Published online Nov 21, 2025. doi: 10.3748/wjg.v31.i43.112483
Revised: September 2, 2025
Accepted: October 13, 2025
Published online: November 21, 2025
Processing time: 115 Days and 1.1 Hours
Distal small bowel resection with preservation of the terminal ileum (DBRPI) sig
To explore the underlying mechanisms of DBRPI in improving glucose meta
Following 8 weeks of a high-fat diet, the rats were randomly divided into the DBRPI group and the sham operation group. After surgery, body weight and glu
DBRPI reduced body weight and improved glucose tolerance. At 6 weeks post-surgery, the abundance of Prevotellaceae_NK3B31_group and the level of 7-keto
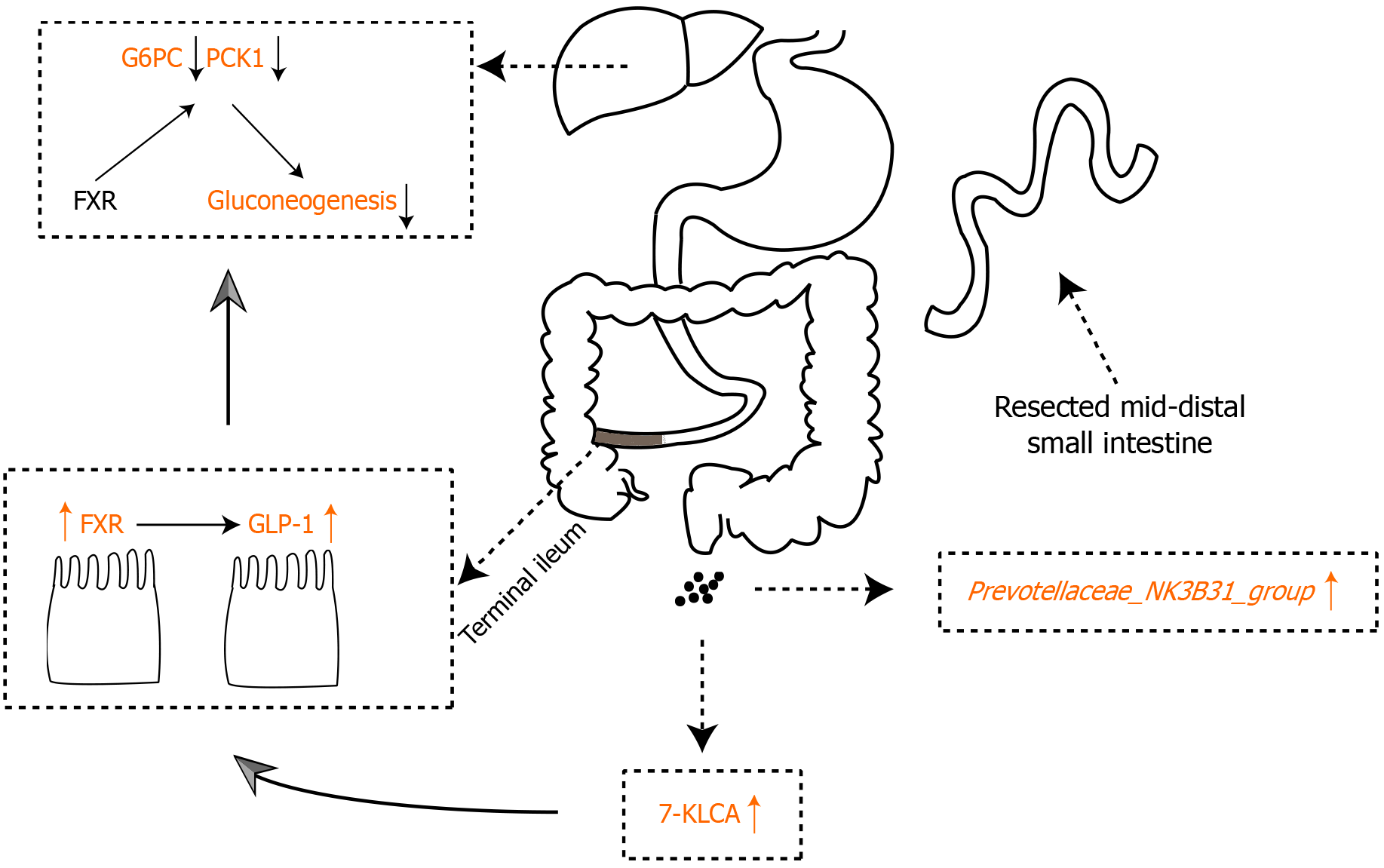
DBRPI inhibits hepatic gluconeogenesis and improves glucose metabolism. The mechanism may be related to activation of the 7-KLCA-FXR signaling pathway mediated by the Prevotellaceae_NK3B31_group.
Core Tip: Distal small bowel resection with preservation of the terminal ileum (DBRPI) improves glucose tolerance and reduces weight in high-fat diet-fed rats. This effect is linked to altered gut microbiota and bile acids: Increased Prevotellaceae_NK3B31_group and 7-ketolithocholic acid (7-KLCA), alongside decreased Desulfovibrio fairfieldensis and α-muricholic acid. DBRPI upregulates ileal farnesoid X receptor (FXR) and glucagon-like peptide-1 (GLP-1) expression, and downregulates hepatic gluconeogenic genes. Critically, Prevotellaceae_NK3B31_group correlates positively with 7-KLCA and FXR, and negatively with glucose intolerance and gluconeogenesis. Thus, DBRPI likely improves glucose metabolism by activating the Prevotellaceae-mediated 7-KLCA-FXR pathway, enhancing GLP-1, and suppressing hepatic glucose production.
- Citation: Xu CY, Zheng ZH, Yang K, Wu RR, Cao JQ, Duan JY. Distal small bowel resection with preservation of the terminal ileum suppresses hepatic gluconeogenesis via the Prevotellaceae_NK3B31_group-mediated 7-KLCA-FXR axis. World J Gastroenterol 2025; 31(43): 112483
- URL: https://www.wjgnet.com/1007-9327/full/v31/i43/112483.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i43.112483
Obesity is a major global health concern, contributing to chronic diseases such as cardiovascular disorders and metabolic dysregulation[1]. Metabolic surgery (MS) demonstrates superior glycemic control compared to pharmacotherapy[2,3], with bile acid signaling playing a pivotal role in its metabolic benefits[4,5]. Bile acids not only act as fat emulsifiers but also as signaling molecules, modulating metabolism via receptors[6], such as the farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5). FXR activation enhances lipid metabolism and suppresses inflammation, while TGR5 stimulates energy expenditure and insulin sensitivity[7-9]. Notably, FXR plays a central role in regulating bile acid synthesis through a negative feedback mechanism. Upon activation by bile acids in the ileum, FXR induces the expression of fibroblast growth factor (FGF) 19 (FGF15 in rodents), which then acts on the liver to suppress the expression of cytochrome P450 7A1 (CYP7A1), the rate-limiting enzyme in the classical bile acid synthesis pathway[10].
Moreover, MS alters the composition of the gut microbiota, thereby influencing bile acid metabolism and glucose homeostasis[5]. Therefore, the activation status of bile acids and their receptors is considered one of the key factors affecting postoperative glucose metabolism, with the gut microbiota potentially playing an important mediating role in this process[11,12]. For example, microbial modification can convert the FXR agonist chenodeoxycholic acid (CDCA) into the antagonist α-muricholic acid (α-MCA)[13,14]. Notably, although 7-ketolithocholic acid (7-KLCA)—a secondary bile acid produced by the oxidative metabolism of lithocholic acid (LCA) via gut microbiota—has been associated with lipid-lowering effects[15], its changes after MS and its impact on glucose metabolism remain unclear. Similarly, the Prevotel
To investigate the role of bile acid metabolism in MS, we developed a surgical model termed distal bowel resection with preservation of the terminal ileum (DBRPI), which involves resection of 60% of the mid-distal small intestine while retaining the terminal 15 cm of ileum[16]. Previous work from our group demonstrated that DBRPI improves glucose metabolism, potentially via the hindgut mechanism—whereby accelerated nutrient delivery to the distal small intestine following MS activates L-cells, stimulating the secretion of GLP-1 and peptide YY, thereby contributing to improved glycemic control[17]. However, the specific roles of gut microbiota and individual bile acid species in this process remain to be elucidated. In this study, we employed DBRPI as a mechanistic model to explore the gut microbiota-bile acid-FXR axis in glucose regulation. We hypothesize that DBRPI enhances glucose metabolism by activating the Prevotel
This study used six-week-old male Sprague-Dawley rats purchased from Beijing Huafukang Biotechnology Co., Ltd (Beijing, China). After one week of acclimation with free access to water and standard chow, the rats were fed a high-fat diet (HFD) for 8 weeks. They were then randomly divided into two groups: DBRPI (n = 6) and sham-operated controls (n = 6). All surgeries were performed in a single batch on the same day to ensure procedural consistency. Postoperatively, all rats were maintained on a HFD until the end of the experiment.
Body weight and glucose tolerance were assessed preoperatively and at 2, 4, and 6 weeks postoperatively. At 6 weeks, portal serum, ileum and liver tissues, and fecal samples were collected for analysis.
Animal experimental protocols followed the ARRIVE guidelines and were approved by the Animal Ethics Committee of the Affiliated Hospital of Nanchang University.
Following overnight fasting, rats were anesthetized with 3% isoflurane and maintained with 1% isoflurane. After abdominal hair removal and povidone-iodine sterilization, a midline laparotomy was performed under aseptic con
In the DBRPI group, a 25-cm jejunal segment (distal to the Treitz ligament) and a 15-cm ileal segment (proximal to the ileocecal junction) were resected, followed by end-to-end anastomosis using 6-0 nylon sutures[16]. Sham-operated rats underwent identical laparotomy and intestinal exposure without resection.
Body weights were recorded after 14-hour fasting weekly. For fasting blood glucose (FBG) and oral glucose tolerance test (OGTT), rats underwent a 14-hour fast followed by tail vein blood sampling. Baseline glucose levels were measured using a glucometer. A 20% glucose solution (2 g/kg body weight) was administered orally, and blood glucose was monitored at 15, 30, 60, 90, and 120 minutes post-administration. Glucose-time curves were analyzed to calculate the area under the curve (AUC-OGTT).
Portal venous blood was collected immediately post-euthanasia and centrifuged (3000 rpm, 15 minutes, 4 °C), and the serum was stored at -80 °C. Serum levels of aminotransferase (ALT), aspartate aminotransferase (AST), total bile acid (TBA), glycosylated serum protein (GSP), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL), and postprandial blood glucose (PBG) were quantified using an automated biochemical analyzer.
Standard stock solutions (1 mg/mL) were prepared by dissolving reference compounds in water, then serially diluted to generate calibration curves. Fecal bile acids were extracted with methanol via ice-bath ultrasonication (10 minutes) followed by centrifugation (12000 rpm, 10 minutes, 4 °C). Supernatants were filtered (0.22 μm) into amber vials for analysis.
Quantification utilized UPLC-ESI-MS/MS with a bile acid-optimized column and gradient elution (0.4 mL/minutes) using aqueous-organic mobile phases. Metabolite concentrations were calculated from standard curve-derived regression equations using characteristic parent-daughter ion transitions.
Fecal DNA was extracted using the MagPure Soil DNA LQ Kit (Magan, China), quantified via NanoDrop 2000 (Thermo Fisher Scientific, United States), and assessed for integrity by agarose gel electrophoresis. The V3-V4 region of bacterial 16S rRNA was amplified with universal primers using ExTaq high-fidelity polymerase (Takara), followed by dual AMPure XP bead purification. Libraries were quantified (Qubit), normalized, and sequenced on the Illumina NovaSeq 6000 platform (250 bp paired-end).
Raw reads were primer-trimmed with Cutadapt. QIIME2 (2020.11) processed data using DADA2 for quality filtering, denoising, chimera removal, and amplicon sequence variant generation. Taxonomic annotation was performed against the SILVA v138 database.
Total RNA was isolated from liver and terminal ileum tissues using RNA extraction solution (Servicebio, China), quantified (NanoDrop 2000, Thermo Fisher), and reverse-transcribed into cDNA with SweScript RT SuperMix (Servicebio) under the following conditions: 25 °C/5 minutes, 42 °C/30 minutes, 85 °C/5 seconds. qPCR reactions containing SYBR Green Master Mix (Servicebio), gene-specific primers, and cDNA were run on a Bio-Rad CFX system with cycling parameters: 95 °C/30 seconds (initial denaturation); 40 cycles of 95 °C/15 seconds and 60 °C/30 seconds. Melt curve analysis was performed from 60 °C to 95 °C (0.5 °C increments) to confirm amplification specificity.
Statistical analyses were conducted using GraphPad Prism 9.5 and R 4.4.0. Intergroup differences were assessed via Student’s t-test or two-way ANOVA with Bonferroni correction for longitudinal data. β-diversity was evaluated using unweighted UniFrac-based principal coordinate analysis. Taxonomic and functional differences were identified by the ANOVA, Kruskal-Wallis tests, and Linear Discriminant Analysis Effect Size. Spearman correlation analysis was conducted to explore relationships, and false discovery rate correction was applied to adjust for multiple comparisons.
Compared with the sham-operated group, DBRPI induced sustained body weight reduction (P < 0.001), with statistically significant divergence from week 3 onward (Figure 1A). Although DBRPI did not reduce FBG levels in rats, there was a significant decrease in PBG (P < 0.05) and glycated serum protein (GSP) levels (P < 0.01) (Figure 1B-D). AUC-OGTT improved progressively, showing enhanced responses at weeks 2 (P < 0.05), 4 (P < 0.01), and 6 (P < 0.001) postoperatively (Figure 1E and F).
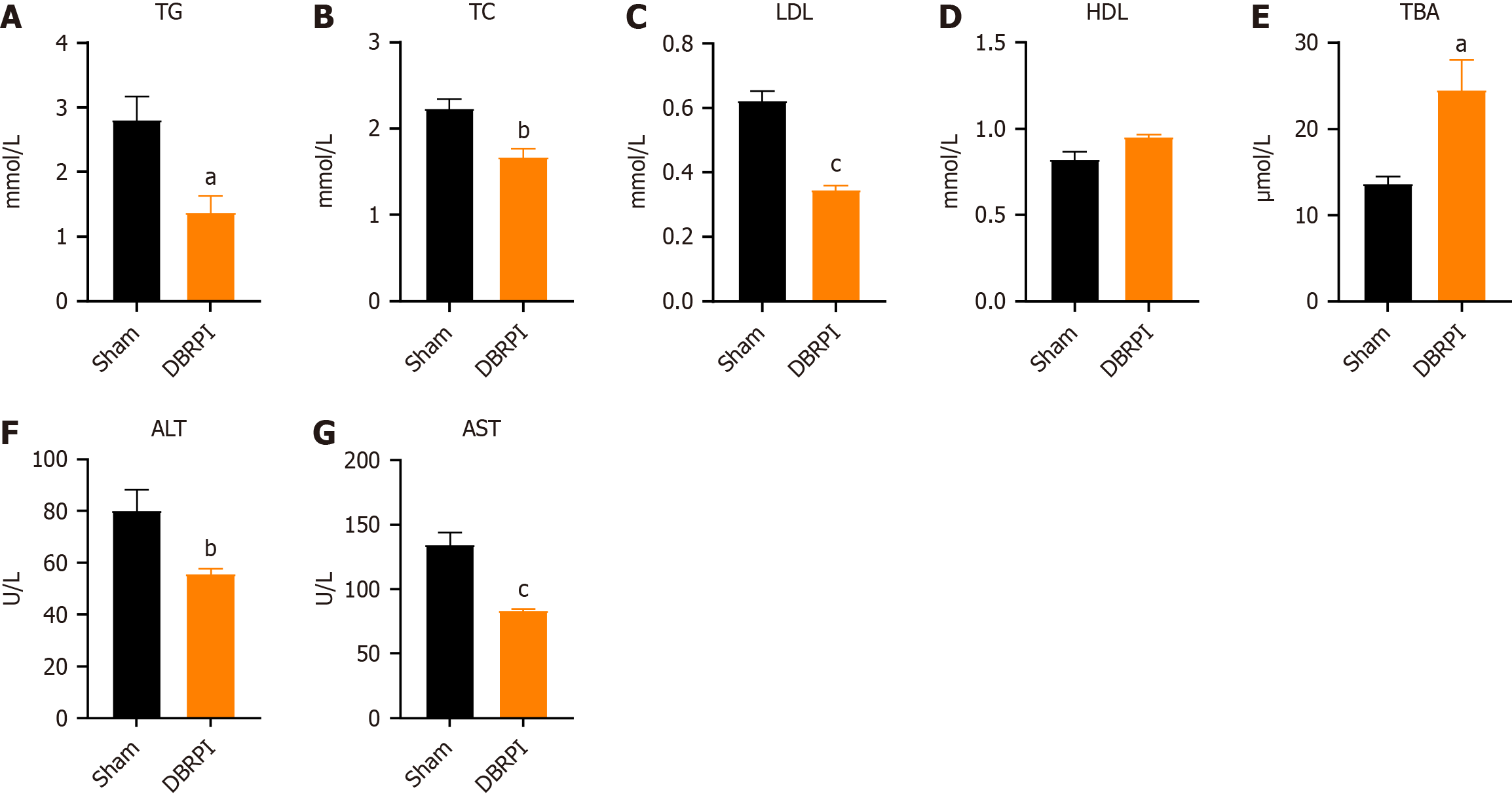
Six weeks post-surgery, the lipid profile of the DBRPI group significantly changed, as shown by reduced levels of TG (P < 0.05), TC (P < 0.01), and LDL (P < 0.001) (Figure 2A-D). Additionally, there was a notable increase in the level of TBA in the serum after DBRPI surgery (P < 0.05) (Figure 2E). The levels of serum ALT (P < 0.01) and AST (P < 0.001) in the DBRPI group also decreased to varying degrees (Figure 2F and G).
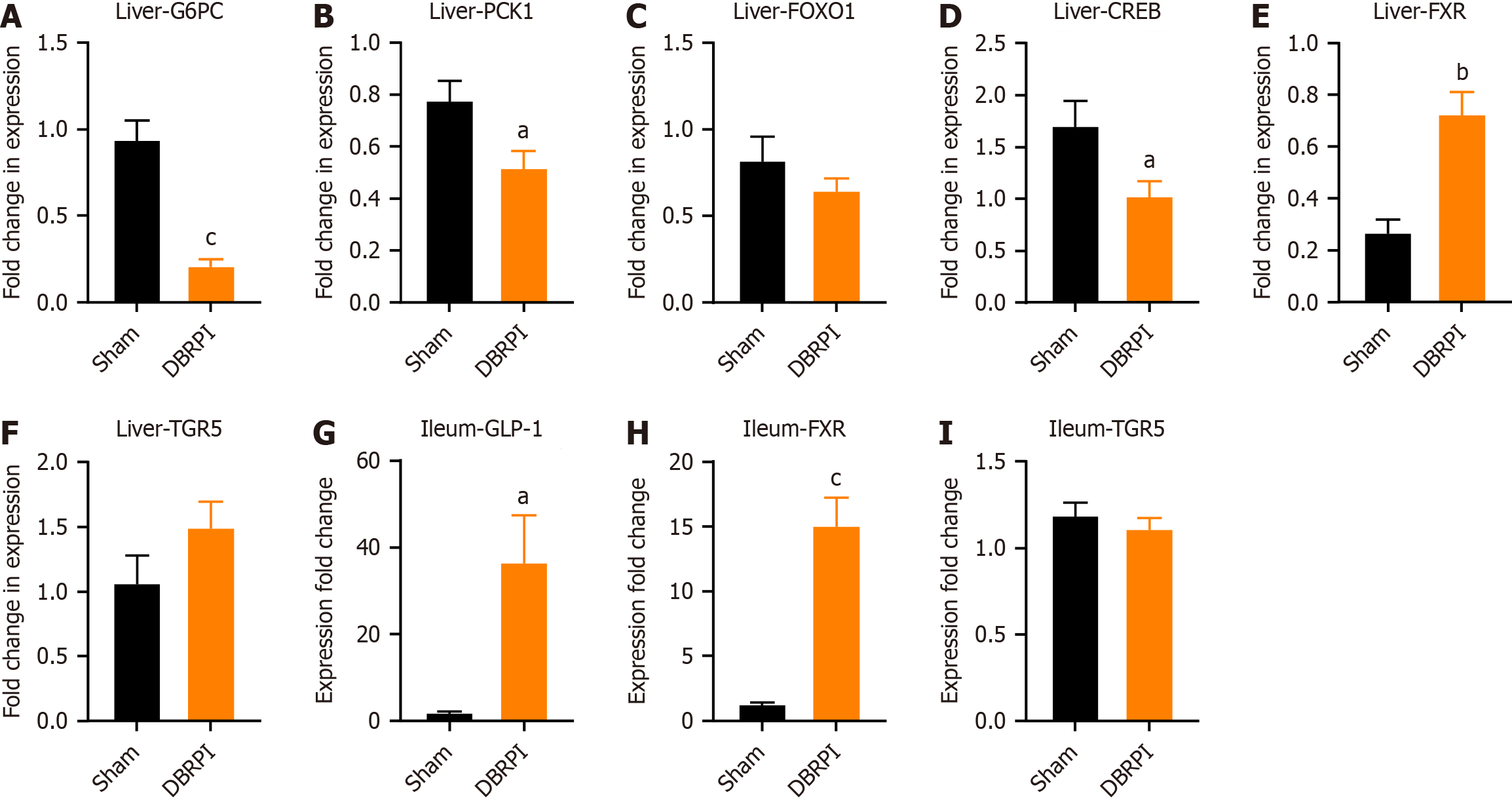
Compared with the sham group, the DBRPI group showed significantly lower expression of the gluconeogenic key enzyme-encoding genes glucose-6-phosphatase (G6PC) (P < 0.001) and phosphoenolpyruvate carboxykinase 1 (PCK1) (P < 0.05), along with significant inhibition of the activity of the transcription factor CREB (P < 0.05), which regulates gluconeogenesis (Figure 3A-D). Additionally, the expression levels of FXR (P < 0.0 and P < 0.001, respectively) and GLP-1 (P < 0.05) were significantly increased, while there was no significant change in the expression level of TGR5 in the terminal ileum (Figure 3E-I).
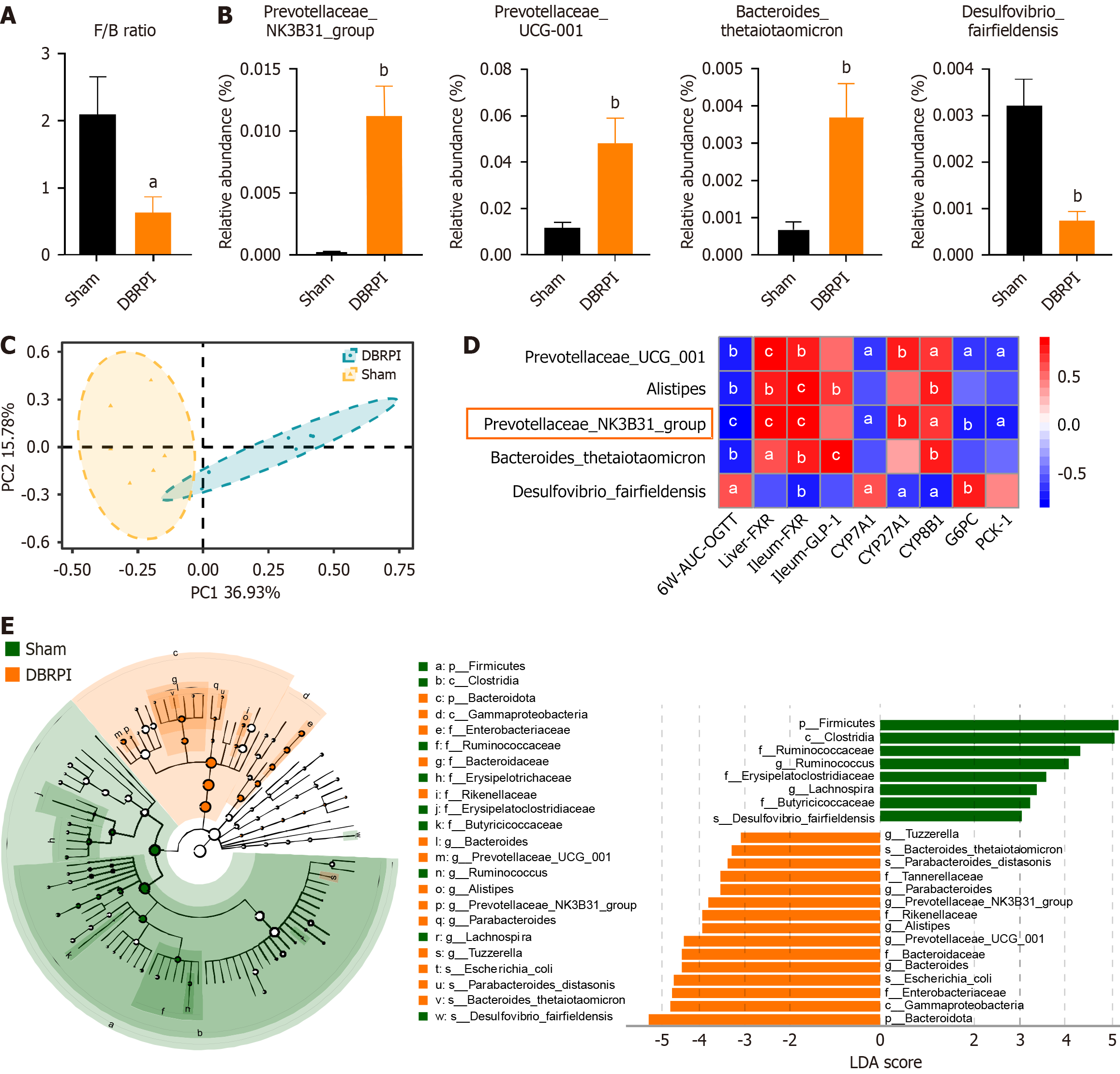
DBRPI decreased the abundance ratio of Firmicutes to Bacteroidetes in obese rats (P < 0.05) (Figure 4A). Compared with that in the sham group, the gut microbial abundance in the DBRPI group significantly changed after surgery, with principal coordinates analysis revealing significant differences in β-diversity between the two groups (P < 0.01) (Figure 4B and C). Furthermore, correlation analysis revealed that the abundance of the Prevotellaceae_NK3B31_group was negatively correlated with AUC-OGTT, G6PC, and PCK1 Levels and positively correlated with liver and terminal ileum FXR, CYP27A1 (key initiating and rate-limiting enzyme in the alternative bile acid synthesis pathway), and CYP8B1 (regulation of the cholic acid to CDCA ratio) levels (Figure 4D). Linear discriminant analysis revealed increased abundances of bacteria such as Prevotellaceae UCG-001, Alistipes, Prevotellaceae_NK3B31_group, Parabacteroides distasonis, and Bacteroides thetaiotaomicron after DBRPI surgery, whereas the abundance of Desulfovibrio fairfieldensis increased in the sham group (Figure 4E).
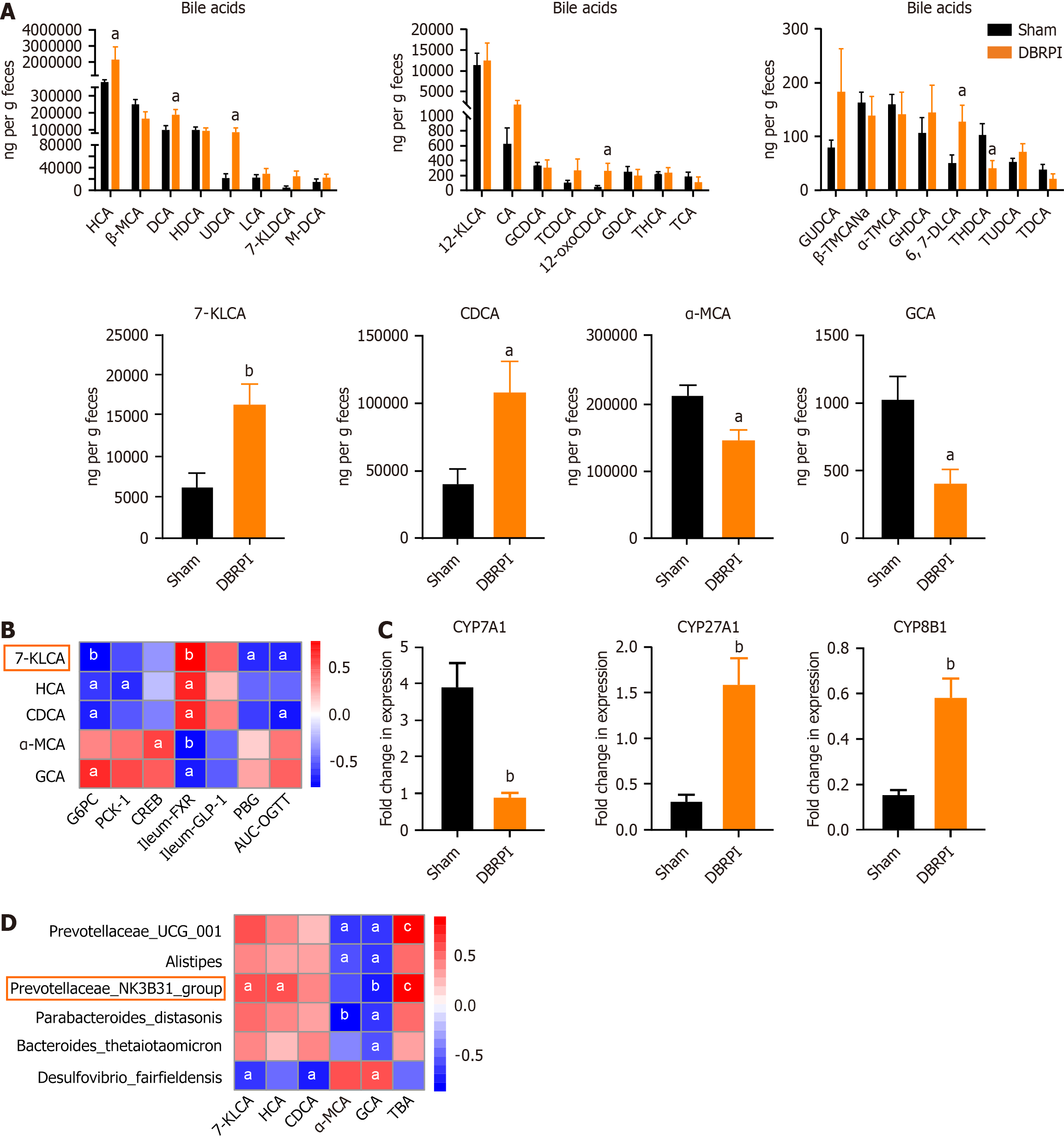
Six weeks after surgery, significant changes in bile acid levels were observed in the feces of rats in the DBRPI group. Specifically, compared with those in the sham group, the levels of 7-KLCA (P < 0.01), hyocholic acid (HCA) (P < 0.05), CDCA (P < 0.05), deoxycholic acid (P < 0.05), ursodeoxycholic acid (UDCA) (P < 0.05), and 12-oxolithocholic acid (P < 0.05) were significantly elevated in the DBRPI group, whereas the levels of α-MCA (P < 0.05), glycocholic acid (GCA) (P < 0.05), and taurohydrodeoxycholic acid (P < 0.05) were significantly reduced (Figure 5A). Further correlation analysis revealed a negative correlation between 7-KLCA and G6PC, PBG, and AUC-OGTT levels and a positive correlation with FXR levels (Figure 5B). Additionally, DBRPI upregulated the expression of the bile acid synthase genes CYP27A1 and CYP8B1, but downregulated the expression of CYP7A1 (all P < 0.01) (Figure 5C). Further correlation analysis revealed a positive correlation between Prevotellaceae_NK3B31_group abundance and 7-KLCA, HCA, and TBA levels and a negative correlation with GCA levels (Figure 5D).
Our study revealed that DBRPI significantly reduced body weight and improved glucose tolerance. DBRPI serves as a simplified research model specifically designed to investigate the "hindgut mechanism". Due to its technical simplicity and ability to isolate specific physiological mechanisms, DBRPI was chosen as the mechanistic model for this study, aiming to elucidate the role of the gut microbiota-bile acid-FXR axis in glucose metabolism with greater clarity.
To explore the underlying mechanisms by which DBRPI increases glucose metabolism, we evaluated the expression of key enzymatic genes involved in gluconeogenesis in the livers of rats. The results showed that DBRPI significantly downregulated the expression of G6PC and PCK1, which are key enzymatic genes in hepatic gluconeogenesis[18], accompanied by a decrease in the mRNA expression of the transcription factor CREB, which regulates gluconeogenesis[19]. These findings suggest that the increase in glucose metabolism following DBRPI may be related to the inhibition of hepatic gluconeogenesis.
To further determine the mechanistic pathway, we evaluated fecal bile acid profiles, the expression of bile acid re
Interestingly, despite the increase in the levels of many bile acids, the levels of α-MCA and GCA decreased in the DBRPI group. α-MCA is considered a potent antagonist of FXR, and high concentrations of GCA may act as an antagonist rather than an agonist of FXR[21,22]. The reduction in these FXR antagonists, combined with the upregulation of ileal and hepatic FXR expression, suggests that DBRPI may shift the bile acid pool toward a more FXR-activating profile. This may be attributed to the role of the gut microbiota in bile acid transformation, as α-MCA, for example, is converted from CDCA by the gut microbiota[13]. In rodents, CDCA is primarily converted to α-MCA, whereas in humans, CDCA is mainly transformed into UDCA and LCA[13]. Despite species differences in bile acid metabolism, the functional role of microbiota-bile acid crosstalk in regulating metabolic health is conserved—highlighting the relevance of our rodent model findings to human metabolic physiology[14].
Given the critical role of gut microbiota in bile acid transformation, we next characterized the gut microbial composition in the DBRPI and sham groups. Following DBRPI, the abundance of the Prevotellaceae_NK3B31_group increased significantly, whereas the abundance of Desulfovibrio fairfieldensis decreased significantly. Correlation analysis revealed that the abundance of the Prevotellaceae_NK3B31_group had the strongest correlation with metabolic indicator levels. It was negatively correlated with AUC-OGTT, G6PC, and PCK1 levels and positively correlated with 7-KLCA. Therefore, we speculate that the Prevotellaceae_NK3B31_group may mediate activation of the 7-KLCA-FXR-gluconeogenesis signaling pathway, but this hypothesis still requires further research for verification. Consistent with previous reports[23], this study also revealed a significant increase in the ratio of Firmicutes to Bacteroidetes in HFD induced obese rats. However, DBRPI intervention decreased this ratio, prompting a shift in the gut microbial community from being dominated by Firmicutes to being dominated by Bacteroidetes at the phylum level. In addition, correlation analysis revealed that Desulfovibrio fairfieldensis abundance was negatively correlated with the levels of bile acids and their receptors FXR and GLP-1, and positively correlated with the levels of G6PC, suggesting that the reduction in Desulfovibrio fairfieldensis abundance in DBRPI may contribute to increased glucose metabolism. Qi et al[24] further confirmed that Desulfovibrio reduces GLP-1 Levels in mice by producing hydrogen sulfide, impairing host metabolism. This finding supports our hypothesis to some extent.
Beyond glucose metabolism, DBRPI also improved lipid profiles and liver function. Six weeks post-surgery, the DBRPI group showed reduced serum TG, TC, and LDL levels. The reasons for the reduction in lipid levels after DBRPI surgery may be diverse. For instance, an increase in lipid uptake and metabolism by the liver or peripheral tissues may lead to enhanced lipid clearance, thereby lowering blood lipid levels. Secondly, bile acids, through activation of the FXR receptor, may regulate the expression of genes involved in lipid metabolism[25]. Additionally, a decrease in lipid absorption due to reduced intestinal capacity following DBRPI surgery could also contribute to lower blood lipid levels. Although the current data cannot fully distinguish the specific contributions of these mechanisms, future research will include more in-depth exploration to further elucidate the precise mechanisms underlying these changes. Additionally, the decrease in ALT and AST levels after DBRPI surgery suggests that the procedure may have improved liver damage induced by a HFD to some extent. Studies have shown that liver fat content decreases significantly after bariatric surgery, leading to a reduction in hepatocyte inflammation and fibrosis, which is closely related to the decrease in transaminase levels[26]. Additionally, MS can significantly reduce the level of systemic inflammatory factors, and reduction of the inflammatory response helps to protect hepatocytes and decrease the release of transaminases[27].
We also observed significant upregulation of ileal GLP-1 expression in the DBRPI group. GLP-1 improves glucose metabolism by stimulating insulin secretion, inhibiting glucagon release, and suppressing appetite, and it may indirectly inhibit hepatic gluconeogenesis via the cAMP/PKA signaling pathway[28]. However, the impact of FXR on GLP-1 ex
Our experimental design had several limitations. First, despite a significant increase in fecal 7-KLCA levels, we cannot confirm whether there is a concomitant trend in serum levels as we did not measure 7-KLCA concentrations in serum samples. The observed changes in these key metabolic regulators occurred at the gene transcription level, and further validation at the protein level is required to determine whether these transcriptional changes truly translate into functional protein expression differences. Moreover, the specific role of the gut microbiota in this process requires more detailed investigation. Specifically, which bacteria play a key role in bile acid metabolism, which bacteria and their metabolites can effectively stimulate L-cells to secrete GLP-1, and how these metabolites are affected by surgery are questions that still need to be elucidated through more in-depth research.
In summary, this study demonstrates that DBRPI improves glucose tolerance and suppresses gene expression of the key hepatic gluconeogenic enzymes G6PC and PCK1. These improvements may be partially associated with activation of the 7-KLCA-FXR axis mediated by the Prevotellaceae_NK3B31_group.
| 1. | Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e984-e1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1976] [Article Influence: 395.2] [Reference Citation Analysis (0)] |
| 2. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, Chamseddine G, Bornstein SR, Rubino F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 3. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2015] [Article Influence: 223.9] [Reference Citation Analysis (0)] |
| 4. | Wei M, Cao WB, Zhao RD, Sun DP, Liang YZ, Huang YD, Cheng ZW, Ouyang J, Yang WS, Yu WB. Fibroblast growth factor 15, induced by elevated bile acids, mediates the improvement of hepatic glucose metabolism after sleeve gastrectomy. World J Gastroenterol. 2023;29:3280-3291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Tu J, Wang Y, Jin L, Huang W. Bile acids, gut microbiota and metabolic surgery. Front Endocrinol (Lausanne). 2022;13:929530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Lin S, Wang S, Wang P, Tang C, Wang Z, Chen L, Luo G, Chen H, Liu Y, Feng B, Wu D, Burrin DG, Fang Z. Bile acids and their receptors in regulation of gut health and diseases. Prog Lipid Res. 2023;89:101210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 7. | Gonzalez FJ, Jiang C, Patterson AD. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology. 2016;151:845-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 302] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 8. | Xu H, Fang F, Wu K, Song J, Li Y, Lu X, Liu J, Zhou L, Yu W, Yu F, Gao J. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 2023;11:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 9. | Li W, Zhuang T, Wang Z, Wang X, Liu L, Luo Y, Wang R, Li L, Huang W, Wang Z, Yang L, Ding L. Red ginseng extracts ameliorate high-fat diet-induced obesity and insulin resistance by activating the intestinal TGR5-mediated bile acids signaling pathway. Phytomedicine. 2023;119:154982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 10. | Chiang JYL, Ferrell JM. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol. 2022;548:111618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 11. | Chen B, Bai Y, Tong F, Yan J, Zhang R, Zhong Y, Tan H, Ma X. Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes. 2023;15:2192155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 12. | Hou Y, Zhai X, Wang X, Wu Y, Wang H, Qin Y, Han J, Meng Y. Research progress on the relationship between bile acid metabolism and type 2 diabetes mellitus. Diabetol Metab Syndr. 2023;15:235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 2019] [Article Influence: 201.9] [Reference Citation Analysis (0)] |
| 14. | Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 15. | Li X, Xiao Y, Huang Y, Song L, Li M, Ren Z. Lactobacillus gasseri RW2014 Ameliorates Hyperlipidemia by Modulating Bile Acid Metabolism and Gut Microbiota Composition in Rats. Nutrients. 2022;14:4945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 16. | Duan J, Zhou J, Ren F, Tan C, Wang S, Yuan L. Mid to distal small bowel resection with the preservation of the terminal ileum improves glucose homeostasis in diabetic rats by activating the hindgut-dependent mechanism. J Gastrointest Surg. 2014;18:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Knop FK. Resolution of type 2 diabetes following gastric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009;52:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Xue Y, Cui A, Wei S, Ma F, Liu Z, Fang X, Huo S, Sun X, Li W, Hu Z, Liu Y, Cai G, Su W, Zhao J, Yan X, Gao C, Wen J, Zhang H, Li H, Liu Y, Lin X, Xu Y, Fu W, Fang J, Li Y. Proline hydroxylation of CREB-regulated transcriptional coactivator 2 controls hepatic glucose metabolism. Proc Natl Acad Sci U S A. 2023;120:e2219419120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Zhao Y, Li S, Chen Y, Wang Y, Wei Y, Zhou T, Zhang Y, Yang Y, Chen L, Liu Y, Hu C, Zhou B, Ding Q. Histone phosphorylation integrates the hepatic glucagon-PKA-CREB gluconeogenesis program in response to fasting. Mol Cell. 2023;83:1093-1108.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 20. | Song L, Hou Y, Xu D, Dai X, Luo J, Liu Y, Huang Z, Yang M, Chen J, Hu Y, Chen C, Tang Y, Rao Z, Ma J, Zheng M, Shi K, Cai C, Lu M, Tang R, Ma X, Xie C, Luo Y, Li X, Huang Z. Hepatic FXR-FGF4 is required for bile acid homeostasis via an FGFR4-LRH-1 signal node under cholestatic stress. Cell Metab. 2025;37:104-120.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 21. | Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1273] [Article Influence: 47.1] [Reference Citation Analysis (7)] |
| 22. | Liu J, Lu H, Lu YF, Lei X, Cui JY, Ellis E, Strom SC, Klaassen CD. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci. 2014;141:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 24. | Qi Q, Zhang H, Jin Z, Wang C, Xia M, Chen B, Lv B, Peres Diaz L, Li X, Feng R, Qiu M, Li Y, Meseguer D, Zheng X, Wang W, Song W, Huang H, Wu H, Chen L, Schneeberger M, Yu X. Hydrogen sulfide produced by the gut microbiota impairs host metabolism via reducing GLP-1 levels in male mice. Nat Metab. 2024;6:1601-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 25. | Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, Barshop W, Wohlschlegel J, Calkin AC, Liu Y, Thorell A, Meikle PJ, Drew BG, Mack JJ, Marschall HU, Tarling EJ, Edwards PA, de Aguiar Vallim TQ. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671-1684.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 26. | Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290-1301.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 436] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 27. | Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 28. | Ip W, Shao W, Chiang YT, Jin T. GLP-1-derived nonapeptide GLP-1(28-36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2013;305:E1348-E1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza J, Briand O, Dehondt H, Vallez E, Dorchies E, Baud G, Spinelli V, Hennuyer N, Caron S, Bantubungi K, Caiazzo R, Reimann F, Marchetti P, Lefebvre P, Bäckhed F, Gribble FM, Schoonjans K, Pattou F, Tailleux A, Staels B, Lestavel S. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 30. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 765] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 31. | Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, Alikhan M, Clements BA, Abumrad NN, Flynn CR. Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology. 2019;156:1041-1051.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/