotto R, Spirli C, Cadamuro M, Mariotti V, Perugorria MJ, Banales JM, Strazzabosco M. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol. 2019;16:497-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 104. | Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, Grompe M, Keller G, Kamath BM, Ghanekar A. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 105. | Sampaziotis F, Segeritz CP, Vallier L. Potential of human induced pluripotent stem cells in studies of liver disease. Hepatology. 2015;62:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Tasnim F, Xing J, Huang X, Mo S, Wei X, Tan MH, Yu H. Generation of mature kupffer cells from human induced pluripotent stem cells. Biomaterials. 2019;192:377-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 107. | Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4108] [Cited by in RCA: 3954] [Article Influence: 247.1] [Reference Citation Analysis (0)] |
| 108. | Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1823] [Article Influence: 151.9] [Reference Citation Analysis (0)] |
| 109. | Fowler JL, Zheng SL, Nguyen A, Chen A, Xiong X, Chai T, Chen JY, Karigane D, Banuelos AM, Niizuma K, Kayamori K, Nishimura T, Cromer MK, Gonzalez-Perez D, Mason C, Liu DD, Yilmaz L, Miquerol L, Porteus MH, Luca VC, Majeti R, Nakauchi H, Red-Horse K, Weissman IL, Ang LT, Loh KM. Lineage-tracing hematopoietic stem cell origins in vivo to efficiently make human HLF+ HOXA+ hematopoietic progenitors from pluripotent stem cells. Dev Cell. 2024;59:1110-1131.e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 110. | Bonnardel J, T'Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, Vanneste B, De Prijck S, Nedospasov SA, Kremer A, Van Hamme E, Borghgraef P, Toussaint W, De Bleser P, Mannaerts I, Beschin A, van Grunsven LA, Lambrecht BN, Taghon T, Lippens S, Elewaut D, Saeys Y, Guilliams M. Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity. 2019;51:638-654.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 111. | Zhang K, Yuan X, Lu S, Shu Y, Wang C, Cen J, Wu B, Hui L. Expansion of human hepatocytes and their application in three-dimensional culture and genetic manipulation. Nat Protoc. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Chi KY, Kim G, Kim H, Kim H, Jo S, Lee J, Lee Y, Yoon H, Cho S, Kim J, Lee JS, Yeon GB, Kim DS, Park HJ, Kim JH. Optimization of culture conditions to generate vascularized multi-lineage liver organoids with structural complexity and functionality. Biomaterials. 2025;314:122898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 113. | Kim HY, Oh JH, Kim HS, Jun DW. Biomarker-Driven Optimization of Saponin Therapy in MASLD: From Mouse Models to Human Liver Organoids. Antioxidants (Basel). 2025;14:943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Hegazy RA. Unraveling Liver Cirrhosis: Bridging Pathophysiology to Innovative Therapeutics. J Gastroenterol Hepatol. 2025;40:2449-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 115. | Chen W, Chen X, Gao F, Yao Q, Cheng S, Pan Q, Yu J, Yang J, Ma G, Gong J, Li Q, Chen Y, Lim LW, Stambler I, Ellison-Hughes GM, Ulfhake B, Zhao RC, Cao H. Mesenchymal stem cell-derived extracellular vesicles attenuate periductal fibrosis by inhibiting Th17 differentiation in human liver multilineage organoids and Mdr2(-/-) mice. J Nanobiotechnology. 2025;23:546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 116. | Zhou H, Zhu L, Zhang Y, Chen L, Gou DM, Zhang H, Hua R, Song J, Qiu C, Yao FW, Wei X, Gu D, Xu Y. Tissue Factor Pathway Inhibitor 2 Enhances Hepatocellular Carcinoma Chemosensitivity by Activating CCAR2-GADD45A-Mediated DNA Damage Repair. Int J Biol Sci. 2025;21:4629-4646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 117. | Kabir TD, Beck S, Stuart LM, Li J, Hou R, Liu P, Margolius S, Kim C, Choi YS, Bastow ER, Beveridge DJ, Spalding L, Li Z, Ginhoux F, Chow P, Phillips M, Redfern AD; Liver Cancer Collaborative, Yeoh GC, Forrest A, Woo AJ, Sharma A, George J, McCaughan G, Leedman PJ. Inhibition of the Caveolin-1 pathway promotes apoptosis and overcomes pan-tyrosine kinase inhibitor resistance in hepatocellular carcinoma. Cell Death Dis. 2025;16:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 118. | Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017;21:2661-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 119. | Ashmore-Harris C, Ayabe H, Yoshizawa E, Arisawa T, Takada Y, Takebe T, Fruhwirth GO. Gene editing enables non-invasive in vivo PET imaging of human induced pluripotent stem cell-derived liver bud organoids. Mol Ther Methods Clin Dev. 2025;33:101406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 120. | Yoshimoto K, Maki K, Adachi T, Kamei KI. Cyclic Stretching Enhances Angiocrine Signals at Liver Bud Stage from Human Pluripotent Stem Cells in Two-Dimensional Culture. Tissue Eng Part A. 2024;30:426-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Peng WC, Kraaier LJ, Kluiver TA. Hepatocyte organoids and cell transplantation: What the future holds. Exp Mol Med. 2021;53:1512-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 122. | Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Álvarez-Varela A, Wu P, Jin Y, Zhu J, Li B, Grompe M, Wang B, Nusse R. Inflammatory Cytokine TNFα Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell. 2018;175:1607-1619.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 123. | Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J, van den Born M, Zou C, Quirk C, Chiriboga L, Rice CM, Ma S, Rios A, Peters PJ, de Jong YP, Clevers H. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175:1591-1606.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 606] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 124. | Rose S, Ezan F, Huot L, Pécot T, Bellamri M, Turesky R, Nesslany F, Platel A, Langouët S. Computerized predictive approaches of genotoxicity and mutagenesis in 3D Hepoid of normal and transformed human hepatocytes. Toxicology. 2025;517:154242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 125. | Berreur E, Lazzaroni G, Roth C, Zihlmann M, Stirn M, Matheis R, Xicluna R, Breous-Nystrom E, Roth AB. iPSC-hepatocyte organoids as a novel platform to predict AAV gene therapy efficacy. Mol Ther Methods Clin Dev. 2025;33:101467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 126. | Luo YH, Ge JY, Chi Y, Ding M, Wu HL, Zou Q, Zheng YW. Generation of human induced pluripotent stem cells with inducible expression of GATA6 (Liver cells from hiPSC-GATA6(eGFP)). Stem Cell Res. 2025;87:103784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Dowbaj AM, Sljukic A, Niksic A, Landerer C, Delpierre J, Yang H, Lahree A, Kühn AC, Beers D, Byrne HM, Seifert S, Harrington HA, Zerial M, Huch M. Mouse liver assembloids model periportal architecture and biliary fibrosis. Nature. 2025;644:473-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 128. | Choi YJ, Kim MS, Rhoades JH, Johnson NM, Berry CT, Root S, Chen Q, Tian Y, Fernandez RJ 3rd, Cramer Z, Adams-Tzivelekidis S, Li N, Johnson FB, Lengner CJ. Patient-Induced Pluripotent Stem Cell-Derived Hepatostellate Organoids Establish a Basis for Liver Pathologies in Telomeropathies. Cell Mol Gastroenterol Hepatol. 2023;16:451-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 129. | Zhong H, Wang J, Liu X, Wei X, Zhou C, Zou T, Han X, Mo L, Qin W, Zhang Y. A multi-dimensional computational framework of drug-induced hepatotoxicity: integrating molecular structure features with disease pathogenesis. Brief Bioinform. 2025;26:bbaf456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 130. | Teng LS, Ying ZD, Sun XH, Hou HC, Qiu SD, Liu P, Li KJ, Zhang L, Sheng XH. Formoterol, a clinically approved drug, inhibits ferroptosis by suppressing lipid peroxidation and attenuates APAP-induced acute liver injury. Chem Biol Interact. 2025;421:111724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Qu Y, Jiang Z, Chen Z, Luo S, Xie B, Wu X, Yuan G, Wu K, Chen L, Tian T, Li S, Luo H, Li Q, Yuan D, Zhang Y, Gao Y, Zhou J, Yan Z, Jiang Y. CpG-oligodeoxynucleotides challenged macrophages ameliorate acetaminophen induced liver injury by activating TLR9/IRG1/itaconate metabolic pathway. Mol Med. 2025;31:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 132. | Chen H, Liu H, Wei J, Liu R, Tian X. Integration of transcriptomics and metabolomics to reveal crizotinib-induced liver injury in mice. Eur J Pharmacol. 2025;1006:178096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 133. | Altowijri MA, Abdelmageed ME, El-Gamal R, Saeedi T, El-Agamy DS. Pristimerin Dampens Acetaminophen-Induced Hepatotoxicity; The Role of NF-κB/iNOS/COX-II/Cytokines, PI3K/AKT, and BAX/BCL-2/Caspase-3 Signaling Pathways. Pharmaceutics. 2025;17:1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 134. | Samy EM, Shaaban EA. Liraglutide Ameliorates Gamma Radiation-Induced Hepatic Damage in Rats: The Role of an Autophagy Flux Activation Via LKB1/AMPK/mTOR Axis. Arch Med Res. 2025;57:103296. [PubMed] [DOI] [Full Text] |
| 135. | Tang Y, Wu P, Li L, Xu W, Jiang J. Mesenchymal Stem Cells and Their Small Extracellular Vesicles as Crucial Immunological Efficacy for Hepatic Diseases. Front Immunol. 2022;13:880523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 136. | Zhao S, Huang M, Yan L, Zhang H, Shi C, Liu J, Zhao S, Liu H, Wang B. Exosomes Derived from Baicalin-Pretreated Mesenchymal Stem Cells Alleviate Hepatocyte Ferroptosis after Acute Liver Injury via the Keap1-NRF2 Pathway. Oxid Med Cell Longev. 2022;2022:8287227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 137. | Lin F, Chen W, Zhou J, Zhu J, Yao Q, Feng B, Feng X, Shi X, Pan Q, Yu J, Li L, Cao H. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022;13:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 138. | Chen F, Che Z, Liu Y, Luo P, Xiao L, Song Y, Wang C, Dong Z, Li M, Tipoe GL, Yang M, Lv Y, Zhang H, Wang F, Xiao J. Invigorating human MSCs for transplantation therapy via Nrf2/DKK1 co-stimulation in an acute-on-chronic liver failure mouse model. Gastroenterol Rep (Oxf). 2024;12:goae016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 139. | Tian Y, Zhang J, Jia Z, Pan X, Hu Z, Kang R, Zhou X, Luo L, Shen Z, Shen Q. Biomimetic mineralized mesenchymal stem cell-derived exosomes for dual modulation of ferroptosis and lactic acid-driven inflammation in acute liver injury therapy. J Colloid Interface Sci. 2025;687:489-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 140. | Cai J, Tang D, Hao X, Liu E, Li W, Shi J. Mesenchymal stem cell-derived exosome alleviates sepsis- associated acute liver injury by suppressing MALAT1 through microRNA-26a-5p: an innovative immunopharmacological intervention and therapeutic approach for sepsis. Front Immunol. 2023;14:1157793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 141. | Wang Z, Zhong D, Yan T, Zheng Q, Zhou E, Ye Z, He X, Liu Y, Yan J, Yuan Y, Wang Y, Cai X. Stem Cells from Human Exfoliated Deciduous Teeth-Derived Exosomes for the Treatment of Acute Liver Injury and Liver Fibrosis. ACS Appl Mater Interfaces. 2025;17:17948-17964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 142. | He J, Du C, Li C, Li W, Qiu J, Ma M, Chen Y, Zhang Q. Ferroptosis in acute liver Failure: Unraveling the hepcidin-ferroportin axis and therapeutic interventions. Redox Biol. 2025;84:103657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 143. | Zhang J, Chen X, Chai Y, Jin Y, Li F, Zhuo C, Xu Y, Wang H, Ju E, Lao YH, Xie X, Li M, Tao Y. Mesenchymal stromal/stem cell spheroid-derived extracellular vesicles advance the therapeutic efficacy of 3D-printed vascularized artificial liver lobules in liver failure treatment. Bioact Mater. 2025;49:121-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 144. | Maiwall R, Kulkarni AV, Arab JP, Piano S. Acute liver failure. Lancet. 2024;404:789-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 145. | Wang H, Yao W, Wang Y, Dong H, Dong T, Zhou W, Cui L, Zhao L, Zhang Y, Shi L, Jiang Y. Meta-analysis on last ten years of clinical injection of bone marrow-derived and umbilical cord MSC to reverse cirrhosis or rescue patients with acute-on-chronic liver failure. Stem Cell Res Ther. 2023;14:267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 146. | Yang Y, Ni M, Zong R, Yu M, Sun Y, Li J, Chen P, Li C. Targeting Notch1-YAP Circuit Reprograms Macrophage Polarization and Alleviates Acute Liver Injury in Mice. Cell Mol Gastroenterol Hepatol. 2023;15:1085-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 147. | Tao Y, Wang Y, Wang M, Tang H, Chen E. Mesenchymal Stem Cells Alleviate Acute Liver Failure through Regulating Hepatocyte Apoptosis and Macrophage Polarization. J Clin Transl Hepatol. 2024;12:571-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 148. | Lu YT, Chen TY, Lin HH, Chen YW, Lin YX, Le DC, Huang YH, Wang AH, Lee CC, Ling TY. Small Extracellular Vesicles Engineered Using Click Chemistry to Express Chimeric Antigen Receptors Show Enhanced Efficacy in Acute Liver Failure. J Extracell Vesicles. 2025;14:e70044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 149. | Yang F, Ni B, Liang X, He Y, Yuan C, Chu J, Huang Y, Zhong H, Yang L, Lu J, Xu Y, Zhang Q, Chen W. Mesenchymal stromal cell-derived extracellular vesicles as nanotherapeutics for concanavalin a-induced hepatitis: modulating the gut‒liver axis. Stem Cell Res Ther. 2025;16:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 150. | Sun M, Li M, Hu M, Fan Y, Liu Y, Sun J, Zhang J. Fully Bioactive Nanodrugs: Stem Cell-Derived Exosomes Engineered with Biomacromolecules to Treat CCl(4)- and Extreme Hepatectomy-Induced Acute Liver Failure. ACS Nano. 2024;18:33907-33921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 151. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 612] [Article Influence: 102.0] [Reference Citation Analysis (3)] |
| 152. | Eslam M, Fan JG, Yu ML, Wong VW, Cua IH, Liu CJ, Tanwandee T, Gani R, Seto WK, Alam S, Young DY, Hamid S, Zheng MH, Kawaguchi T, Chan WK, Payawal D, Tan SS, Goh GB, Strasser SI, Viet HD, Kao JH, Kim W, Kim SU, Keating SE, Yilmaz Y, Kamani L, Wang CC, Fouad Y, Abbas Z, Treeprasertsuk S, Thanapirom K, Al Mahtab M, Lkhagvaa U, Baatarkhuu O, Choudhury AK, Stedman CAM, Chowdhury A, Dokmeci AK, Wang FS, Lin HC, Huang JF, Howell J, Jia J, Alboraie M, Roberts SK, Yoneda M, Ghazinian H, Mirijanyan A, Nan Y, Lesmana CRA, Adams LA, Shiha G, Kumar M, Örmeci N, Wei L, Lau G, Omata M, Sarin SK, George J. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease. Hepatol Int. 2025;19:261-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 153. | Zhang XJ, Fu J, Cheng X, Shen H, Yang H, Wang K, Li W, Tian H, Tian T, Zhou J, Tian S, Wang Z, Wan J, Bai L, Duan H, Zhang X, Tian R, Xu H, Liao R, Zou T, Shi J, Qu W, Fang L, Cai J, Zhang P, She ZG, Jiang J, Hu Y, Wang Y, Li H. Integrated screening identifies GPR31 as a key driver and druggable target for metabolic dysfunction-associated steatohepatitis. J Clin Invest. 2025;135:e173193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 154. | Zou X, Brigstock D. Extracellular Vesicles from Mesenchymal Stem Cells: Potential as Therapeutics in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines. 2024;12:2848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (20)] |
| 155. | Dai Q, Zhu D, Du X, Tan H, Chen Q. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicle in nonalcoholic fatty liver disease: a systematic review and meta-analysis of preclinical evidence. Lipids Health Dis. 2025;24:217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 156. | Ruiz MA, Kaiser Junior RLR, Piron-Ruiz G, de Quadros LG. Are mesenchymal stem/stromal cells a novel avenue for the treatment of non-alcoholic fatty liver disease? World J Stem Cells. 2025;17:99638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (2)] |
| 157. | Hu R, Zhao J, Cheng Y, Su W, Ren R, Zhang H, Zhang Y, Wang A, Mu Y, Yu S. Exogenous Mitochondrial Pretreatment Enhances the Therapeutic Effect of UC-MSCs on NAFLD in Type 2 Diabetic Mice by Mediating Mitochondrial Transfer. Stem Cells Int. 2025;2025:4639115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 158. | Tunstead C, Bitterlich LM, Ankrum JA, Hogan AE, English K. Palmitate enhances MSC immunomodulation of human macrophages via the ceramide/CCL2 axis in vitro. Stem Cell Res Ther. 2025;16:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 159. | Wang J, Bao S, An Q, Li C, Feng J. Roles of extracellular vesicles from different origins in metabolic-associated fatty liver disease: progress and perspectives. Front Immunol. 2025;16:1544012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 160. | Wang Y, Wang C, Yang F, Chen Y, Shi Y, Xu R, Zhang Z, Yan Y. USP9X-enriched MSC-sEV inhibits LSEC angiogenesis in MASH mice by downregulating the IκBα/NF-κB/Ang-2 pathway. Pharmacol Res. 2024;209:107471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 161. | Chen Y, Yang F, Wang Y, Shi Y, Liu L, Luo W, Zhou J, Yan Y. Mesenchymal stem cell-derived small extracellular vesicles reduced hepatic lipid accumulation in MASLD by suppressing mitochondrial fission. Stem Cell Res Ther. 2025;16:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 162. | Kasahara N, Teratani T, Doi J, Yokota S, Shimodaira K, Kaneko Y, Ohzawa H, Sakuma Y, Sasanuma H, Fujimoto Y, Urahashi T, Yoshitomi H, Yamaguchi H, Kitayama J, Sata N. Controlled release of hydrogel-encapsulated mesenchymal stem cells-conditioned medium promotes functional liver regeneration after hepatectomy in metabolic dysfunction-associated steatotic liver disease. Stem Cell Res Ther. 2024;15:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 163. | Chiabotto G, Semnani A, Ceccotti E, Bruno S. Extracellular vesicles: emerging therapeutic agents for liver fibrosis. Extracell Vesicles Circ Nucl Acids. 2025;6:216-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 164. | Sharma S, Le Guillou D, Chen JY. Cellular stress in the pathogenesis of nonalcoholic steatohepatitis and liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:662-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 165. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (10)] |
| 166. | Ebrahimi H, Naderian M, Sohrabpour AA. New Concepts on Reversibility and Targeting of Liver Fibrosis; A Review Article. Middle East J Dig Dis. 2018;10:133-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 167. | Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 168. | Wilujeng IDA, Pramono AP, Tjang YS, Puspita R. Therapeutic evaluation of bone marrow mesenchymal stem cells for hepatitis virus infection: immunoregulation, anti-inflammatory effects, and clinical potential - a systematic review. Mol Biol Rep. 2025;52:819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 169. | Gunardi H, Alatas FS, Antarianto RD, Rahayatri TH. The Effect of Intrahepatic and Intrasplenic Administration of Mesenchymal Stem Cell to Liver Function and Degree of Liver Fibrosis in Common Bile Duct Ligation Model in Rabbit. J Pediatr Surg. 2024;59:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 170. | Ren H, Zhao Q, Cheng T, Lu S, Chen Z, Meng L, Zhu X, Yang S, Xing W, Xiao Y, Ren Q, Chi Y, Gu D, Yang R, Han ZC. No contribution of umbilical cord mesenchymal stromal cells to capillarization and venularization of hepatic sinusoids accompanied by hepatic differentiation in carbon tetrachloride-induced mouse liver fibrosis. Cytotherapy. 2010;12:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Sani F, Soufi Zomorrod M, Azarpira N, Soleimani M. The Effect of Mesenchymal Stem Cell-Derived Exosomes and miR17-5p Inhibitor on Multicellular Liver Fibrosis Microtissues. Stem Cells Int. 2023;2023:8836452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 172. | Zhang Y, Li Y, Li W, Cai J, Yue M, Jiang L, Xu R, Zhang L, Li J, Zhu C. Therapeutic Effect of Human Umbilical Cord Mesenchymal Stem Cells at Various Passages on Acute Liver Failure in Rats. Stem Cells Int. 2018;2018:7159465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 173. | Tian S, Zhou X, Zheng L, Liu J, Zhang M, Ma S, Zheng X, Guo G, Ju R, Yang F, Liu Y, Li B, Hu Y, Xia E, Su R, Sun K, Cui L, Guo C, Zhou X, Wang J, Shang Y, Han Y. Engineered Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Mitigate Liver Fibrosis by Delivering USP10 to Reprogram Macrophage Phenotype. Biomater Res. 2025;29:0244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 174. | Bozorgi V, Babaahmadi M, Salehi M, Vafaeimanesh J, Hajizadeh-Saffar E. From signaling pathways to clinical trials: mesenchymal stem cells as multimodal regenerative architects in liver cirrhosis therapy. Stem Cell Res Ther. 2025;16:421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 175. | Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA. 2023;329:1589-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 220] [Article Influence: 73.3] [Reference Citation Analysis (33)] |
| 176. | Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol. 2015;12:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 177. | Nguyen TL, Nguyen HP, Bui TH, Phan TKT, Ngo DM, Ha TTH, Tran TT, Luu NAT, Phan TK, Ho PD. Outcomes of allogeneic umbilical cord mesenchymal stem cell infusion for liver cirrhosis due to biliary atresia after Kasai operation. J Pediatr Surg. 2025;162624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 178. | Shi M, Li YY, Xu RN, Meng FP, Yu SJ, Fu JL, Hu JH, Li JX, Wang LF, Jin L, Wang FS. Mesenchymal stem cell therapy in decompensated liver cirrhosis: a long-term follow-up analysis of the randomized controlled clinical trial. Hepatol Int. 2021;15:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 179. | Huang WC, Li YC, Chen PX, Ma KS, Wang LT. Mesenchymal stem cell therapy as a game-changer in liver diseases: review of current clinical trials. Stem Cell Res Ther. 2025;16:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 180. | Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, Kim HS, Jang JY, Lee CH, Kim BS, Jang YO, Cho MY, Jung ES, Kim YM, Bae SH, Baik SK. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 181. | Shi Q, Xia Y, Wu M, Pan Y, Wu S, Lin J, Kong Y, Yu Z, Zan X, Liu P, Xia J. Mi-BMSCs alleviate inflammation and fibrosis in CCl(4)-and TAA-induced liver cirrhosis by inhibiting TGF-β/Smad signaling. Mater Today Bio. 2024;25:100958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 182. | Shi L, Zhang Z, Mei S, Wang Z, Xu Z, Yao W, Liu L, Yuan M, Pan Y, Zhu K, Liu K, Meng F, Sun J, Liu W, Xie X, Dong T, Huang L, Meng F, Fu JL, Li Y, Zhang C, Fan X, Shi M, Zhang Y, Li Y, Xie WF, Zhang P, Wang FS. Dose-escalation studies of mesenchymal stromal cell therapy for decompensated liver cirrhosis: phase Ia/Ib results and immune modulation insights. Signal Transduct Target Ther. 2025;10:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 183. | Li ZH, Wang JY, Li XL, Meng SB, Zheng HY, Wang JL, Lei ZY, Lin BL, Zhang J. Mesenchymal stem cell-regulated miRNA-mRNA landscape in acute-on-chronic liver failure. Genomics. 2023;115:110737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 184. | Cuadra B, Silva V, Huang YL, Diaz Y, Rivas C, Molina C, Simon V, Bono MR, Morales B, Rosemblatt M, Silva S, Acuña R, Ezquer F, Ezquer M. The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model. Int J Mol Sci. 2024;25:2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 185. | Sitbon A, Delmotte PR, Goumard C, Turco C, Gautheron J, Conti F, Aoudjehane L, Scatton O, Monsel A. Therapeutic potentials of mesenchymal stromal cells-derived extracellular vesicles in liver failure and marginal liver graft rehabilitation: a scoping review. Minerva Anestesiol. 2023;89:690-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 186. | Heo SK, Yu HM, Kim DK, Seo HJ, Shin Y, Kim SA, Kim M, Kim Y, Lee YJ, Noh EK, Jo JC. LIGHT (TNFSF14) promotes the differentiation of human bone marrow-derived mesenchymal stem cells into functional hepatocyte-like cells. PLoS One. 2023;18:e0289798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 187. | Chen L, Zhang N, Huang Y, Zhang Q, Fang Y, Fu J, Yuan Y, Chen L, Chen X, Xu Z, Li Y, Izawa H, Xiang C. Multiple Dimensions of using Mesenchymal Stem Cells for Treating Liver Diseases: From Bench to Beside. Stem Cell Rev Rep. 2023;19:2192-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 188. | Huang YL, De Gregorio C, Silva V, Elorza ÁA, Léniz P, Aliaga-Tobar V, Maracaja-Coutinho V, Budini M, Ezquer F, Ezquer M. Administration of Secretome Derived from Human Mesenchymal Stem Cells Induces Hepatoprotective Effects in Models of Idiosyncratic Drug-Induced Liver Injury Caused by Amiodarone or Tamoxifen. Cells. 2023;12:636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 189. | Lu W, Yan L, Peng L, Wang X, Tang X, Du J, Lin J, Zou Z, Li L, Ye J, Zhou L. Efficacy and safety of mesenchymal stem cell therapy in acute on chronic liver failure: a systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res Ther. 2025;16:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 190. | Liu Y, Dong Y, Wu X, Xu X, Niu J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: a systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res Ther. 2022;13:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 191. | Lin D, Chen H, Xiong J, Zhang J, Hu Z, Gao J, Gao B, Zhang S, Chen J, Cao H, Li Z, Lin B, Gao Z. Mesenchymal stem cells exosomal let-7a-5p improve autophagic flux and alleviate liver injury in acute-on-chronic liver failure by promoting nuclear expression of TFEB. Cell Death Dis. 2022;13:865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 192. | Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 497] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 193. | Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008;233:901-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 194. | Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, Yang SG, Li LN, Luo WF, Li JP, Chen DD, Du WJ, Cao XC, Zhuo GS, Wang T, Han ZC. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013;8:e59354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 195. | Du W, Li X, Chi Y, Ma F, Li Z, Yang S, Song B, Cui J, Ma T, Li J, Tian J, Yang Z, Feng X, Chen F, Lu S, Liang L, Han ZB, Han ZC. VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity. Stem Cell Res Ther. 2016;7:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 196. | Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song B, Li C, Han Z, Zhang L, Han Z. High-efficient generation of VCAM-1(+) mesenchymal stem cells with multidimensional superiorities in signatures and efficacy on aplastic anaemia mice. Cell Prolif. 2020;53:e12862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 197. | Zhang L, Zhuo Y, Yu H. Spatio-temporal metabolokinetics and therapeutic effect of CD106(+) mesenchymal stem/stromal cells upon mice with acute lung injury. Cell Biol Int. 2023;47:720-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 198. | Zhang X, Sang X, Chen Y, Yu H, Sun Y, Liang X, Zheng X, Wang X, Yang H, Bi J, Zhang L, Wang P. VCAM-1(+) hUC-MSCs Exert Considerable Neuroprotection Against Cerebral Infarction in Rats by Suppression of NLRP3-Induced Pyroptosis. Neurochem Res. 2023;48:3084-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 199. | Liu H, Cui D, Huangfu S, Wang X, Yu X, Yang H, Zheng X, Li Y, Bi J, Zhang L, Wang P. VCAM-1(+) Mesenchymal Stem/Stromal Cells Reveal Preferable Efficacy Upon an Experimental Autoimmune Encephalomyelitis Mouse Model of Multiple Sclerosis Over the VCAM-1(-) Counterpart. Neurochem Res. 2024;50:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 200. | Panchuk I, Kovalskaia V, Balinova N, Ryzhkova O, Smirnikhina S. The Optimization of a Protocol for the Directed Differentiation of Induced Pluripotent Stem Cells into Liver Progenitor Cells and the Delivery of Transgenes. Biology (Basel). 2025;14:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 201. | Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71:970-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (12)] |
| 202. | Yao S, Cen Y, Lou G, Liu Y. Therapeutic potentials of mesenchymal stem cells and their extracellular vesicles on liver diseases by modulating mitochondrial function of macrophages. Int Immunopharmacol. 2025;165:115486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 203. | Le Guillou D, Siao K, Her CL, Duwaerts CC, Maher JJ. iPSC-Derived Hepatocytes from Patients with MASLD Exhibit Early Mitochondrial Dysfunction. bioRxiv. 2025;2025.08.27.672733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 204. | Neves SP, Bomfim LM, Bezerra DP. Hepatocellular carcinoma stem cells: the current state of small molecule-based inhibitors. Cell Death Dis. 2025;16:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 205. | Lekkala VKR, Shrestha S, Al Qaryoute A, Dhinoja S, Acharya P, Raheem A, Jagadeeswaran P, Lee MY. Enhanced Maturity and Functionality of Vascular Human Liver Organoids through 3D Bioprinting and Pillar Plate Culture. ACS Biomater Sci Eng. 2025;11:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 206. | Kim HJ, Kim G, Chi KY, Kim H, Jang YJ, Jo S, Lee J, Lee Y, Woo DH, Han C, Kim SK, Park HJ, Kim JH. Generation of multilineage liver organoids with luminal vasculature and bile ducts from human pluripotent stem cells via modulation of Notch signaling. Stem Cell Res Ther. 2023;14:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 207. | Fitzsimmons RL, Hudson JE. Endothelial specialization and angiocrine factors are critical for vascularized organoid models. Cell Rep. 2025;44:116410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 208. | Sun C, Wu G, Wu D, Wu L, Lu Q, Hu W, Du Q, Wang J, Xie A, Xia M, Hu H, Hu B, Huang J, Wang S. Determining the optimal transplantation window in hepatic organoids via real-time biosensing of vascularization and metabolic maturation utilizing the integrated organoid-on-a-chip platform. Biosens Bioelectron. 2025;292:118057. [PubMed] [DOI] [Full Text] |
| 209. | Yang X, Nie YZ, Lu C, Li Y, Hayashi Y, Plummer R, Luo N, Li Q, Kasai T, Okumura T, Isobe Y, Yamaguchi K, Furukawa Y, Li Y, Taniguchi H. Human pluripotent stem cell-derived fetal hepatic stellate cells promote vascularization and maturation in liver organoids. Dev Cell. 2025;S1534-5807(25)00540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 210. | Chen H, Liang Y, Sun X, Xiong W, Yang T, Liang Y, Ye X, Li X, Wang W, Gu J, Zhang J, Chen L, Chan HF, Chen J. Generation of vascularized retinal organoids containing microglia based on a PDMS microwell platform. Sci Adv. 2025;11:eady6410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 211. | Liu J, Feng Y, Qu P, Luo Y, Shi J, Ma C, Liang Q, Zhao L, Li G, Yang B, Cheng P. Application of biomaterials in vascularization of cardiac organoids. iScience. 2025;28:113531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 212. | Kim H, Kim SK, Oelgeschläger M, Park HJ. Prediction of Acute Hepatotoxicity With Human Pluripotent Stem Cell-Derived Hepatic Organoids. Curr Protoc. 2024;4:e1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 213. | He L, Pu W, Liu X, Zhang Z, Han M, Li Y, Huang X, Han X, Li Y, Liu K, Shi M, Lai L, Sun R, Wang QD, Ji Y, Tchorz JS, Zhou B. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science. 2021;371:eabc4346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 214. | Fan H, Shang J, Li J, Yang B, Zhou D, Jiang S, Fan Y, Zhou Y, Wang Y, Liu P, Li C, Chen Z, Chen P. High-Throughput Formation of Pre-Vascularized hiPSC-Derived Hepatobiliary Organoids on a Chip via Nonparenchymal Cell Grafting. Adv Sci (Weinh). 2025;12:e2407945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 215. | Reshke R, Taylor JA, Savard A, Guo H, Rhym LH, Kowalski PS, Trung MT, Campbell C, Little W, Anderson DG, Gibbings D. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat Biomed Eng. 2020;4:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 216. | Zhang B, Zhang B, Lai RC, Sim WK, Lam KP, Lim SK. MSC-sEV Treatment Polarizes Pro-Fibrotic M2 Macrophages without Exacerbating Liver Fibrosis in NASH. Int J Mol Sci. 2023;24:8092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 217. | Wang J, Liu C, Wang P, Liu Z, Hu W, Lv Z, Huang C, Yao X. Bioengineered Tumor-Derived Extracellular Vehicles Suppressed Colorectal Cancer Liver Metastasis and Bevacizumab Resistance. Adv Sci (Weinh). 2025;12:e2417714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 218. | Xie JH, Li YY, Jin J. The essential functions of mitochondrial dynamics in immune cells. Cell Mol Immunol. 2020;17:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 219. | Huang H, Peng W, Zhou Q, Zhao Y, Liu L, Cui H, Liang J, Cao M, Chen W, Wang R, Chen S, Xiong S, Cheng B, Bai S. Senescent fibroblasts secrete CTHRC1 to promote cancer stemness in hepatocellular carcinoma. Cell Commun Signal. 2025;23:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 220. | Luo S, Wu F, Yang X. Single-cell transcriptome atlas reveals mitophagy dynamics in acute chemical injury model and the role of MSCs transplantation. Stem Cell Res Ther. 2025;16:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 221. | You XY, Li XY, Wang H, Zhao GP. Development of NAFLD-Specific Human Liver Organoid Models on a Microengineered Array Chip for Semaglutide Efficacy Evaluation. Cell Prolif. 2025;e70118. [PubMed] [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/