Published online Nov 14, 2025. doi: 10.3748/wjg.v31.i42.112354
Revised: August 18, 2025
Accepted: October 13, 2025
Published online: November 14, 2025
Processing time: 112 Days and 4.2 Hours
Portosystemic venous invasion (PSVI) depth critically influences prognosis in borderline resectable pancreatic cancer (BRPC), necessitating precise preoperative discrimination for personalized therapy.
To develop and validate a preoperative nomogram integrating computed to
This retrospective cohort study analyzed 167 BRPC patients undergoing radical resection between 2011 and 2023. Patients were stratified by pathological PSVI depth [no venous invasion (VI)/adventitial/muscularis propria/intimal]. Kaplan-Meier and ordinal logistic regression identified preoperative predictors from clinical/laboratory/computed tomography parameters (e.g., circumferential involvement and CA19-9). A nomogram was developed and validated via cali
PSVI depth significantly stratified survival.: Intimal VI showed worst prognosis (median overall survival: 9 months, 5-year overall survival: 0% vs no VI: 17 months, 12.5%; P < 0.001). Independent predictors: CA19-9 [odds ratio (OR) = 3.819, Wald = 14.125, 95% confidence interval (CI): 1.980-7.410], circumferential involvement (OR = 8.271, Wald = 33.352, 95%CI: 3.950-17.320), and luminal compromise (OR = 3.544, Wald = 8.489, 95%CI: 1.818-6.447). The nomogram achieved C-index = 0.928 (95%CI: 0.889-0.967), with 100-250 points indicating high invasiveness risk. Decision curve analysis confirmed clinical utility (threshold: 0-0.7).
This model integrates routine indicators to preoperatively quantify PSVI depth, guiding precision treatment.
Core Tip: This study developed a predictive model for pathological grading of portosystemic venous invasion depth in borderline resectable pancreatic cancer using routine preoperative indicators: Serum carbohydrate antigen 19-9, computed tomography circumferential involvement angle, and luminal compromise. The model achieved high accuracy (C-index 0.928) in stratifying venous invasion depth into adventitial, muscularis propria, or intimal invasion, which significantly affected prognosis (e.g., intimal invasion showed worst median overall survival of 9 months). This innovative tool enables preoperative risk quantification, guiding personalized therapy decisions such as neoadjuvant intensification or surgical planning, and addresses a critical unmet need in precision oncology for borderline resectable pancreatic cancer.
- Citation: Wang FF, Dai XD, Zhao X, He Q, Lyu SC. Development and validation of a predictive model for portal-systemic venous invasion grading in borderline resectable pancreatic cancer. World J Gastroenterol 2025; 31(42): 112354
- URL: https://www.wjgnet.com/1007-9327/full/v31/i42/112354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i42.112354
Pancreatic ductal adenocarcinoma (PDAC) remains a persistent therapeutic challenge, with 5-year survival below 12% even after curative resection, underscoring the critical role of occult metastatic drivers[1,2]. Microscopic venous invasion (VI) depth constitutes a pivotal prognostic determinant with hierarchical biological significance for PDAC. The 9th edition of the American Joint Committee on Cancer (AJCC) staging system categorizes intimal (IT) invasion (ITI) as T4 disease, fundamentally altering therapeutic algorithms[3]. Landmark analysis of 2143 resected specimens revealed stepwise survival decline, median overall survival (OS) was 28.6 months for adventitial (AD) invasion (ADI) vs 23.5 months for muscularis propria (MP) invasion (MPI) vs 12.1 months for ITI.
Current high-resolution computed tomography (CT)/magnetic resonance imaging fails to distinguish microscopic invasion layers (AD/MP/IT), resulting in significant underdiagnosis (ITI missed in 30.7%-41.2% of cases). Undetected ITI correlates with therapeutic inertia (MITO-PAC trial: 84.3% 3-year recurrence vs 39.8% with neoadjuvant therapy), while ADI-only patients showed therapeutic excess, 35.2% undergoing unnecessary venous resection, increasing severe complications (Clavien-Dindo ≥ IIIa) from 12.4% to 41.6%[4,5]. Diagnostic limitations in discriminating microscopic invasion layers directly compromise clinical management, resulting in unwarranted avoidance of neoadjuvant therapy or inadequate margin planning.
Therefore, precise preoperative discrimination of VI depth is imperative. To address this unmet need, we integrated multidimensional preoperative biomarkers, including serum profiles [carbohydrate antigen 19-9 (CA19-9) kinetics, neutrophil-to-lymphocyte ratio], radiological signatures (tumor-vein contact morphology), and clinicopathological variables, to develop a hierarchical portosystemic VI prediction model. This tool quantitatively estimates AD/MP/IT invasion probabilities, enabling precision interventions such as neoadjuvant intensification or en bloc venous resection.
From January 2011 to December 2023, 322 patients with borderline resectable pancreatic cancer (BRPC) were treated at the Department of Hepatobiliary Pancreas and Spleen Surgery, Beijing Chao-Yang Hospital, Capital Medical University. According to relevant inclusion and exclusion criteria, 167 patients with BRPC who underwent radical resection were finally included [based on National Comprehensive Cancer Network (NCCN) criteria].
Inclusion criteria: (1) Combined with preoperative imaging, assessed as BRPC according to the 2024 NCCN guidelines for pancreatic cancer (PC); (2) No distant metastasis on preoperative evaluation; (3) No neoadjuvant therapy performed preoperatively; (4) Intraoperative venous resection and reconstruction feasible for patients with portosystemic VI (PSVI); (5) Informed consent for the surgical method and treatment plan obtained from the patient and family members; and (6) Complete perioperative and follow-up data.
Exclusion criteria: (1) Preoperatively assessed as BRPC, locally advanced PC, or unresectable PC according to the 2024 NCCN guidelines; (2) Receipt of neoadjuvant therapy; (3) Intraoperative detection of distant metastasis; (4) No intraoperative venous resection and reconstruction performed; (5) Postoperative pathology confirmed non-PC; (6) Failure to achieve R0 resection; (7) Abandonment of radical surgery due to patient or family factors; and (8) Loss to follow-up.
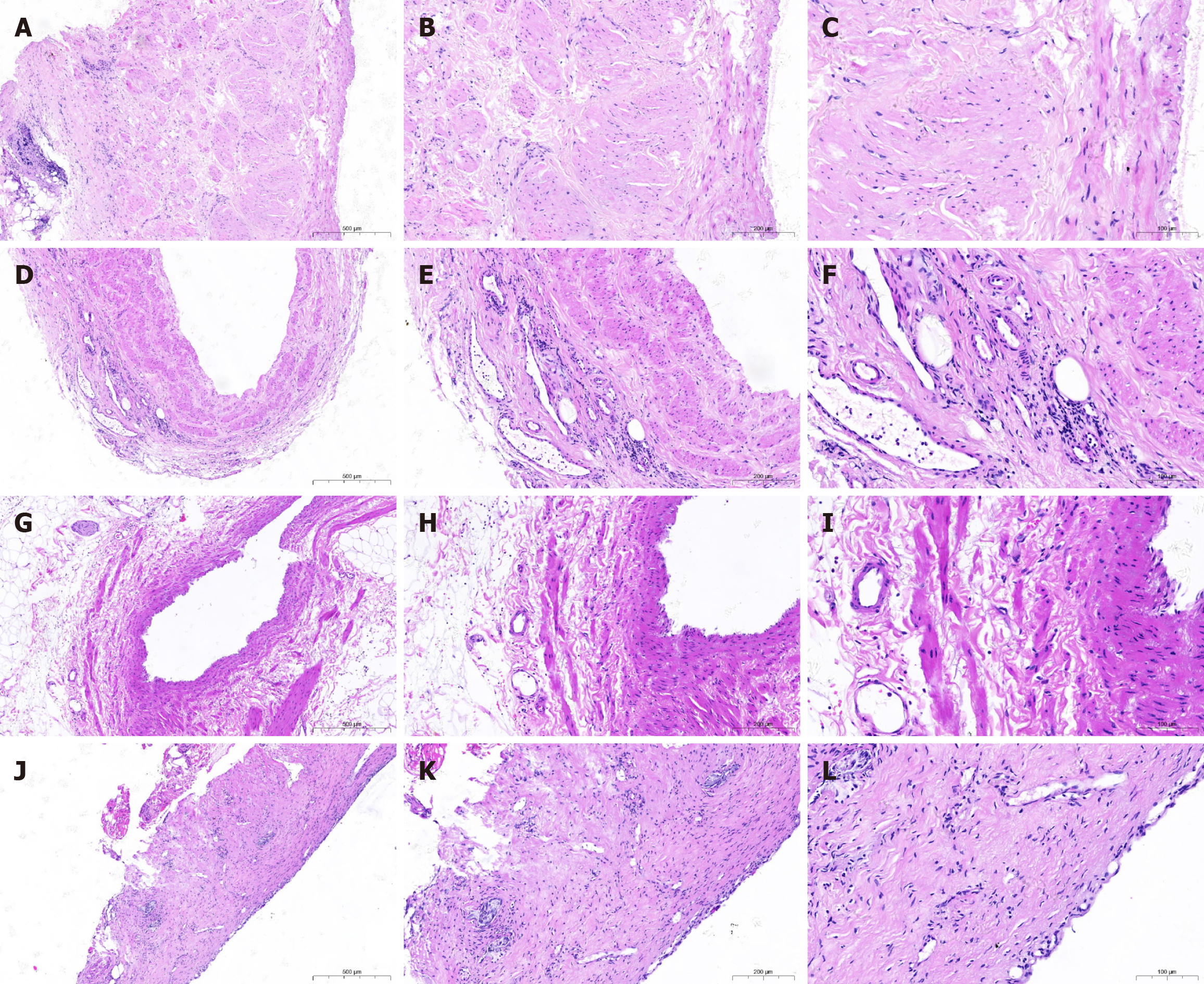
The primary endpoint was pathological PSVI grading, determined by histopathological evaluation of resected specimens using AJCC 9th edition[6] and College of American Pathologists standards[7]. VI was defined as microscopic tumor cells within the venous wall structures (portal, superior mesenteric, and splenic veins). Grading classified invasion depth: AD (confined to adventitia), MP (smooth muscle layer), and IT (endothelial penetration). Assessment utilized hematoxylin and eosin-stained sections; Elastic Van Gieson staining supplemented ITI-grade confirmation by visualizing elastic laminae. Multifocal invasion was classified by the highest grade present (e.g., ITI overrides ADI/MPI).
Two blinded gastrointestinal pathologists independently assessed all slides using hematoxylin and eosin-stained sections, with Elastic Van Gieson staining confirming ITI-grade by visualizing elastic laminae; this histopathological assessment was conducted without access to clinical data, radiological findings, or each other’s evaluations, yielding substantial initial inter-rater agreement (k = 0.78, 95% confidence interval (CI): 0.71-0.85]. Discrepancies (18% of cases) were resolved through consensus meetings referencing AJCC/College of American Pathologists standards, escalating to a third senior arbiter for unresolved cases (5%), with intraobserver reproducibility confirmed as excellent [intraclass correlation coefficient (ICC) = 0.92] through random retesting of 10% samples, establishing robust histological ground truth for model development.
This study collected patient baseline data (age, sex, smoking history, diabetes history); preoperative laboratory indicators (complete blood count, comprehensive biochemistry panel, CA19-9 and carcinoembryonic antigen); contrast-enhanced abdominal CT imaging features (tumor location/size, regional lymph node status, and PSVI parameters: VI length, circumferential involvement, luminal compromise, tumor-vein distance); and postoperative pathological results (primarily including the AJCC 9th edition VI grade, along with tumor-nodes-metastasis stage and differentiation grade). Follow-up was conducted through outpatient review (every 3 months) and telephone calls. The endpoint events were defined as OS (time from surgery to death from any cause/Last follow-up) and recurrence-free survival (time from surgery to radiologically confirmed recurrence). Follow-up ended in June 2025. Patients were divided into four groups based on the pathological degree of PSVI: No invasion group, ADI group, MPI group, and ITI group for survival stratification analysis. The patients’ baseline data, preoperative laboratory indicators, and imaging features were analyzed for their influence on the endpoint of pathological PSVI grading. Independent risk factors identified were used to construct a pathological PSVI model. Its accuracy was validated and the model’s dynamic predictive performance was assessed.
CT imaging features of PSVI parameters were as follows. Luminal compromise was quantified as the percentage reduction in venous cross-sectional area measured on arterial-phase CT orthogonal reformats perpendicular to the vessel axis, using workstation polygon segmentation tools. VI length was measured along the vessel centerline on curved planar reformations, recording the longitudinal tumor-vein contact distance exceeding 180° interface. Tumor-vein distance was the minimal separation on 80° orthogonal reformats. Circumferential involvement was calculated as the maximal tumor-vessel interface arc (°) on arterial-phase axial slices. To assess inter- and intraobserver reliability, two abdominal radiologists (with 8 and 12 years of experience), blinded to pathological outcomes and each other’s assessments, independently re-evaluated key CT parameters (circumferential involvement, luminal compromise, VI length) on a randomly selected subset of 50 studies.
To comprehensively validate the model’s robustness within neoadjuvant therapeutic contexts, we designed a dedicated sensitivity analysis (Supplementary material) to reveal that the model maintained discriminatory capacity (C-index = 0.78, Supplementary Figure 1), leveraging pretreatment baseline parameters from eight consecutive BRPC patients undergoing surgical resection following neoadjuvant therapy at our institution. Model predictions derived from these pre-intervention variables were rigorously benchmarked against postoperative histopathological outcomes, with performance quantified through concordance indices (C-index) and calibration curves. Concurrently, the therapeutic impact on critical predictors was evaluated via paired-sample statistical testing frameworks, incorporating Wilcoxon signed-rank tests for ordinal biomarkers and ICCs for continuous imaging metrics, thereby establishing methodological resilience to treatment-induced biological perturbations.
ICCs for absolute agreement of CT parameters were calculated for continuous variables, and Cohen’s k was used for categorical luminal compromise (none/stenosis/defect). The reliability analysis revealed excellent interobserver and intraobserver agreement for all key CT parameters, with high ICCs and kappa values (Supplementary Table 1). The Bland-Altman analysis confirmed excellent interobserver agreement for all CT parameters, with minimal bias and narrow limits of agreement (Supplementary Figure 2). Serum CA19-9 Levels were log10-transformed to normalize their distribution prior to analysis. Patients with CA19-9 Levels < 2 U/mL (nonsecretors, n = 5) were included, and their value was set to log10(2) for analysis. To address the potential confounding effect of cholestasis, a sensitivity analysis was conducted on the subset of patients with normal total bilirubin levels (< 21 mmol/L, n = 132). The association between log10(CA19-9) and VI grade remained significant in this subpopulation [odds ratio (OR) = 3.211, 95%CI: 1.652-6.412, P = 0.001). The CA19-9/total bilirubin ratio was explored but did not improve model fit compared to log10(CA19-9) alone (ΔAIC = +2.4). There were no missing data for CA19-9. Survival analysis was performed using the Kaplan-Meier method for key grouping variables; differences in survival rates between groups were compared using the log-rank test. Ordinal logistic regression was used for univariate and multivariate analysis of pathological PSVI grade. Variables with P < 0.05 in univariate analysis were included in the multivariate model to calculate Wald and 95%CIs, using SPSS 24.0 software. Prognostic modeling was performed using R language (v4.0.3). A nomogram integrating independent risk factors was generated using the rms package to predict pathological PSVI grade. Bootstrap internal validation with 1000 resamples was performed to plot calibration curves for assessing accuracy, as well as to calculate the optimism-corrected C-index and calibration slope for overfitting adjustment. Decision curve analysis (DCA) was performed using the ggDCA package to quantify clinical net benefit; and the timeROC package was used to evaluate the model’s dynamic predictive performance. All basic statistics were performed using SPSS 24.0, while advanced modeling was implemented on the R platform. P < 0.05 was considered statistically significant.
To address potential confounding factors inherent in the retrospective design and ensure balanced comparison groups for the PSVI depth analysis, propensity score matching (PSM) was used. Propensity scores were calculated using a non-parsimonious multivariable logistic regression model, with pathological PSVI grade (categorized as no invasion, ADI, MPI, ITI) as the dependent variable. Covariates included in the propensity model encompassed key baseline characteristics and preoperative variables identified as potentially influential or showing imbalance: Age, gender, smoking history, diabetes history, leukocyte count, neutrophil count, lymphocyte count, total bilirubin, g-glutamyltransferase, CA19-9 (log-transformed due to skewness), carcinoembryonic antigen, tumor size, regional lymph node enlargement, VI length, tumor location, circumferential involvement, luminal compromise, and tumor-vein distance on CT. Matching was performed using the nearest neighbor method without replacement, with a caliper width set at 0.2 of the standard deviation of the logit of the propensity score and a matching ratio of 1:1. The adequacy of covariate balance after matching was rigorously assessed by comparing standardized differences between groups; a threshold of < 10% for standardized differences was considered indicative of good balance. This PSM approach resulted in well-matched cohorts for subsequent comparative analyses of PSVI depth predictors and outcomes.
Based on postoperative pathological results, the depth of VI was categorized into four grades (Table 1). The appearance of VI at 40 ×, 100 ×, and 200 × is shown in Figure 1.
| Grade | Definition | Cases |
| No invasion | Tumor cells are confined to the perivascular connective tissue surrounding the vein but do not penetrate the adventitial layer | 39 |
| Adventitial invasion | Tumor cells are confined to the adventitial layer of the vein and do not breach the external elastic lamina or invade deeper structures of the venous wall | 47 |
| Muscularis propria invasion | Tumor cells invade and are present within the smooth muscle layer of the venous wall (muscularis propria/muscular layer) | 41 |
| Intimal invasion | Tumor cells penetrate through the endothelial cell layer (endothelium) of the vein, becoming directly exposed to the vascular lumen, or are attached to/floating within the lumen (with or without thrombus formation) | 40 |
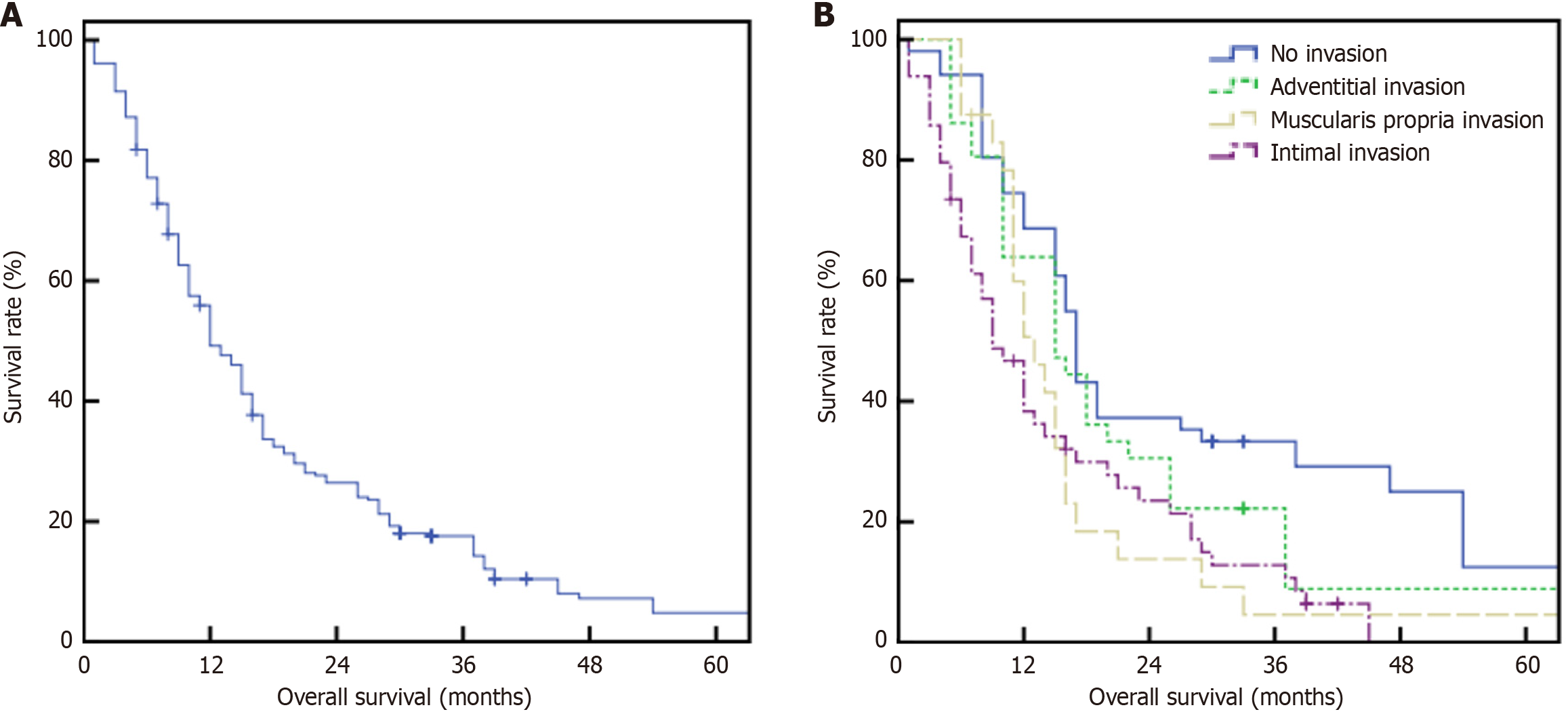
All patients underwent successful surgery, with postoperative pathological examination confirming PDAC and no perioperative mortality. Follow-up extended until June 2025, with a median duration of 15 months. The overall median survival time was 12 months, with 1-year, 2-year, and 5-year OS rates of 49.2%, 26.5%, and 4.8%, respectively (Figure 2A). Median survival times were 17 months (no invasion group), 15 months (ADI group), 13 months (MPI group), and 9 months (ITI group). The corresponding 1-year, 2-year, and 5-year survival rates were: 68.6%, 37.3%, and 12.5% (no invasion) vs 63.9%, 30.6%, and 8.9% (ADI) vs 50.7%, 13.8%, and 4.6% (MPI) vs 38.3%, 23.5%, and 0% (ITI) (P < 0.001, χ2 = 18.139; Figure 2B).
Univariate analysis is presented in Table 2. CA19-9, VI length, circumferential involvement, luminal compromise, and tumor-vein distance were potential risk factors influencing survival. These variables were included in the ordinal logistic regression for multivariate analysis. CA19-9 (OR = 3.819, Wald = 14.125, 95%CI: 1.980-7.410), circumferential involvement (OR = 8.271, Wald = 33.352, 95%CI: 3.950-17.320), and luminal compromise (OR = 3.544, Wald = 8.489, 95%CI: 1.818-6.447) were identified as independent preoperative risk factors for pathological VI grading. Patients with normal CA19-9 Levels and CT features showing no circumferential involvement or luminal compromise had a significantly lower probability of pathological VI.
| Factors | Univariate analysis | Multivariate analysis | ||||
| χ2 value | P value | Odds ratio value | 95% confidence interval | Wald | P value | |
| Age (60 years old) | 0.530 | 0.467 | ||||
| Gender | 0.755 | 0.385 | ||||
| Smoking history | 0.733 | 0.392 | ||||
| Diabetes history | 1.090 | 0.296 | ||||
| Leukocyte count (× 109/L) | 0.010 | 0.922 | ||||
| Neutrophil count (× 109/L) | 0.658 | 0.417 | ||||
| Lymphocyte count (× 109/L) | 0.029 | 0.865 | ||||
| Total bilirubin (μmol/L) | 0.638 | 0.424 | ||||
| Gamma-glutamyl transferase (U/L) | 0.112 | 0.738 | ||||
| Carcinoembryonic antigen (ng/mL) | 0.554 | 0.457 | ||||
| CA19-9 (U/mL) | 23.462 | 0.000 | 3.819 | 1.980-7.410 | 14.125 | 0.000 |
| Regional lymph node enlargement (imaging) | 3.469 | 0.063 | ||||
| Tumor size | 2.086 | 0.149 | ||||
| Tumor location (head/body/tail/uncinate) | 2.591 | 0.107 | ||||
| Venous invasion length | 21.534 | 0.000 | 1.012 | 0.496-2.064 | 0.001 | 0.973 |
| Circumferential involvement | 83.009 | 0.000 | 8.271 | 3.950-17.320 | 33.352 | 0.000 |
| Luminal compromise | 75.556 | 0.000 | 3.544 | 1.818-6.447 | 8.489 | 0.004 |
| Tumor-vein distance | 17.961 | 0.000 | 0.434 | 0.180-1.044 | 3.469 | 0.063 |
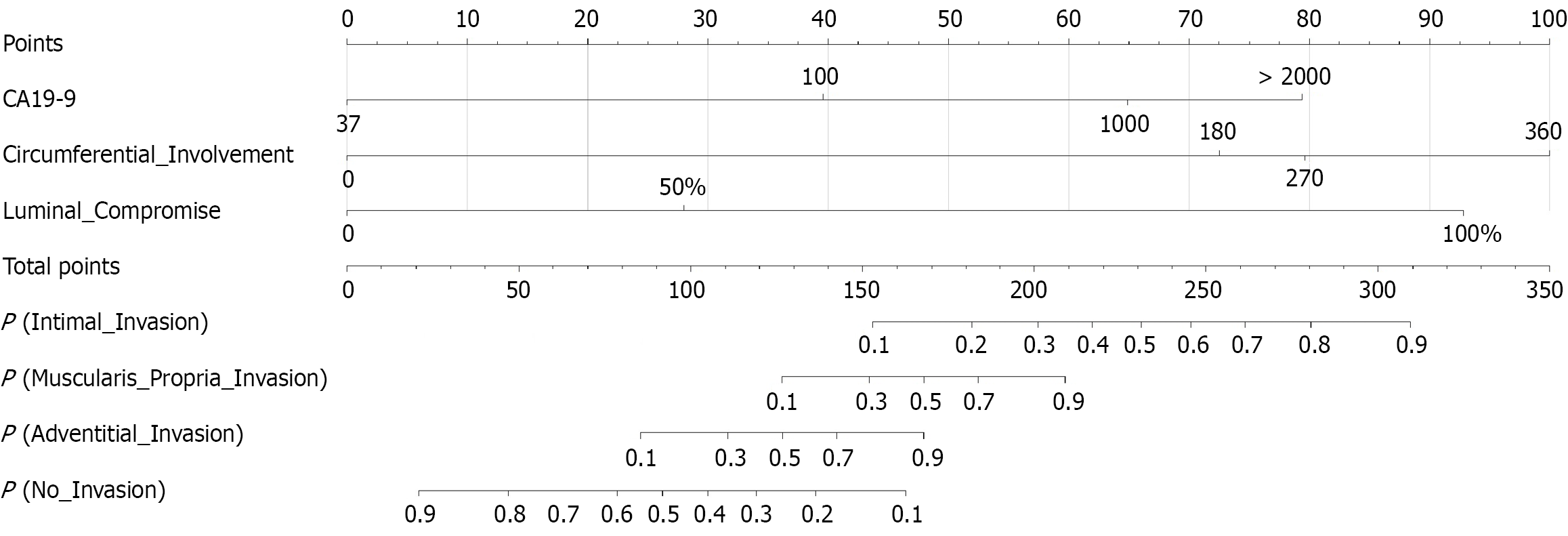
This nomogram-based analysis constructs a predictive model for assessing VI risk in PC patients (Figure 3). The scoring system integrated three key clinical parameters: Serum CA19-9 (range: 37-2000 U/mL), circumferential involvement extent (range: 0°-360°), and luminal compromise (range: 0%-100%). The model generated a total score ranging from 0 to 350 points, corresponding to predicted probabilities for four outcomes: P (No_Invasion), P (ADI), P (MPI), and P (ITI). The scoring procedure involved three sequential steps. First, assigning points for variables where CA19-9 begins scoring at the normal threshold of 37 U/mL (> 100 U/mL yields > 40 points); circumferential involvement at 360° contributes up to 100 points; and luminal compromise of 100% stenosis (defect occlusion) assigned 93 points. Second, calculating the total score. Finally, predicting probabilities where 200 points correspond to approximately 30% probability of ITI and > 80% probability of MPI. Risk stratification revealed that a total score < 20 points indicated high probability of no invasion (> 90%), while a score > 310 points showed significantly elevated probability of ITI (> 90%). The steep probability slope within the 100-250-point interval signified a critical threshold for determining tumor invasiveness, providing substantial clinical decision-making guidance.
Based on the nomogram’s total point score, we propose clinically actionable risk thresholds: Total score < 150 points suggests a low risk of deep invasion [P (ITI) < 10%], potentially favoring upfront surgery. A score > 230 points indicates high risk [P (ITI) > 50%], strongly warranting consideration for intensified neoadjuvant therapy. A score > 280 points signifies very high risk [P (ITI) > 80%], mandating meticulous surgical planning for anticipated en bloc venous resection and reconstruction.
To demonstrate clinical application, consider a representative case. A 58-year-old male BRPC patient with serum CA19-9 = 380 U/mL, circumferential involvement = 240°, and luminal stenosis (defect absent). First, assign points, CA19-9 = 380 U/mL, 50 points (scale: 37-1000 U/mL), circumferential involvement = 240°, 77 points (scale: 0°-360°), luminal compromise = 50%, 28 points. Second, sum points, total score = 50 + 77 + 28 = 155 points. Third, predict probabilities, P (No VI) = 15%, P (ADI) = 80%, P (MPI) = 30%, P (ITI) = 10%. This indicates the highest probability of ADI in this patient. The patient subsequently underwent neoadjuvant FOLFIRINOX, avoiding immediate high-risk venous resection. Final pathology confirmed ADI, validating the prediction.
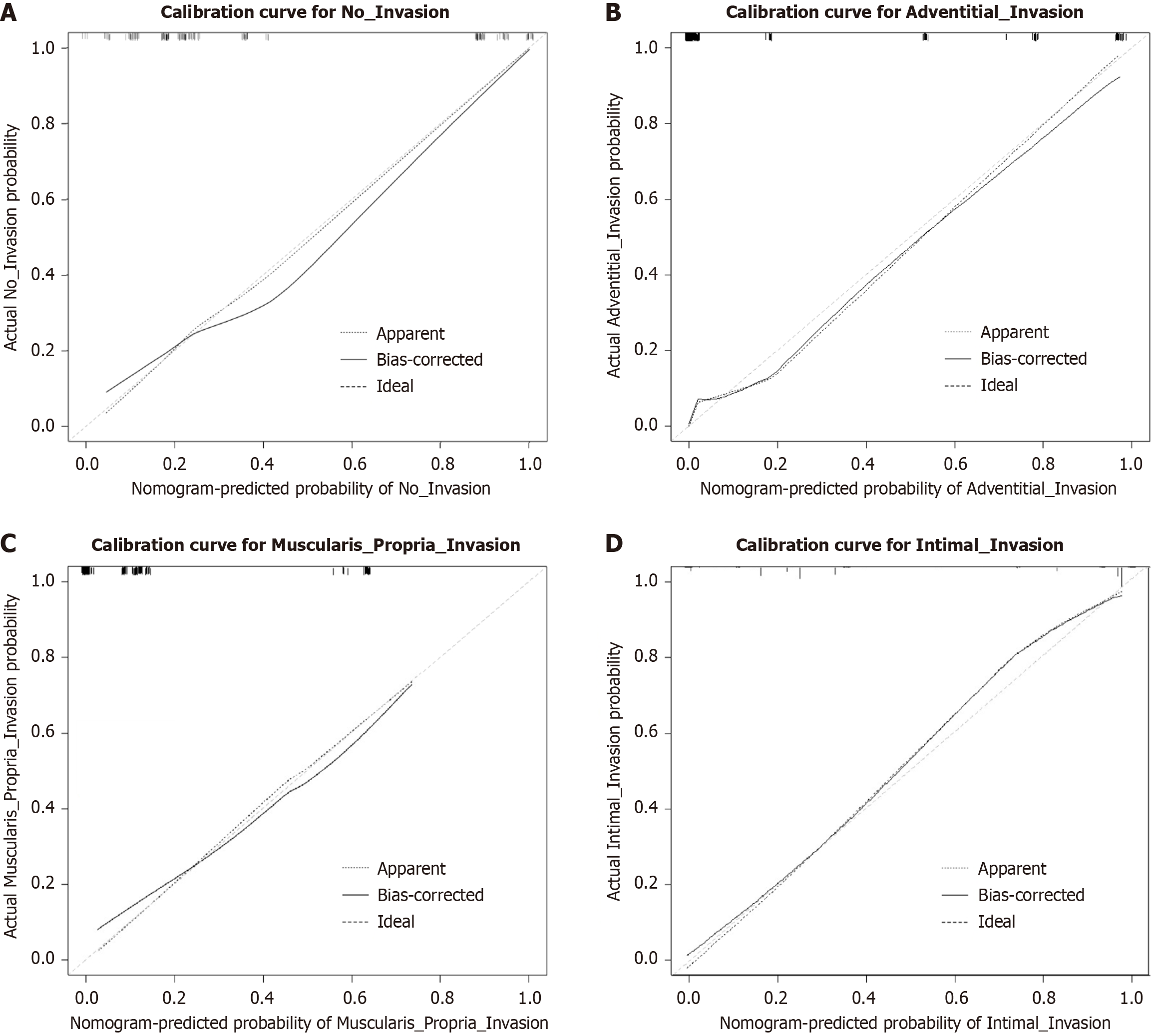
Calibration curve analysis demonstrated that the nomogram model exhibited excellent predictive calibration performance across all VI depths (Figure 4). The bias-corrected calibration curves (solid lines) in each subplot closely aligned with the ideal diagonal lines (dashed-dotted lines), indicating high concordance between model-predicted probabilities and actual observed probabilities. This supports the model’s clinical applicability for decision-making. Validation metrics confirmed a C-index of 0.928 (95%CI: 0.889-0.967), demonstrating robust predictive capability of the nomogram. After bootstrap internal validation (1000 resamples), the optimism-corrected C-index was 0.912 (95%CI: 0.869-0.955) and the calibration slope was 0.941, indicating minimal overfitting and robust internal performance.
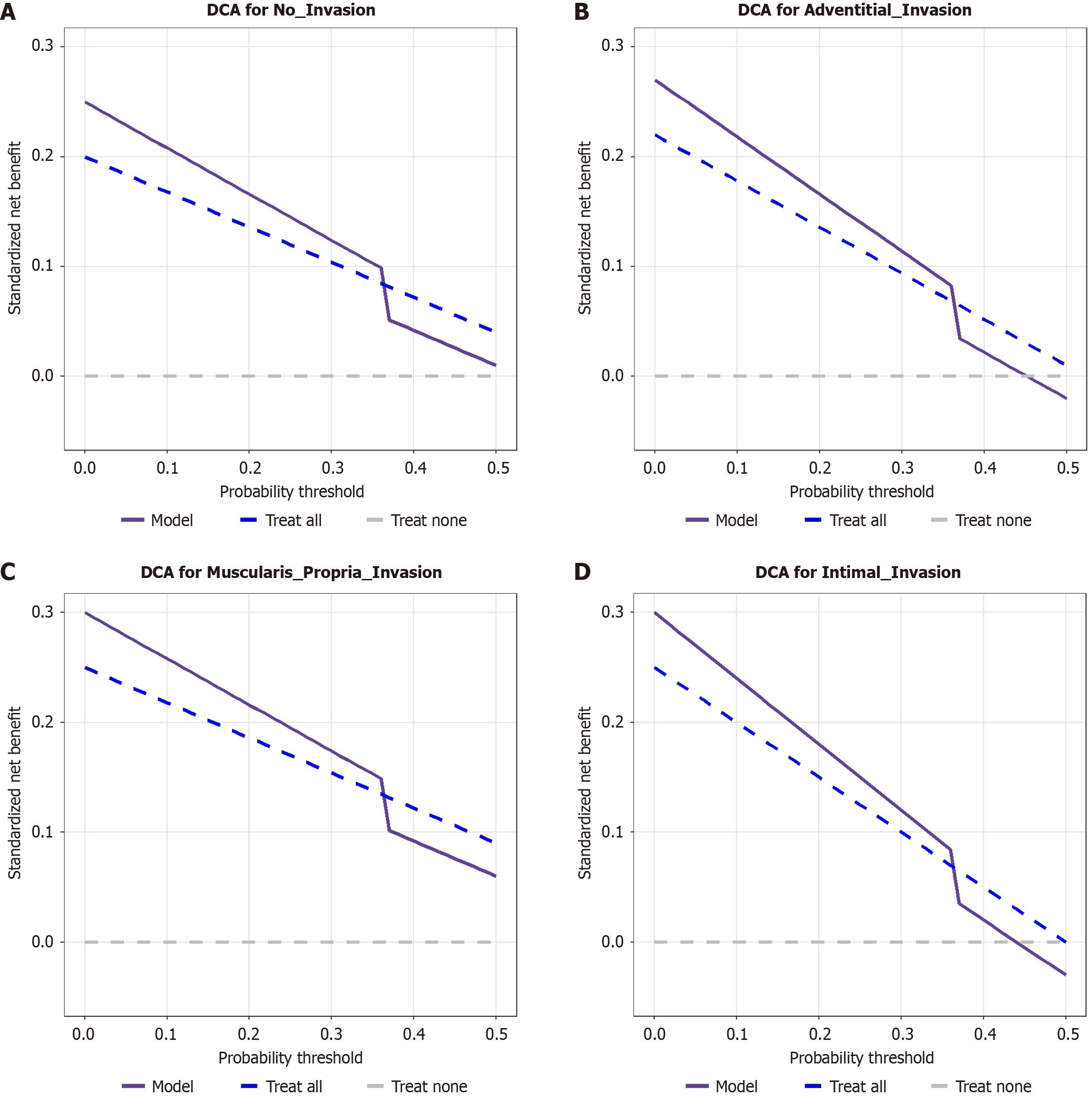
The three curves were plotted in each panel: Model (red solid line), treat all (blue dashed line), and treat none (green dashed line). The horizontal axis represents the threshold probability (0-1.0), and the vertical axis the standardized net benefit (-0.05 to 0.3). Within the threshold probability range of 0-0.7, the net benefit of the model curve exceeded that of the treat all and treat none curves. Beyond the 0.7 threshold, the model curve gradually fell below the treat all curve but remained above the treat none curve in the no-invasion and MPI groups. This demonstrates that the model provides superior clinical utility for predicting all grades of PSVI (no invasion, ADI, MPI, ITI) within the 0-0.7 probability threshold range, yielding higher net benefit than the treat-all or treat-none strategies.
BRPC, defined by 2024 NCCN criteria[8], requires ≤ 180° arterial contact (no encasement) plus reconstructable venous involvement (stenosis ≤ 50% or contact ≤ 180°). This definition reflects a paradigm shift from static anatomy to dynamic multidimensional assessment. Three pivotal advances drove this evolution: (1) Traditional vascular criteria missed 23% of operable patients[9]; (2) New consensus mandates integrated venous stenosis/contact/hemodynamic metrics[10]; and (3) Neoadjuvant response-guided frameworks now direct resectability[11]. Crucially, imaging innovations enable this transition: Multidetector CT quantifies venous angles (0°-360°) and stenosis (0%-100%), while ≤ 1 mm thin-slice re
This study developed a predictive model for pathological vascular invasion grading in BRPC. Innovatively utilizing routine preoperative indicators, serum CA19-9, CT-based venous circumferential contact angle, and luminal stenosis, the model achieves preoperative stratification of vascular invasion depth, demonstrating a C-index of 0.928 (95%CI: 0.889-0.967). First, we confirmed that invasion depth significantly affected prognosis. Patients with ITI exhibited a median OS of only 9 months and 5-year OS of 0%, markedly lower than the no-invasion group (17 months, 12.5%; log-rank P < 0.001). Multivariable Cox regression identified three independent risk factors: CA19-9 > 500 U/mL (OR = 3.819, Wald = 14.125, 95%CI: 1.980-7.410); circumferential involvement > 180° (OR = 8.271, Wald = 33.352, 95%CI: 3.950-17.320); and luminal stenosis (OR = 3.544, Wald = 8.489, 95%CI: 1.818-6.447). The established risk-scoring system delineated 100-250 points as the critical clinical decision window (probability of IT invasion approximately 30% at 200 points), with scores > 310 points indicating > 90% ITI risk. DCA validated significant net clinical benefit across thresholds of 0-0.7. This model serves as a quantitative tool for neoadjuvant therapy selection and surgical planning, advancing PC management into an era of precision decision-making integrating anatomical features, functional evaluation, and treatment response assessment (Figure 5).
This multifactorial analysis aligned with and expanded upon several pivotal recent studies. Regarding CA19-9, our findings demonstrated its superior prognostic value as a continuous variable, corroborating a 2023 study (n = 876)[15], which established that dynamic CA19-9 patterns, particularly post-neoadjuvant decline, outperform single-threshold dichotomization. Our venous circumferential involvement data revealed that patients with > 180° contact exhibited prognostic characteristics closer to ISGPS grade III than grade II; a discovery substantiated by Bhutiani et al[16] in 2022 in a hemodynamic simulation study, which demonstrated that > 180° contact induces significant flow turbulence (P < 0.001), potentially promoting tumor cell shedding. In evaluating luminal stenosis, our quantitative approach converged with the 2023 stenosis index concept of Khoja et al[17] in 2023, echoing their warning that qualitative stenosis percentage assessments risk critical information loss. Our pioneering integration of venous geometric features with CA19-9 kinetics resonates with VI phenotype theory, positing intrinsic links between molecular signatures (e.g., CA19-9 secretion patterns) and VI patterns. Our nomogram demonstrated superior predictive accuracy (area under the curve = 0.928 vs ISGPS 0.791, P = 0.004) and enhanced clinical utility over traditional ISGPS grading.
The clinical translation value of this study manifests in three key domains. First, regarding preoperative assessment, our quantitative model provides surgeons with enhanced risk stratification. For ISGPS grade II patients, a nomogram score > 120 prompts consideration of intensified neoadjuvant therapy rather than immediate surgery, aligning with the 2023 American Society of Clinical Oncology guideline update advocating risk-adapted preoperative strategies. Second, for surgical planning, our luminal defect assessment accurately predicts venous reconstruction complexity. Moazzam et al[18] in 2022 confirmed that precise CT-based stenosis measurement significantly improves venous resection decisions (OR = 3.21, 95%CI: 1.87-5.49). Third, in adjuvant therapy, our risk stratification identified patients who may benefit from novel targeted agents against vascular invasion pathways (e.g., vascular endothelial growth factor/focal adhesion kinase inhibitors). From a technical innovation perspective, this work achieved three breakthroughs: (1) Pioneering multidimensional integration of venous geometric parameters (circumferential contact angle, luminal stenosis) with molecular biomarkers (CA19-9); (2) Developing standardized measurements using routine CT imaging that are more widely deployable than the artificial intelligence-assisted protocol reported by Gupta et al[19]; and (3) Establishing a resource-adaptable risk assessment system addressing the precision medicine resource disparity noted by Kjaer et al[20].
Despite these achievements, several limitations warrant attention. Methodologically, although PSM was used to mitigate confounding factors in this retrospective study, an inherent evidence-level gap persists compared to prospective designs. Technically, although a standardized imaging assessment protocol was established, its sensitivity for detecting microvascular invasion may remain suboptimal due to spatial resolution constraints. Biologically, the absence of single-cell sequencing data precludes exploration of molecular signatures underlying distinct VI patterns. To address these limitations, future research should prioritize multicenter prospective validation, integrate liquid biopsy technologies, and investigate radiogenomic correlations between imaging phenotypes and molecular subtypes. While our model demonstrated robust internal validation (C-index = 0.928), three key limitations warrant consideration. The single-center design at Beijing Chao-Yang Hospital, Capital Medical University (2011-2023) introduces inherent referral bias from its tertiary care population, potentially inflating accuracy through institution-specific factors including homogeneous demographics and standardized protocols. Concurrently, temporal evolution in CT technology (64 to 128-detector transition) and treatment paradigms (FOLFIRINOX adoption post-2015) may affect model transportability to contemporary practice despite standardized post-processing. These constraints limit external applicability where variations in VI assessment or CA19-9 methodologies exist, a challenge we are addressing through multicenter validation, prospective real-world cohort studies with temporal modeling (landmark analysis), and artificial intelligence-driven adaptive learning for enhanced robustness across heterogeneous clinical environments.
The nomogram model developed in this study integrates routine clinical parameters to preoperatively discriminate PSVI depth in BRPC using CT imaging, thereby guiding therapeutic decision-making and providing an evidence-based foundation for personalized surgical planning.
The authors extend sincere gratitude to all participating patients for their essential contributions to this research. We also acknowledge He Q for his valuable scientific guidance and manuscript review.
| 1. | Bolm L, Pisuchpen N, Qadan M, Kambadakone A, Sondermann S, Mueller K, Petruch N, May K, Zelga P, Nebbia M, Michelakos T, Baba T, Roldan J, Harrison JM, Honselmann KC, Keck T, Lillemoe KD, Ferrone CR, Wellner UF, Fernandez-Del Castillo C. Prediction of R Status in Resections for Pancreatic Cancer Using Simplified Radiological Criteria. Ann Surg. 2022;276:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6103] [Article Influence: 3051.5] [Reference Citation Analysis (4)] |
| 3. | Vargas Pecino C, Castellano E, Trujillo H. Factors associated with substance use among Spanish military personnel involved in "Bosnia-Herzegovina". Adicciones. 2017;29:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, Jäger D, Schirmacher P, Hackert T, Büchler MW. Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg. 2017;265:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 5. | Chu LC, Fishman EK. Pancreatic ductal adenocarcinoma staging: a narrative review of radiologic techniques and advances. Int J Surg. 2024;110:6052-6063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Nickel F, Wise PA, Müller PC, Kuemmerli C, Cizmic A, Salg GA, Steinle V, Niessen A, Mayer P, Mehrabi A, Loos M, Müller-Stich BP, Kulu Y, Büchler MW, Hackert T. Short-term Outcomes of Robotic Versus Open Pancreatoduodenectomy: Propensity Score-matched Analysis. Ann Surg. 2024;279:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Garey L, Manning K, Jardin C, Leventhal AM, Stone M, Raines AM, Pang RD, Neighbors C, Schmidt NB, Zvolensky MJ. Smoking Consequences Questionnaire: A reevaluation of the psychometric properties across two independent samples of smokers. Psychol Assess. 2018;30:678-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Montoro-Pérez N, Martínez-González AE, Infante-Cañete L, de Los Ángeles Martínez-González M, Hidalgo-Berutich S, Andreo-Martínez P. Development and validation of the Gastrointestinal Symptom Severity Scale in Spanish children and adolescents. Eur J Pediatr. 2024;183:2703-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Waljee JF, Gunaseelan V, Bicket MC, Brummett CM, Chua KP. Safety and Distribution of Opioid Prescribing by U.S. Surgeons. Ann Surg. 2023;277:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Bae MS, Seo BK. Breast Cancer Screening with Digital Breast Tomosynthesis Improves Performance of Mammography Screening. Radiology. 2023;307:e230306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Turrel E, Chopin N, Meeus P, Blache A, Ray-Coquard I, Tredan O, Treilleux I, Ebring C, Heinemann M, Rossi L. Do surgeons overestimate diaphragmatic peritoneal disease in interval debulking surgery of ovarian cancer? Eur J Surg Oncol. 2023;49:106963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zhang M, Tan Z, Zhang Q, Shen Y, Mao X, Wei L, Sun R, Zhou F, Liu C. Flexible Self-Powered Friction Piezoelectric Sensor Based on Structured PVDF-Based Composite Nanofiber Membranes. ACS Appl Mater Interfaces. 2023;15:30849-30858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 13. | Chao TC, Wang SH, Chen YC, Li TY. Successful transfemoral prosthesis in a patient with haemophilia A and factor VIII inhibitors: A case report. J Int Med Res. 2023;51:3000605231195446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Xu X, Zhang Y, Gan J, Ye X, Yu X, Huang Y. Association Between Storage Time of Transfused Red Blood Cells and Infection After Clean-contaminated Surgery: A Retrospective Cohort Study. Ann Surg. 2024;280:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Vallejos PA, Gonda A, Yu J, Sullivan BG, Ostowari A, Kwong ML, Choi A, Selleck MJ, Kabagwira J, Fuller RN, Gironda DJ, Levine EA, Hughes CCW, Wall NR, Miller LD, Senthil M. ASO Visual Abstract: Plasma Exosome Gene Signature Differentiates Colon Cancer from Healthy Controls. Ann Surg Oncol. 2023;30:3847-3848. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Bhutiani N, Cox DM, Robinson KA, Kim BJ, Mansfield PF, Fournier KF, White MG. Stapled Versus Hand-Sewn Anastomosis in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. J Gastrointest Surg. 2022;26:2365-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Khoja K, Sadiq O, Chisholm PR, Dua KS, Madhavan S, Smith ZL. The incidence of new mental health disorders after acute pancreatitis: A large, propensity-matched, observational study. Pancreatology. 2023;23:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Moazzam Z, Lima HA, Alaimo L, Endo Y, Shaikh CF, Ratti F, Marques HP, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Impact of tumor burden score on timing and patterns of recurrence after curative-intent resection of hepatocellular carcinoma. Surgery. 2022;172:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 19. | Gupta P, Rana P, Marodia Y, Samanta J, Sharma V, Sinha SK, Singh H, Gupta V, Yadav TD, Sreenivasan R, Vaiphei K, Rajwanshi A, Kochhar R, Sandhu M. Contrast-enhanced ultrasound of solid pancreatic head lesions: a prospective study. Eur Radiol. 2022;32:6668-6677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kjaer J, Norlén O, Hellman P, Stalberg P. Correction: Author´s Reply: Overall Survival in Patients with Stage IV Pan-NET Eligible for Liver Transplantation. World J Surg. 2023;47:1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/