Published online Nov 21, 2025. doi: 10.3748/wjg.v31.i43.111358
Revised: September 14, 2025
Accepted: October 21, 2025
Published online: November 21, 2025
Processing time: 144 Days and 22.9 Hours
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s di
Core Tip: This review examines pyroptosis as a pivotal mechanism in inflammatory bowel disease (IBD) pathogenesis, driving inflammation and tissue damage. It evaluates therapeutic strategies targeting pyroptosis pathways, emphasizing their potential to modulate disease progression. Key challenges including drug specificity, delivery, and safety are analyzed, alongside opportunities for combination therapies. The discussion highlights the need for precision approaches and improved translational models to advance pyroptosis-targeted treatments for IBD. Future directions focus on overcoming current limitations to optimize clinical outcomes.
- Citation: Dong WW, Liu T, He LX, He WT. Targeting pyroptosis in inflammatory bowel disease: A potentially effective therapeutic approach. World J Gastroenterol 2025; 31(43): 111358
- URL: https://www.wjgnet.com/1007-9327/full/v31/i43/111358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i43.111358
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), represents a chronic intestinal disorder with escalating global health implications. Known as the “green cancer” for its refractory nature and propensity for lifelong recurrence, IBD imposes substantial morbidity and socioeconomic burdens. IBD is undergoing a dramatic epidemiological shift globally. Although the incidence of IBD has stabilized in early-industrialized countries, the prevalence continues to rise, with more than 1% of their populations projected to be affected within the next decade. In newly industrialized countries such as China, Malaysia, and Brazil, IBD incidence is accelerating, with annual rates ranging from 3.3 to 10.6 per 100000 people. This upward trend is expected to persist over the next two decades. The shift is closely associated with environmental triggers linked to urbanization and the adoption of Westernized diets. Consequently, IBD has transitioned from being considered a “Western disease” to a genuinely global health challenge[1]. This accelerating global burden underscores the imperative for innovative therapeutic paradigms.
The management of IBD continues to face significant challenges. Although the introduction of biologics [e.g., anti-tumor necrosis factor (TNF) agents, anti-integrins, and anti-interleukin (IL)-12/23 therapies] and small-molecule drugs [e.g., Janus tyrosine kinase (JAK) inhibitors] has markedly improved patient outcomes, therapeutic efficacy exhibits substantial interindividual variability. Approximately 30%-50% of patients fail to respond to initial treatment (primary nonresponse), while only about 40% of those who initially respond maintain clinical remission for one year. Furthermore, a “ceiling effect” of current therapies exists, with only a subset of patients achieving mucosal healing or long-term ste
Pyroptosis, a lytic cell death mechanism mediated by gasdermin family proteins, has emerged as a pivotal driver of IBD pathogenesis. It acts upstream of the inflammatory cascades targeted by current therapies, addressing the critical cell death events they fail to sufficiently inhibit. This programmed cell death process is typically triggered by inflammasomes and executed by gasdermin proteins, leading to characteristic features such as cell swelling, membrane pore formation, and the release of cellular contents. Pyroptosis is essential for host defense, but its dysregulation can trigger persistent inflammation and contributing to the progression of inflammatory diseases[3]. This detrimental role is starkly highlighted in IBD. Growing evidence demonstrates that aberrant activation of the gasdermin-mediated pyroptotic pathway contributes to intestinal epithelial barrier dysfunction, excessive inflammation, and disease progression. However, the precise regulatory mechanisms and therapeutic potential of targeting pyroptosis in IBD remain incompletely understood.
This review first outlines the molecular mechanisms of pyroptosis and its role in IBD development. It then analyzes the clinical evidence connecting pyroptosis to IBD features and finally evaluates emerging therapies, ranging from small-molecule inhibitors to combination strategies. By integrating preclinical and clinical data, we highlight challenges in drug development while proposing future directions for precision IBD treatment.
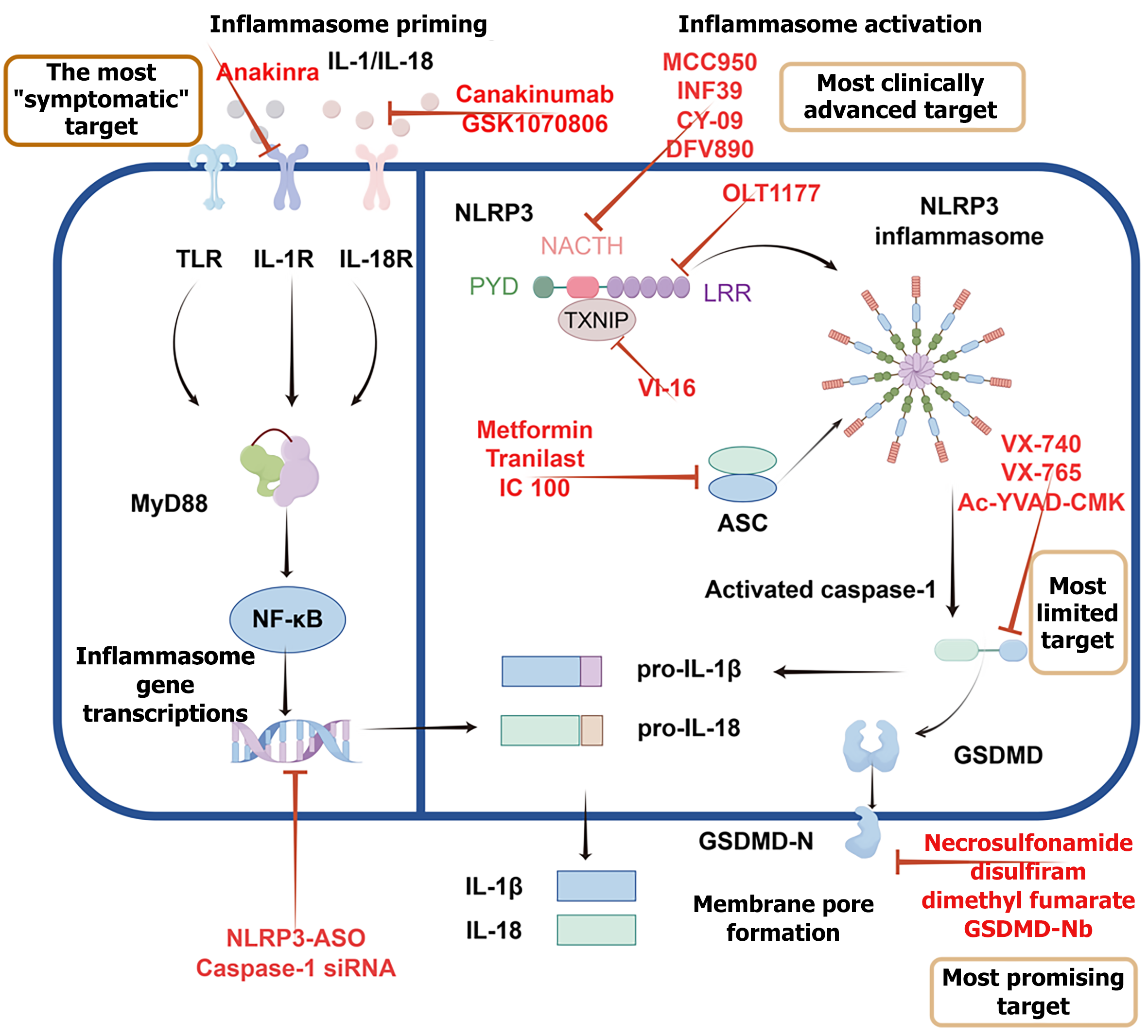
The nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3)-caspase-1-gasdermin D (GSDMD) pathway is the most extensively studied pyroptosis pathway. In the classical pathway, the process begins when Toll-like receptor 4 (TLR4) and NLRP3 detect pathogen-associated molecular patterns [e.g., bacterial lipopolysaccharide (LPS)], damage-associated molecular patterns (e.g., extracellular adenosine triphosphate), or homeostasis-altering molecular processes (e.g., lysosomal rupture). TLR4 then initiates nuclear factor kappa-B (NF-κB)-dependent transcriptional upregulation of NLRP3 and pro-IL-1β. Following activation, NLRP3 oligomerizes and recruits the adapter protein apoptosis-associated speck-like protein containing a CARD (ASC), forming a large multiprotein complex known as the NLRP3 inflammasome. ASC subsequently recruits and activates caspase-1. Within the inflammasome, the precursor of caspase-1 undergoes autocatalytic cleavage to form activated caspase-1, which then exerts protease activity by cleaving multiple substrates, including the precursors of IL-1β and IL-18 (pro-IL-1β and pro-IL-18), as well as GSDMD. Cleavage of GSDMD releases its N-terminal fragment, which oligomerizes and interacts electrostatically with anionic phospholipids (like phosphatidylserine) in the plasma membrane, forming transmembrane pores. These pores disrupt osmotic balance and facilitate the release of mature IL-1β and IL-18 into the extracellular environment. Once released, these cytokines act to amplify the inflammatory response, recruit immune cells and enhance the host’s defense against infection[4] (Figure 1).
The non-canonical pyroptosis pathway can be activated through an atypical inflammasome pathway. Caspase-11 in mice (caspase-4/5 in humans) can directly recognize intracellular bacterial LPS and subsequently cleave GSDMD, leading to pyroptosis. This pathway does not rely on traditional inflammasomes[5] (Figure 1). Emerging evidence indicates that caspase-8 serves as a critical regulator of pyroptosis under specific pathological conditions, such as infection with Yersinia or cellular stress[6]. Notably, caspase-8 can be recruited to atypical inflammasome complexes and directly cleave both GSDMD and gasdermin E (GSDME), thereby triggering lytic cell death. Recent studies further reveal that caspase-8-mediated pyroptosis depends on non-canonical signaling pathways, including the cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes axis and unconventional inflammasomes such as NLRP12-ASC[7,8].
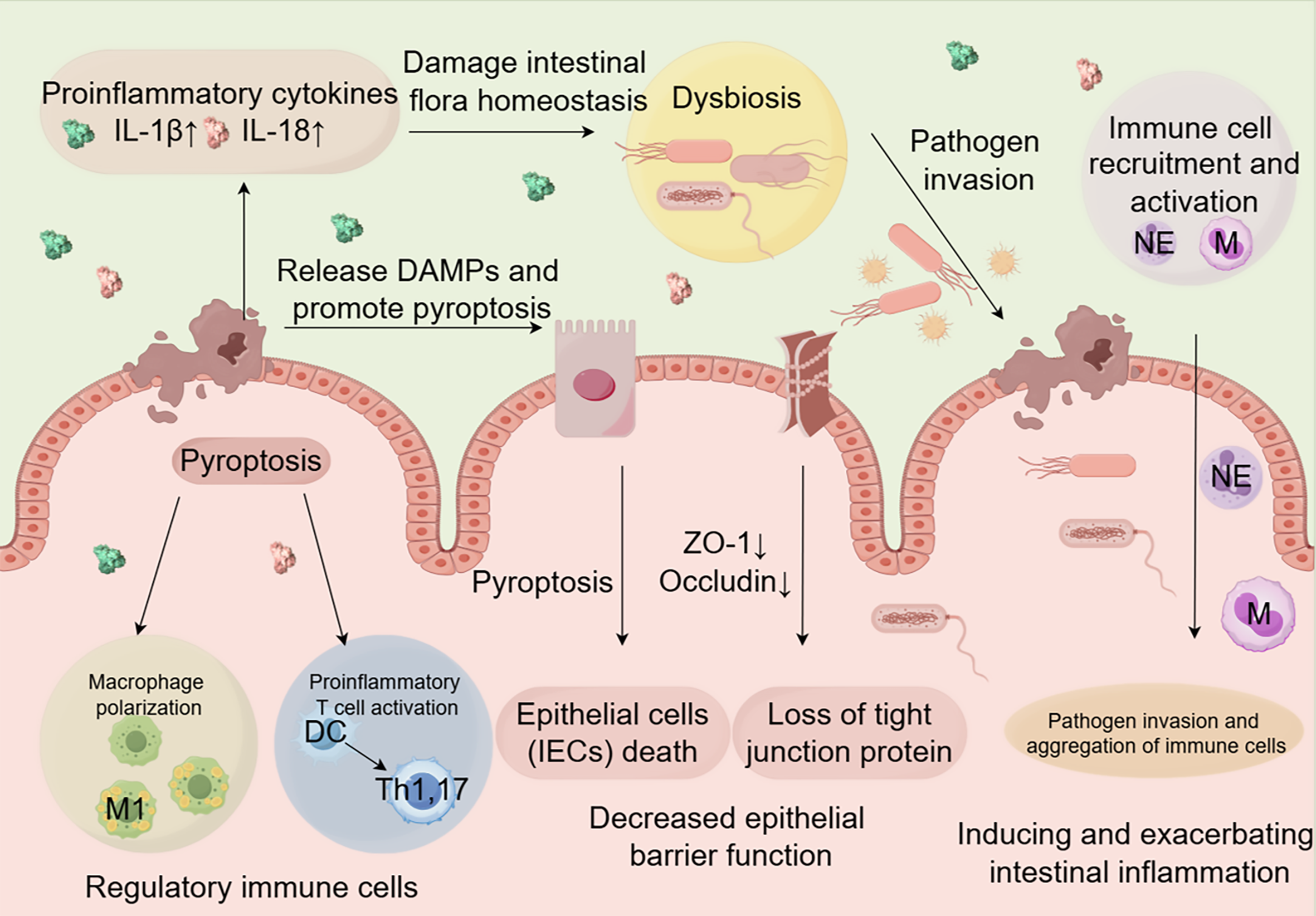
IBD is primarily characterized by intestinal inflammation and epithelial damage. Although its exact etiology is unknown, IBD is thought to originate from a complex interaction of genetic susceptibility and environmental factors that trigger an aberrant immune response[9]. A growing body of evidence indicates that pyroptosis significantly contributes to IBD pathogenesis[10-12]. Emerging research now positions pyroptosis as a central driver of intestinal inflammation through three interconnected mechanisms: Epithelial barrier disruption, immune dysregulation, and microbiota imbalance. Nevertheless, a more comprehensive understanding of the pathogenic role of pyroptosis in intestinal epithelial cells (IECs) is required to fully elucidate its impact on IBD progression.
The impact of pyroptosis on intestinal barrier function: The barrier formed by IECs is crucial for preventing the in
Regulation of intestinal immune cells by pyroptosis: Pyroptosis significantly influences the development of IBD by modulating immune responses. The activation of inflammasomes such as NLRP3 and absent in melanoma 2 (AIM2) during pyroptosis triggers the production and release of pro-inflammatory cytokines, particularly IL-1β and IL-18[4]. These cytokines enhance intestinal inflammation by promoting the recruitment and activation of immune cells (Figure 2)[21]. Pyroptosis-related cytokines also regulate macrophage polarization (Figure 2). IL-1β, for example, promotes the differentiation of macrophages toward pro-inflammatory M1 phenotypes, which release additional inflammatory mediators and exacerbate intestinal damage[22]. Consistent with this, studies using the dextran sulfate sodium (DSS)-induced colitis model have shown that inhibiting pyroptosis signaling pathways suppresses M1 polarization and ameliorates colitis symptoms[23-25]. Furthermore, pyroptosis shapes the intestinal immune microenvironment by modulating interactions between dendritic cells (DCs) and T cells (Figure 2). Pyroptosis-stimulated DCs exhibit enhanced antigen presentation capacity, promoting the activation and expansion of pro-inflammatory T cell subsets such as Th1 and Th17 cells, which contribute to chronic inflammation in IBD[26]. Concurrently, the pyroptosis-induced inflammatory milieu can impair regulatory T cell function, further disrupting immune homeostasis[27]. A recent study revealed that the NLRP3/IL-1β pathway regulates the activation and effector functions of γδT17 cells in UC patients[28]. Inhibition of this pathway significantly reduces Th17 cell differentiation, decreases levels of pro-inflammatory factors in the colons of DSS-induced mice, and ameliorates colitis symptoms[29,30]. The immune activation orchestrated by pyroptosis not only aggravates tissue inflammation but also disrupts the symbiotic relationship with the gut microbiota, shaping a dysbiotic microenvironment.
The impact of pyroptosis on intestinal microbiota homeostasis: The inflammatory response induced by pyroptosis can alter the composition and diversity of the intestinal microbiota (Figure 2). In DSS-induced IBD animal models and IBD patients, the gut microbiota often exhibits a dysbiotic state characterized by a decrease in beneficial bacteria and an increase in harmful bacteria[31,32]. Mechanistically, pyroptotic IECs release nucleotides and amino acids that selectively fuel the expansion of enteric pathogens, such as Salmonella[33]. Therapeutic modulation of this crosstalk is achievable: The NLRP3 inhibitor MCC950 restores microbial homeostasis by increasing the Firmicutes/Bacteroidetes ratio, thereby counteracting inflammation-associated dysbiosis[31].
NLRP3: The NLRP3 inflammasome, a cytosolic multiprotein complex comprising NLRP3, ASC, and caspase-1, functions as a pattern recognition receptor central to the innate immune system. It mediates caspase-1 activation and the secretion of pro-inflammatory cytokines IL-1β and IL-18 in response to microbial infections and cellular damage[3,4]. The normal expression of NLRP3 is crucial for the host’s innate defense against bacterial, fungal, and viral infections[3,11]. However, its dysregulation is associated with the pathogenesis of many inflammation-related diseases, including Alzheimer’s disease, diabetes, and atherosclerosis[34]. The NLRP3 inflammasome is considered a key mechanism of intestinal inflammation in the DSS colitis model[10]. Selective small-molecule inhibitors of NLRP3, such as MCC950 and OLT1177, have shown promising therapeutic potential in IBD animal models[15,35]. Targeting the regulation of NLRP3 inflammasome activation offers new avenues and strategies for the treatment of IBD.
Caspase protein family: Caspase-1, the inflammatory caspase, exacerbates IBD through GSDMD-dependent pyroptosis and IL-1β/IL-18 hyperactivation[4,10]. Preclinical studies demonstrate that caspase-1 inhibitor, belnacasan (VX-765), reduces weight loss, colon shortening, and colonic pathological damage in DSS-induced colitis mice[36]. Non-canonical caspases (human caspase-4/5, murine caspase-11) are critical components of the innate immune pathway. They can sense and respond to intracellular LPS, and also trigger pyroptosis by inducing cell death, leading to a potent inflammatory response. In patients with UC and CD exhibit upregulated caspase-4/5, mirroring caspase-11 overexpression observed in DSS-induced colitis models[5,37]. Inhibition of caspase-11/4-induced macrophage pyroptosis has been shown to alleviate colitis in mice[38]. Caspase-11 not only drives inflammation but may also paradoxically maintain intestinal barrier integrity and immune homeostasis under specific conditions, highlighting its complex dual role in IBD[39]. Furthermore, apoptotic caspases caspase-3/8 have been implicated in mediating pyroptosis through the activation of GSDME[7,40,41].
GSDM protein family: In the intestinal epithelium, gasdermin family proteins play pivotal but divergent roles in IBD. GSDMD drives pyroptosis through caspase-mediated pore formation to amplify intestinal inflammation and, beyond this role, also mediates a novel nonpyroptotic pathway for IL-1β release via small extracellular vesicles in IBD[12]. Pharmacological inhibition of GSDMD attenuates this inflammatory response[42,43]. GSDME, another gasdermin member, similarly executes pyroptosis upon caspase-3/8 activation and exacerbates IBD progression by disrupting the epithelial barrier; studies show that targeting GSDME-mediated pyroptosis mitigates mucosal damage in IBD models[40,42,43]. In contrast, gasdermin B (GSDMB) is genetically associated with IBD but primarily promotes epithelial repair and regeneration instead of causing pyroptosis. According to Zhu et al[44] GSDMB generally does not trigger pyroptosis and may aid mucosal healing by improving cell migration and repair. Although it can be cleaved by some caspases under specific conditions, this usually does not result in pyroptosis and may affect other immune responses. This functional duality presents a therapeutic challenge: Inhibiting GSDMD or GSDME to reduce damaging inflammation may in
Dual challenges in targeting pyroptosis: Targeting key molecular components of pyroptosis, such as NLRP3, caspases, and GSDMD, represents a promising yet challenging therapeutic strategy for IBD. However, the dual roles of certain molecules introduce considerable complexity into treatment design. For instance, caspase-11 contributes not only to inflammatory responses but also to the maintenance of intestinal homeostasis[39]. Similarly, while GSDMD and GSDME primarily promote inflammation, GSDMB exhibits protective functions in epithelial repair[44]. Moreover, systemic targeting of pyroptosis carries broader safety concerns, including an increased risk of infections due to suppressed pathogen clearance and potential off-target effects that may lead to unintended immune modulation[3,11,45]. Therefore, a nuanced therapeutic approach is essential to selectively inhibit detrimental pathways without compromising protective mechanisms.
To enable such precision, the development and implementation of pyroptosis-specific biomarkers are critical. Promising candidates include: (1) Executor markers, such as circulating GSDMD-N, which correlates with disease activity in UC and allows for non-invasive monitoring[46]; (2) Effector markers, including cytokines IL-1β and IL-18 that are directly released during pyroptosis[47]; and (3) Transcriptomic markers (e.g., IL1B, GZMB), which are upregulated in active UC and are associated with macrophage pyroptosis[48]. By identifying pyroptosis-driven patient subgroups and monitoring treatment responses, these biomarkers can help reduce off-target risks and guide personalized interventions, advancing precision IBD medicine.
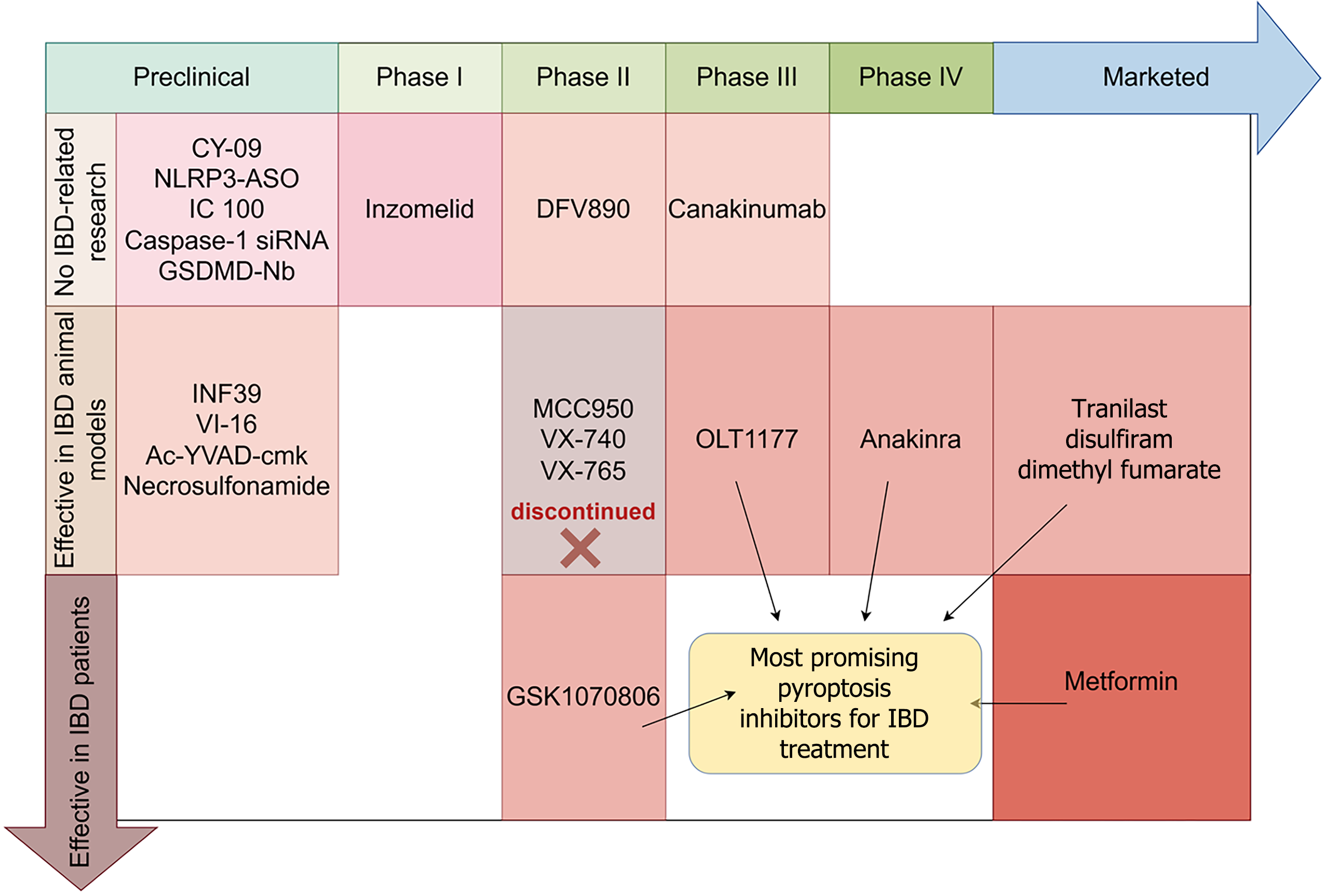
The pathological hyperactivation of pyroptosis in both IBD animal models and patients underscores its potential as a therapeutic target. Small-molecule inhibitors and repurposed drugs targeting key pyroptotic nodes have demonstrated anti-inflammatory efficacy in preclinical and clinical studies (Table 1).
| Name | Target protein | Pharmacological mechanism | IBD disease model | Clinical stage | Ref. |
| MCC950 (CP-456773) | NLRP3 | Binds to the NACHT domain of NLRP3, preventing ATP hydrolysis and oligomerization | Winnie spontaneous colitis, DSS-induced acute colitis | Phase II discontinued (rheumatoid arthritis) | Perera et al[15]; Wang et al[31]; Coll et al[49]; Saber et al[50]; Saber and El-Kader[51]; Li et al[52]; Liu et al[53] |
| OLT1177 (dapansutrile) | NLRP3 | Binds NLRP3 LRR domain, blocks NLRP3 inflammasome assembly | DSS-induced acute colitis | Phase II completed (osteoarthritis; No. NCT02104050); phase III planned (gout; No. NCT05658575) | Oizumi et al[35]; Marchetti et al[54]; Saber et al[55] |
| INF39 | NLRP3 | Irreversibly inhibits NLRP3 ATP activity | DNBS-induced colitis | Preclinical | Shi et al[56]; Pellegrini et al[57] |
| VI-16 | TXNIP-NLRP3 | Disrupts TXNIP-NLRP3 interaction, reduces oxidative stress | DSS-induced colitis, LPS-stimulated THP-1/BMDM | Preclinical | Zhao et al[58] |
| CY-09 | NLRP3 | Binds NLRP3 NACHT domain to block ATP activity | LPS-stimulated BMDM | Preclinical | Jiang et al[59] |
| VX-740 (pralnacasan) | Caspase-1 | Reversible competitive caspase-1 inhibitor | DSS-induced acute colitis | Phase II discontinued (rheumatoid arthritis) | Bauer et al[60]; Dhani et al[61] |
| VX-765 (belnacasa) | Caspase-1 | Prodrug of VRT-043198, a reversible caspase-1 inhibitor | DSS-induced acute colitis | Phase II discontinued (epilepsy; No. NCT01501383) | Wang et al[36]; Dhani et al[61] |
| Ac-YVAD-CMK | Caspase-1 | Irreversible covalent inhibitor (chloromethylketone modification of Cys285) | DNBS-induced colitis | Preclinical | Pellegrini et al[57] |
| Necrosulfonamide | GSDMD | Alkylates GSDMD to prevent pore formation | DSS-induced acute colitis | Preclinical | Rathkey et al[62]; Yang et al[63] |
| Metformin | AMPK/NLRP3 | AMPK activation inhibits NLRP3 inflammasome assembly | DSS-induced acute colitis | Marketed (T2DM); IBD trials ongoing (No. NCT05574387) | Hosseini et al[64]; Cao et al[65]; El-Haggar et al[66] |
| Tranilast | NLRP3 | Inhibits NLRP3 oligomerization and ASC speck formation | DSS/TNBS-induced colitis | Marketed (anti-allergic) | Huang et al[67]; Seto et al[68]; Oshitani et al[69] |
| Disulfiram | GSDMD | Covalent modification of GSDMD to inhibit pore formation | DSS/TNBS-induced colitis | Marketed (alcohol addiction) | Chi et al[70]; Lei et al[71]; Zhou et al[72]; Ou et al[73]; Luo et al[74] |
| Dimethyl fumarate | GSDMD | Covalent modification of GSDMD to inhibit pore formation | DSS-induced colitis | Marketed (multiple sclerosis) | Humphries et al[75]; Li et al[76]; Patel et al[77]; Buscarinu et al[78]; Shah et al[79] |
Development of NLRP3 inhibitors: MCC950 (CP-456773) is a selective NLRP3 inhibitor that targets the NLRP3 ade
OLT1177 (dapansutrile) is a clinically safe, selective NLRP3 inflammasome inhibitor that binds the NLRP3 leucine-rich repeats domain, preventing ASC oligomerization and inflammasome assembly without altering NLRP3 expression or adenosine triphosphate activity[54]. In IBD models, it reduces IL-1β and protects intestinal barrier integrity in DSS-induced colitis[35], and exhibits synergistic efficacy when combined with the P2X7 antagonist brilliant blue G via dual suppression of NLRP3 and MyD88/NF-κB pathways[55]. With a favorable clinical safety profile, OLT1177 has completed phase I (No. NCT02134964) and phase II osteoarthritis trials (No. NCT02104050), and is currently in phase III for gout (No. NCT05658575). Its robust preclinical data, pharmacokinetic properties, and distinct mechanism free from hepatotoxicity risks associated with analogs like MCC950 support its promise as a next-generation IBD therapeutic. Further human studies in IBD are warranted.
The acrylate derivative INF39 irreversibly inhibits NLRP3 by covalently modifying Cys409 in its NACHT domain. Administered at 25 mg/kg, it completely suppresses dinitrobenzene sulfonic acid-induced colitis with no observed toxicity, due to its rapid plasma clearance[56,57]. In contrast, the flavonoid-based VI-16 employs a novel redox me
Limitations of caspase-1 inhibitors: Inhibition of caspase-1 by VX-740 and VX-765 has been shown to attenuate DSS-induced colitis in mice[36,60]. However, both compounds face challenges related to metabolic instability and hepatotoxicity. Structurally, they incorporate a difluorophenol warhead that enables potent caspase-1 binding but is susceptible to extensive glucuronidation, leading to poor metabolic stability. These pharmacokinetic drawbacks have significantly limited their clinical translation. Specifically, VX-740 was discontinued in phase II trials for rheumatoid arthritis due to hepatotoxicity and observations of hepatic fibrosis in primate studies. Similarly, VX-765 failed to achieve efficacy endpoints in a clinical trial for epilepsy (No. NCT01501383), despite evidence of sufficient target engagement[61] (Figure 3). Ac-YVAD-CMK (caspase-1 inhibitor II) irreversibly inhibits caspase-1, reducing IL-1β and myeloperoxidase (MPO) activity in colitis models, though its effects on downstream cytokines remain limited compared to NLRP3 or IL-1-targeted therapies[57].
Pyroptosis execution protein inhibitors: Necrosulfonamide (NSA) represents a mechanistically distinct class of small-molecule pyroptosis inhibitors that directly targets the executioner protein GSDMD. This synthetic sulfonamide compound (molecular weight: 427.5 Da) exerts its therapeutic effects through covalent modification of human GSDMD at Cys191 within the N-terminal pore-forming domain, effectively blocking plasma membrane pore formation (half maximal inhibitory concentration = 1.8 μM)[62]. In DSS-induced murine colitis models, daily NSA administration attenuated disease severity, evidenced by improved disease activity index scores and reduced histopathological inflammation compared to controls. Beyond inhibiting GSDMD-mediated pyroptosis, NSA also directly suppresses MLKL to block necroptosis, reduces macrophage infiltration and T-cell activation, and helps maintain intestinal barrier integrity to prevent bacterial translocation. These pleiotropic immunomodulatory effects collectively contribute to the amelioration of IBD[63].
Small-molecule pyroptosis inhibitors demonstrate considerable therapeutic potential for IBD. Preclinical studies have shown that NLRP3 antagonists (e.g., MCC950, OLT1177) and GSDMD inhibitors (e.g., NSA) effectively attenuate colitis, restore microbial balance, maintain epithelial barrier integrity, and exert pleiotropic immunomodulatory effects. However, their clinical translation has encountered significant obstacles. Although NLRP3 inhibitor MCC950 and caspase-1 inhibitors VX-740/VX-765 exhibited robust efficacy in preclinical models, their development was halted due to safety and pharmacokinetic concerns. MCC950 was discontinued owing to hepatotoxicity, while the VX compounds failed because of metabolic instability resulting from rapid glucuronidation of their difluorophenol warhead[52,61]. These setbacks underscore a critical translational challenge: Strong target engagement and efficacy in animal models are insufficient to ensure clinical success. Major barriers include unforeseen off-target organ toxicity, inadequate predictive toxicity models, poor metabolic stability of compounds, and a lack of biomarkers for patient stratification. Therefore, future research should prioritize comprehensive safety profiling, structural optimization to improve metabolic stability, and biomarker-driven clinical trial designs to facilitate the successful application of pyroptosis inhibitors in IBD treatment.
Drug repurposing (accelerated clinical translation pathways): Beyond novel small-molecule inhibitors, several repurposed pharmacological agents targeting pyroptosis-related proteins have exhibited significant anti-inflammatory efficacy in both in vitro studies and experimental models of IBD.
Metformin exerts anti-inflammatory effects primarily through adenosine 5’-monophosphate-activated protein kinase-dependent inhibition of the NLRP3 inflammasome, leading to reduced IL-1β secretion, oxidative stress, NF-κB activation, and ASC speck formation[64,65]. It also enhances intestinal barrier integrity and attenuates pyroptosis via upregulation of UCP2 and NCF1, thereby suppressing reactive oxygen species[65]. Clinically, as an adjunct to mesalamine, metformin has been shown to improve Mayo scores, pain, inflammatory markers, and zonulin levels in UC patients in a randomized trial (No. NCT05553704)[66]. With an ongoing IBD clinical investigation (No. NCT05574387) and a well-established safety profile, metformin represents a promising repurposed therapeutic for UC.
Originally developed as an anti-allergic agent, tranilast (TL) has been repurposed as a selective NLRP3 inflammasome inhibitor that directly targets the NACHT domain to prevent oligomerization, demonstrating therapeutic potential across multiple NLRP3-driven conditions including gout and autoinflammatory diseases[67]. A study demonstrates that TL alleviates 2,4,6-trinitrobenzenesulfonic acid solution (TNBS)-induced experimental colitis by inhibiting MPO activity and inflammatory cell infiltration. However, pharmacokinetic analysis reveals that TL exhibits poor oral bioavailability (approximately 6.5%) in TNBS-induced colitis rats, suggesting its limited intestinal absorption may restrict clinical efficacy. These findings indicate the need to improve TL’s bioavailability for enhanced therapeutic outcomes[68]. Its clinical application for IBD awaits formal trials despite preliminary evidence of efficacy in CD cases[69].
Disulfiram, an anti-alcoholism drug repurposed for IBD, has demonstrated robust efficacy in preclinical studies by exerting pleiotropic effects. It not only directly inhibits GSDMD-mediated pyroptosis but also activates the nuclear respiratoty factor 2 (Nrf2)/heme oxygenase 1 antioxidant pathway via GSK-3β downregulation, elevating the glu
Dimethyl fumarate (DMF), an oral drug for relapsing multiple sclerosis, inhibits pyroptosis by succinylating cysteine C192 of GSDMD, thereby blocking its activation by inflammatory caspases[75]. In DSS-induced colitis models, DMF demonstrates broad efficacy by simultaneously activating Nrf2-mediated antioxidant pathways and suppressing inflammatory responses, and based on this mechanism, it shows promising potential in the treatment of gastrointestinal disorders such as gastric ulcers and UC[76,77]. A localized delivery strategy may reduce systemic exposure and mitigate adverse effects such as lymphopenia while preserving efficacy[77]. Although clinical studies in MS indicate benefits on gut barrier and microbiota[78,79], further research is needed to validate its efficacy and safety specifically in IBD contexts.
The repurposing of existing drugs such as metformin, TL, disulfiram, and DMF represents a promising approach to modulate pyroptosis in IBD, with each agent employing distinct mechanisms including NLRP3 inhibition, GSDMD suppression, or Nrf2 activation. However, the clinical application of these drugs faces several challenges: TL exhibits low oral bioavailability, disulfiram is associated with systemic side effects including the alcohol-disulfiram reaction, and DMF may lead to systemic adverse reactions such as lymphopenia. These limitations collectively underscore their short
Biologic therapies targeting pyroptosis offer precision intervention for IBD through monoclonal antibodies, nucleic acid-based agents, and engineered proteins, minimizing off-target effects compared to small molecules (Table 2).
| Name | Target protein | Pharmacological mechanism | Pyroptotic diseases | Clinical stage | Ref. |
| Canakinumab (ilaris) | IL-1β | IL-1β monoclonal antibody, inhibits NLRP3 downstream effects | Cryopyrin-associated periodic syndromes | Phase III completed (SJIA; No. NCT02396212) | Shaul et al[81]; Miyamoto et al[82] |
| Anakinra | IL-1 | Recombinant IL-1 receptor antagonist | Rheumatoid arthritis; Winnie-TNF-KO murine model (IBD) | Phase IV (RA; No. NCT00121043) | Liso et al[83]; Dogan et al[84]; Truyens et al[85] |
| GSK1070806 | IL-18 | Humanized monoclonal antibody against IL-18 | Rheumatoid arthritis; Crohn’s disease | Phase II (CD; No. NCT03681067) | Guha et al[86] |
| DFV890 | NLRP3 | Oral NLRP3 inhibitor (small molecule biologic) | Coronary heart disease; COVID-19 | Phase II (COVID-19; No. NCT04382053) | Shen et al[87]; Gatlik et al[88]; Madurka et al[89] |
| NLRP3-ASO | NLRP3 mRNA | Antisense oligonucleotide suppressing NLRP3 | Cryopyrin-associated periodic syndromes; Alzheimer’s disease | Preclinical | Kaufmann et al[90]; Braatz et al[91] |
| IC100 (ASC-mAb) | ASC speck | Monoclonal antibody blocking inflammasome assembly | Retinopathy of prematurity; Parkinson’s disease | Preclinical | de Rivero Vaccari et al[92]; Yuan et al[93]; Cyr et al[94] |
| Caspase-1 siRNA | Caspase-1 mRNA | siRNA silencing caspase-1 expression | Alzheimer’s disease | Preclinical | Han et al[95] |
| GSDMD-Nb | GSDMD | Nanobody binding GSDMD-N terminus to prevent pore formation | Sepsis | Preclinical | Schiffelers et al[96] |
Canakinumab is a selective, fully humanized monoclonal antibody targeting IL-1β, which has been successfully used in the treatment of various autoinflammatory diseases. Clinically approved for CAPS, it also shows potential in very early-onset IBD (VEO-IBD)[81]. A phase III trial in patients with systemic juvenile idiopathic arthritis (No. NCT02396212; Figure 3) demonstrated high efficacy, including 100% American College of Rheumatology pediatric (ACR pedi) 30 response at week 8 and 73.7% corticosteroid tapering by week 28. At week 48, ACR pedi 50/70/90/100 responses were achieved by 100.0%/100.0%/87.5%/68.8% of patients, respectively. However, the safety profile included frequent infections (271.6 per 100 patient-years) and serious adverse events in 42.1% of patients, with two suspected cases of macrophage activation syndrome though no deaths were reported. Importantly, the safety profile of canakinumab appears to vary across indications; while it has shown a confirmed long-term safety record in CAPS[82], its applications in IBD remain investigational, requiring biomarker-driven trials to identify optimal responders.
Anakinra, a recombinant IL-1 receptor antagonist targeting IL-1 signaling, is currently approved for rheumatoid arthritis with established phase IV clinical evidence (No. NCT00121043). Emerging evidence suggests its potential therapeutic role in IBD, particularly in specific subtypes characterized by IL-1 pathway dysregulation. Preclinical studies using the Winnie-TNF-knockout murine model demonstrated that anakinra ameliorates colonic inflammation in TNF-independent UC by blocking IL-1β-mediated effects[83]. Clinically, case reports highlight efficacy in IL-10 receptor β deficiency-associated IBD and refractory UC, yet controlled trials are needed to define its role[84,85].
GSK1070806, an investigational anti-IL-18 monoclonal antibody, is currently undergoing phase II clinical evaluation for CD (No. NCT03681067), demonstrating emerging therapeutic potential in IBD (Figure 3). Notably, this agent has shown clinical efficacy in treating VEO-IBD associated with IL-18opathy[86]. The therapeutic mechanism involves targeted neutralization of IL-18-mediated inflammatory signaling, which appears particularly relevant in certain monogenic IBD forms characterized by IL-18 pathway dysregulation. While these preliminary findings suggest promise for IL-18 inhibition in selected IBD populations, particularly pediatric-onset cases with specific autoinflammatory features, further clinical development is necessary to establish its safety profile, optimal dosing, and efficacy across broader IBD patient subgroups. The ongoing phase II trial in CD may provide more definitive evidence regarding its therapeutic potential in conventional IBD presentations.
DFV890 is a highly selective oral small-molecule NLRP3 inflammasome inhibitor that directly targets the NACHT domain of NLRP3 protein through its unique sulfonimidamide structure, effectively blocking inflammasome oligomerization and activation[87]. Preclinical studies demonstrate that DFV890 significantly inhibits caspase-1 activation and subsequent maturation/release of downstream IL-1β and IL-18, while showing superior selectivity for NLRP3 over other inflammasomes (e.g., AIM2 or NLRC4). In first-in-human trials, DFV890 exhibited favorable safety and tolerability profiles with ideal pharmacokinetic characteristics supporting once-daily dosing[88]. The compound has completed evaluation in a randomized, double-blind, placebo-controlled phase II clinical trial (No. NCT04382053) for coronavirus disease 2019-associated pneumonia, demonstrating superior clinical improvement, viral clearance, and ventilator-free survival, along with a favorable safety profile[89]. Although no clinical studies have yet been conducted for IBD, DFV890 shows promising therapeutic potential in IBD treatment based on the pivotal role of NLRP3 inflammasome. Future preclinical efficacy validation and biomarker-guided clinical studies are warranted to evaluate DFV890’s potential value for IBD therapy.
NLRP3-targeted antisense oligonucleotide (NLRP3-ASO) represents a novel therapeutic modality that specifically suppresses NLRP3 expression through the principle of Watson-Crick base pairing. Preclinical evidence supports the therapeutic potential of NLRP3-ASO in inflammatory diseases[90]. Kaufmann et al[90] showed that NLRP3-ASO treatment significantly reduced IL-1β expression and improved survival in mutant NLRP3 mice, establishing proof-of-concept for this approach in systemic autoinflammatory disorders, while Braatz et al[91] confirmed suppression of microglial activation in neuroinflammatory contexts. NLRP3-ASO remains a preclinical-stage intervention whose efficacy in IBD awaits experimental assessment.
The humanized IgG4 monoclonal antibody IC100 represents a novel therapeutic approach targeting the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), a critical adaptor protein in inflammasome assembly[92]. Current preclinical studies demonstrate its broad anti-inflammatory potential. In oxygen-induced retinopathy, IC100 significantly reduced pathological angiogenesis and inflammation[93], and in Parkinson’s disease models, it effectively attenuated neuroinflammation induced by α-synuclein aggregates[94]. While current research has focused on retinopathy and neurodegenerative applications, the demonstrated ability of IC100 to block multiple inflammasome pathways suggests potential utility in other inflammatory conditions, including possible future exploration in IBD.
A novel therapeutic approach using adeno-associated virus 9-delivered caspase-1 small interfering RNA (siRNA) demonstrated significant neuroprotective effects in APP/PS1 transgenic mice, a model of Alzheimer’s disease. The study revealed that β-amyloid-induced neuronal pyroptosis occurs through caspase-1 activation, establishing a new mechanism of neural injury in Alzheimer’s disease pathogenesis. By specifically silencing caspase-1 expression via siRNA, researchers observed decreased neuroinflammation and improved neuronal survival[95]. While currently in preclinical development for Alzheimer’s disease, this caspase-1 inhibition strategy may have broader implications for other caspase-1-dependent inflammatory conditions, pending further investigation.
Schiffelers et al[96] developed antagonistic nanobodies that specifically target the N-terminal domain of GSDMD, effectively inhibiting pore formation by blocking GSDMD oligomerization, thereby providing a novel therapeutic approach for inflammatory diseases such as sepsis. In LPS- or bacteria-induced septic mouse models, GSDMD-Nb demonstrated significant efficacy in reducing serum levels of IL-1β and IL-18, attenuating systemic inflammatory responses, and markedly improving survival rates. GSDMD-Nb demonstrates significant advantages over small-molecule inhibitors like disulfiram in terms of target specificity, mechanistic precision and sustained efficacy, albeit with higher development costs and unresolved delivery challenges.
A range of novel inhibitors including monoclonal antibodies, small molecule drugs, oligonucleotides, and nanobodies have demonstrated promising therapeutic potential in autoinflammatory diseases and IBD. These agents precisely target key nodes of the pyroptosis pathway and have shown significant efficacy in preclinical models and early clinical studies. However, current clinical evidence suggests that single-target therapies (e.g., canakinumab or anakinra), while effective in some patients, often face limitations due to disease heterogeneity, compensatory inflammatory pathway activation, or feedback mechanisms, in addition to safety concerns such as increased infection risk. These insights highlight the need for multi-target intervention strategies to achieve more robust and sustained anti-inflammatory effects in complex diseases like IBD. Promising approaches include the development of dual-target inhibitors (e.g., simultaneously inhibiting NLRP3 and caspase-1) to broadly suppress pyroptosis signaling and reduce compensatory activation, as well as rationally designed combination therapies incorporating pyroptosis inhibitors with mechanistically complementary agents such as anti-cytokine biologics (anti-TNF-α, anti-IL-23) or JAK inhibitors which may enhance efficacy in refractory cases and allow dose reduction to mitigate potential risks.
Strategic target selection in the pyroptosis pathway represents a critical translational bridge, guiding the rational application of the aforementioned therapeutic agents from small molecules and biologics to repurposed drugs and advanced delivery systems into clinically viable regimens. Selecting the best target in the pyroptosis pathway involves balancing scientific potential with clinical feasibility. Inhibiting the downstream protein GSDMD represents the most promising long-term strategy, as it blocks the final step of inflammation and avoids activation of alternative pathways. Currently, targeting upstream NLRP3 is the most clinically advanced approach, with safe and effective candidates like OLT1177 already in phase II trials, making it suitable for first-line use. Midstream caspases are less ideal targets due to pharmacokinetic issues, functional redundancy, and previous clinical failures. Targeting downstream cytokines such as IL-1β and IL-18 offers an alternative strategy; however, cytokine inhibition may be limited by compensatory pathways and broader immunosuppressive effects, whereas upstream targeting of NLRP3 or executioner GSDMD may provide more comprehensive control of pyroptotic inflammation. Thus, an optimal strategy may involve a tiered approach: Starting with NLRP3 inhibitors for most patients, and adding or switching to GSDMD inhibitors for those with hard-to-treat disease, to achieve better control of gut inflammation (Figure 4).
Pyroptosis has emerged as a critical pathogenic mechanism in IBD, characterized by dysregulated activation of the NLRP3 inflammasome-caspase-GSDMD axis which drives intestinal barrier dysfunction, immune dysregulation and microbial imbalance (Figure 2). Therapeutic targeting of this pathway demonstrates significant promise but faces translational barriers including hepatotoxicity risks with first-generation inhibitors (e.g., MCC950), suboptimal colonic biodistribution, and the absence of validated stratification biomarkers. Leading clinical candidates notably the NLRP3 inhibitor OLT1177 and IL-18 neutralizing antibody GSK1070806 (both in phase II trials) show dual efficacy in suppressing inflammation and promoting epithelial repair (Figure 3). Repurposed drugs including DMF, disulfiram and metformin provide complementary strategies requiring confirmatory clinical validation (Figure 3). While these approaches show considerable potential, key challenges remain in optimizing drug safety profiles, enhancing tissue-specific delivery, and developing biomarkers for patient stratification. Future progress requires a multipronged approach: Developing precision biomarkers for patient stratification, structurally optimizing safer and more selective inhibitors, and designing innovative delivery systems (e.g., colon-targeted nanoparticles) to enhance therapeutic localization. Combination strategies with existing immunomodulators may broaden efficacy in refractory cases while preserving mucosal immunity. Success demands multidisciplinary collaboration to bridge mechanistic insights with clinical innovation specifically through biomarker-stratified phase II trials of lead compounds and real-world validation of monitoring protocols. This paradigm shift toward mechanism-based intervention promises to transform IBD management by achieving durable deep remission in treatment-resistant patients through precision modulation of pyroptotic pathways.
| 1. | Kaplan GG. The global burden of inflammatory bowel disease: from 2025 to 2045. Nat Rev Gastroenterol Hepatol. 2025;22:708-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Xu YH, Zhu WM, Guo Z. Current status of novel biologics and small molecule drugs in the individualized treatment of inflammatory bowel disease. World J Gastroenterol. 2022;28:6888-6899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 2356] [Article Influence: 235.6] [Reference Citation Analysis (0)] |
| 4. | Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1529] [Cited by in RCA: 2627] [Article Influence: 262.7] [Reference Citation Analysis (0)] |
| 5. | Matikainen S, Nyman TA, Cypryk W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J Immunol. 2020;204:3063-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 6. | Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888-E10897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 720] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 7. | Xiao L, Ai YL, Mi XY, Liang H, Zhi X, Wu LZ, Chen QT, Gou T, Chen C, Zhou B, Hong WB, Yao LM, Chen JJ, Deng X, Li FN, Wu Q, Chen HZ. cGAS activation converges with intracellular acidification to promote STING aggregation and pyroptosis in tumor models. J Clin Invest. 2025;135:e188872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Li Y, Sun Y, Xie D, Chen H, Zhang Q, Zhang S, Wen F, Ou JS, Zhang M, Su L, Li X, Wen WP, Chi W. AIP1 Regulates Ocular Angiogenesis Via NLRP12-ASC-Caspase-8 Inflammasome-Mediated Endothelial Pyroptosis. Adv Sci (Weinh). 2024;11:e2405834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 699] [Article Influence: 99.9] [Reference Citation Analysis (2)] |
| 10. | Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 740] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 11. | Bauer C, Duewell P, Lehr HA, Endres S, Schnurr M. Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors. Dig Dis. 2012;30 Suppl 1:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Bulek K, Zhao J, Liao Y, Rana N, Corridoni D, Antanaviciute A, Chen X, Wang H, Qian W, Miller-Little WA, Swaidani S, Tang F, Willard BB, McCrae K, Kang Z, Dubyak GR, Cominelli F, Simmons A, Pizarro TT, Li X. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. J Clin Invest. 2020;130:4218-4234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Davis EM, Kaufmann Y, Goyne H, Wang Y, Chen T, Theus S, Lai K, Glover SC, Claggett BL, Jobin C, Liu JJ. Pyroptosis of Intestinal Epithelial Cells is Crucial to the Development of Mucosal Barrier Dysfunction and Intestinal Inflammation. Gastroenterology. 2017;152:S967. [DOI] [Full Text] |
| 14. | Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CC, Graham M, Elinav E, Flavell RA. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 463] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 15. | Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep. 2018;8:8618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 16. | Li S, Chen M, Zheng S, Abudourexiti W, Zhu F, Wang Z, Guo Y, Yu Z, Yang Z, Zhang L, Ding C, Gong J. ZAKα Induces Pyroptosis of Colonic Epithelium Via the Caspase-11/GSDMD Pathway to Aggravate Colitis. Inflammation. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Kaminsky LW, Al-Sadi R, Ma TY. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front Immunol. 2021;12:767456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 18. | Shao M, Yan Y, Zhu F, Yang X, Qi Q, Yang F, Hao T, Lin Z, He P, Zhou Y, Tang W, He S, Zuo J. Artemisinin analog SM934 alleviates epithelial barrier dysfunction via inhibiting apoptosis and caspase-1-mediated pyroptosis in experimental colitis. Front Pharmacol. 2022;13:849014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 19. | Cai J, Liu J, Fan P, Dong X, Zhu K, Liu X, Zhang N, Cao Y. Dioscin prevents DSS-induced colitis in mice with enhancing intestinal barrier function and reducing colon inflammation. Int Immunopharmacol. 2021;99:108015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Shen P, Zhang Z, Zhu K, Cao H, Liu J, Lu X, Li Y, Jing Y, Yuan X, Fu Y, Cao Y, Zhang N. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed Pharmacother. 2019;110:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | de Oliveira IM, Chaves MM. The NLRP3 Inflammasome in inflammatory diseases: Cellular dynamics and role in granuloma formation. Cell Immunol. 2025;411-412:104961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Zhang K, Guo J, Yan W, Xu L. Macrophage polarization in inflammatory bowel disease. Cell Commun Signal. 2023;21:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 23. | Lv W, Jin W, Lin J, Wang Z, Ma Y, Zhang W, Zhu Y, Hu Y, Qu Q, Guo S. Forsythia suspensa polyphenols regulate macrophage M1 polarization to alleviate intestinal inflammation in mice. Phytomedicine. 2024;125:155336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 24. | Ma S, Yang B, Du Y, Lv Y, Liu J, Shi Y, Huang T, Xu H, Deng L, Chen X. 1,8-cineole ameliorates colon injury by downregulating macrophage M1 polarization via inhibiting the HSP90-NLRP3-SGT1 complex. J Pharm Anal. 2023;13:984-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Wang Z, Li C, He X, Xu K, Xue Z, Wang T, Xu Z, Liu X. Platycodon grandiflorum root fermentation broth reduces inflammation in a mouse IBD model through the AMPK/NF-κB/NLRP3 pathway. Food Funct. 2022;13:3946-3956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Martynova E, Rizvanov A, Urbanowicz RA, Khaiboullina S. Inflammasome Contribution to the Activation of Th1, Th2, and Th17 Immune Responses. Front Microbiol. 2022;13:851835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Liu X, Chen L, Peng W, Deng H, Ni H, Tong H, Hu H, Wang S, Qian J, Liang A, Chen K. Th17/Treg balance: the bloom and wane in the pathophysiology of sepsis. Front Immunol. 2024;15:1356869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Ma J, Wang FY, Tang XD. Involvement of the NLRP3/IL-1β pathway in activation and effector functions of γδT17 cells in patients with ulcerative colitis. World J Gastroenterol. 2025;31:98174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 29. | Zhang Q, Wang S, Ji S. Trifolirhizin regulates the balance of Th17/Treg cells and inflammation in the ulcerative colitis mice through inhibiting the TXNIP-mediated activation of NLRP3 inflammasome. Clin Exp Pharmacol Physiol. 2022;49:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 30. | Liu X, Zhou M, Dai Z, Luo S, Shi Y, He Z, Chen Y. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother Res. 2023;37:367-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 31. | Wang SL, Zhang MM, Zhou H, Su GQ, Ding Y, Xu GH, Wang X, Li CF, Huang WF, Yi LT. Inhibition of NLRP3 attenuates sodium dextran sulfate-induced inflammatory bowel disease through gut microbiota regulation. Biomed J. 2023;46:100580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Gilliland A, Chan JJ, De Wolfe TJ, Yang H, Vallance BA. Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care. Gastroenterology. 2024;166:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 129] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 33. | Anderson CJ, Medina CB, Barron BJ, Karvelyte L, Aaes TL, Lambertz I, Perry JSA, Mehrotra P, Gonçalves A, Lemeire K, Blancke G, Andries V, Ghazavi F, Martens A, van Loo G, Vereecke L, Vandenabeele P, Ravichandran KS. Microbes exploit death-induced nutrient release by gut epithelial cells. Nature. 2021;596:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20:3328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 2593] [Article Influence: 370.4] [Reference Citation Analysis (0)] |
| 35. | Oizumi T, Mayanagi T, Toya Y, Sugai T, Matsumoto T, Sobue K. NLRP3 Inflammasome Inhibitor OLT1177 Suppresses Onset of Inflammation in Mice with Dextran Sulfate Sodium-Induced Colitis. Dig Dis Sci. 2022;67:2912-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Wang L, Dong X, Feng S, Pan H, Jang X, Chen L, Zhao Y, Chen W, Huang Z. VX765 alleviates dextran sulfate sodium-induced colitis in mice by suppressing caspase-1-mediated pyroptosis. Int Immunopharmacol. 2022;102:108405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Smith AP, Creagh EM. Caspase-4 and -5 Biology in the Pathogenesis of Inflammatory Bowel Disease. Front Pharmacol. 2022;13:919567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Xu Y, Tang X, Fang A, Yan J, Kofi Wiredu Ocansey D, Zhang X, Mao F. HucMSC-Ex carrying miR-203a-3p.2 ameliorates colitis through the suppression of caspase11/4-induced macrophage pyroptosis. Int Immunopharmacol. 2022;110:108925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Yi YS. Dual roles of the caspase-11 non-canonical inflammasome in inflammatory bowel disease. Int Immunopharmacol. 2022;108:108739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Tan G, Huang C, Chen J, Chen B, Zhi F. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn's disease by promoting intestinal inflammation. Cell Rep. 2021;35:109265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 41. | Bhat AA, Thapa R, Afzal O, Agrawal N, Almalki WH, Kazmi I, Alzarea SI, Altamimi ASA, Prasher P, Singh SK, Dua K, Gupta G. The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: A Review. Int J Biol Macromol. 2023;242:124832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 42. | Xiao J, Sun K, Wang C, Abu-Amer Y, Mbalaviele G. Compound loss of GSDMD and GSDME function is necessary to achieve maximal therapeutic effect in colitis. J Transl Autoimmun. 2022;5:100162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 43. | Yang W, Wang Y, Wang T, Li C, Shi L, Zhang P, Yin Y, Tao K, Li R. Protective effects of IRG1/itaconate on acute colitis through the inhibition of gasdermins-mediated pyroptosis and inflammation response. Genes Dis. 2023;10:1552-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 44. | Zhu C, Xu S, Jiang R, Yu Y, Bian J, Zou Z. The gasdermin family: emerging therapeutic targets in diseases. Signal Transduct Target Ther. 2024;9:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 45. | Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, Hu J. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death Dis. 2019;10:650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 634] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 46. | Buldukoglu OC, Ocal S, Akca S, Atar GE, Kaya B, Isik MD, Koca O, Harmandar FA, Cekin Y, Cekin AH. A novel inflammatory biomarker in assessing disease activity in ulcerative colitis: gasdermin D. BMC Gastroenterol. 2025;25:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Zhang S, Liang Y, Yao J, Li DF, Wang LS. Role of Pyroptosis in Inflammatory Bowel Disease (IBD): From Gasdermins to DAMPs. Front Pharmacol. 2022;13:833588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Chen K, Shang S, Yu S, Cui L, Li S, He N. Identification and exploration of pharmacological pyroptosis-related biomarkers of ulcerative colitis. Front Immunol. 2022;13:998470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 49. | Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, Schroder K. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 752] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 50. | Saber S, Abd El-Kader EM, Sharaf H, El-Shamy R, El-Saeed B, Mostafa A, Ezzat D, Shata A. Celastrol augments sensitivity of NLRP3 to CP-456773 by modulating HSP-90 and inducing autophagy in dextran sodium sulphate-induced colitis in rats. Toxicol Appl Pharmacol. 2020;400:115075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Saber S, El-Kader EMA. Novel complementary coloprotective effects of metformin and MCC950 by modulating HSP90/NLRP3 interaction and inducing autophagy in rats. Inflammopharmacology. 2021;29:237-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Li H, Guan Y, Liang B, Ding P, Hou X, Wei W, Ma Y. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur J Pharmacol. 2022;928:175091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 53. | Liu Y, Wang Q, Ma J, Li J, Li C, Xie X, Xiao Q, Xie C, Liu H, Hong Y, Wang J. Discovery of Novel Sulfonylurea NLRP3 Inflammasome Inhibitor for the Treatment of Multiple Inflammatory Diseases. J Med Chem. 2025;68:7243-7262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 54. | Marchetti C, Swartzwelter B, Gamboni F, Neff CP, Richter K, Azam T, Carta S, Tengesdal I, Nemkov T, D'Alessandro A, Henry C, Jones GS, Goodrich SA, St Laurent JP, Jones TM, Scribner CL, Barrow RB, Altman RD, Skouras DB, Gattorno M, Grau V, Janciauskiene S, Rubartelli A, Joosten LAB, Dinarello CA. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci U S A. 2018;115:E1530-E1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 459] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 55. | Saber S, Youssef ME, Sharaf H, Amin NA, El-Shedody R, Aboutouk FH, El-Galeel YA, El-Hefnawy A, Shabaka D, Khalifa A, Saleh RA, Osama D, El-Zoghby G, Gobba NA. BBG enhances OLT1177-induced NLRP3 inflammasome inactivation by targeting P2X7R/NLRP3 and MyD88/NF-κB signaling in DSS-induced colitis in rats. Life Sci. 2021;270:119123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 56. | Shi Y, Lv Q, Zheng M, Sun H, Shi F. NLRP3 inflammasome inhibitor INF39 attenuated NLRP3 assembly in macrophages. Int Immunopharmacol. 2021;92:107358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 57. | Pellegrini C, Fornai M, Colucci R, Benvenuti L, D’Antongiovanni V, Natale G, Fulceri F, Giorgis M, Marini E, Gastaldi S, Bertinaria M, Blandizzi C, Antonioli L. A Comparative Study on the Efficacy of NLRP3 Inflammasome Signaling Inhibitors in a Pre-clinical Model of Bowel Inflammation. Front Pharmacol. 2018;9:1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Zhao Y, Guo Q, Zhu Q, Tan R, Bai D, Bu X, Lin B, Zhao K, Pan C, Chen H, Lu N. Flavonoid VI-16 protects against DSS-induced colitis by inhibiting Txnip-dependent NLRP3 inflammasome activation in macrophages via reducing oxidative stress. Mucosal Immunol. 2019;12:1150-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 59. | Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219-3238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 616] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 60. | Bauer C, Loher F, Dauer M, Mayer C, Lehr HA, Schönharting M, Hallwachs R, Endres S, Eigler A. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-alpha mRNA expression. Dig Dis Sci. 2007;52:1642-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Dhani S, Zhao Y, Zhivotovsky B. A long way to go: caspase inhibitors in clinical use. Cell Death Dis. 2021;12:949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 62. | Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, Xiao TS, Li X, Abbott DW. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3:eaat2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 63. | Yang W, Tao K, Wang Y, Huang Y, Duan C, Wang T, Li C, Zhang P, Yin Y, Gao J, Li R. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem Pharmacol. 2022;206:115338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 64. | Hosseini Y, Niknejad A, Sabbagh Kashani A, Gholami M, Roustaie M, Mohammadi M, Momtaz S, Atkin SL, Jamialahmadi T, Abdolghaffari AH, Sahebkar A. NLRP3 inflammasomes pathway: a key target for Metformin. Inflammopharmacology. 2025;33:1729-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 65. | Cao R, Jing J, Ma Y, Qi W, Huang X, Zhang C, Lu Z, He J, Wang G, Ma Y, Zhang H. Metformin Ameliorates Ulcerative Colitis Through Inhibiting NLRP3 Inflammasome Activation. J Inflamm Res. 2025;18:1773-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | El-Haggar SM, Hegazy SK, Maher MM, Bahgat MM, Bahaa MM. Repurposing metformin as adjuvant therapy in patients with ulcerative colitis treated with mesalamine: A randomized controlled double-blinded study. Int Immunopharmacol. 2024;138:112541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 67. | Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, Deng X, Liang G, Zhang H, Jiang W, Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018;10:e8689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 411] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 68. | Seto Y, Kato K, Tsukada R, Suzuki H, Kaneko Y, Kojo Y, Sato H, Onoue S. Protective effects of tranilast on experimental colitis in rats. Biomed Pharmacother. 2017;90:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Oshitani N, Yamagami H, Watanabe K, Higuchi K, Arakawa T. Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn's disease. Gut. 2007;56:599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Chi F, Zhang G, Ren N, Zhang J, Du F, Zheng X, Zhang C, Lin Z, Li R, Shi X, Zhu Y. The anti-alcoholism drug disulfiram effectively ameliorates ulcerative colitis through suppressing oxidative stresses-associated pyroptotic cell death and cellular inflammation in colonic cells. Int Immunopharmacol. 2022;111:109117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Lei Y, Tang L, Chen Q, Wu L, He W, Tu D, Wang S, Chen Y, Liu S, Xie Z, Wei H, Yang S, Tang B. Disulfiram ameliorates nonalcoholic steatohepatitis by modulating the gut microbiota and bile acid metabolism. Nat Commun. 2022;13:6862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Zhou W, Zhang H, Huang L, Sun C, Yue Y, Cao X, Jia H, Wang C, Gao Y. Disulfiram with Cu(2+) alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Theranostics. 2023;13:2879-2895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 73. | Ou AT, Zhang JX, Fang YF, Wang R, Tang XP, Zhao PF, Zhao YG, Zhang M, Huang YZ. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol Sin. 2021;42:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 74. | Luo J, Zeng Y, Chen Z, Luo Y, Shi L, Zhou X. Safety assessment of disulfiram: real-world adverse event analysis based on FAERS database. Front Psychiatry. 2024;15:1498204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 75. | Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, Shaffer SA, Dutta R, Ionete C, Pesiridis S, Yang S, Thompson PR, Fitzgerald KA. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 517] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 76. | Li S, Takasu C, Lau H, Robles L, Vo K, Farzaneh T, Vaziri ND, Stamos MJ, Ichii H. Dimethyl Fumarate Alleviates Dextran Sulfate Sodium-Induced Colitis, through the Activation of Nrf2-Mediated Antioxidant and Anti-inflammatory Pathways. Antioxidants (Basel). 2020;9:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Patel V, Joharapurkar A, Kshirsagar S, Patel M, Savsani H, Patel A, Ranvir R, Jain M. Repurposing dimethyl fumarate for gastric ulcer and ulcerative colitis: Evidence of local efficacy without systemic side effect. Med Drug Discov. 2022;16:100142. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Buscarinu MC, Gargano F, Lionetto L, Capi M, Morena E, Fornasiero A, Reniè R, Landi AC, Pellicciari G, Romano C, Mechelli R, Romano S, Borsellino G, Battistini L, Simmaco M, Fagnani C, Salvetti M, Ristori G. Intestinal Permeability and Circulating CD161+CCR6+CD8+T Cells in Patients With Relapsing-Remitting Multiple Sclerosis Treated With Dimethylfumarate. Front Neurol. 2021;12:683398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Shah S, Locca A, Dorsett Y, Cantoni C, Ghezzi L, Lin Q, Bokoliya S, Panier H, Suther C, Gormley M, Liu Y, Evans E, Mikesell R, Obert K, Salter A, Cross AH, Tarr PI, Lovett-Racke A, Piccio L, Zhou Y. Alterations of the gut mycobiome in patients with MS. EBioMedicine. 2021;71:103557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Ouyang Y, Zhao J, Wang S. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromolecules for the treatment of inflammatory bowel disease: A review. Int J Biol Macromol. 2023;227:505-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 81. | Shaul E, Conrad MA, Dawany N, Patel T, Canavan MC, Baccarella A, Weinbrom S, Aleynick D, Sullivan KE, Kelsen JR. Canakinumab for the treatment of autoinflammatory very early onset- inflammatory bowel disease. Front Immunol. 2022;13:972114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 82. | Miyamoto T, Izawa K, Masui S, Yamazaki A, Yamasaki Y, Matsubayashi T, Shiraki M, Ohnishi H, Yasumura J, Kawabe T, Miyamae T, Matsubara T, Arakawa N, Ishige T, Takizawa T, Shimbo A, Shimizu M, Kimura N, Maeda Y, Maruyama Y, Shigemura T, Furuta J, Sato S, Tanaka H, Izumikawa M, Yamamura M, Hasegawa T, Kaneko H, Nakagishi Y, Nakano N, Iida Y, Nakamura T, Wakiguchi H, Hoshina T, Kawai T, Murakami K, Akizuki S, Morinobu A, Ohmura K, Eguchi K, Sonoda M, Ishimura M, Furuno K, Kashiwado M, Mori M, Kawahata K, Hayama K, Shimoyama K, Sasaki N, Ito T, Umebayashi H, Omori T, Nakamichi S, Dohmoto T, Hasegawa Y, Kawashima H, Watanabe S, Taguchi Y, Nakaseko H, Iwata N, Kohno H, Ando T, Ito Y, Kataoka Y, Saeki T, Kaneko U, Murase A, Hattori S, Nozawa T, Nishimura K, Nakano R, Watanabe M, Yashiro M, Nakamura T, Komai T, Kato K, Honda Y, Hiejima E, Yonezawa A, Bessho K, Okada S, Ohara O, Takita J, Yasumi T, Nishikomori R; Japan CAPS Working Group. Clinical Characteristics of Cryopyrin-Associated Periodic Syndrome and Long-Term Real-World Efficacy and Tolerability of Canakinumab in Japan: Results of a Nationwide Survey. Arthritis Rheumatol. 2024;76:949-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 83. | Liso M, Verna G, Cavalcanti E, De Santis S, Armentano R, Tafaro A, Lippolis A, Campiglia P, Gasbarrini A, Mastronardi M, Pizarro TT, Cominelli F, Lopetuso LR, Chieppa M. Interleukin 1β Blockade Reduces Intestinal Inflammation in a Murine Model of Tumor Necrosis Factor-Independent Ulcerative Colitis. Cell Mol Gastroenterol Hepatol. 2022;14:151-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Dogan S, Selen R, Ozbay Hosnut F, Ozdel S, Dogu F, Ikinciogullari A, Aytekin C. Successful Use of Anakinra in a Patient with IL-10R Beta Deficiency: A Case Report. Pediatr Allergy Immunol Pulmonol. 2025;38:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Truyens M, Hoste L, Geldof J, Hoorens A, Haerynck F, Huis In 't Veld D, Lobatón T. Successful treatment of ulcerative colitis with anakinra: a case report. Acta Gastroenterol Belg. 2023;86:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Guha A, Diaz-Pino R, Fagbemi A, Hughes SM, Wynn RF, Lopez-Castejon G, Arkwright PD. Very Early-Onset IBD-Associated IL-18opathy Treated with an Anti-IL-18 Antibody. J Clin Med. 2024;13:6058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 87. | Shen DM, Byth KF, Bertheloot D, Braams S, Bradley S, Dean D, Dekker C, El-Kattan AF, Franchi L, Glick GD, Ghosh S, Hinniger A, Katz JD, Kitanovic A, Lu X, Olhava EJ, Opipari AW, Sanchez B, Seidel HM, Stunden J, Stutz A, Telling A, Venkatraman S, Winkler DG, Roush WR. Discovery of DFV890, a Potent Sulfonimidamide-Containing NLRP3 Inflammasome Inhibitor. J Med Chem. 2025;68:5529-5550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 88. | Gatlik E, Mehes B, Voltz E, Sommer U, Tritto E, Lestini G, Liu X, Pal P, Velinova M, Denney WS, Fu Y, Opipari A, Dean D, Junge G. First-in-human safety, tolerability, and pharmacokinetic results of DFV890, an oral low-molecular-weight NLRP3 inhibitor. Clin Transl Sci. 2024;17:e13789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 89. | Madurka I, Vishnevsky A, Soriano JB, Gans SJ, Ore DJS, Rendon A, Ulrik CS, Bhatnagar S, Krishnamurthy S, Mc Harry K, Welte T, Fernandez AA, Mehes B, Meiser K, Gatlik E, Sommer U, Junge G, Rezende E; Study group. DFV890: a new oral NLRP3 inhibitor-tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function. Infection. 2023;51:641-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 90. | Kaufmann B, de Los Reyes Jiménez M, Booshehri LM, Onyuru J, Leszczynska A, Uri A, Michel S, Klar R, Jaschinski F, Feldstein AE, Broderick L, Hoffman HM. Antisense Oligonucleotide Therapy Decreases IL-1β Expression and Prolongs Survival in Mutant Nlrp3 Mice. J Immunol. 2023;211:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 91. | Braatz C, Komes MP, Ravichandran KA, de Fragas MG, Griep A, Schwartz S, McManus RM, Heneka MT. NLRP3-directed antisense oligonucleotides reduce microglial immunoactivities in vitro. J Neurochem. 2024;168:3467-3481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | de Rivero Vaccari JP, Mim C, Hadad R, Cyr B, Stefansdottir TA, Keane RW. Mechanism of action of IC 100, a humanized IgG4 monoclonal antibody targeting apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). Transl Res. 2023;251:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Yuan H, Chen S, Duncan MR, de Rivero Vaccari JP, Keane RW, Dalton Dietrich W, Chou TH, Benny M, Schmidt AF, Young K, Park KK, Porciatti V, Elizabeth Hartnett M, Wu S. IC100, a humanized therapeutic monoclonal anti-ASC antibody alleviates oxygen-induced retinopathy in mice. Angiogenesis. 2024;27:423-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Cyr B, Vontell RT, Hadad R, de Rivero Vaccari JP, Keane RW. IC100 blocks inflammasome activation induced by α-synuclein aggregates and ASC specks. NPJ Parkinsons Dis. 2025;11:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 95. | Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y, Wang J, Zhou X, Li W, Guo L, Jiao Q. New mechanism of nerve injury in Alzheimer's disease: β-amyloid-induced neuronal pyroptosis. J Cell Mol Med. 2020;24:8078-8090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 96. | Schiffelers LDJ, Tesfamariam YM, Jenster LM, Diehl S, Binder SC, Normann S, Mayr J, Pritzl S, Hagelauer E, Kopp A, Alon A, Geyer M, Ploegh HL, Schmidt FI. Antagonistic nanobodies implicate mechanism of GSDMD pore formation and potential therapeutic application. Nat Commun. 2024;15:8266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |