Published online Nov 21, 2025. doi: 10.3748/wjg.v31.i43.111976
Revised: August 26, 2025
Accepted: October 17, 2025
Published online: November 21, 2025
Processing time: 120 Days and 0.1 Hours
Patients with acute-on-chronic liver failure (ACLF) experience severe immune dysfunction. Liver transplantation (LT) significantly improves survival outcomes. However, the characteristics of peripheral blood lymphocyte subsets (PBLSs) in this patient population are not well defined, and the dynamics of immune recon
To characterize PBLSs in patients with ACLF prior to LT and to evaluate PBLS re
Clinical data from patients undergoing LT in the Transplantation Center, The Third Xiangya Hospital from January 2022 to December 2023 were analyzed retrospectively. Our cohort comprised 44 patients with ACLF, 16 patients with acute decompensation of cirrhosis, and 23 patients with compensated cirrhosis. Twenty healthy volunteers were included as controls. PBLSs were evaluated across all groups. The relationship between PBLSs and post-LT prognosis was assessed, and dynamic changes in PBLSs among patients with ACLF were analyzed at different time points.
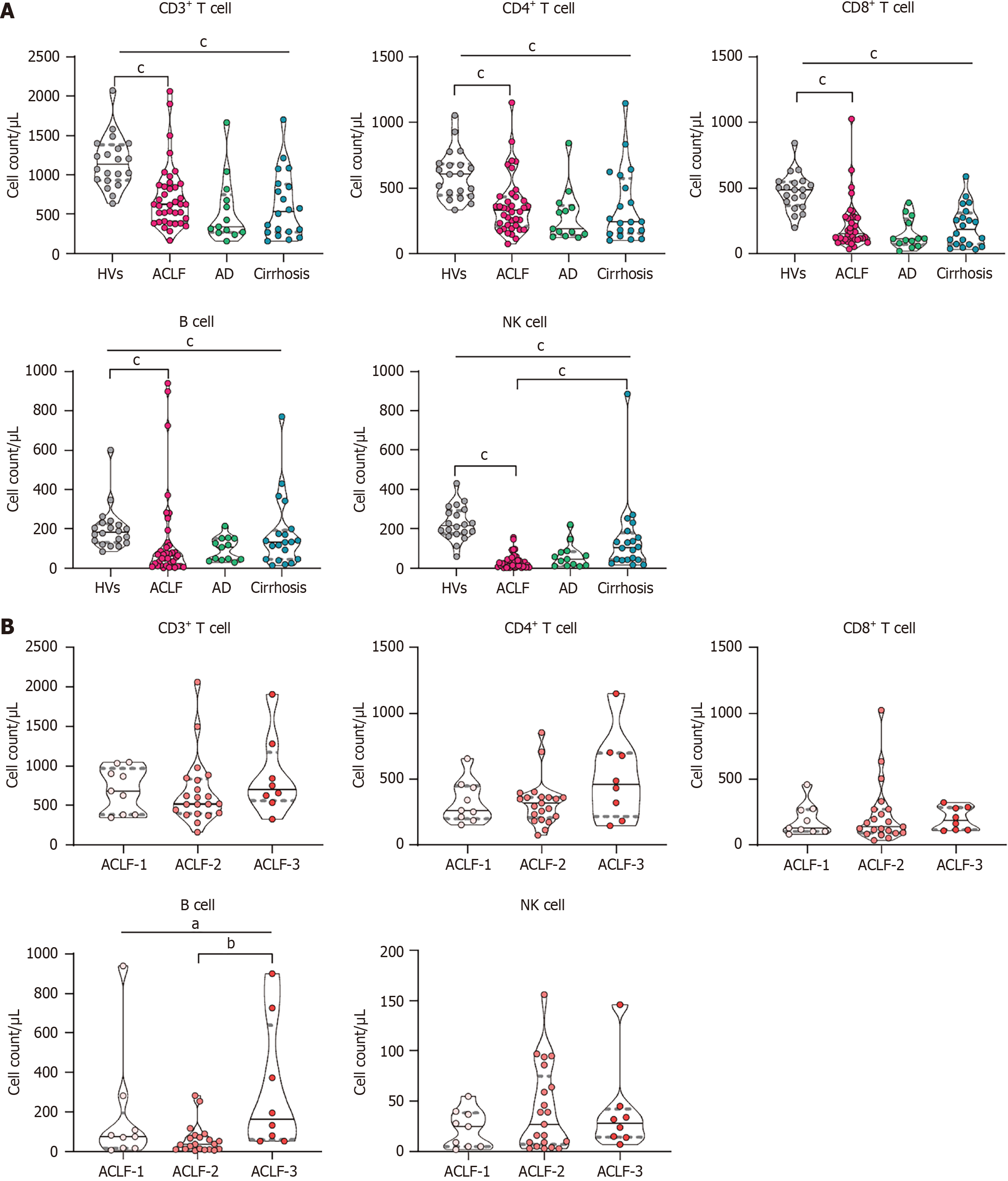
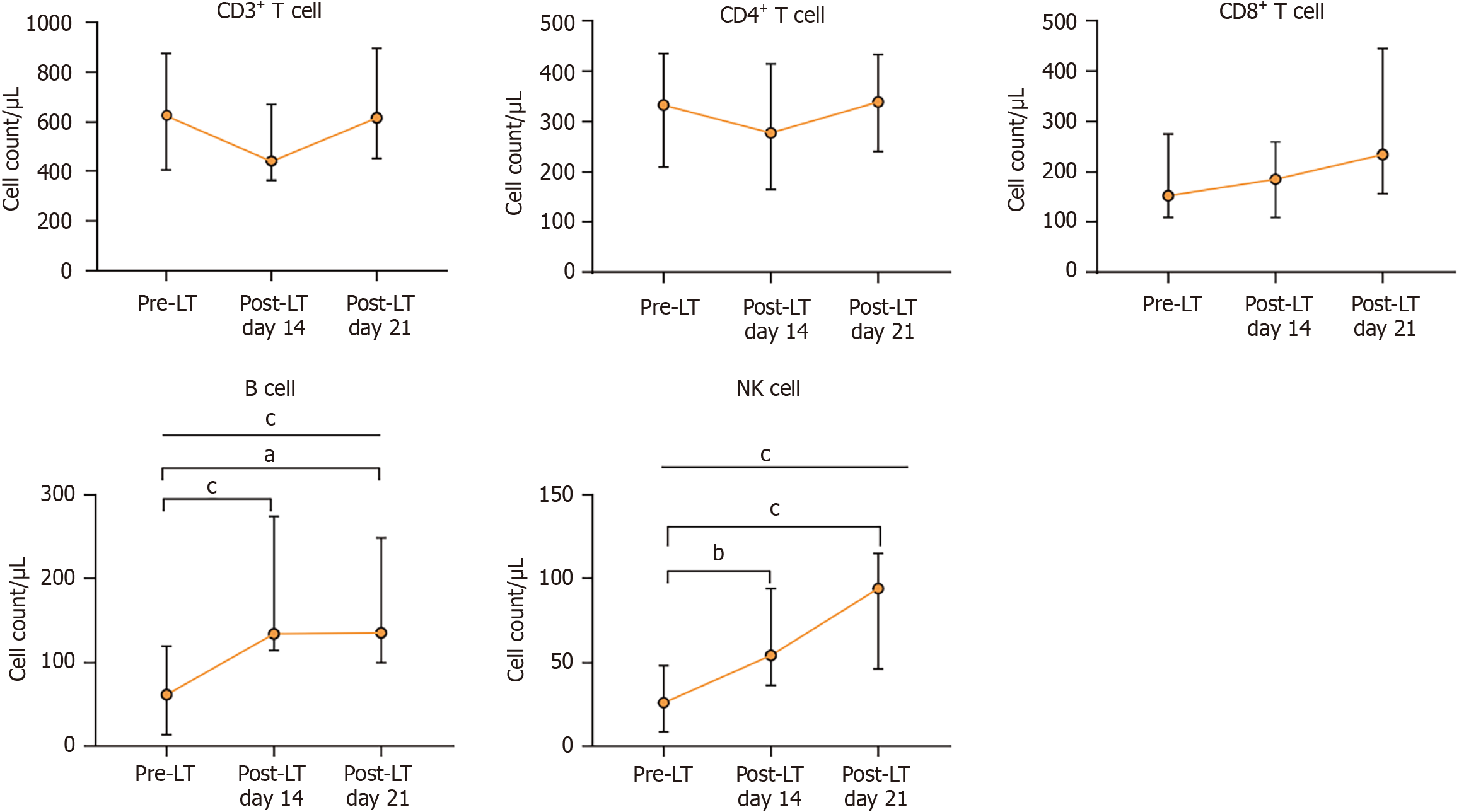
Patients with ACLF exhibited a marked reduction in PBLSs compared with healthy volunteers. Natural killer (NK) cell counts were further reduced in patients with ACLF when compared with patients with compensated cirrhosis. PBLSs did not correlate with the etiology or severity of ACLF or with established liver failure scores. Following LT, a rapid restoration of NK cells and B cells was observed in patients with ACLF. However, the cluster of differentiation (CD) 3+ T cell and CD4+ T cell counts decreased 14 days post-LT and subsequently returned to preoperative levels by day 21.
Patients with ACLF exhibited markedly reduced PBLSs, with decreased NK cells potentially linked to progression from compensated cirrhosis to liver failure. NK and B cell were rapidly restored after LT.
Core Tip: Immune dysfunction is a hallmark characteristic of acute-on-chronic liver failure (ACLF). This study demonstrated that patients with ACLF exhibited a significant reduction in the level of peripheral blood lymphocyte subsets (PBLSs). Notably, natural killer (NK) cells may be associated with the progression of liver failure. Both NK and B cells have the potential to serve as biomarkers for immune recovery after liver transplantation. A thorough assessment of dynamic changes in PBLSs provided valuable insights into the evolving immune status of this population and supported the development of strategies for post-transplantation management.
- Citation: Peng B, Li ZX, Yang M, Liu K, Xiang XY, Zhang Y, Li JH, Zhang PP, Zhuang Q, Cheng K, Ming YZ. Peripheral blood lymphocyte subset deficiency in acute-on-chronic liver failure and reconstitution following liver transplantation. World J Gastroenterol 2025; 31(43): 111976
- URL: https://www.wjgnet.com/1007-9327/full/v31/i43/111976.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i43.111976
Acute-on-chronic liver failure (ACLF) is a severe clinical syndrome with a high 28-day mortality and a substantial global disease burden[1]. The pathogenesis of ACLF is complex and exhibits regional variations in underlying chronic liver diseases and acute precipitating factors. In Asian countries, particularly China, hepatitis B virus (HBV) infection is the predominant chronic etiology and accounts for 60% of ACLF cases[2]. Conversely, alcohol-associated liver disease is the leading cause of ACLF in Europe and the United States.
Severe systemic inflammation is widely recognized as a key characteristic of ACLF across various etiologies[3]. The CANONIC study highlighted that nearly all patients with ACLF exhibited significantly elevated leukocyte counts and C-reactive protein levels[4]. Immune deficiency is also fundamental to the pathophysiology of ACLF. When neutrophil levels are elevated in cases of ACLF, they show functional impairments such as defective chemotaxis, reduced superoxide production, and decreased pathogen clearance[5]. Patients with ACLF display significantly decreased human leukocyte antigen-DR expression on monocytes, have markedly impaired phagocytic capacity, show reduced responsiveness to lipopolysaccharide stimulation, and have a notable increase in interleukin-10 secretion[6,7]. This profound immune deficiency substantially increases the risk of infection that further exacerbates systemic inflammatory responses.
Although the altered immunity in ACLF has become well known, most studies have primarily focused on the innate immune system. There are limited studies addressing the role of peripheral blood lymphocyte subsets (PBLSs), which are critical indicators of adaptive immune competence. Existing evidence indicates that alterations in PBLSs may be associated with impaired immune function and adverse prognosis in ACLF patients[8,9]. To provide further insights, we utilized consecutive patient data from our transplant center to compare preoperative PBLSs profiles among patients with ACLF, acute decompensation (AD) of cirrhosis, and compensated liver cirrhosis. Furthermore, we examined longitudinal changes in PBLSs before and after liver transplantation (LT) in patients with ACLF. This study aimed to provide a comprehensive characterization of immune dysfunction in ACLF prior to transplantation as well as to elucidate the patterns and dynamics of PBLS reconstitution after LT in this patient population.
Consecutive adult patients undergoing LT at the Transplantation Center, The Third Xiangya Hospital, Central South University from January 2022 to December 2023 were enrolled in this study. Patients with a history of previous transplantation (n = 3) or with unavailable pre-LT and post-LT PBLSs data (n = 3) were excluded. To avoid the confounding influence of cancer-related immune factors, patients with primary liver cancer (n = 17) were also excluded. A total of 83 patients were included in the study. Twenty healthy volunteers (HVs) were recruited as controls.
We used the diagnostic criteria of ACLF and AD of cirrhosis from the European Association for the Study of the Liver (EASL)-chronic liver failure (CLIF) guidelines[4]. The severity of ACLF was evaluated using several scoring systems, including the Child-Turcotte-Pugh score, albumin-bilirubin score, model for end-stage liver disease score, model for end-stage liver disease-sodium score, CLIF-consortium (CLIF-C) organ failure (OF) score, and CLIF-C ACLF score. For patients with hepatitis B, the HBV-sequential OF assessment, Chinese Group on the Study of Severe Hepatitis B-ACLF (COSSH-ACLF) score, and COSSH-ACLF II score were also applied. The diagnosis of liver cirrhosis was confirmed through pathological examination.
All patients included in this study underwent LT using grafts from donation after brain death. The modified piggyback technique was applied for the LT procedure. Intraoperatively, patients routinely received methylprednisolone (500 mg) and basiliximab (20 mg and again on postoperative day 4). Postoperatively, a standard triple immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil (or mycophenolate sodium enteric-coated tablets), and prednisone was employed for anti-rejection treatment.
PBLSs were assessed using the BD Multitest 6-color TBNK reagent (Catalog No. 662967; BD Biosciences, Franklin Lakes, NJ, United States) and BD Trucount tubes (Catalog No. 663028) that enabled simultaneous detection of both the percentages and absolute counts of cluster of differentiation (CD) 3+ T cells, CD3+ CD4+ T cells, CD3+ CD8+ T cells, CD19+ B cells, and CD16+/CD56+ natural killer (NK) cells. The assessments were conducted at three time points: Before LT; Day 14 ± 1 after LT; And day 21 ± 2 after LT. All assays were performed in accordance with the manufacturer’s instructions, and data analysis was conducted using the BD FACSCanto clinical software.
The median follow-up period was 1.82 years. The primary outcomes were 1-month, 3-month, and 1-year survival rates after LT. The secondary outcomes included perioperative infection, primary non-function (PNF), and early allograft dysfunction. Perioperative infection was confirmed by positive bacterial or fungal cultures. PNF was defined as graft failure resulting in re-transplantation or death within 14 days following LT[10]. Early allograft dysfunction was diagnosed if recipients met at least one of the following criteria: (1) Total bilirubin ≥ 10 mg/dL at day 7 after LT; (2) International normalized ratio ≥ 1.6 at day 7 after LT; and (3) Alanine aminotransferase or aspartate aminotransferase > 2000 IU/L within the first 7 days after LT[11].
The characteristics of the patients were presented as mean ± SD, median [interquartile ranges (IQR), or number (percentage)]. The normality distribution of the data was examined by the D’Agostino-Pearson test or Shapiro-Wilk test based on the sample size. Correlation analyses were conducted using Spearman’s rank correlation. For comparisons between two independent groups, either a t-test or the Mann-Whitney U test was applied. For multiple group com
A total of 83 participants (mean age: 48.8 years; 80.7% male) undergoing LT was enrolled in this study. There were 44 patients diagnosed with ACLF, 16 patients with AD of cirrhosis, and 23 patients with compensated liver cirrhosis (Table 1). HBV infection was identified as the predominant etiology across all three groups. Patients with ACLF had significantly higher levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin (P < 0.001). These patients also presented with marked coagulation dysfunction and severe systemic inflammation as shown by increased international normalized ratios and elevated white blood cells (WBCs) and neutrophils (P < 0.001).
| Characteristics | Total (n = 83) | ACLF (n = 44) | AD (n = 16) | Cirrhosis (n = 23) | P value |
| Age in years | 48.8 ± 9.4 | 47.0 ± 8.4 | 50.9 ± 9.3 | 50.7 ± 10.9 | 0.057 |
| Male | 67 (80.7) | 41 (93.2) | 12 (75.0) | 14 (60.9) | 0.003 |
| Etiology1 | 0.038 | ||||
| HBV | 55 (66.3) | 35 (79.6) | 9 (56.2) | 11 (47.8) | |
| HCV | 1 (1.2) | 1 (2.3) | 0 | 0 | |
| Alcohol | 8 (9.6) | 2 (4.5) | 3 (18.8) | 3 (13.0) | |
| Autoimmune | 11 (13.3) | 3 (6.8) | 3 (18.8) | 5 (21.8) | |
| Mixed | 5 (6.0) | 3 (6.8) | 1 (6.2) | 1 (4.4) | |
| Other | 3 (3.6) | 0 | 0 | 3 (13.0) | |
| Laboratory data | |||||
| Albumin in g/L | 33.8 ± 5.4 | 34.4 ± 4.9 | 31.5 ± 5.9 | 34.5 ± 5.9 | 0.156 |
| ALT in U/L, median IQR | 42.0 (26.0-86.0) | 56.5 (37.3-98.3) | 37.5 (27.0-87.3) | 26.0 (16.0-38.0) | < 0.001 |
| AST in U/L, median IQR | 70.0 (45.0-115.0) | 99.5 (53.8-120.8) | 86.0 (49.5-183.5) | 41.0 (30.0-52.0) | < 0.001 |
| TB in μmol/L | 301.9 ± 240.7 | 461.1 ± 180.4 | 241.9 ± 195.9 | 39.0 ± 26.0 | < 0.001 |
| Creatinine in mg/dL, median IQR | 0.7 (0.6-1.1) | 0.8 (0.6-1.4) | 0.7 (0.6-0.9) | 0.7 (0.5-1.3) | 0.424 |
| Serum urea in mmol/L, median IQR | 5.6 (3.8-7.9) | 5.7 (3.8-9.1) | 5.5 (4.1-7.5) | 5.5 (3.6-7.1) | 0.833 |
| INR, median IQR | 1.9 (1.4-2.6) | 2.5 (2.0-3.0) | 1.6 (1.4-2.0) | 1.3 (1.2-1.6) | < 0.001 |
| WBC as 109/L, median IQR | 5.0 (3.5-7.9) | 6.7 (4.3-8.5) | 4.4 (2.2-6.6) | 3.6 (2.6-4.2) | < 0.001 |
| Neutrophil as 109/L, median IQR | 3.4 (2.2-5.5) | 4.7 (3.0-6.9) | 2.9 (1.5-4.7) | 2.1 (1.6-2.8) | < 0.001 |
| Perioperative complications | |||||
| Infection | 43 (51.8) | 24 (54.5) | 7 (43.8) | 12 (52.2) | 0.800 |
| PNF | 4 (4.8) | 3 (6.8) | 0 (0) | 1 (4.3) | 0.811 |
| EAD | 31 (37.3) | 20 (45.5) | 2 (12.5) | 9 (39.1) | 0.061 |
Classification according to the EASL-CLIF criteria revealed that 12 patients had ACLF-1, 22 patients had ACLF-2, and 10 patients had ACLF-3 (Table 2). Patients with ACLF-3 experienced a higher incidence of OF, including coagulation, cerebral, and respiratory failure (P < 0.05). Patients with ACLF-3 also demonstrated significantly higher Child-Turcotte-Pugh, CLIF-OF, and CLIF-C ACLF scores, aligning with increased short-term mortality and a higher prevalence of postoperative complications.
| Characteristics | ACLF-1 (n = 12) | ACLF-2 (n = 22) | ACLF-3 (n = 10) | P value |
| Age in years | 43.5 ± 8.2 | 47.9 ± 7.1 | 49.3 ± 10.5 | 0.213 |
| Male | 10 (83.3) | 21 (95.5) | 10 (100.0) | 0.301 |
| Etiology1 | 0.362 | |||
| HBV | 9 (75) | 17 (77.3) | 9 (90.0) | |
| HCV | 1 (8.3) | 0 | 0 | |
| Alcohol | 0 | 1 (4.5) | 1 (10.0) | |
| Autoimmune | 2 (16.7) | 1 (4.5) | 0 | |
| Mixed | 0 | 3 (13.6) | 0 | |
| Other | 0 | 0 | 0 | |
| Laboratory data | ||||
| Albumin in g/L | 34.0 ± 6.0 | 35.1 ± 5.0 | 33.2 ± 3.3 | 0.585 |
| ALT in U/L, median IQR | 70.0 (39.8-103.3) | 49.0 (36.5-78.0) | 93.0 (28.5-163.3) | 0.304 |
| AST in U/L, median IQR | 107.5 (57.5-158.3) | 101.0 (75.3-120.3) | 58.0 (50.8-79.5) | 0.126 |
| TB in μmol/L | 455.3 ± 273.0 | 504.7 ± 133.9 | 372.0 ± 94.71 | 0.155 |
| Creatinine in mg/dL, median IQR | 1.3 (0.7-2.0) | 0.7 (0.6-1.0) | 0.7 (0.5-1.3) | 0.142 |
| Serum urea in mmol/L, median IQR | 10.3 (5.7-18.3) | 5.4 (4.1-7.8) | 4.1 (2.9-6.2) | 0.039 |
| INR, median IQR | 1.9 (1.5-2.2) | 2.6 (2.3-3.0) | 3.3 (2.7-4.9) | < 0.001 |
| WBC as 109/L, median IQR | 5.7 (4.1-7.1) | 6.8 (4.0-8.6) | 8.2 (6.7-9.4) | 0.113 |
| Neutrophil as 109/L, median IQR | 3.9 (2.7-4.7) | 4.8 (2.7-7.0) | 5.7 (4.6-8.2) | 0.183 |
| Organ failure | ||||
| Liver | 10 (83.3) | 22 (100.0) | 10 (100.0) | 0.117 |
| Kidney | 2 (16.7) | 2 (9.1) | 2 (20.0) | 0.614 |
| Coagulation | 0 (0) | 15 (68.2) | 9 (90.0) | < 0.001 |
| Brain | 0 (0) | 5 (22.7) | 9 (90.0) | < 0.001 |
| Respiratory | 0 (0) | 0 (0) | 2 (20.0) | 0.048 |
| Circulation | 0 (0) | 0 (0) | 1 (10.0) | 0.227 |
| Predictive scores | ||||
| CTP | 10.8 ± 1.2 | 11.4 ± 1.0 | 12.7 ± 1.3 | 0.001 |
| ALBI | -1.2 ± 0.4 | -1.2 ± 0.4 | -1.1 ± 0.3 | 0.549 |
| CLIF-C OF | 9.0 (8.3-10.0) | 11.0 (10.0-11.0) | 12.0 (12.0-13.3) | < 0.001 |
| CLIF-C ACLF | 39.3 (33.5-40.9) | 44.3 (42.8-47.4) | 54.3 (52.0-58.8) | < 0.001 |
| MELD | 27.3 ± 5.0 | 31.0 ± 4.0 | 32.5 ± 7.4 | 0.050 |
| MELD-Na | 28.2 ± 5.1 | 32.0 ± 3.6 | 32.5 ± 7.4 | 0.077 |
| Perioperative complications | ||||
| Infection | 8 (66.7) | 8 (36.4) | 8 (80.0) | 0.054 |
| PNF | 0 (0) | 0 (0) | 3 (30.0) | 0.009 |
| EAD | 4 (33.3) | 8 (36.4) | 8 (80.0) | 0.054 |
Patients with ACLF exhibited a significant reduction in the absolute count of preoperative PBLSs compared with the HV group. The decline in NK cell counts was most pronounced [ACLF group: 26 (IQR: 8.5-48.3)/μL; HV group: 216.3 (IQR: 175.5-293.2)/μL, P < 0.001], followed by a notable decrease in CD8+ T cell counts [ACLF group: 152.0 (IQR: 109.0-274.3)/μL; HV group: 486.9 (IQR: 365.1-546.7)/μL, P < 0.001] and B cell counts [ACLF group: 61.5 (IQR: 13.5-119.8)/μL; HV group: 184.1 (IQR: 132.2-227.9)/μL, P < 0.001] (Figure 1A). There were no significant differences in the counts of CD3+ T cells, CD4+ T cells, CD8+ T cells, or B cells among the three disease condition groups. However, patients with ACLF did have lower NK cell counts compared with those with compensated cirrhosis.
Within the ACLF group only the B cell counts exhibited significant differences across the ACLF grades. Although these differences were observed, B cell counts did not display a linear relationship as the severity of ACLF increased (Figure 1B). Furthermore, the correlation analysis demonstrated that the absolute count of PBLSs was not associated with any of the predictive scores (Supplementary Table 1).
Because of the distinct chronic etiology profile in China, we compared patients with ACLF due to HBV infection to those with ACLF arising from other etiologies. The analysis revealed no significant differences in PBLSs across the different etiological groups (Supplementary Figure 1). The correlation analyses between PBLS counts and the severity scores of ACLF in patients with HBV (i.e., HBV-sequential OF assessment, COSSH-ACLF, and COSSH-ACLF II) showed no significant associations (Supplementary Table 2).
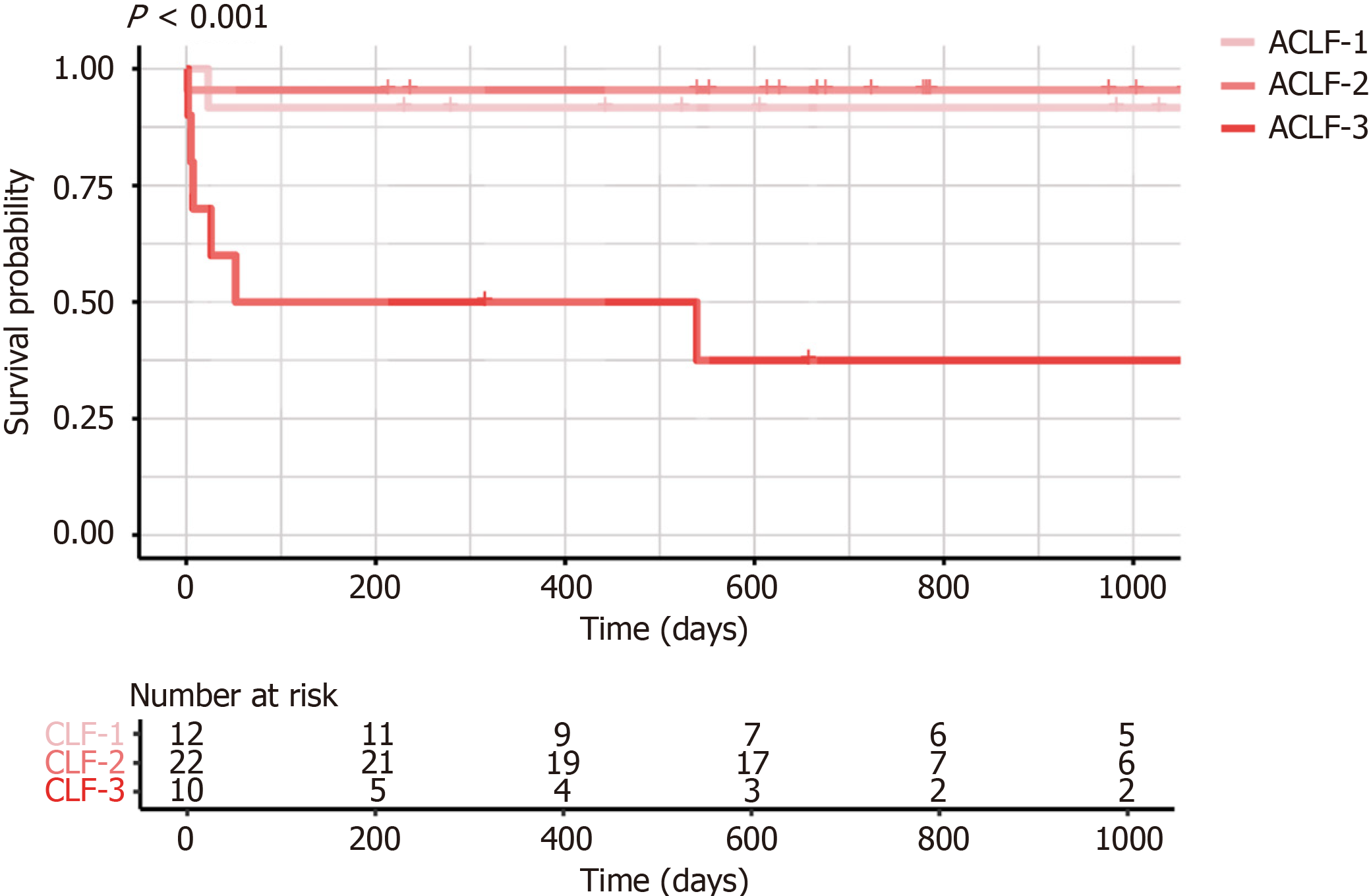
The Kaplan-Meier curves for patients with ACLF are presented in Figure 2. The 1-month survival rates for patients with ACLF grades 1, 2, and 3 were 91.7%, 95.5%, and 60.0%, respectively. The corresponding 3-month and 1-year survival rates for these groups were identical, at 91.7%, 95.5%, and 50.0%, respectively. As shown in Table 3, preoperative PBLS counts were not significantly associated with long-term survival rates. The unadjusted model indicated that a reduced NK cell count was associated with an increased risk of perioperative infection (odds ratio = 0.96, 95% confidence interval: 0.93-0.99, P = 0.016) (Table 4). Comparison of NK cell counts between the infection and non-infection groups revealed a significantly lower absolute NK cell count in the infection group [infection group: 14.5 (IQR: 5.5-35.8)/L, non-infection group: 39.0 (IQR: 14.3-94.3)/L, P = 0.021]. However, after adjusting for potential confounding factors (defined as clinical variables with a P value < 0.1 for their relationship with the respective outcomes), the association between reduced NK cell counts and infections was no longer statistically significant (Supplementary Table 3).
| PBLS | HR (95%CI) | P value |
| CD3+ T cell | 1.00 (1.00-1.00) | 0.773 |
| CD4+ T cell | 1.00 (1.00-1.00) | 0.995 |
| CD8+ T cell | 1.00 (0.99-1.00) | 0.725 |
| B cell | 0.99 (0.96-1.01) | 0.331 |
| NK cell | 1.00 (1.00-1.00) | 0.205 |
| Variables | Univariate analysis | Multivariable analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| EAD | ||||
| CD3+ T cell | 1.00 (1.00-1.00) | 0.329 | ||
| CD4+ T cell | 1.00 (1.00-1.01) | 0.151 | ||
| CD8+ T cell | 1.00 (1.00-1.00) | 0.815 | ||
| B cell | 1.00 (1.00-1.01) | 0.117 | ||
| NK cell | 1.01 (0.99-1.03) | 0.366 | ||
| PNF | ||||
| CD3+ T cell | 1.00 (0.99-1.00) | 0.317 | ||
| CD4+ T cell | 0.99 (0.98-1.00) | 0.205 | ||
| CD8+ T cell | 1.00 (0.99-1.00) | 0.895 | ||
| B cell | 1.00 (1.00-1.01) | 0.051 | ||
| NK cell | 0.95 (0.82-1.01) | 0.275 | ||
| Infection | ||||
| CD3+ T cell | 1.00 (1.00-1.00) | 0.126 | ||
| CD4+ T cell | 1.00 (0.99-1.00) | 0.142 | ||
| CD8+ T cell | 1.00 (0.99-1.00) | 0.120 | ||
| B cell | 1.00 (1.00-1.00) | 0.435 | ||
| NK cell | 0.96 (0.93-0.99) | 0.016 | 0.97 (0.94-1.00) | 0.067 |
The dynamic changes in PBLSs before and after LT are shown in Figure 3. NK cells and B cells progressively increased after LT [NK cells: Changing from 26.0 (IQR: 8.5-48.3)/μL to 94.0 (IQR: 46.0-115.0)/μL, P < 0.001; B cell: Changing from 61.5 (IQR: 13.5-119.8)/μL to 135.0 (IQR: 99.0-248.0)/μL, P < 0.001]. In contrast, CD3+ T cells and CD4+ T initially decreased at postoperative day 14 and returned to preoperative levels by day 21.
The following key findings were observed in this study utilizing consecutive patient data from our transplant center: (1) Patients with ACLF exhibited a significant reduction in PBLSs compare with HVs; (2) NK cell counts were reduced in patients with ACLF compared with patients with compensated cirrhosis; (3) No significant association was found between ACLF severity, etiology, and PBLS counts; and (4) NK cell and B cell counts gradually increased after LT even under the immunosuppressive therapy conditions, while CD3+ T cell and CD4+ T cell counts decreased by day 14 after LT but returned to preoperative levels by day 21.
Systemic inflammation and immune deficiency are hallmark features of ACLF. Consistent with previous studies, our results indicated that patients with ACLF exhibited significantly elevated WBC and neutrophil counts. Both pathogen-associated molecular patterns and damage-associated molecular patterns play an important role in initiating and perpetuating systemic inflammation, progressing to a cytokine storm marked by the excessive release of proinflammatory mediators. Consequently, a large proportion of patients with ACLF fulfill the criteria for systemic inflammatory response syndrome[12]. This immunological profile supports the rationale to include immune-related parameters in prognostic scoring systems to enhance the accuracy of predicting outcomes. As an example, the CLIF-C ACLF score incorporates the WBC count as an immunological marker and offers superior prognostic accuracy compared with the CLIF-OF score, which is based solely on OF assessment[13]. Similarly, the COSSH-II score, which includes the neutrophil count, predicts short-term mortality in patients with HBV-ACLF more accurately than the original COSSH-I score[14].
The progression of uncontrolled inflammation in ACLF leads to a disruption of systemic immune homeostasis and triggers a compensatory anti-inflammatory response[15]. Persistent stimulation by pathogen-associated molecular patterns mechanistically leads to the breakdown of endotoxin tolerance and ultimately drives the transition to an immune paralysis state that is accompanied by quantitative and functional alterations in multiple immune cell populations[7]. For example, when the phagocytic function of neutrophils is impaired, the sustained formation of neutrophil extracellular traps further reduces the activity of antigen-presenting cells. Macrophage populations are also altered in ACLF. Our team previously utilized single-cell sequencing technology to determine that monocyte-derived macrophages in the livers of patients with ACLF expressed high levels of triggering receptor on myeloid cells 2. These macrophages highly expressed anti-inflammatory molecules and contributed to the hepatic anti-inflammatory environment[16]. Moreover, reduced levels of hepatic dendritic cells have been associated with poor prognosis in patients with ACLF[17]. In addition to these innate immune alterations, our study focused particularly on the profile of PBLSs in patients with ACLF. We observed a decrease in all PBLS levels in patients with ACLF, indicating a widespread impairment in adaptive immune responses. Additional investigations into these lymphocyte populations hold significant importance for understanding the mechanisms underlying immune dysfunction in ACLF.
Among the PBLSs, we observed a significant reduction in NK cell numbers in patients with ACLF compared with patients with compensated cirrhosis. Previous studies primarily focused on NK cells within the liver where their numbers exceeded those in peripheral blood[18]. Dysfunction of hepatic NK cells were shown to significantly increase susceptibility to infections in patients with CLIF, thereby impairing their immune defense against pathogens[19]. In the ACLF context, activation of hepatic NK cells was reported to trigger gasdermin-D-dependent hepatocyte pyroptosis, contributing to ACLF development[20]. There is a possibility that circulating NK cells migrate to the liver and promote ACLF progression. Regardless, our findings highlight the need for further investigation into the role of peripheral blood NK cells in the pathogenesis of ACLF.
Intriguingly, ACLF severity, etiology, and common liver failure scores all did not correlate with the PBLS levels. A possible explanation is that immune deficiency is a fundamental feature of ACLF that is independent of dysfunction in other organ systems. Further research should determine the potential of immune dysfunction as an independent marker in the diagnostic criteria for ACLF.
Furthermore, after adjusting for potential confounding factors, preoperative PBLS levels were not significantly associated with prognosis, indicating that outcomes after LT are influenced more by the therapeutic benefits of LT and comprehensive perioperative management. Previous investigations have demonstrated that the 28-day mortality rates for ACLF-1, ACLF-2, and ACLF-3 without LT are 18.2%, 41.7%, and 91.8%, respectively[21]. LT is one of the most effective treatment options for patients with ACLF. The short-term survival rate after LT in our study was 86.0% with greater survival benefits for patients with ACLF-1 or ACLF-2. Therefore, we hypothesize that PBLSs are associated with the natural prognosis of ACLF in patients who do not undergo LT. Existing evidence has shown a relationship between reduced CD8+ T cell counts and increased short-term mortality in patients with ACLF[22]. Our recent work also confirmed the decrease of NK cells and CD8+ T cells in ACLF patients through single-cell multimodal analysis. More importantly, NK cells and CD8+ T cells were found to be further decreased in patients with progression but increased in patients with recovery[23]. Because of the limited number of available organs for transplantation, PBLSs may serve as valuable biomarkers to prioritize the LT wait list and maximize the survival benefits for recipients.
Currently, the standard immunosuppressive regimen for LT includes the perioperative administration of high-dose corticosteroids and basiliximab induction and long-term use of immunosuppressive drugs to prevent rejection. Even under these conditions we observed a significant rebound in NK cells and B cells in patients with ACLF after LT. These findings underscored the profound state of immunosuppression present prior to transplantation and highlighted the great capacity of the immune system for reconstitution following LT. Moreover, basiliximab and tacrolimus primarily inhibit the proliferation and activation of CD4+ T cells, and we noted a marked decrease in CD4+ T cell counts at day 14 after LT. By day 21 after LT, CD4+ T cell counts returned to preoperative levels, demonstrating that the immune system is capable of reconstitution in patients with ACLF. We postulate that dynamic monitoring of PBLSs at multiple preoperative time points warrants further investigation because they could provide a sensitive assessment of disease progression and immune status fluctuations, facilitate the identification of the optimal transplantation window, and ultimately contribute to improved patient outcomes.
Despite the novel results observed in our study, potential limitations must be acknowledged. As a single-center retrospective study, the sample size was relatively small, the PBLS recovery data were not complete, and external validation was lacking. Additionally, the assessment of lymphocytes was limited to quantification of each subset without a detailed evaluation of their functional characteristics. Future research should integrate both the quantitative and functional dynamics of PBLSs to develop a comprehensive immunological profile that will provide deeper insights into the pathophysiology of ACLF.
Significant impairment of PBLSs is a hallmark characteristic of immune dysfunction in ACLF with NK cells potentially linked to the progression of compensated cirrhosis to liver failure. Rapid reconstitution of NK cells and B cells after LT represents a key indicator of immune reconstitution in ACLF.
| 1. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (2)] |
| 2. | Zhang Y, Tan W, Wang X, Zheng X, Huang Y, Li B, Meng Z, Gao Y, Qian Z, Liu F, Lu X, Shi Y, Shang J, Yan H, Zheng Y, Zhang W, Gu W, Qiao L, Deng G, Zhou Y, Hou Y, Zhang Q, Xiong S, Liu J, Duan L, Chen R, Chen J, Jiang X, Luo S, Chen Y, Jiang C, Zhao J, Ji L, Mei X, Li J, Li T, Zheng R, Zhou X, Ren H, Cheng X, Guo L, Li H; Chinese (Acute on) Chronic Liver Failure Consortium (Ch-CLIF. C). Metabolic biomarkers significantly enhance the prediction of HBV-related ACLF occurrence and outcomes. J Hepatol. 2023;79:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Laleman W, Claria J, Van der Merwe S, Moreau R, Trebicka J. Systemic Inflammation and Acute-on-Chronic Liver Failure: Too Much, Not Enough. Can J Gastroenterol Hepatol. 2018;2018:1027152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2258] [Article Influence: 173.7] [Reference Citation Analysis (6)] |
| 5. | Hensley MK, Deng JC. Acute on Chronic Liver Failure and Immune Dysfunction: A Mimic of Sepsis. Semin Respir Crit Care Med. 2018;39:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Berres ML, Schnyder B, Yagmur E, Inglis B, Stanzel S, Tischendorf JJ, Koch A, Winograd R, Trautwein C, Wasmuth HE. Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 2009;29:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM, Matern S, Lammert F. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol. 2005;42:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 404] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 8. | Yu X, Yang F, Shen Z, Zhang Y, Sun J, Qiu C, Zheng Y, Zhao W, Yuan S, Zeng D, Zhang S, Long J, Zhu M, Zhang X, Wu J, Ma Z, Zhu H, Su M, Xu J, Li B, Mao R, Su Z, Zhang J. BTLA contributes to acute-on-chronic liver failure infection and mortality through CD4(+) T-cell exhaustion. Nat Commun. 2024;15:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 9. | Yao J, Ji Y, Liu T, Bai J, Wang H, Yao R, Wang J, Zhou X. Single-Cell RNA Sequencing Shows T-Cell Exhaustion Landscape in the Peripheral Blood of Patients with Hepatitis B Virus-Associated Acute-on-Chronic Liver Failure. Gut Liver. 2024;18:520-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Al-Freah MAB, McPhail MJW, Dionigi E, Foxton MR, Auzinger G, Rela M, Wendon JA, O'Grady JG, Heneghan MA, Heaton ND, Bernal W. Improving the Diagnostic Criteria for Primary Liver Graft Nonfunction in Adults Utilizing Standard and Transportable Laboratory Parameters: An Outcome-Based Analysis. Am J Transplant. 2017;17:1255-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 923] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 12. | Choudhury A, Kumar M, Sharma BC, Maiwall R, Pamecha V, Moreau R, Chawla YK, Duseja A, Mahtab M, Rahman S, Hamid SS, Butt AS, Jafri W, Tan SS, Devarbhavi H, Amarapurkar D, Ning Q, Eapen CE, Goel A, Kim DJ, Ghazinyan H, Shiha G, Lee GH, Abbas Z, Payawal DA, Dokmeci AK, Yuen MF, Lesmana LA, Sood A, Chan A, Lau GK, Jia JD, Duan Z, Yu C, Yokosuka O, Jain P, Bhadoria AS, Kumar G, Sarin SK; APASL ACLF working party. Systemic inflammatory response syndrome in acute-on-chronic liver failure: Relevance of 'golden window': A prospective study. J Gastroenterol Hepatol. 2017;32:1989-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Hareesh GJ, Ramadoss R. Clinical Profile, Short-term Prognostic Accuracies of CLIF-C ACLF Score and Serial CLIF-C OF Scores in Acute-on-chronic Liver Failure Patients: A Prospective Observational Study. Indian J Crit Care Med. 2024;28:126-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Zhou X, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 15. | Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617-625, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | Peng B, Li H, Liu K, Zhang P, Zhuang Q, Li J, Yang M, Cheng K, Ming Y. Intrahepatic macrophage reprogramming associated with lipid metabolism in hepatitis B virus-related acute-on-chronic liver failure. J Transl Med. 2023;21:419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 17. | Zhao J, Zhang JY, Yu HW, He YL, Zhao JJ, Li J, Zhu YK, Yao QW, Wang JH, Liu HX, Shi SY, Zou ZS, Xu XS, Zhou CB, Wang FS, Meng QH. Improved survival ratios correlate with myeloid dendritic cell restoration in acute-on-chronic liver failure patients receiving methylprednisolone therapy. Cell Mol Immunol. 2012;9:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 757] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 19. | Liu F, Duan X, Wan Z, Zang H, You S, Yang R, Liu H, Li D, Li J, Zhang Y, Xin S. Lower number and decreased function of natural killer cells in hepatitis B virus related acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol. 2016;40:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Zhao Q, Chen DP, Chen HD, Wang YZ, Shi W, Lu YT, Ren YZ, Wu YK, Pang YH, Deng H, He X, Kuang DM, Guo ZY. NK-cell-elicited gasdermin-D-dependent hepatocyte pyroptosis induces neutrophil extracellular traps that facilitate HBV-related acute-on-chronic liver failure. Hepatology. 2025;81:917-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C, Solís-Munoz P, Saliba F, Zeuzem S, Albillos A, Benten D, Montero-Alvarez JL, Chivas MT, Concepción M, Córdoba J, McCormick A, Stauber R, Vogel W, de Gottardi A, Welzel TM, Domenicali M, Risso A, Wendon J, Deulofeu C, Angeli P, Durand F, Pavesi M, Gerbes A, Jalan R, Moreau R, Ginés P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 493] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 22. | Li J, Hu CH, Chen Y, Zhou MM, Gao ZJ, Fu MJ, Wang J, Li JZ, Chen TY, Zhao YR, He YL. Characteristics of Peripheral Lymphocyte Subsets in Patients With Acute-On-Chronic Liver Failure Associated With Hepatitis B. Front Med (Lausanne). 2021;8:689865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Liang X, Luo J, Zhou Q, Xin J, Li J, Peng B, Hu M, Jiang J, Qiang W, Li P, Chen P, Yao H, Zhang H, Zhou X, Chen J, Hu W, Li B, Ma S, Wu X, Li X, Zhang J, Cheng J, Liu S, Fu X, Lu Y, Ming Y, Chen X, Shi D, Li J. Single-cell multimodal analysis reveals the dynamic immunopathogenesis of HBV-ACLF progression. Gut. 2025;gutjnl-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |