Published online Dec 7, 2025. doi: 10.3748/wjg.v31.i45.113115
Revised: October 2, 2025
Accepted: November 3, 2025
Published online: December 7, 2025
Processing time: 110 Days and 15.4 Hours
The retrospective study by Hyatt et al reported that metabolic dysfunction-associated steatotic liver disease (MASLD) with fibrosis was associated with poorer sleep, which in turn correlated with worse life quality in patients with human immunodeficiency virus. While their findings were significant, some limi
Core Tip: The recent study by Hyatt et al investigated the prevalence and associations between metabolic dysfunction-associated steatotic liver disease (MASLD), poor sleep, and life quality in patients with human immunodeficiency virus. To further explore the potential causal relationship between specific sleep traits and MASLD, we conducted a Mendelian randomization analysis, which demonstrated that sleep apnea significantly increases the risk of MASLD (P = 0.014), whereas no causal associations were found for other sleep traits (P > 0.05). These findings complement and extend those of Hyatt et al, providing additional insight into the associations between MASLD and sleep.
- Citation: Yao QH, Yang WY. Revisiting the association between metabolic dysfunction-associated steatotic liver disease and sleep: Moving from correlation to causation. World J Gastroenterol 2025; 31(45): 113115
- URL: https://www.wjgnet.com/1007-9327/full/v31/i45/113115.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i45.113115
We read with great interest the article by Hyatt et al[1], which investigated the prevalence and associations of metabolic dysfunction-associated steatotic liver disease (MASLD), poor sleep, and health-related quality of life among individuals with human immunodeficiency virus[1]. The authors reported that MASLD with fibrosis was associated with poorer sleep quality, and poor sleep was associated with worse health-related quality of life. The relationship between sleep and fatty liver disease has attracted increasing attention. A study utilizing data from the National Health and Nutrition Examination Survey examined the association between sleep traits and MASLD in 4731 older adults. The findings indicated that both short and long sleep durations, as well as the presence of sleep disorders, were significantly associated with MASLD[2]. Another Iranian cohort study of 9151 participants, including 1320 with MASLD, demonstrated that those with MASLD had shorter sleep durations, delayed sleep-wake cycles, reduced sleep efficiency, and more frequent daytime napping[3].
While the findings of Hyatt et al[1] are clinically significant, some limitations warrant further discussion. First, the authors acknowledged that the cross-sectional design of their study precludes the establishment of causal relationships. Second, no subgroup analyses stratified by specific sleep characteristics or disorders were performed, making it unclear which types of sleep disturbances may confer a higher risk of MASLD. Third, baseline characteristics differed signi
MR utilizes single-nucleotide polymorphisms (SNPs) as instrumental variables to infer causality between exposures and outcomes[5]. Due to the random allocation of SNPs at conception, MR analyses are less susceptible to confounding and reverse causation[6]. Genome-wide association study (GWAS) data were sourced from the FinnGen[7] and IEU OpenGWAS databases[8] (Supplementary Table 1). The FinnGen project, involving nearly 500000 Finnish participants, is a genomic initiative providing a valuable resource for investigating genetic variations and their clinical implications[9]. The IEU OpenGWAS database is also a publicly accessible database that integrates large-scale GWAS data[8]. SNPs meeting standard MR assumptions were selected as instrumental variables (Supplementary Table 2), while those associated with confounders or outcomes were excluded using the GWAS catalog[10]. The inverse variance weighted method was employed as the primary analytical approach, supplemented by MR-Egger, weighted median, and weighted mode methods. Heterogeneity was assessed using Cochran’s Q test. Horizontal pleiotropy was evaluated using the MR-Egger intercept test and the MR Pleiotropy RESidual Sum and Outlier test global test.
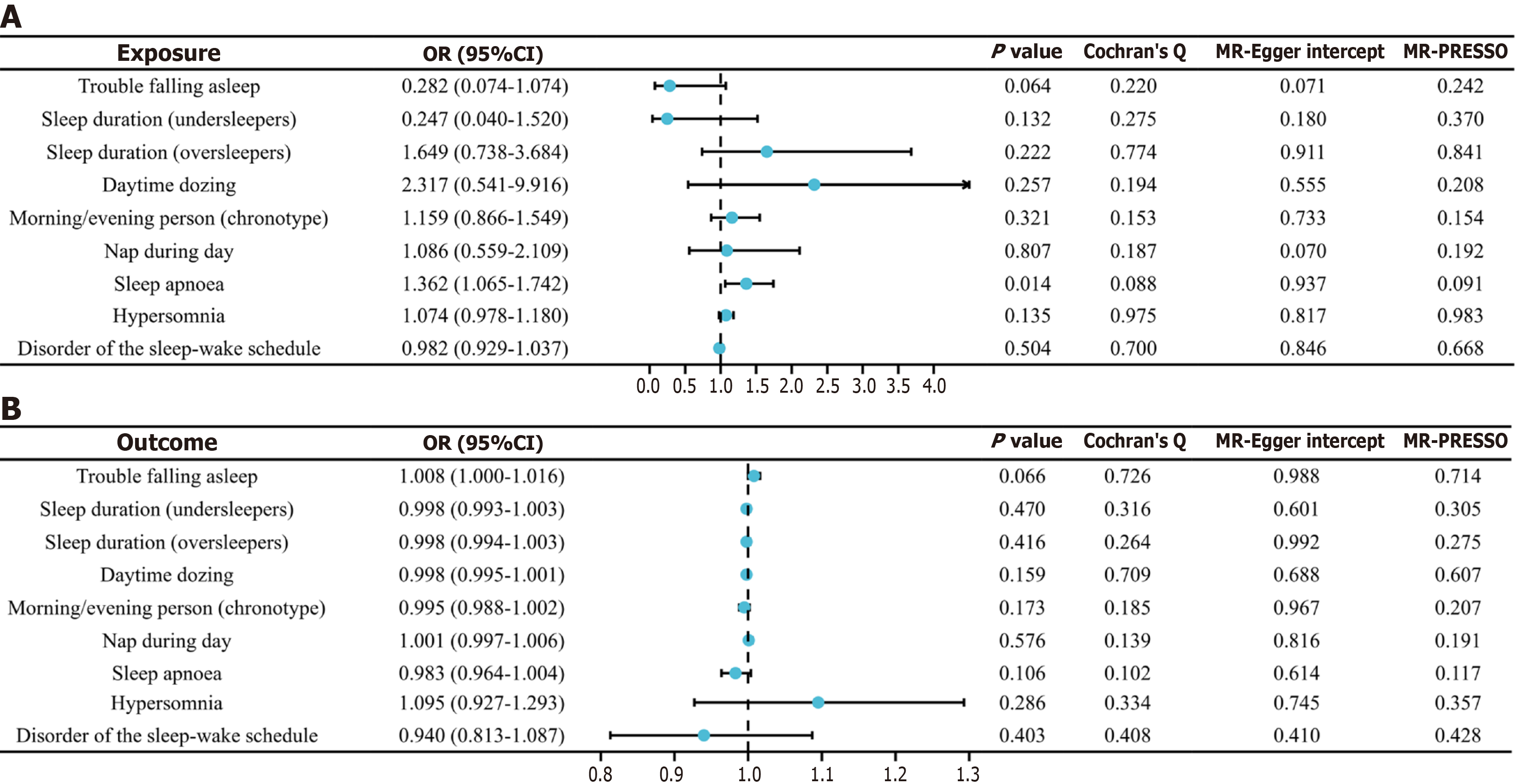
As shown in Figure 1A, MR analysis revealed that sleep apnea significantly increases the risk of MASLD [odds ratio (OR) = 1.362; 95% confidence interval (CI): 1.065-1.742; P = 0.014]. In contrast, no causal associations were observed for other sleep traits, including trouble falling asleep, sleep duration, daytime dozing, morning/evening person, nap during day, hypersomnia, and disorder of the sleep–wake schedule. No significant heterogeneity or horizontal pleiotropy was detected (P > 0.05). Additionally, reverse MR analysis did not reveal any causal relationship from MASLD to sleep traits (Figure 1B). These results provide valuable complementary evidence suggesting that the management of sleep apnea may reduce MASLD risk. The results are consistent with previous studies. A systematic review by Umbro et al[11] revealed that the prevalence of MASLD increased among individuals with sleep apnea. A cross-sectional study by Huang et al[12] also demonstrated that the prevalence of MAFLD increased with the severity of sleep apnea. The latter study further identified body mass index, oxygen desaturation index, and triglyceride (TG) levels as potential contributing factors to MAFLD in sleep apnea patients.
Chronic intermittent hypoxia (IH), a hallmark of sleep apnea, is considered a critical driver of MASLD progression through several molecular pathways[13]. These include the activation of hypoxia-inducible factors, nuclear factor kappa-B signaling, and the upregulation of proinflammatory cytokines, contributing to hepatic inflammation, fibrogenesis, and metabolic dysfunction. Studies demonstrate that IH impairs glucose homeostasis[14]. Glucose-sensing neurons mediate IH-induced insulin resistance by enhancing sympathetic activity, elevating circulating free fatty acids, and stimulating hepatic glycogenolysis. IH also promotes hyperlipidemia by inhibiting the clearance of TG-rich lipoproteins[15]. Furthermore, IH suppresses lipoprotein lipase activity, leading to increased cholesterol and TG levels[16]. Overall, sleep apnea contributes to MASLD progression through IH-induced insulin resistance, which diverts excess free fatty acids into TG and cholesterol synthesis, thereby promoting hyperlipidemia and disease progression.
In addition, IH contributes to MASLD progression via inflammation. Savransky et al[17] found elevated interleukin (IL)-1 beta, IL-6, and tumor necrosis factor alpha levels in mice exposed to IH, accompanied by hepatic lobular inflammation and fibrosis. Schaefer et al[18] found that IH markedly induced IL-6 expression in hepatocytes and macrophages. IH activates inflammasomes and caspase-1 in lipid-laden hepatocytes, while promoting hepatocyte-Kupffer cell crosstalk via extracellular vesicles, thereby amplifying hepatocellular injury. In a biopsy-confirmed study involving 80 children with MASLD, sleep apnea was associated with increased intestinal permeability, endotoxemia, expansion of adiponectin-deficient progenitor cell populations, and upregulation of Toll-like receptor 4 in hepatocytes, Kupffer cells, and hepatic stellate cells, which were key features of steatohepatitis and fibrosis[19].
The identification of biomarkers linking MASLD and sleep disorders is critical for early detection and disease monitoring. In a retrospective study by Li et al[20], the prevalence of MASLD in patients with sleep apnea increased with higher triglyceride-glucose (TyG) index values (P < 0.05), while metabolic score for insulin resistance (METS-IR) exhibited a non-linear association with MASLD risk (P < 0.05). A dose-response relationship was evident between MASLD risk and METS-IR, and a linear association was found for the TyG index. Notably, METS-IR (under the receiver operating characteristic curve = 0.778; 95%CI: 0.716-0.836) outperformed the TyG index (under the receiver operating characteristic curve = 0.775; 95%CI: 0.711-0.828) in predicting MASLD in sleep apnea patients.
Inflammation-related biomarkers have also drawn attention. Yang et al[21] investigated the independent relationships between trouble sleeping and the systemic immune-inflammation (SII) index in patients with MASLD. Their results indicated that both SII (OR: 1.21; 95%CI: 1.08-1.35) and trouble sleeping (OR: 1.24; 95%CI: 1.05-1.47) were independently associated with MASLD. An additive interaction between SII and sleep trouble was observed, with SII partially mediating this relationship. For patients with MASLD and coexisting sleep apnea, interventions to improve sleep-disordered breathing may offer benefits. Effective strategies include positive airway pressure[22], pharmacological treatments[23], physical activity[24], and bariatric surgery[25]. Zhang et al[25] found that bariatric surgery significantly improved sleep apnea, nocturnal hypoxia, hepatic steatosis, and fibrosis (P < 0.05). Clinical decision-making should balance risks and benefits, incorporating patient preferences and the availability of healthcare resources. In conclusion, our MR results indicated that sleep apnea causally increases the risk of MASLD (P = 0.014). We recommend that screening for sleep apnea be included in MASLD risk assessment protocols. We sincerely appreciate the contributions of Hyatt et al[1]. Building on their pronounced contributions, the MR analysis is expected to provide novel insight into the association between MASLD and sleep.
| 1. | Hyatt AN, Kuchana SK, Vilar-Gomez E, Sterling RK, Naggie S, Heath SL, Price JC, Wilson LA, Crandall H, Gawrieh S, Chalasani N, Loomba R, Sulkowski MS, Desai AP, Lake JE. Poor sleep and hepatic steatosis contribute to poorer quality of life in people with human immunodeficiency virus. World J Gastroenterol. 2025;31:109202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zhang F, Xue Y, Li W. Exploring the impact of sleep duration and sleep disorders on metabolic dysfunction-associated steatotic liver disease in older adults. BMC Geriatr. 2025;25:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Zarean E, Looha MA, Amini P, Ahmadi A, Dugué PA. Sleep characteristics of middle-aged adults with non-alcoholic fatty liver disease: findings from the Shahrekord PERSIAN cohort study. BMC Public Health. 2023;23:312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | GebreEyesus FA, Degu FS, Yohanes YB, Azagew AW. Sleep quality and associated factors among adult people living with HIV on follow-up at Dessie Town Governmental Health Facilities Antiretroviral Therapy Clinics, Northeast, Ethiopia, 2020, a multicenter cross-sectional study. BMC Psychiatry. 2023;23:132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Tang B, Lin N, Liang J, Yi G, Zhang L, Peng W, Xue C, Jiang H, Li M. Leveraging pleiotropic clustering to address high proportion correlated horizontal pleiotropy in Mendelian randomization studies. Nat Commun. 2025;16:2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J. 2023;44:4913-4924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 500] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 7. | Räsänen J, Heikkinen S, Mäklin K, Lipponen A, Kuulasmaa T, Mehtonen J, Korhonen VE, Junkkari A, Grenier-Boley B, Bellenguez C, Oinas M, Avellan C, Frantzén J, Kotkansalo A, Rinne J, Ronkainen A, Kauppinen M, von Und Zu Fraunberg M, Lönnrot K, Satopää J, Perola M, Koivisto AM, Julkunen V, Portaankorva AM, Mannermaa A, Soininen H, Helisalmi S, Jääskeläinen JE, Lambert JC, Eide PK; for FinnGen, Palotie A, Kurki MI, Hiltunen M, Leinonen V. Risk Variants Associated With Normal Pressure Hydrocephalus: Genome-Wide Association Study in the FinnGen Cohort. Neurology. 2024;103:e209694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Wang X, Wang C, Liu K, Wan Q, Wu W, Liu C. Association between sleep-related phenotypes and gut microbiota: a two-sample bidirectional Mendelian randomization study. Front Microbiol. 2024;15:1341643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 9. | Li Z, Ren Y, Jiang F, Zhang K, Meng X, Zheng Y, He M. Unveiling biomarkers via plasma metabolome profiling for diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. 2025;24:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Cao T, Li A, Huang Y. pandasGWAS: a Python package for easy retrieval of GWAS catalog data. BMC Genomics. 2023;24:238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J Gastroenterol. 2020;26:2669-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 12. | Huang J, Chen L, Li X, Chen M, Lin T, Chen G. Association Between Metabolic-Associated Fatty Liver Disease and Obstructive Sleep Apnea: A Cross-Sectional Study. Nat Sci Sleep. 2023;15:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 13. | Bu LF, Xiong CY, Zhong JY, Xiong Y, Li DM, Hong FF, Yang SL. Non-alcoholic fatty liver disease and sleep disorders. World J Hepatol. 2024;16:304-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 14. | Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol. 2008;586:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Shimazu T, Minokoshi Y. Systemic Glucoregulation by Glucose-Sensing Neurons in the Ventromedial Hypothalamic Nucleus (VMH). J Endocr Soc. 2017;1:449-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O'Byrne SM, Kroupa O, Olivecrona G, Blaner WS, Polotsky VY. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871-G877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Schaefer E, Wu W, Mark C, Yang A, DiGiacomo E, Carlton-Smith C, Salloum S, Brisac C, Lin W, Corey KE, Chung RT. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol Commun. 2017;1:326-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Nobili V, Alisi A, Cutrera R, Carpino G, De Stefanis C, D'Oria V, De Vito R, Cucchiara S, Gaudio E, Musso G. Altered gut-liver axis and hepatic adiponectin expression in OSAS: novel mediators of liver injury in paediatric non-alcoholic fatty liver. Thorax. 2015;70:769-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Li M, Wan Y, Wei Z, Lin W. Comparison of the predictive value of TyG index and METS-IR for OSA combined with NAFLD: a retrospective observational study based on a physical examination population. BMC Gastroenterol. 2025;25:279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Yang X, Zhuo S, Zhuang H, Fang T. Interaction between the systemic immune-inflammation index and trouble sleeping in nonalcoholic fatty liver disease: a cross-sectional study of the NHANES 2005-2018 data. J Health Popul Nutr. 2024;43:175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Hirono H, Watanabe K, Hasegawa K, Kohno M, Terai S, Ohkoshi S. Impact of continuous positive airway pressure therapy for nonalcoholic fatty liver disease in patients with obstructive sleep apnea. World J Clin Cases. 2021;9:5112-5125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Kim BY, Kang SM, Kang JH, Kim KK, Kim B, Kim SJ, Kim YH, Kim JH, Kim JH, Nam GE, Park JY, Son JW, Shin HJ, Oh TJ, Lee H, Jeon EJ, Chung S, Hong YH, Kim CH; Committee of Clinical Practice Guidelines, Korean Society for the Study of Obesity (KSSO). Current Long-Term Pharmacotherapies for the Management of Obesity. J Obes Metab Syndr. 2020;29:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | da Silva BRD, Nunes PIG, Santos FA, de Bruin PFC, de Bruin VMS. Exercise modifies lipid and glucose metabolism alterations induced by sleep deprivation in mice. Sleep Sci. 2022;15:347-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Zhang YX, Yang L, Yang CC, Wang WY, Shen JH, Shi ML, Yu Y, Dai QC, Gu Y, Yang JJ, Yu WW, Yao K, Hu M, Ni J, Sun JL, Zhang L, Sun HX, Lu XF, Wang B. Correlation between Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Disease before and after Metabolic Bariatric Surgery. Obes Surg. 2020;30:3803-3812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/