Published online Oct 28, 2025. doi: 10.3748/wjg.v31.i40.111406
Revised: August 19, 2025
Accepted: September 16, 2025
Published online: October 28, 2025
Processing time: 120 Days and 19.7 Hours
Endoscopic variceal band ligation (EVBL) represents a pivotal treatment in the prophylaxis of esophageal varices bleeding in patients with cirrhosis, but in some cases a single session of EVBL is unable to eradicate esophageal varices comple
To identify non-invasive predictors of varices eradication (VE) after EVBL through multiparametric ultrasound (US). Secondary aim was to develop a predi
We prospectively enrolled consecutive cirrhotic patients intolerant or with con
We enrolled 41 patients (median age 64 years, 75.6% males). At T1 28 patients (68.3%) reached VE, whereas 13 (31.7%) required a second EVBL. Patients who achieved VE showed a significant decrease in SSM (P = 0.018), and a significant increase in peak enhancement, area under the curve and wash-in rate of both liver parenchyma and portal vein after treatment (P < 0.001). Statistically significant differences between the two groups of patients were incorporated in a multivariate analysis and used to develop three prediction models.
A multimodal US approach based on DCE-US parameters, LSM and SSM might become a reliable predictor of VE and a useful non-invasive alternative to endoscopy.
Core Tip: Follow-up endoscopy is commonly used to assess treatment response after endoscopic variceal band ligation (EVBL) in cirrhotic patients. This study evaluated the usefulness of multiparametric ultrasound to predict the short-term outcome of EVBL in patients with liver cirrhosis. We identified significant parameters associated with variceal eradication such as liver and spleen stiffness and longitudinal changes in dynamic contrast enhanced ultrasound variables. This approach could support the usefulness of multiparametric ultrasound for non-invasive evaluation of EVBL efficacy.
- Citation: Ainora ME, Borriello R, Pecere S, Paratore M, Galasso L, Calvez V, Esposto G, Mignini I, Barbaro F, Del Vecchio LE, Ponziani FR, Annicchiarico BE, Garcovich M, Riccardi L, Pompili M, Spada C, Gasbarrini A, Zocco MA. Multiparametric ultrasound for the prediction of the short-term outcome after esophageal varices band ligation. World J Gastroenterol 2025; 31(40): 111406
- URL: https://www.wjgnet.com/1007-9327/full/v31/i40/111406.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i40.111406
Gastroesophageal variceal bleeding represents one of the most severe and life-threatening complications of portal hypertension with an estimated short-term mortality of around 17%-20% within six weeks[1,2]. Up to 85% of cirrhotic patients may develop esophageal varices (EV) during their life with an incidence of variceal bleeding that ranges from 5% to 15% per year[3,4]. Therefore, predicting the presence and severity of EV is essential in the diagnostic work up of cirrhotic patients. Early identification of patients at risk of this condition is based on non-invasive methods, such as transient elastography and blood platelets count, whereas endoscopic surveillance with esophagogastroduodenoscopy (EGD) is reserved for selected cases[5]. According to current guidelines both, endoscopic variceal band ligation (EVBL) and non-selective beta blockers (NSBB), could be employed as effective treatments in primary prophylaxis. In clinical practice, patients with non-invasive diagnosis of portal hypertension start treatment with NSBB without performing EGD. However, there is a subset of patients who are intolerant or present specific contraindications to pharmacological therapy that require endoscopic treatment. Unfortunately, EVBL is not always effective due to the persistence of residual varices after treatment and to date it is not possible to select these patients with non-invasive tools[3,6]. Thus, in order to minimize the risk of bleeding, a second EGD should be performed after 2-4 weeks, to identify and treat patients with persistent high-risk varices and/or with failure of endoscopic treatment[6,7].
Based on Baveno VII consensus, liver stiffness measurement (LSM) and spleen stiffness measurements (SSM) respectively by different ultrasonographic devices have already proven to be useful in determining the presence and degree of portal hypertension[5,8,9]. Moreover, this condition could be associated with hemodynamic changes in the liver and portal venous system. In particular, the transit time between hepatic vein and hepatic artery or portal vein (PV), detected by contrast enhanced ultrasound (CEUS), has been previously correlated to portal hypertension[10-13].
Finally, we have recently demonstrated that the combination of LSM and perfusion parameters obtained with dynamic CEUS (DCE-US) has an excellent accuracy for diagnosing clinically significant and severe portal hypertension, since both hepatic fibrosis and altered hepatic blood flow are involved in its pathogenesis[14].
We hypothesize that multiparametric ultrasound, combining the evaluation of tissue perfusion and stiffness, could reflect the hemodynamic alterations caused by EVBL and identify non-invasive parameters associated with successful variceal eradication after endoscopic treatment. The aim of the present study was therefore to evaluate the usefulness of multiparametric ultrasound to predict the short-term outcome of EVBL in patients with liver cirrhosis. Secondary aim was to develop a prediction model of successful variceal eradication based on non-invasive parameters.
Between January 2020 and February 2022 all consecutive patients with liver cirrhosis who were scheduled for EVBL for bleeding prophylaxis in our Department of Internal Medicine and Gastroenterology were enrolled in this prospective study. According to international guidelines, EGD was performed in cirrhotic patients with LSM ≥ 20 kPa and platelet count ≤ 150 × 109/L intolerant or with contraindications to NSBB[5]. Patients who underwent EVBL were selected. Additional inclusion criteria were age > 18 years and consent to EVBL. Patients were excluded if they had previous EVBL, history of decompensated liver disease (Child Pugh C or Child Pugh B with grade 2 or 3 ascites or overt hepatic encephalopathy), heart failure, malignant liver tumors, PV thrombosis, cerebrovascular disease, sepsis, transjugular intrahepatic porto-systemic shunt, liver transplantation, hypersensitivity to Sonovue®, treatment with vasoactive drugs within 2 weeks before the enrollment.
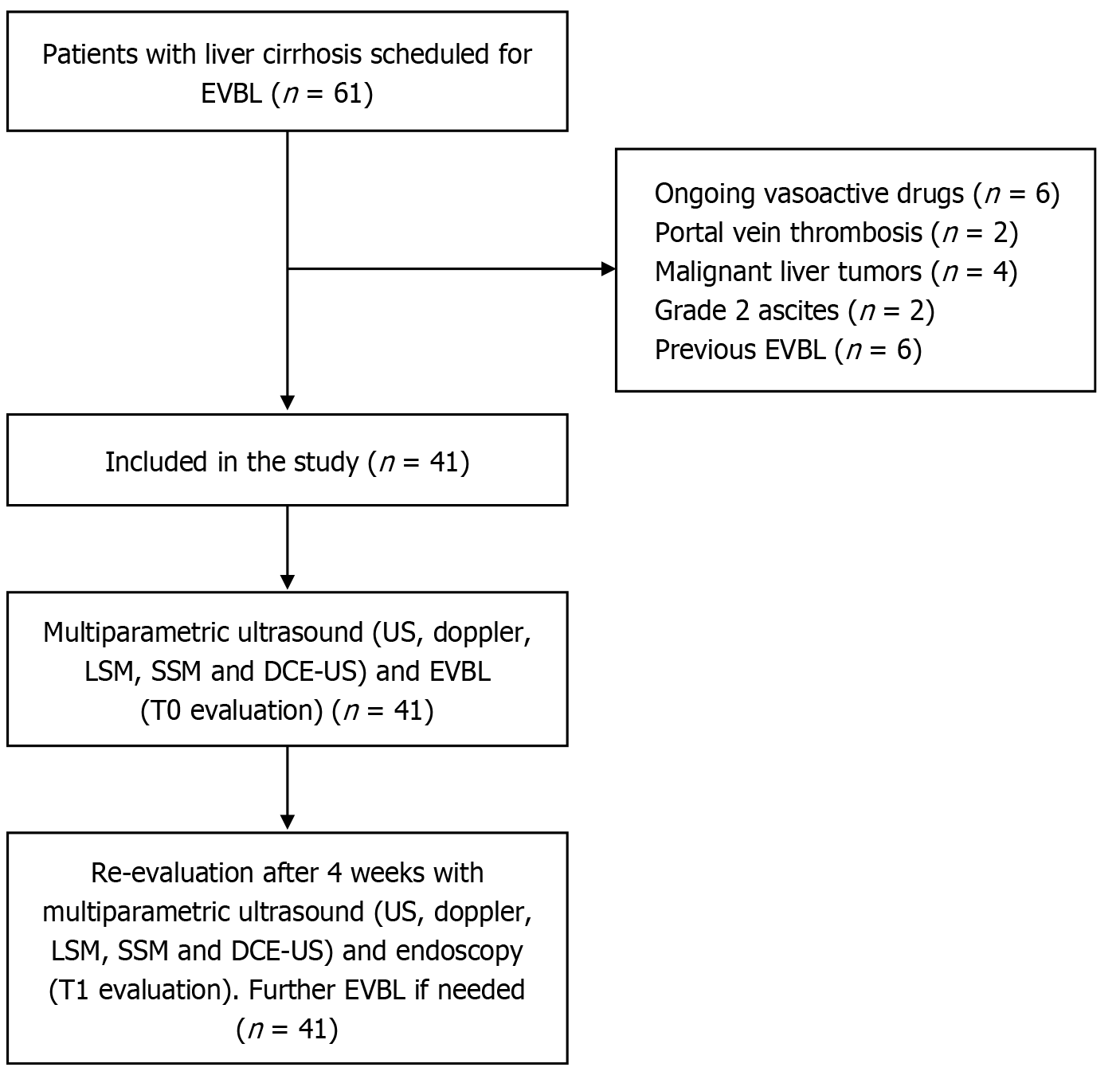
During the enrollment period 61 patients with liver cirrhosis underwent EVBL. Among them 20 participants did not enter the study because of ongoing treatment with vasoactive drugs (6 patients), PV thrombosis (2 patients), malignant liver tumors (4 patients), grade 2 ascites (2 patients) and previous EVBL (6 patients).
The presence and size of EV were characterized by EGD, and location, grade and the presence or absence of red color signs (RCS) were recorded. Patients with gastroesophageal varices categorized as varices grade 2 or RCS 2 or higher were considered at high risk of bleeding and therefore indicated for treatment.
According to clinical practice, patients who underwent EVBL were re-evaluated 4 weeks after treatment to assess the efficacy of treatment or the necessity of additional EVBL. The patients enrolled underwent multiparametric US with doppler, LSM, SSM and DCE-US immediately before endoscopy (T0) and 4 weeks after EVBL on the same day of control endoscopy (T1). The patient selection process and study procedures are summarized in Figure 1.
Liver cirrhosis severity was assessed by Child-Pugh[15] and model for end-stage liver disease (MELD) scores[16]. Based on endoscopic assessment at T1, patients were classified as responders (R) or non-responders (NR) considering the reduction of size and risk of bleeding. The study protocol was approved by the University Hospital “Agostino Gemelli” IRCCS Institutional Review Board (No. 2986) and registered at clinicaltrials.gov (No. NCT05789641). All patients provided written informed consent.
Doppler ultrasound, elastography and DCE-US studies were performed by two experienced physicians (Zocco MA and Ainora ME, respectively with 20 and 15 years of experience in liver imaging) with Aixplorer Mach 30 (SuperSonic Imagine, Aix-en-Provence, France) equipped with a wideband C6-1 convex probe (frequency range, 1.0-6.0 MHz). The examination included a color doppler examination according to a standardized protocol in order to obtain PV blood velocity, hepatic venous waveforms and damping index, hepatic artery resistivity index (RI), splenic artery (SA) RI and renal artery (RA) RI[17,18].
Two-dimensional shear-wave elastography (2D-SWE) was performed with the same equipment. Measurements were obtained with the patient in supine position and suspended normal breathing. The shear-wave measurement box was positioned on the right liver lobe (usually 5th or 6th segment) and on the spleen parenchyma free of large vessels and at least 2 cm below the capsule by moving the region of interest (ROI), perpendicular to the center of the transducer. We considered reliable only the cases with a stability index (SI) of at least 90%. In cases where the initial SI was < 90%, the measurement was repeated until a reliable SI was achieved. In accordance with international guidelines[19], the median value of at least 3 successful measurements was considered for analysis and was expressed in kilopascals (kPa). An interquartile range to the median ratio (medial interquartile range) < 30% was used as a measurement reliability criterion.
After doppler ultrasound and 2D-SWE evaluations, the operator obtained an intercostal scan of the right liver containing the right PV. Thereafter, a 2.4 mL solution of a second-generation ultrasound contrast agent (SonoVue®, Bracco) was injected as an intravenous bolus followed by a flush of 10 mL normal saline. A dedicated, contrast-specific, low mechanical index-technique (mechanical index = 0.08) was used to study the whole vascular phase. To avoid motion artefacts, patients were asked to maintain relaxed breathing during video clip acquisition.
Signal enhancement of liver parenchyma (LP) and PV was evaluated in real time and a dynamic sequence of 3 minutes was recorded continuously on a hard disk and exported as digital imaging and communications in medicine for further analysis.
Finally, digitized quantification of contrast uptake was performed using the quantitative analysis software package VueBox®, Version 7.4 (Bracco, Italy). The analysis can display mean, median and standard deviation of intensity pixel within the ROI drawn on the image for each frame of the sequence acquired. In our study, the time-intensity curves were generated from a manually defined ROI, placed over the LP, and a second ROI was drawn on the adjacent PV at the same level, avoiding large blood vessels.
Six perfusion parameters were extracted from time-intensity curves: Peak enhancement (PE) in arbitrary units (AU); Mean signal intensity (meanLin) in AU, wash-in rate (WiR), time to PE, in AU time to peak (in seconds); Mean transit time local (in seconds); Area under the curve (AUC) (in AU). The variations of each parameter between T0 and T1 was calculated as the difference between the value at two time points.
EGD was performed under moderate sedation according to clinical practice in our center[20]. EVs were diagnosed according to The European Society of Gastrointestinal Endoscopy and Baveno VII guidelines[5,6,21]. According to Japanese classification, EV were classified as F1 (small, straight varices of small caliber), F2 (moderately enlarged, tortuous varices occupying less than one-third of the esophageal lumen), and F3 (large, coil-shaped varices occupying more than one-third of the lumen). This system also includes the evaluation of RCS-such as red wale marks (longitudinal red streaks), cherry-red spots (discrete red lesions on variceal surfaces), and hematocystic spots (rare, blood-filled blisters) which are predictors of bleeding risk[22]. A multiband ligator device, consisting of a transparent cap with preloaded elastic bands (Cook Super Seven) attached to the endoscope’s tip, was used to obtain variceal obliteration[6].
Patients were divided into two groups based on VE at T1 (R and NR). Baseline characteristics and multiparametric US data were compared between the two groups.
The normality of the variables was tested using the Shapiro-Wilk test. Quantitative variables were reported as mean ± SD and compared using the Mann-Whitney U test. The association between categorical variables was assessed using Fisher exact test.
Variables showing significant differences in univariate analysis (P < 0.05) were selected for multivariate modeling. A logistic regression with least absolute shrinkage and selection operator (LASSO) regularization was applied to reduce dimensionality and prevent overfitting, given the limited sample size. Odds ratios (ORs), 95% confidence intervals, and P values were reported for each variable retained in the final logistic regression model.
Based on multivariate results, a point-based clinical score was constructed to stratify patients by VE likelihood. Each variable was dichotomized based on clinically or statistically meaningful thresholds and assigned a score of 1 (risk factor for NR) or -1 (protective factor for R). The total score ranged from -1 to 3. Two simplified 4-variable versions were derived.
Logistic regression models using the 9-variable set and each 4-variable score were trained and tested using a 70/30 stratified split. Model performance was evaluated with accuracy, area under the receiver operating characteristic curve (AUROC), sensitivity, specificity, positive predictive value. VE likelihood was also stratified by risk group according to the score (≤ 0: High likelihood of VE; 1: Intermediate risk; ≥ 2: High risk of no-VE) and observed VE rates were reported for each stratum.
All tests were two-sided, and a P value < 0.05 was considered statistically significant. All statistical analyses were conducted using R (version 4.2.2) and GraphPad Prism (version 9.0).
The study was completed in 41 patients (31 men, 10 women; mean age ± SD: 63 ± 7.5 years). Demographic and clinical data of the study population are provided in Table 1. At baseline (T0), patients who achieved VE (R, n = 28) had significantly lower MELD scores compared to those with persistent varices (NR, n = 13) (mean ± SD in R vs NR: 10.1 ± 2 vs 12.2 ± 1.8; P = 0.0025), and a significantly higher proportion of Child-Pugh class A (R vs NR: 75% vs 7.7%; P = 0.00023). Ascites was more frequently observed in NR group (R vs NR: 10.7% vs 53.8%; P = 0.009). At follow-up (T1), these differences remained significant, with patients in R group still exhibiting lower MELD scores (mean ± SD in R vs NR: 9.68 ± 2.13 vs 12.46 ± 1.94; P = 0.0003) and a more favorable Child-Pugh distribution (P = 0.00019). Regarding elastographic parameters, LSM was significantly lower in the R group at both time points (mean ± SD of LSM in R vs NR: 26.3 kPa vs 39.6 kPa, P = 0.013 at T0; 25.4 kPa vs 38.3 kPa, P = 0.0042 at T1) whereas SSM was significantly lower in the R group at T1 (mean ± SD of SSM in R vs NR: 43.3 kPa vs 52.8 kPa, P = 0.0213). No significant differences were observed in doppler or DCE-US parameters at this stage, although some variables, such as SA RI and LP enhancement features, showed non-significant trends.
| Characteristic | Responders (n = 28) | Non-responders (n = 13) | P value | |
| Age, years | 62.8 ± 7.8 | 64.5 ± 7.1 | 0.475 | |
| Sex, number, M/F | 21/7 | 10/3 | > 0.99 | |
| T0 | MELD | 10.1 ± 2 | 12.2 ± 1.8 | 0.0025 |
| Child (A/B/C), number | 21/7/0 | 1/12/0 | 0.00023 | |
| Spleen size, cm | 15.50 ± 2.74 | 15.79 ± 2.15 | 0.77 | |
| Ascites, yes/no | 3/25 | 7/6 | 0.009 | |
| Collateral vessels, yes/no | 6/22 | 4/9 | 0.797 | |
| SA RI | 0.71 ± 0.12 | 0.69 ± 0.05 | 0.73 | |
| LRA RI | 0.73 ± 0.17 | 0.69 ± 0.06 | 0.292 | |
| RRA RI | 0.71 ± 0.14 | 0.68 ± 0.05 | 0.325 | |
| SMA RI | 0.84 ± 0.09 | 0.84 ± 0.08 | 0.918 | |
| HA RI | 0.73 ± 0.11 | 0.69 ± 0.07 | 0.193 | |
| PVV, cm/second | 23.9 ± 5.79 | 22.5 ± 4.16 | 0.379 | |
| PVD, cm | 1.25 ± 0.17 | 1.27 ± 0.25 | 0.91 | |
| DI | 0.44 ± 0.30 | 0.49 ± 0.14 | 0.450 | |
| LSM, kPa | 26.32 ± 13.46 | 39.62 ± 15.07 | 0.013 | |
| SSM, kPa | 47.92 ± 8.54 | 52.03 ± 10.51 | 0.232 | |
| LP meanLin, AU | 409.6 ± 356.5 | 717.1 ± 669.2 | 0.140 | |
| LP PE, AU | 993.1 ± 920.1 | 1547.9 ± 1430.9 | 0.218 | |
| LP WiR, AU | 424.8 ± 879.0 | 1099.9 ± 2074.1 | 0.278 | |
| LP mTT, second | 276.3 ± 203.2 | 181.8 ± 203.4 | 0.179 | |
| LP TTP, second | 14.86 ± 7.73 | 11.59 ± 6.71 | 0.181 | |
| LP AUC, AU | 45808.7 ± 48761.3 | 45046.8 ± 40751.4 | 0.959 | |
| PV meanLin, AU | 284.4 ± 237.7 | 340.0 ± 238.2 | 0.493 | |
| PV PE, AU | 623.9 ± 582.9 | 772.7 ± 714.5 | 0.519 | |
| PV WiR, AU | 81.3 ± 91.6 | 114.7 ± 145.8 | 0.459 | |
| PV mTTI, second | 193.7 ± 214.1 | 254.4 ± 225.2 | 0.423 | |
| PV TTP, second | 23.71 ± 10.20 | 19.68 ± 6.96 | 0.149 | |
| PV AUC, AU | 30180.9 ± 31763.7 | 45136.8 ± 35684.0 | 0.210 | |
| T1 | MELD | 9.68 ± 2.13 | 12.46 ± 1.94 | 0.0003 |
| Child (A/B/C), number | 23/5/0 | 2/11/0 | 0.00019 | |
| Spleen size, cm | 15.5 ± 2.77 | 15.92 ± 2.25 | 0.611 | |
| Ascites, yes/no | 5/23 | 6/7 | 0.127 | |
| Collateral vessels, yes/no | 6/22 | 4/9 | 0.797 | |
| SA RI | 0.67 ± 0.07 | 0.76 ± 0.15 | 0.070 | |
| LRA RI | 0.69 ± 0.10 | 0.74 ± 0.13 | 0.249 | |
| RRA RI | 0.67 ± 0.06 | 0.74 ± 0.13 | 0.097 | |
| SMA RI | 0.84 ± 0.11 | 0.85 ± 0.05 | 0.745 | |
| HA RI | 1.01 ± 1.71 | 0.72 ± 0.11 | 0.373 | |
| PVV, cm/second | 26.66 ± 6.78 | 23.54 ± 5.19 | 0.279 | |
| PVD, cm | 12.38 ± 2.28 | 12.62 ± 1.45 | 0.690 | |
| DI | 0.46 ± 0.28 | 0.31 ± 0.42 | 0.274 | |
| LSM, kPa | 25.44 ± 10.14 | 38.33 ± 12.59 | 0.0042 | |
| SSM, kPa | 43.29 ± 11.65 | 52.77 ± 11.36 | 0.0213 | |
| LP meanLin, AU | 499.4 ± 444.8 | 521.7 ± 464.9 | 0.886 | |
| LP PE, AU | 1200.8 ± 1058.6 | 983.0 ± 807.9 | 0.474 | |
| LP WiR, AU | 481.4 ± 955.9 | 973.6 ± 1518.2 | 0.298 | |
| LP mTTI, second | 261.6 ± 200.9 | 195.6 ± 198.4 | 0.334 | |
| LP TTP, second | 12.07 ± 5.99 | 15.19 ± 8.68 | 0.191 | |
| LP AUC, AU | 42757.2 ± 43432.2 | 34202.5 ± 30970.2 | 0.477 | |
| PV meanLin, AU | 782.6 ± 2327.1 | 254.8 ± 209.6 | 0.244 | |
| PV PE, AU | 625.5 ± 619.6 | 589.7 ± 622.7 | 0.766 | |
| PV WiR, AU | 75.6 ± 62.3 | 89.8 ± 104.2 | 0.654 | |
| PV mTTI, second | 133.4 ± 150.5 | 256.3 ± 225.8 | 0.092 | |
| PV TTP, second | 22.52 ± 7.91 | 19.38 ± 7.83 | 0.245 | |
| PV AUC, AU | 32951.1 ± 33858.3 | 34981.5 ± 32055.2 | 0.854 |
The analysis of delta (Δ) variations between T1 and T0 revealed several parameters significantly associated with VE (Table 2). Responders showed a greater reduction in the SA RI (ΔSA RI mean ± SD in R vs NR: -0.037 ± 0.12 vs 0.069 ± 0.15; P = 0.033), left RA (LRA) RI (ΔLRA RI mean ± SD in R vs NR: -0.040 ± 0.13 vs 0.046 ± 0.12; P = 0.048), and right RA (RRA) RI (ΔRRA RI mean ± SD in R vs NR: -0.043 ± 0.12 vs 0.054 ± 0.11; P = 0.017). A significant reduction in PV diameter was also observed in patients who achieved VE (P = 0.036). As concerning elastography, patients in the R group showed a substantial decrease in SSM compared to NR (ΔSSM mean ± SD in R vs NR: -4.63 ± 6.71 kPa vs 0.74 ± 6.12 kPa; P = 0.018). Finally, DCE-US derived parameters showed strong discriminatory potential. In particular a significant increase in
| Responders (n = 28) | Non-responders (n = 13) | P value | |
| ΔMELD | -0.39 ± 1.26 | 0.23 ± 0.73 | 0.053 |
| Δ spleen size | -0.00 ± 0.69 | 0.12 ± 0.44 | 0.481 |
| ΔSA RI | -0.037 ± 0.12 | 0.069 ± 0.15 | 0.033 |
| ΔLRA RI | -0.040 ± 0.13 | 0.046 ± 0.12 | 0.048 |
| ΔRRA RI | -0.043 ± 0.12 | 0.054 ± 0.11 | 0.017 |
| ΔSMA RI | 0.005 ± 0.14 | 0.012 ± 0.05 | 0.829 |
| ΔHA RI | 0.284 ± 1.71 | 0.026 ± 0.12 | 0.434 |
| ΔPVV | 1.76 ± 5.53 | 1.05 ± 4.95 | 0.687 |
| ΔPVD | -0.30 ± 0.73 | 0.15 ± 0.55 | 0.036 |
| ΔDI | 0.02 ± 0.15 | -0.18 ± 0.43 | 0.132 |
| ΔLSM | -0.87 ± 8.03 | -1.30 ± 4.81 | 0.836 |
| ΔSSM | -4.63 ± 6.71 | 0.74 ± 6.12 | 0.018 |
| LP Δ meanLin | 89.80 (203.31) | -195.41 (236.54) | 0.000004 |
| LP ΔPE | 207.71 (492.36) | -564.87 (817.97) | 0.000002 |
| LP ΔWiR 1 | 56.60 (115.09) | -126.34 (1130.23) | 0.000354 |
| LP ΔmTT | -14.66 (131.99) | 13.77 (142.85) | 0.084885 |
| LP Δ TTP 1 | 0.55 (4.40) | 0.48 (2.41) | 0.093950 |
| LP Δ AUC | -3051.50 (37324.96) | -10844.24 (12302.78) | 0.000065 |
| PV Δ meanLin | 498.29 (2348.79) | -85.14 (95.27) | 0.000007 |
| PV ΔPE | 28.59 (279.21) | 183.01 (209.36) | 0.000147 |
| PV ΔWiR 1 | -5.70 (68.67) | -24.82 (42.41) | 0.000354 |
| PV ΔmTT | -50.22 (134.15) | 1.88 (48.14) | 0.034408 |
| PV ΔTTP 1 | -1.19 (6.78) | -0.30 (6.45) | 0.022410 |
| PE ΔAUC | 2770.26 (12605.83) | -10155.34 (15630.37) | 0.000052 |
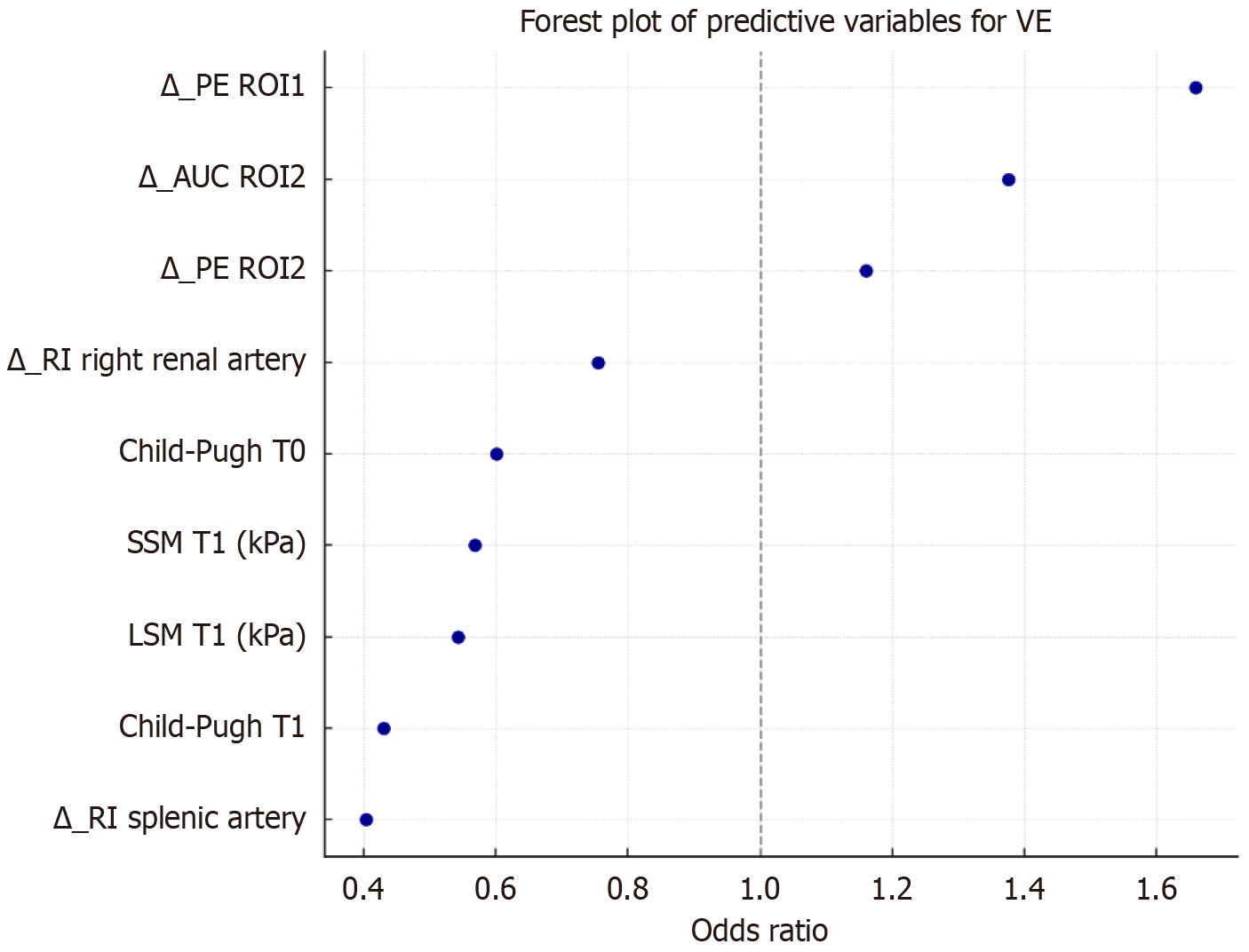
The variables that were significantly associated with VE at univariate analysis were combined in a multivariate logistic regression model. After the elimination of 14 collinear or non-contributory variables we selected 9 independent predictors of VE (Figure 2 and Table 3).
| LASSO coefficient | β coefficient | Odds ratio | |
| T1 Child-Pugh | -0.84 | -0.84 | 0.43 |
| PV ΔAUC | 0.34 | 0.32 | 1.38 |
| T1 LSM | -0.33 | -0.61 | 0.54 |
| LP ΔPE | 0.28 | 0.51 | 1.66 |
| T1 SSM | -0.16 | -0.57 | 0.57 |
| ΔSA RI | -0.14 | -0.91 | 0.40 |
| ΔRRA RI | -0.13 | -0.28 | 0.75 |
| T0 Child-Pugh | -0.11 | -0.51 | 0.60 |
| PV ΔPE | Approximately = 0 | 0.15 | 1.16 |
Among the retained predictors, higher Child-Pugh class, LSM and SSM at follow-up (T1) were strongly associated with a reduced probability of VE (OR = 0.43, 0.54 and 0.57, respectively). Similarly, a greater reduction in ΔSA RI and ΔRRA were correlated with failure of VE (OR = 0.40 and 0.75 respectively). In contrast, an increase in PV ΔAUC and LP ΔPE significantly predicted successful VE (OR = 1.38 and 1.66 respectively).
Finally, a low Child-Pugh score at baseline and an increase of PV ΔPE showed a moderate association with VE. These findings highlight the relevance of both stiffness measurements and dynamic changes of doppler and DCE-US parameters in predicting success in VE.
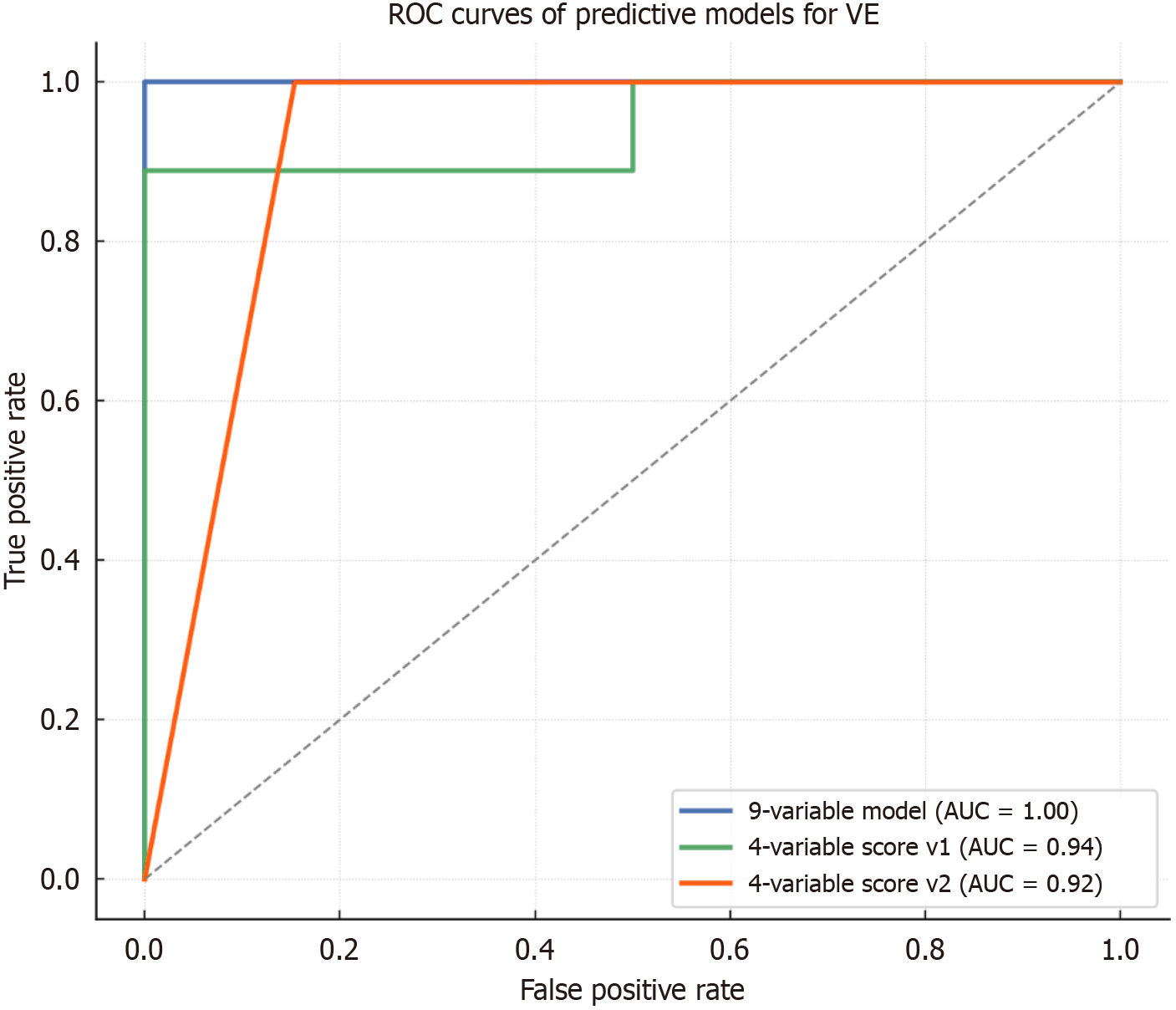
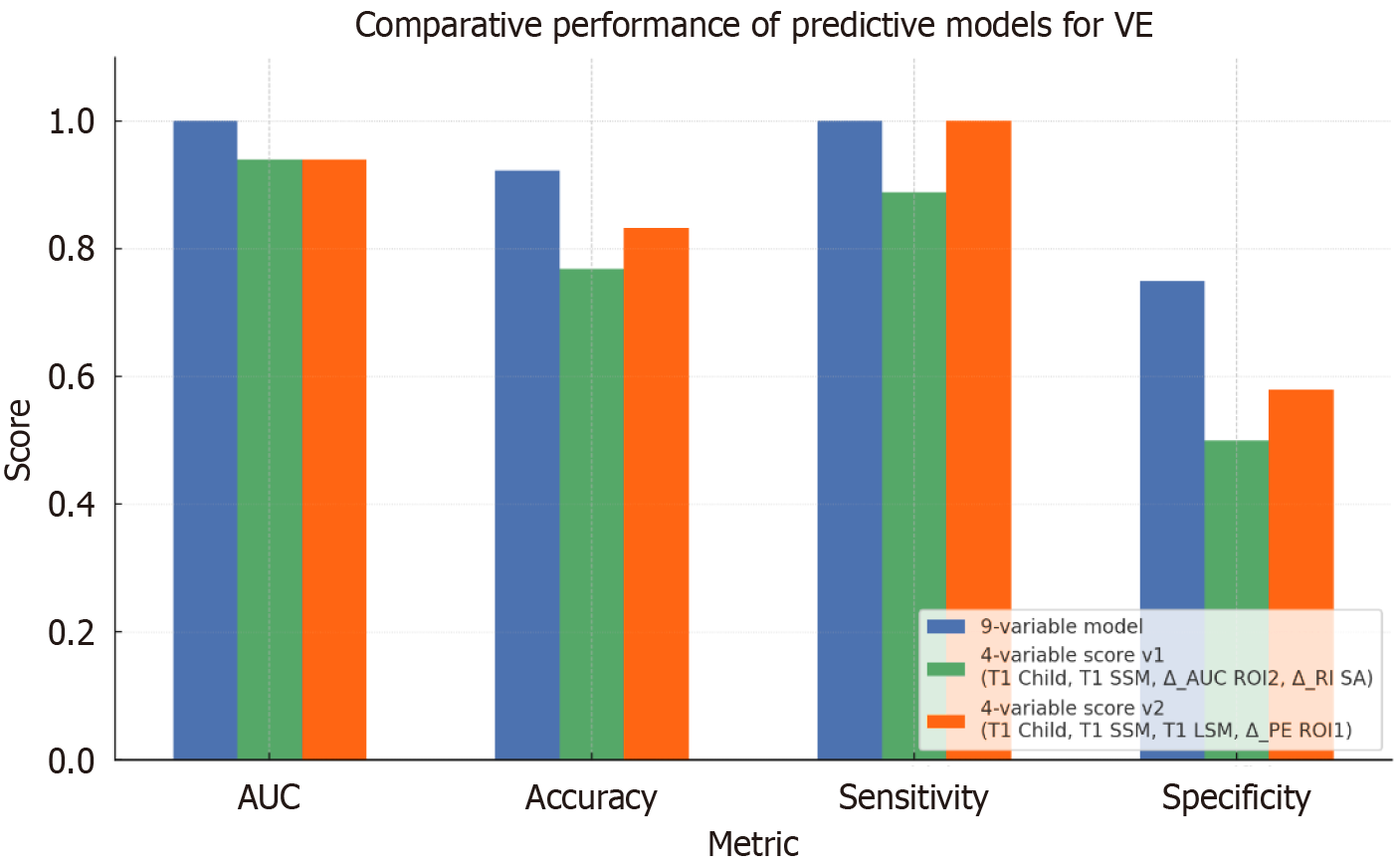
The final 9-variable model demonstrated an excellent performance, with an accuracy of 92.3%, a sensitivity of 100%, a specificity of 75% and an AUROC of 1.00 (Figure 3). Based in this multivariate model, a point-based clinical score was developed by assigning 1 to variables associated with VE failure (e.g., Child-Pugh ≥ B at T1, SSM ≥ 50 kPa at T1 and ΔSA RI ≤ -0.05) and -1 to protective features (e.g., LP ΔPE ≥ 10 AU, PV ΔAUC ≥ 30 AU) (Table 4). The composite score effectively stratified patients by response likelihood. In particular a score ≤ 0 was associated with 100% probability of VE, 1-2 points corresponded to intermediate risk (VE rate 80%), and a score ≥ 3 points identified patients at high risk of treatment failure (VE rate 14.3%).
| Predictor | Points |
| Child-Pugh T1 ≥ B | 1 |
| LSM T1 ≥ 20 kPa | 1 |
| SSM T1 ≥ 50 kPa | 1 |
| ΔSA RI ≤ -0.05 | 1 |
| ΔRRA RI ≤ -0.03 | 1 |
| LP ΔPE ≥ 10 AU | -1 |
| PV ΔAUC ≥ 30 AU | -1 |
| PV ΔPE ≥ 5 AU | -1 |
| Child-Pugh T0 ≥ B | 1 |
In consideration of the limited sample size (n = 40), a full 9-variable logistic model may be prone to overfitting. Therefore, two simplified multivariate models were developed using four representative, non-collinear predictors with strong statistical and clinical relevance to maintain predictive accuracy.
The first simplified model (model v1) included Child-Pugh class and SSM at T1, ΔSA RI and PV ΔAUC. This model achieved an overall accuracy of 76.9%, an AUROC of 0.94, 88.9% sensitivity, and 50% specificity. A corresponding point-based score was constructed, assigning 1 to high-risk features (e.g., Child-Pugh B/C, SSM ≥ 50 kPa, ΔSA RI ≤ -0.05) and -1 to protective markers (PV ΔAUC ≥ 30 AU). According to this score patients with values ≤ 0 had a 100% VE rate, patients with 1-2 points were at intermediate risk (VE rate 33.3%), and subjects with ≥ 3 points were at high risk of treatment failure (VE rate 28.6%).
The second model (model v2) replaced doppler and PV DCE-US parameters with LSM at T1 and LP ΔPE), alongside Child-Pugh class and SSM (Figures 3 and 4). This model achieved higher diagnostic performance, with 83.3% accuracy, 100% sensitivity, 57.9% specificity and an AUROC of 0.94. The derived score showed excellent discriminative ability: A score ≤ 0 was associated with 100% VE rate, a score of 1 corresponded to intermediate risk (VE rate 77.8%), and a score ≥ 2 indicated high risk of treatment failure (VE rate 0%). The etiology of the underlying cirrhosis and the presence of previous decompensation episodes did not influence the performance of the score.
Gastroesophageal variceal bleeding represents the most dangerous complication of portal hypertension and often requires repeated invasive procedures to minimize bleeding risk. It is well established that the grade and the bleeding risk of EV are directly proportional to the entity of the underlying hepatic venous pressure gradient (HVPG), which also defines clinically significant portal hypertension[3,5]. In our study, non-invasive indicators of clinically significant portal hypertension and liver decompensation were found to be predictive of failure to achieve VE after one session of EVBL. Indeed, patients with treatment failure exhibited significantly higher levels of Child-Pugh and MELD scores, higher LSM and more frequent presence of ascites at baseline. These findings suggest that patients with more advanced liver disease or with signs of severe portal hypertension at the time of EVBL could be considered intrinsically at risk of treatment failure.
Another notable finding is represented by the lower SSM found at follow-up (T1) in patients who were successfully treated. SSM has been shown to correlate more closely with HVPG than LSM and it is considered a reliable biomarker to identify clinically significant portal hypertension[5,23,24]. Furthermore, SSM has already shown a potential utility as a biomarker of treatment response in patients with portal hypertension, particularly in those undergoing transjugular intrahepatic porto-systemic shunt[25,26]. However, it is known that EVBL is not able to modify portal pressure gradient[3,27]. Thus, if the observed reduction in SSM after a successful EVBL is validated by larger studies, it would represent a distinctive characteristic of this parameter and may reflect local hemodynamic changes independent from HVPG.
Our study also explored the application of DCE-US for the prediction of VE. Previous studies investigating quantitative parameters from CEUS mainly focused on the time of arrival of the contrast media to PV and hepatic artery to predict the existence and obliteration of varices[13,28]. We examined the time-intensity curves related to the contrast transit through LP and PV, and their modifications after EVBL. Particularly, patients achieving VE showed a significant raise in PE, WiR, and meanLin of both LP and PV compared to those with treatment failure. These changes that reflect the maximum signal intensity and the total amount of contrast agent passing through the ROI, may underline local hemodynamic variations in liver perfusion following EVBL. Therefore, they could be useful for the noninvasive evaluation of variceal ligation outcomes.
We also noted modest but statistically significant reductions in the SA RI and RA RI in patients with successful treatment. While these findings may reflect systemic hemodynamic changes, they should be interpreted cautiously, given the complex interplay of factors influencing doppler measurements and their operator-dependent nature.
To identify predictive factors for VE, we developed multivariate logistic regression models. The multivariate logistic regression using LASSO regularization identified nine independent predictors of VE, including LSM, SSM, Child-Pugh class at T1, and longitudinal DCE-US parameters variations between T0 and T1. The resulting model achieved excellent performance (AUC = 1.00), but its complexity and the presence of several operator-dependent variables may limit direct clinical application. The two simplified models could overcome this limitation, and particularly the second model, which includes Child-Pugh class, LSM and SSM at T1 as well as LP ΔPE, seems to be the most promising for clinical application. In fact, this model relies on highly reproducible variables and showed an excellent discriminative ability (AUROC = 0.94, sensitivity = 100%).
Despite these promising results, this study presents several limitations. First of all, it was a proof-of-concept and monocentric study conducted on a small sample size, with potential selection bias. Therefore, these findings should be validated in larger cohorts of patients. Second, the predominance of patients with Child-Pugh class A or lower-range class B in our cohort may have contributed to the unexpected high prevalence of successful EVBL after just one session and limits the generalizability of our results. In fact, the selected parameters could present a different behavior in Child-Pugh class C patients due to the higher grade of liver decompensation and portal hypertension. Moreover, there were no episodes of further decompensation among the first and the second evaluation of the study, and thus our results may not be generalizable to patients experiencing an episode of decompensation after the first EVBL. Similarly, since our cohort did not include patients with gastric varices, we cannot evaluate their influence on the proposed scores.
Additionally, we excluded patients in therapy with NSBB, which represent the cornerstone in the therapy of portal hypertension[5]. The hemodynamic modifications induced by NSBB in liver and systemic circulation may affect doppler and DCE-US parameters, representing an additional limitation in extrapolating our results to the real-world cirrhotic population. Finally, the US techniques are associated with intrinsic limitations that could interfere with adequate examination such as patients’ characteristics (body habitus, the presence of bowel gas, etc.) and the operator dependency. This is of particular concern for the DCE-US, which requires operators with more specific expertise than that required for a standard US examination. Moreover, for the quantitative analysis of time-intensity curves, current software rely on manual tracing of the ROI, contributing to further subjectivity. In this study we did not assess interobserver variability between the two operators involved in ultrasound acquisitions. However, we have previously demonstrated a good to excellent interobserver agreement in ROI tracing and subsequent perfusion parameters calculation in a comparable clinical setting[29]. Moreover, these limitations could be overcome by the development of standardized protocols of image acquisition and the diffusion of ultrasound machines able to perform quantitative DCE-US analysis in real-time.
Despite these limitations, our findings support the hypothesis of a potential role of noninvasive tools for the prediction of EVBL outcome. In particular, our proposed model based on multiparametric US, that combines elastographic measurements with longitudinal changes in DCE-US parameters, could be useful for preliminary risk stratification. Specifically, the implementation of this technique in clinical practice could be based on a two-steps approach: At the time of EVBL, a baseline multiparametric ultrasound evaluation combined with clinical data (e.g., Child-Pugh class or liver stiffness) could help stratify patients according to their risk of treatment failure. Those with high-risk features could undergo routine follow-up EGD. In contrast, a second ultrasound assessment, performed after EVBL, could be used in low-to-intermediate risk patients to monitor treatment response, avoiding non necessary endoscopic screening in the remaining patients. This stepwise evaluation could be based on a multiparametric score integrating key ultrasound-derived parameters, such as liver and spleen stiffness and variations in DCE-US measurements. For example, a spleen stiffness value below a determined threshold or a measurable decrease from baseline, together with an increase in PV and LP AUC or in PV PE, potentially combined with the other parameters proposed by our study, may predict successful EVBL. However, further larger studies should explore the feasibility of this approach and validate our preliminary results.
In conclusion, our study explored the use of multiparametric US for the noninvasive evaluation of the outcome of EVBL in patients with liver cirrhosis and the development of a predictive model for treatment success. We identified associations between EVBL success and ultrasound-derived parameters such as liver and spleen stiffness values, a reduction of spleen stiffness and longitudinal changes in DCE-US parameters, which could be integrated into multiparametric scores to predict variceal eradication. While preliminary, our findings support the potential of a non-invasive approach based on elastographic and hemodynamic parameters. If these results will be confirmed by larger and multicenter studies, the proposed approach could become an interesting option for the first line evaluation of endoscopic treatment.
Thanks to Fondazione Roma for the continuous support to our scientific research.
| 1. | Jakab SS, Garcia-Tsao G. Evaluation and Management of Esophageal and Gastric Varices in Patients with Cirrhosis. Clin Liver Dis. 2020;24:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Tapper EB, Beste L, Curry M, Bonder A, Waljee A, Saini S. Suboptimal Implementation of Evidence-based Therapy for Acute Variceal Hemorrhage: A Systematic Review of Observational Studies. Clin Gastroenterol Hepatol. 2017;15:1373-1381.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1500] [Article Influence: 166.7] [Reference Citation Analysis (3)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1985] [Article Influence: 248.1] [Reference Citation Analysis (2)] |
| 5. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1848] [Article Influence: 462.0] [Reference Citation Analysis (2)] |
| 6. | Gralnek IM, Camus Duboc M, Garcia-Pagan JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H, Hernandez-Gea V, Tantau M, Ebigbo A, Ibrahim M, Vlachogiannakos J, Burgmans MC, Rosasco R, Triantafyllou K. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:1094-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 7. | Wang J, Chen S, Naga YM, Liu J, Dai M, Yang S, Wang L, Ye B. Esophageal Variceal Ligation Monotherapy versus Combined Ligation and Sclerotherapy for the Treatment of Esophageal Varices. Can J Gastroenterol Hepatol. 2021;2021:8856048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6418] [Article Influence: 802.3] [Reference Citation Analysis (9)] |
| 9. | De Gottardi A, Sempoux C, Berzigotti A. Porto-sinusoidal vascular disorder. J Hepatol. 2022;77:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 10. | Kim MY, Suk KT, Baik SK, Kim HA, Kim YJ, Cha SH, Kwak HR, Cho MY, Park HJ, Jeon HK, Park SY, Kim BR, Hong JH, Jo KW, Kim JW, Kim HS, Kwon SO, Chang SJ, Baik GH, Kim DJ. Hepatic vein arrival time as assessed by contrast-enhanced ultrasonography is useful for the assessment of portal hypertension in compensated cirrhosis. Hepatology. 2012;56:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Zhang CX, Hu J, Hu KW, Zhang C, Wang L, Xu JM. Noninvasive analysis of portal pressure by contrast-enhanced sonography in patients with cirrhosis. J Ultrasound Med. 2011;30:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Jeong WK, Kim TY, Sohn JH, Kim Y, Kim J. Severe portal hypertension in cirrhosis: evaluation of perfusion parameters with contrast-enhanced ultrasonography. PLoS One. 2015;10:e0121601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Li J, Feng JC, Peng XY, Wu XW, Du TT, Wang JJ, Tian SX, Lu GL. Usefulness of Contrast-Enhanced Ultrasonography for Predicting Esophageal Varices in Patients with Hepatitis B Virus (HBV)-Related Cirrhosis. Med Sci Monit. 2017;23:2241-2249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zocco MA, Cintoni M, Ainora ME, Garcovich M, Lupascu A, Iezzi R, Annichiarico BE, Siciliano M, Riccardi L, Rapaccini GL, Grieco A, Pompili M, Gasbarrini A. Noninvasive Evaluation of Clinically Significant Portal Hypertension in Patients with Liver Cirrhosis: The Role of Contrast-Enhanced Ultrasound Perfusion Imaging and Elastography. Ultraschall Med. 2023;44:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 16. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3778] [Article Influence: 151.1] [Reference Citation Analysis (2)] |
| 17. | Kim MY, Baik SK, Park DH, Lim DW, Kim JW, Kim HS, Kwon SO, Kim YJ, Chang SJ, Lee SS. Damping index of Doppler hepatic vein waveform to assess the severity of portal hypertension and response to propranolol in liver cirrhosis: a prospective nonrandomized study. Liver Int. 2007;27:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Sacerdoti D, Gaiani S, Buonamico P, Merkel C, Zoli M, Bolondi L, Sabbà C. Interobserver and interequipment variability of hepatic, splenic, and renal arterial Doppler resistance indices in normal subjects and patients with cirrhosis. J Hepatol. 1997;27:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Igea F, Casellas JA, González-Huix F, Gómez-Oliva C, Baudet JS, Cacho G, Simón MA, De la Morena E, Lucendo A, Vida F; Spanish Society of Digestive Endoscopy. Sedation for gastrointestinal endoscopy. Endoscopy. 2014;46:720-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 558] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Miyaaki H, Ichikawa T, Taura N, Miuma S, Isomoto H, Nakao K. Endoscopic management of esophagogastric varices in Japan. Ann Transl Med. 2014;2:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 23. | Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 24. | Jachs M, Odriozola A, Turon F, Moga L, Téllez L, Fischer P, Saltini D, Kwanten WJ, Grasso M, Llop E, Mendoza YP, Armandi A, Thalhammer J, Pardo C, Colecchia A, Ravaioli F, Maasoumy B, Laleman W, Presa J, Schattenberg JM, Berzigotti A, Calleja JL, Calvaruso V, Francque S, Schepis F, Procopet B, Albillos A, Rautou PE, García-Pagán JC, Puente Á, Fortea JI, Reiberger T, Mandorfer M; SSM-100Hz/ACLD Study Group; Baveno Cooperation. Spleen stiffness measurement by vibration-controlled transient elastography at 100 Hz for non-invasive predicted diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a modelling study. Lancet Gastroenterol Hepatol. 2024;9:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Reiberger T. The Value of Liver and Spleen Stiffness for Evaluation of Portal Hypertension in Compensated Cirrhosis. Hepatol Commun. 2022;6:950-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | Buechter M, Manka P, Theysohn JM, Reinboldt M, Canbay A, Kahraman A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018;50:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Tevethia HV, Pande A, Vijayaraghavan R, Kumar G, Sarin SK. Combination of carvedilol with variceal band ligation in prevention of first variceal bleed in Child-Turcotte-Pugh B and C cirrhosis with high-risk oesophageal varices: the 'CAVARLY TRIAL'. Gut. 2024;73:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Furuichi Y, Moriyasu F, Sugimoto K, Taira J, Sano T, Miyata Y, Sofuni A, Itoi T, Nakamura I, Imai Y. Obliteration of gastric varices improves the arrival time of ultrasound contrast agents in hepatic artery and vein. J Gastroenterol Hepatol. 2013;28:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G, Rapaccini GL, Landolfi R, Siciliano M, Pompili M, Gasbarrini A. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/