Published online Nov 14, 2025. doi: 10.3748/wjg.v31.i42.110717
Revised: July 22, 2025
Accepted: October 9, 2025
Published online: November 14, 2025
Processing time: 153 Days and 18.8 Hours
Helicobacter pylori (H. pylori) is a Gram-negative bacillus classified as a Group I carcinogen by the World Health Organization. However, the efficacy of eradi
To investigate the efficacy of precision-guided first-line therapy for H. pylori in
This single-center randomized controlled trial enrolled 194 H. pylori-positive patients at a tertiary hospital in Qingdao, China (October 2022-August 2024). Participants were randomized to receive either a 14-day bismuth quadruple therapy (BQT: Amoxicillin, clarithromycin, esomeprazole, and bismuth) or a 14-day G-AST-guided regimen with tailored antibiotics (clarithromycin, levofloxacin, or tetracycline). Treatment efficacy and adverse events were compared between groups using intention-to-treat (ITT) and per-protocol (PP) analyses. Primary and secondary outcomes were analyzed with χ² tests.
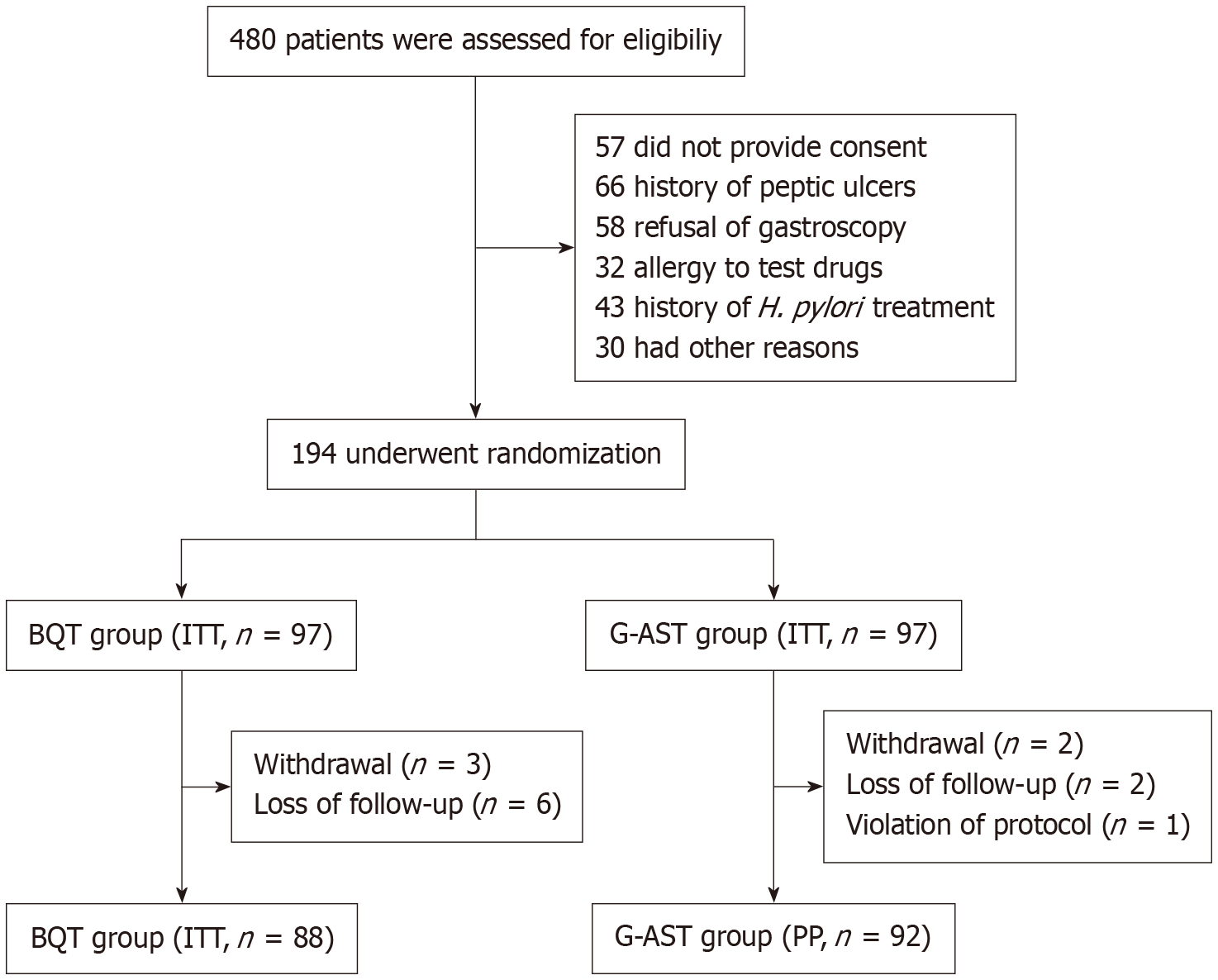
Of 194 patients enrolled, 180 (92.8%) completed the study as planned. In the ITT analysis, the eradication rate was higher in the G-AST group than in the BQT group [92.8% (95%CI: 85.8-96.5) vs 79.4% (95%CI: 70.3-86.2), P = 0.007], with a risk difference of 13.4% (95%CI: 3.7-23.2). In the PP analysis, eradication rates were 97.8% (95%CI: 92.4-99.4) in the G-AST group and 84.1% (95%CI: 75.1-90.3) in the BQT group (P = 0.001), with a risk difference of 13.7% (95%CI: 5.5-23.0). Adverse event incidence did not differ significantly between groups (30.9% vs 28.9%, P = 0.754).
G-AST-guided therapy yielded higher eradication rates than empirical BQT in first-line H. pylori treatment without increasing adverse events, supporting the clinical utility of individualized, resistance-based therapy.
Core Tip: Antibiotic resistance in Helicobacter pylori (H. pylori) has reduced the efficacy of traditional eradication regimens. This randomized controlled trial in northern China used rapid genotypic antibiotic susceptibility testing (G-AST) to guide first-line therapy by tailoring regimens according to detected resistance mutations, including tetracycline for dual-resistant strains. G-AST-guided therapy significantly improved eradication rates compared with standard bismuth quadruple therapy without increasing adverse events. These findings demonstrate the feasibility and clinical benefit of molecular-guided personalized treatment, offering a promising strategy to overcome antibiotic resistance in H. pylori infection.
- Citation: Xu Y, Hao JW, Min CC, Yang L, Ma CP, Shi C, Mao T, Tian ZB, Wang T, Yu YN. Precision therapy guided by genotypic antibiotic resistance for Helicobacter pylori eradication: A prospective, randomized controlled trial. World J Gastroenterol 2025; 31(42): 110717
- URL: https://www.wjgnet.com/1007-9327/full/v31/i42/110717.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i42.110717
With an adult global prevalence of approximately 43.9%, Helicobacter pylori (H. pylori) infection is a significant etiological factor in gastrointestinal disease[1]. H. pylori plays a crucial role in the pathogenesis of peptic ulcers and is closely associated with gastric cancer. Eradication substantially improves gastric health and reduces the risk of peptic ulcers, gastric malignancies, and mucosa-associated lymphoid tissue lymphoma.
In China, the standard approach for H. pylori eradication is bismuth quadruple therapy (BQT), which combines acid inhibitors and antibiotics for optimal results[2]. However, increasing antibiotic resistance, poor patient adherence, and low intragastric antibiotic concentrations have contributed to a gradual decline in the success rate of BQT[3]. Recent data from the European H. pylori Management Registration Center show that 14-day BQT achieves an 83% eradication rate[4]. In China, the average eradication rate for BQT is 81.3%[2]. The Maastricht VI/Florence Consensus Report places strong emphasis on antibiotic stewardship and recommends tailoring treatment strategies to local and individual antimicrobial resistance patterns[5].
Currently, H. pylori antimicrobial susceptibility is assessed with two primary approaches: Phenotypic testing, which relies on H. pylori culture, and genotypic antibiotic susceptibility testing (G-AST). Phenotypic drug resistance testing via H. pylori culture is complex and costly, which limits its widespread clinical application. G-AST has emerged as a promising strategy for personalized H. pylori treatment, offering advantages in efficiency, speed, and diagnostic accuracy[6]. It has demonstrated clinical value across diverse infectious diseases by rapidly detecting resistance mutations to guide therapy. It has been applied in the treatment of tuberculosis and bloodstream infections to optimize treatment and reduce antibiotic misuse[7,8]. This broad applicability supports the use of G-AST for targeted H. pylori eradication. Moreover, there is strong concordance between genotypic and phenotypic resistance to clarithromycin and levofloxacin, indicating that these genetic markers can serve as reliable and practical targets for directing personalized treatment strategies in H. pylori infections[9].
Polymerase chain reaction (PCR) testing was performed on gastric mucosal samples from treatment-naive patients, guiding individualized therapy based on G-AST for levofloxacin and clarithromycin. Previous studies have demonstrated that this PCR method yields results comparable to those of gastric biopsy analysis and Sanger sequencing, while maintaining practicality and ease of use[10]. This study aimed to investigate the efficacy of precision-guided first-line therapy for H. pylori infection using the G-AST method.
This single-center prospective randomized controlled trial was conducted at a tertiary hospital in Qingdao, China, between October 2022 and August 2024. Participants aged 18-70 years who tested positive for H. pylori infection by the 13C/14C urea breath test (UBT) and consented to gastroscopy with biopsy for histopathological evaluation were enrolled. Primary exclusion criteria were active gastrointestinal bleeding; prior upper gastrointestinal surgery; pregnancy or lactation without willingness to use contraception during the study; prior unsuccessful H. pylori eradication therapy; severe comorbidities potentially affecting evaluation (e.g., advanced hepatic, pulmonary, cardiac, or renal disease; malignancy), and known allergy to study medications.
Approval was obtained from the Ethics Committee of the Affiliated Hospital of Qingdao University (Approval No. QYFYKYLL 923711921). All trial procedures complied with Clinical Trial Standards/Code guidelines. Participants pro
Eligible participants were randomized 1:1 to the G-AST or BQT group using a computer-generated random sequence. Allocation was concealed with opaque sealed envelopes labeled with unique subject identifiers. The trial used an open-label design, and all participants and healthcare providers were aware of the assigned treatment after randomization.
All patients underwent gastroscopy at the hospital. Two gastric mucosal biopsy specimens, approximately 0.1 cm in diameter, were obtained from the antrum 1-2 cm proximal to the pylorus. For patients in the G-AST group, an H. pylori drug resistance gene nucleic acid detection kit (Qingdao Jianma Gene Detection Company) was used for PCR amp
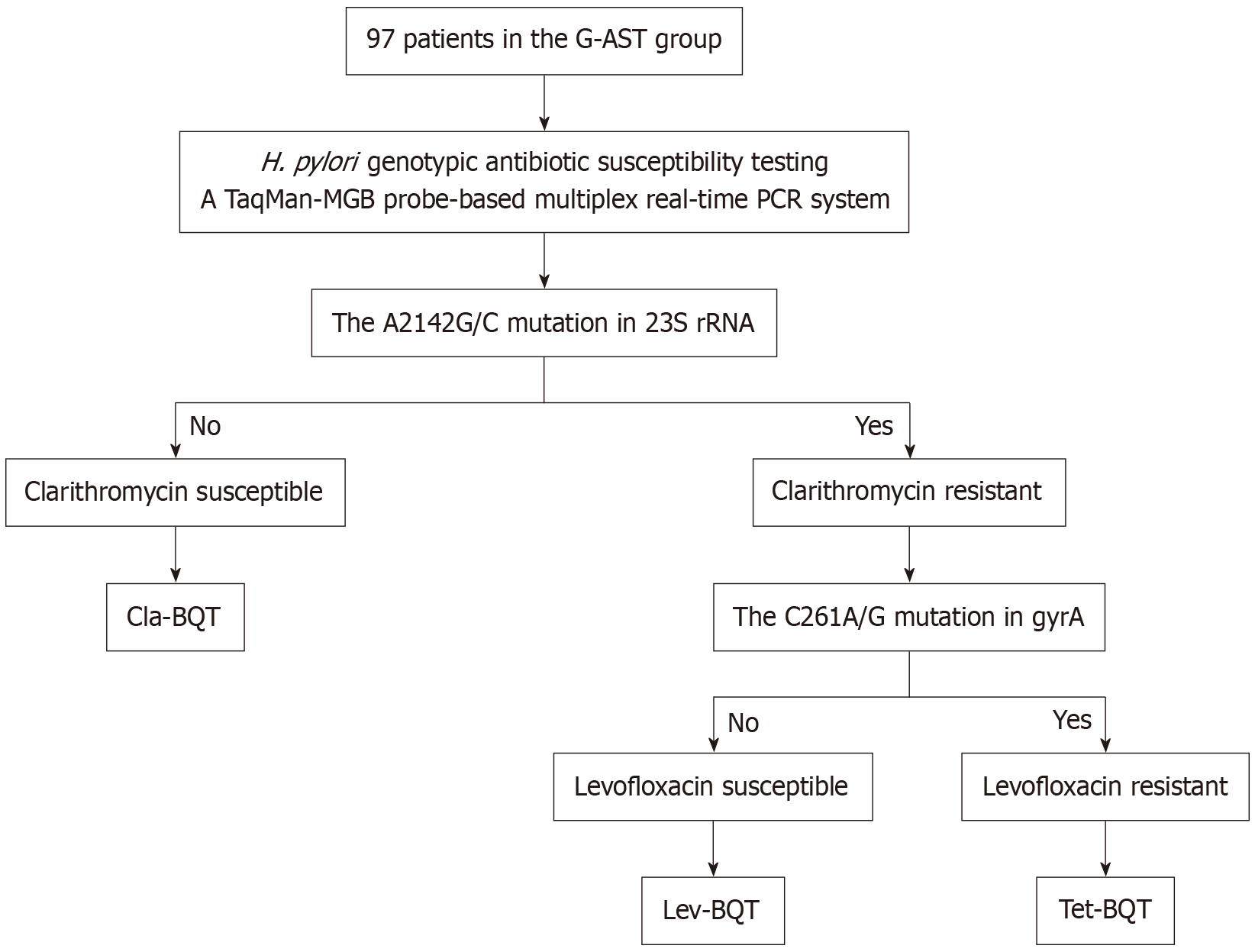
The BQT group received a twice-daily regimen of esomeprazole, bismuth potassium citrate, amoxicillin, and clarithromycin for 14 days. The G-AST group received regimens tailored to G-AST results. The detailed procedure is shown in Figure 1. Because levofloxacin-based quadruple therapy is infrequently used as a primary strategy for H. pylori era
| Regimen | Clarithromycin | Levofloxacin | Drugs | ||
| Clarithromycin | Levofloxacin | Tetracycline | |||
| Cla-BQT | S | - | 500 mg, bid | - | - |
| Lev-BQT | R | S | - | 500 mg, qd | - |
| Tet-BQT | R | R | - | - | 500 mg, tid |
Investigators completed a case report form and recorded baseline demographic data for each patient. After treatment initiation, investigators conducted telephone follow-ups to monitor adherence and document any adverse events that occurred during therapy.
After completing eradication therapy, participants underwent a UBT (13C or 14C) 5 weeks later to assess efficacy (proton pump inhibitors and H2 receptor antagonists were prohibited for 2 weeks before the test; antibiotics or probiotics were not permitted within 4 weeks). A delta over baseline (DOB) value < 4‰ in the 13C-UBT was considered negative (su
Sample size estimation was based on eradication rates reported in earlier studies[11], with expected rates of 80% in the control group and 94% in the trial group. With an 80% power (1 - β), a two-sided α level of 0.05, and calculations per
Demographic characteristics and the incidence of adverse events were recorded. Eradication rates were evaluated by intention-to-treat (ITT) and per-protocol (PP) analyses. ITT included all randomized patients; PP included only those who completed treatment with ≥ 80% adherence and without loss to follow-up. Continuous variables with a normal distribution were expressed as mean ± SD; Categorical variables were presented as frequencies and percentages. For con
Between October 2022 and August 2024, 194 patients were enrolled; 180 completed all required study procedures (Figure 2). Baseline clinical characteristics did not differ significantly between groups (P > 0.05; Table 2).
| Baseline characteristics | BQT group (n = 97) | G-AST group (n = 97) | P value | χ²/t/z |
| Age (years), mean ± SD | 52.81 ± 10.69 | 50.20 ± 9.45 | 0.072 | 1.808 |
| Gender (male) | 46 (47.4) | 48 (49.5) | 0.083 | 0.774 |
| Height (m), mean ± SD | 1.67 ± 0.08 | 1.68 ± 0.09 | 0.230 | -1.2 |
| Weight (kg), mean ± SD | 66.14 ± 10.56 | 68.24 ± 11.62 | 0.189 | -1.318 |
| BMI, mean ± SD | 23.73 ± 2.89 | 24.08 ± 3.24 | 0.432 | -0.787 |
| Smoking | 20 (20.6) | 14 (14.4) | 0.257 | 1.284 |
| Alcohol consumption | 22 (22.7) | 22 (22.7) | 1.0 | 0 |
| Hypertension | 23 (23.7) | 19 (19.6) | 0.486 | 0.486 |
| Diabetes | 22 (22.7) | 13 (13.4) | 0.093 | 2.824 |
| Family history of gastric cancer | 11 (11.3) | 13 (13.4) | 0.663 | 0.190 |
| Bile reflux | 10 (10.3) | 15 (15.5) | 0.284 | 1.148 |
| Atrophic gastritis | 63 (64.9) | 52 (53.6) | 0.108 | 2.584 |
The G-AST group achieved eradication rates of 92.8% (90/97) in the ITT analysis and 97.8% (90/92) in the PP analysis. Eradication rates in the G-AST group were significantly higher than those in the BQT group in both analyses (both P < 0.05; Table 3).
| BQT group | G-AST group | Difference in eradication rate (%) | P value | χ² | |
| ITT | 77 (79.4) | 90 (92.8) | 13.4 | 0.007 | 7.271 |
| 95%CI | 70.3-86.2 | 85.8-96.5 | 3.7-23.2 | ||
| PP | 74 (84.1) | 90 (97.8) | 13.7 | 0.001 | 10.477 |
| 95%CI | 75.1-90.3 | 92.4-99.4 | 5.5-23.0 |
In the G-AST group, 60 patients received clarithromycin-containing BQT (Cla-BQT), 19 received levofloxacin-containing BQT (Lev-BQT), and 18 received tetracycline-containing BQT (Tet-BQT). H. pylori eradication rates in these subgroups were 91.7%, 94.7%, and 94.4% (ITT), and 98.2%, 94.7%, and 100% (PP), respectively. Eradication rates did not differ significantly among the three subgroups (ITT: P = 0.863; PP: P = 0.530; Table 4).
| G-AST group | Eradication rate, n/N [% (95%CI)] | |
| ITT | PP | |
| Overall eradication rates | 90/97 [92.8 (85.8-96.5)] | 90/92 [97.8 (92.4-99.4)] |
| Cla-BQT | 55/60 [91.7 (81.9-96.4)] | 55/56 [98.2 (90.6-99.7)] |
| Lev-BQT | 18/19 [94.7 (75.4-99.1)] | 18/19 [94.7 (75.4-99.1)] |
| Tet-BQT | 17/18 [94.4 (74.2-99.0)] | 17/17 [100 (81.6-100.0)] |
| P value | 0.863 | 0.530 |
| χ² | 0.294 | 1.270 |
In the G-AST group, 60 H. pylori strains were susceptible to clarithromycin (61.9%), and 37 were resistant (38.1%). The levofloxacin resistance rate was 40.2%, and dual resistance to clarithromycin and levofloxacin was 17.5% (Table 5).
| Antibiotics | Susceptible | Resistant |
| Clarithromycin | 60 (61.9) | 37 (38.1) |
| Levofloxacin | 58 (59.8) | 39 (40.2) |
| Clarithromycin and levofloxacin | 38 (39.2) | 17 (17.5) |
Fifty-eight patients reported adverse events: 28.9% (28/97) in the BQT group and 30.9% (30/97) in the G-AST group. All adverse symptoms resolved spontaneously after treatment completion. No significant difference in adverse events was observed between the groups (P = 0.754). Adverse event incidence in the Cla-BQT, Lev-BQT, and Tet-BQT subgroups was 30.0% (18/60), 31.6% (6/19), and 33.3% (6/18), respectively, with no significant differences (P = 0.890). The most common adverse events were bitter taste (16/194) and nausea (9/194) (Table 6).
| Adverse events | BQT group (n = 97) | G-AST group (n = 97) | P value |
| Bitter taste | 9 (9.3) | 7 (7.2) | |
| Nausea | 5 (5.2) | 4 (4.1) | |
| Abdominal pain | 3 (3.1) | 4 (4.1) | |
| Diarrhea | 4 (4.1) | 3 (3.1) | |
| Abdominal discomfort | 2 (2.1) | 2 (2.1) | |
| Bloating | 1 (1.0) | 3 (3.1) | |
| Heartburn | 1 (1.0) | 2 (2.1) | |
| Skin rash | 1 (1.0) | 2 (2.1) | |
| Dizziness | 2 (2.1) | 3 (3.1) | |
| Total | 28/97 (28.9) | 30/97 (30.9) | 0.754 |
Antibiotic resistance has gradually increased, and the effectiveness of eradicating H. pylori infection has steadily declined[12]. A recent systematic review of antibiotic resistance in the Asia-Pacific region from 1990 to 2023 reported primary resistance rates of 30% for clarithromycin and 35% for levofloxacin[13]. Amoxicillin and tetracycline showed substantially lower resistance rates of 2.41% and 2.53%, respectively[14].
The A2142G/C mutation in 23S rRNA and the C261A/G mutation in gyrA are associated with resistance to clarithromycin and levofloxacin, respectively[15,16]. Genotypic resistance correlates strongly with phenotypic resistance to these agents in H. pylori strains[17], and a meta-analysis confirmed the high sensitivity and specificity of genotypic detection for clarithromycin and fluoroquinolone resistance[18]. Moreover, genotypic testing outperforms phenotypic methods in predicting treatment outcomes[19] and is increasingly favored when performed on gastric mucosal samples to guide eradication therapy[20].
According to the Maastricht VI/Florence consensus, the goal of antimicrobial therapy is to reliably cure H. pylori infection in most patients (e.g., ≥ 90%)[5]. Our findings demonstrate that G-AST-guided therapy, tailored to clarithromycin and levofloxacin resistance, meets and exceeds this efficacy threshold. All three G-AST-based regimens—Cla-BQT, Lev-BQT, and Tet-BQT—achieved ITT and PP eradication rates above 90%. These outcomes highlight the clinical effectiveness of individualized therapy guided by G-AST.
The resistance rates to clarithromycin and levofloxacin were both approximately 40%, with a dual resistance rate of 17.5%, consistent with recent Chinese literature[14], reaffirming the relevance of G-AST in settings with substantial antimicrobial resistance. Patients receiving G-AST-guided Cla-BQT or Lev-BQT achieved eradication rates markedly higher than previously reported empirical rates for the same regimens (75.2% and 68.0%, respectively)[21]. Patients with dual resistance were successfully treated with Tet-BQT, reaching 100% eradication in the PP analysis. Although this result likely reflects the small sample size, it is concordant with the low tetracycline resistance reported in Chinese H. pylori strains[14]. Widespread empirical tetracycline use remains challenging due to limited availability, concerns about adverse effects, and dosing complexity[22,23]. Within a G-AST framework, however, tetracycline can be deployed selectively and effectively rather than as a universal empirical option. Overall, the success of G-AST-guided therapy across all subgroups highlights its practical value for optimizing eradication outcomes while advancing antibiotic stewardship. This precision-based strategy is particularly suitable for regions with moderate to high resistance rates where empirical regimens often underperform, and it provides a feasible pathway for integrating molecular diagnostics into routine care.
Adverse event rates were comparable between the G-AST and BQT groups, with no significant differences among G-AST subgroups. The most common adverse reactions were a bitter taste and nausea, likely related to the effects of bismuth potassium citrate or antibiotics. Structured follow-up calls, both during and after therapy, facilitated systematic assessment of adverse events. The adverse reaction rate in the Lev-BQT subgroup was 31.6%. No serious adverse events (e.g., tendinitis, tendon rupture, neuropathy) occurred with levofloxacin, which may be related to the 14-day course, standard dosing (500 mg once daily), and exclusion of high-risk individuals. The predominant side effects were mild gastrointestinal symptoms that resolved spontaneously. These findings align with prior studies[24,25] that support the safety of levofloxacin-based regimens for H. pylori eradication when used appropriately and monitored.
Clinically, G-AST-guided therapy significantly improves eradication outcomes without increasing adverse events, providing a practical and effective approach to personalized treatment. Although PCR-based genotypic testing and gastric biopsy entail higher upfront costs than empirical therapy, long-term benefits—including reduced treatment failures, fewer retreatments, and mitigation of antibiotic resistance—justify the investment. The broader applicability depends on local resistance patterns and the healthcare infrastructure. G-AST is particularly advantageous in areas with moderate to high clarithromycin and levofloxacin resistance, where empirical regimens are less effective. In low-resistance settings, adoption should be guided by local cost-effectiveness assessments and diagnostic capacity.
This study has several limitations. First, the study was conducted at a single tertiary center in Northern China, which may limit its generalizability to regions with different resistance profiles or healthcare systems. Second, all participants were receiving initial H. pylori therapy. Further studies are needed to evaluate the effectiveness of personalized eradi
G-AST-guided precision therapy achieved higher first-line H. pylori eradication rates than empirical BQT (92.8% vs 79.4%, ITT), without increasing adverse events. These findings highlight the clinical utility of individualized, resistance-based therapy and support the integration of G-AST into routine clinical practice to improve treatment outcomes.
We sincerely thank all personnel who supported patient recruitment and sample collection, as well as the laboratory staff for their PCR technical assistance.
| 1. | Chen YC, Malfertheiner P, Yu HT, Kuo CL, Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, Wu CY, Lin JT, O'Morain C, Megraud F, Lee WC, El-Omar EM, Wu MS, Liou JM. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology. 2024;166:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 283] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 2. | Zhou L, Lu H, Song Z, Lyu B, Chen Y, Wang J, Xia J, Zhao Z; on behalf of Helicobacter Pylori Study Group of Chinese Society of Gastroenterology. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin Med J (Engl). 2022;135:2899-2910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 3. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 576] [Article Influence: 192.0] [Reference Citation Analysis (1)] |
| 4. | Nyssen OP, Bordin D, Tepes B, Pérez-Aisa Á, Vaira D, Caldas M, Bujanda L, Castro-Fernandez M, Lerang F, Leja M, Rodrigo L, Rokkas T, Kupcinskas L, Pérez-Lasala J, Jonaitis L, Shvets O, Gasbarrini A, Simsek H, Axon ATR, Buzás G, Machado JC, Niv Y, Boyanova L, Goldis A, Lamy V, Tonkic A, Przytulski K, Beglinger C, Venerito M, Bytzer P, Capelle L, Milosavljević T, Milivojevic V, Veijola L, Molina-Infante J, Vologzhanina L, Fadeenko G, Ariño I, Fiorini G, Garre A, Garrido J, F Pérez C, Puig I, Heluwaert F, Megraud F, O'Morain C, Gisbert JP; Hp-EuReg Investigators. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2021;70:40-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 5. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;gutjnl-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 852] [Article Influence: 213.0] [Reference Citation Analysis (0)] |
| 6. | Chen MJ, Chen PY, Fang YJ, Bair MJ, Chen CC, Chen CC, Yang TH, Lee JY, Yu CC, Kuo CC, Chiu MC, Chou CK, Chen CY, Hu WH, Tsai MH, Hsu YC, Shun CT, Luo JC, Lin JT, El-Omar EM, Wu MS, Liou JM; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Molecular testing-guided therapy versus susceptibility testing-guided therapy in first-line and third-line Helicobacter pylori eradication: two multicentre, open-label, randomised controlled, non-inferiority trials. Lancet Gastroenterol Hepatol. 2023;8:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Banerjee R, Humphries R. Rapid Antimicrobial Susceptibility Testing Methods for Blood Cultures and Their Clinical Impact. Front Med (Lausanne). 2021;8:635831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Aung WW, Ei PW, Nyunt WW, Swe TL, Lwin T, Htwe MM, Kim KJ, Lee JS, Kim CK, Cho SN, Song SD, Chang CL. Phenotypic and genotypic analysis of anti-tuberculosis drug resistance in Mycobacterium tuberculosis isolates in Myanmar. Ann Lab Med. 2015;35:494-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Cui R, Song Z, Suo B, Tian X, Xue Y, Meng L, Niu Z, Jin Z, Zhang H, Zhou L. Correlation Analysis Among Genotype Resistance, Phenotype Resistance and Eradication Effect of Helicobacter pylori. Infect Drug Resist. 2021;14:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Zhao Y, Li Y, Luan Z, Ma C, Yang L, Zhang W, Shi C. Establishment of a TaqMan-MGB probe multiplex real-time PCR system for one-step levofloxacin and clarithromycin resistant Helicobacter pylori detection. J Microbiol Methods. 2022;192:106393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Li CL, Zhou K, Zhang YX, Suo BJ, Tian XL, Zhang YX, Ren XL, Shi YY, Zhou LY, Song ZQ. Tailored therapy guided by genotypic resistance of clarithromycin and levofloxacin detected by polymerase chain reaction in the first-line treatment of Helicobacter pylori infection. J Dig Dis. 2024;25:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 586] [Article Influence: 58.6] [Reference Citation Analysis (2)] |
| 13. | Kuo YT, Liou JM, El-Omar EM, Wu JY, Leow AHR, Goh KL, Das R, Lu H, Lin JT, Tu YK, Yamaoka Y, Wu MS; Asian Pacific Alliance on Helicobacter and Microbiota. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Zeng S, Kong Q, Wu X, Duan M, Nan X, Yang X, Zuo X, Li Y, Li Y. Antibiotic resistance of Helicobacter pylori in Mainland China: A focus on geographic differences through systematic review and meta-analysis. Int J Antimicrob Agents. 2024;64:107325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 15. | Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 16. | Wang LH, Cheng H, Hu FL, Li J. Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. World J Gastroenterol. 2010;16:2272-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Xiong M, Mohammed Aljaberi HS, Khalid Ansari N, Sun Y, Yin S, Nasifu L, Sun H, Xu T, Pan Y, Nie Z, Liu C, Zhang Z, Jiang Z, Wang S, He B. Phenotype and genotype analysis for Helicobacter pylori antibiotic resistance in outpatients: a retrospective study. Microbiol Spectr. 2023;11:e0055023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Wang YH, Li Z, Wang L, Zhu-Ge LY, Zhao RL, Wu S, Wang Y, An Y, Xie Y. A systematic review and meta-analysis of genotypic methods for detecting antibiotic resistance in Helicobacter pylori. Helicobacter. 2018;23:e12467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 19. | Liou JM, Chang CY, Sheng WH, Wang YC, Chen MJ, Lee YC, Hung HW, Chian H, Chang SC, Wu MS, Lin JT. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Vasapolli R, Ailloud F, Spießberger B, Malfertheiner P, Suerbaum S, Schulz C. Real-Time Assessment of H. pylori Infection to Guide Molecular Antibiotic Resistance Testing: A Combined Endoscopy-Gastric Juice Analysis Approach. Aliment Pharmacol Ther. 2025;61:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Cheng J, Fan C, Li Z, Dong Z, Zhao X, Cai Y, Ding H, Dou Y, Zhang X. Real-World Situation of Eradication Regimens and Risk Factors for Helicobacter pylori Treatment in China: A Retrospective Single-Center Study. Clin Exp Gastroenterol. 2024;17:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Graham DY, Lee SY. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol Clin North Am. 2015;44:537-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Sun YC, Zhu MJ, Chen XQ, Yue L, Zhao YR, Wang XJ, Kim JJ, Du Q, Hu WL. Efficacy and safety of modified tetracycline dosing in a quadruple therapy for Helicobacter pylori: A retrospective single center study. World J Gastroenterol. 2023;29:3508-3518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Gisbert JP, Romano M, Gravina AG, Solís-Muñoz P, Bermejo F, Molina-Infante J, Castro-Fernández M, Ortuño J, Lucendo AJ, Herranz M, Modolell I, Del Castillo F, Gómez J, Barrio J, Velayos B, Gómez B, Domínguez JL, Miranda A, Martorano M, Algaba A, Pabón M, Angueira T, Fernández-Salazar L, Federico A, Marín AC, McNicholl AG. Helicobacter pylori second-line rescue therapy with levofloxacin- and bismuth-containing quadruple therapy, after failure of standard triple or non-bismuth quadruple treatments. Aliment Pharmacol Ther. 2015;41:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Gisbert JP, Pérez-Aisa A, Bermejo F, Castro-Fernández M, Almela P, Barrio J, Cosme A, Modolell I, Bory F, Fernández-Bermejo M, Rodrigo L, Ortuño J, Sánchez-Pobre P, Khorrami S, Franco A, Tomas A, Guerra I, Lamas E, Ponce J, Calvet X; H. pylori Study Group of the Asociación Española de Gastroenterología (Spanish Gastroenterology Association). Second-line therapy with levofloxacin after failure of treatment to eradicate helicobacter pylori infection: time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol. 2013;47:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/